Summary
Acute myeloid leukemia (AML) and myeloid neoplasms develop through acquisition of somatic mutations that confer mutation-specific fitness advantages to hematopoietic stem and progenitor cells. However, our understanding of mutational effects remains limited to the resolution attainable within immunophenotypically and clinically accessible bulk cell populations. To decipher heterogeneous cellular fitness to preleukemic mutational perturbations, we performed single-cell RNA sequencing of eight different mouse models with driver mutations of myeloid malignancies, generating 269,048 single-cell profiles. Our analysis infers mutation-driven perturbations in cell abundance, cellular lineage fate, cellular metabolism, and gene expression at the continuous resolution, pinpointing cell populations with transcriptional alterations associated with differentiation bias. We further develop an 11-gene scoring system (Stem11) on the basis of preleukemic transcriptional signatures that predicts AML patient outcomes. Our results demonstrate that a single-cell-resolution deep characterization of preleukemic biology has the potential to enhance our understanding of AML heterogeneity and inform more effective risk stratification strategies.
Keywords: preleukemia, hematopoiesis, acute myeloid leukemia, myeloid malignancies, single-cell RNA-seq
Graphical abstract
Highlights
-
•
Single-cell hematopoietic landscape of eight preleukemic mouse models
-
•
Streamlined pipeline for integrated analysis of single-cell perturbation datasets
-
•
Mutational impact on cell abundance, fate probability, metabolism, and gene expression
-
•
Stem11 lineage perturbation signature predictive of AML patient outcomes
Isobe et al. profiled 269,048 single-cell transcriptomes of hematopoietic stem and progenitor cells from eight preleukemic mouse models, revealing mutation-specific perturbations in cell abundance, differentiation fate, metabolic activity, and gene expression. They further developed Stem11, a preleukemic lineage perturbation signature correlating with AML patient outcomes.
Introduction
Acute myeloid leukemia (AML) is a heterogeneous hematological malignancy that arises from hematopoietic stem and progenitor cells (HSPCs). At least nine functional categories of genes are recurrently mutated and drive the heterogeneity in the process of leukemogenesis.1 At the time of diagnosis, leukemic blasts are often present with multiple driver mutations with characteristic patterns of co-occurrence and mutual exclusivity.1,2 These observations suggest that individual mutations possess unique and complementary functions that cooperate to promote leukemogenesis. Therefore, a deeper understanding of the distinct leukemogenic effects of individual mutations is a crucial next step in deciphering the intra- and inter-patient heterogeneity that results from different combinations of mutations.
Approximately 20% of AML cases are recognized as developing from antecedent myeloid neoplasms, such as myeloproliferative neoplasms and myelodysplastic syndrome,3 following the acquisition of additional functional categories of mutations.4,5 Recent studies on clonal hematopoiesis6,7 have demonstrated that individual mutations confer varying degrees of fitness to HSPCs and modulate the risk for progression to leukemia. Thus, mutation-specific remodeling of hematopoietic clonal architecture plays a role from the earliest preleukemic stage through to full leukemic transformation. However, as early preleukemic changes are clinically silent, how single mutations differently perturb the entire hematopoietic system remains largely elusive.
Over the past half decade, single-cell technologies, including single-cell RNA sequencing (scRNA-seq), have reshaped our understanding of the hematopoietic hierarchy from a tree-like, stepwise model toward a differentiation landscape model.8 More recent advances in computational and mathematical methods have further expanded the application of scRNA-seq beyond gene expression comparison, enabling the inference of more complex biological information, such as cellular fate probabilities9 and metabolic activities.10 As such, scRNA-seq provides a unique advantage in characterizing mutation-driven perturbations in various biological processes.
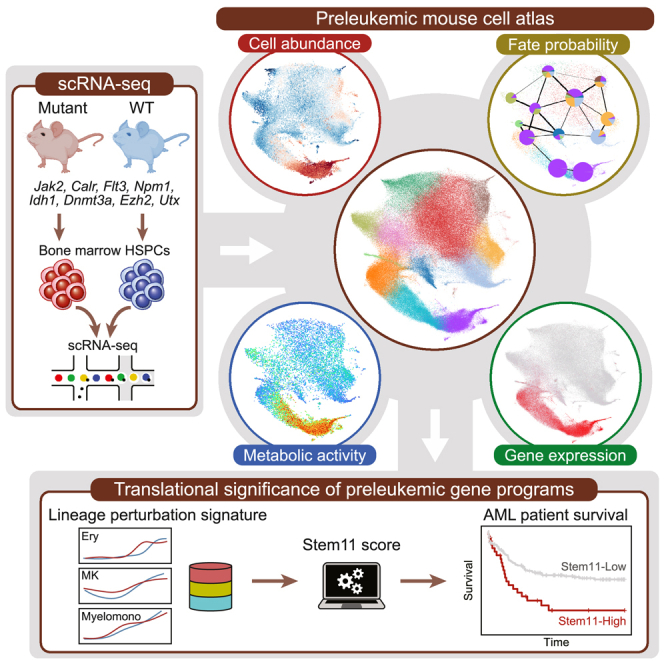
In this study, we perform scRNA-seq on bone marrow HSPCs and computationally infer the preleukemic changes in cell abundance, cellular lineage fate, metabolic activities, and gene expression across eight different mouse models harboring preleukemic mutations in Jak2, Calr, Flt3, Npm1, Idh1, Dnmt3a, Ezh2, and Utx (Figure 1A). Our preleukemic mouse cell atlas (PMCA) can be explored using an interactive web portal at https://gottgens-lab.stemcells.cam.ac.uk/preleukemia_atlas/. This single-cell-resolution multi-scale analysis illustrates mutation-specific mechanisms of hematopoietic perturbation and identifies preleukemic genetic programs predictive of AML patient outcomes (Stem11 signature), thus establishing a novel framework for translating preleukemic biology into an improved treatment stratification strategy for AML patients.
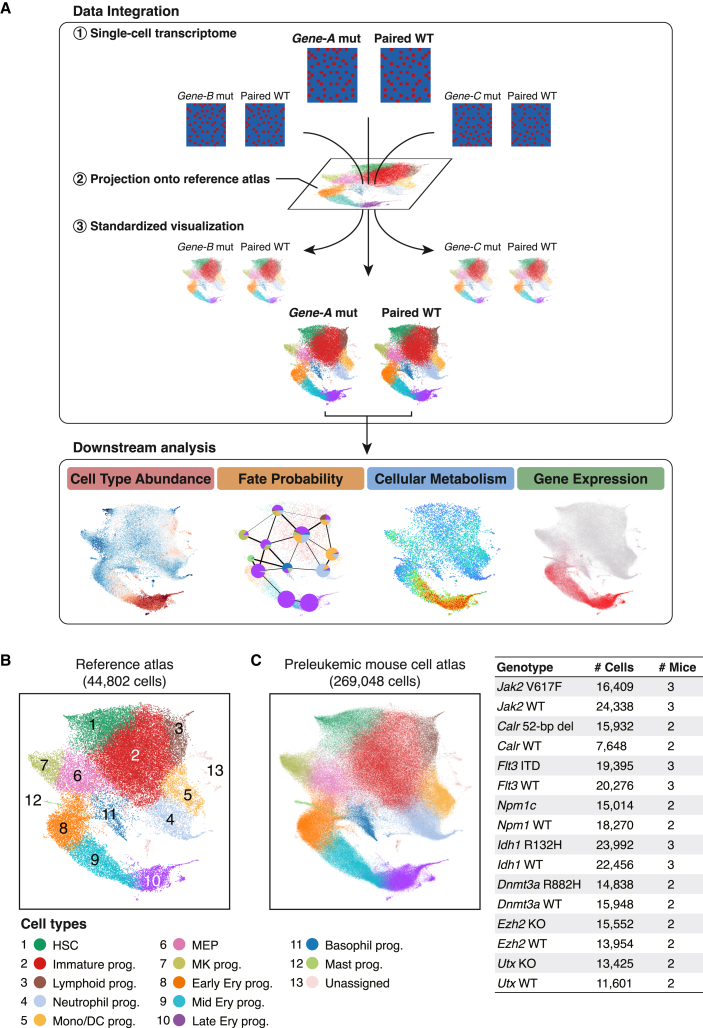
Figure 1.
Standardized visualization across multiple mutant landscapes using reference-based data integration
(A) Schematic overview of the analysis workflow in this study. Data integration: scRNA-seq data of individual animals are first projected onto the reference mouse hematopoietic atlas and all visual representations of the results are shown in the common UMAP space. Downstream analysis: the downstream analysis modules then infer differential abundance, cellular fate probability, cellular metabolic activity and gene expression changes. All results are shown in the common UMAP space to permit comparison among genotypes.
(B) UMAP plot of the reference mouse hematopoietic atlas11 (44,802 cells). HSC, hematopoietic stem cell; prog, progenitors; Mono, monocyte; DC, dendritic cell; MEP, megakaryocyte-erythroid progenitors; MK, megakaryocyte; Ery, erythroid.
(C) Integrated preleukemic mouse hematopoietic atlas (38 animals, 269,048 cells). Cells are color coded according to cell types as in (B). WT, wild type; KO, knockout.
Results
Reference-based integration provides standardized visualization across multiple datasets
To unravel the mutation-specific preleukemic effects on hematopoietic differentiation, we performed scRNA-seq of lineage-negative/c-Kit-positive (LK) cells for 38 animals from eight different mutant mouse models and their wild-type littermates: homozygous Jak2 V617F,12 homozygous Calr 52 bp deletion,13 heterozygous Flt3-ITD,14 heterozygous Npm1c,15 heterozygous Idh1 R132H,16 heterozygous Dnmt3a R882H,17 homozygous Ezh2 knockout (KO)18 and homozygous Utx KO19 (Table S1). A total of 269,048 single-cell transcriptomes passed stringent quality control measures (see STAR Methods), with a median of 2,819 genes detected per cell.
To minimize technical batch effects and enhance comparability between the mutant hematopoietic landscapes, we took advantage of our previously reported single-cell mouse hematopoietic atlas11 (Figures 1B and S1A) and projected all mutant and wild-type samples onto this reference atlas with a reference-based integration and label transfer strategy using Seurat20 (see STAR Methods). This enabled an automatic identification of the hematopoietic differentiation landscape within individual animals within a common uniform manifold approximation and projection (UMAP) space, while mitigating technical batch effects (Figure 1C). Our mutant animals did not develop overt leukemia at the time of analysis and consistently, the scRNA-seq landscapes revealed multi-lineage hematopoiesis in all mutant models (Figures S1B and S1C). Therefore, our data provide a unique view into the “preleukemic” window of various mutations, and our data integration strategy enables a standardized visualization across conditions to facilitate further quantitative comparisons of mutation-driven molecular, cellular, and tissue-scale alterations (Figure 1A).
Preleukemic stem and progenitor cells show skewed abundance under steady state
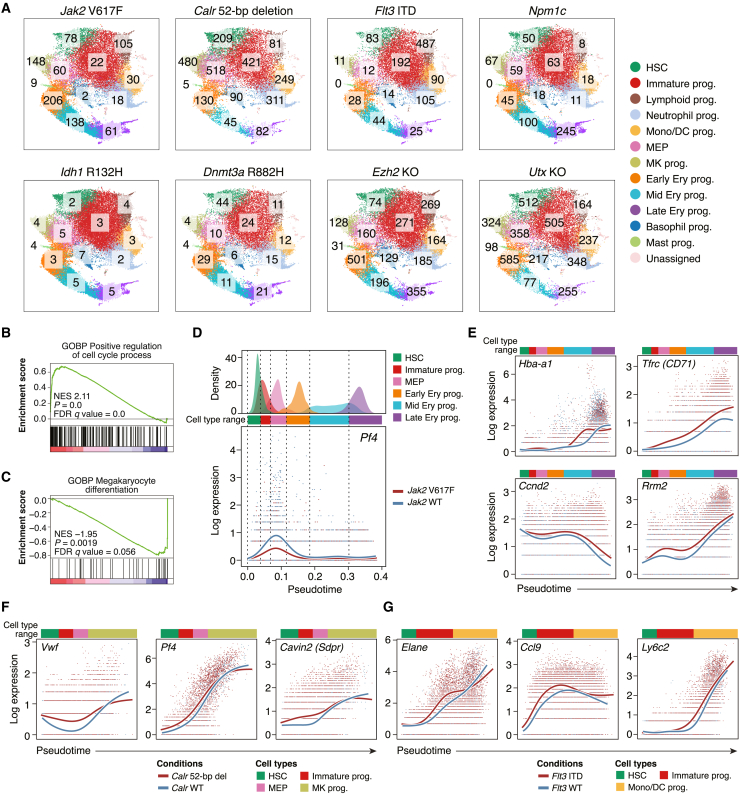
To characterize the tissue-scale effects of individual mutations, we first performed differential cell abundance analysis. Using the Python package MELD,21 we computed single-cell-resolution differential abundance by comparing the cell densities between paired mutant and wild-type samples (Figure S2A; see STAR Methods for details). When plotted in the common UMAP space, this differential abundance score (mutant relative likelihood) illustrated, at single-cell resolution, which transcriptionally defined cell types were increased or depleted in each mutant model (Figure 2A).
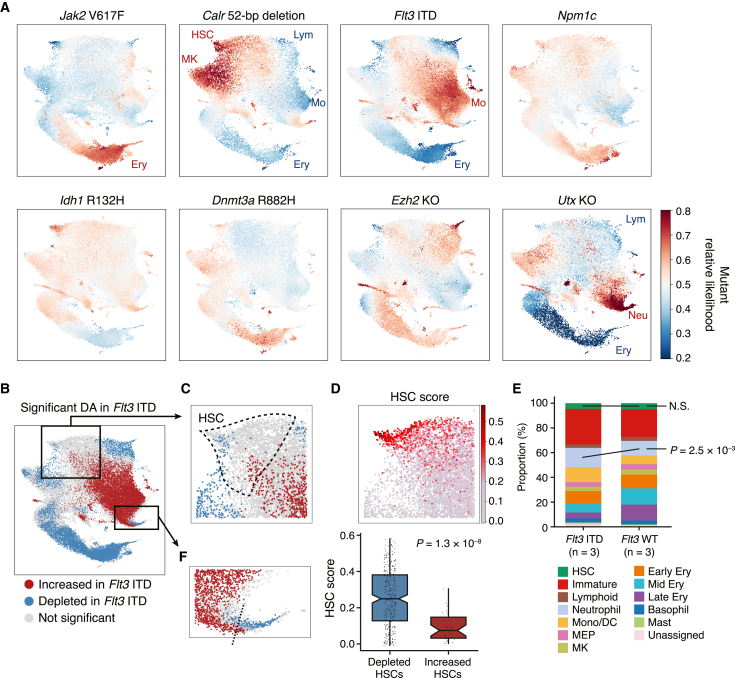
Figure 2.
Depicting tissue-scale subpopulation abundance at single-cell resolution
(A) Differential abundance landscapes of eight preleukemic mutant models. Higher likelihood (red) corresponds to higher abundance and lower likelihood (blue) to lower abundance in each mutant model compared with paired wild-type samples. All results are presented in the same color scale ranging from 0.2 to 0.8 for comparability. Cell types with a median mutant relative likelihood of >0.6 or <0.4 are indicated.
(B) Statistically significant differential abundance (DA) in the Flt3 ITD model. p values were derived using a two-sided t test. Cells with raw p values < 0.05 and BH-adjusted p values < 0.25 are colored red (abundant) or blue (depleted in the Flt3 ITD model).
(C) A magnified section of the HSC cluster region. The boundary of the HSC cluster is outlined with dashed lines.
(D) Top: the HSC score in the Flt3 ITD and wild-type HSCs. Bottom: significant difference in the HSC score between Flt3 ITD-depleted (mutant relative likelihood < 0.4) and increased (mutant relative likelihood > 0.6) HSCs. Boxplots show median and first/third quartiles. The whisker extends from the smallest to the largest values within 1.5 × IQR from the box hinges. The p value is from a two-sided Wilcoxon rank-sum test.
(E) Stacked bar plots showing the cell type proportions in the Flt3 ITD and wild-type animals. Cell types are color coded as in Figure 1B. Statistical significance was determined with two-sided t test.
(F) A magnified section of the neutrophil progenitor cluster region. The boundary between significantly expanded neutrophil progenitors (red) and depleted later neutrophil progenitors (blue) is indicated by a dashed line.
Reassuringly, this analysis was able to recapitulate the phenotype of Jak2 V617F mice, in that in addition to a significant increase in later erythroid progenitors, a depletion of the most quiescent HSCs and megakaryocyte progenitors was detected (Figure S2B).12 Of particular interest were the complex changes observed within the broadly defined hematopoietic stem cell (HSC) cluster of the Flt3 ITD mutant animals (Figures 2B and 2C). To characterize these increased and depleted subpopulations of HSCs, a previously developed HSC score (molecular signature of long-term repopulating HSCs22) was calculated, which showed significantly higher scores in the depleted HSCs (p = 1.3 × 10−8; Figure 2D), indicating that the Flt3 ITD mutation selectively depletes the most quiescent long-term HSCs. Importantly, cluster-wise comparison of population proportions failed to capture significant changes in the Flt3 ITD HSCs (Figure 2E), as discrete population-level summarization loses information about intra-cluster heterogeneity. Moreover, although the bulk neutrophil progenitor population was significantly increased in the Flt3 ITD mice (p = 2.5 × 10−3; Figure 2E), our single-cell-level evaluation revealed a significant depletion of later neutrophil progenitors (Figure 2F), suggesting a differentiation block at the late neutrophilic progenitor stage, which is consistent with the observation of terminal neutrophilic differentiation of FLT3 ITD-positive AML blasts after treatment with FLT3 inhibitors.23,24
Both young (12-week-old) and old (41-week-old) Calr mutant mice showed a consistent increase in HSCs and megakaryocyte progenitors, where the magnitude of changes was significantly greater in the old animal (Figures S2C and S2D). The Utx KO model showed a significant increase in neutrophil progenitors and megakaryocyte progenitors as well as a depletion of erythroid progenitors (Figure S2E), while the Ezh2 KO model had only a few cells with significant changes (Figure S2E), presumably because Ezh2 KO affects later B and T lymphopoiesis in adult hematopoiesis25 and thus the impact on HSPC abundance is relatively small. The Npm1c, Idh1 R132H, and Dnmt3a R882H mutations showed minimal changes in cellular abundance with the cluster median mutant relative likelihood falling within the range of 0.4–0.6 for all cell types (Figure 2A) and with no statistically significant differential abundance, indicating that these mutations contribute to leukemogenesis in a way that does not involve dramatic changes in tissue-wide population abundance at the HSPC level. Altogether, our differential abundance analysis has provided a mutation-specific tissue-wide picture of skewed hematopoiesis at single-cell resolution.
Single-cell transcriptome deciphers mutation-driven fate bias in early HSPCs
Next, to explore whether and how each mutation alters the cell-intrinsic potential of differentiation, we inferred cellular fate probabilities using CellRank.9 On the basis of a cell-to-cell neighborhood graph and diffusion pseudotime,26 cell-to-cell transition probabilities were first computed, and then the fate probabilities toward seven major hematopoietic lineages (megakaryocyte, erythroid, lymphoid, neutrophil, monocyte, basophil, and mast cell) were computed for each cell, allowing visual and statistical evaluation of the fate bias in each mutant model (Figures 3A and S3).
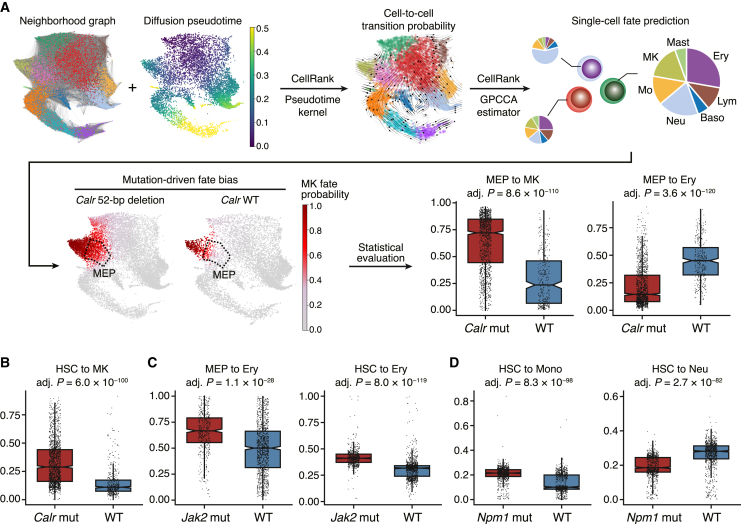
Figure 3.
Mutation-driven fate bias in early HSPCs
(A) Workflow of CellRank-based differential fate probability analysis. A neighborhood graph and diffusion pseudotime were computed (top left) and used to infer cell-to-cell transition probabilities (top middle). On the basis of the transition matrix, the fate probabilities toward seven hematopoietic lineages (megakaryocyte, erythroid, lymphoid, neutrophil, monocyte, basophil, and mast cell) were estimated for the individual cells (top right). The single-cell estimates of fate probabilities were then compared between the paired mutant and wild-type samples (bottom). Ery, erythroid; Lym, lymphoid; Baso, basophil; Neu, neutrophil; Mo, monocyte; MK, megakaryocyte.
(B) Significant difference in the megakaryocyte probability between Calr mutant and wild-type HSCs.
(C) Significant differences in the erythroid probability between Jak2 mutant and wild-type MEPs (left) and HSCs (right).
(D) Significant differences in the monocyte (left) and neutrophil (right) probability between Npm1 mutant and wild-type HSCs. Boxplots show median and first/third quartiles. The whisker extends from the smallest to the largest values within 1.5 × IQR from the box hinges. p values are from logistic regression and likelihood ratio test and are BH adjusted.
Of note, cell fate probability analysis identified a significant increase in megakaryocytic and a decrease in erythroid fate probability in the Calr mutant megakaryocyte-erythroid progenitors (MEPs; Figure 3A), consistent with the higher and lower production of megakaryocyte and erythroid progenitors, respectively (Figure 2A). Intriguingly, megakaryocytic fate bias was detectable even at the level of HSCs (Figure 3B), although the effect was smaller than in MEPs (median fate difference of 17.9% in HSCs (28.9% in Calr mutant vs. 11.0% in wild type) compared with 48.5% in MEPs (72.1% in Calr mutant vs. 23.6% in wild type). Similarly, erythroid fate bias was detected in the Jak2 V617F HSCs and MEPs with a greater difference in MEPs (median fate difference of 9.4% in HSCs (41.1% in Jak2 mutant vs. 31.7% in wild type) compared with 16.6% in MEPs (66.7% in Jak2 mutant vs. 50.0% in wild type); Figure 3C). As the megakaryocytic and erythroid bias in the Calr and Jak2 mutated HSCs has been previously demonstrated experimentally,27,28 our results not only computationally recapitulated these phenotypes but also provided a quantitative view of how hematopoietic lineage fates are progressively biased from HSCs to more differentiated oligo-/bi-potent progenitors. Other lineage bias detected in mutant HSCs included monocytic bias by the Flt3 ITD and Npm1c mutations, lymphoid bias by the Ezh2 KO and neutrophilic and megakaryocytic bias by the Utx KO (Table S2). Of note, Npm1c mutant HSCs showed monocytic bias at the expense of neutrophilic fate (Figure 3D), consistent with the highest incidence of monocytic AML (FAB M4/M5) in the NPM1-mutated patients.29 No positive lineage bias was detected in the Dnmt3a and Idh1 mutant models (Table S2). Overall, our fate probability analysis provided insights into how different mutations may alter HSC fates and have the potential to tilt the balance of hematopoietic lineages.
Neural network modeling reveals distinct metabolic alterations associated with different mutations
As the differential abundance and fate probability analyses have revealed tissue and cellular scale perturbations, we next sought to characterize the molecular level alterations in the preleukemic conditions. For this purpose, we computationally inferred the cellular metabolic activities from scRNA-seq data using a deep neural network model of the Python package scFEA10 and investigated mutation-driven metabolic alterations (Figure 4A).
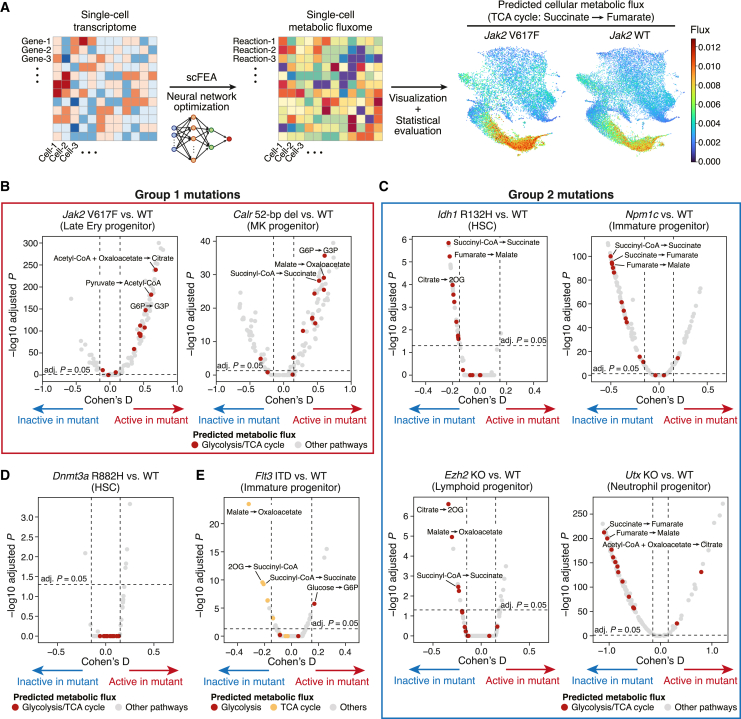
Figure 4.
Transcriptome-based metabolic profiling reveals distinct metabolic consequences of different mutations
(A) Workflow of neural-network-based metabolic profiling. Using the expression levels of enzyme genes as input, a deep neural network model was optimized to estimate the activities of 168 metabolic reactions in the individual cells. The cellular metabolic estimates were then used for statistical comparisons.
(B–E) Volcano plots comparing glycolysis and TCA cycle activities in the mouse models of group 1 mutations (Jak2 and Calr) (B), group 2 mutations (Idh1, Npm1, Ezh2, and Utx) (C), Dnmt3a mutation (D), and Flt3 ITD (E). The x and y axes represent Cohen’s D standardized difference of means and −log10 adjusted p values, respectively. Each dot represents each metabolic reaction module and is colored according to the functional pathways. The horizontal dotted line indicates the adjusted p value of 0.05; the vertical dotted lines indicate the Cohen’s D values of −0.15 and 0.15.
By comparing the paired mutant and wild-type samples, we first asked whether our results recapitulate known metabolic changes in mutant mouse hematopoiesis. MEPs from Jak2 V617F mutant mice are known to upregulate glycolysis, the tricarboxylic acid (TCA) cycle and nucleotide synthesis pathways to fuel their increased energy requirements for overproduction of erythrocytes.30 Consistently, neural-network-based metabolic profiling successfully identified a significant upregulation of these pathways in MEPs within our Jak2 V617F model (Figures S4A and S4B). Moreover, a recent report has shown by isotope tracing that the co-occurrence of Ezh2 KO and Nras G12D mutation enhances the branched-chain amino acid (BCAA) metabolism while Ezh2 KO or Nras G12D mutation alone does not alter the activity of this metabolic pathway.31 Again, our metabolic profiling using the scRNA-seq data from the same mouse models32 successfully identified the enhanced BCAA metabolism only in the double-mutant model (Figures S4C and S4D). These results thus verified the applicability of this method for the hematopoietic system.
Encouraged by the validation results, we further explored the metabolic alterations in our mutant models. As glycolysis and the TCA cycle are the major source of cellular energy, we asked whether the increased cell populations in each mutant model (Figure 2A) are accompanied by increased energy generation. Indeed, the Jak2 and Calr mutations (group 1 mutations) showed global upregulation of these metabolic pathways in the expanded erythroid and megakaryocyte progenitors, respectively (Figure 4B). This is consistent with the constitutive activation of the JAK-STAT signaling pathway and cellular proliferation by these mutations,33,34 which augments cellular energy requirements. In contrast, the mutations in the epigenetic regulators Idh1, Ezh2, and Utx, as well as the Npm1 mutation (group 2 mutations), showed significant downregulation of these energy-generating pathways in their respective targets of cell type accumulation (Figures 4C and S4E), suggesting that accumulation of cells was the consequence of a differentiation block rather than a proliferative push.
The Dnmt3a and Flt3 ITD mutations did not fit into either group; the Dnmt3a mutation did not show significant metabolic changes in these energy-producing pathways (Figure 4D). Interestingly, the Flt3 ITD mutation induced downregulation of the TCA cycle while maintaining or upregulating the glycolysis reactions (Figure 4E). This dissociation between glycolysis and the TCA cycle is consistent with the Warburg effect, a cancerous metabolic alteration in favor of glucose metabolism into lactate rather than harnessing the TCA cycle.35 Altogether, our single-cell metabolic analysis has successfully recapitulated known mutation-driven metabolic alterations in the hematopoietic system and further proposed a classification of leukemogenic mutations on the basis of the potential metabolic modes of action.
Leukemogenic mutations induce cell-type-specific and dynamic changes in gene expression
To further characterize the preleukemic perturbations, we next sought to identify transcriptional signatures associated with the lineage bias in each mutant model. To this end, we performed cell-type-wise differential expression analysis of the paired mutant and wild-type samples. This revealed highly variable numbers of genes differentially expressed in different cell types and in different mutant models (Figure 5A). Reassuringly, the changes in gene expression seen in the mouse models recapitulated the gene expression signatures seen in patients with the respective mutation1,36,37,38,39 (Figure S5A). Assuming the number of differentially expressed genes as a proxy for the perturbing effect size, the Calr deletion mutation, Ezh2 KO and Utx KO showed the largest perturbing effects with >500 genes differentially expressed in one or more cell types. In contrast, the Idh1 R132H mutation induced <10 differentially expressed genes in any cell types in our model. This is consistent with a previous expression microarray analysis of an Idh1 R132H model,40 which identified no significantly differentially expressed genes with false discovery rates < 0.05, suggesting non-transcriptomic mechanisms of the Idh1 R132H mutation in leukemogenesis.
Figure 5.
Perturbed regulation of gene expression in preleukemic mutant models
(A) The number of genes differentially expressed in each cell type from each mutant model compared with the wild-type counterpart. The numbers are shown over the corresponding cell types in the individual UMAP plots. HSC, hematopoietic stem cell; prog, progenitors; Mono, monocyte; DC, dendritic cell; MEP, megakaryocyte-erythroid progenitors; MK, megakaryocyte; Ery, erythroid.
(B) Significant upregulation of cell cycle regulators in Jak2 mutant early erythroid progenitors. Gene Ontology (GO) terms for biological processes (BPs) were evaluated. NES, normalized enrichment score; FDR, false discovery rate.
(C) Significant downregulation of genes regulating megakaryocytic differentiation in Jak2 mutant MEPs.
(D) Differential expression dynamics of Pf4 between the erythroid trajectory of the Jak2 mutant and the paired wild-type samples. The upper panel shows the pseudotime distribution of each cell type, defining the pseudotime ranges of dominant cell types. The lower panel shows the pseudotemporal expression patterns of Pf4. The red (Jak2 V617F) and blue (wild-type) lines show the expression smoothers estimated by a negative binomial generalized additive model.41 Each dot shows the log-normalized expression and the pseudotime of each cell.
(E–G) Significantly altered gene expression patterns in the Jak2 mutant and wild-type erythroid trajectory (E), the Calr mutant and wild-type megakaryocyte trajectory (F), and the Flt3 mutant and wild-type myelomonocytic trajectory (G). The pseudotime ranges of dominant cell types are indicated with colored bars.
Of note, in the Jak2 V617F model, early erythroid progenitors showed the greatest transcriptomic change (Figure 5A, top left panel) with a significant activation of cell cycle regulators (Figure 5B), while population-level abundance was increased only in later erythroid progenitors (Figure 2A). Likewise, MEPs in the Calr mutant model and immature progenitors in the Flt3 ITD model showed a greater number of differentially expressed genes than megakaryocyte progenitors and neutrophil/monocyte progenitors, respectively (Figure 5A), suggesting that transcriptomic alterations precede the expansion of later progenitor stages. Furthermore, consistent with the increase in erythroid fate probability in the Jak2 V617F MEPs (Figure 3C), the regulatory genes of megakaryocyte differentiation were significantly downregulated in the Jak2 V617F MEPs (Figure 5C). Collectively, these results indicate that mutations induce differentiation stage-specific transcriptomic programs that eventually lead to a tissue-scale hematopoietic skew.
To better capture stage-specific dynamic expression changes, we next modeled gene expression as a function of pseudotime and compared the pseudotemporal expression patterns using a generalized additive model.41 By defining lineage trajectories on the basis of lineage fate probability (see STAR Methods), we first compared the pseudotemporal gene expression patterns between the Jak2 V617F and wild-type erythroid trajectory (Figure S6A) and identified 80 genes with significantly altered expression patterns (Table S3). Among them, a megakaryocytic differentiation marker Pf4 showed lower activation in the pseudotime window corresponding to the MEP stage (Figure 5D), while globin genes (Hba-a1, Hba-a2, Hbb-bs, and Hbb-bt) showed an earlier onset of expression from the mid-erythroid progenitor stage (Figure 5E; Table S3). Tfrc (encoding the transferrin receptor or CD71) as well as cell cycle regulators showed constant upregulation throughout the erythroid differentiation (Figure 5E). These results indicate that not a single factor but a combination of (1) lower activation of megakaryocytic differentiation regulators, (2) early activation of erythroid differentiation genes and (3) constant upregulation of cell cycle regulators is the transcriptomic underpinning for the erythroid bias observed within the Jak2 V617F-homozygous HSPCs.12 Early activation of megakaryocytic and myelomonocytic differentiation markers were also observed in the Calr mutant megakaryocytic trajectory and the Flt3-mutant myelomonocytic trajectory, respectively (Figures 5F, 5G, S6B, and S6C; Table S3). Moreover, the unfolded protein response genes, reported to be upregulated in CALR-mutated myeloproliferative neoplasms,37 were significantly upregulated in our Calr mutant model with pseudotemporal expression patterns similar to those in patients with CALR-mutated essential thrombocythemia37 (Figures S5B–S5F). Overall, our differential expression analysis has enabled quantification of the perturbing effects of different mutations and revealed the putative transcriptomic basis of lineage bias.
PMCA pipeline streamlines single-cell analysis of preleukemic mouse models
To test the external applicability of our analysis pipeline, we next analyzed a previously published scRNA-seq dataset of a Tet2 KO mouse model.42 The hematopoietic differentiation landscape was automatically identified (Figure S7A), and differential abundance and fate probability analyses illustrated expansion of HSCs and myeloid-biased hematopoiesis within the Tet2 KO model (Figures S7B and S7C), consistent with previous observations.43,44,45 Furthermore, single-cell metabolic analysis demonstrated reduced activity of glycolysis and the TCA cycle (Figure S7D), classifying the Tet2 mutation as a group 2 mutation. Differential expression analysis identified the largest transcriptomic changes in the immature to myeloid progenitors (Figure S7E), and myeloid differentiation/maturation markers showed significantly altered expression patterns along the neutrophilic differentiation trajectory (Figures S7F and S7G), in concordance with impaired late neutrophilic maturation by the Tet2 mutation.46 As loss-of-function mutations in the TET2 gene are among the most common drivers of human preleukemia,47,48,49 this analysis not only provides complementary results to the original publication but also serves to demonstrate the broad utility of our PMCA pipeline for streamlined characterization of mutational effects.
Preleukemic lineage perturbation signature defines patients with the most immature and refractory AML
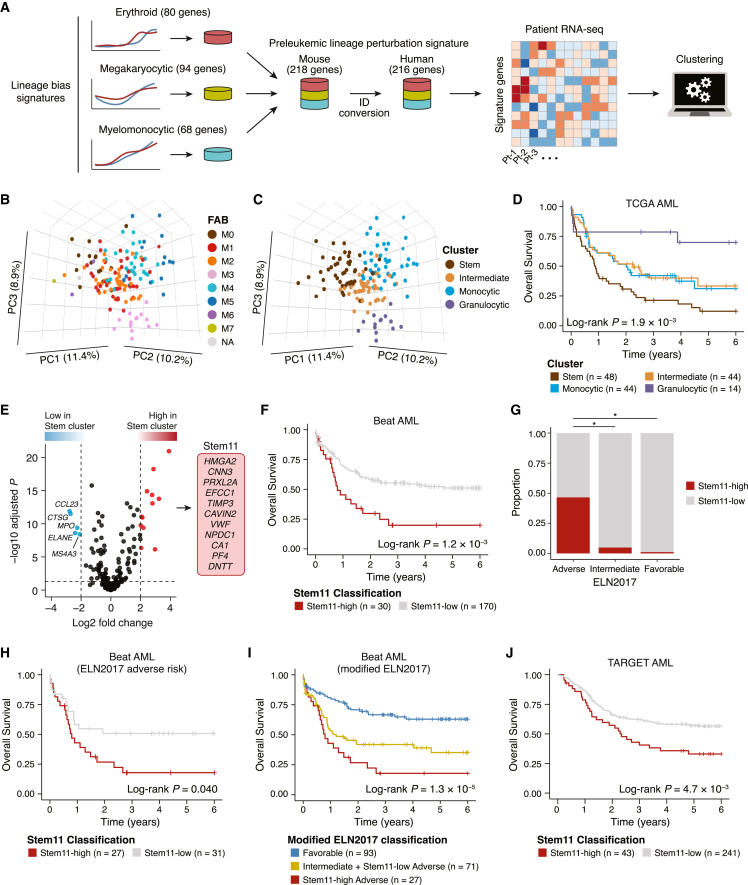
Finally, in order to evaluate the translational applicability of our analyses, we asked whether the molecular signatures derived from our pseudotemporal differential expression analysis can identify clinically relevant patient characteristics. As our analysis and previous reports12,13,50 indicate that the Jak2, Calr, and Flt3 mutations actively drive proliferation and biased differentiation, we combined the erythroid, megakaryocytic, and myelomonocytic bias signatures dysregulated by these mutations (Table S3). This allowed us to develop a comprehensive gene signature representing the biased differentiation toward the major myeloid lineages (hereafter, preleukemic lineage perturbation signature [PLPS]; 216 genes). We then applied this signature to the clustering of gene expression data from AML patients (Figure 6A; Table S4). The PLPS genes effectively separated the TCGA (The Cancer Genome Atlas) AML patient samples1 according to the morphological FAB classification in the principal component space (Figure 6B). Consistent with this, hierarchical clustering on the basis of the PLPS genes identified four clusters (Stem, Intermediate, Monocytic, and Granulocytic) with distinct expression patterns of differentiation marker genes (Figures 6C, S8A, and S8B). The stem-cell-like immature transcriptomic status of the Stem cluster was further confirmed by significant enrichment of the previously developed LSC17 score51 (Figures S8C and S8D). Notably, these four PLPS-based clusters showed significant differences in overall survival, with the Stem cluster having the poorest survival rate (Figure 6D).
Figure 6.
Prognostic relevance of preleukemic lineage perturbation signature genes
(A) Overview of the analysis of patient RNA-seq data. The erythroid, megakaryocytic, and myelomonocytic bias signatures derived from the pseudotemporal differential expression analysis were combined to develop the PLPS (preleukemic lineage perturbation signature) genes, which were then used to cluster the patient data.
(B and C) Principal-component analysis of the TCGA cohort. Samples are colored according to the FAB classification (B) and the patient clusters (C). PC1, PC2, and PC3 represent the first three principal components, with the percentage of variance explained indicated on the axes.
(D) Survival analysis comparing the overall survival of the different clusters of TCGA AML cohort.
(E) Volcano plot showing the differential expression of PLPS genes. Genes with significant upregulation (BH-adjusted p < 0.05 and log2 fold change > 2; red) and downregulation (BH-adjusted p < 0.05 and log2 fold change < −2; blue) are color coded.
(F) Survival analysis comparing the Stem11-high and Stem11-low groups in the Beat AML cohort.
(G) Proportions of Stem11-high patients in each of the ELN2017 risk groups. p values are from Fisher’s exact test. ∗p < 1.0 × 10−5.
(H) Survival analysis comparing the Stem11-high and Stem11-low patients among the ELN2017 adverse risk group in the Beat AML cohort.
(I) Survival analysis comparing our modified ELN2017 risk groups, where the Stem11-low, ELN2017 adverse-risk patients were re-stratified to the intermediate risk group.
(J) Survival analysis comparing the Stem11-high and Stem11-low groups in the TARGET AML cohort.
To further refine the PLPS genes and pinpoint genes associated with poor prognosis, we compared the expression of PLPS genes between the Stem cluster and the other three clusters. Of the 216 PLPS genes, twelve were significantly upregulated in the Stem cluster (Benjamini-Hochberg [BH]-adjusted p < 0.05 and log2 fold change > 2; Figures 6E and S9). To assess the predictive capability of these 12 genes for patient prognosis, we next analyzed the Beat AML cohort,2 in which the PLPS genes also partitioned the patient samples according to their differentiation status (Figures S10A and S10B). Of the 12 genes upregulated in the Stem cluster of the TCGA cohort, eleven were also expressed in the Beat AML cohort (hereafter, Stem11; Figure 6E). The sum of the expression Z scores of the Stem11 genes (Stem11 score) showed a bimodal distribution in which the top 15th percentile formed a peak with higher Stem11 scores (Stem11-high patients; Figure S10C). Importantly, the Stem11-high patients exhibited significantly poorer overall survival than the Stem11-low patients (p = 1.2 × 10−3; Figure 6F), indicating the predictive power of the Stem11 system. Despite the presence of physiological differentiation markers for megakaryocytic (CAVIN2, VWF, and PF4), erythroid (CA1) and lymphoid (DNTT) lineages within the Stem11 genes, the blast content of the sequenced samples did not correlate with the Stem11 score (Figure S10D), suggesting that the higher Stem11 gene expression is due to a specific expression program preferentially seen in patients with poor prognosis.
To further ascertain how the Stem11 score stratifies patient prognosis, we next evaluated the association between the Stem11 score and clinical risk factors. Although the Stem11 score did not correlate with patient age (Figure S10E), Stem11-high patients were significantly more likely to belong to the ELN2017 adverse risk group52 (Figure 6G). Specifically, 27 of 30 (90%) Stem11-high patients belonged to the ELN2017 adverse risk group, almost halving the group (Figure 6G). Additionally, the Stem11 score was significantly associated with overall survival even in the ELN2017 adverse risk group (Figure 6H). This is in contrast to the LSC17 system,51 which broadly divided the entire cohort into two prognostic groups but did not segregate the ELN2017 adverse risk group (Figures S10F and S10G). As previously reported in another independent cohort,53 the ELN2017 intermediate and adverse risk groups did not differ in patient outcomes in the Beat AML cohort (Figure S10H); however, re-stratification of the Stem11-low, ELN2017 adverse-risk patients to the intermediate risk group enabled better stratification of patient prognosis (Figure 6I). Importantly, all cases with KMT2A rearrangements had low Stem11 scores (Figure S11A), suggesting that re-stratification of this group to the intermediate risk group may be beneficial. Moreover, among the adverse risk mutations in the recently updated ELN2022 classification,54 TP53, RUNX1, BCOR, SF3B1, and ZRSR2 mutations were significantly more prevalent among the most refractory Stem11-high patients (Figure S11A), indicating that these mutations maintain characteristics inherited from preleukemic HSPCs. When applied to a cohort of AML patients uniformly treated with intensive chemotherapy,55 the Stem11 classification showed a significant association with overall survival in both univariate (p = 7.2 × 10−3) and multivariate analysis (p = 7.5 × 10−3), thus demonstrating prognostic value independent from both patient age and ELN2017 cytogenetic/molecular risk classification (Figures S10I and S10J). The Stem11 score was also significantly associated with patient outcomes in the TARGET pediatric AML cohort56 (p = 4.7 × 10−3; Figure 6J). As pediatric and adult AML have distinct mutational profiles, the ELN2022 adverse risk mutations developed for adult patients were rare and not significantly enriched among the Stem11-high pediatric patients, while the Stem11-high pediatric patients had significantly higher frequencies of PTPN11 and WT1 mutations (Figure S11B). These results indicate that the PLPS and Stem11 signatures identified through our integrated single-cell analysis of preleukemic hematopoiesis can define patients with the most immature and refractory AML for both adult and pediatric cohorts, regardless of their distinct genetic basis, thus providing improved molecular risk stratification.
Discussion
Premalignant biology is an emerging focus of global research, with the potential to advance our understanding of cancer development and to guide effective detection and intervention strategies for cancers.57 In this study, we present a scRNA-seq-based multi-scale analysis framework for characterizing mutation-driven preleukemic perturbations and demonstrate that the molecular signatures of preleukemic lineage perturbations decipher AML heterogeneity and suggest improved risk stratification strategies.
The initial step of reference-based single-cell data integration systematically anchors new datasets within a hematopoietic differentiation landscape, providing a standardized visualization that facilitates comparison across different perturbation models. Importantly, our characterization of mutation-specific perturbations is conducted by comparing paired mutant and wild-type samples with matched age, sex, genetic background, and pIpC regimens (for the Npm1, Idh1, Dnmt3a, Ezh2, Utx, and Calr [41-week-old] models). In addition, different experimental batches (i.e., different sample collections and library preparations) are included as covariates in the statistical models and adjusted to compute statistical significance. In line with the growing recognition of the importance of accounting for biological and technical variations in single-cell comparative analysis,58,59 our experimental and statistical design enables the robust identification of true mutation-driven perturbations while mitigating non-pathological false positives.
Biologically, the four downstream analysis modules collectively illustrate how individual mutations drive their target molecular programs (i.e., metabolic and expression), bias cellular lineage fates and ultimately skew the tissue-wide landscapes of hematopoiesis. Here, the fate probability analysis and metabolic flux analysis exemplify the transformative capabilities of scRNA-seq beyond gene expression comparisons. On the basis of the recently developed machine learning models,9,10 these analyses successfully recapitulate previously demonstrated experimental observations27,28,30,31 (Figures 3B, 3C, and S4), thereby validating their applicability in the study of hematopoiesis. Notably, our differential metabolic flux analysis identified two groups of mutations with opposing impacts on glycolysis and TCA cycle reactions. As these energy-generating pathways are crucial for cell proliferation and differentiation,60 our results suggest that the group 1 mutations (Jak2 and Calr) skew hematopoiesis through active proliferation and biased differentiation, while the group 2 mutations (Idh1, Npm1, Ezh2, and Utx) lead to reduced energy demands and passive accumulation of specific cell lineages. This is specifically highlighted by the contrasting effects on cell cycle status seen in the Calr mutation and Utx KO models, whereby both have increased abundance of megakaryocyte progenitors (Figure 2A), yet the Calr mutation led to an increased proportion of cycling G2/M-phase cells, while the Utx KO resulted in an increase in G1-phase cells (Figure S4F). Interestingly, all the group 2 mutations are known to act through epigenetic dysregulation.19,40,61,62 As epigenetic processes play a crucial role in normal and malignant stem cell differentiation,63 our results suggest that downregulation of energy-generating metabolic pathways may represent at least part of the metabolic underpinning of the differentiation block caused by the group 2 mutations. Metabolic modulation is a promising therapeutic strategy for targeting cancer cell-specific dependencies by limiting cell growth or inducing differentiation.64,65 Thus, our single-cell metabolic profiling provides novel insights into possible early metabolic intervention by uncovering shared and unique metabolic alterations in preleukemic conditions.
The differential abundance and differential expression analysis modules further highlight the unique advantages of using scRNA-seq in characterizing perturbations. By comparing cell densities in the reference-based common latent space, our differential abundance analysis enables statistical evaluation of mutant cell expansion and depletion at the single-cell resolution. As this approach is not constrained by predefined cell type boundaries, it can pinpoint perturbed cell populations with a level of resolution that is unattainable through discrete cluster-wise comparison or immunophenotype-based quantification, as exemplified by our identification of a late neutrophilic differentiation block by the Flt3-ITD mutation. Likewise, the continuous resolution of gene expression analysis allows for the identification of genes with perturbed dynamic expression patterns, as opposed to discrete comparison of averaged expression in transcriptionally or immunophenotypically defined cell types. Consequently, we show that individual mutations orchestrate diverse transcriptional programs over the course of differentiation, including (1) early activation of specific lineage markers, (2) lowering expression of regulators of alternative lineages, and (3) modulation of cell cycle regulators, collectively perturbing hematopoietic differentiation.
Derived from the preleukemic molecular signatures, our PLPS and Stem11 gene sets are likely to at least in part, signify aberrant lineage priming in mutant HSPCs. Intriguingly, the PLPS genes effectively distinguish the heterogeneous differentiation status of clinical AML samples, suggesting that perturbed lineage priming during the preleukemic phase determines the eventual stages of arrested differentiation in AML blasts. Furthermore, the Stem11 score, derived from a subset of PLPS genes overexpressed in the most immature AML cases, identifies both adult and pediatric AML patients with the poorest survival outcomes. Of the 11 genes, HMGA2 has been implicated in poorer prognosis in various human cancers including AML.66 Otherwise, the Stem11 system has no gene overlap with previously published prognostic systems for AML,51,67,68 thus representing a unique feature of refractory AML reflecting preleukemic perturbations. Considering the presence of multiple lineage markers (e.g., DNTT, PF4, and CA1) in the Stem11 genes, Stem11-high AML may capture features reminiscent of acute leukemia of ambiguous lineage, which is associated with immature cells of origin and inferior prognosis.69 For adult patients, the Stem11 system could have clinical impact by recommending less toxic treatment options without hematopoietic cell transplantation (HCT) for more patients by re-stratifying the Stem11-low, ELN2017 adverse-risk patients to the intermediate risk group. In pediatric cases, HCT is usually reserved for the highest risk children and relapsed cases to balance long-term toxicity against survival risk; nevertheless, molecular genetic risk factors to stratify treatment are not well established and are thus urgently needed.70 Therefore, in the pediatric context, the Stem11 system helps identify patients for whom toxic but curative HCT would be indicated.
Finally, in the present study, we have characterized mutations relevant to varying degrees of preleukemic perturbation and different clinical contexts; DNMT3A and TET2 mutations are the most common drivers of clonal hematopoiesis,47,48,49 while JAK2 and CALR mutations are rather specific to myeloproliferative neoplasms and secondary AML.2,71 IDH1, EZH2, and UTX mutations are known to be rare drivers of clonal HSPC expansion,72 whereas NPM1 and FLT3 mutations are more definitive events toward leukemogenic progression.73 As fully transformed AML patient samples consist of multiple clones with distinct mutation loads, preleukemic mouse models can make unique contribution to disentangling the individual mutational effects despite their obvious drawback of not being human. Furthermore, future studies on other recurrent drivers of human preleukemia, such as ASXL1, would be important for a more comprehensive understanding of preleukemic hematopoiesis. In this growing field of preleukemic biology, our study establishes a novel analysis framework for integrated analysis of comprehensive single-cell genomics datasets and provides new lessons and opportunities for translation into individualized therapy for patients with AML.
Limitations of the study
Although this study provides a powerful computational framework and data resources to understand preleukemic perturbations, we note a few limitations. First, this study’s primary focus on LK HSPCs precluded an assessment of mutational impacts upon more differentiated cell populations. For instance, the known effects of Ezh2 KO on B and T cell differentiation25 are not captured within the confines of the LK gate. When focusing on mature cell populations, a more comprehensive reference atlas (e.g., a total mononuclear cell atlas) is required for robust data projection and subsequent downstream analyses. Second, although a broad spectrum of leukemogenic mutations has been characterized in this study, there still remains additional recurrent drivers of human preleukemia to be characterized (e.g., ASXL1 and TP53).47,48,49 The cooperative mechanisms of multiple mutations (e.g., combined DNMT3A, NPM1, and FLT3 mutations) also need to be investigated to better understand the mechanisms of progression to overt leukemia. Finally, the insights gained from mouse models need to be compared with human preleukemia. To this end, recently developed methods enabling simultaneous single-cell DNA and RNA sequencing37,74 represent promising approaches to extracting mutant-cell-specific features within preleukemic human donors.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-EPCR | STEMCELL technologies | 60038PE |
| Anti-CD48 | eBioscience | 17-0481-82 |
| Anti-CD150 | Biolegend | 115914 |
| Anti-c-Kit | Biolegend | 105826 |
| Anti-Sca1 | Biolegend | 108128 |
| Anti-CD45 | Biolegend | 103108 |
| Lineage Cocktail | STEMCELL technologies | 19856C (part of 19856) |
| Streptavidin | Biolegend | 405233 |
| 7AAD | Life technologies | A1310 |
| Chemicals, peptides, and recombinant proteins | ||
| Ammonium chloride | STEMCELL technologies | 07850 |
| EasySep™ Mouse Hematopoietic Progenitor Cell Isolation Kit | STEMCELL technologies | 19856 |
| Critical commercial assays | ||
| Chromium Single Cell 3′ Reagent Kit v2 | 10x Genomics | N/A |
| Deposited data | ||
| scRNA-seq data | This paper | GEO: GSE227026 |
| Experimental models: Organisms/strains | ||
| Mouse: Jak2 V617F: C57BL6J-Jak2V617F/V617F | Li et al.12 | N/A |
| Mouse: Jak2 WT: C57BL6J-Jak2+/+ | Li et al.12 | N/A |
| Mouse: Calr 52-bp del: C57BL6J-Calrfl/fl; Vav-Cre+/− | Li et al.13 | N/A |
| Mouse: Calr WT: C57BL6J-Calrfl/+; Vav-CreWT | Li et al.13 | N/A |
| Mouse: Calr 52-bp del: C57BL6J-Calrfl/fl; Mx1-Cre+/− | Li et al.13 | N/A |
| Mouse: Calr WT: C57BL6J-Calr+/+; Mx1-Cre+/− | Li et al.13 | N/A |
| Mouse: Flt3 ITD: C57BL6-Flt3+/ITD | Dovey et al.14 | N/A |
| Mouse: Flt3 WT #1: C57BL6-Flt3+/+ | Dovey et al.14 | N/A |
| Mouse: Flt3 WT #2: C57BL6-CRTKOWT; Mx1-CreWT | Mesaeli et al.75 | N/A |
| Mouse: Flt3 WT #3: C57BL6J-Calrfl/+; Vav-CreWT | Li et al.13 | N/A |
| Mouse: Npm1c: C57BL6-Npm1flox-cA/+; Mx1-Cre+/− | Vassiliou et al.15 | N/A |
| Mouse: Npm1 WT: C57BL6- Npm1flox-cA/+; Mx1-CreWT | Vassiliou et al.15 | N/A |
| Mouse: Idh1 R132H: C57BL6-Idh1R132H/+; Mx1-Cre+/− | Gupta et al.16 | N/A |
| Mouse: Idh1 WT: C57BL6-Idh1R132H/+; Mx1-Cre- | Gupta et al.16 | N/A |
| Mouse: Dnmt3a R882H: C57BL6-Dnmt3aflox-R882H/+; Mx1-Cre+/− | Gozdecka et al.17 | N/A |
| Mouse: Dnmt3a WT: C57BL6- Dnmt3aflox-R882H/+; Mx1-CreWT | Gozdecka et al.17 | N/A |
| Mouse: Ezh2 KO: C57BL6-Ezh2fl/fl; Mx1-Cre+/− | Basheer et al.18 | N/A |
| Mouse: Ezh2 WT: C57BL6-Ezh2fl/fl; Mx1-CreWT | Basheer et al.18 | N/A |
| Mouse: Utx KO: C57BL6-Utxfl/fl; Mx1-Cre+/− | Gozdecka et al.19 | N/A |
| Mouse: Utx WT: C57BL6-Utxfl/fl; Mx1-CreWT | Gozdecka et al.19 | N/A |
| Software and algorithms | ||
| Code and algorithms for analysis | This paper | https://doi.org/10.5281/zenodo.8345465 |
| Cell Ranger | 10x Genomics | v6.0.1 |
| Scanpy | Wolf et al.76 | https://scanpy.readthedocs.io/ |
| Scrublet | Wolock et al.77 | https://github.com/swolock/scrublet |
| Seurat | Stuart et al.20 | https://satijalab.org/seurat/ |
| MELD | Burkhardt et al.21 | https://github.com/KrishnaswamyLab/MELD |
| CellRank | Lange et al.9 | https://cellrank.readthedocs.io/ |
| scFEA | Alghamdi et al.10 | https://github.com/changwn/scFEA |
| edgeR | Robinson et al.78 | https://bioconductor.org/packages/release/bioc/html/edgeR.html |
| DESeq2 | Love et al.79 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| tradeSeq | Van den Berge et al.41 | https://github.com/statOmics/tradeSeq |
| GSEA | Subramanian et al.80 | https://www.gsea-msigdb.org/gsea/index.jsp |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Berthold Göttgens (bg200@cam.ac.uk).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Mice
This study included a total of 38 animals from eight different mutant mouse models: homozygous Jak2 V617F12 (n = 3), homozygous Calr 52-bp deletion13 (n = 2), heterozygous Flt3 ITD14 (n = 3), heterozygous Npm1c15 (n = 2), heterozygous Idh1 R132H16 (n = 3), heterozygous Dnmt3a R882H17 (n = 2), homozygous Ezh2 KO18 (n = 2) and homozygous Utx KO19 (n = 2). For each mutant model, paired wild-type mice with the same background (n = 1–3 per model; n = 17 in total) were simultaneously collected and subjected to flow cytometry sorting and sequencing to obtain wild-type comparators with minimal batch effects. For the Flt3 ITD model, one paired and two unpaired wild-type animals were collected.13,14,75 For the Npm1, Idh1, Dnmt3a, Ezh2, Utx and Calr (41-week-old mice) models, Mx1-Cre was induced by intraperitoneal injection of pIpC (Sigma #P1530). All mice were bred and maintained in microisolator cages and provided continuously with sterile food, water, and bedding. All mice were kept in specified pathogen-free conditions, and all procedures were performed according to the United Kingdom Home Office regulations. Details of all animals used in this study, including age, sex and pIpC regimens, are summarized in Table S1. Further details on the individual mouse models are provided in the original reports cited above.
Method details
Flow cytometry sorting of hematopoietic stem and progenitor cells
Mouse bone marrow cells were collected from the femurs, tibiae and iliac crest and depleted of red blood cells by an ammonium chloride lysis step (STEMCELL Technologies). Cells were lineage depleted using EasySep Mouse Hematopoietic Progenitor Cell Isolation Kit (19856, STEMCELL Technologies). Lineage− c-Kit+ (LK) cells were then isolated using the following antibodies (clone and company): streptavidin BV510 (BioLegend), c-kit APC-Cy7 (2B8, BioLegend), Sca1 BV421 (D7, BioLegend), CD45 FITC (30-F11, BioLegend), EPCR (CD201) PE (RMEPCR1560, STEMCELL Tech), CD150 PE/Cy7 (TC15-12F12.2, BioLegend), CD45 FITC (30-F1,1 BD Bioscience) and CD48 APC (HM48-1, eBioscience). Flow cytometry was performed on an LSRII Fortessa (BD) and all data were analyzed using FlowJo (BD). A representative gating strategy is shown in Figure S12.
scRNA-seq data generation and preprocessing
The scRNA-seq data of the Calr mutant (n = 2) and the paired wild-type samples (n = 2) were previously published27 and re-analyzed in this study; all remaining samples were newly sequenced for the current study. Single-cell sequencing libraries were generated using 10x Chromium (10x Genomics, Pleasanton, CA) reagent kit v2 according to the manufacturer’s protocol and sequenced on an Illumina HiSeq 4000 or Illumina Novaseq 6000 platform. Raw reads were mapped to the mm10 genome and quantified using the Cell Ranger pipeline (v6.0.1) with default parameters. Cell-associated barcodes and background-associated barcodes were determined using the EmptyDrops method81 implemented in the Cell Ranger pipeline, and the background-associated barcodes were excluded. Subsequent data analysis was performed using Scanpy.76 Cell libraries with less than 1,000 detected genes or with mitochondrial gene expression exceeding 10% of UMI counts were removed from downstream analysis. Multiplets were estimated using the Python package Scrublet77 and subsequently removed. Cell cycle phase was assigned to each cell using a previously published list of cell cycle-associated genes82 and the Scanpy function ‘tl.score_genes_cell_cycle’. All remaining cells that passed these quality controls (n = 269,048) were used for the downstream analysis. A previously published scRNA-seq dataset from Tet2 KO and wild-type mice (GSE124822)42 was subjected to the same mapping and preprocessing methods as shown above.
Reference atlas of mouse hematopoietic landscape
Our previously published mouse HSPC atlas11 including 44,802 LK and LSK cells was used as the reference hematopoietic landscape. The reference atlas data was log-normalized, and 5,000 highly variable genes were identified using the Scanpy function ‘pp.highly_variable_genes’. Cell cycle-associated genes83 were then removed and the expression values of the remaining 4,713 highly variable genes were scaled and used to compute 50 principal components, which were subsequently used to identify 10 nearest neighbors. The UMAP embedding was computed using the python package umap-learn84 and the fitted model was saved to be applied to the mutant datasets as described below.
Reference-based integration of mutant hematopoietic landscapes
All mutant and paired wild-type samples (n = 38) were log-normalized and projected onto the reference atlas using the reference-based integration method of Seurat.20 First, all detected genes excluding cell cycle-associated genes were used to compute 30 canonical correlation vectors using the Seurat function ‘RunCCA’. Next, integration anchors were identified using the ‘FindIntegrationAnchors’ with the option ‘reference’ set to the reference atlas data and using canonical correlation analysis for dimension reduction (reduction = ‘cca’). Subsequently, each sample was integrated with the reference atlas using the ‘IntegrateData’ function using the canonical correlation vectors for anchor weighting. These integration steps were performed on the basis of the individual samples.
Integrated expression data were then scaled using the gene-wise means and standard deviations derived from the reference atlas data. Finally, 50 principal components and UMAP embeddings were computed using the same model fitted to the reference atlas data to project each sample onto the latent space of the reference atlas. Cell type annotation was transferred from the reference atlas using the Seurat functions ‘FindTransferAnchors’ and ‘TransferData’ with the dimension reduction method set to ‘cca’.
Differential abundance analysis
Mutation-specific changes in subpopulation abundance were quantified using the python package MELD.21 First, a cell similarity graph was constructed based on the Euclidean distances in the common 50-dimensional principal component space after the data integration. Next, a kernel density estimate (KDE) was computed for each biological replicate and smoothed over the cell similarity graph with eight nearest neighbors and the smoothing parameter β = 15. The KDEs of paired mutant and wild-type samples were then compared, and differential abundance was quantified as the relative likelihood of observing each cell in either the mutant or wild-type condition by performing cell-wise L1 normalization of the KDEs as implemented in the MELD package. The mutant relative likelihoods from all pairwise comparisons were averaged to obtain the mutation-specific differential abundance landscapes. Statistical significance was tested by comparing the sample-wise KDEs between the paired mutant and wild-type samples. We used paired t test for the mutant models with paired samples from multiple experimental batches (Jak2, Calr) and independent t test for the other datasets. p values were adjusted with the Benjamini-Hochberg (BH) procedure and cells with raw p values < 0.05 and BH-adjusted p values < 0.25 were considered significant.
Differential fate probability analysis
Cellular fate probability was inferred using the python package CellRank.9 The HSC score (molecular signature of long-term repopulating HSCs22) was first computed for all samples to determine the root cells for pseudotime calculation. The HSC score was then smoothed by taking the mean of 10 nearest neighbors in the diffusion map, and the cell with the highest smoothed HSC score was used as the root cell in each sample. Diffusion pseudotime was computed for each sample using the Scanpy function ‘tl.dpt’. Cells without assigned cell types (cell type = ‘Unassigned’) were removed from the analysis.
Cell-to-cell transition probabilities were then inferred using the CellRank’s pseudotime kernel (‘tl.kernels.PseudotimeKernel’) and the function ‘compute_transition_matrix’ with the ‘soft’ weighting scheme (threshold_scheme = ’soft'). Terminally differentiated cells were first identified in the reference atlas using the ‘compute_macrostates’ function. The terminal cells in each sample were then identified as the 10 nearest neighbors of the reference terminal cells in the common principal component space after the data integration. Estimated terminal cells with only one supporting neighbor or outlier terminal cells were excluded.
The fate probabilities toward these terminal states were computed using the CellRank’s Generalized Perron Cluster Cluster Analysis (GPCCA) estimator and the function ‘compute_absorption_probabilities’. The inferred fate probabilities were compared between the paired mutant and wild-type samples by logistic regression and likelihood ratio test using the Seurat function ‘findMarkers’ with the option ‘LR’ (test.use = ‘LR’). The experimental batch information was included as a covariate in the logistic regression model to account for batch effects in the statistical evaluation. Lineage fate changes with |median fate probability differences| >5% and BH-adjusted p values < 0.05 were considered significant. The fate probabilities in each condition were summarized in pie charts with partition-based graph abstraction (PAGA) connections.85
Differential metabolic flux analysis
Raw expression counts were first normalized to counts per million (CPM), which were then used as input for the python package scFEA.10 Metabolic flux values for the 168 core metabolic reactions implemented in scFEA were inferred using the default parameters. The inferred metabolic flux values were compared between the paired mutant and wild-type samples by logistic regression and likelihood ratio test using the Seurat function ‘findMarkers’ with the option ‘LR’ (test.use = ‘LR’) with the experimental batch information included as a covariate. Constant low-flux reactions with differences between the minimum and maximum flux <1.0 × 10−4 were excluded. The size of differences between two groups was evaluated by Cohen’s D standardized mean differences. The metabolic reactions with |Cohen’s D| > 0.15 and BH-adjusted p values < 0.05 were considered significant. A published scRNA-seq dataset (GSE155763)32 was used to validate the method.
Pseudobulk differential expression analysis
Cell-type-wise differential expression analysis was performed with a pseudobulk method. First, gene-by-cell expression matrices were summed and aggregated for different cell types in different biological replicates to generate gene-by-replicate expression matrices. Single-cell-level mean expression was computed for each cell type, and low-expression genes with mean single-cell expression ≤0.05 counts per 10,000 UMIs (CP10K) were removed before differential expression testing. Differential expression analysis was performed using the likelihood ratio test of edgeR78 with experimental batch information included as a covariate in the additive linear model. Genes with |log2 fold changes| ≥0.5 and BH-adjusted p values < 0.05 were considered significant. Gene set enrichment analysis was performed using the edgeR-derived −log10 adjusted p values with the signs of log2 fold changes as input for the preranked mode of GSEA software.80
Pseudotemporal differential expression analysis
To identify genes with altered expression patterns over the course of differentiation, paired mutant and wild-type samples were combined and a common pseudotime was computed for each pair. The root cell for pseudotime inference was determined according to the highest smoothed HSC score as described above (see ‘Differential fate probability analysis’).
The erythroid, megakaryocytic and myelomonocytic trajectories were extracted from our Jak2, Calr and Flt3 datasets, respectively. The Jak2 mutant or wild-type erythroid trajectory was defined as the cells belonging to the 'HSC', 'Immature prog', 'MEP', 'Early Ery prog', 'Mid Ery prog' or 'Late Ery prog' clusters and with the erythroid fate probability ≥0.20, neutrophil probability <0.3 and Mono/DC probability <0.23. A small number of cells (56 of 24,210 cells, 0.23%) at the end of the trajectory with variable pseudotime ≥0.40 were excluded. For the Calr mutant or wild-type megakaryocytic trajectory, cells belonging to the 'HSC', 'Immature prog', 'MEP' or 'MK prog' clusters and with the megakaryocytic fate probability ≥0.08 and neutrophil probability <0.3 were included. The Flt3 mutant or wild-type myelomonocytic trajectory was defined as the cells belonging to the 'HSC', 'Immature prog' or 'Mono/DC prog' clusters and with the monocytic fate probability ≥0.25 and the pseudotime <0.10.
Low-expression genes with mean CP10K ≤ 0.05 were removed before differential expression testing. A negative binomial generalized additive model was fitted using the ‘fitGAM’ function of the R package tradeSeq41 with six knots (nknots = 6) and with the experimental batch information included as a covariate in the model. The fitted pseudotemporal expression patterns were then compared between the mutant and wild-type samples using the function ‘conditionTest’ with the log2 fold change threshold of 0.5 (‘l2fc = 0.5’). Genes with BH-adjusted p values < 0.05 were considered significant.
CALR-mutated patient dataset
A previously published scRNA-seq dataset (GSE117826)37 of CD34+ bone marrow HSPCs from patients with CALR-mutated essential thrombocythemia was analyzed. Multiplets were first estimated using Scrublet and subsequently removed. Cells with less than 1000 UMI counts or with mitochondrial gene percentage >10% were then removed. Cells with no genotyping UMI information were further removed. The remaining cells (n = 17,975) were log-normalized, and 500 highly variable genes were identified using the Scanpy function ‘pp.highly_variable_genes’. Cell cycle-associated genes86 were then removed and the expression values of the remaining 489 highly variable genes were scaled and used to compute 50 principal components. Five patient samples (ET01-ET05) were then integrated using Harmony87 with a theta of 0. Harmony adjusted principal components were subsequently used to identify 10 nearest neighbors, and the UMAP embedding was computed using the Scanpy function ‘tl.umap’. Leiden clustering was performed using the ‘tl.leiden’ function with a resolution of 0.6, and the cluster cell identity was assigned based on known marker genes. Based on the genotyping information obtained from GSE117826, cells with num.MUT.call ≥ 1 and total genotyping UMI ≥ 2 were labeled as mutant, while cells with num.MUT.call = 0 and total genotyping UMI ≥ 2 were labeled as wild-type.
For pseudotemporal differential expression analysis, the human HSC score88 was first computed to determine the root cell for pseudotime calculation. The HSC score was then smoothed by taking the mean of 10 nearest neighbors in the diffusion map, and the cell with the highest smoothed HSC score was used as the root cell. Diffusion pseudotime was computed using the Scanpy function ‘tl.dpt’. The megakaryocyte trajectory was defined as the HSC and megakaryocyte progenitor clusters. Cells at the end of the trajectory with variable pseudotime ≥0.10 were excluded, and the remaining cells (1,199 mutant and 2,226 wild-type cells) were analyzed for pseudotemporal differential gene expression using tradeSeq.
AML patient datasets
Publicly available TCGA AML,1 Beat AML,2 EGAD0000100848455 and TARGET AML56 RNA-seq datasets were used. Gene expression raw counts and TPM values of the TCGA and Beat AML cohorts were downloaded using the R package TCGAbiolinks. Clinical data for the TCGA cohort were downloaded from the National Cancer Institute Genomic Data Commons (https://gdc.cancer.gov/about-data/publications/laml_2012). Clinical and mutation data for the Beat AML cohort were downloaded from the Beat AML data portal (https://biodev.github.io/BeatAML2). For the Beat AML cohort, de novo AML patients with RNA-seq data and FAB classification available were included. Samples with low tumor cell content (<20%) were excluded. Patients with FAB labels of “M0/M1”, “M4eo”, “M5a” and “M5b” were reannotated in the figures as “M1”, “M4”, “M5” and “M5”, respectively. For the EGAD00001008484 dataset, gene expression data were downloaded from the European Genome-Phenome Archive (EGA), and de novo AML patients were included. For the TARGET AML cohort, gene expression data were downloaded from the National Cancer Institute TARGET data portal (https://target-data.nci.nih.gov/Public/AML/).
Analysis of patient RNA-seq data
The gene expression matrices were filtered to include only the 216 preleukemic lineage perturbation signature genes, and low-expression genes with mean TPM ≤0.5 were further excluded. The expression TPM values were log-normalized, scaled and used to compute the principal components. The first three principal components were used for visualization. For the TCGA cohort, hierarchical clustering was performed using the ‘ward.D2’ method and differential expression analysis were performed using the R package DESeq2.79 Two-group comparisons were performed using the Wald test, and p values were adjusted with the BH procedure. The Stem11 genes were defined based on significant overexpression (log2 fold change > 2 and BH-adjusted p < 0.05) in the Stem cluster of the TCGA cohort, where 12 genes were identified and one gene (RORB) was removed due to low expression in the Beat-AML cohort (mean TPM <0.5). The Stem11 score was calculated as the sum of the Z-scores of the Stem11 genes, and Stem-11 high patients were defined as the top 15th percentile.
Survival analysis
Survival was estimated using the Kaplan–Meier method and the difference was tested using the log rank test. Overall survival was compared over the first 6 years of observation. For multivariate analysis, a Cox proportional hazards regression model was used to identify the risk factors associated with the overall survival. p values of less than 0.05 were considered statistically significant. Statistical analysis was performed using the R package survival.
Quantification and statistical analysis
Statistical analyses were performed using Python (3.8.6 or 3.8.12) or R (3.6.3 or 4.0.3). All statistical methods used in this study are described in the individual figure legends and the METHOD DETAILS. For differential abundance analysis, cells with raw p values < 0.05 and BH-adjusted p values < 0.25 were considered significant. For differential fate probability analysis, differential metabolic flux analysis and differential expression analysis, BH-adjusted p values of less than 0.05 were considered significant. For all the other analyses, raw p values of less than 0.05 were considered significant.
Acknowledgments
Work in the Göttgens laboratory is funded by grants from Wellcome (206328/Z/17/Z), Blood Cancer UK (18002), Cancer Research UK (C1163/A21762), National Institutes of Health (NIDDK DK106766); core support grants from the Cancer Research UK Cambridge Centre (C49940/A25117), the Wellcome Trust (203151/Z/16/Z), and the UKRI Medical Research Council (MC_PC_17230). T.I. is supported by the Funai Foundation for Information Technology. Work in the Green laboratory is funded by grants from Wellcome (RG74909), Cancer Research UK (RG83389), and the William B. Harrison Foundation (RG91681). The authors thank Reiner Schulte, Chiara Cossetti, and Gabriela Grondys-Kotarba from the Cambridge Institute for Medical Research Flow Cytometry Core facility for their assistance with cell sorting. We would also like to thank the Cancer Research UK Cambridge Institute Genomics Core Facility for performing high-throughput sequencing. The graphical abstract was created using BioRender.com. For the purpose of open access, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission.
Author contributions
Conceptualization, T.I., N.K.W., and B.G.; methodology, T.I., I.K., N.K.W., and B.G.; software, T.I., I.K., M.B., and X.W.; validation, T.I., N.K.W., and B.G.; formal analysis, T.I., N.K.W., and B.G.; investigation, T.I., S.C., M.S.V., K.H.S., M.J.W., and N.K.W.; resources, H.P.B., G.G., L.M., J.L., J.R., M.G., D.P., M.S.S., S.W., A.R.G., D.G.K., G.S.V., B.J.P.H., N.K.W., and B.G.; data curation, T.I., R.H., N.K.W., and B.G.; writing – original draft, T.I.; writing – review & editing, T.I., N.K.W., and B.G.; visualization, T.I., N.K.W., and B.G.; supervision, N.K.W. and B.G.; project administration, T.I., N.K.W., and B.G.; funding acquisition, T.I. and B.G.
Declaration of interests
Aspects of this work are included in United Kingdom patent application 2312684.0.
Published: October 27, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xgen.2023.100426.
Contributor Information
Nicola K. Wilson, Email: nkw22@cam.ac.uk.
Berthold Göttgens, Email: bg200@cam.ac.uk.
Supplemental information
Data and code availability
-
•
All raw sequencing data has been deposited on GEO under accession number GSE227026.
-
•
All processed data and analysis results can be explored via our interactive web portal at https://gottgens-lab.stemcells.cam.ac.uk/preleukemia_atlas/.
-
•
All original code has been deposited at https://github.com/TomoyaIsobe/PMCA/. DOI is listed in the key resources table.
References
- 1.Cancer Genome Atlas Research Network Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013;368:2059–2074. doi: 10.1056/nejmoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyner J.W., Tognon C.E., Bottomly D., Wilmot B., Kurtz S.E., Savage S.L., Long N., Schultz A.R., Traer E., Abel M., et al. Functional Genomic Landscape of Acute Myeloid Leukemia. Nature. 2018;562:526–531. doi: 10.1038/s41586-018-0623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granfeldt Østgård L.S., Medeiros B.C., Sengeløv H., Nørgaard M., Andersen M.K., Dufva I.H., Friis L.S., Kjeldsen E., Marcher C.W., Preiss B., et al. Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J. Clin. Oncol. 2015;33:3641–3649. doi: 10.1200/jco.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar A.J., Rampal R.K., Levine R. Leukemia secondary to myeloproliferative neoplasms. Blood. 2020;136:61–70. doi: 10.1182/blood.2019000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J., Kao Y.-R., Sun D., Todorova T.I., Reynolds D., Narayanagari S.-R., Montagna C., Will B., Verma A., Steidl U. Myelodysplastic Syndrome Progression to Acute Myeloid Leukemia at the Stem Cell Level. Nat. Med. 2019;25:103–110. doi: 10.1038/s41591-018-0267-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fabre M.A., de Almeida J.G., Fiorillo E., Mitchell E., Damaskou A., Rak J., Orrù V., Marongiu M., Chapman M.S., Vijayabaskar M.S., et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606:335–342. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson N.A., Latorre-Crespo E., Terradas-Terradas M., Lemos-Portela J., Purcell A.C., Livesey B.J., Hillary R.F., Murphy L., Fawkes A., MacGillivray L., et al. Longitudinal dynamics of clonal hematopoiesis identifies gene-specific fitness effects. Nat. Med. 2022;28:1439–1446. doi: 10.1038/s41591-022-01883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laurenti E., Göttgens B. From haematopoietic stem cells to complex differentiation landscapes. Nature. 2018;553:418–426. doi: 10.1038/nature25022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange M., Bergen V., Klein M., Setty M., Reuter B., Bakhti M., Lickert H., Ansari M., Schniering J., Schiller H.B., et al. CellRank for directed single-cell fate mapping. Nat. Methods. 2022;19:159–170. doi: 10.1038/s41592-021-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alghamdi N., Chang W., Dang P., Lu X., Wan C., Gampala S., Huang Z., Wang J., Ma Q., Zang Y., et al. A graph neural network model to estimate cell-wise metabolic flux using single-cell RNA-seq data. Genome Res. 2021;31:1867–1884. doi: 10.1101/gr.271205.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlin J.S., Hamey F.K., Pijuan-Sala B., Shepherd M., Lau W.W.Y., Nestorowa S., Weinreb C., Wolock S., Hannah R., Diamanti E., et al. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 2018;131:e1–e11. doi: 10.1182/blood-2017-12-821413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Kent D.G., Godfrey A.L., Manning H., Nangalia J., Aziz A., Chen E., Saeb-Parsy K., Fink J., Sneade R., et al. JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood. 2014;123:3139–3151. doi: 10.1182/blood-2013-06-510222. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Prins D., Park H.J., Grinfeld J., Gonzalez-Arias C., Loughran S., Dovey O.M., Klampfl T., Bennett C., Hamilton T.L., et al. Mutant calreticulin knockin mice develop thrombocytosis and myelofibrosis without a stem cell self-renewal advantage. Blood. 2018;131:649–661. doi: 10.1182/blood-2017-09-806356. [DOI] [PubMed] [Google Scholar]
- 14.Dovey O.M., Chen B., Mupo A., Friedrich M., Grove C.S., Cooper J.L., Lee B., Varela I., Huang Y., Vassiliou G.S. Identification of a germline F692L drug resistance variant in cis with Flt3-internal tandem duplication in knock-in mice. Haematologica. 2016;101:e328–e331. doi: 10.3324/haematol.2016.146159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vassiliou G.S., Cooper J.L., Rad R., Li J., Rice S., Uren A., Rad L., Ellis P., Andrews R., Banerjee R., et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat. Genet. 2011;43:470–475. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S., Dovey O.M., Domingues A.F., Cyran O.W., Cash C.M., Giotopoulos G., Rak J., Cooper J., Gozdecka M., Dijkhuis L., et al. Transcriptional variability accelerates preleukemia by cell diversification and perturbation of protein synthesis. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abn4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gozdecka M., Dudek M., Tzelepis K., Damaskou A., Baskar V., Wright P., Duddy G., Meduri E., Mazan M., Yusa K., et al. Genetic Vulnerabilities of DNMT3AR882H in Myeloid Malignancies. Blood. 2019;134:111. doi: 10.1182/blood-2019-126505. [DOI] [Google Scholar]
- 18.Basheer F., Giotopoulos G., Meduri E., Yun H., Mazan M., Sasca D., Gallipoli P., Marando L., Gozdecka M., Asby R., et al. Contrasting requirements during disease evolution identify EZH2 as a therapeutic target in AML. J. Exp. Med. 2019;216:966–981. doi: 10.1084/jem.20181276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gozdecka M., Meduri E., Mazan M., Tzelepis K., Dudek M., Knights A.J., Pardo M., Yu L., Choudhary J.S., Metzakopian E., et al. UTX-mediated enhancer and chromatin remodeling suppresses myeloid leukemogenesis through noncatalytic inverse regulation of ETS and GATA programs. Nat. Genet. 2018;50:883–894. doi: 10.1038/s41588-018-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive Integration of Single-Cell Data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkhardt D.B., Stanley J.S., Tong A., Perdigoto A.L., Gigante S.A., Herold K.C., Wolf G., Giraldez A.J., van Dijk D., Krishnaswamy S. Quantifying the effect of experimental perturbations at single-cell resolution. Nat. Biotechnol. 2021;39:619–629. doi: 10.1038/s41587-020-00803-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson N.K., Kent D.G., Buettner F., Shehata M., Macaulay I.C., Calero-Nieto F.J., Sánchez Castillo M., Oedekoven C.A., Diamanti E., Schulte R., et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015;16:712–724. doi: 10.1016/j.stem.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sexauer A., Perl A., Yang X., Borowitz M., Gocke C., Rajkhowa T., Thiede C., Frattini M., Nybakken G.E., Pratz K., et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood. 2012;120:4205–4214. doi: 10.1182/blood-2012-01-402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nybakken G.E., Canaani J., Roy D., Morrissette J.D., Watt C.D., Shah N.P., Smith C.C., Bagg A., Carroll M., Perl A.E. Quizartinib elicits differential responses that correlate with karyotype and genotype of the leukemic clone. Leukemia. 2016;30:1422–1425. doi: 10.1038/leu.2015.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mochizuki-Kashio M., Mishima Y., Miyagi S., Negishi M., Saraya A., Konuma T., Shinga J., Koseki H., Iwama A. Dependency on the polycomb gene Ezh2 distinguishes fetal from adult hematopoietic stem cells. Blood. 2011;118:6553–6561. doi: 10.1182/blood-2011-03-340554. [DOI] [PubMed] [Google Scholar]
- 26.Haghverdi L., Büttner M., Wolf F.A., Buettner F., Theis F.J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods. 2016;13:845–848. doi: 10.1038/nmeth.3971. [DOI] [PubMed] [Google Scholar]
- 27.Prins D., Park H.J., Watcham S., Li J., Vacca M., Bastos H.P., Gerbaulet A., Vidal-Puig A., Göttgens B., Green A.R. The stem/progenitor landscape is reshaped in a mouse model of essential thrombocythemia and causes excess megakaryocyte production. Sci. Adv. 2020;6 doi: 10.1126/sciadv.abd3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamieson C.H.M., Gotlib J., Durocher J.A., Chao M.P., Mariappan M.R., Lay M., Jones C., Zehnder J.L., Lilleberg S.L., Weissman I.L. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc. Natl. Acad. Sci. USA. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falini B., Nicoletti I., Martelli M.F., Mecucci C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc+ AML): biologic and clinical features. Blood. 2007;109:874–885. doi: 10.1182/blood-2006-07-012252. [DOI] [PubMed] [Google Scholar]
- 30.Rao T.N., Hansen N., Hilfiker J., Rai S., Majewska J.-M., Leković D., Gezer D., Andina N., Galli S., Cassel T., et al. JAK2-mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood. 2019;134:1832–1846. doi: 10.1182/blood.2019000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Z., Liu Y., Cai F., Patrick M., Zmajkovic J., Cao H., Zhang Y., Tasdogan A., Chen M., Qi L., et al. Loss of EZH2 Reprograms BCAA Metabolism to Drive Leukemic Transformation. Cancer Discov. 2019;9:1228–1247. doi: 10.1158/2159-8290.cd-19-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., Gu Z., Cao H., Kaphle P., Lyu J., Zhang Y., Hu W., Chung S.S., Dickerson K.E., Xu J. Convergence of oncogenic cooperation at single-cell and single-gene levels drives leukemic transformation. Nat. Commun. 2021;12:6323. doi: 10.1038/s41467-021-26582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James C., Ugo V., Le Couédic J.P., Staerk J., Delhommeau F., Lacout C., Garçon L., Raslova H., Berger R., Bennaceur-Griscelli A., et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 34.Klampfl T., Gisslinger H., Harutyunyan A.S., Nivarthi H., Rumi E., Milosevic J.D., Them N.C.C., Berg T., Gisslinger B., Pietra D., et al. Somatic Mutations of Calreticulin in Myeloproliferative Neoplasms. N. Engl. J. Med. 2013;369:2379–2390. doi: 10.1056/nejmoa1311347. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen E., Beer P.A., Godfrey A.L., Ortmann C.A., Li J., Costa-Pereira A.P., Ingle C.E., Dermitzakis E.T., Campbell P.J., Green A.R. Distinct Clinical Phenotypes Associated with JAK2V617F Reflect Differential STAT1 Signaling. Cancer Cell. 2010;18:524–535. doi: 10.1016/j.ccr.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam A.S., Kim K.-T., Chaligne R., Izzo F., Ang C., Taylor J., Myers R.M., Abu-Zeinah G., Brand R., Omans N.D., et al. Somatic mutations and cell identity linked by Genotyping of Transcriptomes. Nature. 2019;571:355–360. doi: 10.1038/s41586-019-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valk P.J.M., Verhaak R.G.W., Beijen M.A., Erpelinck C.A.J., Barjesteh van Waalwijk van Doorn-Khosrovani S., Boer J.M., Beverloo H.B., Moorhouse M.J., van der Spek P.J., Löwenberg B., et al. Prognostically Useful Gene-Expression Profiles in Acute Myeloid Leukemia. N. Engl. J. Med. 2004;350:1617–1628. doi: 10.1056/nejmoa040465. [DOI] [PubMed] [Google Scholar]
- 39.Verhaak R.G.W., Goudswaard C.S., van Putten W., Bijl M.A., Sanders M.A., Hugens W., Uitterlinden A.G., Erpelinck C.A.J., Delwel R., Löwenberg B., Valk P.J.M. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood. 2005;106:3747–3754. doi: 10.1182/blood-2005-05-2168. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki M., Knobbe C.B., Munger J.C., Lind E.F., Brenner D., Brüstle A., Harris I.S., Holmes R., Wakeham A., Haight J., et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Berge K., Roux de Bézieux H., Street K., Saelens W., Cannoodt R., Saeys Y., Dudoit S., Clement L. Trajectory-based differential expression analysis for single-cell sequencing data. Nat. Commun. 2020;11:1201. doi: 10.1038/s41467-020-14766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izzo F., Lee S.C., Poran A., Chaligne R., Gaiti F., Gross B., Murali R.R., Deochand S.D., Ang C., Jones P.W., et al. DNA methylation disruption reshapes the hematopoietic differentiation landscape. Nat. Genet. 2020;52:378–387. doi: 10.1038/s41588-020-0595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moran-Crusio K., Reavie L., Shih A., Abdel-Wahab O., Ndiaye-Lobry D., Lobry C., Figueroa M.E., Vasanthakumar A., Patel J., Zhao X., et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self-Renewal and Myeloid Transformation. Cancer Cell. 2011;20:11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko M., Huang Y., Jankowska A.M., Pape U.J., Tahiliani M., Bandukwala H.S., An J., Lamperti E.D., Koh K.P., Ganetzky R., et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakauchi Y., Azizi A., Thomas D., Corces M.R., Reinisch A., Sharma R., Cruz Hernandez D., Köhnke T., Karigane D., Fan A., et al. The Cell Type–Specific 5hmC Landscape and Dynamics of Healthy Human Hematopoiesis and TET2 -Mutant Preleukemia. Blood Cancer Discov. 2022;3:346–367. doi: 10.1158/2643-3230.bcd-21-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huerga Encabo H., Aramburu I.V., Garcia-Albornoz M., Piganeau M., Wood H., Song A., Ferrelli A., Sharma A., Minutti C.M., Domart M.-C., et al. Loss of TET2 in human hematopoietic stem cells alters the development and function of neutrophils. Cell Stem Cell. 2023;30:781–799.e9. doi: 10.1016/j.stem.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Jaiswal S., Fontanillas P., Flannick J., Manning A., Grauman P.V., Mar B.G., Lindsley R.C., Mermel C.H., Burtt N., Chavez A., et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014;371:2488–2498. doi: 10.1056/nejmoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genovese G., Kähler A.K., Handsaker R.E., Lindberg J., Rose S.A., Bakhoum S.F., Chambert K., Mick E., Neale B.M., Fromer M., et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014;371:2477–2487. doi: 10.1056/nejmoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buscarlet M., Provost S., Zada Y.F., Barhdadi A., Bourgoin V., Lépine G., Mollica L., Szuber N., Dubé M.P., Busque L. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. 2017;130:753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- 50.Lee B.H., Tothova Z., Levine R.L., Anderson K., Buza-Vidas N., Cullen D.E., McDowell E.P., Adelsperger J., Fröhling S., Huntly B.J.P., et al. FLT3 Mutations Confer Enhanced Proliferation and Survival Properties to Multipotent Progenitors in a Murine Model of Chronic Myelomonocytic Leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng S.W.K., Mitchell A., Kennedy J.A., Chen W.C., McLeod J., Ibrahimova N., Arruda A., Popescu A., Gupta V., Schimmer A.D., et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540:433–437. doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 52.Döhner H., Estey E., Grimwade D., Amadori S., Appelbaum F.R., Büchner T., Dombret H., Ebert B.L., Fenaux P., Larson R.A., et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eisfeld A.-K., Kohlschmidt J., Mrózek K., Blachly J.S., Walker C.J., Nicolet D., Orwick S., Maharry S.E., Carroll A.J., Stone R.M., et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of Alliance studies. Leukemia. 2018;32:1338–1348. doi: 10.1038/s41375-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Döhner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and Management of AML in Adults: 2022 ELN Recommendations from an International Expert Panel. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 55.Jayavelu A.K., Wolf S., Buettner F., Alexe G., Häupl B., Comoglio F., Schneider C., Doebele C., Fuhrmann D.C., Wagner S., et al. The proteogenomic subtypes of acute myeloid leukemia. Cancer Cell. 2022;40:301–317.e12. doi: 10.1016/j.ccell.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Bolouri H., Farrar J.E., Triche T., Ries R.E., Lim E.L., Alonzo T.A., Ma Y., Moore R., Mungall A.J., Marra M.A., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018;24:103–112. doi: 10.1038/nm.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srivastava S., Ghosh S., Kagan J., Mazurchuk R., National Cancer Institute’s HTAN Implementation The Making of a PreCancer Atlas: Promises, Challenges, and Opportunities. Trends Cancer. 2018;4:523–536. doi: 10.1016/j.trecan.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Squair J.W., Gautier M., Kathe C., Anderson M.A., James N.D., Hutson T.H., Hudelle R., Qaiser T., Matson K.J.E., Barraud Q., et al. Confronting false discoveries in single-cell differential expression. Nat. Commun. 2021;12:5692. doi: 10.1038/s41467-021-25960-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zimmerman K.D., Espeland M.A., Langefeld C.D. A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun. 2021;12:738. doi: 10.1038/s41467-021-21038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su I.H., Basavaraj A., Krutchinsky A.N., Hobert O., Ullrich A., Chait B.T., Tarakhovsky A. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 2003;4:124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 62.Uckelmann H.J., Kim S.M., Wong E.M., Hatton C., Giovinazzo H., Gadrey J.Y., Krivtsov A.V., Rücker F.G., Döhner K., McGeehan G.M., et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. 2020;367:586–590. doi: 10.1126/science.aax5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dawson M.A., Kouzarides T. Cancer Epigenetics: From Mechanism to Therapy. Cell. 2012;150:12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Luengo A., Gui D.Y., Vander Heiden M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017;24:1161–1180. doi: 10.1016/j.chembiol.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madan V., Koeffler H.P. Differentiation therapy of myeloid leukemia: four decades of development. Haematologica. 2021;106:26–38. doi: 10.3324/haematol.2020.262121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S., Mo Q., Wang X. Oncological role of HMGA2 (Review) Int. J. Oncol. 2019;55:775–788. doi: 10.3892/ijo.2019.4856. [DOI] [PubMed] [Google Scholar]
- 67.Li Z., Herold T., He C., Valk P.J.M., Chen P., Jurinovic V., Mansmann U., Radmacher M.D., Maharry K.S., Sun M., et al. Identification of a 24-Gene Prognostic Signature That Improves the European LeukemiaNet Risk Classification of Acute Myeloid Leukemia: An International Collaborative Study. J. Clin. Oncol. 2013;31:1172–1181. doi: 10.1200/jco.2012.44.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marcucci G., Yan P., Maharry K., Frankhouser D., Nicolet D., Metzeler K.H., Kohlschmidt J., Mrózek K., Wu Y.-Z., Bucci D., et al. Epigenetics Meets Genetics in Acute Myeloid Leukemia: Clinical Impact of a Novel Seven-Gene Score. J. Clin. Oncol. 2014;32:548–556. doi: 10.1200/jco.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alexander T.B., Gu Z., Iacobucci I., Dickerson K., Choi J.K., Xu B., Payne-Turner D., Yoshihara H., Loh M.L., Horan J., et al. The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature. 2018;562:373–379. doi: 10.1038/s41586-018-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rasche M., Zimmermann M., Borschel L., Bourquin J.-P., Dworzak M., Klingebiel T., Lehrnbecher T., Creutzig U., Klusmann J.-H., Reinhardt D. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32:2167–2177. doi: 10.1038/s41375-018-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rampal R., Ahn J., Abdel-Wahab O., Nahas M., Wang K., Lipson D., Otto G.A., Yelensky R., Hricik T., McKenney A.S., et al. Genomic and functional analysis of leukemic transformation of myeloproliferative neoplasms. Proc. Natl. Acad. Sci. USA. 2014;111:E5401–E5410. doi: 10.1073/pnas.1407792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feusier J.E., Arunachalam S., Tashi T., Baker M.J., VanSant-Webb C., Ferdig A., Welm B.E., Rodriguez-Flores J.L., Ours C., Jorde L.B., et al. Large-Scale Identification of Clonal Hematopoiesis and Mutations Recurrent in Blood Cancers. Blood Cancer Discov. 2021;2:226–237. doi: 10.1158/2643-3230.bcd-20-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falini B., Brunetti L., Sportoletti P., Martelli M.P. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136:1707–1721. doi: 10.1182/blood.2019004226. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez-Meira A., Buck G., Clark S.-A., Povinelli B.J., Alcolea V., Louka E., McGowan S., Hamblin A., Sousos N., Barkas N., et al. Unravelling Intratumoral Heterogeneity through High-Sensitivity Single-Cell Mutational Analysis and Parallel RNA Sequencing. Mol. Cell. 2019;73:1292–1305.e8. doi: 10.1016/j.molcel.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mesaeli N., Nakamura K., Zvaritch E., Dickie P., Dziak E., Krause K.-H., Opas M., MacLennan D.H., Michalak M. Calreticulin Is Essential for Cardiac Development. J. Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolf F.A., Angerer P., Theis F.J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19:15. doi: 10.1186/s13059-017-1382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolock S.L., Lopez R., Klein A.M. Scrublet: Computational Identification of Cell Doublets in Single-Cell Transcriptomic Data. Cell Syst. 2019;8:281–291.e9. doi: 10.1016/j.cels.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lun A.T.L., Riesenfeld S., Andrews T., Dao T.P., Gomes T., participants in the 1st Human Cell Atlas Jamboree. Marioni J.C. EmptyDrops: distinguishing cells from empty droplets in droplet-based single-cell RNA sequencing data. Genome Biol. 2019;20:63. doi: 10.1186/s13059-019-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., 2nd, Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kucinski I., Wilson N.K., Hannah R., Kinston S.J., Cauchy P., Lenaerts A., Grosschedl R., Göttgens B. Interactions between lineage-associated transcription factors govern haematopoietic progenitor states. EMBO J. 2020;39 doi: 10.15252/embj.2020104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McInnes L., Healy J., Melville J. UMAP: Uniform Manifold Approximation and Projection for Dimension Reduction. arXiv. 2018 doi: 10.48550/arXiv.1802.03426. Preprint at. [DOI] [Google Scholar]
- 85.Wolf F.A., Hamey F.K., Plass M., Solana J., Dahlin J.S., Göttgens B., Rajewsky N., Simon L., Theis F.J. PAGA: graph abstraction reconciles clustering with trajectory inference through a topology preserving map of single cells. Genome Biol. 2019;20:59. doi: 10.1186/s13059-019-1663-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Macosko E.Z., Basu A., Satija R., Nemesh J., Shekhar K., Goldman M., Tirosh I., Bialas A.R., Kamitaki N., Martersteck E.M., et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Korsunsky I., Millard N., Fan J., Slowikowski K., Zhang F., Wei K., Baglaenko Y., Brenner M., Loh P.R., Raychaudhuri S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mende N., Bastos H.P., Santoro A., Mahbubani K.T., Ciaurro V., Calderbank E.F., Quiroga Londoño M., Sham K., Mantica G., Morishima T., et al. Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans. Blood. 2022;139:3387–3401. doi: 10.1182/blood.2021013450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All raw sequencing data has been deposited on GEO under accession number GSE227026.
-
•
All processed data and analysis results can be explored via our interactive web portal at https://gottgens-lab.stemcells.cam.ac.uk/preleukemia_atlas/.
-
•
All original code has been deposited at https://github.com/TomoyaIsobe/PMCA/. DOI is listed in the key resources table.