Abstract
Bisphenol-A (BPA) is a ubiquitous estrogen-like endocrine disrupting compound (EDC). BPA exposure in utero has been linked to breast cancer and abnormal mammary gland development in mice. The recent rise in incidence of human breast cancer and decreased age of first detection suggests a possible environmental etiology. We hypothesized that developmental programming of carcinogenesis may involve an aberrant immune response. Both innate and adaptive immunity play a role in tumor suppression through cytolytic CD8, NK, and Th1 T-cells. We hypothesized that BPA exposure in utero would lead to dysregulation of both innate and adaptive immunity in the mammary gland. CD1 mice were exposed to BPA in utero during gestation (days 9–21) via osmotic minipump. At 6 weeks, the female offspring were ovariectomized and estradiol was given at 8 weeks. RNA and protein were extracted from the posterior mammary glands, and the mRNA and protein levels were measured by PCR array, qRT-PCR, and western blot. In mouse mammary tissue, BPA exposure in utero significantly decreased the expression of members of the chemokine CXC family (Cxcl2, Cxcl4, Cxcl14, and Ccl20), interleukin 1 (Il1) gene family (Il1β and Il1rn), interleukin 2 gene family (Il7 receptor), and interferon gene family (interferon regulatory factor 9 (Irf9), as well as immune response gene 1 (Irg1). Additionally, BPA exposure in utero decreased Esr1 receptor gene expression and increased Esr2 receptor gene expression. In utero exposure of BPA resulted in significant changes to inflammatory modulators within mammary tissue. We suggest that dysregulation of inflammatory cytokines, both pro-inflammatory and anti-inflammatory, leads to a microenvironment that may promote disordered cell growth through inhibition of the immune response that targets cancer cells.
Electronic supplementary material
The online version of this article (doi:10.1007/s12672-016-0254-5) contains supplementary material, which is available to authorized users.
Keywords: Mammary Gland, Breast Cancer Risk, Mammary Tissue, Interferon Regulatory Factor, Estradiol Treatment
Introduction
Bisphenol-A (BPA) is an organic synthetic chemical used in manufacturing of a variety of consumer products [1–3]. This has resulted in the ubiquitous presence of BPA in industrialized environments [2]. BPA is classified as an endocrine-disrupting molecule with weak estrogenic activity, and it exerts its harmful effects when it binds to a variety of physiological receptors including estrogen receptors Esr1and Esr2 [4]. Humans are exposed to BPA daily through food and drinks stored in bottles and cans manufactured with BPA-containing materials, as well as via dermal absorption from thermal receipts [5]. BPA is detected in 90 % of the population in the USA [6] and has become a public health concern, especially for pregnant women. Greater exposure to BPA has been associated with a wide range of reproductive, cardiovascular, and metabolic disorders and cancer [7, 8]. BPA has been detected in the blood of pregnant women, placental tissues, amniotic fluid, umbilical cord, and neonatal blood [9, 10]. The presence of BPA in fetal circulation can additionally reactivate any inactive BPA conjugates and enhance bioactive BPA levels in the developing fetus [11]. In animals, in utero exposure to BPA during pregnancy has been shown to affect the developmental patterning of fetal organs such as the uterus, testes, brain, heart, ovaries, lungs, and mammary glands [12–20].
The mammary gland is an estrogen-responsive tissue, and persistent alterations in the structure and function of these glands have been reported in rodents exposed to BPA during fetal and perinatal stages [21–24]. BPA has been associated with adverse developmental outcomes in mammary gland tissue, in both human and animal models. In human breast cancer cell lines, BPA induces cell proliferation [25, 26]. Mammary glands from mice exposed to BPA as fetuses showed accelerated differentiation of the fat pad, increased adipose maturation, and ductal growth in epithelium altered composition of the stromal matrix and collagen localization in the mesenchyme, delayed lumen formation, and decreased cell size and altered gene expression in the stroma and epithelium [19, 20, 27]. Mice exposed to BPA perinatally that were ovariectomized at a pre-pubertal age demonstrated increased ductal extension and lateral branching, higher numbers of terminal end buds (TEBs), greater TEB density and area, enhanced sensitivity to estradiol at adulthood, and a delay in ductal invasion of the mammary stroma at puberty [21, 27, 28]. Pre-natal exposure to BPA in mouse and rat models resulted in the development of intraductal hyperplasia and carcinoma in situ [29, 30]. In utero exposure to BPA during embryonic development also enhances the sensitivity of the rat mammary gland to chemical carcinogens [31, 32]. The timing of exposure to BPA can determine the long-term outcome with earlier time points of exposure typically exerting a more severe effect [33, 34]. These studies reveal that the fetal mammary gland is a target of BPA and that the effects of this early exposure worsen at puberty and beyond, long after exposure has ended.
Our laboratory previously reported that in utero exposure to BPA leads to decreased methylation of Hoxa10 in the female reproductive tract [35]. Demethylation of the promoter region of Hoxa10 after BPA in utero exposure was shown to modulate the estrogen response of the Hoxa10 estrogen response element (ERE) [36]. Epigenetic changes, such as DNA methylation, are known to regulate gene expression and are thought to be involved in cancer [37]. Despite accumulated evidence linking BPA exposure to mammary carcinogenesis, the mechanisms by which BPA exposure affects mammary tissue development and increases breast cancer risk are unknown. BPA exposure has been previously associated with increased expression of Esr2, which has known immunomodulatory functions [38]. Here, we show that in utero exposure to BPA in a mouse model resulted in significantly altered developmental programming of inflammatory signaling within the mammary tissue of exposed offspring. We suggest that dysregulation of inflammatory cytokines (both pro- and anti-inflammatory) creates a microenvironment that promotes disordered cell growth and an inhibited immune response to abnormal cells. This altered immune status may contribute to an increased risk of breast cancer.
Materials and Methods
Animals
All animal experiments were conducted in accordance with the Yale University Animal Care Committee Guidelines. CD-1 mice were obtained from Charles River Laboratories (Wilmington, MA, USA). Twelve 8-week old female CD-1 mice were mated with four 8-week old male CD-1 mice. Detection of vaginal plug indicated day 1 of gestation. Pregnant mice were treated with either BPA (5.0 mg/kg) or sesame oil (vehicle control) on days 9–21 (inclusive of gestation) via osmotic minipump infusion. Injection of 5 mg/kg daily produced an average serum BPA level similar to human exposure as demonstrated in our prior study (40). However, in our prior model, daily injection resulted in a peak soon after injection and low levels after 24 h. To minimize these fluctuations, we used a miniosmotic pump administering the same total dose per 24 h. On day 9 of gestation, pregnant dams were anesthetized via intraperitoneal injection of ketamine/xylazine. An ALZET (Cupertino, CA, USA) model 1002 osmotic minipump loaded with either 5.0 mg/kg/day BPA (n = 6) or vehicle control (n = 6) was inserted in the peritoneal cavity. Six weeks after birth, female offspring were ovariectomized as follows: mice were anesthetized via intraperitoneal injection using ketamine/xylazine rodent anesthesia mix. Then, using aseptic technique, a lower abdominal longitudinal incision was made. The ovaries were removed bilaterally in all mice. The incision was closed in two layers using 4-0 Vicryl sutures. The mice were monitored at least daily post-surgically and allowed to rest for 14 days before further intervention. At 8 weeks, ovariectomized mice were treated with a single IP injection of 300 ng estradiol (E2) or vehicle, and the mammary glands were collected after 6 h and processed immediately for RNA and DNA isolation. At least two pups from each litter were used in these studies. In each experiment, the data was analyzed for six mice (n = 6). Any remaining tissue sample was stored in 1 mL of RNAlater solution (Qiagen, Valencia, CA, USA) at −80 °C until RNA and DNA isolation.
RNA Isolation
Tissue samples frozen at −80 °C were thawed on ice. Each sample was placed in 1 mL of TRIzol solution (Invitrogen, Carlsbad, CA, USA) and homogenized on ice. After incubating the cell lysate, 0.2 mL of chloroform was added to the samples. The lysates were added to Phase Lock Gel tubes (5 Prime, Gaithersburg, MD, USA) and centrifuged. The aqueous layer was then transferred to a fresh tube, and the RNA was precipitated twice using 75 % ethanol. The pellet was dried at room temperature and resuspended in RNase-free water. The total RNA was purified using the Qiagen RNeasy Plus Mini Kit (Qiagen,Valencia, CA, USA), according to the manufacturer’s instructions and quantified by NanoDrop.
Quantitative Real-Time Polymerase Chain Reaction Analysis
Total RNA (500 ng) from each sample was reverse-transcribed in a 20-μl reaction mixture using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The reaction mix was incubated for 5 min at 25 °C, 30 min at 42 °C, and 5 min at 85 °C using the Eppendorf Mastercycler (Eppendorf North America, Hauppauge, NY, USA). Quantitative real-time polymerase chain reaction (qRT-PCR) reactions were prepared using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). The PCR reaction was carried out using 1 μl of cDNA template, 1 μl of forward and reverse primers (1 μM), 9.5 μl of nuclease-free H2O, and 12.5 μl of iQ SYBR Green Supermix in 40 cycles at 95 °C for 15 s, 58.7 °C for 20 s, and 72 °C for 25 s. The Bio-Rad iCycler iQ system (Bio-Rad, Hercules, CA, USA) was used to quantify fluorescence of PCR products during amplification. Specificity of the amplified products and the absence of primer-dimers were confirmed by melting curve data analysis. Gene expression was normalized to β-actin expression for each sample. Relative mRNA expression for each gene was calculated using the comparative cycle threshold (Ct) method (known as 2ΔΔCT method) [39, 40]. All experiments were conducted in triplicate.
Cytokines and Chemokines RT2 Profiler PCR Array
The Mouse Cytokines and Chemokines RT2 Profiler PCR PAMM-150Z Array (Qiagen, Valencia, CA, USA) profiles the expression of 84 key secreted proteins central to the immune response and other functions. RNA was extracted from tissue samples by the previously stated TRIzol method. First-strand cDNA was prepared by using the RT2 First Strand Kit (Qiagen, Valencia, CA, USA) following the supplier’s protocol. The RT2 SYBR Green/Fluorescein qRT-PCR master mix was used to perform the PCR array using the iQ5 iCycler Multicolor Real-Time PCR Detection System. The array data was analyzed as per the instructions of the suppliers.
Western Blot Analysis
Mammary gland tissue was homogenized in RIPA buffer with a protease inhibitor cocktail and PMSF protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) using tungsten carbide beads in a TissueLyser II (Qiagen, Valencia, CA, USA). The homogenate was centrifuged at 12,000 rpm for 10 min and the supernatant was collected. The protein concentrations of the respective supernatants were assayed by the Bradford method [41]. Protein samples were prepared with 4× Invitrogen Sample Buffer (Thermo Fisher Scientific, Waltham, MA, USA) and placed in a heat block at 95 °C for 6 min. Protein samples (25 μg) were subjected to SDS-PAGE using NuPAGE Novex 4–12 % Bis-Tris Midi protein gels (Life Technologies, Carlsbad, CA, USA) with MOPS Running Buffer (Invitrogen, Carlsbad, CA, USA). The separated proteins were transferred from the gel onto a polyvinylidene fluoride (PVDF) membrane and blocked with 5 % non-fat dry milk. The membranes were then incubated with anti-Esr1 (sc-542) and anti-Esr2 (sc-8974) primary antibodies, followed by a secondary horseradish peroxidase (HRP)-conjugated antibody. After washing the membranes, protein bands were visualized by chemiluminescence using the SuperSignal West Pico and Femto detection kit (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s protocol.
Statistical Analysis
Results are presented as the mean ± S.E. Statistical significance was determined using one-way ANOVA with the Newman–Keuls multiple analysis. P values less than 0.05 were considered statistically significant. All statistical analysis was carried out using GraphPad Prism 4.00 for Macintosh (GraphPad Software for Science Inc., San Diego, CA, USA).
Results
BPA Exposure In Utero Affected Chemokine Gene Expression
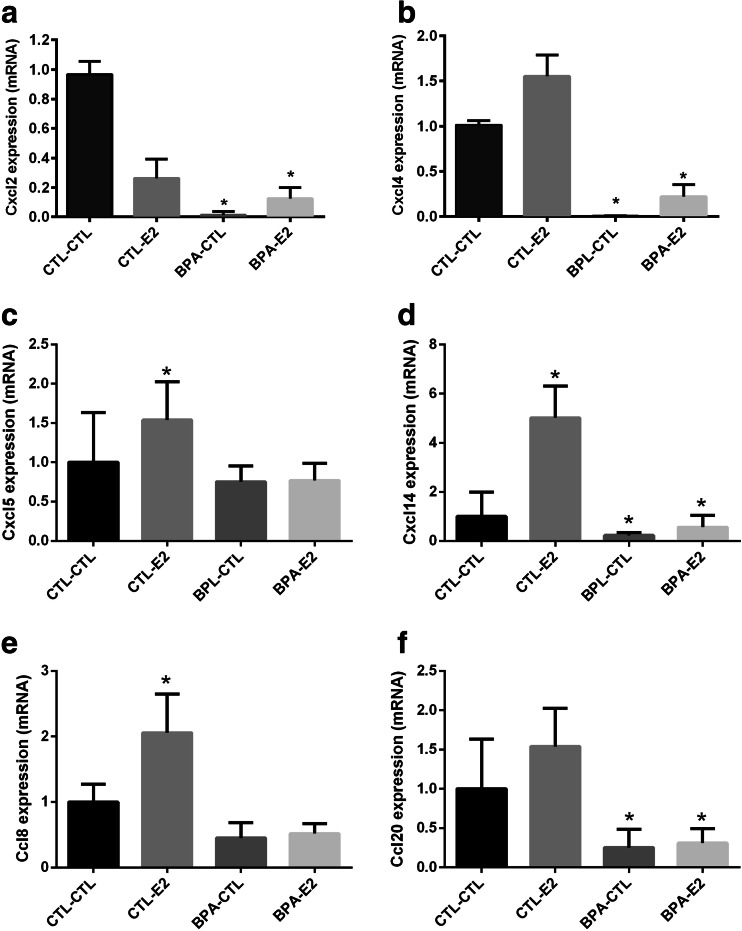
A PCR array and qRT-PCR were used to asses chemokine expression in pre-natal BPA exposed and unexposed animals (Table 1). Chemokine family ligand Cxcl2, Cxcl4, Cxcl5, Cxcl14, Ccl8, and Ccl20 expression in the mammary tissue of mice exposed to BPA in utero and later treated with estradiol was measured. Expression in BPA exposed animals was compared to controls (Fig. 1). The mRNA expression levels were significantly decreased by 15-fold for Cxcl2 (1A, p < 0.0007), 8-fold for Cxcl4 (1B, p < 0.002), 2.5-fold for Cxcl14 (1D, p < 0.005), and 2.8-fold for Ccl20 (1F, p < 0.02) in mice exposed to BPA in utero compared to control group mice. Though not statistically significant, we also observed a trend toward decreased expression of Cxcl5 and Ccl8 chemokines in BPA-exposed mice compared to control mice. We have previously shown that BPA exposure in utero affects subsequent adult estrogen responsiveness. To determine if administration of estradiol could overcome the observed repression of these chemokines, we treated mice with estradiol and subsequently compared their chemokine expression levels with those of untreated mice. In BPA exposed mice, the estradiol treatment did not offset the effects of BPA on the differential expression of all chemokines reported here. Consequently, BPA appears to program the expression of chemokines in an estrogen-independent fashion.
Table 1.
Primer sequence used for qRT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| Cxcl2 | GTTTCTGGGGAGAGGGTGAG | TGTTCTACTCTCCTCGGTGC |
| Cxcl4 | GCTGTGTGTGTGTGAAGACC | TATATAGGGGTGCTTGCCGG |
| Cxcl14 | GCTTCATCAAGTGGTACAAT | CTGGCCTGGAGTTTTTCTTTCCAT |
| Il1β | CTGCCATCACTGAAGAAGCC | TTCTGCCACCCTCACTACAG |
| Il1rn | GCTGGGGATTAGATGCTCCT | CTGTGGTAGGCTTGGAGTGA |
| Il7r | GTCGTATGGCCTAGTCTCCC | GCAGGAAGATCATTGGGCAG |
| Irg1 | ACTGTCCCATCCTTCCACAG | GATCTTCCTGGCTCAGTGGT |
| Irf9 | GGAGCTCTTCAAGACCACCT | GCTCCATCTGCACTGTGATG |
| CD45 | ATGGTCCTCTGAATAAAGCCCA | TCAGCACTATTGGTAGGCTCC |
| CD19 | GGAGGCAATGTTGTGCTGC | ACAATCACTAGCAGATGCCC |
| Ly6G | TGCCCCTTCTCTGATGGATT | TGCTCTTGACTTTGCTTCTGTGA |
| FSP1 | GGCAAGACCCTTGGAGGAG | CCTTTTCCCCAGGAAGCTAG |
| Esr1 | TTACGAAGTGGGCATGATGA | ATAGATCATGGGCGGTTCAG |
| Esr2 | GAAGCTGGCTGACAAGGAAC | GAACGAGGTCTGGAGCAAAG |
Fig. 1.
Pre-natal exposure to BPA alters chemokine gene expression. qRT-PCR shows the decreased expression of genes Cxcl2 (a), Cxcl4 (b), and Cxcl14 (d), while PCR array shows the decreased expression of genes Cxcl5 (c), Ccl8 (e), and Ccl20 (f) in female offspring. Data represent the results obtained from four treatment groups. Mice that had been exposed to BPA in utero were subsequently treated transiently with either E2 or sesame oil as vehicle control (CTL) as adults (BPA-CTL, BPA-E2; i.e, pre-natal exposure-adult treatment). Similarly, animals exposed to vehicle in utero subsequently received transient E2 or vehicle control stimulation (CTL-E2, CTL-CTL). The data are presented as relative mRNA expression in fold change relative to vehicle-treated controls (CTL-CTL). The bars in each graph represent the mean ± S.E. of three individual experiments, each performed in triplicate. *Denotes statistical significance (p < 0.05) compared to control group (CTL-CTL)
Decreased Expression of Interleukin and Interferon Family Members in Mice Exposed to BPA In Utero
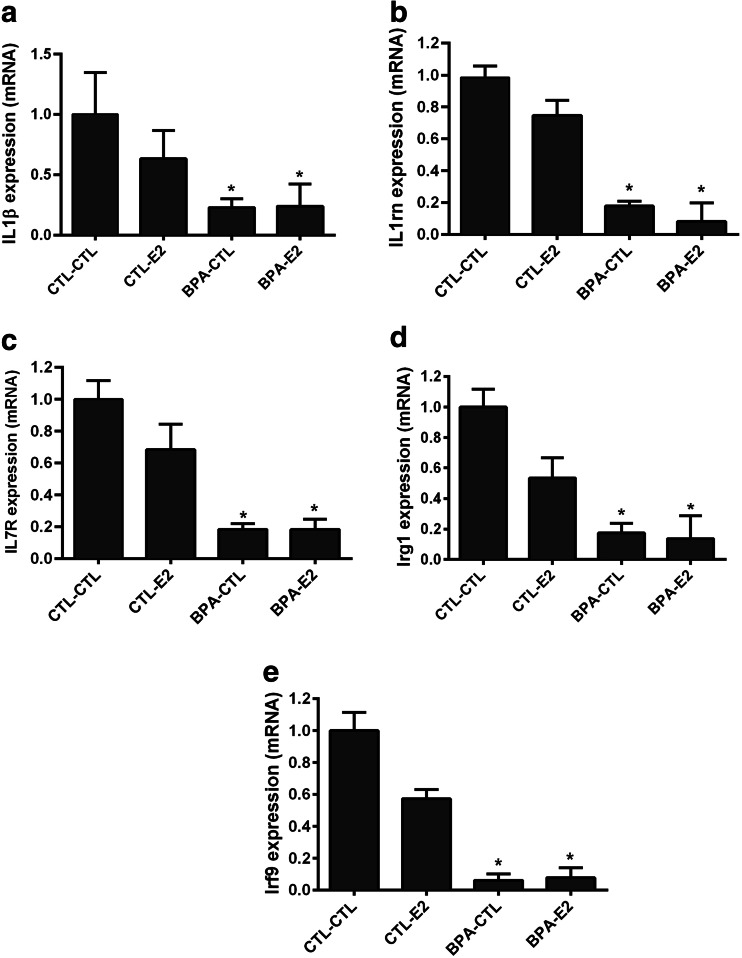
The mRNA expression of interleukin genes Il1β and Il1rn in BPA-exposed mice was significantly decreased by 3.0-fold (p < 0.02) and 3.46-fold (p < 0.0003) compared to controls as shown in Fig. 2a, b, respectively. Il7 receptor, another interleukin 2 family gene, showed a 4.25-fold (p < 0.01) decrease in expression in mice exposed to BPA in utero compared to controls (Fig. 2c). Estrogen treatment did not have a significant effect on expression of these genes. Expression of the interferon family genes Irg1 and Irf9 was significantly decreased in the mammary tissues of mice exposed to BPA in utero by 3.75-fold (p < 0.004) for Irg1 and 7-fold (p < 0.01) for Irf9 compared to controls as shown in Fig. 2d, e. Estrogen treatment did not significantly change the mRNA expression levels of these genes compared to controls.
Fig. 2.
Pre-natal exposure to BPA alters interleukin and interferon family genes. qRT-PCR results show the decreased expression of interleukin genes IL1β (a), IL 1rn (b), IL7 receptor (c), and interferon genes Irg1 (d) and Irf9 (e) in female offspring. Data represent the results obtained from four treatment groups. Mice that had been exposed to BPA in utero were subsequently treated transiently with either E2 or sesame oil as vehicle control (CTL) as adults (BPA-CTL, BPA-E2). Similarly, animals exposed to vehicle in utero subsequently received transient E2 or vehicle control stimulation as adults (CTL-E2, CTL-CTL). The data are presented as relative mRNA expression in fold change relative to vehicle-treated controls (CTL-CTL). The bars in each graph represent the mean ± S.E. of three individual experiments, each performed in triplicate. *Denotes statistical significance (p < 0.05) compared to control group (CTL-CTL)
BPA Exposure In Utero Decreased Expression of the Leukocyte Cell Markers
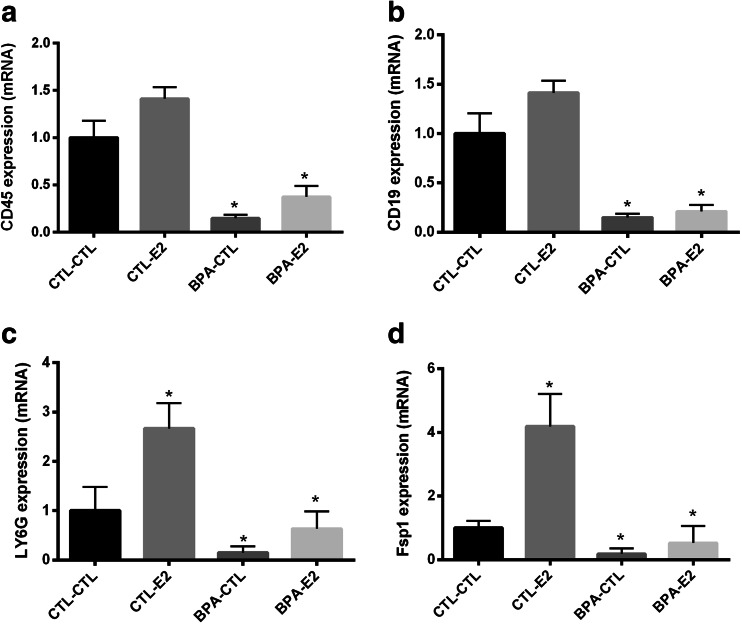
Expression of leukocyte markers in mammary tissue from mice exposed to BPA in utero was analyzed by qRT-PCR as shown in Fig. 3. CD45 (3A), a marker for leukocytes, was significantly decreased in mice exposed to BPA in utero by 7.75-fold (p < 0.001) compared to the control group. In BPA exposed mice, expression of CD19 (3B), a marker for B cell lymphocytes, was reduced in by 7-fold (p < 0.002), Ly6G (3C), a marker for neutrophils, decreased by 6.5-fold (p < 0.02), and FSP1 (3D), a marker for inflammatory macrophages and fibroblasts, decreased by 5.5-fold (p < 0.004) compared to the control group. Estradiol treatment alone did not have a statistically significant effect on mRNA levels of CD45 or CD19, but it did significantly increase expression of Ly6G and FSP1 relative to the control group. Mice exposed to BPA in utero that were also treated with estradiol showed reduced mRNA levels compared to the control group for all four markers (CD45, CD19, Ly6G, and FSP1). Surprisingly, estrogen treatment partially reversed the effect of BPA on Ly6G expression. These data suggest a decrease in leukocytes in the mammary glands of exposed animals with a partial restoration of neutrophil numbers in adults after estrogen exposure.
Fig. 3.
Pre-natal exposure to BPA reduced the presence of immune cells in mammary gland. qRT-PCR results show the decreased mRNA expression of markers for immune cells: CD45 (a) for leukocytes, CD19 (b) for B cells, Ly6G (c) for neutrophils, and FSP1 (d) for inflammatory macrophages and fibroblasts in female offspring. Data represent the results obtained from four treatment groups. Mice exposed to BPA in utero were subsequently treated transiently with either E2 or sesame oil as vehicle control (CTL) as adults (BPA-CTL, BPA-E2). Similarly, animals exposed to vehicle in utero subsequently received transient E2 or vehicle control stimulation as adults (CTL-E2, CTL-CTL). The data are presented as relative mRNA expression in fold change relative to vehicle-treated controls (CTL-CTL). The bars in each graph represent the mean ± S.E. of three individual experiments, each performed in triplicate. *Denotes statistical significance (p < 0.05) compared to control group (CTL-CTL)
BPA Exposure In Utero Decreased Esr1 Expression and Increased Esr2 Expression
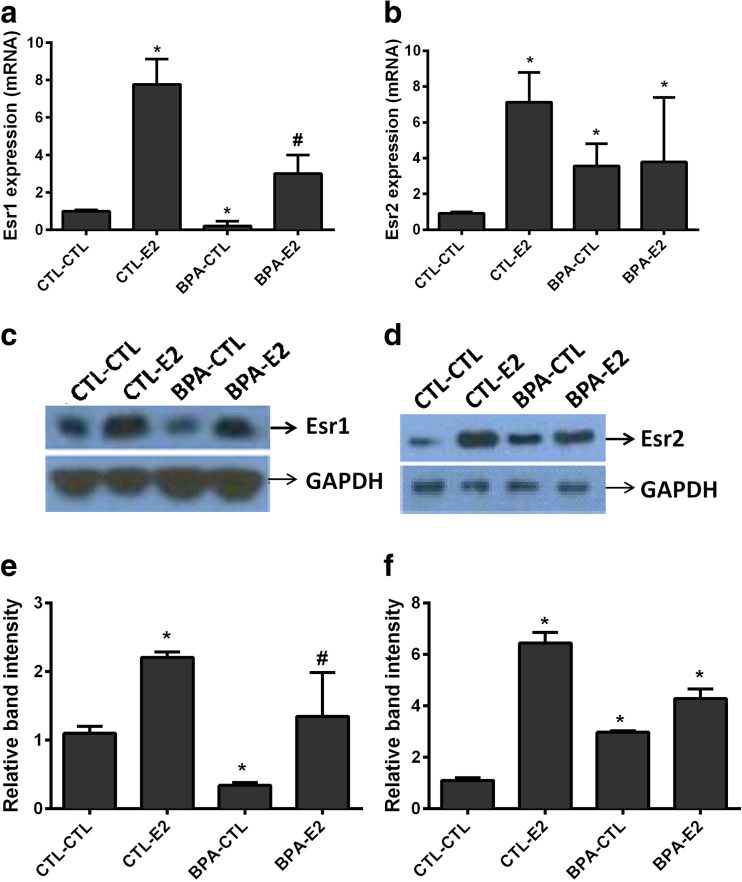
Expression of both estrogen receptors ERα (Esr1) and ERβ (Esr2) was significantly altered in the mammary tissue of CD-1 mice exposed to BPA in utero. Esr1 gene expression was decreased by 2.5-fold (p < 0.03) in BPA-exposed mice compared to controls. Estradiol treatment partially reversed the effect of BPA on Esr1 gene expression, increasing it by 7-fold (p < 0.002) in BPA-exposed mice (Fig. 4a). In contrast to Esr1, Esr2 expression was increased 3-fold (p < 0.04) in BPA-exposed mice compared to controls. No significant change was observed in gene expression following estradiol treatment (Fig. 4b). The intensity of protein bands for Esr1 and Esr2 proteins in all four different groups is shown in Fig. 4e, f, respectively. The Esr1 and Esr2 protein bands were normalized to GAPDH protein bands in each respective group. The mRNA levels measured by qRT-PCR for both Esr1 and Esr2 genes were consistent with changes in protein levels as determined by western blot analysis.
Fig. 4.
a–d Pre-natal exposure to BPA alters the expression of estrogen receptors Esr1 and Esr2 in mouse mammary gland. Results from qRT-PCR show the decreased expression of Esr1 (a) and increased Esr2 (b) mRNA levels in female offspring exposed to BPA in utero compared to controls. The Esr1 expression was increased after estrogen treatment in BPA-exposed mice while Esr2 expression was unchanged. Data represent the results obtained from four treatment groups. Mice exposed to BPA in utero were subsequently treated transiently with either E2 or sesame oil as vehicle control (CTL) as adults (BPA-CTL, BPA-E2). Similarly, animals exposed to vehicle in utero subsequently received transient E2 or vehicle control stimulation as adults (CTL-E2, CTL-CTL). The data are presented as relative mRNA expression in fold change relative to vehicle-treated controls (CTL-CTL). The bars in each graph represent the mean ± S.E. of three individual experiments, each performed in triplicate. *Denotes statistical significance (p < 0.05) compared to control group (CTL-CTL). #Denotes statistical significance (p < 0.05) compared to control group (CTL-CTL) and BPA exposed group (BPL-CTL). c, d The western blot analysis of Esr1 and Esr2, respectively. Protein (25 μg) from four treatment groups was subjected to 10 % SDS-PAGE and immunoblotted against anti-Esr1 and anti-Esr2 antibodies. The intensity of protein bands for Esr1 and Esr2 measured semi-quantitatively shown in e, f, respectively
Discussion
Pre-natal exposure to BPA has been shown to change normal gene expression patterns in estrogen-responsive tissues including human ovarian cells as well as endometrium and mammary glands of CD-1 mice [42, 43]. Specifically, in murine mammary glands, exposure has been shown to result in altered development, tissue organization, and estrogen sensitivity, leading to an increased incidence of carcinoma and ductal hyperplasia [22]. Here, we demonstrated that exposure to the estrogenic endocrine disruptor BPA in utero causes lasting changes in the expression of key inflammatory genes in the mammary gland of CD-1 mice. These alterations endure into adulthood, long after exposure to BPA has been removed. Inflammation is an important mediator of cancer risk, and the changes demonstrated here likely contribute to cancer risk after exposure.
Chemokines are small proteins that regulate leukocyte traffic. Chemokines and their receptors have been demonstrated to be inflammatory mediators in several solid tumor models, with inflammation implicated as a possible mechanism for promotion and progression of tumors [44]. The immune system plays a key role in preventing cancer because it is capable of detecting and destroying transformed cells. Thus, we chose to evaluate inflammatory cytokines in our BPA-exposed mouse model. Expression of members of the CXC family of chemokines (Cxcl2, Cxcl4, Cxcl14, and Ccl20) was reduced significantly in mice exposed to BPA in utero compared to controls, and estrogen treatment did not alter that effect of BPA. These results indicate a possible reduction of immune cells present in mammary glands following BPA exposure due to decreased chemoattraction of polymorphonuclear leukocytes (Cxcl2), neutrophils and fibroblasts (Cxcl4), monocytes and dendritic cells (Cxcl14), and lymphocytes (Ccl20). Chemoattracting, or homing of immune cells, has been previously reported as an important mechanism for identifying and destroying abnormal and diseased cells [45–47]. Reduced expression of cytokines Cxcl2, Cxcl20, and Cxcl14, as seen in the BPA-treated group of this study, may lead to a reduced inflammatory response and hamper an organism’s ability to react to infections or malignant cells.
Interleukin 1β and its complementary anti-inflammatory cytokine, interleukin-1 receptor antagonist, are both key mediators of inflammation and apoptosis [48, 49]. Additionally, secreted interleukin-1 receptor antagonist has been identified as a novel mutp53 target gene, whose suppression may be necessary for a chronic pro-inflammatory tumor microenvironment in mutp53 tumor malignancy [49]. Interleukin 7, a representative member from the interleukin 2 family of cytokines, plays a role in T lymphocyte apoptosis inhibition [50]. Suppressed Il1β and Il1rn could lead to aberrant inflammatory response and regulation of apoptosis, both of which can lead to an increased incidence of breast cancer. Specifically, downregulated Il1rn may lead to a chronic pro-inflammatory state, facilitating mutp53 tumor growth. We found that interleukin 7 receptor expression was decreased in mammary tissue of mice exposed to BPA in utero, suggesting that BPA-induced suppression of immune response leads to subsequent decreased T-cell development and survival [51]. Interferon regulatory factor 9 is a transcription factor responsible for innate immune response regulation via the inhibition of Sirt1 deacetylase activity, resulting in activated p53-mediated apoptosis [52]. Immunoresponsive gene 1, another interferon cytokine, is highly expressed in mammalian macrophages during inflammation and is responsible for linking cellular metabolism with immune defense by catalyzing itaconic acid production [53]. In this study, mammary tissue samples from mice exposed to BPA in utero showed decreased expression of both these immune response factors compared to controls, which could lead to a decrease in cell cycle regulation and apoptosis. Immune responsive gene 1 (Irg1), a key component in immune metabolism, and interferon regulatory factor 9 (Irf9), which can induce hepatocyte apoptosis, are both members of the interferon cytokine family and were shown to be downregulated in BPA-exposed mice versus control mice [54, 55]. Decreased overall expression may further indicate changes in the inflammatory environment of mammary tissue exposed to BPA, resulting in a suppressed immune response.
The expression of cellular markers CD45, Ly6G, FSP1, and CD19 on immune cells, leukocytes, neutrophils, fibroblasts, and B cells, respectively, was decreased significantly in the mammary gland of mice exposed to BPA in utero compared to the mammary gland of control mice. These results reveal a decreased number of immune cells present in the mammary gland of mice exposed to BPA compared to control mice. Stimulation with estradiol did not fully offset any of the effects induced by BPA exposure in utero on expression of these immune cell markers.
The effect of BPA exposure in utero on estrogen receptor expression in the mammary gland was specific to the receptor subtype. This finding concurs with previous studies characterizing the effects of hormone exposure during pregnancy on breast cancer; significantly reduced expression of Esr1 and increased Esr2 expression in the breast tissue of women after pregnancy correlate with a time-dependent effect of pregnancy on breast cancer risk via aberrant estrogen receptor gene expression [56]. Similarly, aberrant high expression of Esr2 was reported in primary breast cancers [57, 58] and shown to function as a carcinogenic factor [59]. The results presented here are in agreement with those findings, as Esr1 expression was decreased while Esr2 expression was increased in the mice exposed to BPA in utero compared to control mice. While exposure to estrogens has long been associated with breast cancer, our data suggest that the action of estrogens mediated through Esr1 may not be the mechanism of BPA-induced breast cancer. Estrogens may mediate the induction of breast cancer through two distinct mechanisms, separately involving Esr1 and Esr2. Esr1 modulates the mitogenic action of estrogens on breast cancer cells. Esr2 has several demonstrated mechanisms of action involving both inflammation and repression of Esr1 [60, 61]. Here, Esr1 expression was repressed while Esr2 expression was increased. Esr2 is implicated in modulating immune responses, suggesting that it is preferentially responsible for estrogen-induced changes to inflammation and immune response cascades [62, 63].
The effect of BPA on Esr2 expression may be central to the altered cytokine profile identified here, given the known immunomodulatory functions of Esr2 [38, 62–64]. Further, common variants of regulatory regions of the Esr2 gene may be involved in pre-disposition to inflammation [65, 66]. An Esr2 isoform (ERβ5) is abundantly expressed in breast tissue [67, 68]. The discovery of five Esr2 isoforms indicates that the mechanism of estrogen action is much more complex than previously understood, and the functions of estrogen receptors in the etiology of breast cancer are still under investigation. Here, BPA exposure caused aberrantly high gene expression of Esr2, likely leading to subsequent changes in the expression of inflammation mediators. These changes could subsequently affect tumor surveillance in addition to estrogen-mediated tumor proliferation.
The precise mechanism responsible for the developmental programming of inflammatory regulators was not explored here. BPA has been shown to exert estrogenic effects as a selective estrogen receptor modulator, both through classic nuclear estrogen receptors and rapid response plasma membrane-associated estrogen receptors [69]. Previously, it has been demonstrated that epigenetic alterations are induced by exposure to BPA and other estrogen-like EDCs [35, 36, 70–75]. BPA exposure specifically affects DNA methylation leading to altered programming of gene expression [35, 36, 76, 77]. BPA exposure has been demonstrated to alter the epigenetic programming of estradiol-regulated RNA HOTAIR, a long non-coding, antisense transcript responsible for gene silencing in breast cancer risk [78]. Histone-modifying enzymes have been shown to be activated by endocrine disruptors including BPA and diethylstilbestrol in cultured breast cancer cells and in the mammary glands of ovariectomized rats [79]. It is likely that epigenetic alterations affecting estrogen receptors as well as inflammatory cytokines are responsible for many of the effects observed in this study.
There are several limitations of this study. We acknowledge that the BPA levels in this model may be higher than typical average human exposure; however, the ability of any dose of BPA to program the immune state of the mammary gland is novel. Future studies will include several doses of maternal circulating BPA to determine dose response and evaluate the epigenetic mechanisms that regulates expression of these inflammatory genes. Further, future studies will fully characterize the leukocyte populations in the mammary gland.
In conclusion, we have demonstrated that BPA exposure in utero leads to modulation of several inflammatory cytokines in the mammary tissue of CD-1 mice. Fetal BPA exposure likely causes epigenetic modifications, resulting in altered developmental programming. Decreased inflammatory gene expression may correlate with a decreased defense against pre-malignant cells. The altered inflammatory cytokine environment and subsequent immune dysfunction may lead to a cellular microenvironment that promotes breast cancer development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 20 kb)
(JPG 324 kb)
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
All animal experiments were conducted in accordance with the Yale University Animal Care Committee Guidelines.
Funding
This work was supported by NIH Grant RO1 HD076422.
Conflict of Interest
The authors declare that they have no conflicts of interests.
Footnotes
Catha Fischer and Ramanaiah Mamillapalli contributed equally to this work.
References
- 1.Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311:859–860. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter CA, Taylor JA, Ruhlen RL, Welshons WV, Vom Saal FS. Estradiol and Bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007;115:902–908. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao C, Kannan K. Widespread occurrence of bisphenol A in paper and paper products: implications for human exposure. Environ Sci Technol. 2011;45:9372–9379. doi: 10.1021/es202507f. [DOI] [PubMed] [Google Scholar]
- 6.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. 2012;2012:713696. doi: 10.1155/2012/713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerona RR, Woodruff TJ, Dickenson CA, Pan J, Schwartz JM, Sen S, Friesen MW, Fujimoto VY, Hunt PA. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in mid gestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47:12477–12485. doi: 10.1021/es402764d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbel T, Gayrard V, Viguie C, Puel S, Lacroix MZ, Toutain PL, Picard-Hagen N. Bisphenol A disposition in the sheep maternal-placental-fetal unit: mechanisms determining fetal internal exposure. Biol Reprod. 2013;89:11. doi: 10.1095/biolreprod.112.106369. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa M, Iwano H, Yanagisawa R, Koike N, Inoue H, Yokota H. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ Health Perspect. 2010;118:1196–1203. doi: 10.1289/ehp.0901575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calhoun KC, Padilla-Banks E, Jefferson WN, Liu L, Gerrish KE, Young SL, Wood CE, Hunt PA, Vandevoort CA, Williams CJ. Bisphenol A exposure alters developmental gene expression in the fetal rhesus macaque uterus. PLoS One. 2014;9:e85894. doi: 10.1371/journal.pone.0085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horstman KA, Naciff JM, Overmann GJ, Foertsch LM, Richardson BD, Daston GP. Effects of transplacental 17-alpha-ethynyl estradiol or bisphenol A on the developmental profile of steroidogenic acute regulatory protein in the rat testis. Birth Defects Res B Dev Reprod Toxicol. 2012;95:318–325. doi: 10.1002/bdrb.21020. [DOI] [PubMed] [Google Scholar]
- 14.Elsworth JD, Jentsch JD, Vandevoort CA, Roth RH, DE, Leranth C. Prenatal exposure to bisphenol A impacts midbrain dopamine neurons and hippocampal spine synapses in non-human primates. Neurotoxicology. 2013;35:113–120. doi: 10.1016/j.neuro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapalamadugu KC, Vandevoort CA, Settles ML, Robison BD, Murdoch GK. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS One. 2014;9:e89096. doi: 10.1371/journal.pone.0089096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hijazi A, Guan H, Cernea M, Yang K. Prenatal exposure to bisphenol A disrupts mouse fetal lung development. FASEB J. 2015;12:4968–4977. doi: 10.1096/fj.15-270942. [DOI] [PubMed] [Google Scholar]
- 19.Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci U S A. 2012;109:8190–8195. doi: 10.1073/pnas.1120488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenberg LN, Maffini MV, Wadia PR, Sonnenschein C, Rubin BS, Soto AM. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz de Toro MM, Markey CM, Wadia PR, Luque EH, Rubin BS, et al. Perinatal exposure to bisphenol A alters peripubertal mammary gland development in mice. Endocrinology. 2005;146:4138–4147. doi: 10.1210/en.2005-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markey CM, Luque EH, Munoz de Toro MM, Sonnenschein C, Soto AM. In utero exposure to bisphenol A alters the development and tissue organization of the mouse mammary gland. Biol Reprod. 2001;65:1215–1223. doi: 10.1093/biolreprod/65.4.1215. [DOI] [PubMed] [Google Scholar]
- 23.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 24.Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, et al. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol Endocrinol. 2011;25:1915–1923. doi: 10.1210/me.2011-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Xie W, Xie C, Huang C, Zhu J, Liang Z, Deng F, Zhu M, Zhu W, Wu R, Wu J, Geng S, Zhong C. Curcumin modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced MCF-7 breast cancer cell proliferation. Phytother Res. 2014;28:1553–1560. doi: 10.1002/ptr.5167. [DOI] [PubMed] [Google Scholar]
- 26.Pupo M, Pisano A, Lappano R, Santolla MF, De Francesco EM, Abonante S, Rosano C, Maggiolini M. Bisphenol A induces gene expression changes and proliferative effects through GPER in breast cancer cells and cancer-associated fibroblasts. Environ Health Perspect. 2012;120:1177–1182. doi: 10.1289/ehp.1104526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wadia PR, Cabaton NJ, Borrero MD, Rubin BS, Sonnenschein C, et al. Low-dose BPA exposure alters the mesenchymal and epithelial transcriptomes of the mouse fetal mammary gland. PLoS One. 2013;8:e63902. doi: 10.1371/journal.pone.0063902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulose T, Speroni L, Sonnenschein C, Soto AM. Estrogens in the wrong place at the wrong time: fetal BPA exposure and mammary cancer. Reprod Toxicol. 2015;54:58–65. doi: 10.1016/j.reprotox.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26:210–219. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal Bisphenol A exposure. Reprod Toxicol. 2007;23:383–390. doi: 10.1016/j.reprotox.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, et al. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect. 2007;115:80–86. doi: 10.1289/ehp.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamartiniere CA, Jenkins S, Betancourt AM, Wang J, Russo J. Exposure to the endocrine disruptor Bisphenol A alters susceptibility for mammary cancer. Horm Mol Biol Clin Invest. 2011;5:45–52. doi: 10.1515/HMBCI.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crain DA, Janssen SJ, Edwards TM, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soto AM, Brisken C, Schaeberle C, Sonnenschein C. Does cancer start in the womb? altered mammary gland development and predisposition to breast cancer due to in utero exposure to endocrine disruptors. J Mammary Gland Biol Neoplasia. 2013;18:199–208. doi: 10.1007/s10911-013-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J. 2010;24:2273–2280. doi: 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacobuzio-Donahue CA. Epigenetic changes in cancer. Annu Rev Pathol. 2009;4:229–249. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 38.Cipelli R, Harries L, Okuda K, et al. Bisphenol A modulates the metabolic regulator oestrogen-related receptor-α in T-cells. Reproduction. 2014;147:419–426. doi: 10.1530/REP-13-0423. [DOI] [PubMed] [Google Scholar]
- 39.Barr A, Manning D (1999) G Proteins Techniques of Analysis, Manning DR, ed. Boca Raton, FL: CRC Press, Inc. 227–245. 48. Mi H, Dong Q, Muruganujan A, Gaudet P, Lewis S, Thomas PD
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 42.Markey CM, Wadia PR, Rubin BS, et al. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–1351. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 43.Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol. 2008;196:101–112. doi: 10.1677/JOE-07-0056. [DOI] [PubMed] [Google Scholar]
- 44.Owen JL, Criscitiello MF, Libreros S, et al. Expression of the inflammatory chemokines CCL2, CCL5 and CXCL2 and the receptors CCR1-3 and CXCR2 in T lymphocytes from mammary tumor-bearing mice. Cell Immunol. 2011;270:172–182. doi: 10.1016/j.cellimm.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biondo C, Mancuso G, Midiri A. The IL-1beta/CXCL1/2/neutrophil axis mediates host protection against group B streptococcal infection. Infect Immun. 2014;82:4508–4517. doi: 10.1128/IAI.02104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med. 2001;194:855–861. doi: 10.1084/jem.194.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shurin GV, Ferris R, Tourkova IL, Perez L, Lokshin A, Balkir L, Collins B, Chatta GS, Shurin MR. Loss of new chemokine CXCL14 in tumor tissue is associated with low infiltration by dendritic cells (DC), while restoration of human CXCL14 expression in tumor cells causes attraction of DC both in vitro and in vivo. J Immun. 2005;174:5490–5498. doi: 10.4049/jimmunol.174.9.5490. [DOI] [PubMed] [Google Scholar]
- 48.Kozai TD, Li X, Bodily LM, et al. Effects of caspase-1 knockout on chronic neural recording quality and longevity: insight into cellular and molecular mechanisms of the reactive tissue response. Biomaterials. 2014;35:9620–9634. doi: 10.1016/j.biomaterials.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ubertini V, Norelli G, D’Arcangelo D, et al. Mutant p53 gains new function in promoting inflammatory signals by repression of the secreted interleukin-1 receptor antagonist. Oncogene. 2014;34:2493–2504. doi: 10.1038/onc.2014.191. [DOI] [PubMed] [Google Scholar]
- 50.Normanton M, Alvarenga H, Hamerschlak N, et al. Interleukin 7 plays a role in T Lymphocyte apoptosis inhibition driven by mesenchymal stem cell without favoring proliferation and cytokines secretion. PLoS One. 2014;9:e106673. doi: 10.1371/journal.pone.0106673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribeiro D, Melao A, Barata JT. IL-7R-mediated signaling in T-cell acute lymphoblastic leukemia. Adv Biol Regul. 2013;53:211–222. doi: 10.1016/j.jbior.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Chen HZ, Guo S, Li ZZ, et al. A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J Neurosci. 2014;34:11897–11912. doi: 10.1523/JNEUROSCI.1545-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michelucci A, Cordes T, Ghelfi J, et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci U S A. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang PX, Zhang R, Huang L. Interferon regulatory factor 9 is a key mediator of hepatic ischemia/reperfusion injury. J Hepat. 2014;62:111–120. doi: 10.1016/j.jhep.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 55.Hall CJ, Boyle RH, Astin JW, et al. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ROS production. Cell Metab. 2013;18:265–278. doi: 10.1016/j.cmet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Asztalos S, Gann PH, Hayes MK, et al. Gene expression patterns in the human breast after pregnancy. Cancer Prev Res (Phila) 2010;3:301–311. doi: 10.1158/1940-6207.CAPR-09-0069. [DOI] [PubMed] [Google Scholar]
- 57.Balfe P, McCann A, McGoldrick A, et al. Estrogen receptor alpha and beta profiling in human breast cancer. Eur J Surg Oncol. 2004;30:469–474. doi: 10.1016/j.ejso.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 58.Baek JM, Chae BJ, Song BJ. The potential role of estrogen receptor β2 in breast cancer. Int J Surg. 2015;14:17–22. doi: 10.1016/j.ijsu.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Hu YF, Lau KM, Ho SM, et al. Increased expression of estrogen receptor beta in chemically transformed human breast epithelial cells. Int J Oncol. 1998;12:1225–1228. doi: 10.3892/ijo.12.6.1225. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Chen L, Li JY, et al. ERbeta and PEA3 co-activate IL-8 expression and promote the invasion of breast cancer cells. Cancer Biol Ther. 2011;11:497–511. doi: 10.4161/cbt.11.5.14667. [DOI] [PubMed] [Google Scholar]
- 61.Williams C, Edvardsson K, Lewandowski SA, et al. A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells. Oncogene. 2008;27:1019–1032. doi: 10.1038/sj.onc.1210712. [DOI] [PubMed] [Google Scholar]
- 62.Polanczyk M, Yellayi S, Zamora A, et al. Estrogen receptor-1 (Esr1) and -2 (Esr2) regulate the severity of clinical experimental allergic encephalomyelitis in male mice. Am J Pathol. 2004;164:1915–1924. doi: 10.1016/S0002-9440(10)63752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yakimchuk K, Jondal M, Okret S. Estrogen receptor α and β in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol. 2013;375:121–129. doi: 10.1016/j.mce.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Melzer D, Harries L, Cipelli R, et al. Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ Health Perspect. 2011;119:1788–1793. doi: 10.1289/ehp.1103809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armstrong CM, Billimek AR, Allred KF, et al. Allred CDA novel shift in estrogen receptor expression occurs as estradiol suppresses inflammation-associated colon tumor formation. Endocr Relat Cancer. 2013;20:515–525. doi: 10.1530/ERC-12-0308. [DOI] [PubMed] [Google Scholar]
- 66.Ashworth JJ, Smyth JV, Pendleton N, et al. Polymorphisms spanning the 0N exon and promoter of the estrogen receptor-beta (ERbeta) gene ESR2 are associated with venous ulceration. Clin Genet. 2008;73:55–61. doi: 10.1111/j.1399-0004.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 67.Moore JT, McKee DD, Slentz-Kesler K, et al. Cloning and characterization of human estrogen receptor beta isoforms. Biochem Biophys Res Commun. 1998;247:75–78. doi: 10.1006/bbrc.1998.8738. [DOI] [PubMed] [Google Scholar]
- 68.Poola I, Fuqua SA, Witty RL, et al. Estrogen receptor alpha-negative breast cancer tissues express significant levels of estrogen-independent transcription factors, ERbeta1 and ERbeta5: potential molecular targets for chemoprevention. Clin Cancer Res. 2005;11:7579–7585. doi: 10.1158/1078-0432.CCR-05-0728. [DOI] [PubMed] [Google Scholar]
- 69.Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147 S:56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- 70.Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer. 2010;1:146–155. doi: 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peretz J, Vrooman L, Ricke WA, et al. Bisphenol a and reproductive health: update of experimental and human evidence 2007-2013. Environ Health Perspect. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril. 2011;96:175–179. doi: 10.1016/j.fertnstert.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- 74.Akbas GE, Song J, Taylor HS. A HOXA10 estrogen response element (ERE) is differentially regulated by 17 beta-estradiol and diethylstilbestrol (DES) J Mol Biol. 2004;340:1013–1023. doi: 10.1016/j.jmb.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 75.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing müllerian system. FASEB J. 2000;200014:1101–1108. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 76.Kim JH, Sartor MA, Rozek LS, et al. Perinatal bisphenol A exposure promotes dose-dependent alterations of the mouse methylome. BMC Genomics. 2014;15:30. doi: 10.1186/1471-2164-15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nahar MS, Kim JH, Sartor MA, Dolinoy DC. Bisphenol A-associated alterations in the expression and epigenetic regulation of genes encoding xenobiotic metabolizing enzymes in human fetal liver. Environ Mol Mutagen. 2014;55:184–195. doi: 10.1002/em.21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ko KP, Kim SW, Ma SH, et al. Dietary intake and breast cancer among carriers and noncarriers of BRCA mutations in the Korean Hereditary Breast Cancer Study. Am J Clin Nutr. 2013;98:1493–1501. doi: 10.3945/ajcn.112.057760. [DOI] [PubMed] [Google Scholar]
- 79.Bhan A, Hussain I, Ansari KI, Bobzean SA, Perrotti LI, Mandal SS. Histone methyltransferase EZH2 is transcriptionally induced by estradiol as well as estrogenic endocrine disruptors bisphenol-A and diethylstilbestrol. J Mol Biol. 2014;426:3426–3441. doi: 10.1016/j.jmb.2014.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 20 kb)
(JPG 324 kb)