Abstract
Objective
Dysregulated appetite control is characteristic of anorexia nervosa (AN), bulimia nervosa (BN), and obesity (OB). Studies using a broad range of methods suggest the cerebellum plays an important role in aspects of weight and appetite control, and is implicated in both AN and OB by reports of aberrant gray matter volume (GMV) compared to nonclinical populations. As functions of the cerebellum are anatomically segregated, specific localization of aberrant anatomy may indicate the mechanisms of its relationship with weight and appetite in different states. We sought to determine if there were consistencies in regions of cerebellar GMV changes in AN/BN and OB, as well as across normative (NOR) variation.
Method
Systematic review and meta‐analysis using GingerALE.
Results
Twenty‐six publications were identified as either case–control studies (n OB = 277; n AN/BN = 510) or regressed weight from NOR data against brain volume (total n = 3830). AN/BN and OB analyses both showed consistently decreased GMV within Crus I and Lobule VI, but volume reduction was bilateral for AN/BN and unilateral for OB. Analysis of the NOR data set identified a cluster in right posterior lobe that overlapped with AN/BN cerebellar reduction. Sensitivity analyses indicated robust repeatability for NOR and AN/BN cohorts, but found OB‐specific heterogeneity.
Discussion
Findings suggest that more than one area of the cerebellum is involved in control of eating behavior and may be differentially affected in normal variation and pathological conditions. Specifically, we hypothesize an association with sensorimotor and emotional learning via Lobule VI in AN/BN, and executive function via Crus I in OB.
Keywords: anorexia nervosa, appetite regulation, cerebellum, eating disorders, meta‐analysis, obesity, review, voxel‐based morphometry
This meta‐analysis reviewed cerebellar volume in 26 publications comprising of case–control and normative cohorts. Findings identified differential reduction of the cerebellum across conditions, suggesting multiple cerebellar regions are implicated in the dysregulation of appetite or feeding behavior.
1. INTRODUCTION
1.1. Importance of studying appetite control mechanisms
Appetite control has a complex and multifaceted nature, and problems with eating behavior arise from a variety of genetic, cognitive, emotional, and physiological factors. Irregular appetite patterns result in abnormal body weight for height (indexed by the body mass index [BMI]) as well as irregular metabolic/mental health (Alhussain et al., 2016; Buyukkurt et al., 2021; Farshchi et al., 2005). For instance, obesity (OB), characterized by a BMI of >30, is a major public health concern pertaining to appetite dysregulation that is on the increase and constitutes as a major risk factor for conditions such as hypertension, diabetes, cardiovascular diseases, and cancer (Abell et al., 2007; Arroyo‐Johnson & Mincey, 2016; Zhang & Rodriguez‐Monguio, 2012). In the past 35 years, worldwide OB prevalence rates have nearly doubled, with 13% classifying with OB (WHO, 2014). At the opposite end of the BMI scale are individuals with anorexia nervosa (AN) who refrain from eating and harbor pathological fears of weight gain and food consumption (APA, 2013). AN is a complex multidimensional eating disorder characterized by pathologically decreased weight‐for‐age/height, with lifetime prevalence as high as 4% (Smink et al., 2013). Recently, publications found an increase in reported AN cases over time, although increased incidence may correlate with increased specificity of reporting protocols (Erskine et al., 2016; Sweeting et al., 2015). AN is relatively uncommon compared to other psychiatric disorders, yet mortality rates are greater, reporting between 2% and 6% (Arcelus et al., 2011; Wakeling, 1996). While AN and OB may not technically be eating disorders at opposite ends of a singular spectrum, there are prominent neuroanatomical (Allen et al., 2005; Amianto, D'Agata, et al., 2013; Augustijn et al., 2019; Boghi et al., 2011; Brooks et al., 2011; García‐García et al., 2019; Kakoschke et al., 2019; Leggio & Olivito, 2018; Martín‐Pérez et al., 2019; Milos et al., 2021; Nagahara et al., 2014; Sanders et al., 2015), metabolic, and genetic (Bulik et al., 2019; Chen et al., 2020; Fawcett & Barroso, 2010; Watson et al., 2019; Yang et al., 2017) factors linking both disorders. It is therefore of significance for research to identify mechanisms and neuroanatomical structures regulating appetite or weight, which may serve as treatment targets.
Despite these priorities, roles of the cerebellum (displayed in Figure S1; Diedrichsen & Zotow, 2015) in body weight or appetite control (Mendoza et al., 2010; Tuulari et al., 2016; Zhu & Wang, 2008) receive surprisingly little attention in appetite‐related research, despite their consistent implication. Traditionally, the cerebellum was thought to solely serve motor coordination and somatic functions, but further investigation reveals that this brain region plays a variety of diverse roles. Researchers have reported the cerebellum demonstrates organizational similarity to that of the cerebral cortex (Herrup, 2000) with anatomically segregated functions. Cerebellar contributions to intrinsic connectivity networks have shown that particular regions of the cerebellum are distinctly involved in different cognitive functions (Habas et al., 2009; Stoodley, 2012; Stoodley & Schmahmann, 2009) and implicated in five intrinsic connectivity networks, including the executive control network (ECN; via Lobule VIIB; Crus I/II), default‐mode network (via Lobule IX), salience network (via Lobule VI), and sensorimotor network (via Lobule VI) (Habas et al., 2009). Contrasting traditional conceptualization, motor tasks are largely consigned to Lobule VIIIa/b and represent limited cerebellar functionality (Stoodley & Schmahmann, 2009). Current views of the cerebellum implicate it in homeostatic regulation (Saker et al., 2014; Supple Jr. & Kapp, 1993), executive/cognitive functionality (Buckner, 2013; Contreras‐Rodríguez et al., 2017; D‘Angelo & Casali, 2013; Habas et al., 2009; Koziol et al., 2014; Leiner et al., 1986; Stoodley & Schmahmann, 2009; Stoodley et al., 2012) (including habit formation [D‘Angelo & Casali, 2013], conditioning behaviors [D‘Angelo & Casali, 2013; Dolan, 1998; Habas et al., 2009, 2013; Molinari et al., 2008; Utz et al., 2015], procedural knowledge storage [Dolan, 1998; Habas et al., 2009, 2013; Zhu et al., 2011], working memory function [Molinari et al., 2008; Stoodley & Schmahmann, 2009], and cravings [Carnell et al., 2014]), and emotional regulation (Adamaszek et al., 2017; Baumann & Mattingley, 2012; Buckner, 2013; Habas et al., 2009, 2013; Lupo et al., 2015; Schmahmann & Pandya, 1997; Shobe, 2014; Stoodley & Schmahmann, 2009; Utz et al., 2015). Importantly, evidence suggests the cerebellum may participate in aspects of food intake and appetite control through multiple mechanisms, including physiological (i.e., feeding circuit connectivity, response/influence on gut hormones/neurotransmitters), cognitive (i.e., food palatability, feeding‐related memories), and emotional (i.e., food‐related cravings) means (Cavdar et al., 2001; Wright et al., 2016).
1.2. A cerebellar role in weight and appetite regulation
On a physiological scale, the cerebellum interacts via extensive signaling networks with the hypothalamus and insula, which both contain networks specific to food intake (Cavdar et al., 2001; Contreras‐Rodríguez et al., 2017) via neural and hormonal mechanisms (Allen et al., 2005; Contreras‐Rodríguez et al., 2017; Zhao et al., 2017). Enteric nervous system gut hormones, such as leptin and ghrelin, interactively modulate regions of the brain associated with food intake control including the cerebellum, hypothalamus, and brainstem (Bouret et al., 2004; Hommel et al., 2006). In response to ghrelin, cerebellar activation decreases and likely works to stimulate appetite via ghrelin‐induced suppression of satiety hormones, such as cholecystokinin, within vagal afferent neurons (Al Massadi et al., 2017; Gavello et al., 2016; Jones et al., 2012). Recently, Choe et al. (2021) investigated synchrony between basal gastric rhythm (governing both vagal activity and peristalsis) and brain activity, identifying the strongest phase‐locked synchrony within the cerebellum (Choe et al., 2021) that suggests a more direct relationship between gastric mechanisms and cerebellar functionality.
The cerebellum is also implicated in genetic aspects associated with body weight disorders. Beyond neuroanatomical/biological aspects of appetite dysregulation, disorders on the extremes of the standard BMI measure (i.e., AN/OB) exhibit shared genetic and metabolic correlations (Chen et al., 2020; Fawcett & Barroso, 2010; Watson et al., 2019; Yang et al., 2017). With genetic associations occurring in opposing directions, they have been termed metabolic “mirror images” of one another (Bulik et al., 2019). Cerebellar tissues and pathways have recently been implicated in aspects of genetic risk for both conditions (Cheng et al., 2020; Watson et al., 2019). Evidence from AN‐ and cerebellum‐related studies suggests that deficits in cerebellar mRNA expression occur in fetal and early‐life AN pathogenesis (Cheng et al., 2020), and altered cerebellar volume may explain body image disturbances in AN (Briatore et al., 2020; Gaudio et al., 2011; Leibovitz et al., 2018; Watson et al., 2019). In OB, a multitude of genes have been associated with increased risk, predominantly the fat mass (FTO) and melanocortin‐4 receptor genes. FTO is expressed in regions such as the hypothalamus, hippocampus, and cerebellum (Madsen et al., 2009; McTaggart et al., 2011; Miller et al., 2009; Wang et al., 2012), and research suggests it is highly associated with OB outcome (Fawcett & Barroso, 2010; Yang et al., 2017).
The cerebellum is also repeatedly reported to participate in neurobiological modulation of dopaminergic and serotonergic signaling (Cutando et al., 2022; Oostland & Hooft, 2016), which becomes dysregulated in conditions of abnormal weight/BMI (Berridge et al., 2010; Bohon, 2017; Carter et al., 2016; Kawakami et al., 2022; Kaye et al., 2013; van Galen et al., 2018). Dopaminergic‐related mechanisms driving both OB and AN have also been associated with addiction‐resembling behavior related to eating behavior (Barbarich‐Marsteller et al., 2011; Berridge, 2009; O'Hara et al., 2015). Recently, Low et al. (2021) used a reverse‐translational approach to identify a cerebellar‐based satiation network. In humans, food cues activate cerebellar output neurons to promote satiation through reductions in phasic dopaminergic responses to food. As such, the cerebellum is likely implicated in altered neurobiological mechanisms associated with dysregulated appetite or weight, such as those with AN/OB.
Importantly, extant literature also suggests that the cerebellum may participate in more conscious domains of appetite, such as emotional and cognitive aspects driving appetitive behaviors. The cerebellum may play a substantive role in cognitive aspects of appetite control via an individuals’ subjective feeling of craving or outcome expectation built off previous experience and memory (Carnell et al., 2014; Garrison et al., 2016; Geliebter et al., 2016; Moreno‐Rius & Miquel, 2017; Noori et al., 2016; Tomasi et al., 2015). The cerebellum is also associated with traits of impulsivity and loss‐of‐control (LOC) eating. Recent investigations also unveiled cerebellar roles in the portion size effect (PSE) (Herman et al., 2015; Rolls et al., 2006; Steenhuis & Poelman, 2017), the phenomenon where more is eaten when large quantities of food are available (English et al., 2019).
1.3. Cerebellar volume and dysregulation of appetite
Structural associations between the cerebellum and conditions of dysregulated BMI and appetite have been consistently documented. This includes evidence from loss‐of‐function studies (Zhu & Wang, 2008), as well as animal studies where lesions or removal of cerebellar hemispheres leads to reduced appetite, pathological weight loss, and increased mortality rate (Colombel et al., 2002). In humans, Oya et al. (2014) report a high proportion of cerebellar tumor detection with associated AN. Similarly, cerebellar degeneration associated with ataxia correlates with increased likelihood of being underweight or experiencing abnormal appetite (Kronemer et al., 2021; Rönnefarth et al., 2020; Ross et al., 2015; Sánchez‐Kuhn et al., 2017). Due to appetite disturbances emerging post‐cerebellar structural abnormalities, these studies indicate that cerebellar deficits could play a causative role in the loss of appetite.
Cerebellar volume has also been directly associated with conditions of under‐ and overeating. Excess weight (BMI > 25.5) or OB (BMI > 30.0) has been associated with both increased (Brooks et al., 2011) and decreased concentrations of gray matter volume (GMV) in the left (Brooks et al., 2011), bilateral (Kakoschke et al., 2019), and bilateral posterior (García‐García et al., 2019) cerebellum. Prefronto‐cerebellar circuits, implicated in cognition, emotion, executive function, and error detection, exhibit volume reduction in those with OB (Allen et al., 2005; Brooks et al., 2011). Recovery from OB is also associated with positive changes in cerebellar volume and is thought to be important in treatment as well as conditioning of eating behavior (Augustijn et al., 2019). Differences in cerebellar volume have been noted in both rat and human models of AN (Fagundo et al., 2012; Moyse et al., 2019; O'Hara et al., 2015). Within humans, cerebellar atrophy and cellular loss are associated with AN disease duration and poor treatment success (Milos et al., 2021), persist after weight recovery, and are suggested to play a role in maintaining a low body weight (Boghi et al., 2011; Nagahara et al., 2014). A multimodal meta‐analysis by Zhang et al. (2018) identified bilateral reduction of the cerebellum in those with AN, suggested to be associated with symptoms or traits of dietary restriction and appetitive inflexibility (Zhang et al., 2018). A systematic volumetric review by Seitz et al. (2018) also demonstrated that volumetric atrophy of the cerebellum upon assessment predicted AN outcome at 1‐year follow‐up.
Altogether, the cerebellum may function as a pivotal point of behavioral or associative tuning in relation to cognitive aspects of appetite regulation. Schmahmann et al. (2019) identified associations between the posterior cerebellar lobes and cognition, proposing “The Dysmetria of Thought” (DoT) theory. This theory suggests that, by receiving multimodal inputs and establishing procedurally optimized behavioral modulation, the cerebellum modulates cognition in a similar way to which it smooths and fine‐tunes coordination of motor behavior. In cases of DoT, the cerebellum may perform similarly regarding altered input and integration of appetite‐related stimuli, such as formation of rigid, repetitive, or inflexible individualized food‐related models, memories, and expectations formed from previous interactions with food stimuli. Cerebellar regions attending to salience of stimuli, such as Lobule VI, may be associated with body weight/appetite changes via formations of negative associations with food that could contribute toward dietary restriction or rigidity. Alternatively, cognitive/executive abnormalities reported in those with OB may suggest associations with cerebellar regions prominent to the ECN, such as Crus I, where the reward value or experiential engagement with food stimuli may be too “positively” integrated, contributing toward repetitive engagement with stimuli to prolong or increase the frequency of a rewarding experience.
1.4. Aims of study
Evidence provided by wide‐ranging cerebellar research demonstrates that the cerebellum is important in modulating aspects of appetite control and implicated in both OB and AN. However, whether identical or differing areas of the cerebellum are associated with AN and OB is unclear. This systematic review aimed to examine cerebellar anatomy reported in case–control AN and OB literature, as well as across the weight range via normative (NOR)/nonclinical populations to investigate whether cerebellar structure differs across body weight states and disorders. We generated two alternative hypotheses: We first hypothesized that a singular area of the cerebellum, such as Crus I (implicated in executive control circuits [Augustijn et al., 2019]), would be associated with abnormal BMI conditions, and that respective regions affected in OB and AN would be similar. Our alternative, competing hypothesis was that separate cerebellar regions would be altered across conditions and shown in distinct regions. For instance, Crus I volume could be primarily affected in OB, while Lobule VI volume (implicated in salience/sensorimotor circuits) could be primarily affected in AN.
2. METHODOLOGY
2.1. Selection of literature
Literature was searched on June 29, 2022 using SCOPUS, PubMed Central (PMC), and Web of Science (WoS) using identical search criteria. SCOPUS identified 2638 publications involving cerebellar volume during OB—search criteria: (cerebell* AND obesity AND MRI) and 572 publications regarding AN (cerebell* AND anorexia AND MRI). PMC identified 4950 publications relating to OB, as well as 461 papers regarding AN. Lastly, 2588 and 233 papers were identified through WoS regarding cerebellar characteristics in those with OB and AN, respectively. Inclusion criteria involved presence of key phrases utilized in search, including “gray literature,” or findings produced outside of traditional publishing. Exclusion criteria were as follows: (1) publications older than 10 years, as CB imaging methods have significantly improved since 2010 (Gutierrez et al., 2014; Sirin et al., 2005); (2) animal studies; (3) publications not reporting case–control studies; (4) inclusion of clinical groups/correlations unrelated to the study scope; (5) publications not utilizing voxel‐based morphometry (i.e., diffusion tensor imaging/functional MRI [fMRI] studies); and (6) publications with results not reported in Talairach/MNI coordinates. Individual studies fitting criteria were additionally collected from meta‐analyses (n = 3).
Two publications (Huang et al., 2019; Frank et al., 2013) could not be further assessed as findings consisted of increased cerebellar volume/positive correlations, and insufficient positive coordinate‐based data were available across cohorts to generate meta‐analytic positive CB findings. Three AN publications (Joos et al., 2010; Amianto, Caroppo, et al., 2013; D'Agata et al., 2015) included individuals with bulimia nervosa (BN) that contributed to AN findings. As both conditions report with significant diagnostic crossover, with 34.1% of AN individuals experiencing crossover to BN over a 7‐year follow‐up (Eddy et al., 2008), these papers were included to form the AN/BN cohort. Due to the limited literature, we were unable to correct for age and gender across data sets.
In summary, six OB (Dommes et al., 2013; Jauch‐Chara et al., 2015; Mueller et al., 2012; Ou et al., 2015; Shan et al., 2019; Wang et al., 2017) and 11 AN/BN (Amianto, Caroppo, et al., 2013; Bomba et al., 2015; D'Agata et al., 2015; Fonville et al., 2014; Gaudio et al., 2011; Joos et al., 2010; Lenhart et al., 2022; Mishima et al., 2021; Phillipou et al., 2018) papers included coordinates and were selected for final analysis (participant numbers: n case–control = 787; n OB = 134 vs. n HC = 143; n AN = 228 and n BN = 48 vs. n HC = 234) (Table 1; Figure 1). As an exploratory assessment of condition‐specific effects, an AN‐only cohort was comprised by excluding three previously included AN papers (Amianto, Caroppo, et al., 2013; D'Agata et al., 2015; Joos et al., 2010) evaluating those with BN in conjunction with AN (eight papers; n AN = 178 vs. n HC = 185).
TABLE 1.
Sociodemographic and statistical data for included studies across AN/BN (n = 11), OB (n = 6), and NOR (n = 10) cohorts.
| First author | Number of subjects | Age, years (mean ± SD) | BMI (mean ± SD) | DOI, M. (mean ± SD) | Correction type | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AN/BN a | n AN | n HC | n BN | M:F ratio | AN | HC | BN | A b | AN | HC | BN | AN | BN | FWE c | Threshold |
| Lenhart, 2022 | 22 | 18 | 0 | 0:40 | 15.2 ± 1.2 | 16.8 ± 0.9 | N/A | 0 | 15.4 ± 1.4 | 21.2 ± 1.0 | N/A | 9.4 ± 6.8 | N/A | 1 | p < .05 |
| Mishima, 2021 | 35 | 35 | 0 | 0:70 | 36.3 ± 10.0 | 36.0 ± 9.6 | N/A | 1 | 14.2 ± 2.5 | 21.0 ± 2.9 | N/A | 188.4 ± 108.0 | N/A | 1 | p < .05 |
| Phillipou, 2018 | 26 | 27 | 0 | 0:53 | 22.8 ± 6.7 | 22.5 ± 3.2 | N/A | 1 | 16.6 ± 1.2 | 22.6 ± 3.5 | N/A | 77.0 ± 89.2 | N/A | 1 | 1.68 |
| D'Agata, 2015 a | 21 | 17 | 18 | 0:56 | 21.0 ± 5.0 | 22.0 ± 5.0 | 23.0 ± 4.0 | 1 | 16.1 ± 0.9 | 21.5 ± 2.3 | 22.0 ± 2.3 | >24.0 | >24.0 | 1 | 1.71 |
| Fonville, 2014 | 33 | 33 | 0 | N/A | 23.0 ± 10.0 | 25.0 ± 4.0 | N/A | 1 | 15.8 ± 1.4 | 21.8 ± 1.8 | N/A | 84.0 ± 120.0 | N/A | 1 | 1.67 |
| Amianto, Caroppo, et al., 2013, a | 17 | 14 | 13 | 0:44 | 20.0 ± 4.0 | 24.0 ± 3.0 | 22.0 ± 3.0 | 1 | 16.0 ± 1.0 | 21.0 ± 2.0 | 22.0 ± 2.0 | 13.0 ± 8.0 | 10.0 ± 5.0 | 1 | 1.7 |
| Bomba, 2013 | 11 | 8 | 0 | 0:19 | 13.6 ± 2.8 | 13.4 ± 2.4 | N/A | 0 | 12.8 ± 0.8 | 19.9 ± 1.5 | N/A | 14.5 ± 10.9 | N/A | 1 | 1.78 |
| Brooks, 2011 | 14 | 21 | 0 | 0:35 | 26.0 ± 1.9 | 26.0 ± 2.1 | N/A | 1 | 15.6 ± 0.4 | 21.4 ± 0.5 | N/A | 110.4 ± 22.8 | N/A | 1 | 1.69 |
| Boghi, 2011 | 21 | 27 | 0 | 0:48 | 29.0 ± 10.0 | 30.8 ± 8.7 | N/A | 1 | 15.5 ± 1.8 | 21.9 ± 1.5 | N/A | 11.3 ± 12.1 | N/A | 1 | 1.7 |
| Gaudio, 2011 | 16 | 16 | 0 | 0:32 | 15.2 ± 1.7 | 15.1 ± 1.5 | N/A | 0 | 14.2 ± 1.4 | 20.2 ± 1.6 | N/A | 5.3 ± 3.2 | N/A | 0 | p < .001 |
| Joos, 2010 a | 12 | 18 | 17 | 0:47 | 25.0 ± 4.8 | 26.9 ± 5.8 | 24.5 ± 4.8 | 1 | 16.0 ± 1.2 | 21.1 ± 2.0 | 21.1 ± 2.5 | 56.4 ± 43.2 | 90.0 ± 68.4 | 0 | 3.1 |
| OB | n OB | n HC | – | M:F ratio | OB | HC | – | A b | OB | HC | – | – | – | FWE c | Threshold |
| Shan, 2019 | 37 | 39 | – | 41:35 | 27.8 ± 6.9 | 26.7 ± 6.8 | – | 1 | 40.0 ± 6.5 | 21.8 ± 1.8 | – | – | – | 1 | 1.67 |
| Wang, 2017 | 31 | 49 | – | 13:7 | 39.6 ± 1.9 | 29.6 ± 1.4 | – | 1 | 34.4 ± 0.7 | 21.9 ± 0.3 | – | – | – | 0 | 2.64 |
| Ou, 2015 | 12 | 12 | – | 1:1 | 9.1 ± 0.9 | 9.8 ± 0.7 | – | 0 | 24.4 ± 3.4 | 5.8 ± 1.0 | – | – | – | 1 | 1.73 |
| Jauch‐Chara, 2015 | 15 | 15 | – | 30:0 | 24.7 ± 0.7 | 24.6 ± 0.7 | – | 1 | 36.3 ± 1.0 | 23.2 ± 0.4 | – | – | – | 1 | 1.7 |
| Dommes, 2013 | 12 | 12 | – | 0:24 | 29.3 ± 7.3 | 27.5 ± 4.3 | – | 1 | 36.3 ± 4.8 | 20.9 ± 1.7 | – | – | – | 1 (FDR) | p < .05 |
| Mueller, 2012 | 27 | 16 | – | 22:21 | 26.4 ± 5.4 | 24.8 ± 3.0 | – | 1 | 33.0 ± 6.4 | 22.5 ± 2.0 | – | – | – | 1 | 1.68 |
| NOR | n OB | n HC | – | M:F ratio | OB | HC | – | A b | OB | HC | BMI range | – | FWE c | Threshold | |
| Weise, 2019 (Cohort a ) | 0 | 136 | – | 11:23 | N/A | 29.6 ± 3.2 | – | 1 | N/A | 27.5 ± 4.9 | N/A | – | – | 1 | 1.66 |
| Weise, 2019 (Cohort b ) | 0 | 306 | – | 20:31 | N/A | 29.4 ± 3.4 | – | 1 | N/A | 26.3 ± 4.7 | N/A | – | – | 1 | 1.65 |
| Huang, 2019 | 0 | 653 | – | 204:449 | N/A | 19.5 ± 1.5 | – | 1 | N/A | 20.8 ± 2.7 | 15.4–34 | – | – | 1 | p < .05 |
| Yao, 2016 | 0 | 109 | – | 62:47 | N/A | 35.2 ± 11.2 | – | 1 | N/A | 27.6 ± 6.1 | <18‐>30 | – | – | 0 | p = .001 |
| Figley, 2016 | 0 | 32 | – | 1:1 | N/A | 26.2 ± 4.4; 23.5 ± 4.2 | – | 1 | N/A | N/A | <18–25 | – | – | 1 | p < .05 |
| Masouleh, 2016 | 0 | 617 | – | 359:258 | N/A | 68.7 ± 4.6 | – | 1 | N/A | 27.5 ± 4 | 16.8‐41.4 | – | – | 1 | 3.1 |
| Janowitz, 2015 | 0 | 2344 | – | 1087:1257 | N/A | 49.8 ± 9.3; 46.3 ± 11.3 | – | 1 | N/A | 27.4 ± 4.5; 27.2 ± 4.4 | N/A | – | – | 1 | 1.65 |
| Kurth, 2013 | 0 | 115 | – | 54:61 | N/A | 45.2 ± 15.5 | – | 1 | N/A | 25.0 ± 4.1 | 18.2–42.3 | – | – | 1 | 1.66 |
| Weise, 2013 | 0 | 76 | – | 13:6 | N/A | 32.1 ± 8.8 | – | 1 | N/A | 29.8 ± 8.9 | <25‐>30 | – | – | 1 | p < .05 |
| Walther, 2010 | 0 | 95 | – | 0:95 | N/A | 69.3 ± 9.3 | – | 1 | N/A | 28.3 ± 2.1 | <22‐>30 | – | – | 0 | p < .001 |
Abbreviations: AN, anorexia nervosa; BMI, body mass index; BN, bulimia nervosa; FDR, false discovery rate; FWE, family‐wise error; HC, healthy control; M/F, male/female; OB, obesity.
Publications utilized in AN/BN cohort analysis, but excluded from exploratory AN‐only analysis.
Adult sample present: 1, yes; 0, no.
Correction for family‐wise error; 1, yes; 0, no.
FIGURE 1.
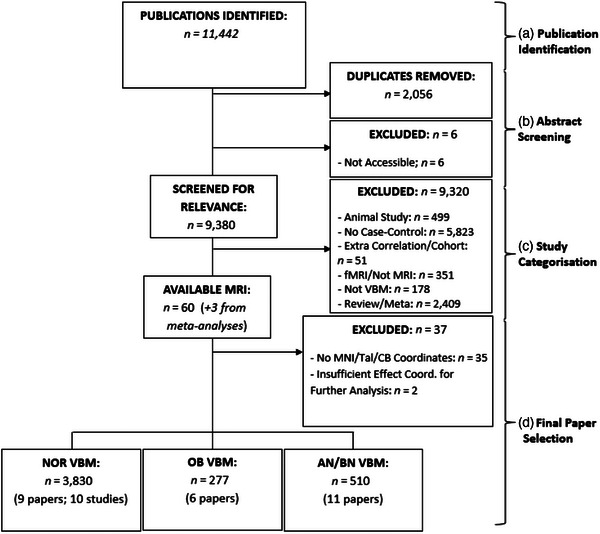
Flowchart depicting the identification (a), abstract screening (b), categorization (c), and final selection (d) of papers. Three papers were also found from meta‐analyses (Dommes et al., 2013; Huang et al., 2019; Weise et al., 2019). AN, anorexia nervosa; BN, bulimia nervosa; CB, cerebellum; Coord., coordinates; fMRI, functional magnetic resonance imaging; MNI, Montreal Neurological Institute; MRI, magnetic resonance imaging; NOR, normative; OB, obesity; Tal, Talairach; VBM, voxel‐based morphometry.
While conducting the OB literature search, publications were identified evaluating correlations between BMI and GMV in nonclinical, NOR populations that fell in line with remaining inclusion criteria (n = 9 papers; 10 studies) (Figley et al., 2016; Janowitz et al., 2015; Kurth et al., 2013; Masouleh et al., 2016; Walther et al., 2010; Weise et al., 2019, 2013; Yao et al., 2016). As authors within the NOR subgroup predominantly conducted recruitment using community‐based methods and data repositories, participant aggregation generated larger sample sizes than the OB and AN/BN literature. These publications evaluated weight states across the BMI spectrum, with all but one (Figley et al., 2016) study (n = 8/9 papers) including individuals with excess weight, and all but three (Figley et al., 2016; Janowitz et al., 2015; Weise et al., 2019) studies (n = 6/9 papers) including those with OB. Papers were included in a separate NOR data set for analysis (participant number = 3830) for a total participant sample size of n = 4617.
2.2. Voxel‐based morphometry and ALE analysis
Cerebellar coordinates (values depicted in Table S1) were respectively incorporated into GingerALE, an activation likelihood estimate (ALE) meta‐analysis software using both Talairach and MNI space (Eickhoff et al., 2009). GingerALE converted Talairach coordinates to MNI space using the icbm2tal transform. The p‐value thresholds for individual analyses were conducted at the whole‐brain level and corrected for family‐wise error (FWE; .05) at 1000 permutations and set to p < .01. First, individual observations on respective AN/BN (n = 510) and OB (n = 277) cohorts were conducted, with a third analysis investigating negative correlations between BMI and cerebellar volume within an NOR data set (n = 3830). As an additional analysis, the AN data set was reassessed upon exclusion of publications (Amianto, Caroppo, et al., 2013; D'Agata et al., 2015; Joos et al., 2010) evaluating volumetric differences in BN (AN‐only) but not further investigated for overlap. Condition‐respective findings were assessed for overlap via logical overlays and conjunction analyses to visualize combinatorial clusters within AN/BN‐OB, AN/BN‐NOR, and OB‐NOR data sets. Jackknife analyses were conducted to assess robustness of findings via identical reassessment of cohort data upon one‐by‐one omission of publications. Additional information regarding cerebellar parcellation, subregion locations, and conjunction analysis thresholding is described within the Supporting Information.
3. RESULTS
3.1. Volumetric reduction in OB
Within OB studies, volume of the cerebellum was found to be decreased in the left cerebellar hemisphere, with no bilateral effect. The cluster contained a volume of 3.58 cm3 with a cluster center of −27, −56, −31. Analysis revealed four peaks of significance partially anteriorly and posteriorly located (Table 2). Specific cerebellar regions affected are Lobule VI, Crus I, and the dentate gyrus (Figure 2).
TABLE 2.
Coordinates of significance in OB studies (n = 6).
| Region (OB < HC) | MNI coordinates | Volume (cm3) | ALE score | p | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L CB | –27 | –56 | –31 | 3.58 | .00960 | 5.89 × 10–5 | 3.85 |
| L CB, anterior L. | –32 | –56 | –28 | .00956 | 5.89 × 10–5 | 3.85 | |
| L CB, anterior L. | –30 | –60 | –26 | .00941 | 6.13 × 10–5 | 3.84 | |
| L CB, posterior L. | –28 | –54 | –34 | .00920 | 1.36 × 10–4 | 3.64 | |
| L CB, posterior L. | –18 | –54 | –34 | .00878 | 2.27 × 10–4 | 3.51 | |
Abbreviations: ALE, activation likelihood estimation; CB, cerebellum; HC, healthy control; L, left; L., lobe; MNI, Montreal Neurological Institute; OB, obesity; R, right.
FIGURE 2.
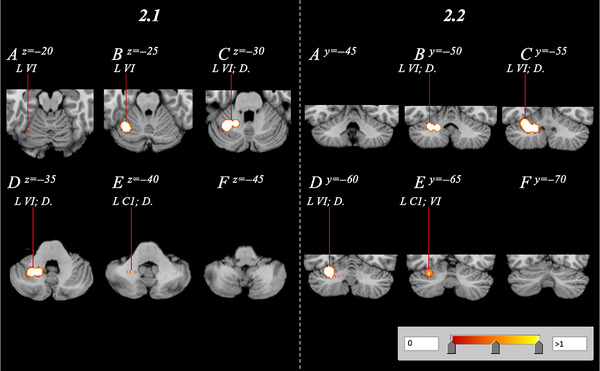
Cerebellar volume reduction of obesity subjects relative to healthy controls, spanning the right cerebellum with a range of z = −20 to −40 and y = −50 to −65 in both axial (2.1A–F) and coronal (2.2A–F) orientations. C1, Crus 1; D., dentate; L, left; R, right; VI, Lobule 6.
3.2. Volumetric reduction in AN/BN
The AN/BN GingerALE analysis revealed two significant clusters displaying bilateral reduction of cerebellar volume. The larger cluster was located within the left posterior lobe and contained a volume of 7.09 cm3 with a cluster center of −25, −55, −31. The region contained an additional seven peaks, with four of these occurring in the posterior lobe (Table 3). The smaller cluster comprising 4.56 cm3 was located in the right hemisphere, with three of five peaks located in the anterior lobe. Identified subregions included Crus I, the bilateral Lobule IV, and the bilateral dentate (Figure 3). Contrasting AN/BN findings, the exploratory AN‐only GingerALE analysis revealed no significant clusters displaying volumetric reduction within the cerebellum.
TABLE 3.
Coordinates of significance in AN/BN studies (n = 11).
| Region (AN/BN < HC) | MNI coordinates | Volume (cm3) | ALE score | p | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| L CB | –25 | –55 | –31 | 7.09 | .0106 | 2.10 × 10–5 | 4.10 |
| L CB, anterior L. | –24 | –54 | –26 | .0106 | 2.10 × 10–5 | 4.10 | |
| L CB, posterior L. | –28 | –56 | –36 | .0105 | 2.18 × 10–5 | 4.09 | |
| L CB, posterior L. | –36 | –48 | –36 | .00983 | 5.41 × 10–5 | 3.87 | |
| L CB, posterior L. | –18 | –58 | –34 | .00973 | 6.88 × 10–5 | 3.81 | |
| L CB, anterior L. | –20 | –48 | –30 | .00960 | 7.23 × 10–5 | 3.80 | |
| L CB, anterior L. | –30 | –54 | –16 | .00923 | 1.37 × 10–4 | 3.64 | |
| L CB, posterior L. | –20 | –68 | –36 | .00916 | 1.72 × 10–4 | 3.56 | |
| R CB | 24 | –56 | –26 | 4.56 | .0105 | 2.20 × 10–5 | 4.09 |
| R CB, anterior L. | 26 | –56 | –34 | .0105 | 2.20 × 10–5 | 4.09 | |
| R CB, anterior L. | 28 | –50 | –16 | .0103 | 2.45 × 10–5 | 4.06 | |
| R CB, anterior L. | 24 | –50 | –26 | .0102 | 2.63 × 10–5 | 4.04 | |
| R CB, posterior L. | 22 | –62 | –12 | .00975 | 5.68 × 10–5 | 3.86 | |
| R CB, posterior L. | 22 | –62 | –40 | .00932 | 1.18 × 10–4 | 3.68 | |
Abbreviations: ALE, activation likelihood estimation; AN, anorexia nervosa; BN, bulimia nervosa; CB, cerebellum; HC, healthy control; L, left; L., lobe; MNI, Montreal Neurological Institute; R, right.
FIGURE 3.
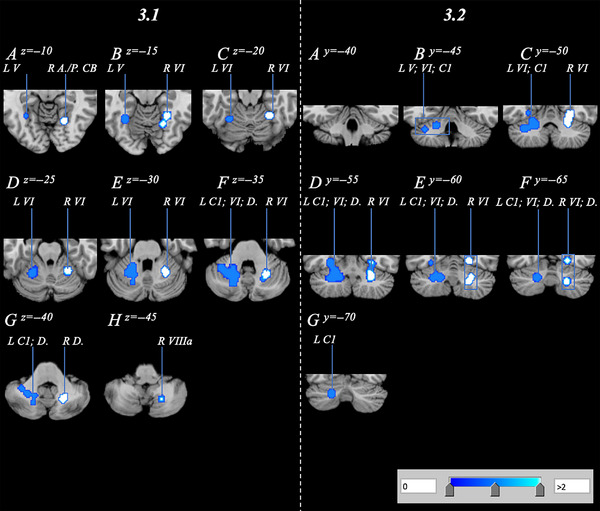
Cerebellar volume reduction of anorexia nervosa/bulimia nervosa subjects relative to healthy controls, spanning the bilateral cerebellum with a range of z = −10 to −45 and y = −45 to −70 in axial (3.1A–H) and coronal (3.2A–G) orientations. A., anterior; C1, Crus 1; CB, cerebellum; D., dentate; L, left; P., posterior; R, right; VI, Lobule 6; VIIIa, Lobule 8a.
3.3. NOR analysis: Cerebellum GMV versus BMI
Analysis of the NOR data set evaluating correlations between volumetric measures and BMI revealed a singular posterior cluster where cerebellum GMV negatively correlated with BMI. The cluster had a volume of 8.02 cm3 with a cluster center at 30, −71, −32 (Table 4; Figure 4). The cluster comprised seven peaks, all located within the posterior lobe affecting regions such as right Crus I/II, Lobule VI, Lobule VIIb, and the dentate.
TABLE 4.
Clusters associated with increased BMI in NOR populations (n = 9/10).
| Region (NOR; CB GMV vs. BMI) | MNI coordinates | Volume (cm3) | ALE score | p | Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| R CB | 30 | –71 | –32 | 8.02 | .0224 | 1.39 × 10–8 | 5.56 |
| R CB, posterior L. | 28 | –70 | –42 | .0224 | 1.39 × 10–8 | 5.56 | |
| R CB, posterior L. | 44 | –72 | –30 | .0207 | 5.54 × 10–8 | 5.31 | |
| R CB, posterior L. | 18 | –66 | –30 | .0189 | 4.14 × 10–7 | 4.93 | |
| R CB, posterior L. | 28 | –80 | –24 | .0138 | 1.50 × 10–5 | 4.17 | |
| R CB, posterior L. | 34 | –78 | –28 | .0133 | 1.91 × 10–5 | 4.12 | |
| L CB, posterior L. | 36 | –72 | –18 | .011 | 6.86 × 10–5 | 3.81 | |
Abbreviations: ALE, activation likelihood estimation; BMI, body mass index; CB, cerebellum; L, left; L., lobe; GMV, gray matter volume; MNI, Montreal Neurological Institute; NOR, normative; R, right.
FIGURE 4.
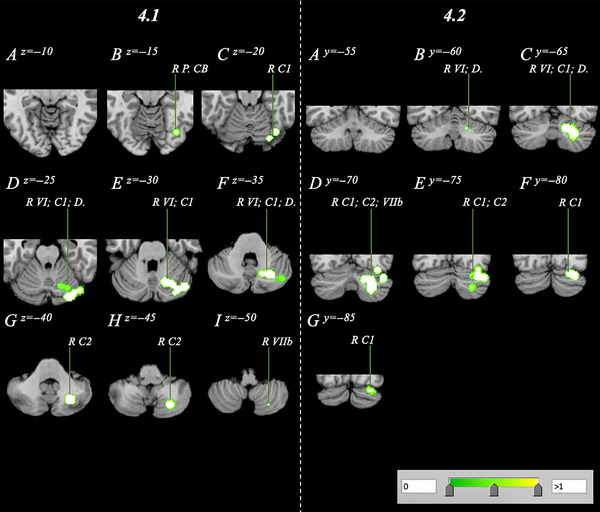
Volume reduction within a normative sample analysis spanning the right cerebellum with a range of z = −15 to −50 and y = 60 to −85 in axial (4.1A–I) and coronal (4.2A–G) orientations. C1, Crus 1; C2, Crus 2; D., dentate; L, left; P., posterior; R, right; VI, Lobule 6; VIIb, Lobule 7b; VIIIa/b, Lobule 8a/b.
3.4. Pooled volumetric reduction and conjunction analyses
Pooling combinations of data (AN/BN‐OB, AN/BN‐NOR, OB‐NOR) showed that cerebellar volume reduction significantly overlapped across cohorts, but these reductions were also largely distinct according to body weight condition as well as across BMI (Figure S2). OB and NOR clusters were exclusive to the left and right hemispheres, respectively, while AN/BN clusters were bilaterally located. Logical overlays on MANGO delineated significant intercondition overlap, which prompted three conjunction analyses (Figure 5). The AN/BN–OB conjunction analysis revealed a large region of overlap. The cluster was located in the left Lobule VI with a volume of 0.214 cm3 and center coordinates of −24, −55, −33 (Figure 6.1; Table 5). Conjunction analyses between clinical cohorts and the NOR cohort conducting correlational analyses between volume and BMI revealed additional findings. The AN/BN–NOR conjunction analysis revealed a unilateral overlap of structural reduction. The cluster was 0.0296 cm3 with a cluster center of 23, −64, −39, and primarily located within right Lobule VI, but also affected the surrounding Crus II and dentate nucleus regions (Figure 6.2; Table 5). No overlap or combinatorial clusters falling within our significance threshold were found in the OB–NOR conjunction analysis.
FIGURE 5.
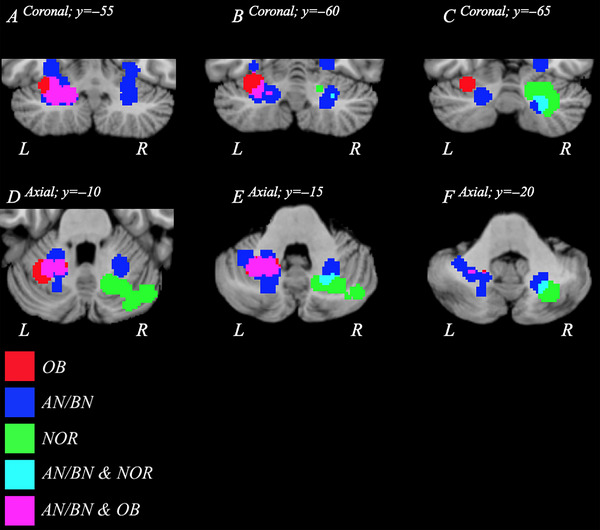
Family‐wise error (FWE)‐corrected overlay clusters visualizing decreases in volume from AN/BN (blue), OB (red), and NOR (green) data. Regions of the cerebellum where AN/BN and OB data overlap are shown in purple, while an overlap in AN/BN and NOR data is displayed in cyan. No overlap between OB and NOR data was present. Panels A–C and D–F depict the logical overlays in axial and coronal orientations, respectively. AN, anorexia nervosa; BN, bulimia nervosa; L, left; NOR, normative; OB, obesity; R, right.
FIGURE 6.
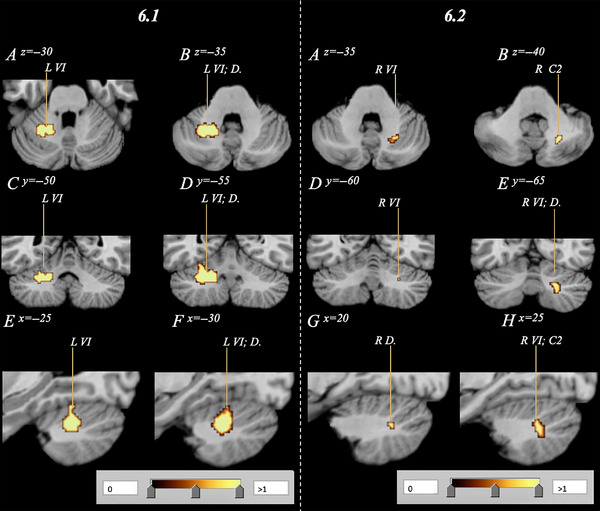
Subsequent conjunction analysis combining respective AN/BN and OB data (6.1) as well as AN/BN and NOR data (6.2) to visualize affected regions of overlap. Analyses confirm an overlap in volumetric decrease in both AN/BN and OB subjects, as well as AN/BN and NOR subjects primarily within Lobule VI (p < .05). Subfigures 6A/B, C/D, and D/E depict the combinatorial clusters in axial, coronal, and sagittal orientations, respectively. No overlap between OB and NOR data was identified. AN, anorexia nervosa; BN, bulimia nervosa; C2, Crus 2; D., Dentate; NOR, normative; OB, obesity; VI, Lobule 6.
TABLE 5.
Conjunction analysis clusters of significance in AN/BN, NOR, and OB cohorts.
| Study | MNI coordinates | Volume (cm3) | Volume breakdown (%) | ALE score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| (AN/BN–OB) | ||||||
| L CB | –24 | –55 | –33 | 0.214 | 54.9% Ant.; 45.1% Post. | .00850 |
| L CB, posterior | –28 | –54 | –34 | .00854 | ||
| L CB, posterior | –18 | –56 | –34 | .00768 | ||
| (AN/BN–NOR) | ||||||
| R CB | 23 | –64 | –39 | 0.029 | 100% Post. | .00690 |
| R CB, posterior | 24 | –64 | –40 | .00689 | ||
| (OB–NOR) | ||||||
| NS | ||||||
Abbreviations: ALE, activation likelihood estimation; AN, anorexia nervosa; Ant., anterior lobe; BN, bulimia nervosa; CB, cerebellum; L, left; NOR, normative; NS, not significant; OB, obesity; Post., posterior lobe; R, right.
3.5. Sensitivity analyses
Sensitivity analyses were conducted on all available cohort data sets (Table S2). One‐by‐one omission of publications identified heterogeneity across OB findings, in which the left posterior and anterior cerebellar clusters were present in three of six analyses. In contrast, NOR and AN/BN findings were robust, with the right posterior cerebellar cluster demonstrating repeatability in 10 of 10 NOR analyses, as well as left and right AN/BN cerebellar clusters appearing in 11 of 11 and nine of 11 analyses, respectively. While the AN‐only cohort did not report findings, the jackknife analysis identified the left posterior CB as reduced upon removal of Lenhart et al. (2022), Phillipou et al. (2018), Fonville et al. (2014), and Bomba et al. (2015).
4. DISCUSSION
4.1. Cerebellar differences: OB versus AN/BN versus HC
Compiling data from OB and AN/BN publications, as well as from correlations between BMI and brain volume, confirm cerebellar structure to be significantly decreased within varying BMI states and on extremes of the spectrum of body weight disorders. However, we are unable to conclude that cerebellar alterations occur in AN alone, with existing differences between AN‐only and AN/BN cohorts potentially due to global atrophy of gray matter associated with AN‐predominant weight loss. As such, subsequent interpretation of identified findings and potential structural mechanisms will focus on the combinatorial appetitive dysregulation presented by the AN/BN cohort. From cohort data, regions Crus I and Lobule VI were most consistently and significantly affected, identifying two cerebellar regions with respective executive and sensorimotor functionality associated with conditions of dysregulated BMI. In addition, findings indicate both condition‐specific differences as well as significant overlap in cerebellar GMV reduction between AN/BN, OB, and NOR cohorts. Reduced GMV in Lobule VI, Crus I, and the dentate nucleus was present in AN/BN, OB, and NOR cohorts but differed according to hemisphere. In the NOR population, affected regions correlating with increased BMI overlapped with cerebellar reduction seen in AN/BN, but not the OB analysis. Differential hemispheric involvement of two regions that reportedly comprise distinct corticocerebellar circuits leads us to suggest that multiple cerebellar regions are reliably and consistently involved across states of appetite dysfunction. Understanding of these regional functions may provide insight as to how the cerebellum may differentially contribute to the dysregulation of body weight.
Crus I, structurally reduced in AN/BN, OB, and NOR cohorts, is reported to play predominant executive‐, memory‐, and some emotional‐related functions. Crus I and II contain specific functional localization that corresponds to cerebral cortical zones (Edge et al., 2003; Guell et al., 2018; Habas et al., 2009; Heinitz et al., 2017; Marron et al., 2019; Stoodley & Schmahmann, 2009) and falls within the ECN (Habas et al., 2009; Stoodley, 2012; Stoodley & Schmahmann, 2009). In typical populations, this network plays roles in executive function, spatial attention (Ciricugno et al., 2020), and verbal working memory (Edge et al., 2003; Guell et al., 2018; Iglói et al., 2015; Shen et al., 2020; Stoodley & Schmahmann, 2009), as well as goal‐directed behavior via communication with the hippocampus (Iglói et al., 2015). Recently, Crus II is reported to serve emotional self‐experience and social mentalizing (Van Overwalle et al., 2020), expanding emotional roles played by posterior cerebellar lobes.
Functional hemispheric differences reported in Crus I may explain how the cerebellum is implicated, but likely plays distinct roles in cases of pathological under‐ and overeating. Bilateral cerebellar reduction has previously been associated in those with AN (Zhang et al., 2018). Despite no cluster‐based findings within the exploratory AN‐only cohort, Crus I reduction within the AN/BN cohort may be associated with general executive and attentional deficits reported in undereating (Gaudio et al., 2018), such as body image disturbance and behavioral rigidity toward food. Alternatively, reduction of Crus I within this cohort may be more strongly associated with BN‐specific symptomatology, such as altered traits of impulsivity or LOC eating. Additionally, volumetric reduction of Crus II was only identified within the NOR and AN/BN findings, suggesting socioemotional functions of Crus II could play a less prominent role in those with OB. Crus I reduction was specific to the left hemisphere in the OB cohort, which interestingly overlaps with previous unilateral cerebellar PSE (English et al., 2019) findings as well as regions activated in spatial attention (Ciricugno et al., 2020) and social processing tasks (Guell et al., 2018). Thus, cerebellar volume reduction in cases of overeating or OB seen in this meta‐analysis may suggest altered measures of food‐related attention, impulsivity, working memory, or loss of control, aspects characteristic of OB (Coppin et al., 2014; Cortese & Vincenzi, 2011; Cortese et al., 2016; Pineda‐Alhucema et al., 2018; Wu et al., 2017; Yang et al., 2018; Zhang et al., 2018). However, sensitivity analyses reported heterogeneity among OB study findings, likely due to lacking study power from limited literature. Further region‐of‐interest structural studies as well as resting‐state and fMRI tasks relating to impulsivity, working memory, and decision making would aid in furthering condition‐specific interpretations from this work.
Within NOR populations, reduction of Crus I was associated with increased BMI, but reduction was unilateral to the right hemisphere and included reduction of Crus II, suggesting that differential mechanisms may underly distinct contributions toward weight gain. However, it is important to note the differential study power between cohorts, with NOR findings derived from a significantly larger sample size than in those with OB. While findings corroborate concepts that the cerebellum is multimodally associated with differing aspects of BMI increase, such as homeostatic‐ versus nonhomeostatic‐associated weight gain, future evaluation of NOR populations excluding those with OB would further solidify interpretations.
Lobule VI consistently reported with structural reduction in AN/BN, OB, and NOR cohorts, and its known functional distinction from Crus I furthers the concept that more than one region of the cerebellum participates in aspects of the dysregulation of body weight. Lobule VI has recently been recognized as serving several cognitive and emotional functions, and plays roles in the salience (Cacciola et al., 2017; Habas et al., 2009; Seeley et al., 2007; Stoodley & Schmahmann, 2009, 2010), somatomotor, ventral attention, and visual networks (Van Overwalle et al., 2020). Roles of the salience network, predominantly represented via Lobule VI, involve autonomic and interoceptive processing in response to various forms of salience such as emotion, reward, and homeostatic regulation (Craig, 2002; Critchley et al., 2004; Eisenberger et al., 2003). Tractography studies demonstrate connections between the motor cortex, Lobule VI, and dentate nucleus (Habas & Manto, 2018; Kelly & Strick, 2003), and fMRI studies report consistent Lobule VI activity in emotional, social, and environmental learning tasks (Bermpohl et al., 2006; Olivo et al., 2018; Takahashi et al., 2004). Computational models also support that circuits implicated in Lobule VI are important for Pavlovian conditioning and emotional learning (Adams et al., 2013; Barrett et al., 1708; Brown et al., 2013; Pezzulo, 2012; Pu et al., 2020), including perceptual inferences such as startle responses and spontaneous behavior (Friston & Herreros, 2016). Such mechanisms may contribute to AN or BN via development of food aversion or negative food/weight associations that tend to exacerbate low dietary intake or restriction. Alternatively, food may be conditioned as an excessively rewarding or positive stimulus, which may be associated with overeating or LOC consumption.
Similar to Crus I findings, differential hemispheric reduction of Lobule VI across cohorts suggests differential cerebellar involvement. While the exploratory AN‐only analysis identified no clusters of interest, the AN/BN analysis identified bilateral reduction of Lobule VI. Those with AN have previously demonstrated reduced bilateral volume of the mid‐posterior cerebellum, which is suggested to contribute toward characteristic AN symptoms such as food aversion (Zhang et al., 2018), but further functional studies focusing on the cerebellum are warranted to further understand this relationship, as well as additional studies are needed to distinguish between AN and BN findings. Reduction of Lobule VI in OB was restricted to the left hemisphere, which contains regions specific to social processing tasks (Guell et al., 2018). In contrast to the OB cohort, reduction of Lobule VI volume within the NOR population was specific to the right hemisphere. While there is currently no specific ascribed role to the right Lobule VI, differing hemisphericity of effect between OB and NOR cohorts similarly suggests differential cerebellar participation in pathological/nonpathological overeating.
Reduction of the dentate nucleus was also seen in AN/BN, OB, and NOR cohorts. This region is highly connected to the hypothalamus and thought to interact with the lateral hypothalamic area, ventromedial nucleus, dorsomedial nucleus, and paraventricular nucleus to modulate forelimb movements in food grasping behavior (Martin et al., 2000). As with previous findings, the recruitment of the dentate nucleus within multiple cohorts not only suggests cerebellar implication in both nonpathological and clinical states of body weight dysregulation, which may functionally differ due to associations with opposing hemispheres, but also that multiple cerebellar regions are associated with the dysregulation of body weight and BMI.
Lastly, a lack of cluster‐based findings within the AN‐only analysis contrast previous reports of cerebellar atrophy in AN (Boghi et al., 2011; Seitz et al., 2018; Zhang et al., 2018) and its structural susceptibility to starvation (Boghi et al., 2011), and do not align with reports of whole‐brain reduction in those with AN (Amianto, D'Agata, et al., 2013; Fonville et al., 2014). Findings from this study suggest aforementioned structural cerebellar contributions underlying AN pathophysiology are unlikely to be distinct to cerebellar subregions and may be partially or fully consequent of whole‐brain reduction characteristic of starvation.
4.2. Cerebellar overlap: OB versus AN/BN versus HC
Volumetric overlap among AN/BN, OB, and NOR cohorts revealed that cases of dysregulated appetite across the BMI‐spectrum exhibit significant reduction in similar cerebellar regions, which have been known for distinct sensorimotor (Lobule VI) and executive (Crus I/II) roles and suggest multimodal cerebellar association across the BMI spectrum. Structural reduction in cases of AN/BN and OB, as well as within NOR populations assessing cerebellar volume alongside increased BMI, was predominant to Lobule VI and Crus I. Findings relating to nonpathological BMI increase via the NOR cohort as well as the AN/BN cohort overlapped within the right Lobule VI, Crus II, and dentate nucleus. Alternatively, Lobule VI and Crus I were both reduced in OB and NOR data sets, but difference of effect according to hemisphere meant no overlap in volume reduction was identified. The implication of similar cerebellar regions yet differing hemisphericity of effect again suggests that while these cerebellar regions participate in differing forms of body weight states, the main sources of variation among NOR populations may be quite different to those driving differences in clinical conditions.
4.3. Limitations
4.3.1. Number of studies
Our review was limited by low study numbers used for some individual analyses, and we were unable to provide sufficient information on race/ethnicity and socioeconomic status. Availability of data also prevented further evaluation into sex, age, hormonal differences, and brain inflammation values across participants. Due to such limitations, we confined our analysis to changes in GMV. Future research would benefit from utilizing more specified imaging technology catered to WMV alterations, such as diffusion tensor imaging and diffusion‐weighted imaging.
Similarly, while there is no definitive instruction on the minimum number of studies needed to conduct an ALE meta‐analysis, the GingerALE (2.0) manual mentions that a minimum of 20–30 coordinates per experiment, as seen in our analyses, is sufficient to produce significant and valid clusters for simple paradigms. Later on, Eickhoff et al. (2016) recommended approximately 15–17 studies for reliability of analysis results, which were not present in our study. While cohort‐specific sensitivity analyses were implemented to attempt to account for heterogeneity across findings, little can be done to correct or adjust for small sample sizes via GingerALE. A method of counteracting potentially unreliable results from future smaller studies would be to analyze small‐cohort findings alongside larger data sets, or to incorporate quantifications of bias from small sample sizes (i.e., Egger test) within GingerALE software utility.
4.3.2. Assumptions
The research question posed by this review—to determine if areas of the cerebellum involved in different disorders of appetite control were the same or distinct, was driven by a review of the literature providing extensive evidence that the cerebellum is implicated in disorders of appetite control (see Section 1). Therefore, our study is based on this assumption and does not independently verify it. This is because we only reviewed studies that included cerebellum findings rather than all MRI studies of eating disorders. Additionally, both AN/BN and OB are associated with elevated inflammation within the brain (Seitz et al., 2019; Spyridaki et al., 2016), which may influence findings related to volumetric reduction of the cerebellum. Future studies would benefit from including brain inflammation factors within analysis.
4.3.3. Causality and direction of effects
Further, we are unable to verify the direction of causation in found associations. A common limitation in neuroimaging studies is their capacity to determine causality. The studies reviewed here are only able to demonstrate associations between volume abnormalities and condition, and it is unclear whether reduced volumes are a cause or subsequent effect.
4.3.4. Bulimia nervosa
Although we excluded the majority of BN patients, approximately 20% of clinical AN data consisted of those with BN that we were unable to remove. As there were insufficient BN papers (n = 3) to conduct individualized cohort analyses for AN and BN, data sets were thus pooled into one AN/BN cohort. While there is a significant overlap in comorbidity and symptomatology between those with AN and BN (D'Agata et al., 2015), including this group may have resulted in volume reduction not linked to states relating to those with AN, and findings from this meta‐analysis are unable to distinguish between those with mixed AN/BN pathology and those with AN alone. We attempted to mitigate limitations regarding AN/BN etiological heterogeneity by conducting an exploratory assessment with AN‐only publications. Nevertheless, our study still focuses on reduced cerebellar volume in eating disorders, which is clearly the case for bulimic individuals.
5. CONCLUSION
While theories proposing cerebellar functions in emotional, cognition, and conditioning behaviors are now receiving wide acceptance, a role in weight and appetite is rarely discussed. In this review, we found utility in exploring cerebellar differences and similarities in states at opposite ends of the body weight dimension, and collated structural evidence from many sources. Altogether, results of our ALE analyses support the concept that the cerebellum is associated with dysregulated appetite, eating disorders, and body weight in both pathological and nonpathological states. We found that while cerebellar associations with bodyweight issues recruited similar regions, hemispherical effects differed according to the type of appetitive condition, suggesting different cerebellar circuitry contributing to eating behavior between nonclinical and pathological populations. Such findings add to a body of evidence theorizing cerebellar participation in appetite‐ and feeding‐related domains. Utilizing our knowledge of the emotional and cognitive functions of the cerebellum will assist in identifying novel remedial approaches to manage disorders of appetite regulation as well as increase efficacy of interventions provided to clinical populations.
AUTHOR CONTRIBUTIONS
Michelle Sader: Conceptualization; methodology; investigation; writing—review and editing. Gordon Waiter: Study visualization; methodology; resources; writing—review and editing; supervision. Justin Williams: Conceptualization; study visualization; methodology; writing—review and editing; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.3286.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
I would like to extend my sincere gratitude to the Northwood Charitable Trust for funding my PhD studentship.
Sader, M. , Waiter, G. D. , & Williams, J. H. G. (2023). The cerebellum plays more than one role in the dysregulation of appetite: Review of structural evidence from typical and eating disorder populations. Brain and Behavior, 13, e3286. 10.1002/brb3.3286
DATA AVAILABILITY STATEMENT
Additional data supporting study findings are available from the corresponding author upon request.
REFERENCES
- Abell, J. E. , Egan, B. M. , Wilson, P. W. , Lipsitz, S. , Woolson, R. F. , & Lackland, D. T. (2007). Age and race impact the association between BMI and CVD mortality in women. Public Health Reports, 122(4), 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamaszek, M. , D'Agata, F. , Ferrucci, R. , Habas, C. , Keulen, S. , Kirkby, K. C. , Leggio, M. , Mariën, P. , Molinari, M. , Moulton, E. , Orsi, L. , Van Overwalle, F. , Papadelis, C. , Priori, A. , Sacchetti, B. , Schutter, D. J. , Styliadis, C. , & Verhoeven, J. (2017). Consensus paper: Cerebellum and emotion. The Cerebellum, 16(2), 552–576. 10.1007/s12311-016-0815-8 [DOI] [PubMed] [Google Scholar]
- Adams, R. A. , Shipp, S. , & Friston, K. J. (2013). Predictions not commands: Active inference in the motor system. Brain Structure and Function, 218(3), 611–643. 10.1007/s00429-012-0475-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhussain, M. H. , Macdonald, I. A. , & Taylor, M. A. (2016). Irregular meal‐pattern effects on energy expenditure, metabolism, and appetite regulation: A randomized controlled trial in healthy normal‐weight women. The American Journal of Clinical Nutrition, 104(1), 21–32. 10.3945/ajcn.115.125401 [DOI] [PubMed] [Google Scholar]
- Allen, G. , McColl, R. , Barnard, H. , Ringe, W. K. , Fleckenstein, J. , & Cullum, C. M (2005). Magnetic resonance imaging of cerebellar–prefrontal and cerebellar–parietal functional connectivity. Neuroimage, 28(1), 39–48. 10.1016/j.neuroimage.2005.06.013 [DOI] [PubMed] [Google Scholar]
- Al Massadi, O. , López, M. , Tschöp, M. , Diéguez, C. , & Nogueiras, R. (2017). Current understanding of the hypothalamic ghrelin pathways inducing appetite and adiposity. Trends in Neurosciences, 40(3), 167–180. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). Author. [Google Scholar]
- Amianto, F. , Caroppo, P. , D'Agata, F. , Spalatro, A. , Lavagnino, L. , Caglio, M. , Righi, D. , Bergui, M. , Abbate‐Daga, G. , Rigardetto, R. , Mortara, P. , & Fassino, S. (2013). Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: A voxel‐based morphometry study. Psychiatry Research: Neuroimaging, 213(3), 210–216. 10.1016/j.pscychresns.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Amianto, F. , D'Agata, F. , Lavagnino, L. , Caroppo, P. , Abbate‐Daga, G. , Righi, D. , Scarone, S. , Bergui, M. , Mortara, P. , & Fassino, S. (2013). Intrinsic connectivity networks within cerebellum and beyond in eating disorders. The Cerebellum, 12(5), 623–631. 10.1007/s12311-013-0471-1 [DOI] [PubMed] [Google Scholar]
- Arcelus, J. , Mitchell, A. J. , Wales, J. , & Nielsen, S. (2011). Mortality rates in patients with anorexia nervosa and other eating disorders: A meta‐analysis of 36 studies. Archives of General Psychiatry, 68(7), 724–731. 10.1001/archgenpsychiatry.2011.74 [DOI] [PubMed] [Google Scholar]
- Arroyo‐Johnson, C. , & Mincey, K. D. (2016). Obesity epidemiology worldwide. Gastroenterology Clinics, 45(4), 571–579. 10.1016/j.gtc.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustijn, M. J. C. M. , D'hondt, E. , Leemans, A. , Van Acker, L. , De Guchtenaere, A. , Lenoir, M. , Deconinck, F. J. A. , & Caeyenberghs, K. (2019). Weight loss, behavioral change, and structural neuroplasticity in children with obesity through a multidisciplinary treatment program. Human Brain Mapping, 40(1), 137–150. 10.1002/hbm.24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarich‐Marsteller, N. C. , Foltin, R. W. , & Walsh, B. T. (2011). Does anorexia nervosa resemble an addiction? Current Drug Abuse Reviews, 4(3), 197–200. 10.2174/1874473711104030197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, L. F. , Quigley, K. S. , & Hamilton, P. (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1708), Article 20160011. 10.1098/rstb.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann, O. , & Mattingley, J. B. (2012). Functional topography of primary emotion processing in the human cerebellum. NeuroImage, 61(4), 805–811. 10.1016/j.neuroimage.2012.03.044 [DOI] [PubMed] [Google Scholar]
- Bermpohl, F. , Pascual‐Leone, A. , Amedi, A. , Merabet, L. B. , Fregni, F. , Gaab, N. , Alsop, D. , Schlaug, G. , & Northoff, G. (2006). Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage, 30(2), 588–600. 10.1016/j.neuroimage.2005.09.040 [DOI] [PubMed] [Google Scholar]
- Berridge, K. C. (2009). ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiology & Behavior, 97(5), 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, K. C. , Ho, C.‐Y. , Richard, J. M. , & Difeliceantonio, A. G. (2010). The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. 10.1016/j.brainres.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boghi, A. , Sterpone, S. , Sales, S. , D'Agata, F. , Bradac, G. B. , Zullo, G. , & Munno, D. (2011). In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Research: Neuroimaging, 192(3), 154–159. 10.1016/j.pscychresns.2010.12.008 [DOI] [PubMed] [Google Scholar]
- Bohon, C. (2017). Brain response to taste in overweight children: A pilot feasibility study. PLoS ONE, 12(2), Article e0172604. 10.1371/journal.pone.0172604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomba, M. , Riva, A. , Morzenti, S. , Grimaldi, M. , Neri, F. , & Nacinovich, R. (2015). Global and regional brain volumes normalization in weight‐recovered adolescents with anorexia nervosa: Preliminary findings of a longitudinal voxel‐based morphometry study. Neuropsychiatric Disease and Treatment, 11, 637–645. 10.2147/NDT.S73239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret, S. G. , Draper, S. J. , & Simerly, R. B. (2004). Trophic action of leptin on hypothalamic neurons that regulate feeding. Science, 304(5667), 108–110. 10.1126/science.1095004 [DOI] [PubMed] [Google Scholar]
- Briatore, F. , Pregno, G. , Di Angelantonio, S. , Frola, E. , De Stefano, M. E. , Vaillend, C. , Sassoè‐Pognetto, M. , & Patrizi, A. (2020). Dystroglycan mediates clustering of essential GABAergic components in cerebellar purkinje cells. Frontiers in Molecular Neuroscience, 13, Article 164. 10.3389/fnmol.2020.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, S. J. , Barker, G. J. , O'Daly, O. G. , Brammer, M. , Williams, S. C. , Benedict, C. , Schiöth, H. B. , Treasure, J. , & Campbell, I. C. (2011). Restraint of appetite and reduced regional brain volumes in anorexia nervosa: A voxel‐based morphometric study. BMC Psychiatry, 11, Article 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H. , Adams, R. A. , Parees, I. , Edwards, M. , & Friston, K. (2013). Active inference, sensory attenuation and illusions. Cognitive Processing, 14(4), 411–427. 10.1007/s10339-013-0571-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. (2013). The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron, 80(3), 807–815. 10.1016/j.neuron.2013.10.044 [DOI] [PubMed] [Google Scholar]
- Bulik, C. M. , Flatt, R. , Abbaspour, A. , & Carroll, I. (2019). Reconceptualizing anorexia nervosa. Psychiatry and Clinical Neurosciences, 73(9), 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyukkurt, A. , Bourguignon, C. , Antinora, C. , Farquhar, E. , Gao, X. , Passarella, E. , Sibthorpe, D. , Gou, K. , Saury, S. , Beaulieu, S. , Storch, K.‐F. , & Linnaranta, O. (2021). Irregular eating patterns associate with hypomanic symptoms in bipolar disorders. Nutritional Neuroscience, 24(1), 23–34. [DOI] [PubMed] [Google Scholar]
- Cacciola, A. , Milardi, D. , Livrea, P. , Flace, P. , Anastasi, G. , & Quartarone, A. (2017). The known and missing links between the cerebellum, basal ganglia, and cerebral cortex. The Cerebellum, 16(3), 753–755. 10.1007/s12311-017-0850-0 [DOI] [PubMed] [Google Scholar]
- Carnell, S. , Benson, L. , Pantazatos, S. P. , Hirsch, J. , & Geliebter, A. (2014). Amodal brain activation and functional connectivity in response to high‐energy‐density food cues in obesity. Obesity, 22(11), 2370–2378. 10.1002/oby.20859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, A. , Hendrikse, J. , Lee, N. , Yücel, M. , Verdejo‐Garcia, A. , Andrews, Z. B. , & Hall, W. (2016). The neurobiology of “food addiction” and its implications for obesity treatment and policy. Annual Review of Nutrition, 36, 105–128. 10.1146/annurev-nutr-071715-050909 [DOI] [PubMed] [Google Scholar]
- Çavdar, S. , Onat, F. , Aker, R. , Şehirli, Ü. , Şan, T. , & Raci Yananli, H. (2001). The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. Journal of Anatomy, 198(4), 463–472. 10.1017/S0021878201007555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Lu, P. , Ma, Q. , Cao, Y. , Chen, N. , Li, W. , Zhao, S. , Chen, B. , Shi, J. , Sun, Y. , Shen, H. , Sun, L. , Shen, J. , Liao, Q. , Zhang, Y. , Hong, J. , Gu, W. , Liu, R. , Ning, G. , … Wang, J. (2020). CTNNB1/β‐catenin dysfunction contributes to adiposity by regulating the cross‐talk of mature adipocytes and preadipocytes. Science Advances, 6(2), Article eaax9605. 10.1126/sciadv.aax9605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, B. , Qi, X. , Liang, C. , Zhang, L. , Ma, M. , Li, P. , Liu, L. , Cheng, S. , Yao, Y. , Chu, X. , Ye, J. , Wen, Y. , Jia, Y. , & Zhang, F. (2020). Integrative genomic enrichment analysis identified the brain regions and development stages related to anorexia nervosa and obsessive‐compulsive disorder. Cerebral Cortex, 30(12), 6481–6489. 10.1093/cercor/bhaa214 [DOI] [PubMed] [Google Scholar]
- Choe, A. S. , Tang, B. , Smith, K. R. , Honari, H. , Lindquist, M. A. , Caffo, B. S. , & Pekar, J. J. (2021). Phase‐locking of resting‐state brain networks with the gastric basal electrical rhythm. PLoS ONE, 16(1), Article e0244756. 10.1371/journal.pone.0244756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciricugno, A. , Ferrari, C. , Rusconi, M. L. , & Cattaneo, Z. (2020). The left posterior cerebellum is involved in orienting attention along the mental number line: An online‐TMS study. Neuropsychologia, 143, Article 107497. 10.1016/j.neuropsychologia.2020.107497 [DOI] [PubMed] [Google Scholar]
- Colombel, C. , Lalonde, R. , & Caston, J. (2002). The effects of unilateral removal of the cerebellar hemispheres on motor functions and weight gain in rats. Brain Research, 950(1‐2), 231–238. 10.1016/S0006-8993(02)03043-3 [DOI] [PubMed] [Google Scholar]
- Contreras‐Rodríguez, O. , Vilar‐López, R. , Andrews, Z. B. , Navas, J. F. , Soriano‐Mas, C. , & Verdejo‐García, A. (2017). Altered cross‐talk between the hypothalamus and non‐homeostatic regions linked to obesity and difficulty to lose weight. Scientific Reports, 7(1), Article 9951. 10.1038/s41598-017-09874-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppin, G. , Nolan‐Poupart, S. , Jones‐Gotman, M. , & Small, D. M. (2014). Working memory and reward association learning impairments in obesity. Neuropsychologia, 65, 146–155. 10.1016/j.neuropsychologia.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S. , Moreira‐Maia, C. R. , St Fleur, D. , Morcillo‐Peñalver, C. , Rohde, L. A. , & Faraone, S. V. (2016). Association between ADHD and obesity: A systematic review and meta‐analysis. American Journal of Psychiatry, 173(1), 34–43. 10.1176/appi.ajp.2015.15020266 [DOI] [PubMed] [Google Scholar]
- Cortese, S. , & Vincenzi, B. (2011). Obesity and ADHD: Clinical and neurobiological implications. In Stanford C. & Tannock R. (Eds.), Behavioral neuroscience of attention deficit hyperactivity disorder and its treatment (pp. 199–218). Springer. [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Critchley, H. D. , Wiens, S. , Rotshtein, P. , Öhman, A. , & Dolan, R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. [DOI] [PubMed] [Google Scholar]
- Cutando, L. , Puighermanal, E. , Castell, L. , Tarot, P. , Belle, M. , Bertaso, F. , Arango‐Lievano, M. , Ango, F. , Rubinstein, M. , Quintana, A. , & Chédotal, A. (2022). Cerebellar dopamine D2 receptors regulate social behaviors. Nature Neuroscience, 25, 900–911. [DOI] [PubMed] [Google Scholar]
- D‘Angelo, E. , & Casali, S. (2013). Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Frontiers in Neural Circuits, 6, Article 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agata, F. , Caroppo, P. , Amianto, F. , Spalatro, A. , Caglio, M. M. , Bergui, M. , Lavagnino, L. , Righi, D. , Abbate‐Daga, G. , Pinessi, L. , Mortara, P. , & Fassino, S. (2015). Brain correlates of alexithymia in eating disorders: A voxel‐based morphometry study. Psychiatry and Clinical Neurosciences, 69(11), 708–716. [DOI] [PubMed] [Google Scholar]
- Diedrichsen, J. , Maderwald, S. , Küper, M. , Thürling, M. , Rabe, K. , Gizewski, E. R. , Ladd, M. E. , & Timmann, D. (2011). Imaging the deep cerebellar nuclei: A probabilistic atlas and normalization procedure. Neuroimage, 54(3), 1786–1794. 10.1016/j.neuroimage.2010.10.035 [DOI] [PubMed] [Google Scholar]
- Diedrichsen, J. , & Zotow, E. (2015). Surface‐based display of volume‐averaged cerebellar imaging data. PLoS ONE, 10(7), Article e0133402. 10.1371/journal.pone.0133402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan, R. (1998). A cognitive affective role for the cerebellum. Brain, 121(4), 545–546. 10.1093/brain/121.4.545 [DOI] [PubMed] [Google Scholar]
- Dommes, E. , Georgiewa, P. , & Klingebiel, R. (2013). Grey matter volume differences in obese as compared to normal‐weight individuals: A voxel‐based morphometric study. Archiv Euromedica, 3(2), 11–16. [Google Scholar]
- Eddy, K. T. , Dorer, D. J. , Franko, D. L. , Tahilani, K. , Thompson‐Brenner, H. , & Herzog, D. B. (2008). Diagnostic crossover in anorexia nervosa and bulimia nervosa: Implications for DSM‐V. American Journal of Psychiatry, 165(2), 245–250. 10.1176/appi.ajp.2007.07060951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge, A. L. , Marple‐Horvat, D. E. , & Apps, R. (2003). Lateral cerebellum: Functional localization within crus I and correspondence to cortical zones. European Journal of Neuroscience, 18(6), 1468–1485. 10.1046/j.1460-9568.2003.02873.x [DOI] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Laird, A. R. , Grefkes, C. , Wang, L. E. , Zilles, K. , & Fox, P. T. (2009). Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping, 30(9), 2907–2926. 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff, S. B. , Nichols, T. E. , Laird, A. R. , Hoffstaedter, F. , Amunts, K. , Fox, P. T. , Bzdok, D. , & Eickhoff, C. R. (2016). Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage, 137, 70–85. 10.1016/j.neuroimage.2016.04.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger, N. I. , Lieberman, M. D. , & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–292. 10.1126/science.1089134 [DOI] [PubMed] [Google Scholar]
- English, L. K. , Masterson, T. D. , Fearnbach, S. N. , Tanofsky‐Kraff, M. , Fisher, J. , Wilson, S. J. , Rolls, B. J. , & Keller, K. L. (2019). Increased brain and behavioural susceptibility to portion size in children with loss of control eating. Pediatric Obesity, 14(2), Article e12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine, H. E. , Whiteford, H. A. , & Pike, K. M. (2016). The global burden of eating disorders. Current Opinion in Psychiatry, 29(6), 346–353. 10.1097/YCO.0000000000000276 [DOI] [PubMed] [Google Scholar]
- Fagundo, A. B. , De La Torre, R. , Jiménez‐Murcia, S. , Agüera, Z. , Granero, R. , Tárrega, S. , Botella, C. , Baños, R. , Fernández‐Real, J. M. , Rodríguez, R. , Forcano, L. , Frühbeck, G. , Gómez‐Ambrosi, J. , Tinahones, F. J. , Fernández‐García, J. C. , Casanueva, F. F. , & Fernández‐Aranda, F. (2012). Executive functions profile in extreme eating/weight conditions: From anorexia nervosa to obesity. PLoS ONE, 7(8), Article e43382. 10.1371/journal.pone.0043382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshchi, H. R. , Taylor, M. A. , & Macdonald, I. A. (2005). Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. The American Journal of Clinical Nutrition, 81(1), 16–24. 10.1093/ajcn/81.1.16 [DOI] [PubMed] [Google Scholar]
- Fawcett, K. A. , & Barroso, I. (2010). The genetics of obesity: FTO leads the way. Trends in Genetics, 26(6), 266–274. 10.1016/j.tig.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley, C. R. , Asem, J. S. A. , Levenbaum, E. L. , & Courtney, S. M. (2016). Effects of body mass index and body fat percent on default mode, executive control, and salience network structure and function. Frontiers in Neuroscience, 10, Article 234. 10.3389/fnins.2016.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonville, L. , Giampietro, V. , Williams, S. C. R. , Simmons, A. , & Tchanturia, K. (2014). Alterations in brain structure in adults with anorexia nervosa and the impact of illness duration. Psychological Medicine, 44(9), 1965–1975. 10.1017/S0033291713002389 [DOI] [PubMed] [Google Scholar]
- Frank, G. K. , Shott, M. E. , Hagman, J. O. , & Mittal, V. A. (2013). Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. American Journal of Psychiatry, 170(10), 1152–1160. 10.1176/appi.ajp.2013.12101294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. , & Herreros, I. (2016). Active inference and learning in the cerebellum. Neural Computation, 28(9), 1812–1839. 10.1162/NECO_a_00863 [DOI] [PubMed] [Google Scholar]
- García‐García, I. , Michaud, A. , Dadar, M. , Zeighami, Y. , Neseliler, S. , Collins, D. L. , Evans, A. C. , & Dagher, A. (2019). Neuroanatomical differences in obesity: Meta‐analytic findings and their validation in an independent dataset. International Journal of Obesity, 43(5), 943–951. 10.1038/s41366-018-0164-4 [DOI] [PubMed] [Google Scholar]
- Garrison, K. A. , Sinha, R. , Lacadie, C. M. , Scheinost, D. , Jastreboff, A. M. , Constable, R. T. , & Potenza, M. N. (2016). Functional connectivity during exposure to favorite‐food, stress, and neutral‐relaxing imagery differs between smokers and nonsmokers. Nicotine & Tobacco Research, 18(9), 1820–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudio, S. , Nocchi, F. , Franchin, T. , Genovese, E. , Cannatà, V. , Longo, D. , & Fariello, G. (2011). Gray matter decrease distribution in the early stages of Anorexia Nervosa restrictive type in adolescents. Psychiatry Research: Neuroimaging, 191(1), 24–30. 10.1016/j.pscychresns.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Gaudio, S. , Olivo, G. , Beomonte Zobel, B. , & Schiöth, H. B. (2018). Altered cerebellar–insular–parietal–cingular subnetwork in adolescents in the earliest stages of anorexia nervosa: A network–based statistic analysis. Translational Psychiatry, 8, Article 127. 10.1038/s41398-018-0173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavello, D. , Carbone, E. , & Carabelli, V. (2016). Leptin‐mediated ion channel regulation: PI3K pathways, physiological role, and therapeutic potential. Channels, 10(4), 282–296. 10.1080/19336950.2016.1164373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter, A. , Benson, L. , Pantazatos, S. P. , Hirsch, J. , & Carnell, S. (2016). Greater anterior cingulate activation and connectivity in response to visual and auditory high‐calorie food cues in binge eating: Preliminary findings. Appetite, 96, 195–202. 10.1016/j.appet.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guell, X. , Schmahmann, J. D. , Gabrieli, J. D. E. , & Ghosh, S. S. (2018). Functional gradients of the cerebellum. eLife, 7, Article e36652. 10.7554/eLife.36652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, C. , Kamdar, N. , Nguyen, D. , Prater, K. , Beckmann, C. F. , Menon, V. , & Greicius, M. D. (2009). Distinct cerebellar contributions to intrinsic connectivity networks. Journal of Neuroscience, 29(26), 8586–8594. 10.1523/JNEUROSCI.1868-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, C. , & Manto, M. (2018). Probing the neuroanatomy of the cerebellum using tractography. In Heilman K. M. & Nadeau S. E. (Eds.), Handbook of clinical neurology (Vol. 154, pp. 235–249). Elsevier. [DOI] [PubMed] [Google Scholar]
- Habas, C. , Shirer, W. R. , & Greicius, M. D. (2013). Delineation of cerebrocerebellar networks with MRI measures of functional and structural connectivity. In Manto M., Schmahmann J. D., Rossi F., Gruol D. L., & Koibuchi N. (Eds.), Handbook of the cerebellum and cerebellar disorders (pp. 571–585). Springer. [Google Scholar]
- Heinitz, S. , Reinhardt, M. , Piaggi, P. , Weise, C. M. , Diaz, E. , Stinson, E. J. , Venti, C. , Votruba, S. B. , Wassermann, E. M. , Alonso‐Alonso, M. , Krakoff, J. , & Gluck, M. E. (2017). Neuromodulation directed at the prefrontal cortex of subjects with obesity reduces snack food intake and hunger in a randomized trial. The American Journal of Clinical Nutrition, 106(6), 1347–1357. 10.3945/ajcn.117.158089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, C. P. , Polivy, J. , Pliner, P. , & Vartanian, L. R. (2015). Mechanisms underlying the portion‐size effect. Physiology & Behavior, 144, 129–136. [DOI] [PubMed] [Google Scholar]
- Herrup, K. (2000). Thoughts on the cerebellum as a model for cerebral cortical development and evolution. Novartis Foundation Symposium, 228, 15–24. discussion 24–9, 46–52. [DOI] [PubMed] [Google Scholar]
- Hommel, J. D. , Trinko, R. , Sears, R. M. , Georgescu, D. , Liu, Z.‐W. , Gao, X.‐B. , Thurmon, J. J. , Marinelli, M. , & Dileone, R. J. (2006). Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron, 51(6), 801–810. 10.1016/j.neuron.2006.08.023 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Li, X. , Jackson, T. , Chen, S. , Meng, J. , Qiu, J. , & Chen, H. (2019). Interaction effect of sex and body mass index on gray matter volume. Frontiers in Human Neuroscience, 13, Article 360. 10.3389/fnhum.2019.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói, K. , Doeller, C. F. , Paradis, A.‐L. , Benchenane, K. , Berthoz, A. , Burgess, N. , & Rondi‐Reig, L. (2015). Interaction between hippocampus and cerebellum crus I in sequence‐based but not place‐based navigation. Cerebral Cortex, 25(11), 4146–4154. 10.1093/cercor/bhu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz, D. , Wittfeld, K. , Terock, J. , Freyberger, H. J. , Hegenscheid, K. , Völzke, H. , Habes, M. , Hosten, N. , Friedrich, N. , Nauck, M. , Domanska, G. , & Grabe, H. J. (2015). Association between waist circumference and gray matter volume in 2344 individuals from two adult community‐based samples. Neuroimage, 122, 149–157. 10.1016/j.neuroimage.2015.07.086 [DOI] [PubMed] [Google Scholar]
- Jauch‐Chara, K. , Binkofski, F. , Loebig, M. , Reetz, K. , Jahn, G. , Melchert, U. H. , Schweiger, U. , & Oltmanns, K. M. (2015). Blunted brain energy consumption relates to insula atrophy and impaired glucose tolerance in obesity. Diabetes, 64(6), 2082–2091. 10.2337/db14-0421 [DOI] [PubMed] [Google Scholar]
- Jones, R. B. , McKie, S. , Astbury, N. , Little, T. J. , Tivey, S. , Lassman, D. J. , McLaughlin, J. , Luckman, S. , Williams, S. R. , Dockray, G. J. , & Thompson, D. G. (2012). Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut, 61(11), 1543–1551. 10.1136/gutjnl-2011-301323 [DOI] [PubMed] [Google Scholar]
- Joos, A. , Klöppel, S. , Hartmann, A. , Glauche, V. , Tüscher, O. , Perlov, E. , Saum, B. , Freyer, T. , Zeeck, A. , & Van Elst, L. T. (2010). Voxel‐based morphometry in eating disorders: Correlation of psychopathology with grey matter volume. Psychiatry Research: Neuroimaging, 182(2), 146–151. 10.1016/j.pscychresns.2010.02.004 [DOI] [PubMed] [Google Scholar]
- Kakoschke, N. , Lorenzetti, V. , Caeyenberghs, K. , & Verdejo‐García, A. (2019). Impulsivity and body fat accumulation are linked to cortical and subcortical brain volumes among adolescents and adults. Scientific Reports, 9, Article 2580. 10.1038/s41598-019-38846-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, I. , Iritani, S. , Riku, Y. , Umeda, K. , Takase, M. , Ikeda, K. , Niizato, K. , Arai, T. , Yoshida, M. , Oshima, K. , & Hasegawa, M. (2022). Neuropathological investigation of patients with prolonged anorexia nervosa. Psychiatry and Clinical Neurosciences, 76(5), 187–194. 10.1111/pcn.13340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye, W. H. , Wierenga, C. E. , Bailer, U. F. , Simmons, A. N. , & Bischoff‐Grethe, A. (2013). Nothing tastes as good as skinny feels: The neurobiology of anorexia nervosa. Trends in Neurosciences, 36(2), 110–120. 10.1016/j.tins.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, R. M. , & Strick, P. L. (2003). Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience, 23(23), 8432–8444. 10.1523/JNEUROSCI.23-23-08432.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharabian Masouleh, S. , Arélin, K. , Horstmann, A. , Lampe, L. , Kipping, J. A. , Luck, T. , Riedel‐Heller, S. G. , Schroeter, M. L. , Stumvoll, M. , Villringer, A. , & Witte, A. V. (2016). Higher body mass index in older adults is associated with lower gray matter volume: Implications for memory performance. Neurobiology of Aging, 40, 1–10. 10.1016/j.neurobiolaging.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Koziol, L. F. , Budding, D. , Andreasen, N. , D'arrigo, S. , Bulgheroni, S. , Imamizu, H. , Ito, M. , Manto, M. , Marvel, C. , Parker, K. , Pezzulo, G. , Ramnani, N. , Riva, D. , Schmahmann, J. , Vandervert, L. , & Yamazaki, T. (2014). Consensus paper: The cerebellum's role in movement and cognition. The Cerebellum, 13(1), 151–177. 10.1007/s12311-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronemer, S. I. , Slapik, M. B. , Pietrowski, J. R. , Margron, M. J. , Morgan, O. P. , Bakker, C. C. , Rosenthal, L. S. , Onyike, C. U. , & Marvel, C. L. (2021). Neuropsychiatric symptoms as a reliable phenomenology of cerebellar ataxia. The Cerebellum, 20, 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth, F. , Levitt, J. G. , Phillips, O. R. , Luders, E. , Woods, R. P. , Mazziotta, J. C. , Toga, A. W. , & Narr, K. L. (2013). Relationships between gray matter, body mass index, and waist circumference in healthy adults. Human Brain Mapping, 34(7), 1737–1746. 10.1002/hbm.22021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio, M. , & Olivito, G. (2018). Topography of the cerebellum in relation to social brain regions and emotions. In Heilman K. M. & Nadeau S. E. (Eds.), Handbook of clinical neurology (Vol. 154, pp. 71–84). Elsevier. [DOI] [PubMed] [Google Scholar]
- Leibovitz, Z. , Mandel, H. , Falik‐Zaccai, T. C. , Ben Harouch, S. , Savitzki, D. , Krajden‐Haratz, K. , Gindes, L. , Tamarkin, M. , Lev, D. , Dobyns, W. B. , & Lerman‐Sagie, T. (2018). Walker‐Warburg syndrome and tectocerebellar dysraphia: A novel association caused by a homozygous DAG1 mutation. European Journal of Paediatric Neurology, 22(3), 525–531. 10.1016/j.ejpn.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Leiner, H. C. , Leiner, A. L. , & Dow, R. S. (1986). Does the cerebellum contribute to mental skills? Behavioral Neuroscience, 100(4), 443–454. [DOI] [PubMed] [Google Scholar]
- Lenhart, L. , Gander, M. , Steiger, R. , Dabkowska‐Mika, A. , Mangesius, S. , Haid‐Stecher, N. , Fuchs, M. , Buchheim, A. , Sevecke, K. , & Gizewski, E. R. (2022). Attachment status is associated with grey matter recovery in adolescent anorexia nervosa: Findings from a longitudinal study. European Journal of Neuroscience, 55(5), 1373–1387. 10.1111/ejn.15614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low, A. Y. T. , Goldstein, N. , Gaunt, J. R. , Huang, K.‐P. , Zainolabidin, N. , Yip, A. K. K. , Carty, J. R. E. , Choi, J. Y. , Miller, A. M. , Ho, H. S. T. , Lenherr, C. , Baltar, N. , Azim, E. , Sessions, O. M. , Ch'ng, T. H. , Bruce, A. S. , Martin, L. E. , Halko, M. A. , Brady, R. O. , … Betley, J. N (2021). Reverse‐translational identification of a cerebellar satiation network. Nature, 600(7888), 269–273. 10.1038/s41586-021-04143-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupo, M. , Troisi, E. , Chiricozzi, F. R. , Clausi, S. , Molinari, M. , & Leggio, M. (2015). Inability to process negative emotions in cerebellar damage: A functional transcranial doppler sonographic study. The Cerebellum, 14(6), 663–669. 10.1007/s12311-015-0662-z [DOI] [PubMed] [Google Scholar]
- Madsen, M. B. , Birck, M. M. , Fredholm, M. , & Cirera, S. (2009). Expression studies of the obesity candidate gene FTO in pig. Animal Biotechnology, 21(1), 51–63. 10.1080/10495390903381792 [DOI] [PubMed] [Google Scholar]
- Marron, E. M. , Viejo‐Sobera, R. , Cuatrecasas, G. , Redolar‐Ripoll, D. , Lorda, P. G. , Datta, A. , Bikson, M. , Magerowski, G. , & Alonso‐Alonso, M. (2019). Prefronto‐cerebellar neuromodulation affects appetite in obesity. International Journal of Obesity, 43(10), 2119–2124. 10.1038/s41366-018-0278-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. H. , Cooper, S. E. , Hacking, A. , & Ghez, C. (2000). Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. Journal of Neurophysiology, 83(4), 1886–1899. 10.1152/jn.2000.83.4.1886 [DOI] [PubMed] [Google Scholar]
- Martín‐Pérez, C. , Contreras‐Rodríguez, O. , Vilar‐López, R. , & Verdejo‐García, A. (2019). Hypothalamic networks in adolescents with excess weight: Stress‐related connectivity and associations with emotional eating. Journal of the American Academy of Child & Adolescent Psychiatry, 58(2), 211–220. [DOI] [PubMed] [Google Scholar]
- McTaggart, J. S. , Lee, S. , Iberl, M. , Church, C. , Cox, R. D. , & Ashcroft, F. M. (2011). FTO is expressed in neurones throughout the brain and its expression is unaltered by fasting. PLoS ONE, 6(11), Article e27968. 10.1371/journal.pone.0027968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza, J. , Pévet, P. , Felder‐Schmittbuhl, M.‐P. , Bailly, Y. , & Challet, E. (2010). The cerebellum harbors a circadian oscillator involved in food anticipation. Journal of Neuroscience, 30(5), 1894–1904. 10.1523/JNEUROSCI.5855-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. L. , Couch, J. , Schwenk, K. , Long, M. , Towler, S. , Theriaque, D. W. , He, G. , Liu, Y. , Driscoll, D. J. , & Leonard, C. M. (2009). Early childhood obesity is associated with compromised cerebellar development. Developmental Neuropsychology, 34(3), 272–283. 10.1080/87565640802530961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milos, G. , Kaufmann, L. K. , Jäncke, L. , Piccirelli, M. , Blatow, M. , Martin‐Soelch, C. , von Känel, R. , Hänggi, J. , & Baur, V. (2021). Does local cerebellar volume predict treatment success in anorexia nervosa? Psychiatry Research: Neuroimaging, 317, Article 111355. [DOI] [PubMed] [Google Scholar]
- Mishima, R. , Isobe, M. , Noda, T. , Tose, K. , Kawabata, M. , Noma, S. , & Murai, T. (2021). Structural brain changes in severe and enduring anorexia nervosa: A multimodal magnetic resonance imaging study of gray matter volume, cortical thickness, and white matter integrity. Psychiatry Research: Neuroimaging, 318, Article 111393. 10.1016/j.pscychresns.2021.111393 [DOI] [PubMed] [Google Scholar]
- Molinari, M. , Chiricozzi, F. R. , Clausi, S. , Tedesco, A. M. , De Lisa, M. , & Leggio, M. G. (2008). Cerebellum and detection of sequences, from perception to cognition. The Cerebellum, 7(4), 611–615. 10.1007/s12311-008-0060-x [DOI] [PubMed] [Google Scholar]
- Moreno‐Rius, J. , & Miquel, M. (2017). The cerebellum in drug craving. Drug and Alcohol Dependence, 173, 151–158. 10.1016/j.drugalcdep.2016.12.028 [DOI] [PubMed] [Google Scholar]
- Moyse, E. , Arsenault, M. , Gaudreau, P. , Ferland, G. , & Ramassamy, C. (2019). Brain region‐specific effects of long‐term caloric restriction on redox balance of the aging rat. Mechanisms of Ageing and Development, 179, 51–59. 10.1016/j.mad.2019.01.002 [DOI] [PubMed] [Google Scholar]
- Mueller, K. , Sacher, J. , Arelin, K. , Holiga, Š. , Kratzsch, J. , Villringer, A. , & Schroeter, M. L. (2012). Overweight and obesity are associated with neuronal injury in the human cerebellum and hippocampus in young adults: A combined MRI, serum marker and gene expression study. Translational Psychiatry, 2(12), Article e200. 10.1038/tp.2012.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara, Y. , Nakamae, T. , Nishizawa, S. , Mizuhara, Y. , Moritoki, Y. , Wada, Y. , Sakai, Y. , Yamashita, T. , Narumoto, J. , Miyata, J. , Yamada, K. , & Fukui, K. (2014). A tract‐based spatial statistics study in anorexia nervosa: Abnormality in the fornix and the cerebellum. Progress in Neuro‐Psychopharmacology and Biological Psychiatry, 51, 72–77. 10.1016/j.pnpbp.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Noori, H. R. , Cosa Linan, A. , & Spanagel, R. (2016). Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta‐analysis. European Neuropsychopharmacology, 26(9), 1419–1430. 10.1016/j.euroneuro.2016.06.013 [DOI] [PubMed] [Google Scholar]
- O'Hara, C. B. , Campbell, I. C. , & Schmidt, U. (2015). A reward‐centred model of anorexia nervosa: A focussed narrative review of the neurological and psychophysiological literature. Neuroscience & Biobehavioral Reviews, 52, 131–152. [DOI] [PubMed] [Google Scholar]
- Olivo, G. , Swenne, I. , Zhukovsky, C. , Tuunainen, A.‐K. , Salonen‐Ros, H. , Larsson, E.‐M. , Gaudio, S. , Brooks, S. J. , & Schiöth, H. B. (2018). Reduced resting‐state connectivity in areas involved in processing of face‐related social cues in female adolescents with atypical anorexia nervosa. Translational Psychiatry, 8(1), Article 275. 10.1038/s41398-018-0333-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostland, M. , & Hooft, J. A. (2016). Serotonin in the cerebellum. In Gruol D., Koibuchi N., Manto M., Molinari M., Schmahmann J., & Shen Y. (Eds.), Essentials of cerebellum and cerebellar disorders. (pp. 243–247). Springer. [Google Scholar]
- Ou, X. , Andres, A. , Pivik, R. T. , Cleves, M. A. , & Badger, T. M. (2015). Brain gray and white matter differences in healthy normal weight and obese children. Journal of Magnetic Resonance Imaging, 42(5), 1205–1213. 10.1002/jmri.24912 [DOI] [PubMed] [Google Scholar]
- Oya, S. , Nejo, T. , Indo, M. , & Matsui, T. (2014). Pearls & Oy‐sters: Anorexia and emaciation in patients with cerebellar hemangioblastoma. Neurology, 83(14), 1298–1300. [DOI] [PubMed] [Google Scholar]
- Pezzulo, G. (2012). An Active Inference view of cognitive control. Frontiers in Psychology, 3, Article 478. 10.3389/fpsyg.2012.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipou, A. , Rossell, S. L. , Gurvich, C. , Castle, D. J. , Abel, L. A. , Nibbs, R. G. , & Hughes, M. E. (2018). Differences in regional grey matter volumes in currently ill patients with anorexia nervosa. European Journal of Neuroscience, 47(2), 177–183. 10.1111/ejn.13793 [DOI] [PubMed] [Google Scholar]
- Pineda‐Alhucema, W. , Aristizabal, E. , Escudero‐Cabarcas, J. , Acosta‐López, J. E. , & Vélez, J. I. (2018). Executive function and theory of mind in children with ADHD: A systematic review. Neuropsychology Review, 28(3), 341–358. 10.1007/s11065-018-9381-9 [DOI] [PubMed] [Google Scholar]
- Pu, M. , Heleven, E. , Delplanque, J. , Gibert, N. , Ma, Q. , Funghi, G. , & Van Overwalle, F. (2020). The posterior cerebellum supports the explicit sequence learning linked to trait attribution. Cognitive, Affective & Behavioral Neuroscience, 20(4), 798–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Gutierrez, D. , Awwad, A. , Meijer, L. , Manita, M. , Jaspan, T. , Dineen, R. A. , Grundy, R. G. , & Auer, D. P. (2014). Metrics and textural features of MRI diffusion to improve classification of pediatric posterior fossa tumors. American Journal of Neuroradiology, 35(5), 1009–1015. 10.3174/ajnr.A3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls, B. J. , Roe, L. S. , & Meengs, J. S. (2006). Reductions in portion size and energy density of foods are additive and lead to sustained decreases in energy intake. The American Journal of Clinical Nutrition, 83(1), 11–17. 10.1093/ajcn/83.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnefarth, M. , Hanisch, N. , Brandt, A. U. , Mähler, A. , Endres, M. , Paul, F. , & Doss, S. (2020). Dysphagia affecting quality of life in cerebellar ataxia—A large survey. The Cerebellum, 19(3), 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, L. J. , Capra, S. , Baguley, B. , Sinclair, K. , Munro, K. , Lewindon, P. , & Lavin, M. (2015). Nutritional status of patients with ataxia‐telangiectasia: A case for early and ongoing nutrition support and intervention. Journal of Paediatrics and Child Health, 51(8), 802–807. 10.1111/jpc.12828 [DOI] [PubMed] [Google Scholar]
- Saker, P. , Farrell, M. J. , Adib, F. R. M. , Egan, G. F. , McKinley, M. J. , & Denton, D. A. (2014). Regional brain responses associated with drinking water during thirst and after its satiation. Proceedings of the National Academy of Sciences of the United States of America, 111(14), 5379–5384. 10.1073/pnas.1403382111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Kuhn, A. , Medina, Y. , Pérez‐García, M. , Martínez‐Sola, M. , De Haro, P. , Flores, P. , & Sánchez‐Santed, F. (2017). Neurorehabilitation treatment of dysphagia after‐stroke with transcranial direct current stimulation: A clinical case. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 10(2), 431. 10.1016/j.brs.2017.01.283 [DOI] [Google Scholar]
- Sanders, N. , Smeets, P. A. M. , Van Elburg, A. A. , Danner, U. N. , Van Meer, F. , Hoek, H. W. , & Adan, R. A. H. (2015). Altered food‐cue processing in chronically ill and recovered women with anorexia nervosa. Frontiers in Behavioral Neuroscience, 9, Article 46. 10.3389/fnbeh.2015.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Guell, X. , Stoodley, C. J. , & Halko, M. A. (2019). The theory and neuroscience of cerebellar cognition. Annual Review of Neuroscience, 42, 337–364. 10.1146/annurev-neuro-070918-050258 [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , & Pandya, D. N. (1997). The cerebrocerebellar system. International Review of Neurobiology, 41, 31–60. 10.1016/S0074-7742(08)60346-3 [DOI] [PubMed] [Google Scholar]
- Seeley, W. W. , Menon, V. , Schatzberg, A. F. , Keller, J. , Glover, G. H. , Kenna, H. , Reiss, A. L. , & Greicius, M. D. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, J. , Belheouane, M. , Schulz, N. , Dempfle, A. , Baines, J. F. , & Herpertz‐Dahlmann, B. (2019). The impact of starvation on the microbiome and gut‐brain interaction in anorexia nervosa. Frontiers in Endocrinology, 10, Article 41. 10.3389/fendo.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, J. , Konrad, K. , & Herpertz‐Dahlmann, B. (2018). Extend, pathomechanism and clinical consequences of brain volume changes in anorexia nervosa. Current Neuropharmacology, 16(8), 1164–1173. 10.2174/1570159X15666171109145651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, H. , Li, P. , Liu, H. , Nie, B. , Yin, X. , Zhang, T. , Sun, X. , Zhang, W. , Feng, T. , Wang, L. , Hu, Y. , Dong, G. , Gao, H. , Du, J. , Ma, L. , Li, D. , & Shan, B. (2019). Gray matter reduction related to decreased serum creatinine and increased triglyceride, Hemoglobin A1C, and low‐density lipoprotein in subjects with obesity. Neuroradiology, 61(6), 703–710. 10.1007/s00234-019-02202-3 [DOI] [PubMed] [Google Scholar]
- Shen, K.‐K. , Welton, T. , Lyon, M. , McCorkindale, A. N. , Sutherland, G. T. , Burnham, S. , Fripp, J. , Martins, R. , & Grieve, S. M. (2020). Structural core of the executive control network: A high angular resolution diffusion MRI study. Human Brain Mapping, 41(5), 1226–1236. 10.1002/hbm.24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe, E. R. (2014). Independent and collaborative contributions of the cerebral hemispheres to emotional processing. Frontiers in Human Neuroscience, 8, Article 230. 10.3389/fnhum.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin, S. , Gonul, E. , Kahraman, S. , & Timurkaynak, E. (2005). Imaging of posterior fossa epidermoid tumors. Clinical Neurology and Neurosurgery, 107(6), 461–467. 10.1016/j.clineuro.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Smink, F. R. E. , Van Hoeken, D. , & Hoek, H. W. (2013). Epidemiology, course, and outcome of eating disorders. Current Opinion in Psychiatry, 26(6), 543–548. 10.1097/YCO.0b013e328365a24f [DOI] [PubMed] [Google Scholar]
- Spyridaki, E. C. , Avgoustinaki, P. D. , & Margioris, A. N. (2016). Obesity, inflammation and cognition. Current Opinion in Behavioral Sciences, 9, 169–175. 10.1016/j.cobeha.2016.05.004 [DOI] [Google Scholar]
- Steenhuis, I. , & Poelman, M. (2017). Portion size: Latest developments and interventions. Current Obesity Reports, 6(1), 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley, C. , & Schmahmann, J. (2009). Functional topography in the human cerebellum: A meta‐analysis of neuroimaging studies. Neuroimage, 44(2), 489–501. 10.1016/j.neuroimage.2008.08.039 [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. (2012). The cerebellum and cognition: Evidence from functional imaging studies. The Cerebellum, 11(2), 352–365. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , & Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex, 46(7), 831–844. 10.1016/j.cortex.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley, C. J. , Valera, E. M. , & Schmahmann, J. D. (2012). Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study. Neuroimage, 59(2), 1560–1570. 10.1016/j.neuroimage.2011.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supple, W. F., Jr. , & Kapp, B. S. (1993). The anterior cerebellar vermis: Essential involvement in classically conditioned bradycardia in the rabbit. Journal of Neuroscience, 13(9), 3705–3711. 10.1523/JNEUROSCI.13-09-03705.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeting, H. , Walker, L. , MacLean, A. , Patterson, C. , Räisänen, U. , & Hunt, K. (2015). Prevalence of eating disorders in males: A review of rates reported in academic research and UK mass media. International Journal of Men's Health, 14(2). 10.3149/jmh.1402.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H. , Koeda, M. , Oda, K. , Matsuda, T. , Matsushima, E. , Matsuura, M. , Asai, K. , & Okubo, Y. (2004). An fMRI study of differential neural response to affective pictures in schizophrenia. Neuroimage, 22(3), 1247–1254. 10.1016/j.neuroimage.2004.03.028 [DOI] [PubMed] [Google Scholar]
- Tomasi, D. , Wang, G. J. , Wang, R. , Caparelli, E. C. , Logan, J. , & Volkow, N. D. (2015). Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors. Human Brain Mapping, 36(1), 120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuulari, J. J. , Karlsson, H. K. , Antikainen, O. , Hirvonen, J. , Pham, T. , Salminen, P. , Helmiö, M. , Parkkola, R. , Nuutila, P. , & Nummenmaa, L. (2016). Bariatric surgery induces white and grey matter density recovery in the morbidly obese: A voxel‐based morphometric study. Human Brain Mapping, 37(11), 3745–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utz, A. , Thürling, M. , Ernst, T. M. , Hermann, A. , Stark, R. , Wolf, O. T. , Timmann, D. , & Merz, C. J. (2015). Cerebellar vermis contributes to the extinction of conditioned fear. Neuroscience Letters, 604, 173–177. 10.1016/j.neulet.2015.07.026 [DOI] [PubMed] [Google Scholar]
- Van Galen, K. A. , Ter Horst, K. W. , Booij, J. , La Fleur, S. E. , & Serlie, M. J. (2018). The role of central dopamine and serotonin in human obesity: Lessons learned from molecular neuroimaging studies. Metabolism, 85, 325–339. 10.1016/j.metabol.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. , Ma, Q. , & Heleven, E. (2020). The posterior crus II cerebellum is specialized for social mentalizing and emotional self‐experiences: A meta‐analysis. Social Cognitive and Affective Neuroscience, 15(9), 905–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeling, A. (1996). Epidemiology of anorexia nervosa. Psychiatry Research, 62(1), 3–9. 10.1016/0165-1781(96)02983-6 [DOI] [PubMed] [Google Scholar]
- Walther, K. , Birdsill, A. C. , Glisky, E. L. , & Ryan, L. (2010). Structural brain differences and cognitive functioning related to body mass index in older females. Human Brain Mapping, 31(7), 1052–1064. 10.1002/hbm.20916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Wen, B. , Cheng, J. , & Li, H. (2017). Brain structural differences between normal and obese adults and their links with lack of perseverance, negative urgency, and sensation seeking. Scientific Reports, 7, Article 40595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Rao, K. , Yuan, L. , Everaert, N. , Buyse, J. , Grossmann, R. , & Zhao, R. (2012). Chicken FTO gene: Tissue‐specific expression, brain distribution, breed difference and effect of fasting. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 163(3‐4), 246–252. [DOI] [PubMed] [Google Scholar]
- Watson, H. J. , Yilmaz, Z. , Thornton, L. M. , Hübel, C. , Coleman, J. R. I. , Gaspar, H. A. , Bryois, J. , Hinney, A. , Leppä, V. M. , Mattheisen, M. , Medland, S. E. , Ripke, S. , Yao, S. , Giusti‐Rodríguez, P. , Hanscombe, K. B. , Purves, K. L. , Adan, R. A. H. , Alfredsson, L. , Ando, T. , … Bulik, C. M. (2019). Genome‐wide association study identifies eight risk loci and implicates metabo‐psychiatric origins for anorexia nervosa. Nature Genetics, 51(8), 1207–1214. 10.1038/s41588-019-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise, C. M. , Bachmann, T. , & Pleger, B. (2019). Brain structural differences in monozygotic twins discordant for body mass index. Neuroimage, 201, Article 116006. 10.1016/j.neuroimage.2019.07.019 [DOI] [PubMed] [Google Scholar]
- Weise, C. M. , Thiyyagura, P. , Reiman, E. M. , Chen, K. , & Krakoff, J. (2013). Fat‐free body mass but not fat mass is associated with reduced gray matter volume of cortical brain regions implicated in autonomic and homeostatic regulation. Neuroimage, 64, 712–721. 10.1016/j.neuroimage.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2014). Global status report on noncommunicable diseases 2014. Author. [Google Scholar]
- Wright, H. , Li, X. , Fallon, N. B. , Crookall, R. , Giesbrecht, T. , Thomas, A. , Halford, J. C. G. , Harrold, J. , & Stancak, A. (2016). Differential effects of hunger and satiety on insular cortex and hypothalamic functional connectivity. European Journal of Neuroscience, 43(9), 1181–1189. 10.1111/ejn.13182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, N. , Chen, Y. , Yang, J. , & Li, F. (2017). Childhood obesity and academic performance: The role of working memory. Frontiers in Psychology, 8, Article 611. 10.3389/fpsyg.2017.00611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q. , Xiao, T. , Guo, J. , & Su, Z. (2017). Complex relationship between obesity and the fat mass and obesity locus. International Journal of Biological Sciences, 13(5), 615–629. 10.7150/ijbs.17051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Shields, G. S. , Guo, C. , & Liu, Y. (2018). Executive function performance in obesity and overweight individuals: A meta‐analysis and review. Neuroscience & Biobehavioral Reviews, 84, 225–244. [DOI] [PubMed] [Google Scholar]
- Yao, L. , Li, W. , Dai, Z. , & Dong, C. (2016). Eating behavior associated with gray matter volume alternations: A voxel based morphometry study. Appetite, 96, 572–579. 10.1016/j.appet.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Zhang, H. , & Rodriguez‐Monguio, R. (2012). Racial disparities in the risk of developing obesity‐related diseases: A cross‐sectional study. Ethnicity & Disease, 22(3), 308–316. [PubMed] [Google Scholar]
- Zhang, S. , Wang, W. , Su, X. , Kemp, G. J. , Yang, X. , Su, J. , Tan, Q. , Zhao, Y. , Sun, H. , Yue, Q. , & Gong, Q. (2018). Psychoradiological investigations of gray matter alterations in patients with anorexia nervosa. Translational Psychiatry, 8, Article 277. 10.1038/s41398-018-0323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J. , Li, M. , Zhang, Y. , Song, H. , Von Deneen, K. M. , Shi, Y. , Liu, Y. , & He, D. (2017). Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: An fMRI study. Brain Imaging and Behavior, 11(1), 264–277. 10.1007/s11682-015-9491-4 [DOI] [PubMed] [Google Scholar]
- Zhu, J.‐N. , & Wang, J.‐J. (2008). The cerebellum in feeding control: Possible function and mechanism. Cellular and Molecular Neurobiology, 28(4), 469–478. 10.1007/s10571-007-9236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Sacco, T. , Strata, P. , & Sacchetti, B. (2011). Basolateral amygdala inactivation impairs learning‐induced long‐term potentiation in the cerebellar cortex. PLoS ONE, 6(1), Article e16673. 10.1371/journal.pone.0016673 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Data Availability Statement
Additional data supporting study findings are available from the corresponding author upon request.


