Abstract
Keratoconus (KC) affects the corneal structure, with thinning and bulging outward into a conelike shape. Irregular astigmatism and decreased visual acuity appear during puberty and progress into the mid-30s, with unpredictable disease severity. The cause of KC is recognized as multifactorial, but remains poorly understood. Hormone imbalances are a significant modulator of the onset of KC. This study sought to investigate the role of gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) in KC, using a three-dimensional, self-assembled matrix in vitro model. Healthy corneal fibroblasts and human KC cells in the corneal stroma were isolated, cultured, and stimulated with stable vitamin C to promote extracellular matrix assembly. Cultures were further stimulated with 2.5 or 10 mIU/mL FSH and 5 or 35 mIU/mL LH. Samples were evaluated for cell proliferation and morphology via BrdU assay and imaging; protein expression was assessed via Western blot analysis. Proliferation was significantly greater in human KC cells compared to healthy corneal fibroblasts with LH stimulation, but no changes were found with FSH stimulation. Additionally, in sex hormone receptors, fibrotic markers, proteoglycans, and members of the gonadotropin signaling pathway were significantly changed, largely driven by exogenous LH. The impact of exogenous FSH/LH in the KC stromal microenvironment was demonstrated. These results highlight the need to further examine the role of FSH/LH in KC and in human corneal homeostasis.
Keratoconus (KC) is a progressive, ectatic corneal disease causing structural and biomechanical changes in the human cornea, leading to significant vision impairment. The disease typically emerges during the adolescent years, progresses during the young-adult years, and generally stabilizes by middle-adult years (mid–late 40s). The pathobiology of the disease is not fully understood, but it is generally considered multifactorial.1, 2, 3, 4 Historically, it was believed that KC patients had a genetic predisposition the disease, compounded by biomechanical factors (eg, eye rubbing) and leading to various manifestations of disease severity. This two-hit hypothesis guided clinical assessment and management of patients with KC for years. Recently, a third component incorporating hormones5, 6, 7, 8 was identified as a key contributor to both the development and severity of KC.1, 2, 3, 4 Specifically, pubertal age and hormone-related corneal biomechanical changes have been increasingly recognized as key factors in KC progression.9, 10, 11, 12 Relevant to sex hormones, progression of KC is exacerbated during pregnancy,13, 14, 15, 16 after in vitro fertilization,17 and during hormone-replacement therapy in post-menopausal patients,18 further supporting the impact of hormones on patients with KC.
We have long posited that hormone imbalances play a vital role in the onset and progression of KC and have found significant changes in plasma and salivary androgens and estrogens in KC patients when compared to their healthy counterparts.7 More recently, reports of modulation in levels of gonadotropins—follicle-stimulating hormone (FSH) and luteinizing hormone (LH)—in KC in vivo, as well as modulation of their receptors (FSHR and LHR) in vitro,19 suggested upstream hormone dysregulation. The discovery of a hormone-regulated, robust biomarker, prolactin-induced protein,20 supports the hypothesis that hormones play a crucial role in KC pathogenesis.
FSH and LH are hormones essential for the growth, development, and maturation of the human reproductive system. They stimulate the gonads in both males (testes) and females (ovaries).21, 22, 23 Gonadotropins are produced by gonadotropic cells in the anterior pituitary gland in response to gonadotropin-releasing hormone (GnRH) secreted by the hypothalamus.24 Regulation of gonadotropins involves positive and negative feedback loops between the different components of the hypothalamic-pituitary-gonadal axis, in which circulating gonad-derived sex hormones act at the level of the hypothalamus and anterior pituitary to modulate the release or inhibition of hypothalamic GnRH and anterior pituitary gonadotropins (FSH and LH).25 FSH and LH are large glycoproteins composed of α and β subunits; the β subunit is unique to each hormone, having the capacity to bind to its own receptor. By puberty, a crucial period in KC manifestation, gonadotropin secretion in boys promotes sperm production by FSH and testosterone secretion by LH in the testes. In girls, gonadotropins are released cyclically, wherein FSH stimulates ovarian follicle growth and estrogen release in the ovaries, and an LH surge stimulates ovulation and progesterone secretion.
In addition to the hypothalamic-pituitary-gonadal axis, the hypothalamic-pituitary-adrenal axis is also involved in KC pathogenesis. The hypothalamic-pituitary-adrenal axis regulates the release of the adrenal gland–derived hormone dehydroepiandrosterone (DHEA), which is present in the tears that coat the cornea.26,27 Based on previous findings, DHEA is increased in KC patients.7,20 Additionally, DHEA increased fibrosis in KC corneal fibroblasts,28 substantiating the involvement of DHEA in the pathogenesis of KC. These effects of DHEA can be exacerbated in the cornea in KC patients, as an up-regulation of enzymes [eg, cytochrome P450 (CYP) isozyme 2B6] suppresses corneal steroidogenesis of estrogens.6 Both basic and clinical studies suggest the existence of a hypothalamic-pituitary-adrenal-corneal axis connecting the brain directly to the cornea. This hypothalamic-pituitary-adrenal-corneal axis could have a significant relationship with the pathogenesis and progression of KC.
The volume of clinical data linking exogenous estrogens to corneal structure/tissue, together with the recent interest in corneal hormone receptors, prompted this investigation of the role of gonadotropins as an exogenous stimulus in vitro. This area is indeed unexplored and could provide significant insights to the KC microenvironment. Herein, an established three-dimensional in vitro model involving primary corneal stroma cells isolated from healthy subjects and KC patients was used to examine the relationships between gonadotropin stimulation and the expression of sex hormone receptors, corneal fibrosis, proteoglycans, and downstream signaling pathway targets. The data highlighted new, previously unknown properties of FSH/LH in the context of the corneal microenvironment and KC. Future in vivo studies are warranted to validate and further determine the role of gonadotropins in cornea and KC.
Materials and Methods
Ethics Approval
The North Texas Regional Institutional Review Board reviewed and approved all studies (protocol approval number 2020-030). Tissue samples from healthy individuals were collected from the National Disease Research Interchange. Tissue samples from severe-stage KC patients undergoing transplantation were collected in accordance with federal and institutional guidelines from the Dean McGee Eye Institute (Oklahoma City, OK; protocol approval #10108 and #3450). All studies adhered to the Declaration of Helsinki; written and informed consent was obtained prior to tissue collection.
Isolation of Corneal Stroma Cells
Primary human corneal stroma cells [healthy corneal fibroblasts (HCFs) and human KC cells (HKCs)] were isolated from healthy subjects and KC donors. In brief, the corneas were scraped using a razor from the top and bottom to remove the epithelial and endothelial layers of the cornea, respectively. The tissue was then rinsed with sterile phosphate-buffered saline (Thermo Fisher Scientific, Waltham, MA) and cut into approximately 2 × 2-mm pieces. Each corneal piece was separated evenly and placed into a T25 flask (Thermo Fisher Scientific) and incubated for 45 minutes at 37°C in 5% CO2 incubator. After incubation, regular media (RM)—Eagle's minimum essential medium (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA) and 1% antibiotic-antimycotic (Life Technologies, Grand Island, NY)—were added, followed by incubation until cells reached approximately 80% confluency. All experiments were executed with cells between passages 3 and 6.
2D Cell Culture and Stimulation
For two-dimensional (2D) studies, both HCFs and HKCs were cultured, and passaged using 0.05% trypsin-EDTA, followed by cell counting (Countess II; Invitrogen/Thermo Fisher Scientific) and seeding. cells were then plated at a seeding density of 0.5 × 106 cells/well in 1 mL of RM onto clear, flat-bottom, 12-well plates, and cultured overnight at 37°C with 5% CO2 incubator. The following morning, cells were stimulated in RM using FSH and LH at two concentrations each: LH, 5 and 35 mIU/mL (catalog number L6420; MilliporeSigma, Burlington, MA); and FSH, 2.5 and 10 mIU/mL (catalog number F4021; MilliporeSigma). HCFs/HKCs with fresh RM only, served as controls. FSH/LH concentrations were selected based on preliminary 2D cell cultures, as well as data from previous studies.19 At 48 hours after stimulation, protein was extracted. All experiments were repeated four times per donor sample.
2D Cell Culture and Morphology
Cell images were captured using an Excelis HDS camera (Accu-Scope, Commack, NY), at original magnification ×4, and cellular morphology was monitored. In brief, HCFs and HKCs were plated at a seeding density of 5 × 104 cells/well in 1 mL of RM onto a clear, flat-bottom, 12-well plate and incubated overnight a 37°C with 5% CO2 incubator. Cells were then imaged before being stimulated with FSH and LH at two concentrations each: FSH, 2.5 and 10 mIU/mL; LH, 5 and 35 mIU/mL. All stimulations and controls (RM only) were done in duplicate. Images were taken at 6, 12, 24, and 48 hours after stimulation.
BrdU Assay and Imaging
Cell proliferation was evaluated at 48 hours after FSH/LH stimulation, using a BrdU assay cell proliferation kit and processing according to the manufacturer's protocol (catalog number QIA58; MilliporeSigma). HCFs and HKCs were plated at a seeding density of 1 × 105 cells/well in 100 μL of RM onto a clear, flat-bottom, 96-well plate. The cells were cultured overnight to allow attachment at 37°C with 5% CO2. The following morning, cells were stimulated with LH 5 mIU/mL and FSH 10 mIU/mL for 24 hours. Two validity controls were used: a blank control that included RM only, and a background control that included cells but no BrdU label. After stimulation, 20 μL of a 1:2000 BrdU label dilution was added to all of the wells except for the background controls, followed by another 24-hour incubation. The following morning, media were removed and carefully blotted on paper towels. A total of 200 μL of the fixative/denaturing solution was added to each well and incubated for 30 minutes at room temperature. After cells were fixed, 100 μL of a 1:100 diluted 100× anti-BrdU antibody solution was added to each well and incubated for 1 hour at room temperature. Wells were then washed three times with a 1× washing buffer solution, and incubated for 30 minutes at room temperature with 100 μL of peroxidase goat anti-mouse IgG horseradish peroxidase conjugate solution. After incubation, wells were washed three times for 1 minute with 1× washing buffer solution. The entire plate was flooded with ultrapure H2O; contents were inverted over a sink and dried by tapping onto clean paper towels. Substrate solution (100 μL) was added to each well, and the plates were covered with foil paper and incubated at room temperature for 15 minutes. Finally, 100 μL of stop solution was added to each well. All experiments were repeated seven times. Absorbance was measured at 450 to 540 nm using an Epoch 2 microplate reader (BioTek, Winooski, VT). BioTek Gen5 software version 3.10.06 was used to calculate and plot absorbance ratios against those of controls.
Three-Dimensional Cell Culture and Stimulation
HCFs and HKCs were plated at a seeding density of 1 × 106 cells/well in 1.5 mL of RM on top of 6-well Transwell plates (Corning Life Sciences, Corning, NY). The following morning, all cells were stimulated at concentrations of 5 (LH) or 10 (FSH) mIU/mL in vitamin C media—Eagle's minimum essential medium supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic, and stable vitamin C (0.5 mmol/L 2-O-α-D-glucopyranosyl-l-ascorbic acid, Sigma-Aldrich, St. Louis, MO)—as previously reported.29, 30, 31, 32 Constructs were stimulated with vitamin C media to stimulate matrix secretion and assembly. Fresh media were provided three times a week for the duration of the study (total of 4 weeks), as previously reported.29, 30, 31, 32
Protein Extraction and Quantification
All samples were lysed using 1× radioimmunoprecipitation buffer assay and protease inhibitor (100 μL; Sigma-Aldrich), which was added to culture wells after being washed twice with cold phosphate-buffered saline, followed by a 30-minute incubation on ice at 4°C. Finally, samples were centrifuged at 4°C for 15 minutes at 16,128 × g and supernatant was collected. Protein quantification was performed using the Pierce bicinchoninic acid protein assay kit (Pierce, Rockford, IL) and bovine serum albumin standards (Thermo Fisher Scientific). In brief, standards and protein samples were vortexed and 10 μL of each was added to Corning Costar 96-well plate (Thermo Fisher Scientific). Pierce bicinchoninic acid reagent A and B solution (200 μL) was then added to each well, mixed for 30 seconds with a rocker, and incubated for 30 minutes at 37°C with 5% CO2. Absorbance was measured at 562 nm with the Epoch 2 microplate reader (BioTek), and values were plotted using linear regression to the standards.
Western Blot Assay
All protein samples were processed by SDS-PAGE at 225 V for 30 minutes in Novex 4% to 20% Tris-glycine Mini Wedge 12-well gels (InvitroGen/Thermo Fisher Scientific), and transferred to nitrocellulose membranes using the iBlot 2 Dry blot analysis system (InvitroGen/Thermo Fisher Scientific). After blocking with 1× Blocker FL fluorescent blocking buffer (Thermo Fisher Scientific) at room temperature for 1 hour on a rocker, the primary antibody was added overnight at 4°C. The following morning, nitrocellulose membranes were washed with Tris-buffered saline with Tween 20 three times for 5 minutes, followed by a 1-hour secondary antibody incubation at room temperature. Primary and secondary antibodies used throughout the studies are listed in Table 1. After washing with Tris-buffered saline with Tween 20 three times for 5 minutes each, all nitrocellulose membranes were imaged with the iBright FL 15000 imaging system (Thermo Fisher Scientific). iBright analysis software version 4.0.1 (Thermo Fisher Scientific) was used to analyze images, and results were normalized to the expression of β-actin housekeeping and plotted.
Table 1.
Western Blot Antibodies Used in 2D and 3D Cell Culture
| Name | Source | Reactive | Dilution | Study | Antibody |
|---|---|---|---|---|---|
| LHR | ab125214 (Abcam, Cambridge, MA) | Rabbit polyclonal | 1:500 | 2D cell culture | Primary |
| FSHR | ab75200 (Abcam) | Rabbit polyclonal | 1:500 | 2D cell culture | Primary |
| GnRHR | ab183079 (Abcam) | Rabbit polyclonal | 1:500 | 2D + 3D cell culture | Primary |
| AR | ab133273 (Abcam) | Rabbit monoclonal | 1:500 | 3D cell culture | Primary |
| PR | ab191138 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| ERβ | ab3576 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| ERα | ab75635 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| α-SMA | ab5694 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| EDA-Fn | SAB4200784 (Millipore Sigma, Burlington, MA) | Mouse monoclonal | 1:700 | 3D cell culture | Primary |
| Keratocan | ab113115 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| Lumican | ab168348 (Abcam) | Rabbit monoclonal | 1:500 | 3D cell culture | Primary |
| CYP3A4 | ab3572 (Abcam) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| PKA | PA5-115763 (Thermo Fisher Scientific, Waltham, MA) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| CRH | SAB1405656 (Millipore Sigma) | Mouse polyclonal | 1:500 | 3D cell culture | Primary |
| GPER | SAB1304967 (Millipore Sigma, Burlington, MA) | Rabbit polyclonal | 1:500 | 3D cell culture | Primary |
| cAMP | SAB1400041 (Millipore Sigma) | Mouse polyclonal | 1:500 | 3D cell culture | Primary |
| CREB | ab178322 (Abcam) | Mouse monoclonal | 1:500 | 3D cell culture | Primary |
| ERK 1/2 | ab184699 (Abcam) | Rabbit monoclonal | 1:500 | 3D cell culture | Primary |
| pan-AKT | ab179463 (Abcam) | Rabbit monoclonal | 1:500 | 3D cell culture | Primary |
| β-Actin | ab184092 (Abcam) | Mouse monoclonal | 1:2000 | 2D + 3D cell culture | Primary |
| Alexa Fluor 568 | ab175470 (Abcam) | Anti-rabbit IgG | 1:2000 | 2D + 3D cell culture | Secondary |
| Alexa Fluor 647 | ab150107 (Abcam) | Anti-mouse IgG | 1:2000 | 3D cell culture | Secondary |
2D, two-dimensional; 3D, three-dimensional; AKT, protein kinase B; CREB, cAMP response element–binding protein; CRH, corticotropin-releasing hormone; CYP, cytochrome P450; EDA-Fn, fibronectin-containing extra domain A; ER, estrogen receptor; FSHR, follicle-stimulating hormone receptor; GnRHR, gonadotropin-releasing hormone receptor; GPER, G protein–coupled estrogen receptor; LHR, luteinizing hormone receptor; pan-AKT, protein kinase B with potential antineoplastic activity; PKA, protein kinase A; SMA, smooth muscle actin.
Statistical Analysis
The means ± SEMs of experimental replicates were plotted with Prism software version 9 (GraphPad Software, San Diego, CA). Significance between groups was assessed by one-way analysis of variance. P < 0.05 was considered to be significant.
Results
2D Cell Culture
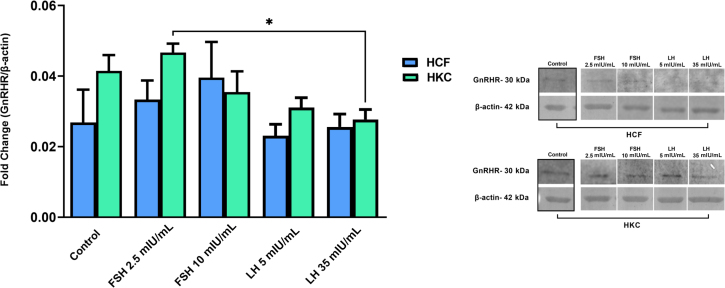
A conventional 2D culture system was used to obtain baseline expression for GnRH receptor (GnRHR) in HCFs and HKCs, and to determine the optimal FSH and LH concentrations for further testing. At 48 hours, neither FSH nor LH stimulation significantly modulated GnRHR protein expression in HCFs or HKCs (Figure 1). However, GnRHR protein expression was significantly greater in HKCs stimulated with FSH 2.5 mIU/mL compared to LH 35 mIU/mL [HKC FSH 2.5 mIU/mL (SEM = 0.002524), LH 35 mIU/mL (SEM = 0.002821); P = 0.0242) (Figure 1).
Figure 1.
Western blot analysis of gonadotropin-releasing hormone receptor (GnRHR) in two-dimensional cell cultures of HCFs and HKCs in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 48 hours. HKC FSH 2.5 mIU/mL (SEM = 0.002524) versus LH 35 mIU/mL (SEM = 0.002821); P = 0.0242. Data are expressed as means ± SEM. n = 4 for all conditions. ∗P < 0.05.
BrdU Assay and Imaging
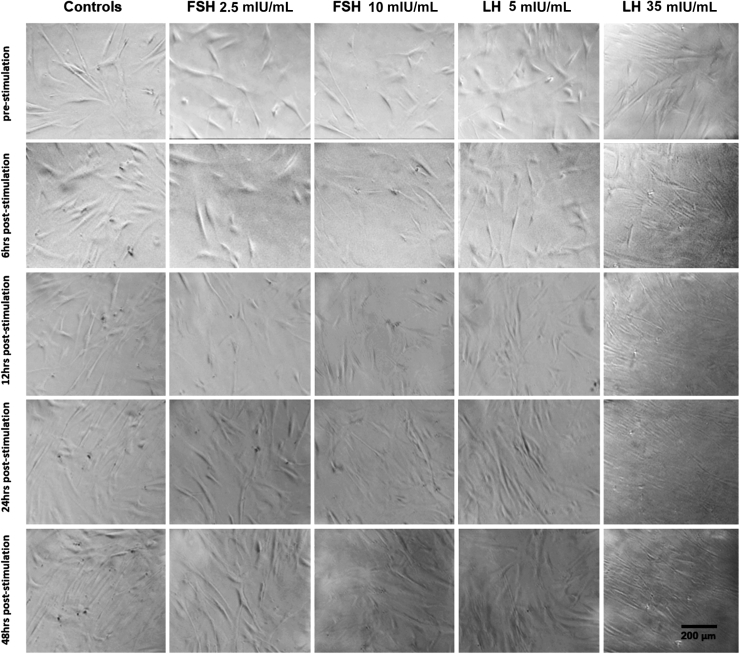
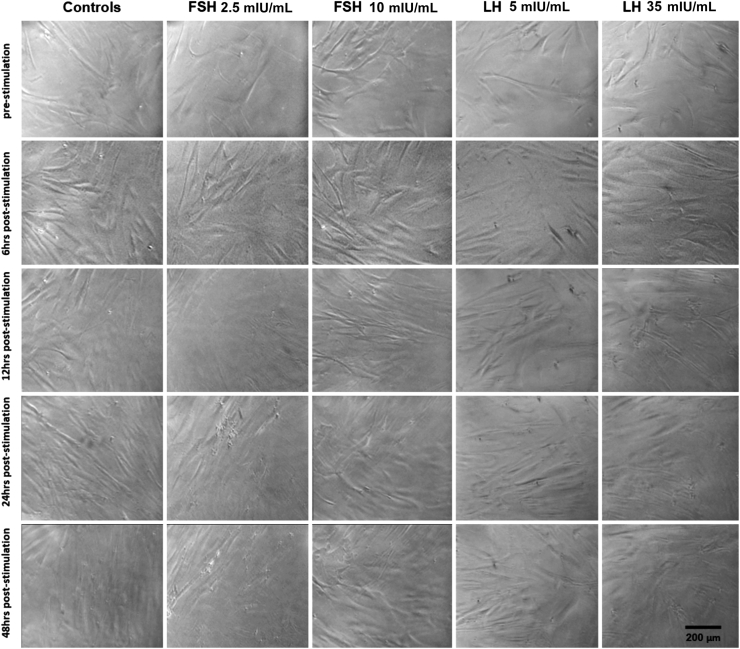
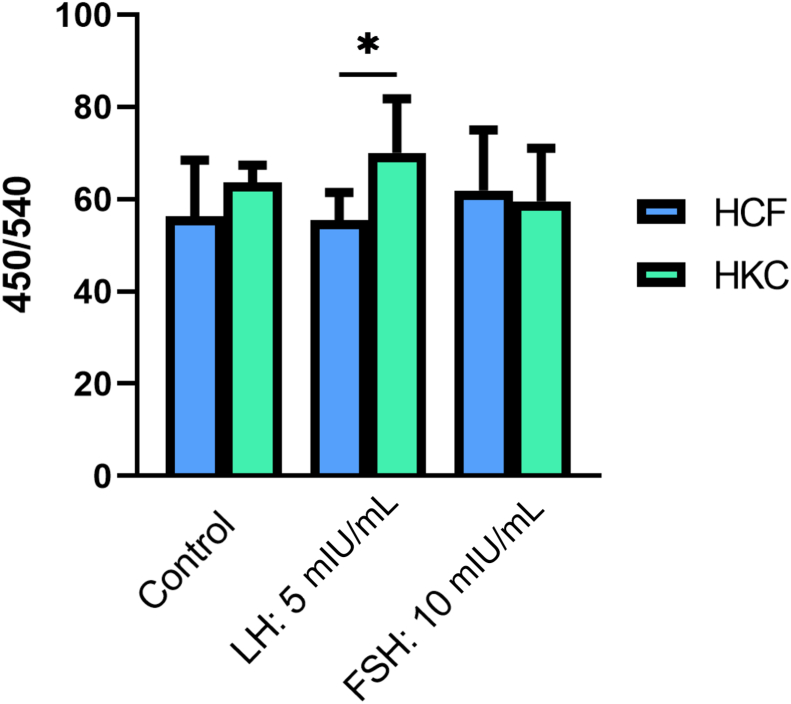
HCF and HKC cellular proliferation and morphology were examined after 6, 12, 24, and 48 hours of FSH/LH stimulation at all concentrations. No morphologic changes in HCFs (Figure 2) or HKCs (Figure 3) with or without FSH/LH were observed. No apoptotic or stressed cells were visualized in any of the conditions (Figures 2 and 3). Therefore, the following concentration of each gonadotropin was used for further analysis: LH 5 mIU/mL and FSH 10 mIU/mL. Levels of proliferation of HCFs and HKCs stimulated with FSH 10 mIU/mL and LH 5 mIU/mL were then examined using BrdU assay. Cultures with RM only served as controls. Proliferation (as measured by 450/540 nm absorbance ratio) was significantly greater in HKCs stimulated with LH 5 mIU/mL of [HKCs (SEM = 4.447) versus HCFs (SEM = 2.288); P = 0.0127], when compared to HCFs (Figure 4). FSH had no effect on the proliferation of HCFs or HKCs. No other statistical significance was noted between LH, FSH, or controls in either HCFs or HKCs.
Figure 2.
BrdU representation of stimulation using LH 5 and 35 mIU/mL or FSH 2.5 and 10 mIU/mL in HCFs for 48 hours. Scale bar = 200 μm.
Figure 3.
BrdU representation of stimulation using LH 5 and 35 mIU/mL or FSH 2.5 and 10 mIU/mL in HKCs for 48 hours. Scale bar = 200 μm.
Figure 4.
BrdU analysis of HCFs and HKCs stimulated using LH 5 mIU/mL or FSH 10 mIU/mL for 48 hours. LH 5 mIU/mL: HKCs (SEM = 4.447) versus HCFs (SEM = 2.288); P = 0.0127. Data are expressed as 450/540 nm absorbance ratios. n = 7 for all conditions. ∗P < 0.05.
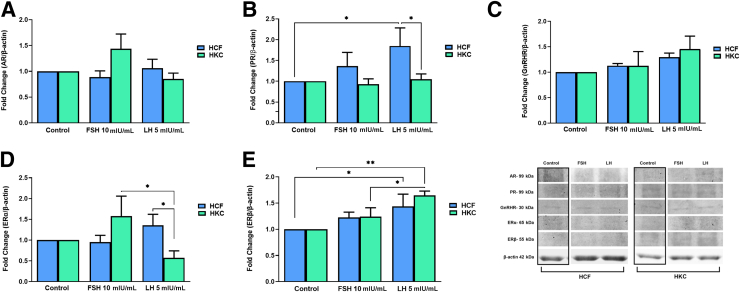
Sex Hormone Receptors
The protein expression of sex hormone receptors [androgen receptor (AR), progesterone receptor (PR), estrogen receptor (ER)-α, ERβ, and GnRHR] was investigated in HCF and HKC constructs, with or without FSH/LH treatment (Figure 5). HCFs showed no significant changes in the expression of AR (Figure 5A), GnRHR (Figure 5C), or ERα (Figure 5D) compared to HCF controls. However, the expression levels of PR [HCF: control versus LH 5 mIU/mL (SEM = 0.4364); P = 0.0259] (Figure 5B) and ERβ [HCF: control versus LH 5 mIU/mL (SEM = 0.2350); P = 0.0356) (Figure 5E) were significantly up-regulated in HCFs following LH 5 mIU/mL treatment compared to HCF controls. In HKCs, AR (Figure 5A), PR (Figure 5B), GnRHR (Figure 5C), and ERα (Figure 5D) showed no significant changes compared to HKC controls; however, HKCs stimulated with LH 5 mIU/mL showed significant up-regulation in ERβ expression when compared to HKC controls [HKC: control versus LH 5 mIU/mL (SEM = 0.08293); P = 0.0043] (Figure 5E).
Figure 5.
Western blot analysis of sex hormone receptors in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: Androgen receptor (AR) expression. FSH 10 mIU/mL: HKC (SEM = 0.1227) versus HCF (SEM = 0.2874); P = 0.0259. HKC: LH 5 mIU/mL (SEM = 0.1135) versus FSH 10 mIU/mL (SEM = 0.2874); P = 0.0195. B: Progesterone receptor (PR) expression. HCF: control versus LH 5 mIU/mL (SEM = 0.4364); P = 0.0259. LH 5 mIU/mL: HKC (SEM = 0.1283) versus HCF (SEM = 0.4364); P = 0.0338. C: Gonadotropin-releasing hormone receptor (GnRHR) expression. D: Estrogen receptor (ER)-α expression. LH 5 mIU/mL: HKC (SEM = 0.1337) versus HCF (SEM = 0.2670); P = 0.0431. HKC: LH 5 mIU/mL (SEM = 0.1667) versus FSH 10 mIU/mL (SEM = 0.4822); P = 0.0133. E: ERβ expression. HCF: control versus LH 5 mIU/mL (SEM = 0.2350); P = 0.0356. HKC: control versus LH 5 mIU/mL (SEM = 0.08293); P = 0.0043. HKC: LH 5 mIU/mL (SEM = 0.08293) versus FSH 10 mIU/mL (SEM = 0.1716); P = 0.0474. Data are expressed as means ± SEM. n = 3 for all conditions. ∗P < 0.05 and ∗∗P < 0.01.
AR expression was significantly up-regulated in HKCs when compared to HCFs with FSH 10 mIU/mL stimulation [HKC (SEM = 0.1227) versus HCF (SEM = 0.2874); P = 0.0259] (Figure 5A), whereas PR [HKC (SEM = 0.1283) versus HCF (SEM = 0.4364); P = 0.0338) (Figure 5B) and ERα [HKC (SEM = 0.1337) versus HCF (SEM = 0.2670); P = 0.0431] (Figure 5D) were significantly down-regulated in HKCs when stimulated with LH 5 mIU/mL compared to HCFs. Lastly, LH treatment was associated with significant down-regulation of AR [HKC: LH 5 mIU/mL (SEM = 0.1135) versus FSH 10 mIU/mL (SEM = 0.2874); P = 0.0195] (Figure 5A) and ERα [HKC: LH 5 mIU/mL (SEM = 0.1667) versus FSH 10 mIU/mL (SEM = 0.4822); P = 0.0133] (Figure 5D), and a significant up-regulation of ERβ [HKC: LH 5 mIU/mL (SEM = 0.08293) versus FSH 10 mIU/mL (SEM = 0.1716); P = 0.0474] (Figure 5E) when compared to FSH stimulation in HKCs.
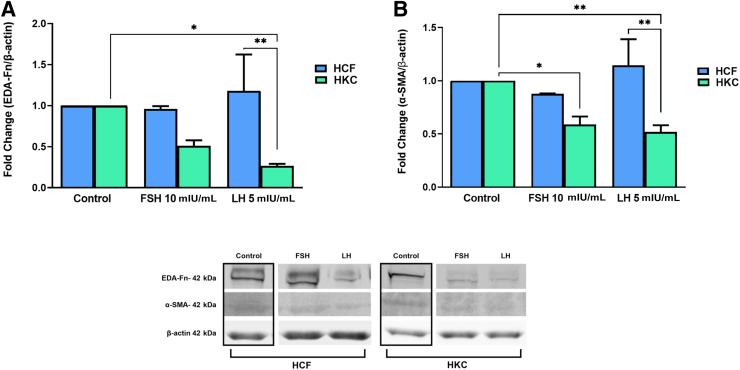
Fibrosis
Corneal fibrotic markers—fibronectin-containing extra domain A (EDA-Fn) and α–smooth muscle actin (α-SMA)—were investigated in all conditions (Figure 6). FSH and LH had no significant effect on EDA-Fn or α-SMA in HCFs (Figure 6, A and B). In HKCs, significant down-regulation in α-SMA [HKC: control versus FSH 10 mIU/mL (SEM = 0.07532) (P = 0.0197); control versus LH 5 mIU/mL (SEM = 0.06353) (P = 0.0084)] (Figure 6B) was noted with both FSH and LH stimulation, while EDA-Fn was down-regulated only with LH 5 mIU/mL [HKC: control versus LH 5 mIU/mL (SEM = 0.02596); P = 0.0150] (Figure 6A). Interestingly, both EDA-Fn [HCF (SEM = 0.4420) versus HKC (SEM = 0.02596); P = 0.042] (Figure 6A) and α-SMA [HCF (SEM = 0.2461) versus HKC (SEM = 0.06353); P = 0.015) (Figure 6B) were significantly higher in LH-stimulated HCFs when compared to their HKC counterparts.
Figure 6.
Western blot analysis of fibrosis markers in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: Fibronectin-containing extra domain A (EDA-Fn) expression. LH 5 mIU/mL: HCF (SEM = 0.4420) versus HKC (SEM = 0.02596); P = 0.042. HKC: control versus LH 5 mIU/mL (SEM = 0.02596); P = 0.0150. B: α–Smooth muscle actin (SMA) expression. LH 5 mIU/mL: HCF (SEM = 0.2461) versus HKC (SEM = 0.06353); P = 0.015. HKC: control versus FSH 10 mIU/mL (SEM = 0.07532); P = 0.0197. HKC: control versus LH 5 mIU/mL (SEM = 0.06353); P = 0.0084. Data are expressed as means ± SEM. ∗P < 0.05 and ∗∗P < 0.01.
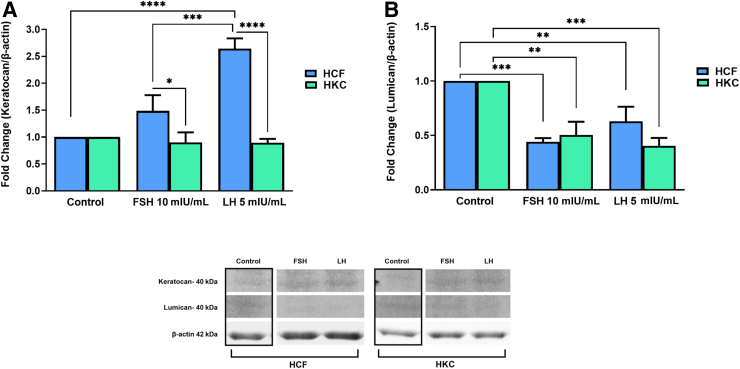
Proteoglycans
Expression levels of key corneal proteoglycans, keratocan and lumican, were examined in all conditions (Figure 7). Keratocan expression was significantly up-regulated in HCFs with LH stimulation when compared to the control, and with both FSH 10 mIU/mL and LH 5 mIU/mL stimulation, both remained significantly greater than in HKCs [HCF: control versus LH 5 mIU/mL (SEM = 0.1943) (P < 0.0001); FSH 10 mIU/mL: HCF (SEM = 0.2940) versus HKC (SEM = 0.1869) (P = 0.0283); LH 5 mIU/mL: HCF (SEM = 0.1943) versus HKC (SEM = 0.07302) (P < 0.0001)] (Figure 7A). On the other hand, keratocan expression was unaffected by FSH/LH in HKCs.
Figure 7.
Western blot analysis of proteoglycans in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: Keratocan expression. HCF: control versus LH 5 mIU/mL (SEM = 0.1943); P < 0.0001. FSH 10 mIU/mL: HCF (SEM = 0.2940) versus HKC (SEM = 0.1869); P = 0.0283. LH 5 mIU/mL: HCF (SEM = 0.1943) versus HKC (SEM = 0.07302); P < 0.0001. B: Lumican expression. HCF: control versus FSH 10 mIU/mL (SEM = 0.03407); P = 0.0004. HCF: control versus LH 5 mIU/mL (SEM = 0.1350); P = 0.0073. HKC: control versus FSH 10 mIU/mL (SEM = 0.1224); P = 0.0010. HKC: control versus LH 5 mIU/mL (SEM = 0.07331); P = 0.0002. Data are expressed as means ± SEM. n = 3 for all conditions. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
Lumican expression was significantly down-regulated with both FSH and LH, in both HCFs and HKCs, with no differences when HCFs were directly compared to HKCs [HCF: control versus FSH 10 mIU/mL (SEM = 0.03407) (P = 0.0004); HCF: control versus LH 5 mIU/mL (SEM = 0.1350) (P = 0.0073); HKC: control versus FSH 10 mIU/mL (SEM = 0.1224); (P = 0.0010); HKC: control versus LH 5 mIU/mL (SEM = 0.07331) (P = 0.0002) (Figure 7B).
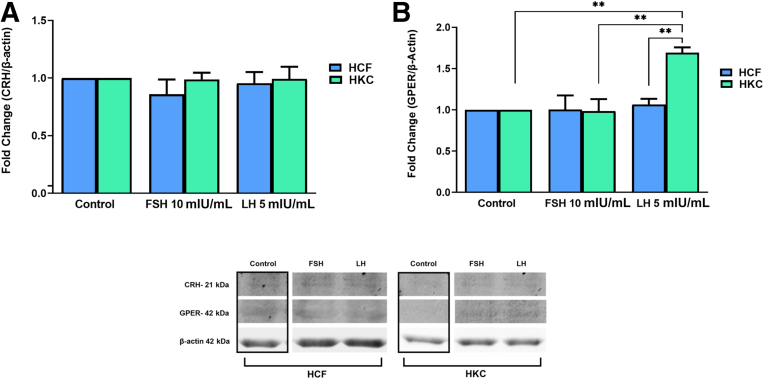
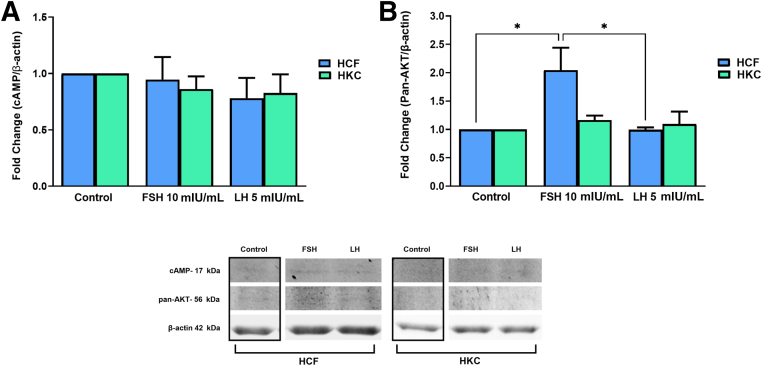
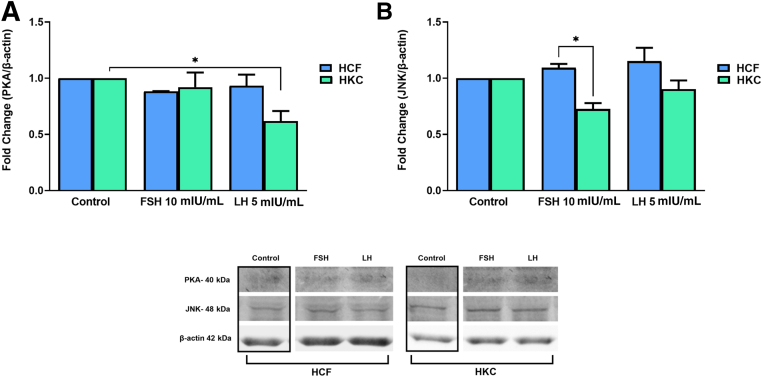
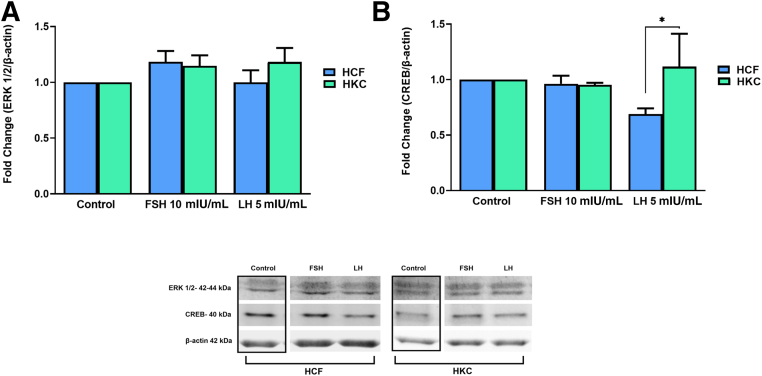
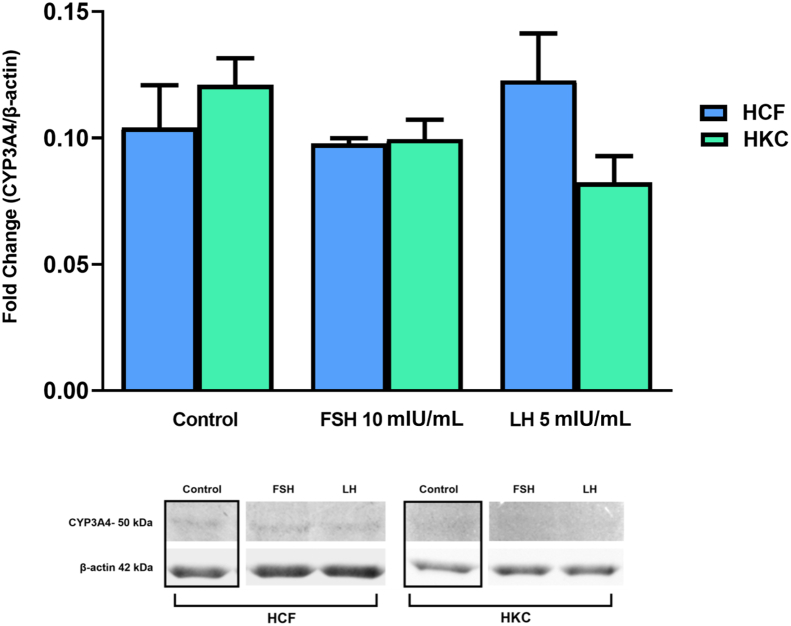
FSH and LH Gonadotropin Signaling Pathways
The gonadotropin signaling pathway was investigated for FSH/LH-induced modulation. Targets examined were corticotropin-releasing hormone (CRH), G protein–coupled (GP)-ER, cAMP, protein kinase B with potential antineoplastic activity (pan-AKT), protein kinase A (PKA), c-Jun N-terminal kinase (JNK), ERK 1/2, cAMP-response element binding protein (CREB) (Figure 8, Figure 9, Figure 10, Figure 11), and CYP3A4 (Figure 12). Statistical analysis was done between controls, LH, and FSH stimulation compared in HCFs and HKCs for each of the gonadotropin-signaling pathway targets. In HCFs, the expression of CRH (Figure 8A), GPER (Figure 8B), cAMP (Figure 9A), PKA (Figure 10A), ERK1/2 (Figure 11A), CREB, and CYP3A4 (Figure 12) showed no statistical changes regardless of FSH or LH stimulation. Pan-AKT was significantly up-regulated with FSH 10 mIU/mL compared to LH 5 mIU/mL and controls in HCFs [HCF: control versus FSH 10 mIU/mL (SEM = 0.3943) (P = 0.0175); LH 5 mIU/mL versus FSH 10 mIU/mL (SEM = 0.04252) (P = 0.0169)] (Figure 9B). HKCs stimulated with FSH 10 mIU/mL showed significant down-regulation of JNK compared to HCFs stimulated with FSH 10 mIU/mL [HKC: FSH 10 mIU/mL (SEM = 0.05283) versus HCF: FSH 10 mIU/mL (SEM = 0.03383); P = 0.0129) (Figure 10B). LH 5 mIU/mL–stimulated HKCs had significant up-regulation in CREB compared to HCFs stimulated with LH 5 mIU/mL [HKC: LH 5 mIU/mL (SEM = 0.2964) versus HCF: LH 5 mIU/mL (SEM = 0.05196); P = 0.0342] (Figure 11B).
Figure 8.
Western blot analysis of gonadotropin signaling pathway in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: Corticotropin-releasing hormone (CRH) expression. B: G protein–coupled estrogen receptor (GPER) expression. HKC: LH 5 mIU/mL (SEM = 0.06333) versus control; P = 0.0030. HKC: LH 5 mIU/mL (SEM = 0.06333) versus FSH 10 mIU/mL (SEM = 0.1441), P = 0.0026, HCF: LH 5 mIU/mL (SEM = 0.06835) versus HKC: LH 5 mIU/mL (SEM = 0.06333); P = 0.0067. Data are expressed as means ± SEM. n = 3 for all conditions. ∗∗P < 0.01.
Figure 9.
Western blot analysis of gonadotropin signaling pathway in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: cAMP expression. B: Protein kinase B with potential antineoplastic activity (Pan-AKT) expression. HCF: control versus FSH 10 mIU/mL (SEM = 0.3943); P = 0.0175. LH 5 mIU/mL versus FSH 10 mIU/mL (SEM = 0.04252); P = 0.0169. Data are expressed as means ± SEM. n = 3 for all conditions. ∗P < 0.05.
Figure 10.
Western blot analysis of gonadotropin signaling pathway in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: Protein kinase A (PKA) expression. HKC: control versus LH 5 mIU/mL (SEM = 0.08882); P = 0.0350. B: c-Jun N-terminal kinase (JNK) expression. HKC: FSH 10 mIU/mL (SEM = 0.05283) versus HCF: FSH 10 mIU/mL (SEM = 0.03383); P = 0.0129. Data are expressed as means ± SEM. n = 3 for all conditions. ∗P < 0.05.
Figure 11.
Western blot analysis of gonadotropin signaling pathway in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. A: ERK 1/2 expression. B: cAMP response element–binding protein (CREB) expression. HKC: LH 5 mIU/mL (SEM = 0.2964) versus HCF: LH 5 mIU/mL (SEM = 0.05196); P = 0.0342. Data are expressed as means ± SEM. n = 3 for all conditions. ∗P < 0.05.
Figure 12.
Western blot analysis of cytochrome P450 (CYP)-3A4 in HCFs and HKCs in three-dimensional cell culture in response to stimulation using LH 5 mIU/mL or FSH 10 mIU/mL for 4 weeks. Data are expressed as means ± SEM. n = 3 for all conditions.
In HKCs, the expression levels of CRH (Figure 8A), cAMP (Figure 9A), pan-AKT (Figure 9B), ERK1/2 (Figure 11A), and CYP3A4 (Figure 12) showed no statistical changes regardless of FSH or LH stimulation. However, GPER expression [HKC: LH 5 mIU/mL (SEM = 0.06333) versus control (P = 0.0030); LH 5 mIU/mL (SEM = 0.06333) versus FSH 10 mIU/mL (SEM = 0.1441) (P = 0.0026); HCF: LH 5 mIU/mL (SEM = 0.06835) versus HKC: LH 5 mIU/mL (SEM = 0.06333) (P = 0.0067)] (Figure 8B) was significantly up-regulated following LH 5 mIU/mL stimulation in HKCs when compared to HKC controls and FSH 10 mIU/mL stimulation. On the other hand, PKA expression [HKC: control versus LH 5 mIU/mL (SEM = 0.08882); P = 0.0350] was significantly down-regulated with LH 5 mIU/mL stimulation when compared to HKC controls (Figure 10A).
Discussion
KC is a progressive, noninflammatory ectatic corneal disorder that is characterized by steepening and thinning of the cornea, irregular astigmatism, myopia, and scarring. Despite the introduction of corneal crosslinking to stop progression and improvements in scleral contact lens designs, cornea transplants remain the holy grail of treating KC. KC is currently one of the most common indications leading to corneal transplantation. However, there is now increasing evidence that KC recurs even after corneal transplantation. To date, the etiology and pathogenesis of KC remain unclear, including the reasons for recurrence. As such, there is an urgent need to understand and define the onset/progression of KC. Despite numerous clinical observations and findings, the impact of hormones in the KC corneal microenvironment is significantly understudied.
Hormones induce effects in cells that express the respective receptors and modulate a variety of processes, including development, metabolism, and growth.33, 34, 35 Hormone balance is sex- and age-dependent, but also largely modulated by the hypothalamic-pituitary-gonadal, hypothalamic-pituitary-adrenal, and hypothalamic-pituitary-thyroid axes. Hormone functions are not limited to inception and maintenance of sexual development, but are also associated with food metabolism, hunger and thirst responses, maintaining body temperature, regulating mood, and cognitive functions.36 Thus, hormone imbalances are often associated with various diseases and conditions such as cardiovascular,37, 38, 39 kidney,40, 41, 42 periodontal,43, 44, 45 and ocular diseases.19,46, 47, 48 Clinicians have increasingly recognized the need to address hormone imbalances in the holistic treatment of patients with systemic diseases.
In recent years the correlation between ocular disease and hormones has gained significant attention, suggesting a pathologic association between hormone imbalances and various diseases/dystrophies. In the context of KC, sex hormone abnormalities have been reported,6, 7, 8,49, 50, 51 but little information exists on the role of gonadotropins as possible regulators of corneal homeostasis. The expression of LH, FSH receptor, and LH receptor in KC stromal/epithelial cells was recently demonstrated in vitro, and modulation of systemic FSH/LH ratio was shown in vivo when compared to healthy controls.19 The current study aimed to delineate the effects of exogenous gonadotropins in the KC microenvironment. A summary of the current three-dimensional cell culture results is shown in Table 2.
Table 2.
Summary of Three-Dimensional Construct Protein Expression in HCF and HKCs Stimulated with FSH and LH
| Name | FSH |
LH |
FSH versus LH |
|||||
|---|---|---|---|---|---|---|---|---|
| HCF | HKC | HCF versus HKC | HCF | HKC | HCF versus HKC | HCF | HKC | |
| GnRHR | ||||||||
| AR | Up ↑ | Down ↓ | ||||||
| PR | Up ↑ | Down ↓ | ||||||
| ERβ | Up ↑ | Up ↑ | Up ↑ | |||||
| ERα | Down ↓ | Down ↓ | ||||||
| α-SMA | Down ↓ | Down ↓ | Down ↓ | |||||
| EDA-Fn | Down ↓ | Down ↓ | ||||||
| Keratocan | Down ↓ | Up ↑ | Down ↓ | Up ↑ | ||||
| Lumican | Down ↓ | Down ↓ | Down ↓ | Down ↓ | ||||
| CYP3A4 | ||||||||
| PKA | Down ↓ | |||||||
| CRH | ||||||||
| GPER | Up ↑ | Up ↑ | Up ↑ | |||||
| cAMP | ||||||||
| CREB | Up ↑ | |||||||
| ERK 1/2 | ||||||||
| pan-AKT | Up ↑ | Down ↓ | ||||||
| JNK | Down ↓ | |||||||
AR, androgen receptor; CREB, cAMP response element–binding protein; CRH, corticotropin-releasing hormone; CYP, cytochrome P450; EDA-Fn, fibronectin-containing extra domain A; ER, estrogen receptor; GnRHR, gonadotropin-releasing hormone receptor; GPER, G protein–coupled estrogen receptor; JNK, c-Jun N-terminal kinase; pan-AKT, protein kinase B with potential antineoplastic activity; PKA, protein kinase A; PR, progesterone receptor; SMA, smooth muscle actin.
KC-associated corneal scarring is largely attributed to oxidative stress and the modulation of several factors, including collagens I, III,52 and V and keratocan, all crucial in myofibroblast differentiation and degradation of the extracellular matrix.32 The present data showed that LH exhibited a protective effect in only keratoconic stromal cells (HKCs), as evidenced by increased proliferation and decreased expression of fibrosis markers (EDA-Fn and α-SMA). FSH did not impact these measures in HKCs or healthy corneal stromal cells (HCFs). Interestingly, GPER expression was also increased with LH and not FSH in only HKCs. GPER is a membrane ER that is involved in nongenomic estrogen activity and is associated with cell growth and proliferation.53, 54, 55, 56, 57 Although LH has been shown to increase GPER expression in other cell types,58, 59, 60, 61 this was the first evidence of LH impacting GPER in the cornea. Based on these results, the effects of gonadotropins in KC were determined to be primarily LH driven. To date, no studies have linked exogenous gonadotropins to corneal fibrotic markers. It is plausible that cornea exposure to gonadotropins can affect corneal extracellular matrix and scarring in those with KC. Systemic KC hormone dysregulation, which ultimately damages the corneal microenvironment, originates via the lacrimal artery and lacrimal gland, where tear content is derived by circulating blood. It is then sensed by the corneal hormone-specific gonadotropin receptors. Long-term exposure to such abnormal sex hormones and gonadotropins (ie, bathing in tear fluid) leads to cellular damage, abnormal extracellular matrix remodeling, and ultimately KC onset. Furthermore, the content of aqueous humor can also be a source of LH/FSH for the cornea that can ultimately play a role in KC. The content of the aqueous humor in KC has been sparsely investigated62, 63, 64; thus it is difficult to make unequivocal connections at this point, and additional studies are warranted to fully elucidate the interplay/mechanism.
While hormone receptors have also been identified in ocular tissue, including the corneal endothelium, stroma, and epithelium,65,66 the exact function of these receptors in the human cornea remains unclear and rather understudied. The present study shed light for the first time on the role of FSH and LH gonadotropins and sex hormone receptors in KC. However, it is still unclear whether modulation of these receptors can significantly impact the pathobiology of KC and which stage of its progression receptors needs to be controlled. There have been contradicting findings on the expression of both ERα and ERβ in KC.67 Thus, their role in the corneal microenvironment could not be definitively and conclusively defined. More studies are certainly warranted for a full understanding of the role and function of ERs, gonadotrophins, and KC pathobiology. However, given the absence of an accurate animal model of KC, translational efforts with clinical studies in human subjects may be necessary to further explore the effects of sex hormones in KC pathogenesis and disease severity. Understanding these relationships may improve diagnosis, monitoring, and treatment strategies for KC patients who are at risk for significant visual dysfunction.
Footnotes
Supported by NIH/National Eye Institute grant EY028888 (D.K.).
Disclosures: None declared.
References
- 1.Crawford A.Z., Zhang J., Gokul A., McGhee C.N.J., Ormonde S.E. The enigma of environmental factors in keratoconus. Asia Pac J Ophthalmol (Phila) 2020;9:549–556. doi: 10.1097/APO.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 2.Galvis V., Sherwin T., Tello A., Merayo J., Barrera R., Acera A. Keratoconus: an inflammatory disorder? Eye (Lond) 2015;29:843–859. doi: 10.1038/eye.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kok Y.O., Tan G.F., Loon S.C. Review: keratoconus in Asia. Cornea. 2012;31:581–593. doi: 10.1097/ICO.0b013e31820cd61d. [DOI] [PubMed] [Google Scholar]
- 4.Moussa S., Grabner G., Ruckhofer J., Dietrich M., Reitsamer H. Genetics in keratoconus - what is new? Open Ophthalmol J. 2017;11:201–210. doi: 10.2174/1874364101711010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jamali H., Heydari M., Masihpour N., Khosravi A., Zare M., Shams M., Omrani G.R. Serum androgens and prolactin levels in patients with keratoconus. Clin Exp Optom. 2022:1–5. doi: 10.1080/08164622.2022.2081067. [DOI] [PubMed] [Google Scholar]
- 6.Karamichos D., Escandon P., Vasini B., Nicholas S.E., Van L., Dang D.H., Cunningham R.L., Riaz K.M. Anterior pituitary, sex hormones, and keratoconus: beyond traditional targets. Prog Retin Eye Res. 2022;88:101016. doi: 10.1016/j.preteyeres.2021.101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKay T.B., Hjortdal J., Sejersen H., Asara J.M., Wu J., Karamichos D. Endocrine and metabolic pathways linked to keratoconus: implications for the role of hormones in the stromal microenvironment. Sci Rep. 2016;6:25534. doi: 10.1038/srep25534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X., Yuan Y., Sun T., Zhang Y., Chen Y. Associations between keratoconus and the level of sex hormones: a cross-sectional study. Front Med (Lausanne) 2022;9:828233. doi: 10.3389/fmed.2022.828233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aydin E., Demir H.D., Demirturk F., Caliskan A.C., Aytan H., Erkorkmaz U. Corneal topographic changes in premenopausal and postmenopausal women. BMC Ophthalmol. 2007;7:9. doi: 10.1186/1471-2415-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghahfarokhi N.A., Vaseghi A., Ghahfarokhi N.A., Ghoreishi M., Peyman A., Dehghani A. Evaluation of corneal thickness alterations during menstrual cycle in productive age women. Indian J Ophthalmol. 2015;63:30–32. doi: 10.4103/0301-4738.151463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuffre G., Di Rosa L., Fiorino F., Bubella D.M., Lodato G. Variations in central corneal thickness during the menstrual cycle in women. Cornea. 2007;26:144–146. doi: 10.1097/01.ico.0000244873.08127.3c. [DOI] [PubMed] [Google Scholar]
- 12.Spoerl E., Zubaty V., Raiskup-Wolf F., Pillunat L.E. Oestrogen-induced changes in biomechanics in the cornea as a possible reason for keratectasia. Br J Ophthalmol. 2007;91:1547–1550. doi: 10.1136/bjo.2007.124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilgihan K., Hondur A., Sul S., Ozturk S. Pregnancy-induced progression of keratoconus. Cornea. 2011;30:991–994. doi: 10.1097/ICO.0b013e3182068adc. [DOI] [PubMed] [Google Scholar]
- 14.Hoogewoud F., Gatzioufas Z., Hafezi F. Transitory topographical variations in keratoconus during pregnancy. J Refract Surg. 2013;29:144–146. doi: 10.3928/1081597X-20130117-11. [DOI] [PubMed] [Google Scholar]
- 15.Soeters N., Tahzib N.G., Bakker L., Van der Lelij A. Two cases of keratoconus diagnosed after pregnancy. Optom Vis Sci. 2012;89:112–116. doi: 10.1097/OPX.0b013e318238c3f2. [DOI] [PubMed] [Google Scholar]
- 16.Naderan M., Jahanrad A. Topographic, tomographic and biomechanical corneal changes during pregnancy in patients with keratoconus: a cohort study. Acta Ophthalmol. 2017;95:e291–e296. doi: 10.1111/aos.13296. [DOI] [PubMed] [Google Scholar]
- 17.Yuksel E., Yalinbas D., Aydin B., Bilgihan K. Keratoconus progression induced by in vitro fertilization treatment. J Refract Surg. 2016;32:60–63. doi: 10.3928/1081597X-20151207-10. [DOI] [PubMed] [Google Scholar]
- 18.Coco G., Kheirkhah A., Foulsham W., Dana R., Ciolino J.B. Keratoconus progression associated with hormone replacement therapy. Am J Ophthalmol Case Rep. 2019;15:100519. doi: 10.1016/j.ajoc.2019.100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karamichos D., Barrientez B., Nicholas S., Ma S., Van L., Bak-Nielsen S., Hjortdal J. Gonadotropins in keratoconus: the unexpected suspects. Cells. 2019;8:1494. doi: 10.3390/cells8121494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharif R., Bak-Nielsen S., Sejersen H., Ding K., Hjortdal J., Karamichos D. Prolactin-induced protein is a novel biomarker for keratoconus. Exp Eye Res. 2019;179:55–63. doi: 10.1016/j.exer.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filatov M., Khramova Y., Parshina E., Bagaeva T., Semenova M. Influence of gonadotropins on ovarian follicle growth and development in vivo and in vitro. Zygote. 2017;25:235–243. doi: 10.1017/S0967199417000168. [DOI] [PubMed] [Google Scholar]
- 22.Messinis I.E., Messini C.I., Dafopoulos K. The role of gonadotropins in the follicular phase. Ann N Y Acad Sci. 2010;1205:5–11. doi: 10.1111/j.1749-6632.2010.05660.x. [DOI] [PubMed] [Google Scholar]
- 23.Olive D.L. The role of gonadotropins in ovulation induction. Am J Obstet Gynecol. 1995;172(2 Pt 2):759–765. doi: 10.1016/0002-9378(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser U.B., Jakubowiak A., Steinberger A., Chin W.W. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- 25.Plant T.M. 60 years of neuroendocrinology: the hypothalamo-pituitary-gonadal axis. J Endocrinol. 2015;226:T41–T54. doi: 10.1530/JOE-15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson E.J., Bucknall M.P., Golebiowski B., Stapleton F. Comparative limitations and benefits of liquid chromatography - mass spectrometry techniques for analysis of sex steroids in tears. Exp Eye Res. 2019;179:168–178. doi: 10.1016/j.exer.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Ducasse A., Delattre J.F., Flament J.B., Hureau J. The arteries of the lacrimal gland. Anat Clin. 1984;6:287–293. doi: 10.1007/BF01654461. [DOI] [PubMed] [Google Scholar]
- 28.Sharif R., Bak-Nielsen S., Hjortdal J., Karamichos D. Pathogenesis of keratoconus: the intriguing therapeutic potential of prolactin-inducible protein. Prog Retin Eye Res. 2018;67:150–167. doi: 10.1016/j.preteyeres.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKay T.B., Priyadarsini S., Rowsey T., Karamichos D. Arginine supplementation promotes extracellular matrix and metabolic changes in keratoconus. Cells. 2021;10:2076. doi: 10.3390/cells10082076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif R., Fowler B., Karamichos D. Collagen cross-linking impact on keratoconus extracellular matrix. PLoS One. 2018;13:e0200704. doi: 10.1371/journal.pone.0200704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay T.B., Hjortdal J., Sejersen H., Karamichos D. Differential effects of hormones on cellular metabolism in keratoconus in vitro. Sci Rep. 2017;7:42896. doi: 10.1038/srep42896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karamichos D., Zareian R., Guo X., Hutcheon A.E., Ruberti J.W., Zieske J.D. Novel in vitro model for keratoconus disease. J Funct Biomater. 2012;3:760–775. doi: 10.3390/jfb3040760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiller-Sturmhofel S., Bartke A. The endocrine system: an overview. Alcohol Health Res World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- 34.Vijayakumar A., Yakar S., Leroith D. The intricate role of growth hormone in metabolism. Front Endocrinol (Lausanne) 2011;2:32. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Symonds M.E., Mostyn A., Stephenson T. Cytokines and cytokine receptors in fetal growth and development. Biochem Soc Trans. 2001;29(Pt 2):33–37. doi: 10.1042/bst0290033. [DOI] [PubMed] [Google Scholar]
- 36.Lechan R.M., Toni R. In: Endotext [Internet] Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dhatariya K., et al., editors. MDText.com, Inc.; South Dartmouth, MA: 2000. Functional anatomy of the hypothalamus and pituitary. Endotext.org. [Google Scholar]
- 37.Cruz-Topete D., Oakley R.H., Cidlowski J.A. Glucocorticoid signaling and the aging heart. Front Endocrinol (Lausanne) 2020;11:347. doi: 10.3389/fendo.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corona G., Croce L., Sparano C., Petrone L., Sforza A., Maggi M., Chiovato L., Rotondi M. Thyroid and heart, a clinically relevant relationship. J Endocrinol Invest. 2021;44:2535–2544. doi: 10.1007/s40618-021-01590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attanasio P., Anker S.D., Doehner W., von Haehling S. Hormonal consequences and prognosis of chronic heart failure. Curr Opin Endocrinol Diabetes Obes. 2011;18:224–230. doi: 10.1097/MED.0b013e3283469505. [DOI] [PubMed] [Google Scholar]
- 40.Waller S. Parathyroid hormone and growth in chronic kidney disease. Pediatr Nephrol. 2011;26:195–204. doi: 10.1007/s00467-010-1614-y. [DOI] [PubMed] [Google Scholar]
- 41.Maric C., Sullivan S. Estrogens and the diabetic kidney. Gend Med. 2008;5(Suppl A):S103–S113. doi: 10.1016/j.genm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh P., Deng A., Weir M.R., Blantz R.C. The balance of angiotensin II and nitric oxide in kidney diseases. Curr Opin Nephrol Hypertens. 2008;17:51–56. doi: 10.1097/MNH.0b013e3282f29a8b. [DOI] [PubMed] [Google Scholar]
- 43.Opeodu O.I., Dosumu E.B., Arowojolu M.O. Periodontal condition and treatment needs of some pregnant women in Ibadan, Nigeria. Ann Med Health Sci Res. 2015;5:213–217. doi: 10.4103/2141-9248.157514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shcherba V., Krynytska I., Marushchak M., Korda M. Does thyroid dysfunction influence inflammatory mediators in experimental periodontitis? Endocr Regul. 2021;55:131–141. doi: 10.2478/enr-2021-0014. [DOI] [PubMed] [Google Scholar]
- 45.Guncu G.N., Tozum T.F., Caglayan F. Effects of endogenous sex hormones on the periodontium--review of literature. Aust Dent J. 2005;50:138–145. doi: 10.1111/j.1834-7819.2005.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 46.Rocha E.M., Mantelli F., Nominato L.F., Bonini S. Hormones and dry eye syndrome: an update on what we do and don't know. Curr Opin Ophthalmol. 2013;24:348–355. doi: 10.1097/ICU.0b013e32836227bf. [DOI] [PubMed] [Google Scholar]
- 47.Versura P., Giannaccare G., Campos E.C. Sex-steroid imbalance in females and dry eye. Curr Eye Res. 2015;40:162–175. doi: 10.3109/02713683.2014.966847. [DOI] [PubMed] [Google Scholar]
- 48.Mantelli F., Moretti C., Macchi I., Massaro-Giordano G., Cozzupoli G.M., Lambiase A., Bonini S. Effects of sex hormones on ocular surface epithelia: lessons learned from polycystic ovary syndrome. J Cell Physiol. 2016;231:971–975. doi: 10.1002/jcp.25221. [DOI] [PubMed] [Google Scholar]
- 49.McKay T.B., Priyadarsini S., Karamichos D. Sex hormones, growth hormone, and the cornea. Cells. 2022;11:224. doi: 10.3390/cells11020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X.R., Yuan Y.F., Zhang Y., Chen Y.G. [Effect of hormones on keratoconus and its mechanism] Zhonghua Yan Ke Za Zhi. 2022;58:309–314. doi: 10.3760/cma.j.cn112142-20210425-00189. [DOI] [PubMed] [Google Scholar]
- 51.El-Massry A., Doheim M.F., Iqbal M., Fawzy O., Said O.M., Yousif M.O., Badawi A.E., Tawfik A., Abousamra A. Association between keratoconus and thyroid gland dysfunction: a cross-sectional case-control study. J Refract Surg. 2020;36:253–257. doi: 10.3928/1081597X-20200226-03. [DOI] [PubMed] [Google Scholar]
- 52.Karamichos D., Hutcheon A.E., Rich C.B., Trinkaus-Randall V., Asara J.M., Zieske J.D. In vitro model suggests oxidative stress involved in keratoconus disease. Sci Rep. 2014;4:4608. doi: 10.1038/srep04608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang H., Wang C., Liao H., Wang Q. Activation of GPER by E2 promotes proliferation, invasion and migration of breast cancer cells by regulating the miR-124/CD151 pathway. Oncol Lett. 2021;21:432. doi: 10.3892/ol.2021.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R., Wang Y., Chen P., Meng J., Zhang H. G-protein coupled estrogen receptor activation protects the viability of hyperoxia-treated primary murine retinal microglia by reducing ER stress. Aging (Albany NY) 2020;12:17367–17379. doi: 10.18632/aging.103733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li R., Wang Y., Chen P., Meng J., Zhang H. G-protein-coupled estrogen receptor protects retinal ganglion cells via inhibiting endoplasmic reticulum stress under hyperoxia. J Cell Physiol. 2021;236:3780–3788. doi: 10.1002/jcp.30149. [DOI] [PubMed] [Google Scholar]
- 56.Luo H., Yang G., Yu T., Luo S., Wu C., Sun Y., Liu M., Tu G. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr Relat Cancer. 2014;21:355–369. doi: 10.1530/ERC-13-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imam Aliagan A., Madungwe N.B., Tombo N., Feng Y., Bopassa J.C. Chronic GPER1 activation protects against oxidative stress-induced cardiomyoblast death via preservation of mitochondrial integrity and deactivation of mammalian sterile-20-like kinase/yes-associated protein pathway. Front Endocrinol (Lausanne) 2020;11:579161. doi: 10.3389/fendo.2020.579161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavlik R., Wypior G., Hecht S., Papadopoulos P., Kupka M., Thaler C., Wiest I., Pestka A., Friese K., Jeschke U. Induction of G protein-coupled estrogen receptor (GPER) and nuclear steroid hormone receptors by gonadotropins in human granulosa cells. Histochem Cell Biol. 2011;136:289–299. doi: 10.1007/s00418-011-0846-7. [DOI] [PubMed] [Google Scholar]
- 59.Heublein S., Mayr D., Vrekoussis T., Friese K., Hofmann S.S., Jeschke U., Lenhard M. The G-protein coupled estrogen receptor (GPER/GPR30) is a gonadotropin receptor dependent positive prognosticator in ovarian carcinoma patients. PLoS One. 2013;8:e71791. doi: 10.1371/journal.pone.0071791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walczak-Jedrzejowska R., Forma E., Oszukowska E., Brys M., Marchlewska K., Kula K., Slowikowska-Hilczer J. Expression of G-protein-coupled estrogen receptor (GPER) in whole testicular tissue and laser-capture microdissected testicular compartments of men with normal and aberrant spermatogenesis. Biology (Basel) 2022;11:373. doi: 10.3390/biology11030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu J., Yao R., Lu S., Xu R., Zhang H., Wei J., Zhao C., Tang Y., Li C., Liu H., Zhao X., Wei Q., Ma B. Synergistic effect between LH and estrogen in the acceleration of cumulus expansion via GPR30 and EGFR pathways. Aging (Albany NY) 2020;12:20801–20816. doi: 10.18632/aging.104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y., Che D., Cao Y., Yue Y., He T., Zhu Y., Zhou J. MicroRNA profiling in the aqueous humor of keratoconus eyes. Transl Vis Sci Technol. 2022;11:5. doi: 10.1167/tvst.11.12.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stachon T., Stachon A., Hartmann U., Seitz B., Langenbucher A., Szentmary N. Urea, uric acid, prolactin and ft4 concentrations in aqueous humor of keratoconus patients. Curr Eye Res. 2017;42:842–846. doi: 10.1080/02713683.2016.1256413. [DOI] [PubMed] [Google Scholar]
- 64.Soria J., Villarrubia A., Merayo-Lloves J., Elortza F., Azkargorta M., Alvarez de Toledo J., Rodriguez-Agirretxe I., Suarez T., Acera A. Label-free LC-MS/MS quantitative analysis of aqueous humor from keratoconic and normal eyes. Mol Vis. 2015;21:451–460. [PMC free article] [PubMed] [Google Scholar]
- 65.Rocha E.M., Wickham L.A., da Silveira L.A., Krenzer K.L., Yu F.S., Toda I., Sullivan B.D., Sullivan D.A. Identification of androgen receptor protein and 5alpha-reductase mRNA in human ocular tissues. Br J Ophthalmol. 2000;84:76–84. doi: 10.1136/bjo.84.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki T., Kinoshita Y., Tachibana M., Matsushima Y., Kobayashi Y., Adachi W., Sotozono C., Kinoshita S. Expression of sex steroid hormone receptors in human cornea. Curr Eye Res. 2001;22:28–33. doi: 10.1076/ceyr.22.1.28.6980. [DOI] [PubMed] [Google Scholar]
- 67.Ayan B., Yuksel N., Carhan A., Gumuskaya Ocal B., Akcay E., Cagil N., Asik M.D. Evaluation estrogen, progesterone and androgen receptor expressions in corneal epithelium in keratoconus. Cont Lens Anterior Eye. 2019;42:492–496. doi: 10.1016/j.clae.2018.11.015. [DOI] [PubMed] [Google Scholar]