Abstract
Introduction
Demographics are important prognostic factors in malignant diseases. A nationwide analysis concerning the prognostic impact of demographics in head and neck cancer (HNC) patients (HNCP) has not been performed previously.
Methods
A retrospective analysis of data from the Center for Cancer Registry Data (ZfKD) and the Federal Statistical Office (Destatis) between 2002 and 2017 was performed. A total of 212′920 HNCP were included. Incidence, tumor stage, age development, sex distribution, age‐, residence‐, and diagnosis‐time‐specific survival were examined.
Results
Mean age of HNCP increased more rapidly than in the general population (slope coefficient: 0.29 vs. 0.20; p < 0.0001). Higher age and male sex were associated with a worse prognosis. Whereas overall survival (OS) increased from the early to the later observation period for HNCP <70 years, no OS improvement for HNCP >70 years was found. Furthermore, an OS disadvantage was observed for East Germany compared to West Germany (median 47 vs. 60 months; p < 0.0001). This disparity was associated with a disproportionately high ratio of men in East Germany (men/women: 4.4 vs. 3.1; p < 0.0001) and a lower mean age (61 vs. 63 years; p < 0.0001). In addition to stage, age and sex, residence in East Germany were confirmed as an independent factor for OS in a multivariate analysis.
Conclusion
Finally, three decades after the German reunion, a survival disadvantage for patients in East Germany still exists. This discrepancy may be a result of socioeconomic disparities.
Keywords: age, cancer registry data, demographic change, socioeconomic health disparity
1. INTRODUCTION
According to data from the Global Cancer Observatory (GLOBOCAN) in 2020, there were 921′462 reported cases of head and neck cancer (HNC), resulting in 447′307 deaths worldwide. 1 The disease exhibited a male‐to‐female ratio of 3:1. 1 In addition to well‐known risk factors such as smoking, alcohol consumption, and human papillomavirus (HPV) infections, 2 , 3 , 4 age plays a significant role in increasing the susceptibility of various tissues to cancer development due to cellular damage accumulation and alterations in the endocrine and immune systems. 5
For the purposes of our study, we focused specifically on Germany, a central European country bordered by nine nations. The country's geography varies, with the northern part characterized by low‐lying plains and coastal areas along the Baltic and North Seas, while the southern regions are dominated by the Bavarian hills and Alps. Prior to the reunification in 1990, Germany was divided into two entities: the Federal Republic of Germany (BRD) in the West and the German Democratic Republic (DDR) in the East. Consequently, different political, economic, and social systems evolved in the East and West over the years.
Presently, high German smoking rates (27% of men and 19% of women smoked daily in 2019 6 ) and an increasing prevalence of HPV (up to 50%) in oropharyngeal cancers have been observed in East Germany. 7 The percentage of elderly and multimorbid patients is rising, resulting in higher healthcare costs throughout Germany. 8 Additionally, older patients face a greater risk of cancer‐related mortality and may not receive the full standard treatment due to various factors. As a result, they may not benefit from advancements in treatment that have occurred over the past two decades. 9 Recently, our research revealed a significant increase in the annual incidence rates and mean age of patients at a tertiary cancer center in Germany, particularly among those aged over 70 years. 10 Gender has been recognized as a prognostic factor, 11 and the region of residence may be associated with socioeconomic status, which is also considered an important prognostic factor. 12 However, despite available data from specific regions of Germany or previous evaluations of cancer registry data, nationwide analyses exploring the prognostic association of demographic factors in Germany are lacking. 13 , 14 , 15
Thus, the objective of our study was to determine the impact of age, gender, and region of residence on the overall survival (OS) of HNC patients in a comprehensive German nationwide dataset.
2. PATIENTS AND METHODS
2.1. Data collection
We requested anonymized data from the German Center of Cancer Registry (‘Zentrum für Krebsregisterdaten’ (ZFKD)) of patients newly diagnosed with HNC between 2002 and 2017. 16 We received data of 212′920 HNCP including sex, anonymized date of birth, county affiliation, rounded date of diagnosis, TNM (tumor, nodal, metastasis) status, and date of mortality follow‐up (East Germany: December 2015, West Germany: December 2017) with the endpoint vital status. Information on Epstein–Barr virus or human papillomavirus was not available. Furthermore, we requested data concerning age and sex of the general German population in between 2002 and 2017 at the central information service of the State Statistical Office Baden‐Wuertemberg (‘Statistisches Bundesamt, Zentraler Auskunftsdienst’), which afterwards coordinated the data retrieval from the 16 individual countries and their statistical offices.
2.2. Data exclusion
For the analysis of HNC incidence, TNM status and demographics of HNCP we were able to use the major part of the data set (n = 212′920). Nevertheless, sometimes data of TNM status was missing (Table 1, Table S1). For 45.1% of HNCP no UICC stage could be created because of at least partly missing TNM status (mostly M status). The UICC stage calculation was based on the 8th version, which was simplified to four stages without subgroups and in which all oropharyngeal carcinomas were included according to p16‐negative classification (because of missing information of HPV status in registry data). It is also of importance that the federal state of Baden‐Wuerttemberg started reporting data to the cancer registry beginning in 2009 after reporting of cancer registry data became obligatory, legal requirement (Federal Cancer Registry Data Law; 18.08.2009). A delay in reporting seemed likely to occur in 2016 and 2017. In fact, no HNC reports were received from East Germany in 2017. Therefore, we have not analyzed TNM status, mean age and sex of HNCP development in relation to residence during the years 2016 and 2017.
TABLE 1.
Cohort characteristics. An overview of the entirety of all included HNCP in terms of their primary sites, stage, gender, mean age, age group and residence is provided.
| Total number and percent | Gender | Mean age and standard deviation | Age group | Residence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | <50 years | 50–69 years | ≥70 years | East | West | |||||
| Primary site | |||||||||||
| Oropharynx | 66,276 | 31.1 | 49,985 | 16,291 | 61.9 | 10.6 | 7938 | 43,627 | 14,711 | 16,910 | 49,366 |
| Oral cavity | 30,011 | 14.1 | 21,135 | 8876 | 62.9 | 11.8 | 3947 | 17,961 | 8103 | 7473 | 22,538 |
| Larnyx | 52,480 | 24.6 | 45,527 | 6953 | 65.6 | 10.8 | 3817 | 30,264 | 18,399 | 11,431 | 41,049 |
| Hypopharynx | 25,080 | 11.8 | 21,574 | 3506 | 62.3 | 10.0 | 2676 | 16,697 | 5707 | 6181 | 18,899 |
| Nasopharynx | 5240 | 2.5 | 3781 | 1459 | 58.5 | 15.3 | 1346 | 2677 | 1217 | 1099 | 4141 |
| Others | 33,833 | 15.9 | 22,132 | 11,701 | 63.4 | 12.9 | 4759 | 18,625 | 10,449 | 7139 | 26,694 |
| Stage | |||||||||||
| UICC I | 22,041 | 10.4 | 16,787 | 5254 | 63.0 | 11.3 | 2720 | 13,054 | 6267 | 6936 | 15,105 |
| UICC II | 14,199 | 6.7 | 10,784 | 3415 | 62.6 | 11.2 | 1762 | 8744 | 3693 | 4566 | 9633 |
| UICC III | 19,164 | 9.0 | 14,646 | 4518 | 61.9 | 11.1 | 2588 | 12,053 | 4523 | 5938 | 13,226 |
| UICC IV | 61,536 | 28.9 | 49,600 | 11,936 | 61.4 | 10.3 | 7974 | 40,845 | 12,717 | 20,289 | 41,247 |
| Missing (no full TNM) | 95,980 | 45.1 | |||||||||
Note: ‘Others’ include for example, salivary glands or sinunasal cancers.
For survival analyses HNCP with missing data on survival time and status were excluded. In addition, patients marked as deceased, but without a date of death and those alive, but without a date of last follow‐up or DCO (death certificate only) cases were excluded for survival analysis too. Hence, the cohort available was reduced to n = 193′877.
2.3. Data analysis
Inhomogeneous data sets from the statistical offices were harmonized and combined in Microsoft Excel 2019. The German Center for Cancer Registry delivered summarized data in an excel file. Data were imported to IMB SPSS Statistics 26 and GraphPad Prism 9 for statistical testing. Figures were arranged in GraphPad Prism 9 and Microsoft Power Point 2019 and the tables were created in Microsoft Excel 2019.
2.4. Statistical analysis
To check whether mean values among two groups were significantly different, we first tested normality by Shapiro–Wilk test (n ≤ 50) or Kolmogorov–Smirnov test (n > 50) and homoscedasticity by Levene's test. Unpaired t‐test was performed if normal distribution and homoscedasticity were present or alternatively a Welch test if normal distribution was given but homoscedasticity was absent. In cases of missing normality, we applied nonparametric Mann–Whitney U test to compare scaled values among two groups. Differences of slopes, assuming a linear relationship, were tested with Deming regression analysis. Deviation of the slope coefficient from zero was then tested by F test. Standard deviation was abbreviated with ‘SD’. Chi‐squared test was used to examine differences in distribution of categorial variables among large sample sizes. Survival data was generated by Kaplan–Meier estimator. Differences in survival data were tested with (pairwise) Log rank tests. Standard error was abbreviated with ‘SEM’. 95% confidence intervals (CIs) were given. Multivariate analysis was performed by Cox regression with backward selection of variables after model fitting by Omnibus tests providing hazard ratios (HR) with 95% CI and level of significance (p).
3. RESULTS
3.1. Patients' characteristics
A total of 212′920 cases were included in the analysis of German cancer registry data between the years 2002 and 2017 (Table 1). The mean age in the cohort was 63.2 years (SD = 11.4). The ratio of men to women was 3.4. Most patients had oropharyngeal (31.1%) and laryngeal cancers (24.6%), locally limited (T1, T2) primary tumor status (41.9%), presence of regional lymph node metastases (38.3%) and no distant metastases (54.8%) at initial diagnosis (Table S1). However, incomplete data were frequent (T: 23.1%; N: 32.8%; M: 41.8%). The largest group of HNCP (28.9%) had UICC Stage IV at initial diagnosis (missing data in 45.1%). Median survival in the total cohort was 57 months (SEM = 0.4; CI: 56.3–57.7).
3.2. Increasing proportion of older HNCP
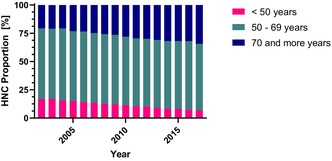
The incidence per 100′000 inhabitants was calculated in relation to data from the State Statistical Office for the general population in Germany for each year between 2002 and 2017. The incidence of HNC was rising from 11.5 in 2002 to 18.3 in 2015 and followed by a decrease to 12.6 in 2017 (Figure 1A). Due to late initiation of data reporting from Baden Württemberg to the cancer registry and a reporting delay renders incidence rates after 2015 unreliable. A clear and steady increase in the proportion of older HNCP (≥ 70 years) between 2002 and 2017 from 20.6% to 34.4% (Figure 1B) was observed. In the same time period, the proportion of younger HNCP (< 50 years) decreased from 16.7% to 6.8%, whereas the proportion of HNCP 50–69 years old remained stably between 62.7% and 58.9%. Meanwhile, OS was significantly shorter in the two older age groups compared to younger patients (Figure 1C). The median survival is 109 months (SEM = 2.1; CI: 104.8–113.2) for young (<50 years), 63 months (SEM = 0.5; CI: 62.0–64.0) for medium old (50–69 years) and 35 months (SEM = 0.4; CI: 34.2–35.8) for old (≥70 years) HNCP (p < 0.0001). The mean incidence in relation to the population numbers of the 2011 census did not differ significantly (p = 0.33) between the western and eastern federal states (Figure 1D). However, certain regional differences in the incidence rate can be noted.
FIGURE 1.
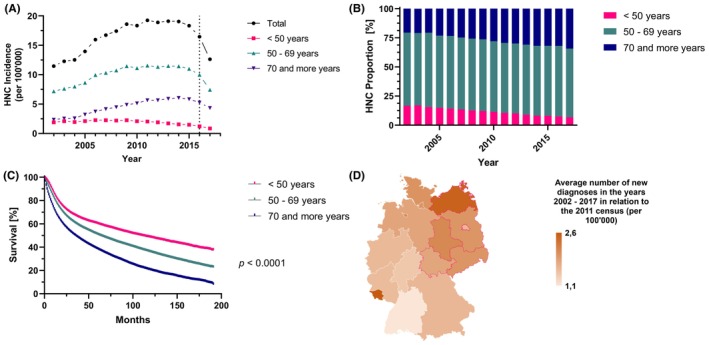
Head and neck cancer incidence and age‐specific survival in Germany. (A) Newly diagnosed cases of HNC per 100,000 inhabitants in Germany between the years 2002 and 2017 are displayed with a subdivision into three age‐related groups. Incomplete data transmission to the cancer registry is assumed since 2016 (indicated with a dotted, vertical line). (B) The relative, age‐group specific proportion of HNC incidence during the observation period is shown. (C) Age‐group specific overall survival is presented. (D) The mean number of newly diagnosed HNC per federal state is demonstrated in relation with the resident population numbers from the census in 2011.
3.3. Outcomes in patients diagnosed after 2009 improved in younger patients only
To assess the impact of changes in standard treatment during the first half of the observation period, the cohort was divided by the year of initial diagnosis (period 1: 2002–2009 vs. period 2: 2010–2017). The mean OS of all HNCP diagnosed between 2002 and 2009 considering a follow‐up period of 5 years was 39.3 months (SEM = 0.1; CI: 39.3–39.6)) compared to 39.5 months (SEM = 0.1; CI: 39.4–39.7) in patients diagnosed between 2010 and 2017. However, when analyzed separately for the three age groups within these two intervals a significant improvement in mean survival was observed for young HNCP (< 50 years; period 1: 43.1 months vs. period 2: 45.5 months p <; 0.0001; Figure 2A) and for middle aged HNCP (50–69 years; period 1: 40.2 months vs. period 2: 41.0 months; p < 0.0001; Figure 2B). But no significant difference was detected between the two time periods for old HNCP (≥70 years; period 1: 34.6 months vs. period 2: 34.6 months; p = 0.76; Figure 2C).
FIGURE 2.
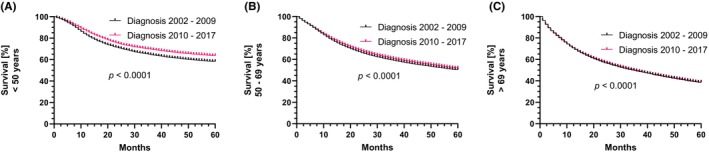
Comparison of time‐specific survival. Age‐specific survival over 5 years is displayed for patients diagnosed between 2002 and 2009 in comparison to those diagnosed between 2010 and 2017 for (A) patients <50 years of age at diagnosis, (B) patients > = 50 years, but <70 years of age at diagnosis and (C) patients >70 years of age at diagnosis.
3.4. TNM and stage at diagnosis was constant over time
During the whole observation period, the ratio of advanced to early primary tumor status (T3 + 4 / T1 + 2), N+ to N0 and M1 to M0 of newly diagnosed HNC did not change significantly (Figure S1A). However, HNCP in East Germany consistently presented with higher T status (p < 0.0001) and N status (p < 0.0001), but not M status (p = 0.15). Differences in UICC stage between East and West (Figure S1B) were not observed (p = 0.22). Moreover, the portions of different HNSCC entities differed only marginally, but still significantly (p < 0.0001) by a maximum of 3% in East and West Germany. As expected, UICC stage was strongly associated with prognosis (Figure S1c). Median survival was 127 months (SEM = 1.6; CI: 123.8–130.2) in Stage I, 94 months (SEM = 1.5; CI: 91.0–97.0) in Stage II, 71 months (SEM = 1.2; CI: 68.6–73.4) in Stage III and 29 months (SEM = 0.3; CI: 28.4–29.6) in Stage IV HNCP (p < 0.0001).
3.5. Mean age of HNCP was rising, especially in West Germany
The mean age of all included HNCP (Figure 3A,D) during the observation period was 63.2 years (SD = 11.4). In the meantime, the age of the general population was between 41.5 and 44.4 years (Figure 3C). The highest mean age was observed in laryngeal cancer patients (65.6 years; SD = 10.8) and the lowest mean age in nasopharyngeal cancer patients (58.5 years; SD = 15.3). Mean age increased significantly over time in all HNC primary tumor sites, whereas the lowest increase was observed in nasopharyngeal cancer patients (Figure 3B). In comparison to the age increment in the general population, mean age among all HNCP was increasing at a significantly higher rate (slope coefficient 0.3 vs. 0.2; p < 0.0001; Figure 3C). The increase in mean age among HNCP was significantly faster in the West than in the East of Germany (slope coefficient: 0.3 vs. 0.2; p < 0.0001; Figure 3C). Consistent with this observation, mean age among HNCP in the East was 2.5 years lower than in the West (61.3 years (SD = 11.2) vs. 63.8 (SD = 11.4); p < 0.0001; Figure 3D).
FIGURE 3.
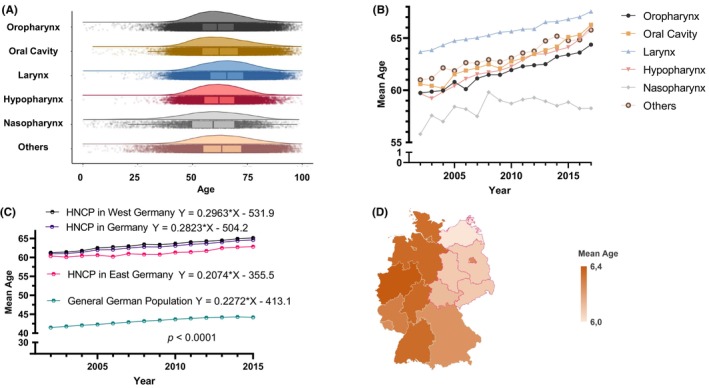
Mean age development by tumor entity and residence. (A) The age distribution of HNCP is displayed as box and violin plots by primary tumor site. (B) Mean age during the observation period is shown by primary site. (C) Mean age of all HNCP per year during the observation period compared to those diagnosed in East or West Germany and age development among the general population. The significance level (p) for the comparisons between slope coefficients is displayed as well. (D) A geographical illustration of the regional mean age of HNCP in Germany for the 16 federal states is shown.
3.6. Male sex and residence in East Germany were associated with poor survival
The sex ratio (men/women) among HNCP was significantly higher in East than in West Germany (mean ratio: 4.4 vs. 3.3; p < 0.0001; Figure 4A,B). However, the sex ratio has decreased in both parts of Germany evenly (p = 0.95) over time (Figure 4A). Male sex was significantly associated with shorter OS (male: 52 months vs. female: 76 months; p < 0.0001; Figure 4C). Residence in East Germany was also associated with significantly shorter median OS (East Germany: 47 months vs. West Germany: 60 months; p < 0.0001; Figure 4D).
FIGURE 4.
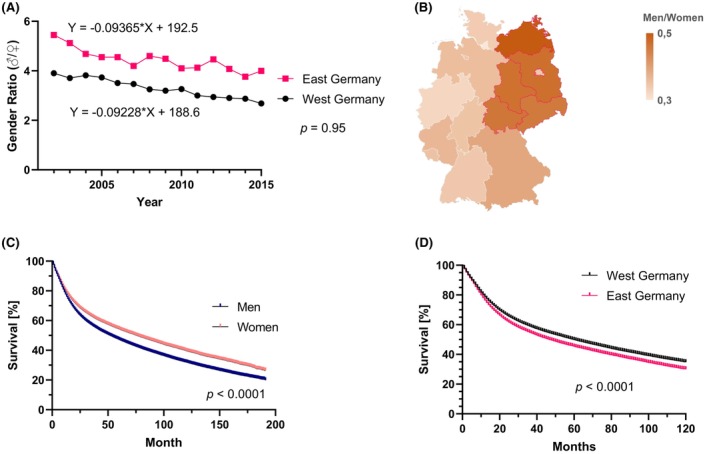
Gender distribution and association with imbalance in survival in Eastern and Western Germany. (A) The gender ratio (men/women) in East and West Germany is displayed over time between 2002 and 2015. The significance level (p) for the comparison between those two slope coefficients is displayed. (B) The mean gender ratio is illustrated geographically for each of the 16 federal states. (C) The overall survival in relation to patient sex is shown with p‐value resulting from log rank test. (D) The survival over 10 years by region of residence is shown with the p‐value from log rank testing.
3.7. Multivariate analysis confirmed impact of demographic factors on prognosis
Finally, we performed a multivariate cox regression analysis considering UICC stage, age, sex and residence (East vs. West). All factors in the model were confirmed as independent factors for prognosis (Figure 5). The highest HR was found for advanced UICC Stages III, IV (HR: 2.43; 95% CI: 2.39, 2.49; p < 0.0001), followed by male sex (HR: 1.26; 95% CI: 1.24, 1.29; p < 0.0001), residence in East Germany (HR: 1.20; 95% CI: 1.18, 1.22; p < 0.0001) and higher age at diagnosis (HR: 1.03; 95% CI: 1.03, 1.03); p < 0.001). It is worth emphasizing that survival was inferior in the East Germany for each of the tumor entities studied here (Table S2).
FIGURE 5.
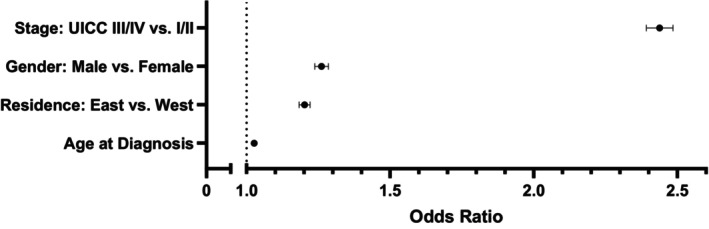
Multivariate Cox regression of stage and demographic factors. Hazard ratios with 95% CI (whiskers) for tumor stage, gender, place of residence and age at diagnosis are shown from a multivariate cox regression analysis. A hazard ratio of 1 is indicated by the dotted, vertical line.
4. DISCUSSION
This is the first comprehensive, nation‐wide analysis of demographic factors on the prognosis in head and neck cancer. Our study confirms reports about increasing age in HNCP from the United States and parts of Germany. 10 , 17 This poses a challenge for health care systems due to a higher degree of morbidity and mortality associated with treatment 9 , 18 , 19 , 20 and is associated with a significant financial burden on health care systems. 8 Frailty is a predictor of severity for complications in HNCP undergoing surgery 21 and surgery is less often performed in older patients. 9 A greater increase in mean age in comparison to the demographic development in the general population underlines the need for age‐specific assessments to improve cancer care 22 , 23 and addresses the described outcome disparities by age. At the same time, the decreasing proportion of younger HNCP may be a result of primary cancer prevention efforts such as anti‐smoking and anti‐alcohol abuse campaigns. 24 Unfortunately, there is no HNC screening tool yet, which is also reflected in the constant tumor burden at initial diagnosis during our observation period.
There were significant improvements in the clinical management of HNC during the first half of the observation period (2002–2009) with regard to definitive chemoradiation, 25 , 26 , 27 , 28 adjuvant (chemo‐)radiation 29 , 30 , 31 and palliative chemotherapy. 32 These innovations may explain the increased OS in younger and middle‐aged patients. However, most of these innovations consisted of treatment intensification, from which older patients seem not to have had a similar benefit. 33 , 34 This is also in line with earlier (1996–2005) evaluations of the Thuringian cancer registry. 15 In older patients, molecular biology, treatment goals and available options may differ significantly from younger patients. 9 , 35 , 36 Thus, there is a definitive need for clinical trials focusing on treatment of older patients.
An additional prognostic factor was sex, increasingly emphasized by the emerging field of sex medicine, also in oncology. 11 , 37 The ratio of male to female patients has been decreasing over the observation period. Potentially due to higher degrees of smoking and alcohol abuse in male patients, these seem to have a higher risk of death. Primary prevention strategies include prophylactic HPV vaccination which will in the future contribute to a decreasing incidence in both male and female patients, especially if vaccination rates can be improved further (approval in Germany for boys since 2018 and for girls already since 2007 38 ).
An alarming result of our analysis is the outcome disparity between East and West Germany. More than 30 years after the German reunion (joining of the DDR to the BRD: 03.10.1990), such a difference in OS among patients indicates the need for health policy action. Both, a more advanced tumor stage at diagnosis and a higher fraction of male patients seem to contribute to this survival difference, whereas the younger mean age of patients in East Germany could counterbalance these influences. Nevertheless, residence in Eastern Germany was confirmed as an additional, independent factor by multivariate analysis.
Thereupon, persisting gaps in general life expectancy between East and West have been reported, but seem to be slowly vanishing after reunification. 39 , 40 At the beginning of our study in 2002, the difference in life expectancy in the general population amounted to 0.4 years for women and 1.5 years for men. 39 A big part of the improved life expectancy in the East since then is considered to be a result of a decline in cardiovascular mortality. 39 , 40 However, socioeconomic factors also exist that may have a strong impact on HNCP and other cancer patients' mortality. In fact, socioeconomic disadvantages such as differences in education, occupational status and income can lead to differences in experienced health burden (e.g. work related) and resilience. These effects may be amplified by differences in access to health care and personal behavior (nutrition, substance abuse, compliance etc.). 41 , 42 In addition, the healthcare system in the East is particularly dependent on modernization. 43 Inequalities in health may then even further increase socioeconomic imbalance. 42 In Germany, the mean household income among inhabitants in East Germany is lower and there is a higher rate of unemployment 39 indicating an elevated level of socioeconomic stress which may result in a higher risk for substance abuse. 44 Similar difficulties seem to be experienced in the USA, here, data on reduced survival of black patients are available; the reasons are very diverse as well. 45 Further, the migration of well‐educated inhabitants to other parts of Germany, a shrinking population with a higher proportion of older inhabitants and an underdeveloped public transport infrastructure, especially in the rural areas may further destabilize comprehensive health care. 39 , 46 , 47 , 48 Current efforts to equalize these disparities between East and West Germany need to be continued and expanded. In this manner, the consolidation of a welfare state, facilized health care access, and an enhanced political inclusion of citizens are thought to be helpful. 49 In addition, a subsequent extension of this study to urban and rural regions in Germany could provide further insights. 50
Finally, this study shows the need to enhance cancer data availability and accuracy of documentation. Despite of the mandatory reporting of cancer‐related data to cancer registries in Germany since 2009, collected data still have a high prevalence of missing data. 51 However, the relations between T, N, M status collected here are comparable to U.S. cancer registry evaluations. 45 Efforts to specialize care within dedicated head and neck cancer centers may in the future improve data completeness. However, this constrained data recording is similar to other countries. 52 In addition, available data from the statistical offices of the federal were not structured uniformly and represent a significant hurdle for data analysis in public health research.
5. CONCLUSIONS
Age, sex, and patient residence contribute independently to the prognosis of HNCP. Outcome disparities between East and West Germany need to be put on the agenda by health policy makers. Supporting well‐networked head and neck cancer centers to improve access to specialized care, the use of geriatric assessment tools and addressing socioeconomic imbalances may be key to improve treatment outcomes.
AUTHOR CONTRIBUTIONS
Julius M. Vahl: Conceptualization (equal); data curation (lead); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – original draft (equal). Gabriele Nagel: Formal analysis (equal); investigation (equal); methodology (equal); writing – review and editing (equal). Tsima Abou Kors: Investigation (supporting); visualization (equal); writing – review and editing (equal). Matthias Brand: Writing – review and editing (supporting). Adrian von Witzleben: Writing – review and editing (equal). Michael Sonntag: Writing – review and editing (equal). Ayla Grages: Writing – review and editing (equal). Marie N. Theodoraki: Writing – review and editing (equal). Jens Greve: Writing – review and editing (equal). Michael Denkinger: Conceptualization (supporting); investigation (supporting); methodology (supporting); writing – review and editing (supporting). Dhayana Dallmeier: Conceptualization (supporting); investigation (supporting); methodology (supporting); writing – review and editing (supporting). Christian Idel: Conceptualization (supporting); investigation (supporting); writing – review and editing (supporting). Stephan Stilgenbauer: Writing – review and editing (equal). Thomas K. Hoffmann: Resources (lead); supervision (supporting); writing – review and editing (equal). Simon Laban: Conceptualization (lead); data curation (supporting); formal analysis (equal); investigation (equal); methodology (lead); project administration (lead); resources (equal); supervision (lead); validation (lead); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
No funding was received.
CONFLICT OF INTEREST STATEMENT
Simon Laban: Advisory Boards: Merck Sharp & Dohme (M.S.D.), Bristol Myers, Squibb (B.M.S.), Astra Zeneca (A.Z.). Honoraria: M.S.D., B.M.S., A.Z., Merck Serono. Thomas K. Hoffmann: Advisory Boards: M.S.D., B.M.S., Sanofi. Honoraria: M.S.D., B.M.S., Merck Serono. All other authors declared no conflict of interests in conjunction with this work.
ETHICS STATEMENT
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. In agreement with our ethics committee of the University Ulm, Germany, no designated ethic vote was needed because data was anonymized before analysis.
INFORMED CONSENT
Epidemiological cancer registration in Germany is regulated by state laws and data received were anonymized facilized. The Federal Cancer Registry Data Act of 2009 defines the tasks of the Center for Cancer Registry Data at the Robert Koch Institute as the national evaluation center. This means that all physicians and dentists in the state are required to report cancer cases they are involved in diagnosing, treating or following up to the state cancer registry. Patients consent is not required for this.
Supporting information
Figure S1.
Table S1.
Table S2.
ACKNOWLEDGEMENTS
We would like to thank the German Center for Cancer Registry (General‐Pape‐Street 62 – 66, 12101 Berlin, Germany) for providing the data on HNC in Germany. We are also thankful to the Statistical State Office Baden‐Wuerttemberg (Böblinger Street 68, 70199 Stuttgart, Germany) for providing and coordinating the cross‐country data retrieval in cooperation with the rest of the statistical offices concerning the general German population. Open Access funding enabled and organized by Projekt DEAL.
Vahl JM, Nagel G, Abou Kors T, et al. Regional outcome disparities in German head and neck cancer patients: Shorter survival in Eastern Germany. Cancer Med. 2023;12:21426‐21435. doi: 10.1002/cam4.6690
DATA AVAILABILITY STATEMENT
The original dataset cannot be shared. The data on HNCP in Germany have been requested in a multistep process from the German cancer registry for a dedicated purpose and specific group of persons only and must not be shared. The data on the general population was ordered from de federal statistical office and suboffices at a charge and may only be used applicant‐related too.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Miranda‐Galvis M, Loveless R, Kowalski LP, Teng Y. Impacts of environmental factors on head and neck cancer pathogenesis and progression. Cell. 2021;10(2):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du E, Mazul AL, Farquhar D, et al. Long‐term survival in head and neck cancer: impact of site, stage, smoking, and human papillomavirus status. Laryngoscope. 2019;129(11):2506‐2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anisimov VN. Biology of aging and cancer. Cancer Control. 2007;14(1):23‐31. [DOI] [PubMed] [Google Scholar]
- 6. Fazel A, Quabius ES, Fabian A, et al. The impact of smoking habit alteration on prognosis of head and neck cancer patients. Laryngorhinootologie. 2021;100(8):634‐643. [DOI] [PubMed] [Google Scholar]
- 7. Tinhofer I, Jöhrens K, Keilholz U, et al. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur J Cancer. 2015;51(4):514‐521. [DOI] [PubMed] [Google Scholar]
- 8. Nowossadeck E. Population aging and hospitalization for chronic disease in Germany. Dtsch Arztebl Int. 2012;109(9):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mountzios G. Optimal management of the elderly patient with head and neck cancer: issues regarding surgery, irradiation and chemotherapy. World J Clin Oncol. 2015;6(1):7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vahl JM, Wigand MC, Denkinger M, et al. Increasing mean age of head and neck cancer patients at a German tertiary referral center. Cancers (Basel). 2021;13(4):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dittberner A, Friedl B, Wittig A, et al. Gender disparities in epidemiology, treatment, and outcome for head and neck cancer in Germany: a population‐based long‐term analysis from 1996 to 2016 of the Thuringian cancer registry. Cancers (Basel). 2020;12(11):3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woods LM, Rachet B, Coleman MP. Origins of socio‐economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5‐19. [DOI] [PubMed] [Google Scholar]
- 13. Wienecke A, Kraywinkel K. Epidemiologie von Kopf‐Hals‐Tumoren in Deutschland. Der Onkologe. 2019;25(3):190‐200. [Google Scholar]
- 14. Bayer O, Krüger M, Koutsimpelas D, et al. Veränderung von Inzidenz und Mortalität von Kopf‐Hals‐Malignomen in Rheinland‐Pfalz, 2000–2009. Laryngorhinootologie. 2015;94(7):451‐458. [DOI] [PubMed] [Google Scholar]
- 15. Guntinas‐Lichius O, Wendt T, Buentzel J, et al. Head and neck cancer in Germany: a site‐specific analysis of survival of the Thuringian cancer registration database. J Cancer Res Clin Oncol. 2010;136(1):55‐63. [DOI] [PubMed] [Google Scholar]
- 16. Zentrum für Krebsregisterdaten (ZfKD) im Robert Koch‐Institut . Datensatz des ZfKD auf Basis der epidemiologischen Landeskrebsregisterdaten, verfügbare Diagnosejahre Bis 2017. 2020. Epi2019_2. 2020.
- 17. Cline BJ, Simpson MC, Gropler M, et al. Change in age at diagnosis of oropharyngeal cancer in the United States, 1975–2016. Cancer. 2020;12(11):3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashim D, Carioli G, Malvezzi M, et al. Cancer mortality in the oldest old: a global overview. Aging (Albany NY). 2020;12(17):16744‐16758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gollnitz I, Inhestern J, Wendt TG, et al. Role of comorbidity on outcome of head and neck cancer: a population‐based study in Thuringia, Germany. Cancer Med. 2016;5(11):3260‐3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kouka M, Buentzel J, Kaftan H, et al. Early mortality among patients with head and neck cancer diagnosed in Thuringia, Germany, between 1996 and 2016‐a population‐based study. Cancers (Basel). 2022;14(13):3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldstein DP, Sklar MC, de Almeida JR, et al. Frailty as a predictor of outcomes in patients undergoing head and neck cancer surgery. Laryngoscope. 2020;130(5):E340‐E345. [DOI] [PubMed] [Google Scholar]
- 22. Li D, Sun C‐L, Kim H, et al. Geriatric assessment–driven intervention (GAIN) on chemotherapy‐related toxic effects in older adults with cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):e214158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster‐randomised study. Lancet. 2021;398(10314):1894‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richter M, Pförtner T‐K, Lampert T. Veränderungen im Tabak‐, Alkohol‐und Cannabiskonsum von Jugendlichen im Zeitraum von 2002 bis 2010 in Deutschland. DasGesundheitswesen. 2012;74(S 01):S42‐S48. [DOI] [PubMed] [Google Scholar]
- 25. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091‐2098. [DOI] [PubMed] [Google Scholar]
- 26. Forastiere AA, Zhang Q, Weber RS, et al. Long‐term results of RTOG 91‐11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermorken JB, Stohlmacher‐Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous‐cell carcinoma of the head and neck (SPECTRUM): an open‐label phase 3 randomised trial. Lancet Oncol. 2013;14(8):697‐710. [DOI] [PubMed] [Google Scholar]
- 28. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous‐cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567‐578. [DOI] [PubMed] [Google Scholar]
- 29. Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high‐risk squamous‐cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937‐1944. [DOI] [PubMed] [Google Scholar]
- 30. Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843‐850. [DOI] [PubMed] [Google Scholar]
- 31. Bernier J, Domenge C, Ozsahin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945‐1952. [DOI] [PubMed] [Google Scholar]
- 32. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116‐1127. [DOI] [PubMed] [Google Scholar]
- 33. Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta‐analysis of chemotherapy in head and neck cancer (MACH‐NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92(1):4‐14. [DOI] [PubMed] [Google Scholar]
- 34. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5‐year survival data from a phase 3 randomised trial, and relation between cetuximab‐induced rash and survival. Lancet Oncol. 2010;11(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 35. White MC, Holman DM, Boehm JE, Peipins LA, Grossman M, Henley SJ. Age and cancer risk: a potentially modifiable relationship. Am J Prev Med. 2014;46(3 Suppl 1):S7‐S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Derks W, De Leeuw J, Hordijk G, Winnubst J. Reasons for non‐standard treatment in elderly patients with advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2005;262(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 37. Baggio G, Corsini A, Floreani A, Giannini S, Zagonel V. Gender medicine: a task for the third millennium. Clin Chem Lab Med. 2013;51(4):713‐727. [DOI] [PubMed] [Google Scholar]
- 38. Takla A, Wiese‐Posselt M, Harder T, et al. Background paper for the recommendation of HPV vaccination for boys in Germany. Bundesgesundheitsblatt‐Gesundheitsforschung‐Gesundheitsschutz. 2018;61(9):1170‐1186. [DOI] [PubMed] [Google Scholar]
- 39. Razum O, Altenhöner T, Breckenkamp J, Voigtländer S. Social epidemiology after the German reunification: East vs. West or poor vs. rich? Int J Public Health. 2008;53(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 40. Vogt TC. How many years of life did the fall of the Berlin Wall add? A projection of East German life expectancy. Gerontology. 2013;59(3):276‐282. [DOI] [PubMed] [Google Scholar]
- 41. Wenau G, Grigoriev P, Shkolnikov V. Socioeconomic disparities in life expectancy gains among retired German men, 1997–2016. J Epidemiol Community Health. 2019;73(7):605‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mielck A, Helmert U. Soziale Ungleichheit und Gesundheit: na. 2000.
- 43. Hurst JW. Reform of health care in Germany. Health Care Financ Rev. 1991;12(3):73. [PMC free article] [PubMed] [Google Scholar]
- 44. Redonnet B, Chollet A, Fombonne E, Bowes L, Melchior M. Tobacco, alcohol, cannabis and other illegal drug use among young adults: the socioeconomic context. Drug Alcohol Depend. 2012;121(3):231‐239. [DOI] [PubMed] [Google Scholar]
- 45. Goodwin WJ, Thomas GR, Parker DF, et al. Unequal burden of head and neck cancer in the United States. Head Neck. 2008;30(3):358‐371. [DOI] [PubMed] [Google Scholar]
- 46. Vahl JM, von Witzleben A, Welke C, et al. Influence of travel burden on tumor classification and survival of head and neck cancer patients. European archives of Oto‐rhino‐laryngology. Eur Arch Otorhinolaryngol. 2021;278(11):4535‐4543. [DOI] [PubMed] [Google Scholar]
- 47. BIB . Regionale Unterschiede in der Bevölkerungsentwicklung: Bundesinstitut für Bevölkerungsforschung. 2022. Available from: https://www.bib.bund.de/DE/Fakten/Regional/Bevoelkerungsentwicklung.html.
- 48. Staab A. Xenophobia, ethnicity and national identity in eastern Germany. German Politics. 1998;7(2):31‐46. [Google Scholar]
- 49. Bambra C. Levelling up: global examples of reducing health inequalities. Scand J Public Health. 2021;50:908‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vahl JM, Nagel G, Grages A, et al. Demographics and access to head and neck cancer care in rural areas compared to urban areas in Germany. Cancer Med. 2023;12:18826‐18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gurung‐Schönfeld I, Kraywinkel K. Krebsregistrierung heute: zwischen Epidemiologie. Qualitätssicherung Und Forschung. 2021;4:3‐9. [Google Scholar]
- 52. dos Santos MF, Fernandes GA, Antunes JLF, Villa LL, Toporcov TN. Global incidence trends in head and neck cancer for HPV‐related and‐unrelated subsites: a systematic review of population‐based studies. Oral Oncol. 2021;115:105177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.
Table S2.
Data Availability Statement
The original dataset cannot be shared. The data on HNCP in Germany have been requested in a multistep process from the German cancer registry for a dedicated purpose and specific group of persons only and must not be shared. The data on the general population was ordered from de federal statistical office and suboffices at a charge and may only be used applicant‐related too.


