Abstract
Motile group N streptococci, classified as Vagococcus fluvialis, have been isolated from cows’ udders, human and animal feces, river water, and seawater. They possess an unusual membrane lipid and fatty acid pattern. We isolated and characterized 13 polar lipids, 8 of them also found in other gram-positive bacteria: mono- and dihexosyldiacylglycerol, an acylated and a glycerophosphate-substituted derivative of the latter, cardiolipin, phosphatidylglycerol, d-alanylphosphatidylglycerol, and l-lysylphosphatidylglycerol. Besides them, we characterized two rare compounds, bis(acylglycero)phosphate and α-d-glucopyranosylcardiolipin, and two compounds so far not detected in nature, d-alanylbis(acylglycero)phosphate and d-alanylcardiolipin. The concomitant occurrence of four aminoacyl phospholipids in one organism is another unique finding. Substituted cardiolipins represent a novel lipid class: in vagococci, d-alanylcardiolipin is a major membrane lipid component, contributing 11 and 26 mol% of total lipids in the exponential and stationary phases of growth, respectively. The vagococcal lipids contain even-numbered straight-chain saturated and cis-monounsaturated fatty acids, but the cis-monoenic acids belong to the ω-9 series and not the ω-7 series, found in enterococci, lactococci, and streptococci.
Chemical and molecular systematic studies have recently been done to clarify the phylogenetic relationship of the group N streptococci (42, 43). Nucleic acid hybridization studies and immunological relationships of superoxide dismutases demonstrated that “Streptococcus lactis” and its subspecies are closely related to each other but not to other streptococci, which led to the formation of a new genus, Lactococcus (43). When during this study the membrane lipids were investigated, group N streptococcus strain Kiel 48809 displayed a pattern that differed greatly from the lipid pattern of the S. lactis group. This strain, which had been isolated from a cow’s udder in Germany (19a), was motile and formed a group with other motile group N streptococci (NCDO 2497, NCDO 2498, and NCDO 2499) (43) which had been isolated in Japan from feces of humans and animals and from river water and seawater (20, 21). Although the motile strains possess the group N antigen, they are not genetically related to Lactococcus or to other streptococci examined. The polar-lipid patterns and their long-chain fatty acid compositions reinforced their distinctiveness and, along with genetic data, suggested that these strains may represent the nucleus of a new taxon (43). This was confirmed by 16S RNA sequence analyses which located the motile group N streptococci on a phylogenetic tree and led to their classification in a new genus, Vagococcus, as Vagococcus fluvialis sp. nov. (7). By using seven isolates, molecular characterization was done and evidence of a possible connection between V. fluvialis and human infections was provided (46).
In this report, we describe the polar-lipid pattern of V. fluvialis, which was the same for all the four strains investigated. We isolated and characterized 13 polar lipids and found, in addition to the ubiquitous membrane lipids of gram-positive bacteria, rare and so-far-unknown structures. Of particular interest is d-alanylcardiolipin, which is a major component among the membrane lipids of vagococci. It is a novel representative of the lipid class of substituted cardiolipins. Other examples are α-d-glucopyranosylcardiolipin, found in this study and earlier in group B streptococci (10), and l-lysylcardiolipin, isolated from species of the genus Listeria (16a). So far, substituted cardiolipins have been found only in gram-positive bacteria.
MATERIALS AND METHODS
Materials.
Reference glyceroglycolipids and phospholipids were isolated and characterized in previous work (10, 11, 15, 29, 35). Enzymes and cosubstrates were purchased from Boehringer Mannheim GmbH and Sigma Aldrich Chemie GmbH, and the reference fatty acids were obtained from Sigma Aldrich Chemie.
Bacteria and growth.
The motile group N streptococci Kiel 48809, NCDO 2497, NCDO 2498, and NCDO 2499 were from previous work (43). The bacteria were stored at −80°C in growth medium containing 10% (mass/vol) glycerol. The growth medium contained, per liter, 7.5 g of yeast extract, 12.5 g of casein peptone, 5 g of sodium citrate, 5 g of NaCl, 5 g of K2HPO4, 0.14 g of MgCl2, 0.8 g of MgSO4, 0.04 g of FeSO4, and 15 g of glucose. The medium was sterilized by filtration. Twenty-liter batches were inoculated with 50 ml of an overnight culture, and the bacteria were grown at 37°C with moderate stirring and without aeration. Growth was monitored by measuring optical density at 578 nm (OD578) and pH. Bacteria were harvested in the exponential phase of growth (OD578 ≈ 0.8) or in the stationary phase of growth (OD578 ≈ 2.5) with a refrigerated continuous-flow centrifuge.
Extraction and purification of lipids.
All extraction and purification steps were performed at 4°C and at low pH (0.1 M sodium acetate, pH 4.7 [buffer A]). Immediately after harvesting, the bacteria were suspended in buffer A (10 g [wet weight]/25 ml) and disintegrated with glass beads in a Braun disintegrator as described elsewhere (14). After removal of the glass beads by filtration, the lipids were extracted by a modified Bligh-Dyer procedure (24) in which water was replaced by buffer A containing 0.2% (mass/vol) BaCl2. The crude lipid extracts were fractionated by column chromatography on DEAE-cellulose, as described in Results. Ammonium acetate-containing fractions (CHCl3-MeOH, 2:1 [by volume]) were extracted twice each with a one-fourth volume of 0.9% (mass/vol) NaCl containing 0.2% CaCl2. Purified lipids were taken several times to dryness with benzene, dissolved in CHCl3-MeOH (2:1 [by volume], slightly acidified with acetic acid), and stored at −20°C.
Analytical procedures.
For compositional analysis, lipids were hydrolyzed in 2 M HCl (100°C, 2.5 h). Fatty acids were extracted with light petroleum-chloroform (4:1, by volume). Amounts of d-galactose (4), d-glucose (28), glycerol (34), sn-glycero-3-phosphate (30), and phosphorus (44) were measured as described in the references. Glycerol, released by hydrolysis as glycerophosphate, was measured after treatment with acid phosphomonoesterase (2.5 U/ml of 0.05 M citrate buffer [pH 5.5], 37°C, 4 h). d-Alanine was measured by a specific enzymatic procedure (19). It separated clearly from l-alanine on a stereospecific high-performance liquid chromatography (HPLC) procedure (6). l-Lysine was distinguished from the d- form and measured by this HPLC procedure (6) by using taurine as an internal standard.
Fatty acids were determined essentially as described by Moss et al. (33). Washed cells were heated in 0.5 M NaOH in 50% aqueous methanol at 100°C for 30 min. The mixture was acidified with HCl to pH 2, and the fatty acids were extracted twice with CHCl3-petroleum benzene (1:4 [by volume]). The extract was washed with water and dried. After addition of 10% BCl3 (Merck), the mixture was heated for 5 min at 85°C. A fivefold volume of water was added, and the fatty acid methyl esters were extracted with petroleum benzene. Unsaturated fatty acid methyl esters were differentiated by gas-liquid chromatography (GLC) by cochromatography with standards (methyl esters of cis-9- or trans-9-hexadecenoate or of cis-9-, trans-9-, cis-11-, and trans-11-octadecenoate) on two fused silica capillary columns, HP-5 (5% diphenylpolysiloxane–95% dimethylpolysiloxane; 25 m; internal diameter, 0.32 mm; film thickness, 0.33 μm) and DB 225 (50% cyanopropylmethyl–50% methylphenyl-polysiloxane; 30 m; internal diameter, 0.24 mm; film thickness, 0.25 μm), which were run isothermally at 180 and 150°C, respectively. cis-Isomers emerged ahead of the respective trans-isomers from the apolar column (HP-5); on the polar column (DB 225), the order was reversed. For location of the double bonds, the unsaturated species in the fatty acid methyl ester mixture were cis dihydroxylated with Woodward’s reagent and analyzed as trimethylsilyl derivatives by GLC-mass spectrometry (MS) (32) by using the HP-5 capillary column isothermally at 205°C. GLC-MS was performed as described elsewhere (2).
For quantification of fatty acids, di-O-heptadecanoyl glycerophosphocholine was added as an internal standard, the mixture was hydrolyzed with HCl (2 M, 100°C, 3 h), and the fatty acids were extracted and methylated as described above.
Thin-layer chromatography (TLC) was performed on silica gel plates (Merck 60). The following solvents (by volume) were used: solvent A, chloroform-methanol-water, 65:25:4; solvents B through D, chloroform-acetone-methanol-acetic acid-water, 50:20:10:10:4, 55:20:10:10:3, and 80:50:10:10:4, respectively; solvent E, chloroform-methanol-acetic acid-water, 80:18:12:5; solvent F, propanol-pyridine-water, 7:4:2; solvent G, propan-2-ol–25% (mass/vol) ammonia–water, 7:2:2; solvent H, propanol–25% (mass/vol) ammonia–water, 6:3:1; solvent I, petroleum benzene-diethylether-acetic acid, 40:60:4; solvent K, butanol-acetic acid-water, 60:15:15; and solvent L, chloroform-methanol, 9:1. Deacylated phospholipids were identified by TLC on cellulose plates (Merck) by using solvent H and the Hanes-Isherwood reagent for visualization. The references to the staining methods can be found in previous work (10, 17). Preparative TLC was performed as described elsewhere (11).
Mild alkaline deacylation.
Mild alkaline deacylation was performed according to the method of Kates (24).
Enzymic hydrolyses.
Deacylated glycolipids were treated with α-galactosidase (1 U/ml) and/or α-glucosidase (5 U/ml) in 0.2 M sodium acetate, pH 6, containing 1.35 mM EDTA at 30°C overnight.
Hydrolysis of phospholipids with stereoselective phospholipase A2 (9) from hog pancreas was carried out as described elsewhere (10).
Selective phosphodiester cleavage.
Hydrolysis in 98% (vol/vol) acetic acid (100°C, 30 to 90 min) cleaves phosphodiester bonds via a cyclic intermediate on an adjacent hydroxyl and leaves fatty acid ester, amino acid ester, and glycosidic bonds intact (10, 15). On prolonged heating, amino acid esters are also hydrolyzed (this work). After hydrolysis, acetic acid was removed by repeated evaporation with CCl4. Water-soluble and lipid products were separated by phase partitioning.
Selective amino acid ester cleavage.
Aminoacylphospholipid (200 to 500 nmol) was dissolved in 0.5 ml of CHCl3-MeOH (2:1 [by volume]), containing 0.01% (mass/vol) Triton X-100. The mixture was taken to dryness, suspended in 0.1 M sodium borate, pH 9.5 (1 ml), and incubated at 37°C for 4 to 24 h. The lipid products were extracted with CHCl3. Fatty acid ester and phosphodiester remain intact.
Stereochemical analysis of glycerophosphates.
Deacylated phospholipids (200 to 500 nmol) were hydrolyzed in 0.5 M NaOH (100°C, 2 h). The hydrolysates were passed through small columns (Pasteur pipettes) of cation-exchange resin, NH4+, and taken to dryness. α-Glycerophosphate was measured after periodate oxidation and subsequent hydrazinolysis as inorganic phosphate (18, 31), sn-glycero-3-phosphate was quantified enzymatically (30), and sn-glycero-1-phosphate was calculated as the difference between α-glycerophosphate and sn-glycero-3-phosphate.
RESULTS
Polar-lipid composition.
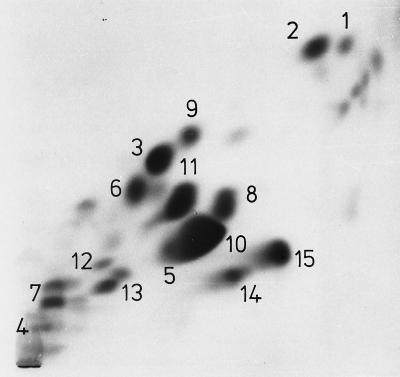
Figure 1 shows a chromatogram of the polar lipids of the type strain NCDO 2497. Identification and relative abundances of the individual lipids during exponential growth and in the stationary phase of growth are summarized in Table 1. The motile group N streptococcus Kiel 48809 and the reference collection strains NCDO 2498 and NCDO 2499 displayed the same lipid pattern as the type strain.
FIG. 1.
Polar-lipid pattern of V. fluvialis NCDO 2497 during exponential growth. For identification of the lipids, see Table 1. Lipids 14 and 15 reacted neither with the Dittmer-Lester reagent for lipid phosphorus nor with 1-naphthol–H2SO4 for carbohydrate and were not further studied. Two-dimensional TLC was performed on silica gel plates (Merck 60). The first dimension (upward) was developed with solvent A, and the second dimension was developed with solvent C. Visualization was done with iodine vapor. For quantitative determination, lipids 5 and 10 were separated in solvent E.
TABLE 1.
Composition of membrane lipids of exponentially growing and stationary-phase cells of the type strain NCDO 2497a
| Com- pound | Proposed structure | Abundance (mol%) at phase:
|
|
|---|---|---|---|
| Exponential | Stationary | ||
| 1 | Glc(α1-3)acyl2Gro | 1.7 | 0.5 |
| 2 | Acyl[Gal(α1-2)Glc(α1-3)]acyl2Gro | 3.8 | 0.2 |
| 3 | Gal(α1-2)Glc(α1-3)acyl2Gro | 8.8 | 12.5 |
| 4 | sn-Gro-1-P-6Gal(α1-2)Glc(α1-3)]acyl2Gro | NDc | ND |
| 5 | Phosphatidylglycerol | 4.3 | 5.8 |
| 6 | d-Ala-phosphatidylglycerolb | 9.8 | 4.5 |
| 7 | l-Lys-phosphatidylglycerolb | 10.1 | 21.2 |
| 8 | Bis(acylglycero)phosphate | 6.5 | 7.9 |
| 9 | d-Ala-bis(acylglycero)phosphate | 4.3 | 2.7 |
| 10 | Bis(phosphatidyl)glycerol (cardiolipin) | 37.4 | 12.0 |
| 11 | d-Ala-cardiolipin | 10.6 | 26.4 |
| 12 | α-d-Glc-cardiolipin | 0.5 | 3.7 |
| 13 | Lysocardiolipin | 1.9 | 2.6 |
The sugars belong to the d- series and are in the pyranose form.
d-Ala- and l-Lys-phosphatidylglycerol migrate as double spots (Fig. 1), presumably isomers carrying the aminoacyl residue at different positions (O-1 and O-2) of the glycerol moiety.
ND, not determined.
Fatty acid composition.
As shown in Table 2, the four Vagococcus strains had identical fatty acid compositions of even-numbered straight-chain saturated and cis-monounsaturated types. The fatty acid pattern of vagococci compared to that of Lactococcus lactis strains is characterized by the absence of cis-11,12-methylene octadecenoic acid, a larger fraction of octadecenoic and hexadecenoic acids, and, most notably, by the different location of the cis double bonds: predominantly Δ5-14:1, Δ7-16:1, and Δ9-18:1 (oleic acid) versus Δ9-16:1 and Δ11-18:1 (cis-vaccenic acid) in L. lactis strains. The same fatty acid composition, including the absence of cis-11,12-methylene octadecenoic acid, was observed when the fatty acids were released by alkaline methanolysis (24). In order to prove the location of the double bonds, the unsaturated species in the fatty acid methyl ester mixture were dihydroxylated, the dihydroxy derivatives were trimethylsilylated, and the whole mixture was analyzed by GLC-MS (32). As shown in Table 3, the major and minor isomers were characterized by ions at m/z 215 and m/z 187, respectively. These ions represent the fragments containing the CH3 terminus and therefore prove that the major isomers belong to the ω-9 series and the minor isomers belong to the ω-7 series. The increasing m/z values in both columns represent the fragments containing the carboxy methyl termini.
TABLE 2.
Percentages of long-chain fatty acids of V. fluvialis and L. lactisa
| Strain | % Fatty acid
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cis-Δ5 14:1 | 14:0 | cis-Δ7 16:1 | cis-Δ9 16:1 | 16:0 | cis-Δ9 18:1 | cis-Δ11 18:1 | 18:0 | 19cy | 20:1 | |
| V. fluvialis | ||||||||||
| Kiel 48809 | 2.2 | 4.7 | 12.1 | 4.1 | 21.0 | 44.6 | 3.5 | 5.5 | 0 | 2.2 |
| NCDO 2497 | 2.2 | 4.6 | 12.6 | 4.0 | 21.3 | 43.7 | 3.1 | 5.8 | 0 | 2.6 |
| NCDO 2498 | 2.3 | 4.7 | 12.6 | 4.4 | 21.9 | 42.5 | 2.8 | 6.6 | 0 | 2.5 |
| NCDO 2499 | 2.6 | 6.1 | 13.6 | 4.2 | 23.8 | 38.9 | 2.7 | 6.1 | 0 | 1.9 |
| L. lactis NCDO 712 | 0 | 7.7 | 1.0 | 1.9 | 41.7 | 0.9 | 11.7 | 3.3 | 30.6 | 0 |
The fatty acids were released from crude lipid extracts with HCl and converted to methyl esters as described in the text. The abbreviations of fatty acids are illustrated by the following examples: 18:0, octadecanoic acid; 18:1, octadecenoic acid; 19cy, cis-11,12 methyleneoctadecanoic acid. 0, not detected.
TABLE 3.
GLC-MS analysis of the trimethylsilyl derivatives of cis-dihydrooxylated monounsaturated fatty acids from vagococcia
| Fatty acid |
m/z of isomer
|
|
|---|---|---|
| Major | Minor | |
| 14:1 | 203, 215 | 231, 187 |
| 16:1 | 231, 215 | 259, 187 |
| 18:1 | 259, 215 | 287, 187 |
The isomers of the same chain length did not separate, but, as shown by MS analysis, the ω-9 isomer appeared in the ascending part of the peak, accompanied by small amounts of the ω-7 isomer in the descending part. For abbreviations of fatty acids, see footnote a to Table 2.
Palmitoleic acid and oleic acid were synthesized by the bacteria, because after growth in a chemically defined fatty acid-free medium (48) both fatty acids were unchangeably demonstrable.
Purification of lipids.
Crude lipid extracts were fractionated by column chromatography on DEAE-cellulose as summarized in Table 4. Purification of individual lipids was achieved by chromatography on small columns of silica gel (latrobeads) and/or by preparative TLC (data not shown).
TABLE 4.
Fractionation of a crude lipid extract from stationary-phase cells of the type strain, NCDO 2497, by column chromatography on DEAE-cellulose acetatea
| Fraction | Eluant
|
Effluent
|
||
|---|---|---|---|---|
| CHCl3/MeOH ratio (vol/vol) | NH4OAc (g/100 ml) | Phosphorus (μmol) | Lipidb | |
| 1 | 1:0 | <1 | Pigments | |
| 2 | 95:5 | 1 | 1, 2, 3 | |
| 3 | 9:1 | 42 | 3, 7 | |
| 4 | 8:1 | 16 | 3, 7 | |
| 5 | 2:1 | 9 | 7, polar pigments | |
| 6 | 2:1 | 0.05 | 17 | 5, 6, 9, 11 |
| 7 | 2:1 | 0.1 | 141 | 5, 9, 11 |
| 8 | 2:1 | 0.4 | 92 | 8, 10, 12, 14, 15 |
| 9 | 2:1 | 0.6 | 20 | 4, 8, 10, 12, 13 |
| 10 | 2:1 | 0.8 | 4 | 4 |
The column was 2.5 by 20 cm, and 350 μmol of lipid phosphorus was applied. Elution was performed at a flow rate of 200 ml · h−1 by a stepwise gradient of MeOH in CHCl3, followed by a stepwise gradient of ammonium acetate in CHCl3-MeOH (2:1, by volume). Fractions of 200 ml were collected and analyzed for phosphorus and lipid composition (one-dimensional TLC, solvents A and B).
For structures of the lipids, see Table 1. Boldface indicates the elution maximum of the respective lipid.
Glyceroglycolipids.
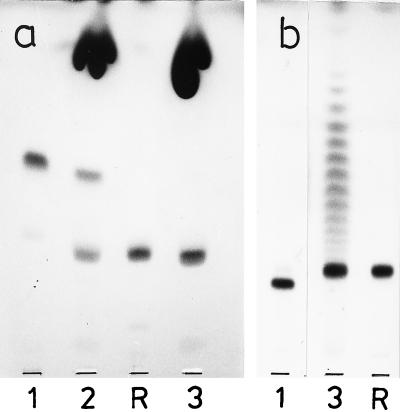
Lipids 1 to 3 had the composition of diacylglyceroglycolipids, and lipid 2 contained in addition a third sugar-linked fatty acid (Table 5). Figures 2 and 3 show the chromatographic mobilities of the purified glycolipids and their deacylation products in comparison with those of reference compounds. Deacylated lipid 1 had the same mobility as Glc(α1-3)Gro and was readily hydrolyzed by α-glucosidase. The deacylation products of lipids 2 and 3 were chromatographically identical, had the same mobility as Gal(α1-2)Glc(α1-3)Gro (Fig. 3), and were hydrolyzed in sequence by α-galactosidase and α-glucosidase with intermediate formation of Glc(α1-2)Gro. The structure of the disaccharide moiety was confirmed by methylation analysis (2), which yielded equimolar amounts of 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl galactitol and 1,2,5-tri-O-acetyl-3,4,6-tri-O-methyl glucitol. The structures proposed for lipids 1 to 3 are shown in Table 1. The location of the third fatty acid of lipid 2 remains to be established. In previously studied acyldihexosyl diacylglycerols, the third fatty acid was uniformly linked to O-6 of the glycerol-linked hexosyl moiety (15, 17, 35, 36).
TABLE 5.
Compositions of the glyceroglycolipidsa
| Lipid | Ratio for:
|
||||
|---|---|---|---|---|---|
| d-Galactose | d-Glucose | Glycerol | Fatty acids | Phosphorus | |
| 1 | None | 1.1 | 1 | 2.0 | None |
| 2 | 1.0 | 1.0 | 1 | 2.8 | None |
| 3 | 0.9 | 0.9 | 1 | 1.9 | None |
| 4 | 0.9 | 0.9 | 2.1 | 2.1 | 1.0 |
Values are molar ratios with respect to glycerol or phosphorus.
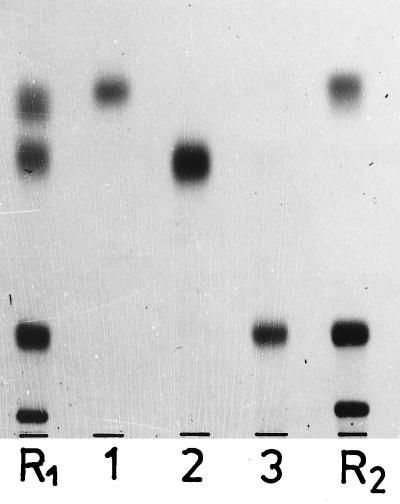
FIG. 2.
Chromatographic behavior of the purified glyceroglycolipids 1 to 3, in comparison with the following reference lipids (from top to bottom): R1, Glc(α1-3)acyl2Gro, Glc(α1-2),acyl-6Glc(α1-3)acyl2Gro, Glc(α1-2)Glc(α1-3)acyl2Gro, and Glc(α1-2)Glc(α1-2)Glc(α1-3)acyl2Gro; R2, Glc(α1-3)acyl2Gro, Gal(α1-2)Glc(α1-3)acyl2Gro, and Glc(β1-6)Gal(α1-2)Glc(α1-3)acyl2Gro. TLC was performed on silica gel plates (Merck 60) by using solvent D, and visualization was done with 1-naphthol–H2SO4.
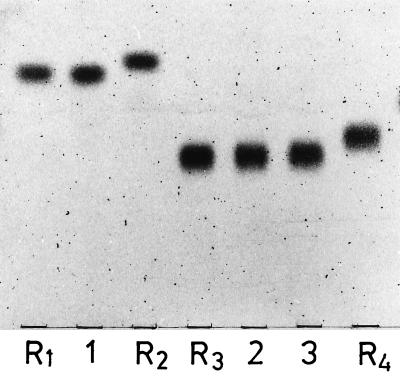
FIG. 3.
Chromatographic behavior of deacylated glyceroglycolipids 1 to 3 in comparison with the following reference glycosylglycerols: R1, Glc(α1-3)Gro; R2, Glc(α1-2)Gro; R3, Gal(α1-2) Glc(α1-3)Gro; R4, Glc(α1-2)Glc(α1-3)Gro. TLC was performed on silica gel plates (Merck 60) by using solvent F, and visualization was done with 1-naphthol–H2SO4.
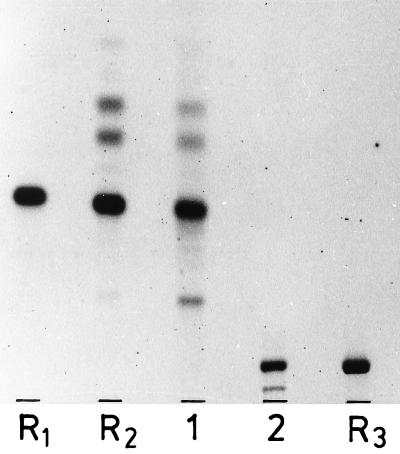
Lipid 4 had the composition (Table 5) and the chromatographic mobility of a glycerophosphodihexosyldiacylglycerol (Fig. 4). Rapid reaction with the periodate-Schiff reagent (45) suggested a terminal nonsubstituted 1(3)-glycerophosphate residue. Hydrolysis with 98% (vol/vol) acetic acid (100°C, 1 h) resulted in the formation of water-soluble glycerophosphates and a glycolipid which had the chromatographic mobility of Gal(α1-2)Glc(α1-3)acyl2Gro (Fig. 4). The deacylation product became susceptible to hydrolysis with α-galactosidase after the glycerophosphate moiety had been removed by alkali hydrolysis. The α-glycerol phosphate, released by alkali, did not react with sn-glycero-3-phosphate dehydrogenase, which suggests the presence of sn-glycero-1-phosphate in the parent compound. In order to locate the point of attachment on the galactopyranosyl residue, we followed a previous protocol (18): periodate oxidation of deacylated lipid 4, followed by treatment at pH 10 (β-elimination) and subsequent hydrazinolysis, released 80% of the total phosphorus as inorganic phosphate. No inorganic phosphate was formed when the treatment at pH 10 was omitted. We interpret this result to indicate that the glycerophosphate was linked to O-6 of the galactopyranosyl residue. It was oxidized to glycolaldehyde phosphate, and this was released from the oxidized sugar moiety at pH 10 by β-elimination and converted by hydrazinolysis to inorganic phosphate. We propose for lipid 4 the structure sn-Gro-1-P-6Gal(α1-2)Glc(α1-3)acyl2Gro.
FIG. 4.
Hydrolysis of glycerophosphoglycolipid 4 with 98% (vol/vol) acetic acid (100°C, 30 min). Lanes 1 and 2, lipid 4 after and before treatment with acetic acid, respectively; lanes R1 and R2, Gal(α1-2)Glc(α1-3)acyl2Gro before and after treatment with acetic acid, respectively; lane R3, GroP-6Glc(α1-2)Glc(α1-3)acyl2Gro. The two faster-moving bands in lane R2 are partially acetylated derivatives; the more slowly moving band in lanes 1 and 2 is the monodeacyl derivative. TLC was performed on silica gel plates (Merck 60) by using solvent A, and visualization was done with 1-naphthol–H2SO4.
Phosphatidylglycerol, bis(acylglycero)phosphate, and aminoacyl derivatives.
Lipids 5 through 9 contained on average 2 mol of glycerol and 2 mol of fatty acid ester per mol of phosphorus (Table 6). Lipids 6 and 9 contained in addition approximately 1 mol equivalent of d-alanine ester, and lipid 7 contained 1 mol equivalent of l-lysyl ester. Lipid 5, but not lipids 6 through 9, showed the reaction with the periodate-Schiff reagent for terminal glycol groups (45), which suggests that, in contrast to lipid 5, lipids 6 through 9 did not contain an unsubstituted glycerophosphate residue. When by mild alkaline treatment (pH 9.5, 37°C, 4 h) the alanine and lysine esters were released from lipids 6 and 7, in both cases a single ninhydrine-negative lipid appeared which had the chromatographic mobility of phosphatidylglycerol (solvents C and E) and reacted positively with the periodate-Schiff reagent. Deacylation of lipids 5 through 9 resulted in the formation of one and the same compound which had the chromatographic mobility of glycerophosphoglycerol (solvent H).
TABLE 6.
Characterization of phosphatidylglycerol (lipid 5) and bis(acylglycero)phosphate (lipid 8) and their aminoacyl derivatives (lipids 6, 7, and 9)
| Lipid | Molar ratio to phosphorus
|
sn-Gro-3-Pa α-Gro-P | |||
|---|---|---|---|---|---|
| Glycerol | Fatty acids | d-Ala | l-Lys | ||
| 5 | 2.0 | 2.2 | 0.5 | ||
| 6 | 2.0 | 1.7 | 0.9 | 0.45 | |
| 7 | 2.0 | 2.0 | 1.0 | 0.4 | |
| 8 | 2.0 | 1.9 | 0.6 | ||
| 9 | 2.0 | 1.9 | 0.9 | 0.6 | |
Glycerophosphates, released from deacylated lipids by NaOH (0.5 M, 100°C, 2 h). For interpretation, see the text.
The deacylation products of lipids 5 through 9 were subjected to alkali hydrolysis (0.5 M NaOH, 100°C, 2 h), which cleaves phosphodiester bonds via a cyclic intermediate on an adjacent hydroxyl to give a 3:2 mixture of β-and α-glycerophosphate. Since in alkali no phosphate migration occurs (1), the released α-glycerophosphates retain the stereochemical configuration of the glycerophosphate residue in the parent compound (13, 16). As shown in Table 6, in all cases approximately one-half of the released α-glycerophosphate reacted with sn-glycero-3-phosphate dehydrogenase; therefore, the other half was the sn-1 isomer. This result indicates sn-glycero-3-phospho-sn-1-glycerol as the basic structure for all five lipids. Lipid 5 and the aminoacyl-free derivatives of lipids 6 and 7 were susceptible to fatty acid ester cleavage by the stereospecific phospholipase A2 from hog pancreas (9), which enables the sn-3 configuration to be assigned to the phosphatidyl moiety. Lipids 5, 6, and 7 are therefore identified as phosphatidylglycerol and the d-alanyl and l-lysyl derivatives of it, respectively.
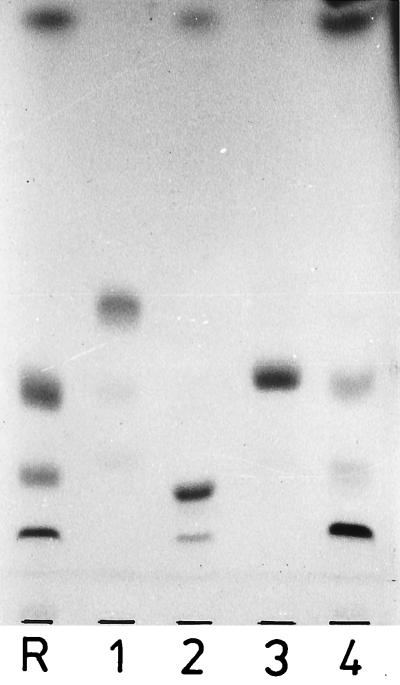
Lipid 8 showed the same chromatographic mobility (silica gel, solvent A) as bis(acylglycero)phosphate (relative mobility to phosphatidylglycerol, Rphosphatidylglycerol = 1.26), which was prepared from acylphosphatidylglycerol (Rphosphatidylglycerol = 1.89) with phospholipase A2 (29). Lipid 9 was converted by alanine ester cleavage into a ninhydrine-negative lipid with the chromatographic mobility of lipid 8 (Fig. 5). Neither lipid 8 nor the alanine-free derivative of lipid 9 was attacked by phospholipase A2. Hydrolysis of lipid 8 with 98% (mass/vol) acetic acid (100°C, 40 min) released acylglycerol and lysophosphatidic acid, which were identified by TLC (acylglycerol, solvent I; lysophosphatidic acid, solvents A and E). Lipid 9 was hydrolyzed by 98% (vol/vol) acetic acid more slowly (100°C, >6 h), and after hydrolysis acylglycerol, lysophosphatidic acid, and free alanine, but no alanyl-acylglycerol, were found. Most likely the positively charged alanyl ester rendered by electrostatic interaction the phosphodiester resistant to cleavage by acetic acid, and the observed cleavage possibly occurred after hydrolysis of the alanine ester. Hydrolysis of lipid 9 with 48% (by mass) HF (hydrofluoric acid) released acylglycerol and a ninhydrine-positive compound migrating more slowly on TLC with solvent L (RacylGro = 0.38). This compound was hydrolyzed on the plate in a moist chamber by ammonia vapor. On subsequent development of the plate with solvent L, acylglycerol was detected as well as nonmigrating ninhydrine-positive material.
FIG. 5.
Dealanylation of d-Ala(acylGro)2P (lipid 9) by treatment with 0.1 M sodium borate, pH 9.5, containing Triton X-100. Lane R, (acylGro)2P (lipid 8); lane 1, lipid 9, untreated; lanes 2 and 3, lipid 9 after treatment at pH 9.5 for 4 and 24 h, respectively. The heavy spot near the front in panel a and the ladderlike pattern in panel b are Triton X-100. TLC was performed on silica gel plates (Merck 60) by using solvents A (a) and D (b); visualization was done with iodine vapor.
On the basis of these results, lipid 8 possesses the structure 1(2)-O-acyl-sn-3-glycerophospho-[3′(2′)-O-acyl]-sn-1′-glycerol and lipid 9 is the d-alanyl derivative of lipid 8.
Cardiolipin [bis(phosphatidyl)glycerol] and its d-alanyl and α-d-glucopyranosyl derivatives.
Lipids 10, 11, and 12 contained glycerol, fatty acids, and phosphorus in molar ratios of approximately 3:4:2; lipid 11 contained in addition 1 mol equivalent of d-alanine; and lipid 12 contained 1 mol equivalent of d-glucose. Lipid 13 displayed a molar ratio of glycerol, fatty acids, and phosphorus of 3:3:2, consistent with it being a monodeacyl derivative of cardiolipin. Mild alkaline treatment (0.1 M sodium borate [pH 9.8], 37°C, 4 h) of lipid 11 released the alanine residue and a ninhydrine-negative lipid product with the chromatographic mobility of cardiolipin (solvents C and E).
Hydrolysis of lipid 10 and dealanylated lipid 11 with 98% (by volume) acetic acid (100°C, 1 h) yielded diacylglycerol and glycero-1,3-bisphosphate, which were separated by phase partitioning and identified by compositional analysis. Native lipid 11 and lipid 12 resisted hydrolysis with 98% (by volume) acetic acid, indicating that the alanine ester and the glucosyl residue occupied the hydroxyl group on the middle glycerol moiety. Accordingly, hydrolysis of lipid 11 with 48% (by mass) HF (2°C, 36 h) led to the formation of inorganic phosphate, alanylglycerol, and diacylglycerol. Alanylglycerol was identified by TLC (solvent K) by using alanylglycerol, released from lipoteichoic acid (LTA) by HF, as a standard.
Alkali hydrolysis (0.5 M NaOH, 100°C, 3 h) of deacylated lipids 10 and 11 released glycerol, glycerobisphosphate, and β-and α-glycerophosphate in molar proportions of 0.56:0.08:0.54:0.44. Approximately two-thirds of the α-glycerophosphate was oxidizable by sn-glycero-3-phosphate dehydrogenase, which suggests that, as in ox heart cardiolipin, three of the four glycerophosphate bonds are linked to the sn-3 position (31) and one is linked to the sn-1 position. Accordingly, both phosphatidyl residues of lipids 10 and 11 were hydrolyzed by the stereospecific phospholipase A2 (9), yielding the monodeacyl and dideacyl derivatives (Fig. 6). On the basis of these results, we propose for lipids 10 and 11 the structures bis(sn-3-phosphatidyl)-1′,3′-glycerol and 2′-O-d-alanyl-bis(sn-3-phosphatidyl)-1′,3′-glycerol.
FIG. 6.
Hydrolysis of d-Ala-cardiolipin (lipid 11) and cardiolipin (lipid 10) with phospholipase A2 (room temperature, 30 min). Lane R (from top to bottom), fatty acids, cardiolipin, and monodeacyl and dideacyl derivatives; lanes 1 and 2, lipid 11 before and after enzymic treatment, respectively; lanes 3 and 4, lipid 10 before and after enzymic treatment, respectively. The heavy band in lane 2 is the dideacyl derivative. TLC was performed on silica gel plates (Merck 60) by using solvent A, and visualization was done with iodine vapor.
Native and deacylated lipid 12 had the same chromatographic mobility as the recently described (10) α-d-glucopyranosylcardiolipin (solvent B) and its deacylated derivative (solvents F and G). Alkali hydrolysis (see above) released neither glycerol nor glycerobisphosphate but a mixture of α- and β-glycerophosphate and glucosylglycerol. The glucosylglycerol had the chromatographic mobility of 2-O-α-d-glucopyranosylglycerol, which differed from that of the 1(3) isomer (Fig. 2), and was readily hydrolyzed by α-glucosidase. The released α-glycerophosphate was completely oxidized by sn-glycero-3-phosphate dehydrogenase, which indicates the presence of two sn-3-phosphatidyl residues. The proposed structure is 2′-O-α-d-glucopyranosyl-bis(sn-3-phosphatidyl)-1′,3′-glycerol.
Molecular weight of d-alanylcardiolipin.
On fast atom bombardment (FAB)-MS in the negative-ion mode, molecular ions [M − H+]− of d-alanylcardiolipin were observed at m/z 1474, 1448, and 1446, which correspond to the fatty acid combinations shown in Table 7. A detailed description of the fragmentation pattern in negative- and positive-ion FAB-MS will be described elsewhere. The structure, derived from the MS data, is in complete harmony with the chemically established structure described in this report.
TABLE 7.
d-Alanylcardiolipin species, determined by negative-ion FAB-MS
| [M − H+]− m/z |
Fatty acid combinationa |
|---|---|
| 1474 | 18/18/16/16, 2Δ |
| 1448 | 18/16/16/16, 1Δ |
| 1446 | 18/16/16/16, 2Δ |
| 18/18/16/14, 2Δ |
The number before the “Δ” denotes the number of monoenic acids; their assignment to C-18 or C-16 would be arbitrary.
Growth phase-dependent changes in lipid composition.
Compared with levels in exponential-phase growth, in the stationary phase there was a moderate increase in levels of Gal(α1-2)Glc(α1-3)acyl2Gro, phosphatidylglycerol, and bis(acylglycero)phosphate (Table 1). l-Lysylphosphatidylglycerol and d-alanylcardiolipin levels increased drastically, and similarly drastic was the decrease in the level of cardiolipin. A decrease by about one-half the amount present during exponential growth was observed for the d-alanyl derivatives of phosphatidylglycerol and bis(acylglycero)phosphate.
DISCUSSION
V. fluvialis contains diacylglyceroglycolipids, phosphatidylglycerol, and cardiolipin, which are common membrane components of gram-positive bacteria (see the review by O’Leary and Wilkinson [38]). Less widespread are l-lysylphosphatidylglycerol, d-alanylphosphatidylglycerol (38), and diacylglyceroglycolipids carrying a third fatty acid ester on their carbohydrate moiety (15, 17, 35, 36). Bis(acylglycero)phosphate has so far been detected only in alkalophilic bacilli (26, 37), and to our knowledge, its d-alanine ester derivative has not yet been described.
This is also true for d-alanylcardiolipin, which is a major component of the vagococcal cytoplasm membrane. The concentration increased from 10.6 mol% during exponential growth to 26.4 mol% in the stationary phase. Since the concentration of cardiolipin concomitantly decreased from 37.4 to 12 mol%, one is tempted to speculate that on transition to the stationary phase a major part of cardiolipin is converted to the d-alanyl derivative and, from the values in Table 1, a minor part is converted to the glucosyl derivative. Taking the changes in the lipid composition together, the ratio of negatively charged to zwitterionic groups decreased from 2.65 during growth to 1.28 in the stationary phase. It will be of interest to investigate whether and how this change in net charge influences the physicochemical and physiological properties of the cytoplasmic membrane. Since in the stationary phase the pH of the medium dropped below 5, one effect of the changed surface charge may be to repell the protons from the surface of the cell.
Whereas aminoacyl esters of phosphatidylglycerol have been known for a long time (see the review by van den Bosch et al. [47]), substituted cardiolipins represent a more recently discovered lipid class. As shown here, d-glucopyranosylcardiolipin is a minor component in vagococci (Table 1). It was detected earlier in group B streptococci, where it represents 7 to 8 mol% of the polar lipids and accounts for approximately 18% of the lipid phosphorus (10). Another novel lipid of this class is l-lysylcardiolipin, which was isolated from species of the genus Listeria. Its concentration varied between 7 and 26 mol% of the polar lipids and between 12 and 38% of the lipid phosphorus. Since l-lysylcardiolipin was present in the four listeria species tested, it may serve as a taxonomic marker for the genus Listeria (16a).
The stereochemical analysis of the glycerophosphate residues revealed phosphatidylglycerol and cardiolipin of vagococci to be stereochemically identical to phosphatidylglycerol and cardiolipin of other eubacteria, plants, and higher organisms (3, 8, 11, 22, 27, 31). Therefore, the classical biosynthetic pathway of phospholipids is apparently also operative in vagococci: it leads from sn-glycero-3-phosphate through phosphatidic acid→CDP-diacylglycerol→phosphatidylglycerophosphate to phosphatidylglycerol and finally to cardiolipin (see reviews by van den Bosch et al. [47], Pieringer [39], and Raetz [40]). The stereochemical analysis further revealed that d-glucosyl- and d-alanylcardiolipin, as well as l-lysyl- and d-alanylphosphatidylglycerol, are apparently biosynthetic derivatives of the respective phospholipids. As shown with other gram-positive bacteria, the biosynthesis of d-alanyl- and l-lysylphosphatidylglycerol is unique, as the aminoacyl residues are transferred from d-alanyl-tRNA and l-lysyl-tRNA, respectively (see the review by van den Bosch et al. [47]).
Due to their sn-glycero-3-phospho-sn-1-glycerol structure, bis(acylglycero)phosphate and its d-alanyl derivative are presumably derivatives of phosphatidylglycerol. The same stereochemical configuration was established for the bis(acylglycero)phosphate of alkalophilic bacteria, and the results of pulse-chase experiments, performed with these bacteria, are compatible with phosphatidylglycerol being the biosynthetic precursor (26, 37). In contrast to bacterial bis(acylglycero)phosphate, the analogous lyso-bis-phosphatidic acid isolated from mammalian cells (41) had the unusual sn-glycero-1-phospho-1-sn-glycerol configuration and requires other biosynthetic reactions (5, 23).
Like bacilli, enterococci, lactobacilli, lactococci, listeriae, staphylococci, and certain streptococci, vagococci possess a poly(glycerophosphate) LTA (see the review by Fischer [12]). Like other poly(glycerophosphate) LTAs, it is substituted with d-alanine ester. It lacks, however, α-d-galactopyranosyl substituents that occur on the LTAs of lactococci (43) as the antigenic determinant of the Lancefield serological group N (49, 50). Unpublished results from our laboratory showed that the lipid anchor of vagococcal LTA consists of Gal(α1-2)Glc(α1-3)acyl2Gro and acyl[Gal(α1-2)Glc(α1-3)]acyl2Gro, both of which occur in the free state among the membrane lipids (Table 1). This finding is consistent with the biosynthetic pathway of poly(glycerophosphate) LTAs, in which a selected glyceroglycolipid, occasionally along with its acylated or phosphatidyl derivative, serves as the starter molecule and definite glycolipid anchor. Attached to O-6 of the terminal hexosyl residue, the linear chain is polymerized by successive transfer of sn-glycero-1-phosphate from phosphatidylglycerol (see the review by Fischer [12]). In this pathway, the sn-glycero-1-phospho-6Gal(α1-2)Glc(α1-3)acyl2Gro detected among vagococcal lipids (Table 1) may be the first intermediate in LTA biosynthesis. sn-Glycero-1-phosphoglyceroglycolipids have been discovered in a large number of poly(glycerophosphate) LTA-synthesizing bacteria. Consistent with the existence of a metabolic interrelationship, the glycerophosphate residues were regularly linked to the same position on the same glycolipid as the poly(glycerophosphate) chain of the respective LTA (12). For Staphylococcus aureus, pulse-chase experiments supported the proposed role of glycerophosphoglycolipids as intermediates in LTA synthesis (25).
ACKNOWLEDGMENT
This work was supported by grant Fi-218/7-1 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Baer E, Kates M. Migration of esters of glycerophosphoric acid. II. The acid and alkaline hydrolysis of l-α-lecithins. J Biol Chem. 1950;185:615–623. [PubMed] [Google Scholar]
- 2.Behr T, Fischer W, Peter Katalinic J, Egge H. The structure of pneumococcal lipoteichoic acid. Improved preparation, chemical and mass spectrometric studies. Eur J Biochem. 1992;207:1063–1075. doi: 10.1111/j.1432-1033.1992.tb17143.x. [DOI] [PubMed] [Google Scholar]
- 3.Benson A A, Miyano M. The phosphatidylglycerol and sulfolipid of plants: asymmetry of the glycerol moiety. Biochem J. 1961;81:31. p. [Google Scholar]
- 4.Beutler H O. Lactose and galactose. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VI. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 104–112. [Google Scholar]
- 5.Brotherus J, Renkonen O, Herrmann J, Fischer W. Novel stereoconfiguration in lyso-bis-phosphatidic acid of cultured BHK-cells. Chem Phys Lipids. 1974;13:178–182. doi: 10.1016/0009-3084(74)90034-6. [DOI] [PubMed] [Google Scholar]
- 6.Brückner H, Wittner R, Godel H. Fully automated separation of dl-amino acids derivatized with O-phthaldialdehyde together with N-isobutyryl-cysteine. Application to food samples. Chromatographia. 1991;32:383–388. [Google Scholar]
- 7.Collins M D, Ash C, Farrow J A E, Wallbanks S, Williams A M. 16S ribosomal ribonucleic acid sequence analyses of lactococci and related taxa. Description of Vagococcus fluvialis gen. nov., sp. nov. J Appl Bacteriol. 1989;67:453–460. doi: 10.1111/j.1365-2672.1989.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 8.de Haas G H, Bonsen P P M, van Deenen L L M. Studies on cardiolipin III. Structural identity of ox-heart cardiolipin and synthetic diphosphatidyl glycerol. Biochim Biophys Acta. 1966;116:114–124. doi: 10.1016/0005-2760(66)90097-x. [DOI] [PubMed] [Google Scholar]
- 9.de Haas G H, Postema N M, Nieuwenhuizen W, van Deenen L L M. Purification and properties of phospholipase A from porcine pancreas. Biochim Biophys Acta. 1968;159:103–117. doi: 10.1016/0005-2744(68)90248-9. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W. The polar lipids of group B streptococci. I. Glycosylated diphosphatidylglycerol, a novel glycophospholipid. Biochim Biophys Acta. 1977;487:74–88. doi: 10.1016/0005-2760(77)90045-5. [DOI] [PubMed] [Google Scholar]
- 11.Fischer W. The polar lipids of group B streptococci. II. Composition and positional distribution of fatty acids. Biochim Biophys Acta. 1977;487:89–104. doi: 10.1016/0005-2760(77)90046-7. [DOI] [PubMed] [Google Scholar]
- 12.Fischer W. Bacterial phosphoglycolipids and lipoteichoic acids. In: Kates M, editor. Glycolipids, phosphoglycolipids and sulfoglycolipids. New York, N.Y: Plenum Press; 1990. pp. 123–234. [Google Scholar]
- 13.Fischer W, Ishizuka I, Landgraf H R, Herrmann J. Glycerophosphoryl diglucosyl diglyceride, a new phosphoglycolipid from streptococci. Biochim Biophys Acta. 1973;296:527–545. doi: 10.1016/0005-2760(73)90113-6. [DOI] [PubMed] [Google Scholar]
- 14.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 15.Fischer W, Laine R A, Nakano M, Schuster D, Egge H. The structure of acyl-α-kojibiosyldiacylglycerol from Streptococcus lactis. Chem Phys Lipids. 1978;21:103–112. [Google Scholar]
- 16.Fischer W, Landgraf H R. Glycerophosphoryl phosphatidyl kojibiosyl diacylglycerol, a novel phosphoglucolipid from Streptococcus faecalis. Biochim Biophys Acta. 1975;380:227–244. doi: 10.1016/0005-2760(75)90009-0. [DOI] [PubMed] [Google Scholar]
- 16a.Fischer, W., and K. Leopold. Unpublished data.
- 17.Fischer W, Peter-Katalinic J, Hartmann R, Egge H. S-2-amino-1,3-propandiol-3-phosphate-carrying diradylglyceroglycolipids. Novel major membrane lipids of Clostridium innocuum. Eur J Biochem. 1994;223:879–892. doi: 10.1111/j.1432-1033.1994.tb19065.x. [DOI] [PubMed] [Google Scholar]
- 18.Fischer W, Schmidt M A, Jann B, Jann K. Structure of the Escherichia coli K2 capsular antigen. Stereochemical configuration of the glycerophosphate and distribution of galactopyranosyl and galactofuranosyl residues. Biochemistry. 1982;21:1279–1284. doi: 10.1021/bi00535a027. [DOI] [PubMed] [Google Scholar]
- 19.Grassl M, Supp M. d-Alanine. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VIII. Metabolites 3: lipids, amino acids and related compounds. Weinheim, Germany: Verlag Chemie; 1985. pp. 336–340. [Google Scholar]
- 19a.Hahn, G. Personal communication.
- 20.Hashimoto H, Kawakami H, Tomokane K, Yoshii Z, Hahn G, Tolle A. Isolation and characterization of motile group N streptococci. J Fac Appl Biol Sci Hiroshima Univ. 1979;18:207–216. [Google Scholar]
- 21.Hashimoto H, Noborisaka R, Yanagawa R. Distribution of motile streptococci in feces of man and animals and in river and sea water. Jpn J Bacteriol. 1978;29:387–393. [PubMed] [Google Scholar]
- 22.Haverkate F, van Deenen L L M. Isolation and chemical characterization of phosphatidylglycerol from spinach leaves. Biochim Biophys Acta. 1965;106:78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- 23.Joutti A, Brotherus J, Renkonen O, Laine R A, Fischer W. The stereochemical configuration of lyso-bisphosphatidic acid from rat liver, rabbit lung and pig lung. Biochim Biophys Acta. 1976;450:206–209. doi: 10.1016/0005-2760(76)90092-8. [DOI] [PubMed] [Google Scholar]
- 24.Kates M. Techniques of lipidology, 2nd rev. ed. New York, N.Y: American Elsevier; 1986. pp. 396–399. [Google Scholar]
- 25.Koch H U, Haas R, Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur J Biochem. 1984;138:357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- 26.Koga Y, Nishihara M, Morii H. Lipids of alkalophilic bacteria: identification, composition and metabolism. J UOEH. 1982;4:227–240. [Google Scholar]
- 27.Komaratat P, Kates M. The lipids of a halotolerant species of Staphylococcus epidermidis. Biochim Biophys Acta. 1975;398:464–484. doi: 10.1016/0005-2760(75)90197-6. [DOI] [PubMed] [Google Scholar]
- 28.Kunst A, Draeger B, Ziegenhorn J. d-Glucose. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VI. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 163–172. [Google Scholar]
- 29.Landgraf H R. Fettsäuremuster und positionsspezifische Verteilung der Fettsäuren in den polaren Lipiden von Streptococcen. Ph.D. thesis. Erlangen, Germany: University Erlangen-Nürnberg; 1976. [Google Scholar]
- 30.Lang G. l-(−)Glycerol 3-phosphate. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VI. Metabolites 1: carbohydrates. Weinheim, Germany: Verlag Chemie; 1984. pp. 525–531. [Google Scholar]
- 31.LeCocq J, Ballou C E. On the structure of cardiolipin. Biochemistry. 1964;3:976–980. doi: 10.1021/bi00895a023. [DOI] [PubMed] [Google Scholar]
- 32.Mayberry W R. Relative simple methodology for the determination of configuration of unsaturation of bacterial monounsaturated fatty acids: application of the unsaturates of Legionella spp. J Microbiol Methods. 1984;2:177–187. [Google Scholar]
- 33.Moss C W, Lambert M A, Merwin W H. Comparison of rapid methods for analysis of bacterial fatty acids. Appl Microbiol. 1974;28:80–85. doi: 10.1128/am.28.1.80-85.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nägele U, Wahlefeld A W, Ziegenhorn J. Triglycerides. In: Bergmeyer H U, Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd ed. VIII. Metabolites 3: lipids, amino acids and related compounds. Weinheim, Germany: Verlag Chemie; 1985. pp. 2–12. [Google Scholar]
- 35.Nakano M, Fischer W. The glycolipids of Lactobacillus casei DSM 20021. Hoppe Seyler’s Z Physiol Chem. 1977;358:1439–1453. doi: 10.1515/bchm2.1977.358.2.1439. [DOI] [PubMed] [Google Scholar]
- 36.Niepel T, Meyer H, Wray V, Abraham W-R. A new type of glycolipid, 1-[α-mannopyranosyl-(1α-3)-(6-O-acyl-α-mannopyranosyl)]-3-O-acylglycerol, from Arthrobacter atrocyaneus. Tetrahedron. 1997;35:3593–3602. [Google Scholar]
- 37.Nishihara M, Morii H, Koga Y. Bis(monoacylglycero)phosphate in alkalophilic bacteria. J Biochem. 1982;92:1469–1479. doi: 10.1093/oxfordjournals.jbchem.a134071. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary W M, Wilkinson S G. Gram-positive bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 1. London, United Kingdom: Academic Press; 1988. pp. 117–201. [Google Scholar]
- 39.Pieringer R A. Formation of bacterial glycerolipids. In: Boyer P D, editor. The enzymes. XVI. New York, N.Y: Academic Press; 1983. pp. 255–306. [Google Scholar]
- 40.Raetz C R H. Enzymology, genetics and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978;42:614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rouser G, Krichevsky G, Yamamoto A, Knudson A G, Jr, Simon G. Accumulation of a glycerophospholipid in classical Nieman-Pick disease. Lipids. 1968;3:287–290. doi: 10.1007/BF02531203. [DOI] [PubMed] [Google Scholar]
- 42.Schleifer K H, Killper-Bälz R. Molecular and chemotaxonomic approaches to the classification of streptococci, enterococci and lactococci: a review. Syst Appl Microbiol. 1987;10:1–19. [Google Scholar]
- 43.Schleifer K H, Kraus J, Dvorak C, Killper-Bälz R, Collins M D, Fischer W. Transfer of Staphylococcus lactis and related streptococci to the genus Lactococcus gen. nov. Syst Appl Microbiol. 1985;6:183–195. [Google Scholar]
- 44.Schnitger H, Papenberg K, Ganse E, Czok R, Bücher T, Adam H. Chromatographie phosphathaltiger Metabolite eines menschlichen Leberpunktates. Biochem Z. 1959;332:167–185. [PubMed] [Google Scholar]
- 45.Shaw N. The detection of lipids on thin-layer chromatograms with the periodate Schiff reagent. Biochim Biophys Acta. 1968;164:435–438. doi: 10.1016/0005-2760(68)90171-9. [DOI] [PubMed] [Google Scholar]
- 46.Teixeira L M, Carvalho M D G, Merquior V L C, Steigerwalt A G, Brenner D J, Facklam R R. Phenotypic and genotypic characterization of Vagococcus fluvialis, including strains isolated from human sources. J Clin Microbiol. 1997;35:2778–2781. doi: 10.1128/jcm.35.11.2778-2781.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Bosch H, van Golde L M G, van Deenen L L M. Dynamics of phosphoglycerides. Ergeb Physiol Biol Chem Exp Pharmacol. 1972;66:13–145. doi: 10.1007/3-540-05882-6_2. [DOI] [PubMed] [Google Scholar]
- 48.Van de Rijn I, Kessler R E. Growth characteristics in group A streptococci in a new chemically defined medium. Infect Immun. 1980;27:444–448. doi: 10.1128/iai.27.2.444-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wicken A J, Knox K W. Lipoteichoic acids: a new class of bacterial antigen. Membrane lipoteichoic acids can function as surface antigens of gram-positive bacteria. Science. 1975;187:1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- 50.Wicken A J, Knox K W. Characterization of group N Streptococcus lipoteichoic acid. Infect Immun. 1975;11:973–981. doi: 10.1128/iai.11.5.973-981.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]