Abstract
Background:
Integrated addiction treatment in HIV clinics is associated with improved outcomes yet is offered inconsistently and with variable models of care. We sought to evaluate the impact of Implementation Facilitation (“Facilitation”) on clinician and staff preference for provision of addiction treatment in HIV clinics with on-site resources (all trained or designated on-site specialist) vs. outside resources (outside specialist or refer out).
Methods:
From July 2017 to July 2020, surveys assessed clinician and staff preferences for addiction treatment models during control (i.e., baseline), intervention, evaluation, and maintenance phases in four HIV clinics in the Northeast United States.
Results:
During the control phase, among 76 respondents (response rate=58%), the proportion that preferred treatment with on-site resources for opioid use disorder (OUD), alcohol use disorder (AUD), and tobacco use disorder (TUD) was 63%, 55%, and 63%, respectively. Compared to control, there were no significant differences in preferred model during the intervention and evaluation phases except for AUD where there was an increased preference for treatment with on-site resources in the intervention vs. control phase. Compared to control, during the maintenance phase, a higher proportion of clinicians and staff preferred providing addiction treatment with on-site resources vs. outside resources: OUD: 75% (OR [95% CI] =1.79 (1.06, 3.03)]; AUD: 73% (OR [95% CI] =2.23 [1.36, 3.65]); and TUD: 76% (OR [95% CI] =1.88 [1.11,3.18].
Conclusion:
The findings from this study lend support for “Facilitation” as a strategy to enhance clinician and staff preference for integrated addiction treatment in HIV clinics with on-site resources.
Keywords: Implementation facilitation, HIV, Tobacco, Alcohol, Opioids, Addiction, Substance use
INTRODUCTION
Evidence-based addiction treatments can be integrated into HIV care settings to improve HIV and substance use-related outcomes.1–5 These treatments include buprenorphine and injectable naltrexone for opioid use disorder (OUD); naltrexone, acamprosate and disulfiram for alcohol use disorder (AUD); and nicotine replacement therapy, bupropion and varenicline for tobacco use disorder (TUD).
Despite evidence, guidelines, and unmet needs, addiction treatment is inconsistently provided in HIV clinical settings.6–8 How clinician and staff preferences regarding the preferred model for integrating addiction treatment into HIV clinical settings vary based on substance and change with implementation efforts is not known.9 To address this literature gap, we conducted a secondary analysis of data from the Working with HIV clinics to adopt Addiction Treatment using Implementation Facilitation (WHAT-IF?) study.10
METHODS
WHAT-IF? was a four-site stepped wedge clinical trial with a hybrid type 3 effectiveness-implementation approach to evaluating the impact of Implementation Facilitation (“Facilitation”) on promoting provision of evidence-based OUD, AUD and TUD care. The protocol,10 results from the formative evaluation,11 and primary outcome analyses are available elsewhere.12 Following a baseline formative evaluation, Facilitation activities during the intervention period primarily consisted of 1) academic detailing targeting clinicians’ knowledge of evidence-based treatments followed by 2) learning collaboratives including case-based learning, as well as efforts to 3) promote program marketing, 4) stimulate and support local champions, and 5) promote processes for audit and feedback.10 After crossing over from the control period to 6-month intervention period, sites then entered a 6-month evaluation period, followed by the maintenance period that lasted for the duration of the study. Learning collaboratives and as needed consultations continued during the evaluation and maintenance phases. Institutional Review Boards at Yale University and each participating site approved the study protocol.
Study activities occurred at: 1) Haelen Center at Yale‐New Haven Hospital, New Haven, CT; 2) the Community Care Center at Hartford Hospital’s HIV Clinic, Hartford, CT; 3) The Miriam Hospital Immunology Center, The Miriam Hospital, Providence, RI; and 4) The Special Treatment and Research (STAR) Health Center, SUNY Downstate Health Sciences University, Brooklyn, NY. All clinicians and staff at each of the participating clinics who had been employed at the given site for ≥6 months at the time of survey collection were invited to complete a confidential, web based Qualtrics™ survey. Surveys were administered every 6 months (July 2017 to July 2020), starting prior to initiation of Facilitation activities at any sites (i.e., control), and then during each of the following study periods (i.e., intervention, evaluation, maintenance).
Clinicians and staff were asked to indicate their preferred model of care for improving addiction treatment in their HIV clinic in response to: “In your opinion, which approach do you think would be most feasible to improve treatment for [opioid/alcohol/tobacco] use disorder?” Response options included: each clinician provides treatment to patients on their panel for the given substance use disorder (“all trained”); one current clinician is appointed as the specialist (“designated onsite specialist”); a specialist is brought into the clinic (“outside onsite specialist”); no treatment is provided onsite, and patients are referred out (“refer out”); or other (option for free text).10
Responses were categorized into a two-level variable: preferring provision of addiction treatment at the clinic with on-site resources (defined as endorsing “all trained” or “designated onsite specialist” or both) vs. outside resources (defined as endorsing “outside onsite specialist” or “refer out”) as the study’s overall goal was to improve integrated care delivered by in-house clinicians. Two researchers (SBM and EJE) independently reviewed “other” free text responses into one of the two categories; discrepancies were resolved by discussion with other team members to reach consensus.
Analyses were conducted with an intention‐to‐treat approach based on the time clinics were intended to cross over from control condition to Facilitation. Generalized estimating equation (GEE) models were used with fixed effects for site, site by study phase interaction, and natural time to generate adjusted odds ratios (OR) and associated 95% confidence intervals (95% CI) to measure the effect of Facilitation on preference of on-site care at each study period (i.e., intervention, evaluation, maintenance) compared to the control period; compound symmetry working correlation matrix was specified to control for correlation of repeated measures within subjects. Two-sided p values <0.05 were considered statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Among all invited clinicians and staff, 76/131 (58%) responded to the survey in the control phase, 70/126 (56%) in the intervention phase, 81/123 (65%) in the evaluation phase and 95/133 (71%) in the maintenance phase. During the control phase, the proportion that preferred provision of addiction treatment with on-site resources for OUD, AUD, and TUD was 63%, 55%, and 63%, respectively (Table 1). Compared to the control phase, preference to provide addiction treatment with on-site resources only increased for AUD (71% (OR [95% CI] = 1.98 [1.13,3.49]) during the intervention phase (Table 1 and Figure 1)). By the maintenance phase, the proportion of clinicians and staff who preferred provision of addiction treatment with on-site resources vs. outside resources had increased for all addiction treatments compared to control phase: OUD: 75% (OR [95% CI] =1.79 (1.06, 3.03)]; AUD: 73% (OR [95% CI] =2.23 [1.36, 3.65]); and TUD: 76% (OR [95% CI] =1.88 [1.11,3.18] (Figure 1). No other significant findings were observed.
Table 1:
Proportion of clinicians and staff preference for integrating addiction treatment with on-site resources vs. outside resources
| Preference of treatment by substance use disorder and study phase | OUD | AUD | TUD | |||
|---|---|---|---|---|---|---|
| Percent | n | Percent | n | Percent | n | |
| Control | 62.5% | 76 | 55% | 76 | 63.1% | 76 |
| Intervention | 67.7% | 70 | 70.8% | 70 | 61.8% | 69 |
| Evaluation | 66.7% | 81 | 64.9% | 81 | 73.9% | 81 |
| Maintenance | 74.9% | 94 | 73.2% | 95 | 76.3% | 95 |
Note: OUD=opioid use disorder; AUD=alcohol use disorder; TUD=tobacco use disorder
Figure 1:
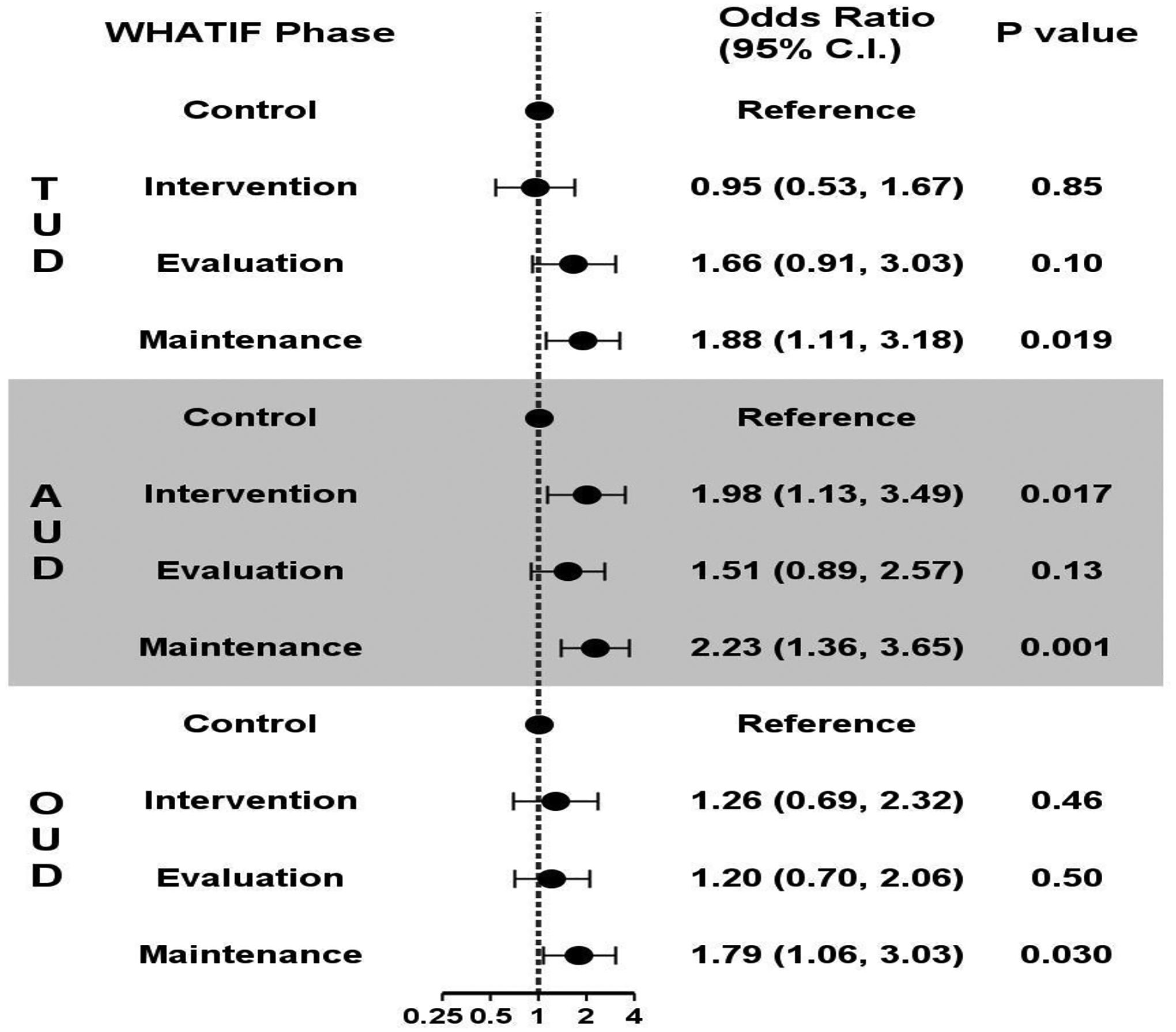
Preference for integrating addiction treatment with on-site resources vs. outside resources at each phase of study compared to control period, results from generalized estimating equations models*
*TUD=tobacco use disorder, AUD=alcohol use disorder, OUD=opioid use disorder. Adjusted for site, site by study phase interaction, and natural time.
Discussion
To our knowledge, this is the first study to examine clinician and staff preferences for delivering addiction treatments in HIV clinics and changes in response to Facilitation. Results show that Facilitation activities, including academic detailing and learning collaboratives, can influence stated preferences of clinicians and staff in models for addiction treatment delivery. While the evaluation phase (six months following intervention) did not show significant change, there was a trend in the positive direction with multi-component facilitation interventions. With ongoing collaborative learning in the maintenance phase, there was significant improvement in clinician and staff preferences to integrate addiction treatments. Despite these interventions, one in four continued to prefer outside referral or onsite treatment with outside resources. Another stepped wedge study in HIV clinics that looked at academic detailing and peer to peer education showed significant improvement in the confidence and readiness to prescribe buprenorphine in the six-month post intervention phase followed by sustained improvement in the 12-month follow up. There were no meaningful improvements in buprenorphine prescribing.13
Further research should focus on understanding the barriers to preferring onsite treatment. Our baseline formative evaluation identified difficulty with determining treatment eligible patients, variable experiences with prior medications for addiction treatment, perceived complexity of treatments, perceived need for robust behavioral services, and inconsistency in availability of trained onsite specialists as barriers.11
Our study has limitations. The COVID-19 pandemic caused major disruptions to health care systems that may also have affected willingness to adopt new treatments in clinical settings during the final period of study observation. The study was conducted in four urban HIV treatment centers in the Northeast US, potentially limiting generalizability. Although the clinicians and staff were told that the surveys were anonymous, there could have been social desirability bias.
Conclusion
Our findings lend support for Facilitation as a strategy for enhancing clinician and staff support for integrated addiction treatment delivery for in HIV clinics with on-site resources. Future efforts that evaluate different approaches for delivering addiction treatment with onsite resources are needed.
Acknowledgments
Drs. Muvvala, Edelman and Fiellin had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: Muvvala, Dziura, Esserman, Fiellin, Edelman. Acquisition, analysis, or interpretation of data: Muvvala, Gan, Dziura, Esserman, Porter, Chan, Cornman, Reynolds, Yager, Morford, Edelman, Fiellin. Drafting of the manuscript: Muvvala. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Gan, Dziura, Esserman, Reynolds. Obtained funding: Fiellin. Administrative, technical, or material support: Porter, Cornman, Reynolds, Yager, Morford, Fiellin. Supervision: Edelman, Yager, Chan, Cornman, Fiellin.
Source of funding:
This work was funded by the National Institute on Drug Abuse (grant #R01DA041067) and the National Center for Advancing Translational Sciences (UL1 TR001863). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
ClinicalTrials.gov Identifier: NCT02907944
Conflict of Interest Disclosures: Dr. Muvvala reported receiving personal fees from Alkermes for participating in an advisory board meeting in 2020 outside the submitted work. Dr. Chan is also on staff at the Rhode Island Department of Health and the Rhode Island Public Health Institute; to the best of our knowledge, there are no additional conflicts of interest, financial or otherwise, exist.
References
- 1.Oldfield BJ, Muñoz N, Boshnack N, et al. “No more falling through the cracks”: A qualitative study to inform measurement of integration of care of HIV and opioid use disorder. J Subst Abuse Treat. 2019;97:28–40. [DOI] [PubMed] [Google Scholar]
- 2.Altice FL, Bruce RD, Lucas GM, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56 Suppl 1(Suppl 1):S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartzler B, Dombrowski JC, Williams JR, et al. Influence of Substance Use Disorders on 2-Year HIV Care Retention in the United States. AIDS Behav. 2018;22(3):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health. 2017;5(6):e578–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40(10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldfield BJ, McGinnis KA, Edelman EJ, et al. Predictors of initiation of and retention on medications for alcohol use disorder among people living with and without HIV. J Subst Abuse Treat. 2020;109:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahrir S, Crothers K, McGinnis KA, et al. Receipt and predictors of smoking cessation pharmacotherapy among veterans with and without HIV. Prog Cardiovasc Dis. 2020;63(2):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyse JJ, Robbins JL, McGinnis KA, et al. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chander G, Monroe AK, Crane HM, et al. HIV primary care providers--Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016;161:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelman EJ, Dziura J, Esserman D, et al. Working with HIV clinics to adopt addiction treatment using implementation facilitation (WHAT-IF?): Rationale and design for a hybrid type 3 effectiveness-implementation study. Contemp Clin Trials. 2020;98:106156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman EJ, Gan G, Dziura J, et al. Readiness to provide medications for opioid, alcohol and tobacco use disorder in HIV clinics: A multi-site mixed-methods formative evaluation. J Acquir Immune Defic Syndr. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelman EJ, Gan G, Dziura J, et al. Effect of Implementation Facilitation to Promote Adoption of Medications for Addiction Treatment in US HIV Clinics: A Randomized Clinical Trial. JAMA Netw Open. 2022;5(10):e2236904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jawa R, Walley AY, Wilson DJ, et al. Prescribe to Save Lives: Improving Buprenorphine Prescribing Among HIV Clinicians. J Acquir Immune Defic Syndr. 2022;90(5):546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]


