Abstract
Background
Primary adrenal leiomyosarcoma is a rare and aggressive mesenchymal tumor derived from the smooth muscle wall of a central adrenal vein or its tributaries; therefore, tumors tend to invade the inferior vena cava and cause thrombosis. The great majority of tumors grow rapidly, which makes the disease difficult to diagnose in its early clinical stages and needs differentiation from adrenocortical carcinomas for the selection of chemotherapy including mitotane which causes adrenal insufficiency.
Case presentation
We presented two patients with adrenal leiomyosarcoma who were referred to our hospital with abdominal pain and harboring large adrenal tumors and inferior vena cava thrombosis. The endocrine findings, including serum catecholamine levels, were unremarkable. These two patients were considered clinically inoperable, and CT-guided core needle biopsy was performed to obtain the definitive histopathological diagnosis and determine the modes of therapy. The masses were subsequently diagnosed as primary adrenal leiomyosarcoma based on the histological features and positive immunoreactivity for SMA (smooth muscle actin), desmin, and vimentin.
Conclusions
Adrenal leiomyosarcoma derived from the smooth muscle wall of a central adrenal vein or its tributaries is rare but should be considered a differential diagnosis in the case of nonfunctioning adrenal tumors extending directly to the inferior vena cava. CT-guided biopsy is considered useful for histopathological diagnosis and clinical management of patients with inoperable advanced adrenal tumors without any hormone excess.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-023-01530-z.
Keywords: Primary adrenal leiomyosarcoma, CT-guided core needle biopsy, Vena cava thrombosis, Nonfunction, Case reports
Background
Primary adrenal leiomyosarcoma is a rare mesenchymal tumor, representing 0.1% to 0.2% of all retroperitoneal soft tissue sarcomas of adults [1]. Primary adrenal leiomyosarcoma is well known to be derived from the smooth muscle wall of a central adrenal vein or its tributaries [2, 3], and the presence of tumor invasion extending from the central vein to the inferior vena cava resulting in thrombosis has been reported in those patients [4–12]. The great majority of adrenal leiomyosarcomas grow rapidly usually with nonspecific abdominal pain, and inoperable [13–17]. Therefore, differential diagnosis of the lesions is considered mandatory, especially needing differentiation from adrenocortical carcinomas for the selection of effective chemotherapy drugs including mitotane which causes adrenal insufficiency. We herein report two rare cases of inoperable primary adrenal leiomyosarcoma which were difficult to distinguish from adrenocortical carcinomas by clinical and imaging findings but only diagnosed by histopathological evaluation of CT-guided core needle biopsy.
Case presentation
Case 1
A 71-year-old woman with no past history was admitted to another hospital due to abdominal pain, and revealed to have a right retroperitoneal mass, thereby referred to our hospital. She has been treated with acetaminophen. At presentation, her blood pressure was slightly high (BP 144/78 mmHg) and body temperature was elevated (37.7 °C). Physical examination revealed a mild abdominal discomfort upon palpation. Routine laboratory investigation showed an average blood count (WBC 5800/µl) but high C-reactive protein levels (CRP: 20.16 mg/dL). Her HIV antibody test was negative. Only the tumor marker neuron-specific enolase (NSE: 44.6 ng/mL) was increased. All blood hormonal parameters were within normal limits: plasma cortisol 15.3 µg/dl (normal range 7.1–19.6), plasma adrenocorticotropic hormone (ACTH) 22.9 pg/ml (normal range 7.2–63.3), dehydroepiandrosterone sulfate (DHEAS) 29 µg/dl (normal range 7–177), aldosterone (RIA) 84.3 pg/ml (normal range 29.9–159), plasma renin activity 0.5 ng/ml/h (normal range 0.3–2.9), adrenaline 6 pg/ml (normal range < 100), noradrenaline 200 pg/ml (normal range 100–450) and dopamine 9 pg/ml (normal range < 20). 24-h urine collection for cortisol (50.3 µg/day: normal range 11.2–80.3), aldosterone (6.37 µg/day: normal range < 10), metanephrine (0.1 mg/day: normal range 0.04–0.19), normetanephrine (0.22 mg/day: normal range 0.09–0.33), and 17-ketosteroids (17-KS) were also within normal limits. Computed tomography (CT) demonstrated a poorly enhanced and heterogeneous mass measuring 10 × 6 × 11 cm in the right suprarenal area with a continuous normal adrenal gland on its dorsal side (Fig. 1A). In addition, there was an infiltration shadow on the bottom of the right lung (Fig. 1B), lymphadenopathy in the longitudinal, bilateral hilum, hilar, para-aorta, and inguinal regions (Fig. 1C), and obstruction of the inferior vena cava. Subsequent adrenal magnetic resonance imaging (MRI) showed an 11 cm heterogeneous mass with a lack of signal drop on out-of-phase imaging. A T1-weighted image showed a high signal from the inferior vena cava to the right common iliac vein and internal iliac vein, suggesting thrombosis (Fig. 1D). The metaodobenzylguanidine (MIBG) scintigram was negative. Because of distant metastasis to the lung and poor general condition, adrenalectomy could not be performed, and a CT-guided core needle biopsy using 16-gauge was subsequently performed. Histopathologically, spindle-shaped atypical cells harboring a high nuclear/cytoplasmic ratio were detected (Fig. 1E). The areas of necrosis and slight tumor heterogeneity and mitosis were seen in the pleomorphic areas. These atypical cells were immunohistochemically positive for smooth muscle actin (SMA) (Fig. 1F), desmin (Fig. 1G), and vimentin (Fig. 1H), consistent with the diagnosis of primary adrenal leiomyosarcoma. The Ki-67 proliferation index was 40% (Fig. 1I). The complete absence of immunoreactivity of SF-1 (Supplemental Fig. 1A), inhibin-alpha (Supplemental Fig. 1B), and calretinin (Supplemental Fig. 1C) ruled out adrenocortical carcinoma. In addition, the lack of synaptophysin, AE1/AE3 cytokeratin, S-100 and CD34 expression (Supplementary Fig. 1D-G) ruled out pheochromocytoma, metastatic carcinoma, malignant peripheral nerve sheath tumor, and angiosarcoma, respectively. The patient was also negative for Epstein–Barr virus (EBV), as demonstrated by EBV-encoded RNA (EBER) in situ hybridization (ISH) (Fig. 1J) and latent membrane protein 1 (LMP1) immunoreactivity (Supplemental Fig. 1H) despite the enlargement of multiple lymph nodes. The tumor cells were also immunohistochemically negative for p53 (Supplementary Fig. 1I). Doxorubicin chemotherapy was suggested, but the patient and her family chose not to undergo any additional chemotherapy or radiotherapy, and refractory pain control was performed by palliative care staff. The patient died 8 months after diagnosis.
Fig. 1.
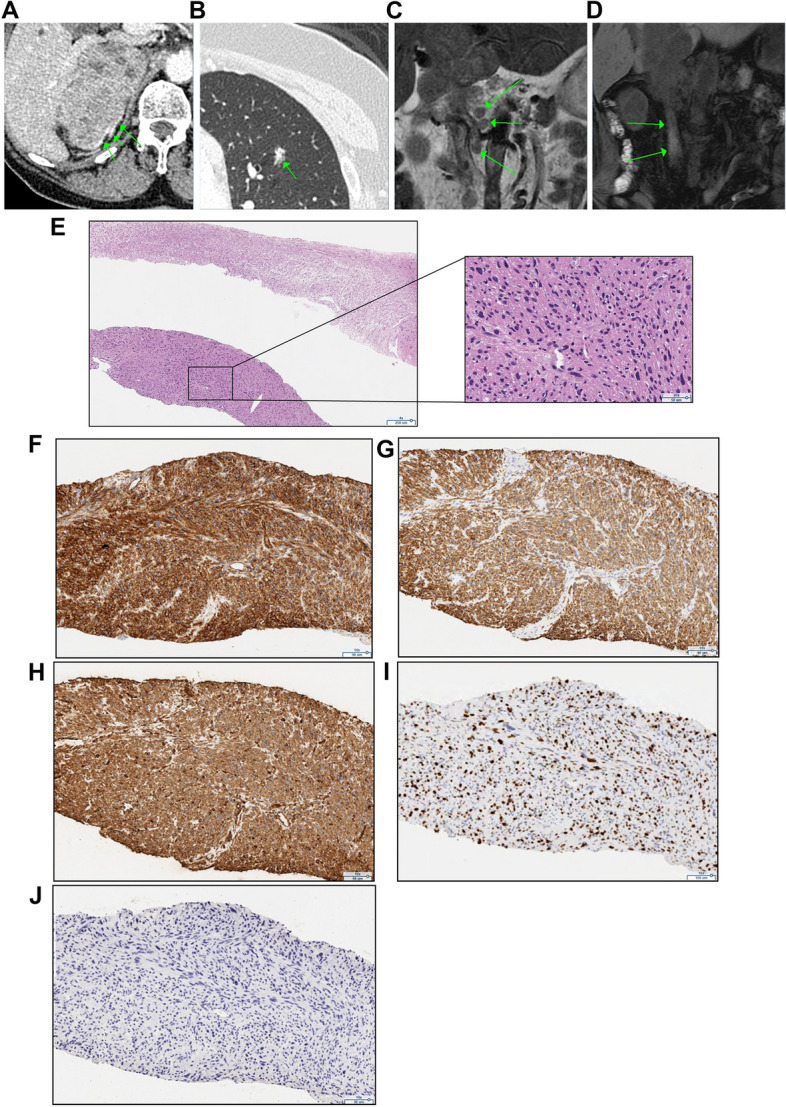
Radiological and histopathological characteristics in case 1. A-B An enhanced CT scan showing a 10 × 6 × 11 cm right-sided adrenal tumor with a normal adrenal gland (green arrow) on the dorsal side (A) and metastatic lesions in the lung (B). C T2-weighted MRI images showing lymphadenopathy in the hilum and para-aorta. D High signal in T1-weighted MRI images reveal venous thrombosis from the inferior vena cava to the right common iliac vein and the right internal iliac vein. E Hematoxylin eosin staining showing atypical spindle cells in adrenal biopsies (40 x). F-H Tumor cell staining positive for smooth muscle actin SMA (F 100 x), desmin (G 100 x) and vimentin (H 100 x). I Immunohistochemical staining of the cells with the Ki-67 marker (100 x). J EBV-encoded RNA in situ hybridization (EBER-ISH) staining for Epstein–Barr virus (EBV) was negative (100 x)
Case 2
A 45-year-old woman with type 2 diabetes, hypertension and dyslipidemia was admitted to our hospital by ambulance with back pain, nausea, and cold sweats. At presentation, she had low blood pressure (BP 96/36 mmHg) and tachypnea (RR 48/min). She had an elevated body temperature (37.8 °C) and elevated white blood cells (10,200 cells/µL), CRP levels (16.84 mg/dL), and D-dimer (5.3 µg/ml). CT demonstrated a 7 × 5 × 5 cm solid mass with blurred boundaries and partial intratumoral bleeding in the left upper abdomen (Fig. 2A and B), accompanied by lymphadenopathy in the para-aortic region (Fig. 2C) and a tumor thrombus extending from the left adrenal vein to the renal and inferior vena cava, as demonstrated by MRI (Fig. 2D). All blood hormonal parameters were within normal limits: plasma cortisol 11.6 µg/dl (normal range 7.1–19.6), ACTH 10.0 pg/ml (normal range 7.2–63.3), DHEAS 125 µg/dl (normal range 19–231), plasma aldosterone (RIA) 74.3 pg/ml (normal range 29.9–159), plasma renin activity 1.0 ng/ml/hr (normal range 0.3–2.9), adrenaline 22 pg/ml (normal range < 100), noradrenaline 222 pg/ml (normal range 100–450) and dopamine 10 pg/ml (normal range < 20). 24-h urine collection for cortisol (97.7 µg/day: normal range 11.2–80.3), aldosterone (4.77 µg/day: normal range < 10), metanephrine (0.11 mg/day: normal range 0.04–0.19), normetanephrine (0.33 mg/day: normal range 0.09–0.33), as well as overnight 1-mg dexamethasone suppression test (1.7 µg/dl), were also within normal limits. The MIBG scintigram result was negative. The mass could not be surgically resected due to the tumor thrombus clinically detected in the left renal vein and inferior vena cava. A CT-guided core needle biopsy using a 16-gauge needle was subsequently performed to definitively diagnose the lesion. The tumor was composed of tumor heterogeneity of spindle-shaped atypical cells harboring a high nuclear/cytoplasmic ratio as well as nuclear pleomorphism with many multinucleated giant cells (Fig. 2E) immunohistochemically positive for SMA (Fig. 2F), desmin (Fig. 2G), and vimentin (Fig. 2H), consistent with the diagnosis of primary adrenal leiomyosarcoma. The absence of SF-1 (Supplemental Fig. 2A), chromogranin A/synaptophysin, AE1/AE3 cytokeratin, S-100, CD34, and HMB-45 (Supplemental Fig. 2B-G) ruled out pheochromocytoma, metastatic carcinoma, malignant peripheral nerve sheath tumor, angiosarcoma, and malignant melanoma, respectively. The Ki-67 index was 30% (Fig. 2I). p53 was detected in some tumor cells (Supplemental Fig. 2H). Chemotherapy with doxorubicin and ifosfamide was scheduled, but discontinued 1 month after diagnosis due to pulmonary embolus.
Fig. 2.
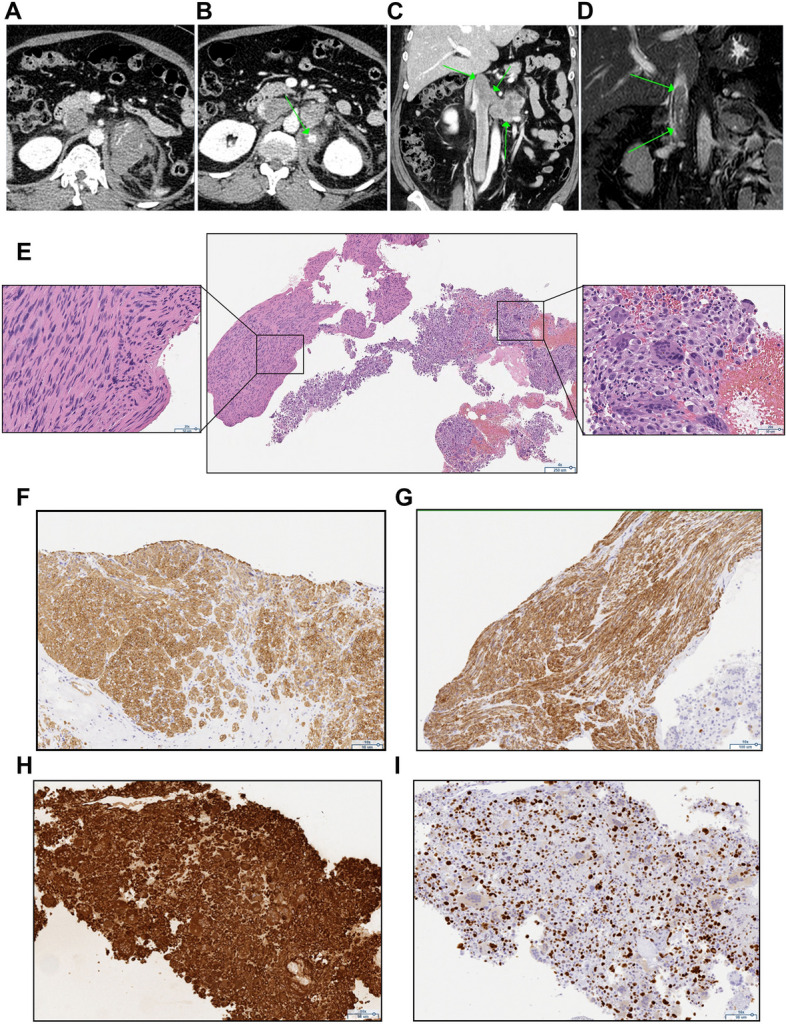
Radiological and histopathological characteristics in case 2. A-C An enhanced CT scan showing a 7 × 5 × 5 cm left-sided adrenal tumor with surrounding adipose tissue opacity, extravasation (A) and partial bleeding (B), as well as lymphadenopathy in the para-aortic region (C). D MRI revealed venous thrombosis extending from the left renal vein to the subhepatic vena cava. E Hematoxylin eosin staining showing atypical spindle cells and multinucleated cells in adrenal biopsies (40 x). F-H Tumor cell staining positive for smooth muscle actin SMA (F 100 x), desmin (G 100 x), and vimentin (H 100 x). I Immunohistochemical staining of the cells with the Ki-67 marker (100 x)
Discussion and conclusions
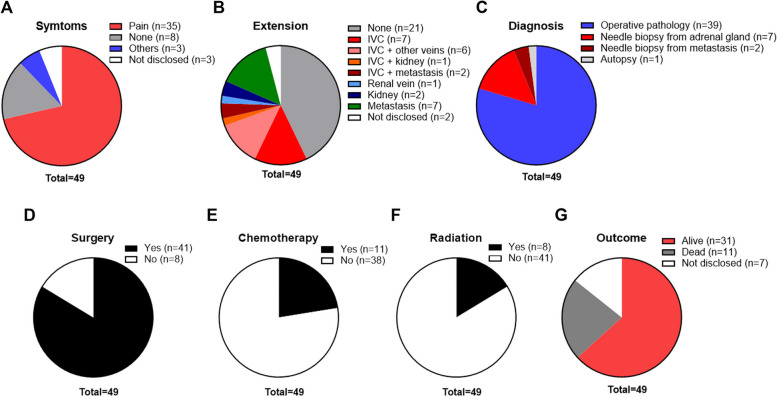
We presented two rare cases of primary adrenal leiomyosarcoma with an extremely poor prognosis. The primary adrenal leiomyosarcomas showed direct extension to the inferior vena cava, and could be distinguished from other retroperitoneal tumors including adrenocortical carcinoma, malignant pheochromocytoma and renal cell carcinoma [18] by only CT-guided core needle biopsy. The clinical and pathological features of the reported cases including our two cases are summarized in Table 1. The ages of the patients ranged from 14 to 79 years, with a mean of 54 years. The patients with primary adrenal leiomyosarcoma occur in 27 women and 22 men. There were 22 right-sided, 25 left-sided, and two bilateral tumors. The size at presentation has ranged from 0.8 to 27 cm (mean, 9.5 cm). The most common presenting symptom is pain (abdominal, flank, back, or groin) in 71.4% of patients (Fig. 3A). Primary adrenal leiomyosarcoma is well known to be derived from the smooth muscle wall of a central adrenal vein or its tributaries [2, 3]. The extension into the IVC was 32.6%: IVC alone 14.3%, IVC with right atrium or other veins (renal, iliac or hepatic) 12.2%, IVC with metastasis (lung, thoracic wall, femur or muscle) 4.1%, and IVC with a kidney 2% (Fig. 3B). The apparent invasion to the kidney and renal vein were 4.1% and 2%, respectively (Fig. 3B). The distal metastasis (liver, lung, bone, pancreas, and brain) was observed in 14.3% (Fig. 3B). The diagnosis of primary adrenal leiomyosarcoma was based on histopathological and immunohistochemical, showing neoplasm consisting of spindle cells that stain positively for SMA, desmin, vimentin, h-caldesmon or others (actin, keratin, cytokeratin, HHF, calpinin, NSE, CD163, MAK6, WT1 or S100). Most histopathological evaluation was performed after surgery in 79.6% of cases, but the needle biopsy from adrenal tumors was also performed in 14.3% and needle biopsy from metastasis (liver or lung) in 4.1% of cases (Fig. 3C). As for treatment, 16.3% of patients could not perform any surgery (Fig. 3D). The chemotherapy and/or radiation were performed in 22.4% and 16.3% of patients, respectively (Fig. 3E and F). During 11 days to 52 months follow-up duration, 63.3% of patients were alive and 22.4% were dead (Fig. 3G).
Table 1.
Summary of the clinical and pathological features of primary adrenal leiomyosarcoma, including previously reported cases and our two cases
| References | Patients’ characteristics | Tumors | Diagnosis | Pathology | Treatment | Outcome | Follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (name) | (year) | Age (year) | Sex | symptoms | Side | Size (cm) | Extension | Procedure | Positive staining | Months | ||
| Our case | 71 | F | Pain (abdominal) | R | 10 | IVC | Needle biopsy (adrenal gland) | SMA, desmin and vimentin | None | Dead | 8 | |
| Our case | 45 | F | Pain (back) and nausea | L | 7 | IVC and renal vein | Needle biopsy (adrenal gland) | SMA, desmin and vimentin | None | Dead | 1 | |
| Lin H. [19] | 2023 | 56 | M | ND | R | 7.4 | IVC and renal vein | Needle biopsy (liver) | SMA and desmin | Adrenalectomy | Alive | 6 |
| Wang YH. [20] | 2023 | 74 | F | Pain (abdominal) | L | 3.4 | None | Operative pathology | SMA, desmin and h-caldesmon | Adrenalectomy | Alive | ND |
| Oshidari B. [21] | 2022 | 32 | F | Pain (abdominal) | R | 10.6 | IVC | Operative pathology | SMA, desmin and vimentin | Adrenalectomy | Alive | 2 |
| Waack A. [22] | 2022 | 58 | F | Pain (abdominal) | L | 5.5 | None | Operative pathology | SMA, desmin, vimentin, and h-caldesmon | Adrenalectomy | Alive | 30 |
| Wang Y. [23] | 2020 | 29 | F | None | R | 3.4 | None | Operative pathology | SMA, desmin, vimentin, and h-caldesmon | Adrenalectomy | Alive | 12 |
| Jabarkhel F. [24] | 2020 | 58 | F | Pain (abdominal) | L | 6 | Metastasis (liver) | Operative pathology | SMA, desmin, vimentin, and h-caldesmon | Chemotherapy | Alive | 13 |
| 66 | M | None | R | 7.5 | IVC and metastasis (thoracic wall, femur and muscle) | Needle biopsy (adrenal gland) | SMA, desmin, h-caldesmon and CD163 | Adrenalectomy and chemotherapy | Alive | 23 | ||
| 72 | M | Pain (abdominal) | L | 10 | None | Operative pathology | SMA, desmin and h-caldesmon | Adrenalectomy and radiation | Alive | 48 | ||
| Sakellariou M. [25] | 2020 | 62 | M | None | L | 10.3 | Metastasis (bone, liver and pulmonary) | Operative pathology | SMA and desmin | Adrenalectomy, chemotherapy and radiation | Alive | 31 |
| Doppalapudi SK. [12] | 2019 | 70 | M | Abdominal varices | R | 9 | IVC and metastasis (lung) | Operative pathology | SMA, desmin, vimentin and h-caldesmon | Adrenalectomy + nephrectomy + thrombectomy | Dead | 14 |
| Nerli RB. [26] | 2019 | 27 | M | Pain (back) | L | 9 | None | Operative pathology | Desmin and h-caldesmon | Adrenalectomy | ND | ND |
| Mulani SR. [27] | 2018 | 50 | M | Pain (abdominal) | L | 8.1 | Metastasis (liver and lung) | Needle biopsy (adrenal gland) | Desmin, cytokeratin, MAK6, WT1 and S100 | Chemotherapy and radiation | ND | ND |
| Onishi T. [28] | 2016 | 34 | M | Pain (abdominal) | R | 5 | IVC | Operative pathology | SMA | Adrenalectomy | Alive | 10 |
| Zhou Y. [2] | 2015 | 49 | F | Pain (back) | L | 6 | None | Operative pathology | SMA, desmin and vimentin | Adrenalectomy | Alive | 6 |
| Quildrian S. [29] | 2015 | 44 | F | Pain (abdominal) | R | 12 | None | Operative pathology | SMA, desmin, vimentin, h-caldesmon and HHF | Adrenalectomy | Alive | 36 |
| Nagaraj V. [1] | 2015 | 61 | M | Pain (flank) | L | 17 | None | Operative pathology | Desmin and vimentin | Adrenalectomy | ND | ND |
| Ozturk H. [9] | 2014 | 70 | F | Pain (flank) | R | 8 | IVC | Operative pathology | SMA and desmin | Adrenalectomy and chemotherapy | Alive | 6 |
| Lee S. [30] | 2014 | 28 | M | Pain (flank) | R | 15 | None | Operative pathology | SMA and desmin | Adrenalectomy | Alive | 8 |
| Gulpinar MT. [31] | 2014 | 48 | M | Lower urinary tract symptom | R | 11 | None | Operative pathology | SMA and vimentin | Adrenalectomy | Alive | 8 |
| Bhalla A. [17] | 2014 | 45 | M | Pain (abdominal) | R | 11 | Metastasis (liver) | Needle biopsy (adrenal gland) | Desmin and actin | Chemotherapy | Alive | 9 |
| Wei J. [32] | 2014 | 57 | F | None | L | 8 | None | Operative pathology | SMA, desmin, vimentin and actin | Adrenalectomy | Alive | 29 |
| Alam MM. [33] | 2014 | 35 | F | Pain (flank) | L | 8.5 | None | Operative pathology | ND | Adrenalectomy | ND | ND |
| Deshmukh SD. [34] | 2013 | 60 | F | Pain (flank) | L | 5 | None | Operative pathology | SMA, desmin and vimentin | Adrenalectomy | Alive | 21 |
| Liu SV. [35] | 2012 | 79 | F | Pain (abdominal) | L | 6.3 | ND | Operative pathology | ND | Adrenalectomy | Alive | 12 |
| Shao IH. [8] | 2012 | 66 | M | Abdominal fullness and nausea | L | 10 | Renal vein | Operative pathology | SMA and desmin | Adrenalectomy | Alive | 18 |
| Kanthan R. [36] | 2012 | 28 | F | Pain (abdominal) | L | 16 | Kidney | Operative pathology | SMA and vimentin | Adrenalectomy + nephrectomy | ND | ND |
| Karaosmanoglu A. [16] | 2010 | 63 | M | Pain (abdominal) | R | ND | IVC | Needle biopsy (adrenal gland) | Desmin, vimentin, actin and keratin | Chemotherapy | Dead | 3 |
| Hamada S. [37] | 2009 | 62 | F | Pain (flank) | Bil | 8 | None | Operative pathology | SMA | Adrenalectomy, chemotherapy and radiation | Dead | 16 |
| Van Laarhoreu HW. [15] | 2009 | 78 | M | Pain (abdominal) | L | ND | Metastasis (lung, pancreas, bone and brain) | Needle biopsy (adrenal gland) | SMA, vimentin and actin | Radiation | Dead | 11 days |
| Goto J | 2008 | 73 | F | Pain (flank) | R | 8 | IVC and kidney | Operative pathology | SMA and NSE | Adrenalectomy + nephrectomy | Alive | 10 |
| Mencoboni M. [38] | 2008 | 75 | F | None | R | 8 | None | Operative pathology | SMA, desmin and actin | Adrenalectomy | Alive | 12 |
| Mohanty SK. [39] | 2007 | 47 | F | Pain (abdominal) | L | 10 | None | Operative pathology | Desmin, calpinin and actin | Adrenalectomy + nephrectomy and radiation | Alive | 9 |
| Wang TS. [7] | 2007 | 64 | F | None | R | 14 | IVC and right atrium | Operative pathology | SMA and desmin | Adrenalectomy + thrombectomy | Alive | 10 |
| Lee CW. [40] | 2006 | 49 | M | Pain (flank) | L | 3 | None | Operative pathology | Desmin | Adrenalectomy | Alive | 10 |
| Wong C. [6] | 2005 | 57 | M | Pain (groin) | L | ND | IVC and both iliac veins | Operative pathology | ND | Adrenalectomy + nephrectomy + thrombectomy | Dead | 6 |
| Candanedo-Gonzalez FA. [41] | 2005 | 59 | F | Pain (flank) and weight loss | L | 16 | Metastasis (liver) | Operative pathology | Desmin, vimentin and actin | Adrenalectomy, chemotherapy and radiation | Alive | 36 |
| Kato T. [5] | 2004 | 59 | M | ND | L | 10 | IVC | Operative pathology | SMA, desmin and vimentin | Adrenalectomy + nephrectomy + thrombectomy | Dead | 6 |
| Linos D. [42] | 2004 | 14 | F | None | Bil | 3.5 | None | Operative pathology | SMA, vimentin, actin and HHF | Adrenalectomy | ND | ND |
| Thamboo TP. [43] | 2003 | 68 | F | Pain (loin) and fever | R | 13 | None | Operative pathology | SMA, desmin, vimentin and actin | Adrenalectomy + nephrectomy + hepatic lobectomy + cholecystectomy and chemotherapy | Alive | 12 |
| Lujan MG. [44] | 2003 | 63 | M | None | R | 25 | Metastasis (lung, liver and kidney) | Needle biopsy (lung) | ND | Adrenalectomy + nephrectomy | Dead | shortly |
| Matsui Y. [4] | 2002 | 61 | F | Pain (flank) and fever | R | ND | IVC and right atrium | Operative pathology | SMA | Adrenalectomy + nephrectomy + thrombectomy | Dead | 1 |
| Etten B. [14] | 2001 | 73 | F | Pain (abdominal) | R | 27 | IVC | Operative pathology | SMA | Exploratory laparotomy | Dead | 3 weeks |
| Boman F. [13] | 1997 | 29 | M | Autopsy | L | 0.8 | None | Autopsy | SMA and HHF | None | ND | ND |
| Zetler PJ. [45] | 1995 | 30 | M | Pain (abdominal) | L | 11 | ND | Operative pathology | SMA | Adrenalectomy | Alive | 20 |
| Hayashi J. [46] | 1995 | 55 | F | Pain (abdominal) and fever | R | ND | IVC, hepatic vein and right atrium | Operative pathology | ND | Adrenalectomy + nephrectomy | Alive | 52 |
| Lack EE. [3] | 1991 | 49 | M | Pain (flank) | R | 11 | None | Operative pathology | SMA, vimentin and actin | Adrenalectomy + nephrectomy, chemotherapy and radiation | Alive | 9 |
| Choi SH. [47] | 1981 | 50 | F | Pain (flank) | L | 16 | Kidney | Operative pathology | ND | Adrenalectomy + nephrectomy | Alive | 12 |
F Female, M Male, R Right, L Left, Bil Bilateral, ND Not disclosed, IVC Inferior vena cava, SMA Smooth muscle actin
Fig. 3.
Analysis of the clinical features of the primary adrenal leiomyosarcoma, including previously reported cases and our two cases. A number of patients were analyzed in each clinical parameter. A Symptoms. B Metastatic lesions or local extensions. C Diagnosis procedures. D-F Patients treated with surgery (in D), chemotherapy (in E), and radiation (in F). G Patient outcomes
The clinical utility of adrenal biopsy including CT-guided core needle biopsy for other than adrenal lymphoma has been in dispute for a number of years. For instance, The European Society of Endocrinology Clinical Practice guidelines recommend against the use of an adrenal biopsy in the diagnostic work-up of patients suspected to harbor adrenocortical carcinoma unless there is sufficient evidence of metastatic disease that precludes surgery, and histopathologic proof is definitively required to determine the clinical management of the patients [48] because of its relatively high nondiagnostic rate (8.7%) and the overall rate of complications such as pneumothorax, pain, and adrenal hemorrhage (2.5%) [49, 50]. However, some patients with newly diagnosed single, large adrenal masses without other primary cancers have obtained enormous clinical benefits after undergoing adrenal biopsy [51]. Notably, approximately 30–50% of patients with adrenocortical carcinoma have no endocrinological abnormalities [51], and in those cases, the differential diagnosis of the lesions is mandatory to define their clinical management. For instance, mitotane therapy in conjunction with chemotherapy can be administered only for adrenocortical carcinoma patients and not for those with other adrenal lesions [52, 53], although its side effects are clinically not negligible [54]. In our present study, the two patients were deemed clinically inoperable, and appropriate diagnosis and subsequent therapeutic decisions could be achieved only by CT-guided core needle biopsy results. The tumor cells were composed of intersecting and sharply margined fascicles of atypical spindled immunohistochemically positive for SMA, desmin and vimentin, yielding the final diagnosis of primary adrenal leiomyosarcoma. Some cases of primary adrenal leiomyosarcoma were reported to be associated with high serum NSE levels [55–57]; however, serum NSE levels were measured in only one case in this study and were not high, and further investigations are required for clarification. In addition, of particular interest, HIV or EBV infection has been reported to be involved in the development of primary adrenal leiomyosarcoma because some primary adrenal leiomyosarcoma occurred in an immunosuppressive situation [13, 45, 58]. However, case 1 in our present study harboring bilaterally symmetric lymphadenopathy was negative for HIV and EBV; thus, the involvement of these infections requires further investigation.
In conclusion, adrenal leiomyosarcomas are malignant tumors derived from the smooth muscle cells in the wall of the central adrenal vein or its tributaries, and should be considered in cases of nonfunctioning adrenal tumors associated with direct extension from adrenals to the inferior vena cava. Primary adrenal leiomyosarcoma proliferates rapidly and is generally difficult to diagnose early; therefore, CT-guided core needle biopsy is considered a clinically useful approach for patient management.
Supplementary Information
Additional file 1: Supplemental Figure 1. The immunohistochemical analysis of samples from case 1. A-I Negative staining of SF-1 (A 100 x), inhibin-alpha (B 100 x), calretinin (C 100 x), synaptophysin (D 100 x), CK AE1/AE3 (E 100 x), S-100 (F 100 x), CD34 (G 100 x), latent membrane protein 1 (LMP1) (H 100 x), and p53 (I 100 x). Supplemental Figure 2. The immunohistochemical analysis of samples from case 2. A-H The negative staining of SF-1 (A 100 x), chromogranin A (B 100 x), synaptophysin (C 100 x), CK AE1/AE3 (D 100 x), S-100 (E 100 x), CD34 (F 100 x), and HMB-45 (G 100 x). p53 staining was positive in some tumour cells (H 100 x).
Acknowledgements
The authors would like to thank all members of the study team, the patients and their family.
Abbreviations
- SMA
Smooth muscle actin
- CRP
C-reactive protein levels
- NSE
Neuron-specific enolase
- MIBG
Metaodobenzylguanidine
- EBV
Epstein–Barr virus
- EBER
EBV-encoded RNA
- ISH
In situ hybridization
- LMP1
Mlatent membrane protein 1
Authors’ contributions
SS, NT, MS, KI, AI, KI, YR, KN, MF, HK, YI, SS, TI, YK, and TW treated the patient and contributed to the data collection. JII, YY and HS performed the pathological analysis. SS drafted the manuscript, and HS, IT and KY commented and revised the manuscript. All the authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the study.
Declarations
Ethics approval and consent to participate
Ethics approval was not necessary for the reported investigations, as they were performed in a routine clinical setting and therapeutic intention. Written informed consent was obtained from the patient before undergoing all clinical procedures.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nagaraj V, Mustafa M, Amin E, Ali W, NajiSarsam S, Darwish A. Primary adrenal leiomyosarcoma in an arab male: a rare case report with immunohistochemistry study. Case Rep Surg. 2015;2015:702541. doi: 10.1155/2015/702541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Tang Y, Tang J, Deng F, Gong G, Dai Y. Primary adrenal leiomyosarcoma: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(4):4258–4263. [PMC free article] [PubMed] [Google Scholar]
- 3.Lack EE, Graham CW, Azumi N, Bitterman P, Rusnock EJ, O’Brien W, et al. Primary leiomyosarcoma of adrenal gland. Case report with immunohistochemical and ultrastructural study. Am J Surg Pathol. 1991;15(9):899–905. doi: 10.1097/00000478-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Matsui Y, Fujikawa K, Oka H, Fukuzawa S, Takeuchi H. Adrenal leiomyosarcoma extending into the right atrium. Int J Urol. 2002;9(1):54–56. doi: 10.1046/j.1442-2042.2002.00413.x. [DOI] [PubMed] [Google Scholar]
- 5.Kato T, Kato T, Sakamoto S, Kobayashi T, Ikeda R, Nakamura T, et al. Primary adrenal leiomyosarcoma with inferior vena cava thrombosis. Int J Clin Oncol. 2004;9(3):189–192. doi: 10.1007/s10147-004-0383-7. [DOI] [PubMed] [Google Scholar]
- 6.Wong C, Von Oppell UO, Scott-Coombes D. Cold feet from adrenal leiomyosarcoma. J R Soc Med. 2005;98(9):418–420. doi: 10.1177/014107680509800910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TS, Ocal IT, Salem RR, Elefteriades J, Sosa JA. Leiomyosarcoma of the adrenal vein: a novel approach to surgical resection. World J Surg Oncol. 2007;5:109. doi: 10.1186/1477-7819-5-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao IH, Lee WC, Chen TD, Chiang YJ. Leiomyosarcoma of the adrenal vein. Chang Gung Med J. 2012;35(5):428–431. doi: 10.4103/2319-4170.105475. [DOI] [PubMed] [Google Scholar]
- 9.Oztürk H. Vena cava invasion by adrenal leiomyosarcoma. Rare Tumors. 2014;6(2):5275. doi: 10.4081/rt.2014.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonoda H, Minamimura K, Endo Y, Irie S, Hirata T, Kobayashi T, et al. Complete surgical resection of a leiomyosarcoma arising from the inferior vena cava. Case Rep Med. 2015;2015:342148. doi: 10.1155/2015/342148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Wang YX, Li B, Jiang YY, Miao CL, Liao DX, et al. Surgical management of leiomyosarcoma of the inferior vena cava. Vascular. 2015;23(3):329–332. doi: 10.1177/1708538114547755. [DOI] [PubMed] [Google Scholar]
- 12.Doppalapudi SK, Shah T, Fitzhugh VA, Bargman V. Primary adrenal leiomyosarcoma with inferior vena cava extension in a 70-year-old man. BMJ Case Rep. 2019;12(3):e227670. doi: 10.1136/bcr-2018-227670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boman F, Gultekin H, Dickman PS. Latent Epstein-Barr virus infection demonstrated in low-grade leiomyosarcomas of adults with acquired immunodeficiency syndrome, but not in adjacent Kaposi’s lesion or smooth muscle tumors in immunocompetent patients. Arch Pathol Lab Med. 1997;121(8):834–838. [PubMed] [Google Scholar]
- 14.Etten B, van Ijken MG, Mooi WJ, Oudkerk M, van Geel AN. Primary leiomyosarcoma of the adrenal gland. Sarcoma. 2001;5(2):95–99. doi: 10.1155/S1357714X01000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Laarhoven HW, Vinken M, Mus R, Flucke U, Oyen WJ, Van der Graaf WT. The diagnostic hurdle of an elderly male with bone pain: how 18F-FDG-PET led to diagnosis of a leiomyosarcoma of the adrenal gland. Anticancer Res. 2009;29(2):469–472. [PubMed] [Google Scholar]
- 16.Karaosmanoglu AD, Gee MS. Sonographic findings of an adrenal leiomyosarcoma. J Ultrasound Med. 2010;29(9):1369–1373. doi: 10.7863/jum.2010.29.9.1369. [DOI] [PubMed] [Google Scholar]
- 17.Bhalla A, Sandhu F, Sieber S. Primary adrenal leiomyosarcoma: a case report and review of the literature. Conn Med. 2014;78(7):403–407. [PubMed] [Google Scholar]
- 18.Chesson JP, Theodorescu D. Adrenal tumor with caval extension–case report and review of the literature. Scand J Urol Nephrol. 2002;36(1):71–73. doi: 10.1080/003655902317259409. [DOI] [PubMed] [Google Scholar]
- 19.Lin H, Yang W, Zhou X, Liu Q. A rare metastatic site of primary adrenal leiomyosarcoma. Am J Med Sci. 2023;365(5):e82–e83. doi: 10.1016/j.amjms.2022.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Wang YH, Hu X, Wu K, Li X. Primary adrenal leiomyosarcoma: a case report. Asian J Surg. 2023;46(2):965–966. doi: 10.1016/j.asjsur.2022.07.081. [DOI] [PubMed] [Google Scholar]
- 21.Oshidari B, Zamani A, Bahrami-Motlagh H, Jamali E, Mahmoodi S, Ebrahimian M. Primary leiomyosarcoma of the adrenal; a case report. Int J Surg Case Rep. 2022;90:106707. doi: 10.1016/j.ijscr.2021.106707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waack A, Jaggernauth S, Vattipally V. Primary adrenal leiomyosarcoma. Radiol Case Rep. 2023;18(3):741–744. doi: 10.1016/j.radcr.2022.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Teng Y, Na S, Yuan Y. Pleomorphic leiomyosarcoma of the adrenal gland in a young woman: a case report and review of the literature. Onco Targets Ther. 2020;13:4705–4713. doi: 10.2147/OTT.S254162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabarkhel F, Puttonen H, Hansson L, Muth A, Ragnarsson O. Primary adrenal leiomyosarcoma: clinical, radiological, and histopathological characteristics. J Endocr Soc. 2020;4(6):bvaa055. doi: 10.1210/jendso/bvaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakellariou M, Dellaportas D, Grapsa E, Tzikanoulas M, Dellis A, Theodosopoulos T, et al. Primary adrenal leiomyosarcoma: a case report. Mol Clin Oncol. 2020;12(4):317–320. doi: 10.3892/mco.2020.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nerli RB, Ghagane S, Dixit NS, Hiremath MB, Deole S. Adrenal leiomyosarcoma in a young adult male. Int Cancer Conf J. 2020;9(1):14–17. doi: 10.1007/s13691-019-00387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulani SR, Stoner P, Schlachterman A, Ghayee HK, Lu L, Gupte A. First reported case of endoscopic ultrasound-guided core biopsy yielding diagnosis of primary adrenal leiomyosarcoma. Case Rep Gastrointest Med. 2018;2018:8196051. doi: 10.1155/2018/8196051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onishi T, Yanagihara Y, Kikugawa T, Miura N, Noda T, Kakuda T, et al. Primary adrenal leiomyosarcoma with lymph node metastasis: a case report. World J Surg Oncol. 2016;14(1):176. doi: 10.1186/s12957-016-0936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quildrian S, Califano I, Carrizo F, Daffinoti A, Calónico N. Primary adrenal leiomyosarcoma treated by laparoscopic adrenalectomy. Endocrinol Nutr. 2015;62(9):472–473. doi: 10.1016/j.endonu.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Tanawit GD, Lopez RA, Zamuco JT, Cheng BG, Siozon MV. Primary leiomyosarcoma of adrenal gland with tissue eosinophilic infiltration. Korean J Pathol. 2014;48(6):423–425. doi: 10.4132/KoreanJPathol.2014.48.6.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulpinar MT, Yildirim A, Gucluer B, Atis RG, Canakci C, Gurbuz C, et al. Primary leiomyosarcoma of the adrenal gland: a case report with immunohistochemical study and literature review. Case Rep Urol. 2014;2014:489630. doi: 10.1155/2014/489630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei J, Sun A, Tao J, Wang C, Liu F. Primary adrenal leiomyosarcoma: case report and review of the literature. Int J Surg Pathol. 2014;22(8):722–726. doi: 10.1177/1066896914526777. [DOI] [PubMed] [Google Scholar]
- 33.Alam MM, Naser MF, Islam MF, Rahman MA. Primary adrenal leiomyosarcoma in an adult female. Mymensingh Med J. 2014;23(2):380–383. [PubMed] [Google Scholar]
- 34.Deshmukh SD, Babanagare SV, Anand M, Pande DP, Yavalkar P. Primary adrenal leiomyosarcoma: a case report with immunohistochemical study and review of literature. J Cancer Res Ther. 2013;9(1):114–116. doi: 10.4103/0973-1482.110394. [DOI] [PubMed] [Google Scholar]
- 35.Liu SV, Lenkiewicz E, Evers L, Holley T, Kiefer J, Ruiz C, et al. Genomic analysis and selected molecular pathways in rare cancers. Phys Biol. 2012;9(6):065004. doi: 10.1088/1478-3975/9/6/065004. [DOI] [PubMed] [Google Scholar]
- 36.Kanthan R, Senger JL, Kanthan S. Three uncommon adrenal incidentalomas: a 13-year surgical pathology review. World J Surg Oncol. 2012;10:64. doi: 10.1186/1477-7819-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamada S, Ito K, Tobe M, Otsuki H, Hama Y, Kato Y, et al. Bilateral adrenal leiomyosarcoma treated with multiple local therapies. Int J Clin Oncol. 2009;14(4):356–360. doi: 10.1007/s10147-008-0844-5. [DOI] [PubMed] [Google Scholar]
- 38.Mencoboni M, Bergaglio M, Truini M, Varaldo M. Primary adrenal leiomyosarcoma: a case report and literature review. Clin Med Oncol. 2008;2:353–6. doi: 10.4137/cmo.s627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohanty SK, Balani JP, Parwani AV. Pleomorphic leiomyosarcoma of the adrenal gland: case report and review of the literature. Urology. 2007;70(3):591.e5–7. doi: 10.1016/j.urology.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Lee CW, Tsang YM, Liu KL. Primary adrenal leiomyosarcoma. Abdom Imaging. 2006;31(1):123–124. doi: 10.1007/s00261-005-0343-3. [DOI] [PubMed] [Google Scholar]
- 41.Candanedo-González FA, Vela Chávez T, Cérbulo-Vázquez A. Pleomorphic leiomyosarcoma of the adrenal gland with osteoclast-like giant cells. Endocr Pathol. 2005;16(1):75–81. doi: 10.1385/EP:16:1:075. [DOI] [PubMed] [Google Scholar]
- 42.Linos D, Kiriakopoulos AC, Tsakayannis DE, Theodoridou M, Chrousos G. Laparoscopic excision of bilateral primary adrenal leiomyosarcomas in a 14-year-old girl with acquired immunodeficiency syndrome (AIDS) Surgery. 2004;136(5):1098–1100. doi: 10.1016/j.surg.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Thamboo TP, Liew LC, Raju GC. Adrenal leiomyosarcoma: a case report and literature review. Pathology. 2003;35(1):47–49. [PubMed] [Google Scholar]
- 44.Lujan MG, Hoang MP. Pleomorphic leiomyosarcoma of the adrenal gland. Arch Pathol Lab Med. 2003;127(1):e32–e35. doi: 10.5858/2003-127-e32-PLOTA. [DOI] [PubMed] [Google Scholar]
- 45.Zetler PJ, Filipenko JD, Bilbey JH, Schmidt N. Primary adrenal leiomyosarcoma in a man with acquired immunodeficiency syndrome (AIDS). Further evidence for an increase in smooth muscle tumors related to Epstein-Barr infection in AIDS. Arch Pathol Lab Med. 1995;119(12):1164–7. [PubMed] [Google Scholar]
- 46.Hayashi J, Ohzeki H, Tsuchida S, Fujita Y, Tatebe S, Namura O, et al. Surgery for cavoatrial extension of malignant tumors. Thorac Cardiovasc Surg. 1995;43(3):161–164. doi: 10.1055/s-2007-1013791. [DOI] [PubMed] [Google Scholar]
- 47.Choi SH, Liu K. Leiomyosarcoma of the adrenal gland and its angiographic features: a case report. J Surg Oncol. 1981;16(2):145–148. doi: 10.1002/jso.2930160205. [DOI] [PubMed] [Google Scholar]
- 48.Fassnacht M, Dekkers OM, Else T, Baudin E, Berruti A, de Krijger R, et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–g46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 49.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–g34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 50.Bancos I, Tamhane S, Shah M, Delivanis DA, Alahdab F, Arlt W, et al. Diagnosis of endocrine disease: the diagnostic performance of adrenal biopsy: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(2):R65–80. doi: 10.1530/EJE-16-0297. [DOI] [PubMed] [Google Scholar]
- 51.Williams AR, Hammer GD, Else T. Transcutaneous biopsy of adrenocortical carcinoma is rarely helpful in diagnosis, potentially harmful, but does not affect patient outcome. Eur J Endocrinol. 2014;170(6):829–835. doi: 10.1530/EJE-13-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Przytulska J, Rogala N, Bednarek-Tupikowska G. Current and emerging therapies for adrenocortical carcinoma–review. Adv Clin Exp Med. 2015;24(2):185–193. doi: 10.17219/acem/30645. [DOI] [PubMed] [Google Scholar]
- 53.Fassnacht M, Terzolo M, Allolio B, Baudin E, Haak H, Berruti A, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366(23):2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 54.Paragliola RM, Torino F, Papi G, Locantore P, Pontecorvi A, Corsello SM. Role of mitotane in adrenocortical carcinoma - review and state of the art. Eur Endocrinol. 2018;14(2):62–66. doi: 10.17925/EE.2018.14.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goto J, Otsuka F, Kodera R, Miyoshi T, Kinomura M, Otani H, et al. A rare tumor in the adrenal region: neuron-specific enolase (NSE)-producing leiomyosarcoma in an elderly hypertensive patient. Endocr J. 2008;55(1):175–181. doi: 10.1507/endocrj.K07E-020. [DOI] [PubMed] [Google Scholar]
- 56.Taniguchi A, Ujike T, Fujita K, Uemura M, Kiuchi H, Imamura R, et al. A case of adrenal leiomyosarcoma. Hinyokika Kiyo. 2017;63(11):465–469. doi: 10.14989/ActaUrolJap_63_11_465. [DOI] [PubMed] [Google Scholar]
- 57.Ueno H, Asami M, Yoneda R, Ogura M, Muraoka A, Oribe T, et al. A neuron-specific enolase (NSE) positive leiomyosarcoma. Gan No Rinsho. 1990;36(15):2616–2622. [PubMed] [Google Scholar]
- 58.Suankratay C, Shuangshoti S, Mutirangura A, Prasanthai V, Lerdlum S, Shuangshoti S, et al. Epstein-Barr virus infection-associated smooth-muscle tumors in patients with AIDS. Clin Infect Dis. 2005;40(10):1521–1528. doi: 10.1086/429830. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Figure 1. The immunohistochemical analysis of samples from case 1. A-I Negative staining of SF-1 (A 100 x), inhibin-alpha (B 100 x), calretinin (C 100 x), synaptophysin (D 100 x), CK AE1/AE3 (E 100 x), S-100 (F 100 x), CD34 (G 100 x), latent membrane protein 1 (LMP1) (H 100 x), and p53 (I 100 x). Supplemental Figure 2. The immunohistochemical analysis of samples from case 2. A-H The negative staining of SF-1 (A 100 x), chromogranin A (B 100 x), synaptophysin (C 100 x), CK AE1/AE3 (D 100 x), S-100 (E 100 x), CD34 (F 100 x), and HMB-45 (G 100 x). p53 staining was positive in some tumour cells (H 100 x).
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the study.