Abstract
Background and objective
Recently, we demonstrated that staging Parkinson's disease (PD) with a novel simple classification called MNCD, based on four axes (motor, non‐motor, cognition, and dependency) and five stages, correlated with disease severity and patients’ quality of life. Here, we analyzed the correlation of MNCD staging with PD caregiver's status.
Patients and methods
Data from the baseline visit of PD patients and their principal caregiver recruited from 35 centers in Spain from the COPPADIS cohort from January 2016 to November 2017 were used to apply the MNCD total score (from 0 to 12) and MNCD stages (from 1 to 5) in this cross‐sectional analysis. Caregivers completed the Zarit Caregiver Burden Inventory (ZCBI), Caregiver Strain Index (CSI), Beck Depression Inventory‐II (BDI‐II), PQ‐10, and EUROHIS‐QOL 8‐item index (EUROHIS‐QOL8).
Results
Two hundred and twenty‐four PD patients (63 ± 9.6 years old; 61.2% males) and their caregivers (58.5 ± 12.1 years old; 67.9% females) were included. The frequency of MNCD stages was 1, 7.6%; 2, 58.9%; 3, 31.3%; and 4–5, 2.2%. A more advanced MNCD stage was associated with a higher score on the ZCBI (p < .0001) and CSI (p < .0001), and a lower score on the PQ‐10 (p = .001), but no significant differences were observed in the BDI‐II (p = .310) and EUROHIS‐QOL8 (p = .133). Moderate correlations were observed between the MNCD total score and the ZCBI (r = .496; p < .0001), CSI (r = .433; p < .0001), and BDI‐II (r = .306; p < .0001) in caregivers.
Conclusion
Staging PD according to the MNCD classification is correlated with caregivers’ strain and burden.
Keywords: burden, caregiver, non‐motor symptoms, Parkinson's disease, stage
Recently, it has been reported that staging Parkinson's disease (PD) with a novel simple classification called MNCD, based on 4 axes (Motor; Non‐motor; Cognition; Dependency) and 5 stages, correlated with disease severity and patients' quality of life. In this new manuscript, we observed that staging PD according to the MNCD classification correlated with caregivers' strain and burden.

1. INTRODUCTION
Parkinson's disease (PD) is a complex and very heterogeneous progressive neurodegenerative disorder causing not only motor but also non‐motor symptoms (NMSs) that result in loss of patient autonomy for activities of daily living (ADL) and worse quality of life (QoL) impairment, but second, caregiver's strain and burden as well (Greenland et al., 2019; Mosley et al., 2017; Zhao et al., 2021). Moreover, caregiver's status impacts on patient's status as well, which has been named the “vicious circle of illness” (Santos‐García et al., 2023b). From a clinical point of view and with the aim of defining exhaustively the status of a patient with PD at each moment, a novel simple classification called “MNCD” has been proposed (Santos‐García et al., 2021a). The MNCD is based on four major axes (M, motor; N, non‐motor; C, cognition; D, dependency) and proposes five stages, from MNCD stage 1, no relevant symptoms, to MNCD stage 5, dementia and dependency for basic ADL, and a total score from 0 (0 + 0 + 0 + 0 = 0; the best possible status) to 12 (4 + 4 + 2 + 2 = 12; the worst possible status). Recently, we demonstrated using data from the COPPADIS cohort (Santos‐García et al., 2016, 2019) that staging PD with the MNCD classification correlated with disease severity and patients’ QoL (Santos‐García et al., 2023a). Based on these findings and awaiting the results of an ongoing validation study of the MNCD classification and of its application in a prospective follow‐up cohort, early observations suggest that the MNCD classification could be a useful tool to apply in PD patients to identify the main symptoms of the disease and to monitor the progression of the disorder. Neuropsychiatric symptoms, such as visual hallucinations, depression, cognitive impairment and/or dementia, falls, disability, and a more advanced stage disease, have been identified as patient factors associated with caregiver burden in PD (Aamodt et al., 2023). All these data are collected in the MNCD classification with also a proposed staging and scoring that indicates the degree of severity, from least to most. That is why we think that the MNCD classification can quickly provide us with information about the patient's condition that affects his/her QoL as well as the status of his/her principal caregiver. Importantly, with the aim to stop the vicious circle of illness in PD (Santos‐García et al., 2023b), the detection of burden in the principal caregiver of the patient is important and should be acted on as earlier as possible.
The aim of this new analysis was to study the correlation of the MNCD staging with PD caregiver's status. Data from the COPPADIS cohort (Santos‐García et al., 2021a) used in the previous publication (Santos‐García et al., 2023a) were used in this analysis. Our hypothesis was that caregivers’ status would be different between the different PD stages according to the MNCD classification, with a better QoL and mood when the patient has a low stage with a low score and vice versa, a worse mood and QoL with greater burden and stress when the patient has a higher stage and score (i.e., a more advanced MNCD stage, a worse caregiver's status).
2. MATERIALS AND METHODS
Data collected at the baseline visit from PD patients and their principal caregiver recruited from 35 hospitals in Spain from the COPPADIS cohort (Santos‐García et al., 2016) from January 2016 to November 2017 were used in this cross‐sectional transversal study. Methodology about COPPADIS‐2015 study has been published and can be consulted (Santos‐García et al., 2019). All patients included were diagnosed according to UK PD Brain Bank criteria (Hughes et al., 1992). Exclusion criteria were: non‐PD parkinsonism; a mini‐mental state examination <26; age <18 or >75 years; inability to read or understand the questionnaires; to be receiving any advanced therapy (continuous infusion of levodopa or apomorphine, and/or with deep brain stimulation); and the presence of comorbidity, sequelae, or any disorder that could interfere with the assessment.
2.1. PD patient assessment
Information on sociodemographic aspects, factors related to PD, comorbidity, and treatment, including levodopa equivalent daily dose (Schade et al., 2020), were collected at baseline. As this analysis was focused on the caregiver status and the results about the relationship between the MNCD staging and patients’ symptoms have been already published (Santos‐García et al., 2023a), all the information collected about motor, NMS, QoL, and autonomy for ADL in PD patients (Santos‐García et al., 2019) were not provided on this occasion.
2.2. Caregiver assessment
A person who, without being a professional and/or receiving money in exchange for services, lived with the patient and was responsible for his/her was included as a primary caregiver (Santos‐García et al., 2016). His/her participation was voluntary, and a written informed consent was collected for all participants. In caregivers, four aspects were analyzed using validated specific scales: burden (Zarit Caregiver Burden Inventory [ZCBI]), strain (Caregiver Strain Index [CSI]), mood (Beck Depression Inventory‐II [BDI‐II]), and global QoL (PQ‐10 and EUROHIS‐QOL 8‐item index [EUROHIS‐QOL8]). The ZCBI (Novak & Guest, 1989) contains 22 items that rate the impact of the disease on the caregiver's physical, emotional, and socioeconomic status. Responses are scored on a scale from 0 (never) to 4 (nearly always). The maximum total score, indicative of the highest burden, is 88. The CSI (Robinson, 1983) is a 13‐item questionnaire designed to assess the level of stress experienced by caregivers. There are two possible responses for each item: “yes” or “no”. The total score is the result of adding all positive responses (from 0, no stress, to 13, maximum level of stress). Mood was assessed with the BDI‐II (Beck et al., 1996). This is a self‐administered, 21‐item instrument. It has been designed to assess the severity of depression symptoms in adults and adolescents with a minimum age of 13 years. The evaluated subject must choose one of four alternatives (ordered from lesser to greater severity), in each item, which best describes his/her status over the previous 2 weeks. The score ranges from 0 (minimum) to 63 (maximum). Higher scores will reflect, a priori, a worse mood. Finally, global QoL was measured with the PQ‐10 and the EUROHIS‐QOL8. The PQ‐10 (Santos‐García et al., 2019) is a rating of global perceived QoL based on a scale from 0 (worst) to 10 (best). The EUROHIS‐QOL8 (Da Rocha et al., 2012) is an 8‐item QoL questionnaire (QoL; health status; energy; autonomy in ADL; self‐esteem; social relationships; economic capacity; habitat) derived from the WHOQOL‐100 and the WHOQOL‐BREF. For each item, the score ranges from 0 (not at all) to 5 (completely). The total score is expressed as the mean of the individual scores. A higher score indicates a higher QoL. Sociodemographic data about the principal caregiver of the PD patient was also collected.
2.3. MNCD classification
The MNCD classification was applied using the same criteria recently reported in the COPPADIS cohort (Santos‐García et al., 2023). This new classification (Santos‐García et al., 2021a) is based on four axes: (1) motor symptoms, (2) NMSs, (3) cognition, and (4) dependency for ADL. The first axis (motor symptoms) is subdivided into four defined sub‐axes: (1) motor fluctuations, (2) dyskinesia, (3) axial symptoms, and (4) tremor. The second axis (NMSs) is subdivided into four defined sub‐axes: (1) neuropsychiatric symptoms, (2) autonomic dysfunction, (3) sleep disturbances and fatigue, and (4) pain and sensory disorders. Regarding the third axis (cognition), patients are classified as having normal cognition, with mild cognitive impairment or dementia. Finally, patients are classified according to the fourth axis (dependency) as having independency for ADL, with dependency for instrumental or with dependency for basic activities. Patients were classified in four groups according to the MNCD stage: stage 1 (the patient has no relevant symptoms); stage 2 (there is at least one motor symptom or one NMS scoring in the MNCD classification); stage 3 (there is mild cognitive impairment and/or dependency for instrumental ADL); stages 4–5 (there is dementia and/or dependency for basic ADL). Moreover, the MNCD total score (from 0 to 12) was calculated according to the sum of the score of all axes of the MNCD classification: M (from 0 to 4) + N (from 0 to 4) + C (from 0 to 2) + D (from 0 to 2).
2.4. Data analysis
Data were processed using SPSS 20.0 for Windows. Only PD patients and their caregivers from the COPPADIS cohort with data collected about the MNCD classification and the ZCBI, CSI, BDI‐II, PQ‐10, and EUROHIS‐QOL8, respectively, were included in the analysis. ZCBI and CSI scores were expressed as quantitative and qualitative variables. Relative to the ZCBI score (from 0 to 88), four groups were defined (Santos‐García & Añón, 2012; Santos‐García & de la Fuente‐Fernández, 2015): little or no burden (0–20); mild‐to‐moderate burden (21–40); moderate‐to‐severe burden (41–60); severe burden (61–88). Two groups were considered according to CSI score (from 0 to 13) (Santos‐García & Añón, 2012; Santos‐García & de la Fuente‐Fernández, 2015): no high level of stress (0–6); high level of stress (7–13). With regard to mood (assessed by BDI‐II]), caregivers were classified as with major depression, minor depression, subthreshold depression, or non‐depression (Santos‐García et al., 2019). Specifically, regarding items 1, 4, 5, 9, 13, 15, 16, 17, and 18 of the BDI‐II, depression was defined: major depression, ≥5 symptoms with the presence of item 1 (feeling of sadness) and/or item 4 (anhedonia) (DSM‐IV criteria); minor depression, from 2 to 4 symptoms with the presence of item 1 and/or item 4 (DSM‐IV criteria); subthreshold depression, from 2 to 4 symptoms without the presence of item 1 and item 4 (Judd criteria) (American Psychiatric Association, 1994; Judd et al., 1994).
For comparison of caregivers’ burden (ZCBI), strain (CSI), mood (BDI‐II), and QoL (PQ‐10 and EUROHIS‐QOL8) between patients with a different MNCD stage (all stages together or two consecutive stages), the Student t‐test, the Mann–Whitney U test, Chi‐square test, Fisher test, ANOVA test, or the Kruskal–Wallis test were used as appropriate (distribution for variables was verified by one‐sample Kolmogorov–Smirnov test). Spearman's or Pearson's correlation coefficient, as appropriate, was used for analyzing the relationship between the MNCD total score (from 0 to 12) and the total score on ZCBI, CSI, BDI‐II, PQ‐10, and EUROHIS‐QOL8 on caregivers. Correlations were considered weak for coefficient values ≤.29, moderate for values between .30 and .59, and strong for values ≥.60. Linear regression models were used to determine in what grade the MNCD stage and the MNCD total score were associated with caregivers’ burden (ZCBI), strain (CSI), mood (BDI‐II), and QoL (PQ‐10 and EUROHIS‐QOL8). Age, gender, disease duration (years from symptoms onset), and caregiver's sociodemographic variables (age, gender, relationship with the patient, civil status, habitat, time as a caregiver, living with the patient or not, full‐time care or not and care of others or not) were included as covariates. With the aim of having a very strong evidence against the null hypothesis, the p‐value was considered significant (highly significant) when it was ≤.001 (Krikwood & Sterne, 2003).
2.5. Standard protocol approvals, registrations, and patient consents
Approval from the Comité de Ética de la Investigación Clínica de Galicia (2014/534; 02/DEC/2014) and a written informed consent from all participants in this study were obtained. COPPADIS‐2015 was classified by the AEMPS (Agencia Española del Medicamento y Productos Sanitarios) as a post‐authorization prospective follow‐up study with the code COH‐PAK‐2014‐01.
2.5.1. Data availability
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
3. RESULTS
Two hundred and twenty‐four PD patients (63 ± 9.6 years old; 61.2% males) and their caregivers (58.5 ± 12.1 years old; 67.9% females) were included. The mean disease duration was 5.6 ± 4.1 years. Regarding MNCD stages, the distribution was stage 1, 7.6% (N = 17); stage 2, 58.9% (N = 132); stage 3, 31.3% (N = 70); stages 4–5, 2.2% (N = 5; four patients in stage 4 and only one patient in stage 5 from the MNCD classification according to the original description [Santos‐García et al., 2021a]). Nearly 70% of the caregivers were females (67.9%) and up to 92.4% were living with the patient and 21.9% taking care of the patient full time (33.5% of the patients had cognitive impairment and/or needed some help for some ADL). No significant differences were observed in sociodemographic aspects between caregivers of PD patients in the different stages except in age (p = .002), being younger the caregivers of those patients in stages 4–5 (in this group, 40% of the caregivers were the son or the daughter) (Table 1).
TABLE 1.
Sociodemographic details and data about the status of the principal caregiver of patients with Parkinson's disease (PD) according to the motor, non‐motor, cognition, and dependency (MNCD) stage (N = 224).
| Stage 1 (N = 17; 7.6%) | Stage 2 (N = 132; 58.9%) | Stage 3 (N = 70; 31.3%) | Stages 4–5 (N = 5; 2.2%) | Total (N = 224) | p | |
|---|---|---|---|---|---|---|
| Patients | ||||||
| Age | 63.2 ± 8.3 | 60.2 ± 10.2 | 68.3 ± 6.6 | 63.8 ± 8.3 | 63 ± 9.6 | <.0001 |
| Males (%) | 64.7 | 61.4 | 58.6 | 80 | 61.2 | .855 |
| Disease duration (years) | 3.4 ± 1.9 | 5.4 ± 4.1 | 6.3 ± 4.4 | 7.5 ± 1.9 | 5.6 ± 4.2 | .062 |
| l‐Dopa eq. daily dose (mg) | 303.4 ± 255.1 | 541.9 ± 391.9 | 666.2 ± 427.9 | 1218 ± 775.9 | 574.8 ± 420.9 | <.0001 |
| Number of non antip. drugs | 3 [0, 4] | 2 [1, 4] | 3 [2, 6.25] | 5.5 [1.25, 6] | 2 [1, 5] | <.0001 |
| Hoehn & Yahr | 2 [1.5, 2] | 2 [1.5, 2] | 2 [2, 2.5] | 3.5 [2.25, 4] | 2 [2, 2] | <.0001 |
| MNCD—total score | 0 | 2.6 ± 1.5 | 4.5 ± 1.9 | 7.6 ± 1.3 | 3.1 ± 2.1 | <.0001 |
| Caregivers | ||||||
| Age | 60.7 ± 10.1 | 56.4 ± 11.5 | 62.5 ± 11.6 | 49.8 ± 9.1 | 58.5 ± 12.1 | .002 |
| Females (%) | 76.5 | 68.2 | 64.3 | 80 | 67.9 | .761 |
| Relationship (%): | .191 | |||||
| Spouse | 94.1 | 87.1 | 82.9 | 60 | 85.7 | |
| Son or daughter | 5.9 | 7.6 | 14.3 | 40 | 10.3 | |
| Other | 0 | 5.3 | 2.6 | 0 | 4 | |
| Civil status (%): | .938 | |||||
| Married | 88.2 | 86.4 | 88.6 | 100 | 87.5 | |
| Single | 5.9 | 6.8 | 8.6 | 0 | 7.1 | |
| Other | 5.9 | 6.8 | 2.8 | 0 | 5.6 | |
| Habitat (%): | .911 | |||||
| Rural | 17.6 | 13.6 | 12.9 | 20 | 13.8 | |
| Semiurban | 29.4 | 22 | 20 | 20 | 21.9 | |
| Urban | 52.9 | 64.4 | 67.1 | 60 | 64.3 | |
| Time as a caregiver (%): | .135 | |||||
| <1 year | 29.4 | 12.1 | 8.6 | 0 | 12.1 | |
| 1–5 years | 35.3 | 44.7 | 30 | 20 | 38.8 | |
| >5, <10 years | 17.6 | 19.7 | 24.3 | 20 | 21.9 | |
| ≥10 years | 17.6 | 23.5 | 37.1 | 60 | 27.2 | |
| Living with the patient (%) | 100 | 93.2 | 90 | 80 | 92.4 | .283 |
| Full time care (%) | 11.8 | 20 | 25.7 | 40 | 21.9 | .411 |
| Care of others (%) | 52.9 | 34.8 | 27.1 | 60 | 34.4 | .111 |
| ZCBI | 7.4 ± 7.7 | 11.7 ± 10.6 | 18.1 ± 14.2 | 33.6 ± 14.5 | 13.9 ± 12.6 | <.0001 |
| Moderate‐to‐severe burden (%) | 0 | 3 | 7.1 | 20 | 4.4 | .001 |
| CSI | 1.1 ± 1.5 | 1.6 ± 2.1 | 2.8 ± 2.8 | 5.8 ± 2.3 | 2 ± 2.5 | <.0001 |
| High level of stress (%) | 0 | 3.8 | 8.6 | 40 | 5.8 | .022 |
| BDI‐II | 4.4 ± 3.7 | 7.1 ± 8.5 | 8.2 ± 8.1 | 9.8 ± 5.7 | 7.3 ± 8.1 | .31 |
| Depressive symptoms (%): | 35.3 | 40.8 | 47.1 | 60 | .344 | |
| Major depression | 5.9 | 13.6 | 18.6 | 20 | ||
| Minor depression | 23.5 | 13.6 | 21.4 | 40 | ||
| Subthreshold depression | 5.9 | 13.6 | 7.1 | 0 | ||
| EUROHIS‐QOL8 | 4.1 ± 0.5 | 3.9 ± 0.6 | 3.8 ± 0.5 | 3.6 ± 0.6 | 3.9 ± 0.6 | .133 |
| Quality of life | 4.2 ± 0.8 | 3.9 ± 0.8 | 3.6 ± 0.8 | 2.8 ± 1.3 | 3.8 ± 0.8 | .001 |
| Health status | 3.7 ± 0.9 | 3.7 ± 0.9 | 3.7 ± 0.8 | 3.6 ± 1.1 | 3.7 ± 0.9 | .968 |
| Energy | 4 ± 0.9 | 3.9 ± 0.9 | 3.8 ± 0.8 | 3.4 ± 1.1 | 3.9 ± 0.9 | .357 |
| Autonomy for ADL | 4.1 ± 0.8 | 4 ± 0.8 | 4 ± 0.9 | 3.8 ± 0.8 | 4 ± 0.8 | .917 |
| Self‐esteem | 4.2 ± 0.4 | 3.9 ± 0.7 | 3.9 ± 0.6 | 3.4 ± 0.5 | 3.9 ± 0.7 | .064 |
| Social relationships | 4.2 ± 0.5 | 4 ± 0.7 | 4 ± 0.7 | 3.9 ± 0.7 | 4 ± 0.7 | .529 |
| Economic capacity | 4.2 ± 0.7 | 3.9 ± 0.8 | 3.6 ± 0.8 | 3.4 ± 0.9 | 3.8 ± 08 | .016 |
| Habitat | 4.4 ± 0.7 | 4.3 ± 0.8 | 4.3 ± 0.5 | 4.2 ± 0.4 | 4.3 ± 0.7 | .959 |
| PQ‐10 | 8.4 ± 1.4 | 7.4 ± 1.4 | 7 ± 1.6 | 5.6 ± 2.5 | 7.3 ± 1.6 | .001 |
Note: The results represent percentages, mean ± SD, or median [p25, p75]. Fisher exact test and ANOVA test were applied. Data about H&Y are during the OFF state (first thing in the morning without taking medication in the previous 12 h).
Abbreviations: ADL, activities of daily living scale; BDI‐II, Beck Depression Inventory‐II; EUROHIS‐QOL8, EUROHIS‐QOL 8‐item index; PD, Parkinson's disease.
A more advanced MNCD stage was associated with a higher score on the ZCBI (from 7.4 ± 7.7 at stage 1 to 33.6 ± 14.5 at stages 4–5; p < .0001) and CSI (from 1.1 ± 1.5 at stage 1 to 5.8 ± 2.3 at stages 4–5; p < .0001) (Table 1). One out of four caregivers (24.6%) had burden (mild‐to‐moderate, 20.1%; moderate‐to‐severe, 4%; severe burden 0.4%) and 5.8% high level of stress (CSI > 6). At stage 1, only 11.8% of the caregivers had burden (mild‐to‐severe; ZCBI 21‐88) compared to 18.2% at stage 2, 34.3% at stage 3, and 100% at stages 4–5 (p = .001) (Figure 1). The frequency of the high level of stress in the different groups was 0% at stage 1; 0% at stage 2; 8.6% at stage 3; 40% at stages 4–5 (p = .022) (Figure 1). Moderate correlations were observed between the MNCD total score and the ZCBI (r = .496; p < .0001) and between the MNCD total score and the CSI (r = .433; p < .0001).
FIGURE 1.
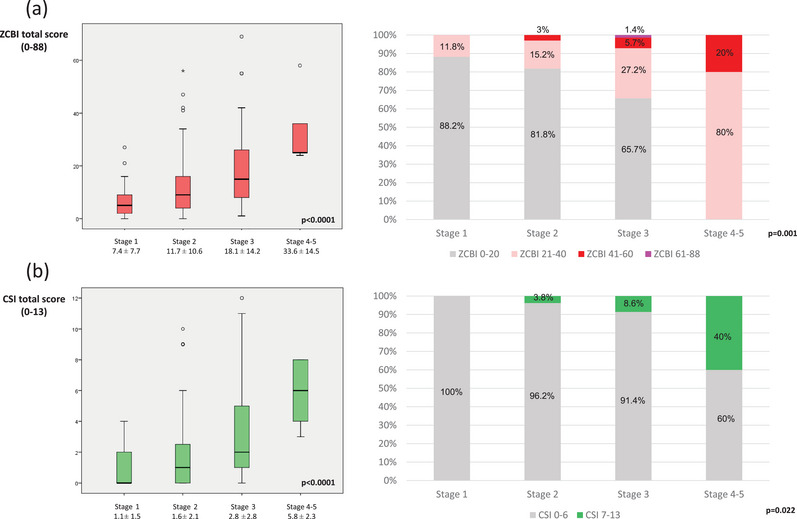
(a) Zarit Caregiver Burden Inventory (ZCBI) total score (on the left) and frequency of caregivers with different burden degree (on the right) regarding to the motor, non‐motor, cognition, and dependency (MNCD) stage, from stage 0 to stages 4–5. (b) Caregiver Strain Index (CSI) total score (on the left) and frequency of caregivers with high level (CSI > 6) versus no high level of stress (on the left) regarding to the MNCD stage, from stage 0 to stages 4–5.
Regarding mood, no significant differences were detected between different stages neither in the BDI‐II total score (p = .310) nor groups according to depressive symptoms (p = .330) (Table 1 and Figure 2). However, the correlation between the MNCD stage and the BDI‐II total score was moderate (r = .306; p < .0001). Moderate correlations were observed between the BDI‐II and the ZCBI (r = .541; p < .0001) and the BDI‐II and the CSI (r = .599; p < .0001). Global QoL was related to MNCD stage when the PQ‐10 was used, with a lower score at a higher stage (from 8.4 ± 1.4 at stage 1 to 5.6 ± 2.5 at stage 4; p = .001) (Table 1 and Figure 2). However, no significance was detected when the EUROHIS‐QOL8 was used (p = .133) (Table 1). By domains, significant differences were observed in “quality of life” and “economic capacity” between groups (from MNCD stage 1 to stages 4–5), with a lower score at a higher stage (Table 1 and Figure 2). Correlations between the MNCD total score and the PQ‐10 and between the MNCD total score and the EUROHIS‐QOL8 were weak (Table 2). As the proof of the utility of the PQ‐10, the correlation between the PQ‐10 and the EUROHIS‐QOL8 was strong (r = .735; p < .0001).
FIGURE 2.
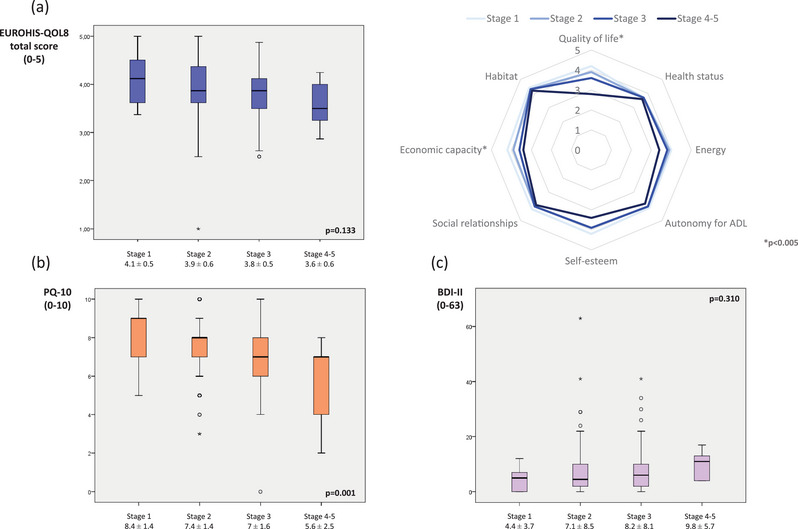
(a) EUROHIS‐QOL 8‐item index (EUROHIS‐QOL8) total score (on the left) and mean value of each domain of the scale (on the right) regarding to the motor, non‐motor, cognition, and dependency (MNCD) stage, from stage 0 to stages 4–5. (b) PQ‐10 score regarding to the MNCD stage, from stage 0 to stages 4–5. (c) Beck Depression Inventory II (BDI‐II) total score regarding to the MNCD stage, from stage 0 to stages 4–5.
TABLE 2.
Correlations between the motor, non‐motor, cognition, and dependency (MNCD) total score (from 0 to 12) and the other variables about the status of the caregiver (Zarit Caregiver Burden Inventory [ZCBI], Caregiver Strain Index [CSI], Beck Depression Inventory‐II [BDI‐II], PQ‐10, EUROHIS‐QOL 8‐item index [EUROHIS‐QOL8]).
| MNCD | ZCBI | CSI | BDI‐II | PQ‐10 | EUROHIS‐QOL8 | |
|---|---|---|---|---|---|---|
| MNCD | N.A. |
r = .496 p < .0001 |
r = .433 p < .0001 |
r = .306 p < .0001 |
r = −.238 p < .0001 |
r = −.193 p = .004 |
| ZCBI |
r = .496 p < .0001 |
N. A. |
r = .651 p < .0001 |
r = .541 p < .0001 |
r = −.421 p < .0001 |
r = −.441 p < .0001 |
| CSI |
r = .433 p < .0001 |
r = .651 p < .0001 |
N. A. |
r = .599 p < .0001 |
r = −.446 p < .0001 |
r = −.486 p < .0001 |
| BDI‐II |
r = .306 p < .0001 |
r = .651 p < .0001 |
r = .599 p < .0001 |
N. A. |
r = −.497 p < .0001 |
r = −.557 p < .0001 |
| PQ‐10 |
r = −.238 p < .0001 |
r = −.421 p < .0001 |
r = −.446 p < .0001 |
r = −.497 p < .0001 |
N. A |
r = .704 p < .0001 |
| EUROHIS‐QOL8 |
r = −.193 p = .004 |
r = −.441 p < .0001 |
r = −.486 p < .0001 |
r = −.557 p < .0001 |
r = .704 p < .0001 |
N. A. |
Note: Spearman correlation test were applied. Correlations between all variables are also shown.
After controlling for covariates mentioned in methods (age; gender; disease duration; caregiver's sociodemographic variables), the MNCD stage was significantly associated to ZCBI (β = .36; 95%CI 4.42–10.25; R 2 = 0.34; p < .0001), CSI (β = .27; 95%CI .44–1.59; R 2 = .31; p = .001), and PQ‐10 (β = −.32; 95%CI −1.27 to −.39; R 2 = .12; p < .0001). Regarding the MNCD total score, the significance was observed to ZCBI (β = .36; 95%CI 1.14–2.98; R 2 = .32; p < .0001) and CSI (β = .3; 95%CI.15–0.51; R 2 = .25; p < .0001), with a trend of significance to BDI‐II (β = .23; 95%CI .25–1.6; R 2 = .17; p = .007), EUROHIS‐QOL8 (β = −.21; 95%CI −.11 to −.09; R 2 = .03; p = .024) and PQ‐10 (β = −.32; 95%CI −.37 – −.1; R 2 = .03; p = .01).
4. DISCUSSION
The present study observed that a more advanced stage in PD patients according to the MNCD classification was associated with greater strain and burden (ZCBI; CSI) and a worse QoL (PQ‐10) in the principal caregiver. This finding, combined with previous reports (Santos‐García et al., 2023), supports that the MNCD classification could be a useful tool to monitor the progression of PD.
PD is a complex and very heterogeneous disorder with a variable prognosis between patients. From a clinical point of view and looking at the disease as a whole, four cardinal aspects in the patient linked to outcome are motor symptoms, NMS, cognition, and the ability to perform day‐to‐day activities. Throughout the evolution of the disease, motor and NMS burden increase, and progressive cognitive impairment and dependency for ADL appear. However, many phenotypes in PD have been reported, and disability can appear very early in some patients or late in the disease course in others (Lawton et al., 2018; Fereshtehnejad et al., 2015; De Pablo‐Fernández et al., 2019). In this context, a classification to monitor PD is required. Although the Hoehn and Yahr (1967) stage is used very frequently in clinical practice because it is very simple, it provides information only about the motor stage. As NMS or cognitive dysfunction can be present at the first steps of the disease or even before motor symptoms development and they impact on QoL (Santos‐García et al., 2021c; Goldman & Postuma, 2014), they should be identified and monitored from the first moment (i.e., at diagnosis) (Zis et al., 2015). In fact, NMSs have gained in importance in the latest proposed diagnostic criteria of PD (Postuma et al., 2015; Berg et al., 2015; Heinzel et al., 2019). These four capitals’ aspects commented are the basement of a new proposed classification for PD called MNCD (Santos‐García et al., 2023). The MNCD classification includes four major axes (M, motor symptoms; N, NMS; C, cognition; D, dependency for ADL) and five stages with the aim to categorize and differentiate patients in a different evolutionary stage, being the first PD classification taking into account key aspects of the disease, such as axial symptoms, motor fluctuations, NMS, cognitive problems, and their impact on disability.
Although it is necessary to apply the MNCD in clinical practice and demonstrate good inter‐ and intra‐variability, a very recent application of this tool using retrospectively the database of the baseline visits from the Spanish Cohort COPPADIS found that the MNCD staging was associated with disease severity and patient's QoL (Santos‐García et al., 2023). That is, a more advanced MNCD stage corresponded to a greater disease severity in terms of motor symptoms and NMS and a worse QoL and autonomy for ADL. On the other hand, the MNCD total score (from 0 to 12) correlated strongly with health‐related (PDQ‐39) and global (EUROHIS‐QOL8) QoL in PD patients (Santos‐García et al., 2023). In this new analysis using the same database and with the aim of gathering more information about the potential role of the MNCD classification in PD, we wanted to know what the status of the principal caregiver of a PD patient with respect to the MNCD classification was. Many studies have identified symptoms related to caregiver burden, such as depression, visual hallucinations, sleep disturbances, cognitive impairment, falls, a more advanced stage disease, and a greater disability (Santos‐García & de la Fuente‐Fernández, 2015; Schrag et al., 2006; Martinez‐Martin et al., 2007; D'Amelio et al., 2009; Martinez Martin et al., 2012; Smith et al., 2019; Peters et al., 2011; Santos‐García et al., 2022). Based on this, our hypothesis was that caregivers’ status would be different between the different MNCD stages, with a better mood and QoL in stage 1 and a worse QoL and mood and a greater strain and burden in caregivers at a more advanced stage (i.e., a more advanced MNCD stage, a worse caregiver's status). We found differences for caregiver burden (ZCBI), strain (CSI), and QoL (PQ‐10) but not for mood (BDI‐II). All caregivers of PD patients in stages 4–5 had some degrees of burden compared to only 11.8% at stage 1. Additionally, up to 40% of caregivers of PD patients in stages 4–5 reported a high level of stress compared to none of the caregivers of stage 1 PD patients. However, it is important to be cautious because although the sample size was 224 caregivers, only 5 were of PD patients in the most advanced stage (stages 4 and 5). The very small sample size of the group with MNCD stages 4–5 (N = 5) suggests that these findings need to be replicated and are not entirely reliable. On the other hand, some of the non‐statistically significant differences (EUROHIS‐QOL8; BDI‐II) may be due to a small sample size (e.g., 60% of caregivers of patients at stages 4–5 had depressive symptoms compared to 35.3% at stage 1). Of note, a moderate correlation was observed between the caregiver's BDI‐II total score and the MNCD total score (from 0 to 12), and between mood and burden and mood and strain in the caregiver, suggesting the necessity of being alert about depressive symptoms due to its important implications (Santos‐García et al., 2023) in the principal caregiver of a patient with a greater disease burden according to the MNCD classification. An ideal scenario could be to apply the MNCD classification in clinical practice to the patient at the first visit (i.e., a naïve patient) and to monitor its changes in each visit in the short‐, medium‐, and long‐term (i.e., >15 years) in a big cohort of PD patients and caregivers (i.e., >500) in a prospective follow‐up study. As an alternative, applying the MNCD classification in a big cohort (i.e., >1,000) in only one visit (cross‐sectional study) with many PD patients in all stages (i.e., from 1 to 5 of Hoehn & Yahr, 1967) would avoid the frequent bias of not including very advanced PD patients (e.g., with dementia and institutionalized). Finally, and regarding QoL, the PQ‐10 was a good marker of global QoL related to the MNCD staging. It is a simple question about the global QoL perception (from 0, the worst, to 10, the best), and in‐line with many previous observations in the COPPADIS cohort (Santos‐García et al., 2019; Santos‐García et al., 2021b), we recommend using it as a very simple and quick marker of global QoL for PD patients and their caregivers as an alternative to others. Indeed, the correlation between the PQ‐10 and the EUROHIS‐QOL8 was strong (r > .7).
This study has important limitations; many of them commented in the recent report about the correlation between disease severity and QoL in PD patients from the COPPADIS cohort and the MNCD staging (Santos‐García et al., 2023): The MNCD classification was applied retrospectively and not directly applied by the neurologist during a face‐to‐face assessment; a bias toward less advanced PD in this cohort with only five caregivers of PD patients in stages 4–5; a cross‐sectional instead of a prospective follow‐up analysis; a study to demonstrate good inter‐ and intra‐variability is needed. On the contrary, the results of this study are again novel and support the idea to consider the MNCD classification as a simple tool to use in PD patients for identifying the main symptoms and monitor the progression of PD.
In conclusion, we observed in this study that staging PD according to the MNCD classification correlates with caregiver burden. More studies are needed to demonstrate the potential value of this new simple proposed tool. The backing of a scientific society may be key to its prosperous use by the scientific community.
AUTHOR CONTRIBUTIONS
Diego Santos‐García: Conceptualization; investigation; writing—original draft; methodology; validation; software; formal analysis; data curation; supervision; resources. Teresa de Deus Fonticoba: Investigation; methodology; writing—review and editing. Carlos Cores Bartolomé: Investigation; writing—review and editing. María J. Feal Painceiras: Investigation; writing—review and editing. Iago García Díaz: Investigation; writing—review and editing. María Cristina Íñiguez Alvarado: Investigation; writing—review and editing. Jose Manuel Paz: Investigation; writing—review and editing. Silvia Jesús: Investigation; writing—review and editing; methodology. Marina Cosgaya: Investigation; writing—review and editing; methodology. Juan García Caldentey: Investigation; writing—review and editing; methodology. Nuria Caballol: Investigation; writing—review and editing; methodology. Ines Legarda: Investigation; writing—review and editing; methodology. Jorge Hernández Vara: Investigation; writing—review and editing; methodology. Iria Cabo: Investigation; writing—review and editing; methodology. Lydia López Manzanares: Investigation; writing—review and editing; methodology. Isabel González Aramburu: Investigation; writing—review and editing; methodology. Maria A. Ávila Rivera: Investigation; writing—review and editing; methodology. Víctor Gómez Mayordomo: Investigation; writing—review and editing; methodology. Víctor Nogueira: Investigation; writing—review and editing; methodology. Julio Dotor García‐Soto: Investigation; writing—review and editing; methodology. Carmen Borrué: Investigation; writing—review and editing; methodology. Berta Solano Vila: Investigation; writing—review and editing; methodology. María Álvarez Sauco: Investigation; methodology; writing—review and editing. Lydia Vela: Investigation; writing—review and editing; methodology. Sonia Escalante: Investigation; methodology; writing—review and editing. Esther Cubo: Investigation; writing—review and editing; methodology. Zebenzui Mendoza: Investigation; writing—review and editing; methodology. Juan C. Martínez Castrillo: Investigation; writing—review and editing; methodology. Pilar Sánchez Alonso: Investigation; writing—review and editing; methodology. Maria G. Alonso Losada: Investigation; methodology; writing—review and editing. Nuria López Ariztegui: Investigation; writing—review and editing; methodology. Itziar Gastón: Investigation; writing—review and editing; methodology. Jaime Kulisevsky: Investigation; writing—review and editing; methodology. Manuel Seijo: Investigation; writing—review and editing; methodology. Caridad Valero: Investigation; writing—review and editing; methodology. Ruben Alonso Redondo: Investigation; writing—review and editing; methodology. Maria Teresa Buongiorno: Investigation; writing—review and editing; methodology. Carlos Ordás: Investigation; writing—review and editing; methodology. Manuel Menéndez‐González: Investigation; writing—review and editing; methodology. Darrian McAfee: Writing—review and editing. Pablo Martinez‐Martin: Writing—review and editing; supervision. Pablo Mir: Investigation; conceptualization; writing—review and editing; methodology; supervision.
CONFLICT OF INTEREST STATEMENT
Diego Santos‐García has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, Italfarmaco, Teva, Archímedes, Esteve, Stada, Merz, and grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co‐founded by ISCIII (Concesión de subvenciones de Proyectos de Investigación en Salud de la convocatoria 2020 de la Acción Estratégica en Salud 2017–2020 por el proyecto “PROGRESIÓN NO MOTORA E IMPACTO EN LA CALIDAD DE VIDA EN LA ENFERMEDAD DE PARKINSON”).
Teresa de Deus Fonticoba: None.
Carlos Cores Bartolomé has received honoraria for educational presentations and advice service by Lundbeck and UCB Pharma.
María J. Feal Painceiras: None.
Iago García Díaz has received support for educational activity from Bial.
María Cristina Íñiguez Alvarado: None.
Jose Manuel Paz has received honoraria/support for educational presentations and attending meetings from UCB, Abbvie, Zambon, Bial and KRKA.
Silvia Jesús has received honoraria from AbbVie, Bial, Merz, UCB, and Zambon and holds the competitive contract “Juan Rodés” supported by the Instituto de Salud Carlos III. She has received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI‐0459‐2018).
Marina Cosgaya: None.
Juan García Caldentey has received honoraria for educational presentations and advice service by Qualigen, Nutricia, Abbvie, Italfarmaco, UCB Pharma, Lundbeck, Zambon, Bial, and Teva.
Nuria Caballol has received honoraria from Bial, Italfármaco, Qualigen, Zambon, UCB, Teva and KRKA and sponsorship from Zambon, TEVA and Abbvie for attending medical conferences.
Ines Legarda has received honoraria for educational presentations and advice service by
Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Jorge Hernández Vara has received travel bursaries and educational grants from Abbvie and has received honoraria for educational presentations from Abbvie, Teva, Bial, Zambon, Italfarmaco, and Sanofi‐Genzyme.
Iria Cabo: has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Lydia López Manzanares compensated advisory services, consulting, research grant support, or speaker honoraria: AbbVie, Acorda, Bial, Intec Pharma, Italfarmaco, Pfizer, Roche, Teva, UCB, and Zambon.
Isabel González Aramburu: None.
Maria A. Ávila Rivera has received honoraria from Zambon, UCB Pharma, Qualigen, Bial, and Teva, and sponsorship from Zambon and Teva for attending conferences.
Víctor Gómez Mayordomo has received honoraria from Bial, Merz and Zambon for educational lectures.
Víctor Nogueira: None.
Julio Dotor García‐Soto compensated advisory services, consulting, research grant support, or speaker honoraria: Merck, Sanofi‐Genzyme, Allergan, Biogen, Roche, UCB and Novartis.
Carmen Borrué: None.
Berta Solano Vila has received honoraria for educational presentations and advice service by UCB, Zambon, Teva, Abbvie, Bial.
María Álvarez Sauco has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Zambon, Bial, and Teva.
Lydia Vela has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Sonia Escalante has received honoraria for educational presentations and advice service by Abbvie, Zambon, and Bial.
Esther Cubo: Travel grants: Abbvie, Allergan, Boston; Lecturing honoraria: Abbvie, International Parkinson's disease Movement Disorder Society.
Zebenzui Mendoza: None.
Juan C. Martínez Castrillo has received research support from Lundbeck, Italfarmaco, Allergan, Zambon, Merz, and Abbvie. He has received speaking honoraria from AbbVie, Bial, Italfarmaco, Lundbeck, Krka, TEVA, UCB, Zambon, Allergan, Ipsen, and Merz.
Pilar Sánchez Alonso has received honoraria for educational presentations and advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva.
Maria G. Alonso Losada has received honoraria for educational presentations and advice service by Zambon and Bial.
Nuria López Ariztegui has received honoraria for educational presentations and advice service by Abbvie, Italfarmaco, Zambon, and Bial.
Itziar Gastón has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts, and Zambon.
Jaime Kulisevsky: (1) Consulting fees: Roche, Zambon; (2) Stock / allotment: No; (3) Patent royalties / licensing fees: No; (4) Honoraria (e.g. lecture fees): Zambon, Teva, Bial, UCB; (5) Fees for promotional materials: No; (6) Research funding: Roche, Zambon, Ciberned; Instituto de SaludCarlos III; FundacióLa Maratóde TV3; (7) Scholarship from corporation: No; (8) Corporate laboratory funding: No; (9) Others (e.g. trips, travel, or gifts): No.
Manuel Seijo has received honoraria for educational services from KRKA, UCB, Zambon, Bial; travel grants from Daiichi and Roche.
Caridad Valero has received honoraria for educational services from Zambon, Abbvie and UCB.
Kurtis M. has received honoraria from Bial, the Spanish Neurology Society, and the International and Movement Disorders Society.
Ruben Alonso Redondo: None.
Maria Teresa Buongiorno: None.
Carlos Ordás: None.
Manuel Menéndez‐González has received consulting honoraria from Eisai Spain and research grants from by Instituto de Salud Carlos III (ISCIII), Fondo Europeo de Desarrollo Regional (FEDER), and Fundación Alimerka.
Darrian McAfee: None.
Pablo Martinez‐Martin has received honoraria from Bial for lecturing in course and from the Parkinson and Movement Disorder Society (MDS) for management of the COA International Program of the Society.
Pablo Mir has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon and have received grants from the Spanish Ministry of Economy and Competitiveness [ PI16/01575] co‐founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación) and by Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI‐02526, CTS‐7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [ PI‐0437‐2012, PI‐0471‐2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, the Fundación Mutua Madrileña.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.3295.
ACKNOWLEDGMENTS
We would like to thank all patients and their caregivers who collaborated in this study. Many thanks also to Fundación Española de Ayuda a la Investigación en Enfermedades Neurodegenerativas y/o de Origen Genético (https://fundaciondegen.org/), Alpha Bioresearch (www.alphabioresearch.com), Instituto de Salud Carlos III, and other institutions helping us.
APPENDIX 1.
1.1.
COPPADIS STUDY GROUP
Adarmes AD, Almeria M, Alonso Losada MG, Alonso Cánovas A, Alonso Frech F, Alonso Redondo R, Álvarez I, Álvarez Sauco M, Aneiros Díaz A, Arnáiz S, Arribas S, Ascunce Vidondo A, Aguilar M, Ávila MA, Bernardo Lambrich N, Bejr‐Kasem H, Blázquez Estrada M, Botí M, Borrue C, Buongiorno MT, Cabello González C, Cabo López I, Caballol N, Cámara Lorenzo A, Canfield Medina H, Carrillo F, Carrillo Padilla FJ, Casas E, Catalán MJ, Clavero P, Cortina Fernández A, Cosgaya M, Cots Foraster A, Crespo Cuevas A, Cubo E, de Deus Fonticoba T, de Fábregues‐Boixar O, Díez‐Fairen M, Dotor García‐Soto J, Erro E, Escalante S, Estelrich Peyret E, Fernández Guillán N, Gámez P, Gallego M, García Caldentey J, García Campos C, García Díez C, García Moreno JM, Gastón I, Gómez Garre MP, Gómez Mayordomo V, González Aloy J, González‐Aramburu I, González Ardura J, González García B, González Palmás MJ, González Toledo GR, Golpe Díaz A, Grau Solá M, Guardia G, Hernández Vara J, Horta‐Barba A, Idoate Calderón D, Infante J, Jesús S, Kulisevsky J, Kurtis M, Labandeira C, Labrador MA, Lacruz F, Lage Castro M, Lastres Gómez S, Legarda I, López Ariztegui N, López Díaz LM, López Domínguez D, López Manzanares L, López Seoane B, Lucas del Pozo S, Macías Y, Mata M, Martí Andres G, Martí MJ, Martínez Castrillo JC, Martinez‐Martin P, McAfee D, Meitín MT, Mendoza Plasencia Z, Menéndez González M, Méndez del Barrio C, Mir P, Miranda Santiago J, Morales Casado MI, Moreno Diéguez A, Nogueira V, Novo Amado A, Novo Ponte S, Ordás C, Pagonabarraga J, Pareés I, Pascual‐Sedano B, Pastor P, Pérez Fuertes A, Pérez Noguera R, Planas‐Ballvé A, Planellas L, Prats MA, Prieto Jurczynska C, Puente V, Pueyo Morlans M, Puig Daví A, Redondo Rafales N, Rodríguez Méndez L, Rodríguez Pérez AB, Roldán F, Ruíz De Arcos M, Ruíz Martínez J, Sánchez Alonso P, Sánchez‐Carpintero M, Sánchez Díez G, Sánchez Rodríguez A, Santacruz P, Santos‐García D, Segundo Rodríguez JC, Seijo M, Sierra Peña M, Solano Vila B, Suárez Castro E, Tartari JP, Valero C, Vargas L, Vela L, Villanueva C, Vives B.
| Name (last Name, first Name) | Location | Role | Contribution |
|---|---|---|---|
| Astrid Adarmes, Daniela | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Almeria, Marta | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Alonso Losada, Maria Gema | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Alonso Cánovas, Araceli | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Frech, Fernando | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Alonso Redondo, Ruben | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Aneiros Díaz, Ángel | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Álvarez, Ignacio | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Álvarez Sauco, María | Hospital General Universitario de Elche, Elche, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Arnáiz, Sandra | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Arribas, Sonia | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Ascunce Vidondo, Arancha | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Aguilar, Miquel | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Ávila Rivera, Maria Asunción | Consorci Sanitari Integral, Hospital General de L´Hospitalet, L´Hospitalet de Llobregat, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Bernardo Lambrich, Noemí | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator | Evaluation of participants and/or data management |
| Bejr‐Kasem, Helena | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Blázquez Estrada, Marta | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator | Evaluation of participants and/or data management |
| Botí González, Maria Ángeles | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Borrué, Carmen | Hospital Infanta Sofía, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Buongiorno, Maria Teresa | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Cabello González, Carolina | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Scheduling of evaluations |
| Cabo López, Iria | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Caballol, Nuria | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Cámara Lorenzo, Ana | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Nurse study coordinator |
| Canfield Medina, Héctor | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo, Fátima | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Carrillo Padilla, Francisco José | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Casas, Elena | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Catalán, Maria José | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Clavero, Pedro | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cortina Fernández, A | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Coordination of blood extractions |
| Cosgaya, Marina | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cots Foraster, Anna | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Crespo Cuevas, Ane | Hospital del Mar, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Cubo, Esther | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| De Deus Fonticoba, Teresa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator |
Nurse study coordinator Evaluation of participants and/or data management |
| De Fábregues‐Boixar, Oriol | Hospital Universitario Vall d´Hebron, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Díez Fairen, M | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Dotor García‐Soto, Julio | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| Erro, Elena | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Escalante, Sonia | Hospital de Tortosa Verge de la Cinta (HTVC), Tortosa, Tarragona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Estelrich Peyret, Elena | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Fernández Guillán, Noelia | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Gámez, Pedro | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Gallego, Mercedes | Hospital La Princesa, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| García Caldentey, Juan | Centro Neurológico Oms 42, Palma de Mallorca, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| García Campos, Cristina | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| García Díez, Cristina | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator (from MAY/22) | neuropsychologist; evaluation of participants |
| García Moreno, Jose Manuel | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator/PI (until MAR/21) |
Coordination at the center Evaluation of participants and/or data management |
| Gastón, Itziar | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Gómez Garre, María del Pilar | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Genetic studies coordination |
| Gómez Mayordomo, Víctor | Hospital Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aloy, Javier | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| González Aramburu, Isabel | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| González Ardura, Jessica | Hospital Universitario Lucus Augusti (HULA), Lugo, Spain | Site investigator/PI (until FEB/21) | Evaluation of participants and/or data management |
| González García, Beatriz | Hospital La Princesa, Madrid, Spain | Site investigator | Nurse study coordinator |
| González Palmás, Maria Josefa | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| González Toledo, Gabriel Ricardo | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Golpe Díaz, Ana | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Laboratory analysis coordination |
| Grau Solá, Mireia | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Guardia, Gemma | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Hernández Vara, Jorge | Hospital Universitario Vall d´Hebron, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Horta Barba, Andrea | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Idoate Calderón, Daniel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigaor (until MAY/22) | neuropsychologist; evaluation of participants |
| Infante, Jon | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Jesús, Silvia | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Kulisevsky, Jaime | Hospital de Sant Pau, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Kurtis, Mónica | Hospital Ruber Internacional, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Labandeira, Carmen | Hospital Álvaro Cunqueiro, Complejo Hospitalario Universitario de Vigo (CHUVI), Vigo, Spain | Site investigator | Evaluation of participants and/or data management |
| Labrador Espinosa, Miguel Ángel | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging data analysis |
| Lacruz, Francisco | Complejo Hospitalario de Navarra, Pamplona, Spain | Site investigator | Evaluation of participants and/or data management |
| Lage Castro, Melva | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Evaluation of participants and/or data management |
| Lastres Gómez, Sonia | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator | Neuropsychologist; evaluation of participants |
| Legarda, Inés | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| López Ariztegui, Nuria | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator/PI | Evaluation of participants and/or data management |
| López Díaz, Luis Manuel | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| López Domínguez, Daniel | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| López Manzanares, Lydia | Hospital La Princesa, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| López Seoane, Balbino | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Lucas del Pozo, Sara | Hospital Universitario Vall d´Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Macías, Yolanda | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Mata, Marina | Hospital Infanta Sofía, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí Andres, Gloria | Hospital Universitario Vall d´Hebron, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Martí, Maria José | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Martínez Castrillo, Juan Carlos | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Martinez‐Martin, Pablo | Centro Nacional de Epidemiología y CIBERNED, Instituto de Salud Carlos III. Madrid | Collaborator in statistical and methods analysis | Methods and statistical reviewer |
| McAfee, Darrian | University of Pennsylvania, Philadelphia | Collaborator in english style | English style reviewer |
| Meitín, Maria Teresa | Hospital Da Costa de Burela, Lugo, Spain | Site investigator | Evaluation of participants and/or data management |
| Menéndez González, Manuel | Hospital Universitario Central de Asturias, Oviedo, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Méndez del Barrio, Carlota | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Mendoza Plasencia, Zebenzui | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Mir, Pablo | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Miranda Santiago, Javier | Complejo Asistencial Universitario de Burgos, Burgos, Spain | Site investigator | Evaluation of participants and/or data management |
| Morales Casado, Maria Isabel | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Moreno Diéguez, Antonio | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Nogueira, Víctor | Hospital Da Costa de Burela, Lugo, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Novo Amado, Alba | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Novo Ponte, Sabela | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Ordás, Carlos | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site Investigator | Evaluation of participants and/or data management |
| Pagonabarraga, Javier | Hospital de Sant Pau, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pareés, Isabel | Hospital Ruber Internacional, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Pascual‐Sedano, Berta | Hospital de Sant Pau, Barcelona, Spain | Site Investigator | Evaluation of participants and/or data management |
| Pastor, Pau | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Pérez Fuertes, Aída | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Pérez Noguera, Rafael | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Planas‐Ballvé, Ana | Consorci Sanitari Integral, Hospital Moisés Broggi, Sant Joan Despí, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Planellas, Lluís | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator (until DEC/19) | Evaluation of participants and/or data management |
| Prats, Marian Ángeles | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator | Evaluation of participants and/or data management |
| Prieto Jurczynska, Cristina | Hospital Rey Juan Carlos, Madrid, Spain, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Puente, Víctor | Hospital del Mar, Barcelona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Pueyo Morlans, Mercedes | Hospital Universitario de Canarias, San Cristóbal de la Laguna, Santa Cruz de Tenerife, Spain | Site investigator | Evaluation of participants and/or data management |
| Puig Daví, Arnau | Hospital de Sant Pau, Barcelona, Spain | Site einvestigator | Evaluation of participants and/or data management |
| Redondo, Nuria | Hospital La Princesa, Madrid, Spain | Site Investigator | Evaluation of participants and/or data management |
| Rodríguez Méndez, Luisa | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Blood analysis |
| Rodríguez Pérez, Amparo Belén | Hospital General Universitario de Elche, Elche, Spain | Site investigator | Evaluation of participants and/or data management |
| Roldán, Florinda | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Neuroimaging studies |
| Ruíz de Arcos, María | Hospital Universitario Virgen Macarena, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Ruíz Martínez, Javier | Hospital Universitario Donostia, San Sebastián, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Alonso, Pilar | Hospital Universitario Puerta de Hierro, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez‐Carpintero, Macarena | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Neuroimaging studies |
| Sánchez Díez, Gema | Hospital Universitario Ramón y Cajal, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Sánchez Rodríguez, Antonio | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Santacruz, Pilar | Hospital Clínic de Barcelona, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Santos‐García, Diego | CHUAC, Complejo Hospitalario Universitario de A Coruña | Coordinator of the Project | Coordination of the COPPADIS‐2015 |
| Segundo Rodríguez, José Clemente | Complejo Hospitalario de Toledo, Toledo, Spain | Site investigator | Evaluation of participants and/or data management |
| Seijo, Manuel | Complejo Hospitalario Universitario de Pontevedra (CHOP), Pontevedra, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Sierra, María | Hospital Universitario Marqués de Valdecilla, Santander, Spain | Site investigator | Evaluation of participants and/or data management |
| Solano, Berta | Institut d'Assistència Sanitària (IAS)—Instituí Cátala de la Salud. Girona, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Suárez Castro, Ester | Complejo Hospitalario Universitario de Ferrol (CHUF), Ferrol, A Coruña, Spain | Site investigator | Evaluation of participants and/or data management |
| Tartari, Juan Pablo | Hospital Universitari Mutua de Terrassa, Terrassa, Barcelona, Spain | Site investigator | Evaluation of participants and/or data management |
| Valero, Caridad | Hospital Arnau de Vilanova, Valencia, Spain | Site investigator | Evaluation of participants and/or data management |
| Vargas, Laura | Hospital Universitario Virgen del Rocío, Sevilla, Spain | Site investigator | Evaluation of participants and/or data management |
| Vela, Lydia | Fundación Hospital de Alcorcón, Madrid, Spain | Site investigator/PI |
Coordination at the center Evaluation of participants and/or data management |
| Villanueva, Clara | Hospital Universitario Clínico San Carlos, Madrid, Spain | Site investigator | Evaluation of participants and/or data management |
| Vives, Bárbara | Hospital Universitario Son Espases, Palma de Mallorca, Spain | Site investigator | Evaluation of participants and/or data management |
Santos‐García, D. , de Deus Fonticoba, T. , Cores Bartolomé, C. , Feal Painceiras, M. J.., García Díaz, I. , Alvarado, M. C. Í. , Paz, J. M. , Jesús, S. , Cosgaya, M. , Caldentey, J. G. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , González Aramburu, I. , Ávila Rivera, M. A. , Gómez Mayordomo, V. , … Mir, P. , COPPADIS Study Group . (2023). Staging Parkinson's disease according to the MNCD classification correlates with caregiver burden. Brain and Behavior, 13, e3295. 10.1002/brb3.3295
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Aamodt, W. W. , Kluger, B. M. , Mirham, M. , Job, A. , Lettenberger, S. E. , Mosley, P. E. , & Seshadri, S. (2023). Caregiver burden in Parkinson disease: A scoping review of the literature from 2017–2022. Journal of Geriatric Psychiatry and Neurology, (ahead of print). 10.1177/08919887231195219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (1994). Diagnostic and statical manual of mental disorders (4th ed.). American Psychiatric Association. [Google Scholar]
- Beck, A. T. , Steer, R. A. , & Brown, G. K. (1996). Beck Depression inventory–Second edition (Manual). The Psychological Corporation. [Google Scholar]
- Berg, D. , Postuma, R. B. , Adler, C. H. , Bloem, B. R. , Chan, P. , Dubois, B. , Gasser, T. , Goetz, C. G. , Halliday, G. , Joseph, L. , Lang, A. E. , Liepelt‐Scarfone, I. , Litvan, I. , Marek, K. , Obeso, J. , Oertel, W. , Olanow, C. W. , Poewe, W. , Stern, M. , & Deuschl, G. (2015). MDS research criteria for prodromal Parkinson's disease. Movement Disorders, 30, 1600–1611. 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- D'Amelio, M. , Terruso, V. , Palmeri, B. , Di Benedetto, N. , Famoso, G. , Cottone, P. , Aridon, P. , Ragonese, P. , & Savettieri, G. (2009). Predictors of caregiver burden in partners of patients with Parkinson's disease. Neurological Sciences, 30, 171–174. 10.1007/s10072-009-0024-z [DOI] [PubMed] [Google Scholar]
- Da Rocha, N. S. , Power, M. J. , Bushnell, D. M. , & Fleck, M. P. (2012). The EUROHIS‐QOL 8‐item index: Comparative psychometric properties to its parent WHOQOL‐BREF. Value in Health: The Journal of the International Society for Pharmacoeconomics and Outcomes Research, 15, 449–457. 10.1016/j.jval.2011.11.035 [DOI] [PubMed] [Google Scholar]
- De Pablo‐Fernández, E. , Lees, A. J. , Holton, J. L. , & Warner, T. T. (2019). Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurology, 76, 470–479. 10.1001/jamaneurol.2018.4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fereshtehnejad, S. M. , Romenets, S. R. , Anang, J. B. , Latreille, V. , Gagnon, J. F. , & Postuma, R. B. (2015). New clinical subtypes of Parkinson disease and their longitudinal progression: A prospective cohort comparison with other phenotypes. JAMA Neurology, 72, 863–873. 10.1001/jamaneurol.2015.0703 [DOI] [PubMed] [Google Scholar]
- Goldman, J. G. , & Postuma, R. (2014). Premotor and nonmotor features of Parkinson's disease. Current Opinion in Neurology, 27, 434–441. 10.1097/WCO.0000000000000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland, J. C. , Williams‐Gray, C. H. , & Barker, R. A. (2019). The clinical heterogeneity of Parkinson's disease and its therapeutic implications. European Journal of Neuroscience, 49, 328–338. 10.1111/ejn.14094 [DOI] [PubMed] [Google Scholar]
- Heinzel, S. , Berg, D. , Gasser, T. , Chen, H. , Yao, C. , & Postuma, R. B. (2019). MDS task force on the definition of Parkinson's disease. Update of the MDS research criteria for prodromal Parkinson's disease. Movement Disorders, 34, 1464–1470. 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- Hoehn, M. M. , & Yahr, M. D. (1967). Parkinsonism: Onset, progression and mortality. Neurology, 17, 427–442. 10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- Hughes, A. J. , Daniel, S. E. , Kilford, L. , & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry, 55, 181–184. 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd, L. L. , Rapaport, M. H. , Paulus, M. P. , & Brown, J. L. (1994). Subsyndromal symptomatic depression: A new mood disorder? Journal of Clinical Psychiatry, 55, (Suppl), 18–28. [PubMed] [Google Scholar]
- Krikwood, B. R. , & Sterne, J. A. C. (2003). Medical statistics (2nd ed.). Oxford Blackwell Science Ltd. [Google Scholar]
- Lawton, M. , Ben‐Shlomo, Y. , May, M. T. , Baig, F. , Barber, T. R. , Klein, J. C. , Swallow, D. M. A. , Malek, N. , Grosset, K. A. , Bajaj, N. , Barker, R. A. , Williams, N. , Burn, D. J. , Foltynie, T. , Morris, H. R. , Wood, N. W. , Grosset, D. G. , & Hu, M. T. M. (2018). Developing and validating Parkinson's disease subtypes and their motor and cognitive progression. Journal of Neurology, Neurosurgery, and Psychiatry, 89, 1279–1287. 10.1136/jnnp-2018-318337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Martin, P. , Forjaz, M. J. , Frades‐Payo, B. , Rusiñol, A. B. , Fernández‐García, J. M. , Benito‐León, J. , Arillo, V. C. , Barberá, M. A. , Sordo, M. P. , & Catalán, M. J. (2007). Caregiver burden in Parkinson´s disease. Movement Disorders, 22, 924–931. 10.1002/mds.21355 [DOI] [PubMed] [Google Scholar]
- Martinez Martin, P. , Rodriguez Blazquez, C. , & Forjaz, M. J. (2012). Quality of life and burden in caregivers for patients with Parkinson's disease: Concepts, assessment and related factors. Expert Review of Pharmacoeconomics & Outcomes Research, 12, 221–230. 10.1586/erp.11.106 [DOI] [PubMed] [Google Scholar]
- Mosley, P. E. , Moodie, R. , & Dissanayaka, N. (2017). Caregiver burden in Parkinson disease: A critical review of recent literature. Journal of Geriatric Psychiatry and Neurology, 30, 235–252. 10.1177/0891988717720302 [DOI] [PubMed] [Google Scholar]
- Novak, M. , & Guest, C. (1989). Application of a multidimensional caregiver burden inventory. Gerontology, 29, 798–803. 10.1093/geront/29.6.798 [DOI] [PubMed] [Google Scholar]
- Peters, M. , Fitzpatrick, R. , Doll, H. , Playford, D. , & Jenkinson, C. (2011). Does self‐reported well‐being of patients with Parkinson's disease influence caregiver strain and quality of life? Parkinsonism & Related Disorders, 17, 348–352. 10.1016/j.parkreldis.2011.02.009 [DOI] [PubMed] [Google Scholar]
- Postuma, R. B. , Berg, D. , Stern, M. , Poewe, W. , Olanow, C. W. , Oertel, W. , Obeso, J. , Marek, K. , Litvan, I. , Lang, A. E. , Halliday, G. , Goetz, C. G. , Gasser, T. , Dubois, B. , Chan, P. , Bloem, B. R. , Adler, C. H. , & Deuschl, G. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Movement Disorders, 30, 1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- Robinson, B. C. (1983). Validation of a caregiver strain index. Journal of Gerontology, 38, 344–348. 10.1093/geronj/38.3.344 [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , Álvarez Sauco, M. , Calopa, M. , Carrillo, F. , Escamilla Sevilla, F. , Freire, E. , García Ramos, R. , Kulisevsky, J. , Gómez Esteban, J. C. , Legarda, I. , Luquín, M. R. I. , Castrillo, J. C. M. , Martínez‐Martin, P. , Martínez‐Torres, I. , Mir, P. , & Ignacio, Á. S. (2021a). MNCD: A new tool for classifying Parkinson's disease in daily clinical practice. Diagnostics (Basel), 12, 55. 10.3390/diagnostics12010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , & Añón, M. J. , Fuster Sanjurjo, L. , & de la Fuente‐fernández, R. (2012). Duodenal levodopa/carbidopa infusion therapy in advanced Parkinson´s disease patients leads to improvement in caregivers’ stress and burden. European Journal of Neurology, 259, 1668–1672. [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , de Deus Fonticoba, T. , Cores, C. , Muñoz, G. , Paz González, J. M. , Martínez Miró, C. , Suárez, E. , Jesús, S. , Aguilar, M. , Pastor, P. , Planellas, L. , Cosgaya, M. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , Aramburu, I. G. , … Ávila Rivera, M. A. , COPPADIS Study Group . (2021b). Predictors of clinically significant quality of life impairment in Parkinson's disease. npj Parkinson's Disease, 7, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , de Deus Fonticoba, T. , Cores Bartolomé, C. , Feal Painceiras, M. J. , Íñiguez‐Alvarado, M. C. , García Díaz, I. , Jesús, S. , Buongiorno, M. T. , Planellas, L. , Cosgaya, M. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , González Aramburu, I. , Ávila Rivera, M. A. , … Gómez Mayordomo, V. , COPPADIS Study Group . (2023a). Staging Parkinson's disease according to the MNCD (motor/non‐motor/cognition/dependency) classification correlates with disease severity and quality of life. Journal of Parkinson's Disease, 13, 379–402. 10.3233/JPD-225073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , de Deus Fonticoba, T. , Cores Bartolomé, C. , Feal Painceiras, M. J. , Íñiguez‐Alvarado, M. C. , García Díaz, I. , Jesús, S. , Buongiorno, M. T. , Planellas, L. , Cosgaya, M. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , González Aramburu, I. , Ávila Rivera, M. A. , Gómez Mayordomo, V. , … Nogueira, V. , COPPADIS Study Group . (2023b). Changes in principal caregiver mood affects the mood of the Parkinson's disease patient: The vicious cycle of illness. Journal of Parkinson's Disease, 13, 219–231. 10.3233/JPD-225014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , de Deus Fonticoba, T. , Cores Bartolomé, C. , Íñiguez Alvarado, M. C. , Feal Panceiras, M. J. , Suárez Castro, E. , Canfield, H. , Martínez Miró, C. , Jesús, S. , Aguilar, M. , Pastor, P. , Planellas, L. , Cosgaya, M. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , … González Aramburu, I. , COPPADIS Study Group . (2022). Predictors of the change in burden, strain, mood, and quality of life among caregivers of Parkinson's disease patients. International Journal of Geriatric Psychiatry, 37(6), 10.1002/gps.5761 [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , De Deus Fonticoba, T. , Paz González, J. M. , Cores Bartolomé, C. , Valdés Aymerich, L. , Muñoz Enríquez, J. G. , Suárez, E. , Jesús, S. , Aguilar, M. , Pastor, P. , Planellas, L. L. , Cosgaya, M. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , González Aramburu, I. , … Ávila Rivera, M. A. , Coppadis Study Group . (2021c). Staging Parkinson's disease combining motor and nonmotor symptoms correlates with disability and quality of life. Parkinsons Disease, 2021, 8871549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos‐García, D. , de Deus Fonticoba, T. , Suárez Castro, E. , Borrué, C. , Mata, M. , Solano Vila, B. , Cots Foraster, A. , Álvarez Sauco, M. , Rodríguez Pérez, A. B. , Vela, L. , Macías, Y. , Escalante, S. , Esteve, P. , Reverté Villarroya, S. , Cubo, E. , Casas, E. , Arnaiz, S. , Carrillo Padilla, F. , Pueyo Morlans, M. , … Mir, P. , Coppadis Study Group . (2019). Non‐motor symptoms burden, mood, and gait problems are the most significant factors contributing to a poor quality of life in non‐demented Parkinson's disease patients: Results from the COPPADIS Study Cohort. Parkinsonism & Related Disorders, 66, 151–157. 10.1016/j.parkreldis.2019.07.031 [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , & de la Fuente‐Fernández, R. (2015). Factors contributing to caregivers’ stress and burden in Parkinson's disease. Acta Neurologica Scandinavica, 131, 203–210. 10.1111/ane.12305 [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , Jesús, S. , Aguilar, M. , Planellas, L. L. , García Caldentey, J. , Caballol, N. , Legarda, I. , Hernández Vara, J. , Cabo, I. , López Manzanares, L. , González Aramburu, I. , Ávila Rivera, M. A. , Catalán, M. J. , López Díaz, L. , Puente, V. , García Moreno, J. M. , Borrué, C. , Solano Vila, B. , Álvarez Sauco, M. , Vela, L. , … COPPADIS Study Group . (2019). COPPADIS‐2015 (COhort of patients with Parkinson's disease in Spain, 2015): An ongoing global Parkinson's disease project about disease progression with more than 1000 subjects included. Results from the baseline evaluation. European Journal of Neurology, 26, 1399–1407. 10.1111/ene.14008 [DOI] [PubMed] [Google Scholar]
- Santos‐García, D. , Mir, P. , Cubo, E. , Vela, L. , Rodríguez‐Oroz, M. C. , Martí, M. J. , Arbelo, J. M. , Infante, J. , Kulisevsky, J. , & Martínez‐Martín, P. , COPPADIS Study Group . (2016). COPPADIS‐2015 (cohort of patients with Parkinson's disease in Spain, 2015), a global–clinical evaluations, serum biomarkers, genetic studies and neuroimaging—Prospective, multicenter, non‐interventional, long‐term study on Parkinson's disease progression. BMC Neurology [Electronic Resource], 16, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade, S. , Mollenhauer, B. , & Trenkwalder, C. (2020). Levodopa equivalent dose conversion factors: An updated proposal including opicapone and safinamide. Movement Disorders Clinical Practice, 7, 343–345. 10.1002/mdc3.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag, A. , Hovris, A. , Morley, D. , Quinn, N. , & Jahanshahi, M. (2006). Caregiver‐burden in Parkinson's disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism & Related Disorders, 12, 35–44. 10.1016/j.parkreldis.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Smith, E. R. , Perrin, P. B. , Tyler, C. M. , Lageman, S. K. , & Villaseñor, T. (2019). Parkinson's symptoms and caregiver burden and mental health: A cross‐cultural mediational model. Behavioural Neurology, 2019, 1396572. 10.1155/2019/1396572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, N. , Yang, Y. , Zhang, L. , Zhang, Q. , Balbuena, L. , Ungvari, G. S. , Zang, Y. F. , & Xiang, Y. T. (2021). Quality of life in Parkinson's disease: A systematic review and meta‐analysis of comparative studies. CNS Neuroscience & Therapeutics, 27, 270–279. 10.1111/cns.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zis, P. , Erro, R. , Walton, C. C. , Sauerbier, A. , & Chaudhuri, K. R. (2015). The range and nature of non‐motor symptoms in drug‐naive Parkinson's disease patients: A state‐of‐the‐art systematic review. NPJ Parkinsons Disease, 1, 15013. 10.1038/npjparkd.2015.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The protocol and the statistical analysis plan are available on request. Deidentified participant data are not available for legal and ethical reasons.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


