Abstract
Purpose of Review
While guidelines exist for the evaluation and management of esophageal dysphagia in the general population, dysphagia disproportionately affects the elderly. In this article, we reviewed the literature on evaluating esophageal dysphagia in elderly patients and proposed a diagnostic algorithm based on this evidence.
Recent Findings
In older patients, dysphagia is often well compensated for by altered eating habits and physiologic changes, underreported by patients, and missed by healthcare providers. Once identified, dysphagia should be differentiated into oropharyngeal and esophageal dysphagia to guide diagnostic workup. For esophageal dysphagia, this review proposes starting with endoscopy with biopsies, given its relative safety even in older patients and potential for interventional therapy. If endoscopy shows a structural or mechanical cause, then further cross-sectional imaging should be considered to assess for extrinsic compression, and same session endoscopic dilation should be considered for strictures. If biopsies and endoscopy are normal, then esophageal dysmotility is more likely, and high-resolution manometry and additional workup should be performed following the updated Chicago Classification. Even after diagnosis of the root cause, complications including malnutrition and aspiration pneumonia should also be assessed and monitored, as they both result from and can further contribute to dysphagia.
Summary
The successful evaluation of esophageal dysphagia in elderly patients requires a thorough, standardized approach to collecting a history, selection of appropriate diagnostic workup, and assessment of risk of potential complications, including malnutrition and aspiration.
Keywords: Geriatrics, Presbyphagia, Esophagogastroduodenoscopy, Barium esophagram, Balloon dilation, Achalasia
Introduction
Dysphagia, broadly characterized as difficulty or trouble swallowing, is a prevalent and common symptom encountered in clinical practice, reported in approximately 1 in 6 adults [1]. Dysphagia can be classified based on anatomic location, which is important to guide clinical workup and management. Oropharyngeal dysphagia refers to difficulty initiating a swallow by forming and moving a food bolus from the mouth to the pharynx and esophagus, whereas esophageal dysphagia refers to the sensation of food or liquid “getting caught” due to pathology within the esophagus [2]. While guidelines exist for the evaluation and management of esophageal dysphagia in the general population [3–5, 6••, 7••], dysphagia is a condition that disproportionately affects the elderly [1, 8–12]. Dysphagia in older patients tends to have different etiologies than in younger age patients [13]. During evaluation, the impact of co-morbidities, frailty [14], malnutrition [15], age [16], and risk of complications of dysphagia such as aspiration pneumonia [17] should be taken into account. In this review, we summarize the current literature on and propose an approach for the evaluation of esophageal dysphagia in the elderly.
Epidemiology and Economic Burden of Dysphagia in the Elderly
The prevalence of dysphagia is difficult to define because many studies use different definitions and screening tools. The word dysphagia is derived from Greek terminology for disordered eating and is broadly defined as difficulty swallowing [18]. Since there is no standardized definition or screening tool for dysphagia, epidemiologic studies utilize variable definitions including screening questions such as “having the feeling that food gets stuck in your throat or chest, or coughing or choking with swallowing?” [8] or the number of swallow referrals [19]. This variability in the literature results in wide ranges of reported prevalence of dysphagia. Furthermore, dysphagia is likely significantly underreported, with around 50% of patients not reporting their symptoms to their physicians [1, 8, 20].
Beyond these challenges, patients above 60 years old are a heterogeneous population. For example, acuity of care can range from healthy, community-dwelling patients, to those with skilled needs living at nursing homes, to those in the intensive care unit with multiple neurocognitive deficits. One of the more commonly studied elderly populations are nursing home residents, in which about 52.7–55% are found to have some degree of dysphagia [11, 21]. Although the prevalence of dysphagia in community dwelling elderly is much lower around 15% according to a systematic review by Madhavan et al., these patients could be a source of missed diagnoses given less frequent clinical surveillance [22]. In hospitalized patients, signs of dysphagia were found in 30.7%-43.1% of patients and found that nursing staff often missed diagnoses without use of the screening tool [23, 24]. In critically ill patients, studies have shown postextubation dysphagia to be anywhere from 3 to 93% [25].
Not only is dysphagia common in the elderly population, but its prevalence may also be increasing over time. One study by Leder et al. showed that referrals for swallowing evaluations in patients older than 60 years increased by 64% from 2007 to 2014, despite only a 23% increase in inpatient discharges in the same population [19]. There are multiple possible explanations for this increased prevalence including general aging of people in the world [26] and increased awareness of the implications of dysphagia in the medical field [19].
Dysphagia is also associated with increased costs to the healthcare system and use of hospital resources. A study in 2018 by Patel et al. found hospitalized patients with dysphagia compared to those without dysphagia to have 33% higher total charges and longer lengths of stay by approximately 3.8 additional days [10]. The combination of the high and increasing prevalence of dysphagia with its burden on the healthcare system highlights the importance of diagnosing and evaluating patients for dysphagia to guide management.
Clinical Presentation
Symptoms
Symptoms can be helpful in determining the cause of dysphagia and in creating a differential diagnosis. For esophageal dysphagia, a majority of cases can be categorized into structural disorders and esophageal dysmotility. The traditional teaching was that dysphagia to only solids suggested a structural etiology whereas dysphagia to both solids and liquids suggested motility disorders. History can also point to specific disorders, such as a history of atopic conditions suggesting a differential of eosinophilic esophagitis (EoE) [27, 28].
Not all presentations reliably correlate to a differential diagnosis though. For example, weight loss is commonly associated with esophageal cancer because up to 78.9% of patients are found to be malnourished [29]. However, weight loss is found in multiple other etiologies of dysphagia such as achalasia [30], which makes it difficult to separate weight loss due to difficulty swallowing and poor nutrition versus sarcopenia secondary to malignancy.
Clinical Assessment Tools
Given the variability in consistent definitions for dysphagia, clinical assessment tools were created to standardize diagnosis and symptom tracking over time (Table 1). To assess esophageal dysphagia in general, the Brief Esophageal Dysphagia Questionnaire (BEDQ), Mayo Dysphagia Questionnaire (MDQ), and Eating Assessment Tool (EAT-10) have been validated [31–35]. The problem is that dysphagia can be caused by a variety of etiologies. These various etiologies can present differently, which is why specific assessment tools have been created for specific disease etiologies and general assessment tools had to be validated for each condition. For achalasia, the Eckardt score can be used to track disease progression and improvement after intervention, but is still recommended to be used in conjunction with objective diagnostic studies such as manometry [3, 4, 30]. For EoE, the Eosinophilic Esophagitis Activity Index (EEsAI) [36] used to be one of the only patient-reported outcome (PRO) based assessment tool that was specifically made for EoE, but other general tools have been validated for EoE as well, including the BEDQ, MDQ, Straumann Dysphagia Index, and Dysphagia Symptom Questionnaire [37]. Because the EEsAI has been found to only have sensitivity of endoscopic and histologic remission in up to 67.7% depending on score cutoff [38], more diagnostic PRO tools have been in development in recent years, including the Index of Severity for Eosinophilic Esophagitis (I-SEE) [39]. Despite dysphagia affecting about 4 out of 5 patients with Parkinson’s disease, it is often underreported by these patients [20, 40]. For this reason, multiple questionnaires have been validated with the goal of earlier detection of dysphagia in patients with Parkinson’s disease, including the Swallowing Disturbance Questionnaire (SDQ), Munich Dysphagia Test – Parkinson’s Disease (MDT-PD), and DYPARK questionnaire [41–43].
Table 1.
| General | Achalasia | Eosinophilic Esophagitis | Parkinson's Disease |
|---|---|---|---|
|
| |||
| Brief Esophageal Dysphagia Question-naire (BEDQ) | Eckardt Score | Eosinophilic Esophagitis Activity Index (EEsAI) | Swallowing Disturbance Questionnaire (SDQ) |
| Mayo Dysphagia Questionnaire (MDQ) | Straumann Dysphagia Index | Munich Dysphagia Test - Parkinson’s Disease (MDT-PD) | |
| Eating Assessment Tool (EAT-10) | Dysphagia Symptom Questionnaire Index of Severity for Eosinophilic Esophagitis (I-SEE) Brief Esophageal Dysphagia Questionnaire (BEDQ) Mayo Dysphagia Questionnaire (MDQ) |
DYPARK questionnaire | |
Differential Diagnoses
Presbyphagia versus Dysphagia
As patients age, they naturally develop changes in their swallowing mechanics [44]. Presbyphagia describes these physiologic changes with age, but without impairment in swallowing [44, 45]. This must be distinguished from overt dysphagia, in which patients experience symptoms and consequences of dysfunctional swallowing [45]. Presbyphagia is thought to predispose the elderly to developing dysphagia in the future, and could potentially be a continuum of disease [45–47]. The issue is the clinical overlap between presbyphagia and dysphagia. At the beginning stages of the development of presbyphagia, patients may not have symptoms, but can develop symptoms as their physiology transitions to dysphagia [46].
While swallowing is a complex orchestration of multiple processes from neuronal pathways to muscular coordination, studies have suggested these mechanisms may change during the development of presbyphagia. For example, in the central nervous system, Labeit et al., through magnetoencephalography, identified increased sensorimotor cortical activation in patients with presbyphagia [47]. Videofluoroscopic swallowing studies combined with manometric data in patients with presbyphagia show increased oral transit time, post-swallow aspiration and duration of pharyngeal swallow delay, as well as decreased duration of pharyngeal swallow response, duration of cricopharyngeal opening, peristaltic amplitude, and peristaltic velocity [48, 49]. Modified barium swallow studies in healthy older individuals showed worse composite scores for oral and pharyngeal swallowing function, with the worst scores in function of: tongue control during bolus hold, hyolaryngeal movement, laryngeal closure, pharyngeal contraction, and pharyngoesophageal segment opening [44]. Studies on tongue mechanics have found that older patients have weaker maximal tongue strength as well as lower anterior and posterior tongue pressures while swallowing [50, 51]. Using M-mode ultrasound, Nienstedt et al. studied tongue movement and found less vertical lingual movement and shorter time to reach maximum amplitude in older women compared to younger women [52]. Despite the identification of multiple physiologic changes in presbyphagia, these patients often do not have symptoms, perhaps because they develop compensatory mechanisms.
Oropharyngeal versus Esophageal Dysphagia
Because oropharyngeal and esophageal dysphagia can present similarly, it can be difficult to differentiate them to guide appropriate workup and specialty referrals. Unfortunately, patient localization of symptoms does not reliably correlate to location of pathology. For example, in a review of 100 patients, localization of their symptoms to the proximal or mid-esophageal regions were rarely correlated to a proximal etiology [53]. However, in the same study, distal localization of dysphagia correlated to a distal esophageal etiology in 80% of cases. In a retrospective study on 3,668 patients with dysphagia, only 48% of them were able to correctly identify the location of pathology as pharyngeal, midsternal, or lower sternal with pharyngeal pathologies being the most accurately identified [54]. This inaccuracy was also tested by Smith et al. by having patients with lower esophageal mucosal rings swallow a marshmallow bolus and report the location of their symptoms. Of the 16 patients, 12 (75%) of them reported symptoms in their upper neck [55]. Only 9% of patients with esophageal dysmotility on manometry reported diffuse symptoms [53]. Thus, it is recommended to tailor the history to ask other questions to differentiate oropharyngeal and esophageal dysphagia (Table 2) [56–58]. Though this review focuses on esophageal dysphagia from a gastroenterological perspective, it is important to consider that not all etiologies of oropharyngeal dysphagia need to be definitively managed by otolaryngology. Some common causes including Zenker’s diverticulum and cricopharyngeal bar have newer treatment modalities that can be performed by a gastroenterologist, such as Zenker’s peroral endoscopic myotomy (z-POEM) for the former and endoscopic dilation or cricopharyngeal peroral endoscopy myotomy (c-POEM) for the latter [59–62].
Table 2.
| Oropharyngeal | Esophageal | |
|---|---|---|
|
| ||
| Sensation | Difficult initiating a swallow | Food getting stuck |
| Timing | Within 1 s of swallowing | A few seconds after swallowing |
| Associated symptoms | Nasal regurgitation | Chest pain (retrosternal) |
| Coughing | Odynophagia | |
| Drooling | Regurgitation (often after lying flat) | |
| Swallowing or breathing with gurgling | ||
| Past Medical History | Neurologic Conditions (Stroke, Parkinson's disease, ALS) | Connective Tissue Diseases (Systemic Sclerosis) |
| Thyrotoxicosis | Autoimmune Conditions (Rheumatoid arthritis, SLE, Sjogren's syndrome) | |
| Head/Neck Surgery or Radiation | Complications of Chronic GERD (reflux esophagitis, peptic strictures, Barretťs esophagus/esophageal cancer, upper GI bleed) | |
| Prolonged Intubation | ||
ALS Amyotrophic Lateral Sclerosis, SLE Systemic Lupus Erythematosus, GERD Gastroesophageal Reflux Disease, GI gastrointestinal
A Broad Differential Diagnosis of Esophageal Dysphagia
Esophageal dysphagia can be caused by a variety of conditions that can be categorized into structural/mechanical etiologies, esophageal dysmotility, or disordered brain-gut interaction. Structural/mechanical causes include malignancy, stricture, and inflammatory conditions such as esophagitis or EoE. Neuromuscular etiologies involve disruption of esophageal peristalsis, such as achalasia or ineffective esophageal motility (Table 3). Disordered brain-gut interactions include a wide-breadth of functional gastrointestinal disorders, one of which is functional dysphagia [63]. Rome IV criteria define functional dysphagia by four main criteria for the prior 3 months: symptoms of dysphagia at least once a week for more than 6 months, absence of esophageal mucosal or structural abnormality, absence of GERD or EoE, and absence of esophageal motility disorders [64]. When evaluating patients of any age with dysphagia, it is important to consider all causes. However, different age groups are disproportionately affected by different etiologies [13]. In particular, neurologic and oncologic etiologies more often affect those > 60 years old [13].
Table 3.
Etiologies of Dysphagia [56]
| Mechanism of Dysphagia | Specific Etiology | |
|---|---|---|
|
| ||
| Structural/Mechanical | Intrinsic | Esophageal Tumors |
| Anatomic Structures: Rings, Webs, Strictures | ||
| Inflammation (Esophagitis): Pill, Infectious (CMV, HSV, Candida), Eosinophilic, Caustic, Reflux, Foreign Body, Dissecans Superficialis | ||
| Ischemia: Acute Esophageal Necrosis | ||
| Surgeries: Laryngeal/Esophagogastric Resections, Fundoplication | ||
| Extrinsic | Mediastinal Masses | |
| Vascular Rings | ||
| Cardiomegaly | ||
| Esophageal Dysmotility | Primary | Primary Disorder of LES relaxation (Achalasia; EGJOO) |
| Hypomotility in the Esophageal Body (Absent Contractility; Ineffective Esophageal Motility) | ||
| Spastic Esophageal Conditions (Distal Esophageal Spasm; Hypercontractile Esophagus) | ||
| Secondary | Muscular involvement: Sarcopenia | |
| Connective Tissue Disease: Systemic Sclerosis (limited and diffuse) | ||
| Neuronal Involvement: Parkinson's Disease, Chagas disease, Diabetes, Medications/Drugs (eg Opioids) | ||
| Autoimmune Disease: Rheumatoid Arthritis, Systemic Lupus Erythematosus, Sjogren's Syndrome | ||
CMV cytomegalovirus, HSV herpes simplex virus, LES lower esophageal sphincter, EGJOO esophagogastric junction outflow obstruction
Mechanical/Structural Etiologies of Esophageal Dysphagia in the Elderly
Esophageal Stricture
Among patients undergoing upper endoscopy for dysphagia, the most common finding was esophageal stricture, found in 40.8% of patients [65]. Esophageal stricture becomes even more common with increased age [66]. Given its prevalence in elderly populations with dysphagia and the possibility of immediate endoscopic intervention on diagnosis, differential diagnoses should always consider stricture. However, esophageal stricture itself can be caused by multiple etiologies. Inflammatory causes include multiple forms of esophagitis including reflux, infectious etiologies, EoE as well as inflammatory conditions including pemphigoid and Crohn’s disease [67–69]. Iatrogenic causes include radiation, prior esophageal interventions and surgeries, and prolonged intubation [69]. Importantly, malignant etiologies need to be ruled out as goals of care, risks of palliative stenting, and multiple other options including resection, chemotherapy, and radiation may need to be discussed before endoscopic intervention [70].
Pill-Induced Esophagitis
The prevalence of polypharmacy in the elderly is reported as high as 96.5% in elderly hospitalized patients [71]. This makes elderly patients a high risk population for pill-induced esophagitis as risk factors include older age, decreased esophageal peristalsis, and larger pills [72]. Pill-induced esophagitis typically presents with chest pain, odynophagia, or dysphagia [73]. While some patients will experience sudden onset of self-limiting retrosternal pain after taking medications, some can present with gradually progressive dysphagia due to the pill lodging in the esophageal mucosa and slowly causing mucosal injury [74]. The latter subset of patients may not associate their symptoms to their medications, which necessitates a careful history and keeping pill esophagitis as a differential diagnosis, even with atypical presentations.
Esophageal Cancer
Dysphagia can be one of the presenting signs of esophageal cancer and should be considered particularly in the elderly population. In the US, esophageal adenocarcinoma (EAC) is one of the fastest growing epithelial malignancies with a sevenfold increase in incidence from 1973 to 2017 [75]. As of 2021, esophageal cancer has an incidence of 4.6 per 100,000, death rate of 3.8 per 100,000, and a 5-year survival 20.6% [76]. Among malignancies, esophageal cancer is also associated with the highest risk of malnutrition [29]. Since EAC has a known precursor, Barrett’s esophagus (BE), that can be treated, the early evaluation, appropriate screening, and endoscopy of patients is crucial [77]. ACG 2022 guidelines recommend screening for BE in those with chronic gastroesophageal reflux disease (GERD) and two or more risk factors (male sex, age above 50 years old, white race, tobacco use, obesity, and family history of EAC or BE in a first degree relative) [77]. Notably, among the elderly population, about half are male in sex, and a significant number of these patients likely have at least one of the other risk factors.
Acute Esophageal Necrosis
Acute esophageal necrosis (AEN) is an acute syndrome characterized by circumferential blackened mucosa of the distal esophagus that classically abruptly transitions to normal mucosa at the GE junction [78]. Some gastroenterologists propose a two-hit hypothesis for the development of AEN [79]. The first hit involves patients having chronic risk factors such as cardiovascular co-morbidities, cirrhosis, malnutrition, and malignancy that confer susceptibility to esophageal ischemia or excess gastric acid in the esophagus, and the second hit is an acute event that triggers AEN through even worse ischemia or gastric acid buildup [79, 80]. AEN typically presents as an upper gastrointestinal (GI) bleed, but atypical presentations can present with dysphagia alone [78, 81]. Given the high rate of perforation at 5% and mortality at 32–38% [80–82], AEN must be diagnosed quickly with endoscopy showing characteristic blackened mucosa.
Etiologies of Esophageal Dysmotility in the Elderly
Sarcopenic Dysphagia
About a third of elderly patients with dysphagia can be associated with sarcopenia [83•]. Though there is no consensus diagnostic criteria for sarcopenia, it is broadly defined as loss of strength and muscle mass [84]. Depending on the criteria used, prevalence of sarcopenia ranges between 10–27% in patients over 60 years old [84]. In a multivariable analysis including sarcopenia and sarcopenia-related conditions, by Maeda et al., sarcopenia independently predicted development of swallowing disorders [85]. “Sarcopenic dysphagia” refers to the loss of coordinated and sufficient deglutition secondary to sarcopenia of the swallowing muscles [86]. Due to the wide prevalence and association between sarcopenia and dysphagia [46], it is important to differentiate patients with sarcopenic dysphagia from those with sarcopenia with dysphagia caused by other etiologies. Mori et al. proposed a potential diagnostic algorithm to make this distinction based on 5 factors including: presence of dysphagia, whole body sarcopenia, supporting imaging, exclusion of other causes of dysphagia, and sarcopenia being the primary cause [87].
Achalasia
A Medicare data analysis found the prevalence of achalasia in those older than 65 to be 162.1 in 100,000 and increasing with advanced age [16]. The same study estimated the national economic burden of achalasia to be 408 million dollars, with patients older than 65 accounting for about 151 million dollars [16]. Among patients with achalasia, it is recommended to identify subtypes of achalasia following the diagnostic algorithm proposed by the Chicago Classification in order to assess prognosis after treatment and direct therapeutic management [6••]. For example, type II achalasia shows the best response to botulinum toxin injection, pneumatic dilation (PD), and laparoscopic Heller myotomy (LHM) compared to other subtypes [88–90]. Whereas PD, peroral endoscopic myotomy (POEM), and LHM are comparable definitive therapies for type I and type II, POEM is first line for type III [4]. Type III also requires a longer myotomy with POEM than other subtypes [91].
Parkinson’s-related Esophageal Dysphagia
Especially in secondary etiologies of dysphagia, it is critical to identify the root cause in order to direct treatment. For Parkinson’s related esophageal dysphagia, consensus guidelines support the efficacy of optimizing the treatment of Parkinson’s disease itself as a first priority in preventing and improving dysphagia. For this reason, identifying Parkinson’s as the etiology becomes critical in management. For patients with Parkinson’s, validated questionnaires have been developed to diagnose dysphagia, such as the SDQ and MDT-PD [41, 42, 92]. On the other hand, for patients with dysphagia, it is often difficult to identify Parkinson’s disease in the early, premotor stage of disease given the lack of reliable diagnostic tools [93], even though esophageal manometry is abnormal in 40–60% of these patients [94].
Opioids
In the United States in 2019, about one in five adults had chronic pain and 12.3% of adults have had used opioids for pain in the prior 12 months [95]. Given this susceptible population especially among the elderly, identifying opioid-induced esophageal dysmotility (OIED) can alter the management of both pain and dysphagia. Both acute and chronic opioid use has been associated with esophageal dysmotility, including impaired lower esophageal sphincter (LES) relaxation and increased nonperistaltic contractions [96•, 97]. Among patients with OIED, diffuse esophageal spasm, esophagogastric junction outflow obstruction (EGJOO), jackhammer esophagus, and achalasia type III were the most common of the Chicago Classification diagnoses [98]. Given that increased doses of opioids are associated with higher likelihood of developing OIED, decreasing dosing could improve OIED [98].
Systemic Sclerosis
Around 50% of patients with systemic sclerosis experience dysphagia [99] and up to 90% have manometric abnormalities, typically slower or lower pressure peristalsis [100, 101]. When comparing limited and diffuse systemic sclerosis, the diffuse subtype was found have increased risk of esophageal hypomotility compared to the limited subtype (85.5% vs. 64%, p < 0.01) [102]. Although systemic sclerosis can directly affect esophageal motility, most commonly resulting in ineffective esophageal motility and absent contractility [103], it is important to keep a broad differential and workup for dysphagia in the population. For example, patients with systemic sclerosis are treated with immunosuppressive medications that could place them at risk of infectious etiologies, such as cytomegalovirus or candidal esophagitis. Additionally, systemic sclerosis can also cause decreased LES pressure and chronic GERD [99], predisposing patients to reflux esophagitis, strictures, and esophageal cancer.
Diagnostic Testing
EGD
Currently, esophagogastroduodenoscopy (EGD) with biopsy while off proton pump inhibitor therapy for two weeks is first line in the evaluation of dysphagia as a diagnostic and potentially therapeutic intervention [3, 104]. Visualization of the esophagus plays a key role in ruling out structural etiologies of dysphagia, and biopsies allow the diagnosis of a wide variety of conditions including EAC and EoE (Fig. 1) [3]. Further, strictures should be assessed for potential dilation during the same session endoscopy. EGD has been shown to be more cost effective than barium swallow as an initial diagnostic test, especially considering most initial encounters for dysphagia are in primary care [105].
Fig. 1.
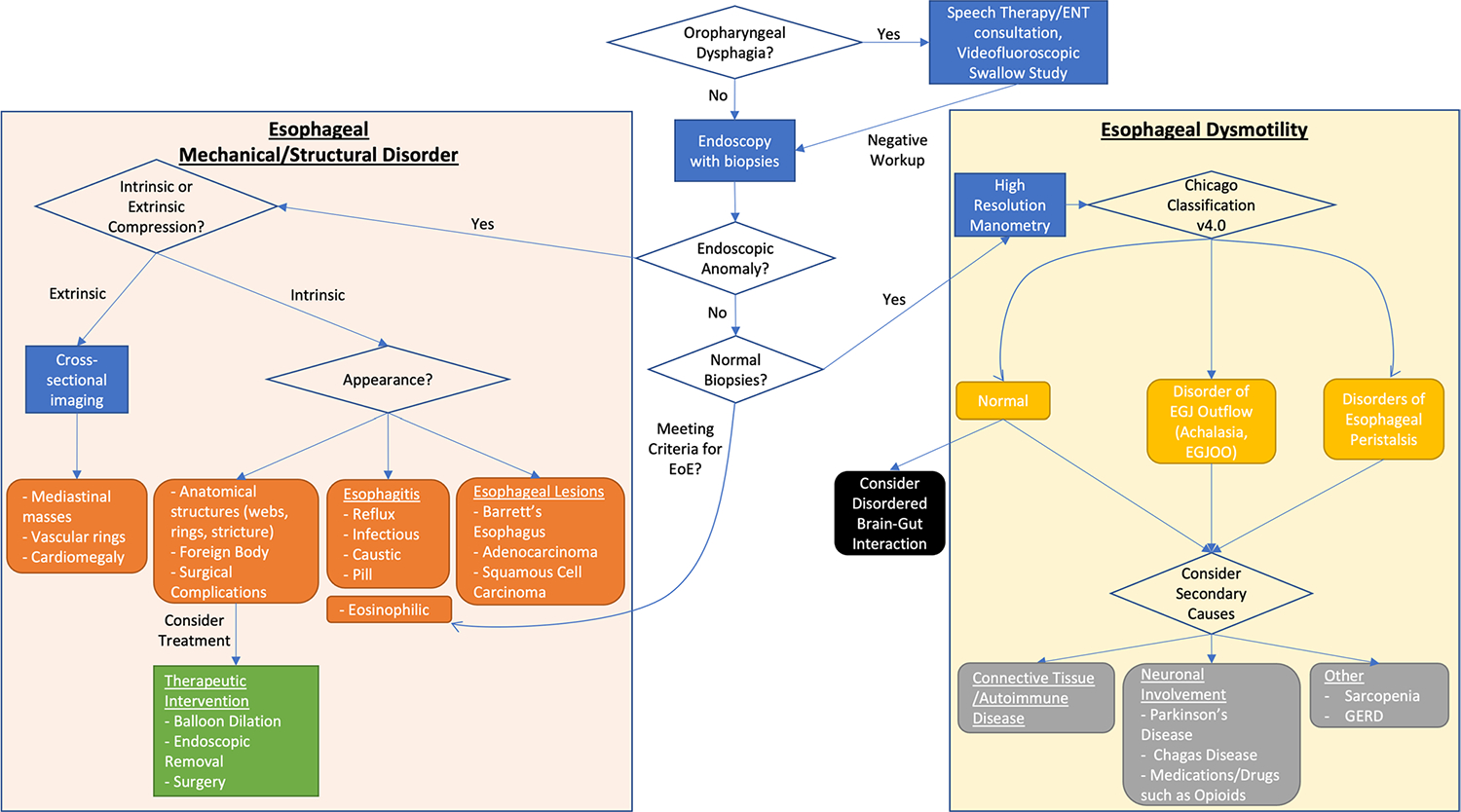
A Diagnostic Algorithm for Evaluating Esophageal Dysphagia in the Elderly
In elderly populations, endoscopy tends to have higher diagnostic yield. EGD can have relevant findings including ulcers or malignancies detected in 10–20% of elderly patients [106, 107] and change management in up to 50% of elderly patients [108]. EGD is also relatively safe in the older populations. While increased age is associated with more frequent hypotension and hypoxia, these changes are usually associated with sedation and tend to be transient [106, 109, 110]. In a large retrospective study, Jang et al. found no difference in 30-day complications between young and older patients [111•]. Given its diagnostic and therapeutic value in addition to its relative safety, EGD is a valuable tool for evaluating dysphagia in elderly patients.
Esophageal Physiologic Testing
After being confirmed to have no obstructive etiology on EGD with normal biopsies, ACG guidelines recommend esophageal high-resolution manometry (HRM) as the gold standard for diagnosing esophageal dysmotility [3]. The Chicago classification version 4.0 provides a standardized procedure for HRM with positioning and provocative maneuvers as well as consideration for follow-up testing for patients with equivocal results for EGJOO [6•]. These patients are recommended to undergo further diagnostic testing with a timed barium esophagram (TBE) or functional lumen imaging probe (FLIP) (Fig. 1).
Barium esophagram can be performed in a variety of ways. Although timed and untimed barium esophagram have not been directly compared, guidelines recommend a standardized, timed procedure [3]. Diagnostic metrics significantly improve when adding a barium tablet swallow to the study [112], but even with a tablet, sensitivity for esophageal dysmotility in general is about 69% with specificity at 58% [113]. FLIP is recommended in the Chicago classification under the same indication as TBE [6••] and allows the visualization of multiple properties not seen on HRM, including response to esophageal distension and nonocclusive esophageal contractions [114].
Other Imaging Modalities
Considering the low correlation between patient localization of symptoms and true location of dysmotility [53], it is important to consider and rule out oropharyngeal dysphagia. For this reason, in most patients with dysphagia, a modified barium swallow, which is a videofluoroscopic study performed with a speech therapist, is recommended as “usually appropriate” for initial workup of dysphagia not related to recent operation by American College of Radiology (ACR) guidelines [5]. However, this study only reaches down to the cervical esophagus and does not assess for etiologies of esophageal dysphagia.
Many patients with dysphagia have already had a computed tomography (CT) of chest and/or neck with or without intravenous (IV) contrast for various co-morbidities or related symptoms, such as chest pain. While CT may be able to identify advanced disease and especially metastases to the lung, it is not sensitive for identifying primary esophageal tumors or locoregional disease [115]. As such, in ACR guidelines it is designated as “usually not appropriate” for initial workup of dysphagia that is not associated with recent surgery [5]. Interestingly, a recent study by Sui et al. applied deep learning to non-contrast CT scans in order to significantly improve sensitivity and specificity for identifying esophageal cancer [116]. In the future, enhancements in artificial intelligence could potentially change diagnostic management.
Complications of Dysphagia
Malnutrition and Dehydration
Dysphagia is independently associated with mortality in nursing home residents, but dysphagia with weight loss is associated with an even higher risk of mortality [15]. Malnutrition and sarcopenia can cause dysphagia [47, 85, 87], but dysphagia can also cause malnutrition. Specifically, modified diets can result in decreased caloric intake and calories [117]. In a study by Wright et al., texture modified diet decreased caloric intake by almost 40% (3877 versus 6115 kJ, p < 0.0001) as well as finding a significantly greater caloric deficit in the modified diet group [117]. Dysphagia is also associated with dehydration because of restrictions on thin liquids to prevent aspiration [118]. Some have considered free water protocols to prevent dehydration and improve quality of life [119]. The most data exists to support the Frazier Free Water Protocol, which has low quality evidence supporting that no association with a higher risk of aspiration pneumonia if patients are carefully selected [120]. Due to the lack of quality evidence, more research and standardized protocols are needed before widespread implementation of a free water protocol [121•]. Both malnutrition and dehydration can be caused by dysphagia, but also potentially worsen dysphagia. This cyclical effect supports the importance of the evaluation and optimization of patients’ nutritional and hydration status when managing dysphagia.
Aspiration Pneumonia
In 2020, pneumonia was the 9th leading cause of death in the general population [122], but for nursing home residents specifically, pneumonia was the leading cause of death [17]. Aspiration is defined as the misdirection of oropharyngeal or gastric contents into the larynx and lower respiratory tract [123]. Aspiration pneumonia occurs when those contents contain pathogens, which usually colonize the oropharynx or stomach and cause pneumonia [17]. Pneumonia, aspiration, and dysphagia are all closely tied. In nursing homes about 50–75% of residents have dysphagia, half of those aspirate, and one third of those who aspirate develop pneumonia [124]. A study by Feng et al. found dysphagia patients were 4.69 times more likely to develop aspiration pneumonia [125]. In stroke patients who aspirate, pneumonia developed 7 times more often than in stroke patients without aspiration [17].
Multiple interventions have been suggested to prevent aspiration pneumonia. For example, Hinchey et al. recommends the use of a formal dysphagia screening protocol, which was associated with statistically significant increased adherence to screening at 78% compared to 57% without the protocol and decreased risk of pneumonia to 2.4% compared to 5.4% [126]. A systematic review by Khadka et al. found multiple studies showing that weekly professional oral care also reduced the risk of aspiration pneumonia [127•].
A review by Ebihara et al. hypothesized that the presence of chronic lung inflammation in elderly individuals could be due to sterile chronic microaspiration and that this microaspiration causes a “vicious cycle” that leads to more inflammation, sarcopenia, dysphagia, and frailty, which all causes further repeated microaspiration [128]. This concept only further strengthens the importance of properly screening and evaluating the elderly for aspiration and dysphagia as well as managing risk factors for their development.
Complications of Endoscopy in the Elderly—Should this Change our Approach to Diagnostic Evaluation?
As previously described, esophagogastroduodenoscopy (EGD) is a valuable diagnostic and therapeutic tool for esophageal dysphagia, but EGD could potentially have more risk for elderly patients who are more frail and have more co-morbidities. In the general population, risk of cardiorespiratory complications are as low as 0.54% and rate of mortality as low as 0.03% [129]. Some studies support the safety of EGD in elderly patients [106, 130, 111•] while others suggest higher risk in very elderly patients [110, 131]. For example, a retrospective study on 62,804 patients by Jang et al. compared EGD in elderly patients ≥ 65 years old and younger patients 18–64 years old and found no difference in GI and non-GI complications after EGD, regardless of the type of sedation used [111•]. In a study comparing nonagenarians (aged 90–94) to octogenarians (80–89), there were no differences in immediate complications or 30-day mortality after EGD [132]. On the contrary, in a study by Ryoichi et al., very elderly patient populations older than 85 years old, as compared to younger patients with a mean age of 40.5 were found to have more adverse events (6.3% vs 1.1% p < 0.01), independent of comorbidities [110]. A nationwide population-based study by Kim et al. on 1,943,150 patients found age from 70–99 years to be an independent risk factor for increased cardiocerebrovascular disease-related adverse effects after EGD [131]. Of the adverse events after EGD in the elderly, many complications are related to sedation and not the procedure itself [106, 110]. Although elderly patients have different co-morbidities, medication profiles, and frailty that potentially increase the risk of EGD, the literature generally seems to support the safety of EGD in elderly population, though with potential increased risk above 85 years.
Conclusion
Esophageal dysphagia is a common clinical symptom that disproportionately impacts elderly patients in prevalence, quality of life, and complications. While some etiologies of esophageal dysphagia are common in the elderly, the methods of diagnostic evaluation should not significantly differ from younger patients. Given the high prevalence of oropharyngeal dysphagia in the elderly, the significant rate of under-reporting symptoms in this population, and the difficulty of differentiating oropharyngeal and esophageal etiologies, older patients with any suspicion for oropharyngeal dysphagia should be screened by a speech language pathologist before evaluation of esophageal dysphagia. Given the relative safety and high diagnostic yield of EGD, it should not be avoided in the elderly. However, due to complications of dysphagia in older patients, it is even more important to evaluate and diagnose malnutrition and aspiration pneumonia.
Given the high prevalence of oropharyngeal dysphagia in the elderly population and the difficulty in differentiating oropharyngeal and esophageal pathology [53, 55], if patients do not have clear risk factors or symptoms suggestive of esophageal dysphagia, they should undergo oropharyngeal evaluation first, which is also supported by ACR guidelines. Once determined to be esophageal in origin, endoscopy with biopsies should be performed given it has been found to be more cost effective than barium swallow as an initial diagnostic study [105] and its relative safety even in older individuals [106, 130, 111•]. If endoscopy shows obstruction, then further cross-sectional imaging can be considered to assess for extrinsic compression, and same session endoscopic dilation should be considered for strictures [104]. If biopsies and endoscopy are normal, then esophageal dysmotility is more likely, and high-resolution manometry and following workup should be performed according to the Chicago Classification [6••].
Funding
This work was supported by NIH K23 DK125266 (PI: Yadlapati).
Abbreviations
- EoE
Eosinophilic esophagitis
- BEDQ
Brief Esophageal Dysphagia Questionnaire
- MDQ
Mayo Dysphagia Questionnaire
- EAT-10
Eating Assessment Tool
- EesAI
Eosinophilic Esophagitis Activity Index
- PRO
Patient-reported outcome
- I-SEE
Index of Severity for Eosinophilic Esophagitis
- SDQ
Swallowing disturbance questionnaire
- MDT-PD
Munich Dysphagia Test—Parkinson’s Disease
- z-POEM
Zenker’s peroral endoscopic myotomy
- c-POEM
Cricopharyngeal peroral endoscopic myotomy
- EAC
Esophageal adenocarcinoma
- BE
Barrett’s esophagus
- GERD
Gastroesophageal reflux disease
- AEN
Acute esophageal necrosis
- GI
Gastrointestinal
- PD
Pneumatic dilation
- LHM
Laparoscopic Heller myotomy
- POEM
Peroral endoscopic myotomy
- OIED
Opioid-induced esophageal dysmotility
- LES
Lower esophageal sphincter
- EGJOO
Esophagogastric junction outflow obstruction
- EGD
Esophagogastroduodenoscopy
- HRM
High-resolution manometry
- TBE
Timed barium esophagram
- FLIP
Functional lumen imaging probe
- ACR
American College of Radiology
- CT
Computed tomography
- IV
Intravenous
- EGD
Esophagogastroduodenoscopy
Footnotes
Conflict of Interest
KHNL: None.
RY: Consultant for Medtronic, Phathom Pharmaceuticals, StatLink-MD, Reckitt Benckiser Healthcare Ltd, Medscape; Research Support: Ironwood Pharmaceuticals; Advisory Board with Stock Options: RJS Mediagnostix.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Adkins C, Takakura W, Spiegel BMR, et al. Prevalence and Characteristics of Dysphagia Based on a Population-Based Survey. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2020;18(9):1970–1979.e2. 10.1016/j.cgh.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiyagalingam S, Kulinski AE, Thorsteinsdottir B, Shindelar KL, Takahashi PY. Dysphagia in Older Adults. Mayo Clin Proc. 2021;96(2):488–97. 10.1016/j.mayocp.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Gyawali CP, Carlson DA, Chen JW, Patel A, Wong RJ, Yadlapati RH. ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing. Off J Am Coll Gastroenterol ACG. 2020;115(9):1412. 10.14309/ajg.0000000000000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RT. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Off J Am Coll Gastroenterol ACG. 2020;115(9):1393–411. 10.14309/ajg.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Expert Panel on Gastrointestinal Imaging:, Levy AD, Carucci LR, et al. ACR Appropriateness Criteria® Dysphagia. J Am Coll Radiol JACR. 2019;16(5S):S104–S115. 10.1016/j.jacr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 6. Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal Motility Disorders on High Resolution Manometry: Chicago Classification Version 4.0©. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2021;33(1):e14058. 10.1111/nmo.14058. •• Although the practice-changing implications of the updated Chicago Classification were not directly discussed in this article, the entire esophageal motility side of our proposed diagnostic algorithm relies on the recommendations from this guideline.
- 7. Patel DA, Yadlapati R, Vaezi MF. Esophageal Motility Disorders: Current Approach to Diagnostics and Therapeutics. Gastroenterology. 2022;162(6):1617–34. 10.1053/j.gastro.2021.12.289. •• This review by Patel et al. served as a strong framework in assessing the utility of each diagnostic test that was discussed in this manuscript and helped guide the development of our diagnostic algorithm.
- 8.Wilkins T, Gillies RA, Thomas AM, Wagner PJ. The prevalence of dysphagia in primary care patients: a HamesNet Research Network study. J Am Board Fam Med JABFM. 2007;20(2):144–50. 10.3122/jabfm.2007.02.060045. [DOI] [PubMed] [Google Scholar]
- 9.Wolf U, Eckert S, Walter G, et al. Prevalence of oropharyngeal dysphagia in geriatric patients and real-life associations with diseases and drugs. Sci Rep. 2021;11(1):21955. 10.1038/s41598-021-99858-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel DA, Krishnaswami S, Steger E, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus Off J Int Soc Dis Esophagus. 2018;31(1):1–7. 10.1093/dote/dox131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YH, Han HR, Oh BM, et al. Prevalence and associated factors of dysphagia in nursing home residents. Geriatr Nurs N Y N. 2013;34(3):212–7. 10.1016/j.gerinurse.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Leder SB, Suiter DM. An epidemiologic study on aging and dysphagia in the acute care hospitalized population: 2000–2007. Gerontology. 2009;55(6):714–8. 10.1159/000235824. [DOI] [PubMed] [Google Scholar]
- 13.Roden DF, Altman KW. Causes of Dysphagia Among Different Age Groups: A Systematic Review of the Literature. Otolaryngol Clin North Am. 2013;46(6):965–87. 10.1016/j.otc.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Sakai K, Nakayama E, Yoneoka D, et al. Association of Oral Function and Dysphagia with Frailty and Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Cells. 2022;11(14):2199. 10.3390/cells11142199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wirth R, Pourhassan M, Streicher M, et al. The Impact of Dysphagia on Mortality of Nursing Home Residents: Results From the nutritionDay Project. J Am Med Dir Assoc. 2018;19(9):775–8. 10.1016/j.jamda.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Gaber CE, Eluri S, Cotton CC, et al. Epidemiologic and Economic Burden of Achalasia in the United States. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2022;20(2):342–352.e5. 10.1016/j.cgh.2021.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marik PE, Kaplan D. Aspiration Pneumonia and Dysphagia in the Elderly. Chest. 2003;124(1):328–36. 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A, Stacey B. Dysphagia: Aspects of assessment and management for the Acute Physician. Acute Med. 2008;7(3):107–12. [PubMed] [Google Scholar]
- 19.Leder SB, Suiter DM, Agogo GO, Cooney LM. An Epidemiologic Study on Ageing and Dysphagia in the Acute Care Geriatric-Hospitalized Population: A Replication and Continuation Study. Dysphagia. 2016;31(5):619–25. 10.1007/s00455-016-9714-x. [DOI] [PubMed] [Google Scholar]
- 20.Miller N, Allcock L, Hildreth AJ, Jones D, Noble E, Burn DJ. Swallowing problems in Parkinson disease: frequency and clinical correlates. J Neurol Neurosurg Psychiatry. 2009;80(9):1047–9. 10.1136/jnnp.2008.157701. [DOI] [PubMed] [Google Scholar]
- 21.Kayser-Jones J, Pengilly K. Dysphagia among nursing home residents. Geriatr Nurs N Y N. 1999;20(2):77–82; quiz 84. 10.1053/gn.1999.v20.97011. [DOI] [PubMed] [Google Scholar]
- 22.Madhavan A, Lagorio LA, Crary MA, Dahl WJ, Carnaby GD. Prevalence of and risk factors for dysphagia in the community dwelling elderly: A systematic review. J Nutr Health Aging. 2016;20(8):806–15. 10.1007/s12603-016-0712-3. [DOI] [PubMed] [Google Scholar]
- 23.Spronk PE, Spronk LEJ, Lut J, et al. Prevalence and characterization of dysphagia in hospitalized patients. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2020;32(3):e13763. 10.1111/nmo.13763. [DOI] [PubMed] [Google Scholar]
- 24.Olesen MD, Modlinski RM, Poulsen SH, Rosenvinge PM, Rasmussen HH, Holst M. Prevalence of signs of dysphagia and associated risk factors in geriatric patients admitted to an acute medical unit. Clin Nutr ESPEN. 2021;41:208–16. 10.1016/j.clnesp.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Brodsky MB, Nollet JL, Spronk PE, González-Fernández M. Prevalence, Pathophysiology, Diagnostic Modalities, and Treatment Options for Dysphagia in Critically Ill Patients. Am J Phys Med Rehabil. 2020;99(12):1164–70. 10.1097/PHM.0000000000001440. [DOI] [PubMed] [Google Scholar]
- 26.Ageing and health. n.d. https://www.who.int/news-room/fact-sheets/detail/ageing-and-health. Accessed February 20, 2023.
- 27.Lehman HK, Lam W. Eosinophilic Esophagitis. Pediatr Clin North Am. 2019;66(5):955–65. 10.1016/j.pcl.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Durrani SR, Mukkada VA, Guilbert TW. Eosinophilic Esophagitis: An Important Co-Morbid Condition of Asthma? Clin Rev Allergy Immunol. 2018;55(1):56–64. 10.1007/s12016-018-8670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larrea J, Vega S, Martínez T, Torrent JM, Vega V, Núñez V. The nutritional status and immunological situation of cancer patients. Nutr Hosp. 1992;7(3):178–84. [PubMed] [Google Scholar]
- 30.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103(6):1732–8. 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 31.Belafsky PC, Mouadeb DA, Rees CJ, et al. Validity and reliability of the Eating Assessment Tool (EAT-10). Ann Otol Rhinol Laryngol. 2008;117(12):919–24. 10.1177/000348940811701210. [DOI] [PubMed] [Google Scholar]
- 32.Taft TH, Riehl M, Sodikoff JB, et al. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2016;28(12):1854–60. 10.1111/nmo.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cisternas D, Taft T, Carlson DA, et al. The Brief Esophageal Dysphagia Questionnaire shows better discriminative capacity for clinical and manometric findings than the Eckardt score: Results from a multicenter study. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2022;34(4):e14228. 10.1111/nmo.14228. [DOI] [PubMed] [Google Scholar]
- 34.Umay E, Sakin YS, Ates MP, et al. Esophageal dysphagia in neuromuscular disorder patients with validity and reliability study of the brief esophageal dysphagia questionnaire. Acta Neurol Belg. 2022;122(2):315–24. 10.1007/s13760-020-01563-4. [DOI] [PubMed] [Google Scholar]
- 35.McElhiney J, Lohse MR, Arora AS, et al. The Mayo Dysphagia Questionnaire-30: documentation of reliability and validity of a tool for interventional trials in adults with esophageal disease. Dysphagia. 2010;25(3):221–30. 10.1007/s00455-009-9246-8. [DOI] [PubMed] [Google Scholar]
- 36.Schoepfer AM, Straumann A, Panczak R, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. 2014;147(6):1255–1266.e21. 10.1053/j.gastro.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schoepfer A, Safroneeva E, Straumann A. How to measure disease activity in eosinophilic esophagitis. Dis Esophagus. 2016;29(8):959–66. 10.1111/dote.12391. [DOI] [PubMed] [Google Scholar]
- 38.Safroneeva E, Straumann A, Coslovsky M, et al. Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis. Gastroenterology. 2016;150(3):581–590.e4. 10.1053/j.gastro.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellon ES, Khoury P, Muir AB, et al. A Clinical Severity Index for Eosinophilic Esophagitis: Development, Consensus, and Future Directions. Gastroenterology. 2022;163(1):59–76. 10.1053/j.gastro.2022.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalf JG, de Swart BJM, Bloem BR, Munneke M. Prevalence of oropharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Parkinsonism Relat Disord. 2012;18(4):311–5. 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2007;22(13):1917–21. 10.1002/mds.21625. [DOI] [PubMed] [Google Scholar]
- 42.Simons JA, Fietzek UM, Waldmann A, Warnecke T, Schuster T, Ceballos-Baumann AO. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(9):992–8. 10.1016/j.parkreldis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Dagna C, Avenali M, De Icco R, et al. From DYMUS to DYPARK: Validation of a Screening Questionnaire for Dysphagia in Parkinson’s Disease. Dysphagia. 2022;37(4):824–30. 10.1007/s00455-021-10332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garand KL, Beall J, Hill EG, et al. Effects of Presbyphagia on Oropharyngeal Swallowing Observed during Modified Barium Swallow Studies. J Nutr Health Aging. 2022;26(11):973–80. 10.1007/s12603-022-1854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azzolino D, Damanti S, Bertagnoli L, Lucchi T, Cesari M. Sarcopenia and swallowing disorders in older people. Aging Clin Exp Res. 2019;31(6):799–805. 10.1007/s40520-019-01128-3. [DOI] [PubMed] [Google Scholar]
- 46.de Lima Alvarenga EH, Dall’Oglio GP, Murano EZ, Abrahão M. Continuum theory: presbyphagia to dysphagia? Functional assessment of swallowing in the elderly. Eur Arch Otorhinolaryngol. 2018;275(2):443–449. 10.1007/s00405-017-4801-7. [DOI] [PubMed] [Google Scholar]
- 47.Labeit B, Muhle P, von Itter J, et al. Clinical determinants and neural correlates of presbyphagia in community-dwelling older adults. Front Aging Neurosci. 2022;14:912691. 10.3389/fnagi.2022.912691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehraban-Far S, Alrassi J, Patel R, et al. Dysphagia in the elderly population: A Videofluoroscopic study. Am J Otolaryngol. 2021;42(2):102854. 10.1016/j.amjoto.2020.102854. [DOI] [PubMed] [Google Scholar]
- 49.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4(2):90–4. 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 50.Park JS, Oh DH, Chang M. Comparison of maximal tongue strength and tongue strength used during swallowing in relation to age in healthy adults. J Phys Ther Sci. 2016;28(2):442–5. 10.1589/jpts.28.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin WY, Chen YM, Wu KM, Chen PK, Hwu YJ. Age and Sex-Related Differences in the Tongue Pressure Generated during Maximum Isometric and Swallowing Tasks by Healthy Chinese Adults. Int J Environ Res Public Health. 2021;18(10):5452. 10.3390/ijerph18105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nienstedt JC, Müller F, Rösler A, Pflug C. Presbyphagia Diagnostics Using M-Mode Ultrasound: Changes in the Tongue Movement Pattern. Dysphagia. 2020;35(4):696–701. 10.1007/s00455-019-10076-z. [DOI] [PubMed] [Google Scholar]
- 53.Be R, Ja M, Ra D. Patient localization of esophageal dysphagia. Dig Dis Sci. 2004;49(4). 10.1023/b:ddas.0000026321.02927.39. [DOI] [PubMed] [Google Scholar]
- 54.Ashraf HH, Palmer J, Dalton HR, et al. Can patients determine the level of their dysphagia? World J Gastroenterol. 2017;23(6):1038–43. 10.3748/wjg.v23.i6.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol. 1998;171(5):1361–5. 10.2214/ajr.171.5.9798879. [DOI] [PubMed] [Google Scholar]
- 56.Aslam M, Vaezi MF. Dysphagia in the Elderly. Gastroenterol Hepatol. 2013;9(12):784–95. [PMC free article] [PubMed] [Google Scholar]
- 57.Ney D, Weiss J, Kind A, Robbins J. Senescent Swallowing: Impact, Strategies and Interventions. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2009;24(3):395–413. 10.1177/0884533609332005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malagelada JR, Bazzoli F, Boeckxstaens G, et al. World Gastroenterology Organisation Global Guidelines: Dysphagia—Global Guidelines and Cascades Update September 2014. J Clin Gastroenterol. 2015;49(5):370. 10.1097/MCG.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 59.Upchurch B Cricopharyngeal Bar Dysphagia Treated Successfully with Endoscopic Dilation: 432. Off J Am Coll Gastroenterol ACG. 2011;106:S169. [Google Scholar]
- 60.Elkholy S, El-Sherbiny M, Delano-Alonso R, et al. Peroral endoscopic myotomy as treatment for Zenker’s diverticulum (Z-POEM): a multi-center international study. Esophagus Off J Jpn Esophageal Soc. 2021;18(3):693–9. 10.1007/s10388-020-00809-7. [DOI] [PubMed] [Google Scholar]
- 61.Elmunzer BJ, Moran RA. Peroral endoscopic myotomy for cricopharyngeal bar. VideoGIE Off Video J Am Soc Gastrointest Endosc. 2020;5(8):378–9. 10.1016/j.vgie.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marston AP, Maldonado FJ, Ravi K, Kasperbauer JL, Ekbom DC. Treatment of oropharyngeal dysphagia secondary to idiopathic cricopharyngeal bar: Surgical cricopharyngeal muscle myotomy versus dilation. Am J Otolaryngol. 2016;37(6):507–12. 10.1016/j.amjoto.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Drossman DA, Hasler WL. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150(6):1257–61. 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 64.Schmulson M How to use Rome IV criteria in the evaluation of esophageal disorders. Curr Opin Gastroenterol. 2018;34(4):258. 10.1097/MOG.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 65.Krishnamurthy C, Hilden K, Peterson KA, Mattek N, Adler DG, Fang JC. Endoscopic findings in patients presenting with dysphagia: analysis of a national endoscopy database. Dysphagia. 2012;27(1):101–5. 10.1007/s00455-011-9346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sonnenberg A, Massey BT, Jacobsen SJ. Hospital discharges resulting from esophagitis among medicare beneficiaries. Dig Dis Sci. 1994;39(1):183–8. 10.1007/BF02090080. [DOI] [PubMed] [Google Scholar]
- 67.Ruiz Manzanera JJ, Ruiz de Angulo Martín D, Fernández Pérez J, Munitiz Ruiz V. Esophageal stricture as an initial manifestation of Crohn’s disease. Rev Esp Enferm Dig. 2022;114(8):501–502. 10.17235/reed.2022.8766/2022. [DOI] [PubMed] [Google Scholar]
- 68.Wang SY, Wang Z, Yang JL. Esophageal mucosal pemphigoid and esophageal stricture. Gastrointest Endosc. 2020;92(4):962–3. 10.1016/j.gie.2020.04.056. [DOI] [PubMed] [Google Scholar]
- 69.Ravich WJ. Endoscopic Management of Benign Esophageal Strictures. Curr Gastroenterol Rep. 2017;19(10):1–8. 10.1007/s11894-017-0591-8. [DOI] [PubMed] [Google Scholar]
- 70.ASGE Standards of Practice Committee, Evans JA, Early DS, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77(3):328–334. 10.1016/j.gie.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Pazan F, Wehling M. Polypharmacy in older adults: a narrative review of definitions, epidemiology and consequences. Eur Geriatr Med. 2021;12(3):443–52. 10.1007/s41999-021-00479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hey H, Jørgensen F, Sørensen K, Hasselbalch H, Wamberg T. Oesophageal transit of six commonly used tablets and capsules. Br Med J Clin Res Ed. 1982;285(6356):1717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim SH, Jeong JB, Kim JW, et al. Clinical and endoscopic characteristics of drug-induced esophagitis. World J Gastroenterol WJG. 2014;20(31):10994–9. 10.3748/wjg.v20.i31.10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kikendall JW. Pill Esophagitis. J Clin Gastroenterol. 1999;28(4):298. [DOI] [PubMed] [Google Scholar]
- 75.Hang TVP, Spiritos Z, Gamboa AM, et al. Epidemiology of early esophageal adenocarcinoma. Clin Endosc. 2022;55(3):372–80. 10.5946/ce.2021.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cancer of the Esophagus - Cancer Stat Facts. NIH National Cancer Institute: Surveillance, Epidemiology, and End Results Program. n.d. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed February 21, 2023 [Google Scholar]
- 77.Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Off J Am Coll Gastroenterol ACG. 2022;117(4):559. 10.14309/ajg.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richards J, Wei R, Anjum F. Esophageal Necrosis. In: Stat-Pearls. StatPearls Publishing. 2022. http://www.ncbi.nlm.nih.gov/books/NBK572075/. Accessed 15 Mar 2023 [PubMed]
- 79.Dias E, Santos-Antunes J, Macedo G. Diagnosis and management of acute esophageal necrosis. Ann Gastroenterol. 2019;32(6):529–40. 10.20524/aog.2019.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gurvits GE. Black esophagus: acute esophageal necrosis syndrome. World J Gastroenterol. 2010;16(26):3219–25. 10.3748/wjg.v16.i26.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Day A, Sayegh M. Acute oesophageal necrosis: A case report and review of the literature. Int J Surg. 2010;8(1):6–14. 10.1016/j.ijsu.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 82.Abdullah HM, Ullah W, Abdallah M, Khan U, Hurairah A, Atiq M. Clinical presentations, management, and outcomes of acute esophageal necrosis: a systemic review. Expert Rev Gastroenterol Hepatol. 2019;13(5):507–14. 10.1080/17474124.2019.1601555. [DOI] [PubMed] [Google Scholar]
- 83.Yiğman ZA, Umay E, Cankurtaran D, Güzel Ş. Swallowing difficulty in the older adults: presbyphagia or dysphagia with sarcopenia? Int J Rehabil Res Int Z Rehabil Rev Int Rech Readaptation. 2021;44(4):336–42. 10.1097/MRR.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 84.Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. 10.1002/jcsm.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maeda K, Akagi J. Sarcopenia is an independent risk factor of dysphagia in hospitalized older people. Geriatr Gerontol Int. 2016;16(4):515–21. 10.1111/ggi.12486. [DOI] [PubMed] [Google Scholar]
- 86.de Sire A, Ferrillo M, Lippi L, et al. Sarcopenic Dysphagia, Malnutrition, and Oral Frailty in Elderly: A Comprehensive Review. Nutrients. 2022;14(5):982. 10.3390/nu14050982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mori T, Fujishima I, Wakabayashi H, et al. Development, reliability, and validity of a diagnostic algorithm for sarcopenic dysphagia. JCSM Clin Rep. 2017;2(2):1–10. 10.17987/jcsm-cr.v2i2.17. [DOI] [Google Scholar]
- 88.Pratap N, Kalapala R, Darisetty S, et al. Achalasia Cardia Subtyping by High-Resolution Manometry Predicts the Therapeutic Outcome of Pneumatic Balloon Dilatation. J Neurogastroenterol Motil. 2011;17(1):48–53. 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rohof WO, Salvador R, Annese V, et al. Outcomes of Treatment for Achalasia Depend on Manometric Subtype. Gastroenterology. 2013;144(4):718–25. 10.1053/j.gastro.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 90.Salvador R, Costantini M, Zaninotto G, et al. The Preoperative Manometric Pattern Predicts the Outcome of Surgical Treatment for Esophageal Achalasia. J Gastrointest Surg. 2010;14(11):1635–45. 10.1007/s11605-010-1318-4. [DOI] [PubMed] [Google Scholar]
- 91.Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc. 2020;91(2):213–227.e6. 10.1016/j.gie.2019.04.231. [DOI] [PubMed] [Google Scholar]
- 92.Schindler A, Pizzorni N, Cereda E, et al. Consensus on the treatment of dysphagia in Parkinson’s disease. J Neurol Sci. 2021;430. 10.1016/j.jns.2021.120008. [DOI] [PubMed] [Google Scholar]
- 93.Suttrup I, Warnecke T. Dysphagia in Parkinson’s Disease. Dysphagia. 2016;31(1):24–32. 10.1007/s00455-015-9671-9. [DOI] [PubMed] [Google Scholar]
- 94.Sung HY, Kim JS, Lee KS, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov Disord Off J Mov Disord Soc. 2010;25(14):2361–8. 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- 95.Mullins PM, Gilligan CJ, Bhattacharyya N. The prevalence of opioid medication use among adults in the United States. J Opioid Manag. 2022;18(6):503–9. 10.5055/jom.2022.0745. [DOI] [PubMed] [Google Scholar]
- 96. Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113(2):409–14. 10.1053/gast.1997.v113.pm9247457. • Some studies have provided data supporting free water protocols for dysphagic patient, but this study highlights that physicians hesitate to implement them despite this evidence due to the lack of robust applicable data, a single standardized protocol, and guideline recommendations.
- 97.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31(5):601–6. 10.1111/j.1365-2036.2009.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Snyder DL, Crowell MD, Horsley-Silva J, Ravi K, Lacy BE, Vela MF. Opioid-Induced Esophageal Dysfunction: Differential Effects of Type and Dose. Am J Gastroenterol. 2019;114(9):1464–9. 10.14309/ajg.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 99.Abu-Shakra M, Guillemin F, Lee P. Gastrointestinal manifestations of systemic sclerosis. Semin Arthritis Rheum. 1994;24(1):29–39. 10.1016/0049-0172(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 100.Lepri G, Guiducci S, Bellando-Randone S, et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann Rheum Dis. 2015;74(1):124–8. 10.1136/annrheumdis-2013-203889. [DOI] [PubMed] [Google Scholar]
- 101.Markus J, Pinto R de MC, Matoso AGB, Ranza R. Esophageal manometry in systemic sclerosis: findings and association with clinical manifestations. Rev Assoc Medica Bras 1992. 2020;66(1):48–54. 10.1590/1806-9282.66.1.48. [DOI] [PubMed] [Google Scholar]
- 102.Ostojić P, Damjanov N. Different clinical features in patients with limited and diffuse cutaneous systemic sclerosis. Clin Rheumatol. 2006;25(4):453–7. 10.1007/s10067-005-0041-0. [DOI] [PubMed] [Google Scholar]
- 103.Aggarwal N, Lopez R, Gabbard S, Wadhwa N, Devaki P, Thota PN. Spectrum of esophageal dysmotility in systemic sclerosis on high-resolution esophageal manometry as defined by Chicago classification. Dis Esophagus Off J Int Soc Dis Esophagus. 2017;30(12):1–6. 10.1093/dote/dox067. [DOI] [PubMed] [Google Scholar]
- 104.Pasha SF, Acosta RD, Chandrasekhara V, et al. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest Endosc. 2014;79(2):191–201. 10.1016/j.gie.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 105.Esfandyari T, Potter JW, Vaezi MF. Dysphagia: a cost analysis of the diagnostic approach. Am J Gastroenterol. 2002;97(11):2733–7. 10.1016/S0002-9270(02)05478-3. [DOI] [PubMed] [Google Scholar]
- 106.Ergenç M, Uprak TK. Esophagogastroduodenoscopy in Patients Aged 75 Years and Older: A Single-Center Study. Cureus. 2022;14(2):e21846. 10.7759/cureus.21846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Buri L, Zullo A, Hassan C, et al. Upper GI endoscopy in elderly patients: predictive factors of relevant endoscopic findings. Intern Emerg Med. 2013;8(2):141–6. 10.1007/s11739-011-0598-3. [DOI] [PubMed] [Google Scholar]
- 108.Lockhart SP, Schofield PM, Gribble RJ, Baron JH. Upper gastrointestinal endoscopy in the elderly. Br Med J Clin Res Ed. 1985;290(6464):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jafri SM, Monkemuller K, Lukens FJ. Endoscopy in the Elderly: A Review of the Efficacy and Safety of Colonoscopy, Esophagogastroduodenoscopy, and Endoscopic Retrograde Cholangiopancreatography. J Clin Gastroenterol. 2010;44(3):161. 10.1097/MCG.0b013e3181c64d64. [DOI] [PubMed] [Google Scholar]
- 110.Miyanaga R, Hosoe N, Naganuma M, et al. Complications and outcomes of routine endoscopy in the very elderly. Endosc Int Open. 2018;6(2):E224–9. 10.1055/s-0043-120569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jang JM, Park SB, Yoon JY, Kwak MS, Cha JM. Gastrointestinal and non-gastrointestinal complication rates associated with diagnostic esophagogastroduodenoscopy under sedation. Medicine (Baltimore). 2022;101(19):e29266. 10.1097/MD.0000000000029266. • This new observational study with a very large cohort, including 39,910 patients, provides robust data supporting the safety of EGD, even in older patients.
- 112.Blonski W, Kumar A, Feldman J, Richter JE. Timed Barium Swallow: Diagnostic Role and Predictive Value in Untreated Achalasia, Esophagogastric Junction Outflow Obstruction, and Non-Achalasia Dysphagia. Am J Gastroenterol. 2018;113(2):196–203. 10.1038/ajg.2017.370. [DOI] [PubMed] [Google Scholar]
- 113.O’Rourke AK, Lazar A, Murphy B, Castell DO, Martin-Harris B. Utility of Esophagram versus High-Resolution Manometry in the Detection of Esophageal Dysmotility. Otolaryngol-Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg. 2016;154(5):888–91. 10.1177/0194599816629379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility not Observed with Manometry in Patients with Achalasia. Gastroenterology. 2015;149(7):1742–51. 10.1053/j.gastro.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iyer R, DuBrow R. Imaging of esophageal cancer. Cancer Imaging. 2004;4(2):125–32. 10.1102/1470-7330.2004.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sui H, Ma R, Liu L, Gao Y, Zhang W, Mo Z. Detection of Incidental Esophageal Cancers on Chest CT by Deep Learning. Front Oncol. 2021;11:700210. 10.3389/fonc.2021.700210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wright L, Cotter D, Hickson M, Frost G. Comparison of energy and protein intakes of older people consuming a texture modified diet with a normal hospital diet. J Hum Nutr Diet Off J Br Diet Assoc. 2005;18(3):213–9. 10.1111/j.1365-277X.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 118.Leibovitz A, Baumoehl Y, Lubart E, Yaina A, Platinovitz N, Segal R. Dehydration among long-term care elderly patients with oropharyngeal dysphagia. Gerontology. 2007;53(4):179–83. 10.1159/000099144. [DOI] [PubMed] [Google Scholar]
- 119.Bernard S, Loeslie V, Rabatin J. Use of a Modified Frazier Water Protocol in Critical Illness Survivors With Pulmonary Compromise and Dysphagia: A Pilot Study. Am J Occup Ther Off Publ Am Occup Ther Assoc. 2016;70(1):7001350040p1–5. 10.5014/ajot.2016.016857. [DOI] [PubMed] [Google Scholar]
- 120.Gillman A, Winkler R, Taylor NF. Implementing the Free Water Protocol does not Result in Aspiration Pneumonia in Carefully Selected Patients with Dysphagia: A Systematic Review. Dysphagia. 2017;32(3):345–61. 10.1007/s00455-016-9761-3. [DOI] [PubMed] [Google Scholar]
- 121. Murray J, Maloney S, Underdown K, Doeltgen S. Patient suitability for free water protocols in acute stroke and general medicine: a qualitative study of clinician perceptions. Int J Lang Commun Disord. 2022;57(3):630–44. 10.1111/1460-6984.12713. • Some studies have provided data supporting free water protocols for dysphagic patient, but this study highlights that physicians hesitate to implement them despite this evidence due to the lack of robust applicable data, a single standardized protocol, and guideline recommendations.
- 122.Murphy SL. Mortality in the United States, 2020. 2021;(427):8. [PubMed] [Google Scholar]
- 123.Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–71. 10.1056/NEJM200103013440908. [DOI] [PubMed] [Google Scholar]
- 124.Sue EE. Dysphagia and aspiration pneumonia in older adults. J Am Acad Nurse Pract. 2010;22(1):17–22. 10.1111/j.1745-7599.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 125.Feng MC, Lin YC, Chang YH, et al. The Mortality and the Risk of Aspiration Pneumonia Related with Dysphagia in Stroke Patients. J Stroke Cerebrovasc Dis. 2019;28(5):1381–7. 10.1016/j.jstrokecerebrovasdis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 126.Hinchey JA, Shephard T, Furie K, et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–6. 10.1161/01.STR.0000177529.86868.8d. [DOI] [PubMed] [Google Scholar]
- 127. Khadka S, Khan S, King A, Goldberg LR, Crocombe L, Bettiol S. Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: a systematic review. Age Ageing. 2021;50(1):81–7. 10.1093/ageing/afaa102. • This systematic review collected the evidence supporting that pathogenic microorganisms in the mouth are associated with aspiration pneumonia and that oral hygiene can reduce both the oral load of these pathogens as well as risk of aspiration pneumonia. Given there are effective ways to prevent aspiration pneumonia, it is even more important to diagnose dysphagia and risk stratify patients at risk of aspiration during evaluation.
- 128.Ebihara S, Sekiya H, Miyagi M, Ebihara T, Okazaki T. Dysphagia, dystussia, and aspiration pneumonia in elderly people. J Thorac Dis. 2016;8(3):632–9. 10.21037/jtd.2016.02.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Arrowsmith JB, Gerstman BB, Fleischer DE, Benjamin SB. Results from the American Society for Gastrointestinal Endoscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest Endosc. 1991;37(4):421–427. 10.1016/s0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 130.Celik M Efficacy of early endoscopy and colonoscopy in very elderly patients with gastrointestinal bleeding. Pak J Med Sci. 2017;33(1):187–90. 10.12669/pjms.331.11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim SY, Moon CM, Kim MH, et al. Impacts of age and sedation on cardiocerebrovascular adverse events after diagnostic GI endoscopy: a nationwide population-based study. Gastrointest Endosc. 2020;92(3):591–602.e16. 10.1016/j.gie.2020.03.3864. [DOI] [PubMed] [Google Scholar]
- 132.Ellis R, Livovsky DM, Shapiro DS, et al. Safety of oesophagogastroduodenoscopy in a nonagenarian population. Age Ageing. 2021;50(5):1840–4. 10.1093/ageing/afab129. [DOI] [PubMed] [Google Scholar]


