Abstract
Dimethyl fumarate 1 is approved for the treatment of multiple sclerosis but is also associated with off-target activation of the niacin receptor. By using a tetrazolone or triazolone bioisostere approach to the fumarate and vinyl sulfone series of Nrf2 activators, we have optimized the electrophilicity of the double bond to tune the on-target Nrf2 activation with PK properties to achieve efficacy in animal models of multiple sclerosis. The study linked highly potent, highly electrophilic molecules to low plasma stability and, subsequently, limited efficacy. By contrast, a sulfonylvinyltriazolone 17 retains on-target potency but shows much weaker electrophilic potential. As a consequence, in vivo high exposures of 17 are obtained, resulting in efficacy in the EAE model similar to that observed for DMF. 17 (R079) is Ames negative, is not cytotoxic to cells, and shows little inhibition of either the niacin receptor or a panel of off-target receptors.
Keywords: Nrf2, Electrophilicity, Multiple sclerosis, Michael acceptors, Bioisosteres
Dimethyl fumarate 1 (DMF, marketed as Tecfidera) is approved for the treatment of relapsing remitting multiple sclerosis in the US. DMF 1 is thought to be a prodrug of methyl fumarate 2 (MMF), which circulates at high exposures in patients, while 1 is not detected. The mechanism of action is believed to be through the activation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), which regulates the phase II antioxidant response element through the biosynthesis of several antioxidant enzyme genes including heme oxygenase 1 (HO-1), NAD(P)H quinone oxidoreductase 1, and enzymes regulating glutathione biosynthesis including glutamate cystine ligase. In this way, cells are able to neutralize excess levels of reactive oxygen species (ROS).1,2 Indeed in animal models of multiple sclerosis, Nrf2–/– animals resulted in a more severe clinical course and earlier onset of disease.3 In the resting state, Nrf2 is bound to Kelch-like erythroid cell-derived protein 1 (Keap1), which induces its ubiquitination and ultimately proteosome-mediated degradation. Thus, the cellular levels of Nrf2 remain low under normal conditions. However, under highly oxidative conditions, cysteine residues on the surface of Keap1 undergo oxidation, resulting in a conformational change of the Keap1 protein. This change results in Nrf2 being released.4 MMF 2 reacts with the cysteine residues of Keap1 through an irreversible Michael addition reaction, resulting in a conformational change and release of Nrf2 similar to those observed in the presence of ROS.
On top of the neuroprotective effects, DMF 1 has been known to show anti-inflammatory properties for many years. Indeed, a mixture of 1 and three monoethyl fumarate salts is marketed in Germany (Fumaderm) for psoriasis, where it is believed to reduce cytokine production. In dendritic cells stimulated with the endotoxin lipopolysaccharide (LPS), 1 is able to inhibit the production of multiple cytokines including TNF-α, IL-6, and IL-23.5 LPS signals through toll-like receptor (TLR) 4, resulting in activation of the transcriptional regulator nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB). While cytokine inhibition may be linked to Nrf2, there appears to be an independent pathway for inhibition of TLR signaling through inhibition of the NFκB and ERK1/2 activation.7 Interestingly, it has been shown that the endogenous ligand itaconate can react through an analogous Michael addition mechanism to activate Nrf2 and reduce the inflammatory response through inhibition of NFκB signaling in a similar manner to DMF/MMF.6
In addition to itaconic acid 3 (Figure 1), there are several naturally occurring Nrf2 activators that react with Keap1 in an analogous manner, including the sulforaphanes found in cruciferous vegetables, and oleanane triterpenoids including the semisynthetic omaveloxolone 4 that has been approved for the treatment of Friedreich’s ataxia, a rare disease which causes progressive damage to the spinal cord.8 There have been several efforts to identify specific disrupters of the Keap1–Nrf2 protein–protein interaction as a method of increasing the level of cellular Nrf2. Both a peptide approach and a fragment screening approach have been investigated with many having potent Keap1 binding.9−11 However, to date, there are no clinical stage direct Nrf2–Keap1 inhibitors. Efforts to modify MMF 2 have resulted in a prodrug approach to attenuate the physiochemical properties as in diroximel fumarate 5.12 Several structural classes of molecules with a similar mechanism of action to MMF 2 have been investigated, including chalcone derivatives13 and their vinyl sulfone analogues for example 6, which was shown to increase expression of the Nrf2 dependent genes and alleviate motor deficits in an animal model of Parkinson’s Disease.14 Further efforts have generated molecules with improved drug-like properties,15 as well as sulfonamide and sulfonate derivatives.16
Figure 1.
Structure of DMF 1, MMF 2, itaconic acid 3, semisynthetic bardoxolone derivative omaveloxolone 4, diroximel fumarate 5, and vinyl sulfone Nrf2 activator 6.
Our own efforts toward Nrf2 activators grew from using the tetrazolone as a bioisostere of the carboxylic acid. In recent times the tetrazolone has been underrepresented, with the tetrazole and acylsulfonamides being the more typical bioisosteres employed by medicinal chemists.17,18 However, as part of a medicinal chemistry program, using tetrazolone as a carboxylic acid bioisostere, we had identified molecules with useful properties. We went on to show that the tetrazolone isostere could be extended to several marketed carboxylic acid containing pharmaceuticals to obtain analogues which retained potency and showed good pharmacokinetic properties.19,20 Indeed, as part of these efforts, an MMF analogue 7 was generated.
Initial studies began by investigating the tetrazolone containing analogues of MMF and DMF 7, 8, and the N-(3-pyridyl)tetrazolone compound 9.21 In addition, the tetrazolone was modified to triazolones 10 and 11. The molecules, along with 1 and 2, were profiled in our cellular assay screening for inhibition of IL-23 in response to LPS in line with the reports discussed above.7 The compounds were screened initially in both the reported dendritic cells and THP-1 monocytes. There was a good correlation between the two assays, with the THP-1 cell line being more sensitive (see Supporting Information a comparison of IC50s in the two assays), and for our screening of new analogues the THP-1 assay was used since it was being run routinely in our laboratories.22 In addition, the analogues were profiled in an Nrf2 translocation assay,15 and in the case of 7 and 8 for their off-target agonist activity against the GRP109A-niacin receptor, a known off-target of MMF 2. Activating the niacin receptor can result in flushing, gastrointestinal toxicity, and skin rash (itching), which were observed in the clinical trials of DMF 1.1 The tetrazolone compounds 7 and 8 were comparable to MMF/DMF (2 and 1, respectively) in both on-target assays. The methyl tetrazolone 8 was approximately 2-fold more potent than 7, similar to the trend observed with 1 and 2 (Table 1). The N-(3-pyridyl)tetrazolone 9 appears to be the most potent of the three tetrazolones, particularly in the translocation assay. The triazolone analogues 10 and 11 showed only weak inhibition of IL-23, which translated into very weak Nrf2 translocation activity. In general, the two assays correlate well, particularly in terms of the rank order. As expected, the tetrazolones analogues 7 and 8 did not show the off-target niacin activity seen with 2, thought to be due to the carboxylic acid moiety, and the three compounds 7, 8, and 9 showed little inhibition of hERG (see Supporting Information).
Table 1. Compounds in the Study, On-Target Potency, and Stability in the Presence of Glutathione.
Given the similarity in biological profile of the free tetrazolone 7 to that of MMF 2, it was further profiled for mutagenicity, where it was unfortunately found to be Ames positive. However, this property is not shared by the series because the N-(3-pyridyl)tetrazolone analogue 9 did not show mutagenicity. Disappointingly, in mouse plasma, the methyl ester of 9 was rapidly cleaved to yield the corresponding acid, which was inactive in both on-target assays.
The results obtained from the small selection of analogues encouraged us to investigate the tetrazolone and triazolone moieties in the context of the vinyl sulfone Nrf2 activators, where ester hydrolysis observed with 9 is not possible, removing a potential PK liability. A small set of analogues 12–18 was synthesized to probe the SAR (Table 1).23
The vinyl sulfone series was consistently more potent in the biological assays. N-Methyltetrazolones 12 and 13 showed an increase in potency of greater than 10-fold in both assays relative to 8. Moving to N-(3-pyridyl)tetrazolone 14 further improved the potency, again mirroring the results observed with 9. Indeed, 14 was the most potent molecule in this study, showing low nanomolar potency in both assays. 13 was also screened for activation of the off-target niacin receptor and shown to be inactive. Unfortunately, these high potency molecules 12–14 also displayed short half-lives when incubated with glutathione (<10 min half-life), indicative of poor pharmacokinetic properties.
The improved potency of the vinyl sulfone series is assumed to arise from the compounds being more reactive Michael acceptors due to the increased electrophilicity of the double bond. This results in a more efficient reaction with cysteines on the Keap1 protein, leading to increased Nrf2 translocation. Consistent with this hypothesis, it is interesting to note that for this vinyl sulfone series 12–18, the position of the carbon beta to the sulfone in the 13C NMR spectrum correlates well with Nrf2 translocation potency (see Supporting Information). However, as the electron deficiency of the double bond increases, the molecules are more susceptible to glutathione addition. Therefore, compounds need to balance the ability to react with the target against the stability in plasma needed to reach that target. Since we had observed increased potency with the vinyl sulfone series, it was proposed that combining with the triazolone might result in improved PK properties without eliminating on-target potency. Two series of triazolones 4-linked 5-oxo-1,2,4-triazolones 15 and 16 and 1-linked 5-oxo-1,2,4-triazolones 17 and 18 were generated that fitted the SAR trends seen with 10 and 11 above, that is, a 10-fold drop in potency relative to the tetrazolones; however, they are still in the low micromolar range making them similar to DMF 1. Of the two triazolone series, 4-methyl-1,2,4-trizaol-3-ones 17 and 18 showed weaker on-target activity but much improved stability in the presence of glutathione, likely reflecting a difference in the electron withdrawing effects of the two triazolone isomers.
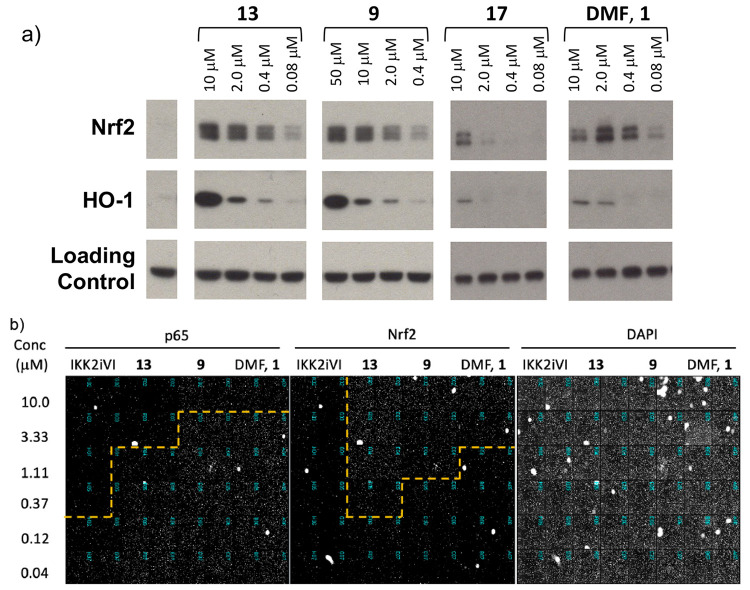
We chose to investigate the mechanistic effects of compounds 9, 13, 17, and 1 in THP-1 cells, probing Nrf2 activation and expression of the antioxidant enzyme HO-1. Cellular levels of Nrf2 increased in a concentration-dependent manner, in line with the translocation assay results (Figure 2a). As levels of Nrf2 rose, the expression of HO-1 increased consistently with the proposed mechanism. Further, we carried out three-way nuclei staining of HUVEC cells in the presence of 9, 13, and 1. Release of cytokines (eg IL-23) in response to TNF-α requires NFκB translocation into the nucleus, which is inhibited by 13, 9, 1, and the control IKK inhibitor (IKKiVI) shown through staining for NFκB subunit p65. Analyzing for nuclear Nrf2 shows concentration dependent levels with the compounds and no nuclear Nrf2 with the IKK inhibitor. DAPI staining confirms the cells are viable (Figure 2b and full plate in the Supporting Information). Finally, a protein labeling experiment showed that several cysteine residues of purified Keap1 were modified after incubation with 13 (see Supporting Information for details).
Figure 2.
(a) Nrf2 activators 13, 9, 17, and DMF 1 increase Nrf2 and expression of HO-1 in THP-1 cells. (b) Nuclear staining of HUVEC cells in the presence of TNF-α and 13, 9, 1, and IKK inhibitor IKK2iVI. IKK2iVI = 2-[(aminocarbonyl)amino]-5-phenyl-3-thiophenecarboxamide.
We prioritized understanding the mutagenicity potential of the vinyl sulfone series given the result with tetrazolone 7. Encouragingly, both tetrazolone 13 and triazolone 17 were found to be Ames negative (result for 17 in the Supporting Information). In a lead profiler screen of off-target transporters and receptors screened at 500 μM, only adenosine A3 and serotonin 5-HT2B inhibition (82% and 74%, respectively) were observed. In this screen hERG was inhibited by 50% at the 500 μM concentration Further, in our assay of cell toxicity, 17 was found to be inactive, while 13 showed weak activity in both cell lines (A549 = 8.81 μM, H1299 = 2.32 μM).24
Both the potent tetrazolone 13 and the weaker triazolone 17 were further investigated in liver microsomes across species. The results were in line with the glutathione result, that is, tetrazolone 13 had a very short half-life (<5 min in rodents and 10 min in human), while triazolone 17 displayed a half-life of greater than 1 h across all species (see Supporting Information).
Compounds 13 and 17 were taken into mouse PK where reasonable exposures were achieved following oral dosing (Table 2). As expected, the more stable 17 results in significantly higher exposures; however, in each case, even at the low doses used in the PK study, the Cmax plasma concentrations are above the EC50 of the compounds in the Nrf2 translocation assay. Clearance of both molecules is low, with 13 being lower than would have been expected from the microsomal stability study and glutathione experiment (see Supporting Information for the PK curves).
Table 2. PK Parameters for 13 and 17 in Balb/c Mice.
| property | 13 | 17 |
|---|---|---|
| clearance (mL/min/kg) | 17.4 | 2.01 |
| half-life (h) | 0.4 | 1.0 |
| Vss (L/kg) | 0.20 | 0.14 |
| oral dose (mg/kg) | 10 | 5 |
| oral AUC (ng·h/mL) | 5,780 | 22,900 |
| oral Cmax (ng/mL) | 2,500 (8.93 μM) | 19,600 (73.96 μM) |
| bioavailability (%F) | 60 | 56 |
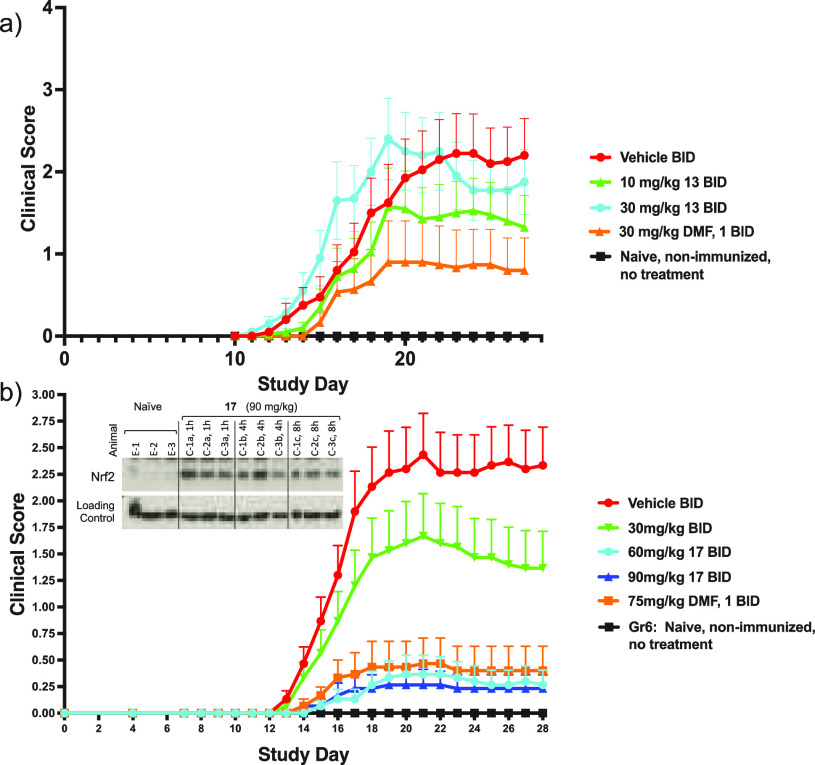
In order to understand the balance between biological activity and PK in vivo, both 13 and 17 were investigated in a mouse experimental autoimmune encephalomyelitis (EAE) model by immunization with myelin oligodendrocyte glycoprotein (MOG), which mimics the symptoms of multiple sclerosis through degradation of the myelin sheath around the nerves, resulting in paralysis.25−27 Given the short half-lives of the molecules in mouse PK, we dosed two times daily (BID) and used DMF 1 (dosed BID) as a control in each experiment. The more potent vinyl sulfonyl tetrazolone 13 was dosed at 10 and 30 mg/kg, while for vinyl sulfonyl triazolone 17, doses of 30, 60, and 90 mg/kg were chosen. For 13, while at the lower dose we did see signs of efficacy (40% reduction in clinical score), it was not dose dependent, with the higher dose not showing any meaningful effect (Figure 3a). By contrast, 17, despite being 10-fold less potent, demonstrated a robust dose-dependent reduction in disease severity (41% reduction in clinical score at 30 mg/kg BID to 89% at 60 mg/kg) similar to that of DMF dosed at 75 mg/kg (83% reduction) (Figure 3b). The improved efficacy of 17 is likely due to the improvement in PK, resulting in significantly greater exposures that maintain concentrations above the Nrf2 translocation EC50. Indeed, in the EAE study, AUC’s of 17 were 247 000, 371 000, and 444 000 ng·h/mL at 30, 60, and 90 mg/kg BID, equivalent to average concentrations of 38.8, 58.3, and 69.8 μM, respectively. These concentrations would be expected to be sufficient for activation of Nrf2 (EC50 = 32.4 μM) despite the moderate potency. For the EAE study with 13, analysis of drug concentrations 15 min after dosing (potentially close to Cmax) showed 786 ng/mL at 30 mg/kg and 425 ng/mL at 10 mg/mL, equivalent to 2.81 μM and 1.52 μM, respectively, which would be sufficient to activate Nrf2 (EC50 0.30 μM) but likely not for sufficient duration for efficacy, given that by 8 h no drug could be detected.
Figure 3.
Mouse MOG-EAE study with 13 (a) and 17 (b) showing clinical scores and SEM during the study.
In the study with triazolone 17, analysis of the thymus showed that both the 60 and 90 mg/kg doses increased levels of Nrf2 for an 8 h period, consistent with the mechanism of action. Dosing with DMF 1 had a similar effect (Figure 3b shows 17 at 90 mg/kg, see Supporting Information for full details).
The compounds in the study were synthesized using procedures reported in the patent literature.21,23 Briefly, 7 was converted to 8 through alkylation with iodomethane. The 3-pyridyl substituted tetrazolone 19 was synthesized from nicotinoyl chloride by heating with (trimethylsilyl)azide as reported.19 Reaction with methyl propiolate in the presence of catalytic DABCO yielded 9 as the major isomer (Scheme 1).
Scheme 1. Synthesis of Tetrazolone Fumarate Analogues 8 and 9.
(a) CH3I, K2CO3, DMF, 0 °C to rt, 14 h. (b) 20% DABCO, CH3CN, rt, 3 h.
For the synthesis of vinyl sulfone analogues, two procedures were employed. In the case of the phenyl sulfone compounds, it was possible to affect a displacement of the symmetrical commercially available 1,2-bis(phenylsulfonyl)ethane 20 again using DABCO at elevated temperature, a method analogous to that reported.28 Alternatively, reaction of the ethynyl sulfone 21 with triazolone 22 in the presence of DABCO resulted in the desired vinyl sulfone 17 (Scheme 2).23
Scheme 2. Synthesis of the Vinyl Sulfone Analogues.
(a) 19, DABCO, CH3CN, rt to 50 °C. (b) DABCO, CH3CN, 0 °C to rt.
In this study, it has been shown that the tetrazolone can replace the carboxylic acid of methyl fumarate in a bioisosteric manner to obtain compounds with superior potency to the Nrf2 activator MMF 1 and prodrug DMF 2. We have been able to expand the use of this moiety to the more active vinyl sulfone series of Nrf2 activators. However, as the potency increases the stability of the molecules decreases because both properties are related to the reactivity of the electron deficient double bond. When the reactivity is too great, there is little exposure in animals, resulting in reduced efficacy as observed with 13. Instead, we have developed a working model for identifying Nrf2 activators in which moderate activity in combination with high systemic exposure is optimal (Figure 4). Indeed, this matches the situation observed with DMF/MMF 1/2 and also the endogenous ligand itaconate 3, which are all in the micromolar range.6,29
Figure 4.
Working model for identifying covalent modifiers is shown with Nrf2 activators.
In 17, the electron withdrawing sulfone group is balanced by the triazolone to tune the electron deficiency of the vinyl group. In this way, 17, known as R079, demonstrated good pharmacokinetic properties, including plasma stability and bioavailability, which can activate Nrf2 in vivo and results in robust efficacy in the EAE model of multiple sclerosis. Further, 17 shows a clean off-target profile; it does not have an effect on the niacin receptor thought to be responsible for some of the side effects of DMF 1, it shows little inhibition of a panel of off-target transporters or receptors, no cell toxicity, and is Ames negative. The clinical efficacy of 1 in multiple sclerosis suggests that 17 has the potential for further development.
Acknowledgments
The work described was solely funded by Rigel Pharmaceuticals, Inc. We thank Mark Irving and Duayne Tokushige for assistance in isolating some compounds, Chi Young for assistance with assays, and Vanessa Taylor for helpful discussions.
Glossary
Abbreviations Used
- BID
bis in die (twice daily)
- DABCO
1,4-diazabicyclo[2.2.2]octane
- DAPI
(4′,6-diamino-2-phenylindole
- DMF
dimethyl fumarate
- EAE
experimental autoimmune encephalomyelitis
- HO-1
heme oxygenase 1
- HUVEC
human umbilical vein endothelial cells
- Keap1
Kelch-like erythroid cell-derived protein 1
- MMF
monomethyl fumarate
- MOG
myelin oligodendrocyte glycoprotein
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2
nuclear factor erythroid 2-related factor 2
- TLR
toll-like receptor
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications Web site. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00336.
Microsomal stability data across species and PK profiles for 13 and 17 in mice. Full Western blot analysis of Nrf2 levels in mouse thymus at conclusion of EAE study with 17 (60, 90 mg/kg), 1 (75 mg/kg). Full plate view of the translocation of p65 and Nrf2 into the nucleus in the presence of compounds 13, 9, and 1. A Keap1 labeling experiment. Comparison of potencies in the IL-23 assay between THP-1 and dendritic cells. Analysis in vinyl sulfone series of 13C NMR shift with Nrf2 translocation potency. Biological assay procedures and representative experimental procedures for the synthesis of molecules described and characterization of the final molecules 8–18 (PDF)
Author Contributions
The manuscript was written through contributions of all authors, who have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): All authors were employed by Rigel Pharmaceuticals, Inc at the time of this work.
Supplementary Material
References
- Phillips J. T.; Fox R. J. BG-12 in Multiple Sclerosis. Semin. Neurol. 2013, 33, 56. 10.1055/s-0033-1343796. [DOI] [PubMed] [Google Scholar]
- Linker R. A.; Lee D.-H.; Ryan S.; van Dam A. M.; Conrad R.; Bista P.; Zeng W.; Hronowsky X.; Buko A.; Chollate S.; Ellrichmann G.; Brück W.; Dawson K.; Goelz S.; Wiese S.; Scannevin R. H.; Lukashev M.; Gold R. Fumaric Acid Esters Exert Neuroprotective Effects in Neuroinflammation via Activation of the Nrf2 Antioxidant Pathway. Brain 2011, 134 (3), 678–692. 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- Johnson D. A.; Amirahmadi S.; Ward C.; Fabry Z.; Johnson J. A. The Absence of the Pro-antioxidant Transcription Factor Nrf2 Exacerbates Experimental Autoimmune Encephalomyelitis. Toxicol. Sci. 2010, 114 (2), 237–246. 10.1093/toxsci/kfp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso R.; Maccallini C.; Bellezza I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants 2023, 12 (3), 778. 10.3390/antiox12030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H.; Guerau-de-Arellano M.; Mehta V. B.; Yang Y.; Huss D. J.; Papenfuss T. L.; Lovett-Racke A. E.; Racke M. K. Dimethyl Fumarate Inhibits Dendritic Cell Maturation via Nuclear Factor κB (NF-κB) and Extracellular Signal-regulated Kinase 1 and 2 (ERK1/2) and Mitogen Stress-activated Kinase 1 (MSK1) Signaling. J. Biol. Chem. 2012, 287 (33), 28017–28026. 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E. L.; Ryan D. G.; Prag H. A.; Dikovskaya D.; Menon D.; Zaslona Z.; Jedrychowski M. P.; Costa A. S. H.; Higgins M.; Hams E.; Szpyt J.; Runtsch M. C.; King M. S.; McGouran J. F.; Fischer R.; Kessler B. M.; McGettrick A. F.; Hughes M. M.; Carroll R. G.; Booty L. M.; Knatko E. V.; Meakin P. J.; Ashford M. L. J.; Modis L. K.; Brunori G.; Sévin D. C.; Fallon P. G.; Caldwell S. T.; Kunji E. R. S.; Chouchani E. T.; Frezza C.; Dinkova-Kostova A. T.; Hartley R. C.; Murphy M. P.; O’Neill L. A. Itaconate is an Anti-inflammatory Metabolite that Activates Nrf2 via Alkylation of KEAP1. Nature 2018, 556, 113. 10.1038/nature25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire V. A.; Ruiz-Zorrilla Diez T.; Emmerich C. H.; Strickson S.; Ritorto M. S.; Sutavani R. V.; Weiβ A.; Houslay K. F.; Knebel A.; Meakin P. J.; Phair I. R.; Ashford M. L. J.; Trost M.; Arthur J. S. C. Dimethyl Fumarate Blocks Inflammatory Cytokine Production via Inhibition of TLR Induced M1 and K63 Ubiquitin Chain Formation. Sci. Rep. 2016, 6, 31159. 10.1038/srep31159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R. R.; Casaubon R. L.; Ott G. R.. Small-Molecule Modulators of KEAP1-Nrf2. In Medicinal Chemistry Reviews; Desai M. C., Ed.; American Chemical Society, 2016; Vol. 51, pp 165–182. 10.29200/acsmedchemrev-v51.ch11. [DOI] [Google Scholar]
- Steel R.; Cowan J.; Payerne E.; O’Connell M. A.; Searcey M. Anti-inflammatory Effect of a Cell-Penetrating Peptide Targeting the Nrf2/Keap1 Interaction. ACS Med. Chem. Lett. 2012, 3 (5), 407–410. 10.1021/ml300041g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. G.; Wixted W. E.; Coyle J. E.; Griffiths-Jones C.; Hearn K.; McMenamin R.; Norton D.; Rich S. J.; Richardson C.; Saxty G.; Willems H. M. G.; Woolford A. J.-A.; Cottom J. E.; Kou J.-P.; Yonchuk J. G.; Feldser H. G.; Sanchez Y.; Foley J. P.; Bolognese B. J.; Logan G.; Podolin P. L.; Yan H.; Callahan J. F.; Heightman T. D.; Kerns J. K. Monoacidic Inhibitors of the Kelch-like ECH-Associated Protein 1:Nuclear Factor Erythroid 2-Related Factor 2 (KEAP1:NRF2) Protein-Protein Interaction with High Cell Potency Identified by Fragment-Based Discovery. J. Med. Chem. 2016, 59 (8), 3991–4006. 10.1021/acs.jmedchem.6b00228. [DOI] [PubMed] [Google Scholar]
- Norton D.; Bonnette W. G.; Callahan J. F.; Carr M. G.; Griffiths-Jones C. M.; Heightman T. D.; Kerns J. K.; Nie H.; Rich S. J.; Richardson C.; Rumsey W.; Sanchez Y.; Verdonk M. L.; Willems H. M. G.; Wixted W. E.; Wolfe L. III; Woolford A. J.-A.; Wu Z.; Davies T. G. Fragment-Guided Discovery of Pyrazole Carboxylic Acid Inhibitors of the Kelch-like ECH-Associated Protein 1: Nuclear Factor Erythroid 2 Related Factor 2 (KEAP1:NRF2) Protein-Protein Interaction. J. Med. Chem. 2021, 64 (21), 15949–15972. 10.1021/acs.jmedchem.1c01351. [DOI] [PubMed] [Google Scholar]
- Zeidan T. A.; Duncan S.; Hencken C. P.; Wynn T. A.; Sanrame C. N.. Prodrugs of Fumarates and Their Use in Treating Various Diseases. WO Patent. WO2014152494 A1, 2014.
- Kumar V.; Kumar S.; Hassan M.; Wu H.; Thimmulappa R. K.; Kumar A.; Sharma S. K.; Parmar V. S.; Biswal S.; Malhotra S. V. Novel Chalcone Derivatives as Potent Nrf2 Activators in Mice and Human Lung Epithelial Cells. J. Med. Chem. 2011, 54 (12), 4147–4159. 10.1021/jm2002348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S. Y.; Kim J. H.; Moon M. K.; Han S.-H.; Yeon S. K.; Choi J. W.; Jang B. K.; Song H. J.; Kang Y. G.; Kim J. W.; Lee J.; Kim D. J.; Hwang O.; Park K. D. Discovery of Vinyl Sulfones as a Novel Class of Neuroprotective Agents toward Parkinson’s Disease Therapy. J. Med. Chem. 2014, 57 (4), 1473–1487. 10.1021/jm401788m. [DOI] [PubMed] [Google Scholar]
- Choi J. W.; Kim S.; Park J.-H.; Kim H. J.; Shin S. J.; Kim J. W.; Woo S. Y.; Lee C.; Han S. M.; Lee J.; Pae A. N.; Han G.; Park K. D. Optimization of Vinyl Sulfone Derivatives as Potent Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Activators for Parkinson’s Disease Therapy. J. Med. Chem. 2019, 62 (2), 811–830. 10.1021/acs.jmedchem.8b01527. [DOI] [PubMed] [Google Scholar]
- Choi J. W.; Shin S. J.; Kim H. J.; Park J.-H.; Kim H. J.; Lee E. H.; Pae A. N.; Bahn Y. S.; Park K. D. Antioxidant, Anti-inflammatory, and Neuroprotective Effects of Novel Vinyl Sulfonate Compounds as Nrf2 Activator. ACS Med. Chem. Lett. 2019, 10 (7), 1061–1067. 10.1021/acsmedchemlett.9b00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell N. A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54 (8), 2529–2591. 10.1021/jm1013693. [DOI] [PubMed] [Google Scholar]
- Horgan C.; O’Sullivan T. P. Recent Developments in the Practical Application of Novel Carboxylic Acid Bioisosteres. Current Med. Chem. 2022, 29 (13), 2203–2234. 10.2174/0929867328666210820112126. [DOI] [PubMed] [Google Scholar]
- Duncton M. A. J.; Singh R. A One-pot Synthesis of Tetrazolones from Acid Chlorides: Understanding Functional Group Compatibility, and Application to the Late-stage Functionalization of Marketed Drugs. Org. Biomol. Chem. 2016, 14 (39), 9338–9342. 10.1039/C6OB01644H. [DOI] [PubMed] [Google Scholar]
- Duncton M. A. J.; Murray R. B.; Park G.; Singh R. Tetrazolone as an Acid Bioisostere: Application to Marketed Drugs Containing a Carboxylic Acid. Org. Biomol. Chem. 2016, 14 (39), 9343–9347. 10.1039/C6OB01646D. [DOI] [PubMed] [Google Scholar]
- Duncton M.; Bhamidipati S.; Yu J.; Darwish I.; Singh R.. Fumarate Analogs and Uses Thereof in the Treatment of an Autoimmune Disease or an Inflammatory Disease. WO Patent WO2016022434 A1, 2016.
- Chen Y.; Singh R.; Lin N.; Taylor V.; Masuda E. S.; Payan D. G. Discovery of 5-Aryl-2,4-diaminopyrimidine Compounds as Potent and Selective IRAK4 Inhibitors. ACS Med. Chem. Lett. 2022, 13 (4), 714–719. 10.1021/acsmedchemlett.2c00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncton M.; Singh R.. Nrf2 Activating Compounds and Uses Thereof. US Patent US20170217922 A1, 2017.
- Catalano S. M.; McLaughlin J.; Hitoshi Y.; Payan D. Discovery and Development of an Aurora Kinase Inhibitor Clinical Candidate Using an Image-Based Assay for Measuring Proliferation, Apoptosis, and DNA Content. Assay Drug Dev. Technol. 2009, 7 (2), 180–190. 10.1089/adt.2007.086. [DOI] [PubMed] [Google Scholar]
- Mendel I.; de Rosbo N. K.; Ben-Nun A. A Myelin Oligodendrocyte Glycoprotein Peptide Induces Typical Chronic Experimental Autoimmune Encephalomyelitis in H-2b Mice: Fine Specificity and T Cell Receptor Vβ expression of encephalitogenic T cells. Eur. J. Immunol. 1995, 25 (7), 1951–1959. 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- Rangachari M.; Kuchroo V. K. Using EAE to Better Understand Principles of Immune Function and Autoimmune Pathology. J. Autoimmun. 2013, 45, 31–39. 10.1016/j.jaut.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes I. M.; Goverman J. M. Active Induction of Experimental Allergic Encephalomyelitis. Nat. Protoc. 2006, 1 (4), 1810–1819. 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Petit E.; Bosch L.; Font J.; Mola L.; Costa A. M.; Vilarrasa J. Tosvinyl and Besvinyl as Protecting Groups of Imides, Azinones, Nucleosides, Sultams, and Lactams. Catalytic Conjugate Additions to Tosylacetylene. J. Org. Chem. 2014, 79, 8826. 10.1021/jo501647w. [DOI] [PubMed] [Google Scholar]
- Muri J.; Wolleb H.; Broz P.; Carreira E. M.; Kopf M. Electrophilic Nrf2 Activators and Itaconate Inhibit Inflammation at Low Dose and Promote IL-1β Production and Inflammatory Apoptosis at High Dose. Redox Biol. 2020, 36, 101647. 10.1016/j.redox.2020.101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.