Abstract
Multidrug efflux pumps with a broad substrate specificity make a major contribution to intrinsic and acquired multiple antibiotic resistance in Pseudomonas aeruginosa. Using genetically defined efflux pump mutants, we investigated the involvement of the three known efflux systems, MexA-MexB-OprM, MexC-MexD-OprJ, and MexE-MexF-OprN, in organic solvent tolerance in this organism. Our results showed that all three systems are capable of providing some level of tolerance to organic solvents such as n-hexane and p-xylene. Expression of MexAB-OprM was correlated with the highest levels of tolerance, and indeed, this efflux system was a major contributor to the intrinsic solvent tolerance of P. aeruginosa. Intrinsic organic solvent tolerance was compromised by a protonophore, indicating that it is substantially energy dependent. These data suggest that the efflux of organic solvents is a factor in the tolerance of P. aeruginosa to these compounds and that the multidrug efflux systems of this organism can accommodate organic solvents, as well as antibiotics.
Many organic solvents are toxic to microorganisms. Generally, toxicity of an organic solvent correlates inversely with the logarithm of its partition coefficient with n-octanol and water (log Pow) (7, 14), at least for compounds with log Pow values between 1 and 5 (11). The toxicity of these compounds appears to be related to their ability to dissolve into biological membranes, disturbing the integrity of these structures and ultimately compromising their physiological function (for a review, see reference 11). Despite this, there have been numerous reports, particularly on members of the family Pseudomonadaceae, of strains demonstrating high-level tolerance of organic solvents (3, 14, 22), and in at least one case, this tolerance was attributed to active efflux of the organic solvent (15). Solvent tolerance has also been reported in Escherichia coli (2, 4), where it appears to be closely aligned with expression of the low-level multidrug resistance mediated by transcriptional activators encoded by the marA (6), soxS (24), and robA (23) genes. The marA gene forms part of an operon, marRAB, which is linked to the so-called multiple antibiotic resistance (Mar) phenotype (1). MarA-mediated multidrug resistance is known to involve the AcrAB-To1C multidrug efflux system (6, 8, 28), indicating that solvent tolerance in E. coli, too, likely involves solvent export (35). Consistent with this, increased expression of both AcrA and To1C has been demonstrated in organic solvent-tolerant mutants of E. coli (5).
Pseudomonas aeruginosa is an opportunistic human pathogen characterized by innate resistance to a variety of antimicrobial agents. This property is recognized to result mainly from the activity of broadly specific drug efflux systems (17, 18, 26, 31). Three such efflux systems have been described in P. aeruginosa, and they are encoded by the mexA-mexB-oprM (10, 19, 30, 31), mexC-mexD-oprJ (29), and mexE-mexF-oprN (16) operons. The MexA-MexB-OprM system has been demonstrated to contribute to the high intrinsic antibiotic resistance of this organism, and hyperexpression of the efflux genes is responsible for the elevated multidrug resistance of nalB mutants (19, 31, 32). MexC-MexD-OprJ and MexE-MexF-OprN are apparently not expressed during growth under normal laboratory conditions but are expressed in nfxB (12, 29) and nfxC (9, 16) multidrug-resistant mutants, respectively. In light of the homology between AcrAB-TolC and the P. aeruginosa multidrug efflux systems (25), then, it was of interest to assess the involvement of the latter in organic solvent tolerance. We report here that the MexAB-OprM efflux system mediates intrinsic organic solvent tolerance in P. aeruginosa and that hyperexpression of this system in nalB mutants enhances such tolerance. Similarly, expression of the multidrug efflux systems MexCD-OprJ and MexEF-OprN also enhances solvent tolerance in this organism.
To study the role of multidrug efflux pumps in organic solvent tolerance, we used genetically defined efflux pump mutants (Table 1) and three organic solvents, n-hexane, p-xylene, and toluene (Table 2). Two approaches were employed to assess solvent tolerance. The first involved overlaying solvent (100%) onto 25-ml Luria-Bertani (LB) agar plates inoculated with bacteria as previously described (4). Briefly, stationary-phase LB broth cultures were diluted into the same medium to yield a suspension of approximately 107 cells/ml. A 5-μl aliquot of the cell suspension was placed in duplicate on LB agar and allowed to dry before organic solvent was overlaid to a depth of approximately 2 mm. A variation of this method, termed efficiency of plating (EOP), was also employed (35). In this assay, 100 μl of a cell suspension (107 cells/ml) was spread over the surface of an LB agar plate which was subsequently overlaid with 1 ml of organic solvent. In both cases, the plates were sealed and growth was assessed following incubation at 30°C for 24 h. The second approach involved assessment of cell growth by measuring the increase in optical density at 660 nm (OD660) of a liquid culture supplemented with organic solvent. Briefly, stationary-phase cells were diluted into 30 ml of prewarmed (37°C) LB broth and incubated (with shaking) for 2 to 2.5 h at 37°C. At the early exponential phase of growth, organic solvent was added at a final concentration of 0.08 to 20% (vol/vol) and growth was monitored for a further 5 to 6 h. In some experiments, the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) was included in the growth medium (20 μM final concentration) to assess the influence of this energy inhibitor on the growth of P. aeruginosa in the presence and absence of organic solvents.
TABLE 1.
P. aeruginosa strains used in this study
| Strain | Description | Reference |
|---|---|---|
| PAO1 | Prototroph | |
| K1119 | PAO1 ΔmexAB-oprM | 20 |
| OCR1 | PAO1 nalB | 21 |
| ML5087 | ilv-220 thr-9001 leu-9001 met-9001 pur-67 aphA | 27 |
| K1112 | ML5087 nalB | 33 |
| K1110 | ML5087 ΔoprM | 20 |
| K1121 | ML5087 ΔmexAB-oprM | 34 |
| K1131 | K1121 nfxB | 20 |
| K1115 | ML5087 ΔmexAB-oprM ΔmexCD-oprJ | 20 |
| K1117 | MexEF-OprN-hyperexpressing derivative of K1115 | 20 |
| PAO6609 | met-9011 amiE200 rpsL pvd-9 | 13 |
| K1032 | PAO6609 ΔmexAB-oprM | 36 |
TABLE 2.
Organic solvent tolerance of P. aeruginosa on agar plates
| Strain | Relevant efflux phenotypeb | Growth ona:
|
||
|---|---|---|---|---|
| n-Hexane | p-Xylene | Toluene | ||
| PAO1 | MexAB-OprM+ | + (S) | + (S) | − |
| K1119 | MexAB-OprM− | − | − | − |
| OCR1 | MexAB-OprM++c | + (C) | + (C) | − |
| ML5087 | MexAB-OprM+ | + (S) | + (S) | − |
| K1110 | MexAB+ OprM− | − | − | − |
| K1112d | MexAB-OprM++ | + (C) | + (C) | − |
| K1131 | MexAB-OprM− MexCD-OprJ+ | − | − | − |
| K1117 | MexAB-OprM− MexEF-OprN+ | − | − | − |
| PAO6609 | MexAB-OprM+ | + | + | − |
| K1032 | MexAB-OprM− | − | − | − |
Bacteria (5 μl) were spotted onto LB agar, overlaid with organic solvent, and incubated overnight at 30°C as outlined in the text. +, growth; −, no growth. In parentheses are the results of EOP experiments in which 100-μl cell cultures were plated on the surfaces of LB agar plates, overlaid with organic solvent, and likewise incubated overnight. C, confluent growth; S, single-colony growth. The log Pow values of the solvents were as follows: n-hexane, 3.9; p-xylene, 3.1; toluene, 2.8.
The status of those efflux systems (components) that are known to be expressed and, thus, potentially contribute to the organic solvent tolerance of the indicated strains is highlighted.
High-level expression of MexAB-OprM.
This strain yielded scattered colonies after 72 h of incubation in the presence of toluene.
As shown in Table 2, all strains expressing wild-type levels of MexAB-OprM (PAO1, ML5087, and PAO6609), as well as the nalB mutants hyperexpressing MexAB-OprM (OCR1, and K1112), showed tolerance to n-hexane and p-xylene, but not to toluene, on agar plates. In EOP experiments, the latter strains elicited confluent growth while strains PAO1 and ML5087 yielded isolated colonies (Table 2), suggesting that nalB strains are better able to tolerate these solvents and, thus, that solvent tolerance in P. aeruginosa correlates with the level of expression of the MexAB-OprM efflux system. These isolated colonies did not appear to be solvent-tolerant mutants, as they elicited growth properties indistinguishable from those of the parental strains in liquid medium containing solvent (data not shown). The absence of a functional MexAB-OprM efflux system, due to deletion of either the entire mexA-mexB-oprM operon (in K1119 and K1032) or the oprM gene alone (in K1110), however, rendered these strains incapable of growth in the presence of any of the organic solvents tested (Table 2). Indeed, subsequent testing of the inoculation site after exposure to the solvents revealed that the bacteria applied to the plates were no longer viable, indicating that the solvents were bactericidal for these mutants. This was consistent with observations that exposure of these mutants to solvents in liquid medium precipitated a rapid decline in viable cell numbers, as assessed by using viable plate counts (data not shown).
Growth upon exposure to toluene occurred only for the nalB mutant P. aeruginosa K1112, a few toluene-tolerant colonies of which arose on plates after 72 h of incubation (Table 2). These were obviously mutants in that they subsequently displayed ready growth in the presence of toluene. Nonetheless, the fact that such mutants only arose from a strain already expressing elevated levels of MexAB-OprM suggests that this efflux system is able to accommodate toluene, thereby providing bacteria with a basal low-level tolerance from which mutants with high-level tolerance could be selected. Consistent with this, elevated production of MexAB-OprM was maintained in these mutants (assessed by Western immunoblotting of isolated cell envelopes with antiserum to OprM [20; data not shown]). That the solvent tolerance of so-called solvent-tolerant mutants can arise as a result of expression of multidrug efflux systems in P. aeruginosa was subsequently confirmed by the isolation of several mutants tolerant to 20% hexane (following serial passage of ML5087 in LB broth containing 3, 5, 7, 10, and, finally, 20% hexane) and the demonstration that >90% of these exhibited a multidrug resistance pattern indistinguishable from that of previously described nalB mutants (data not shown).
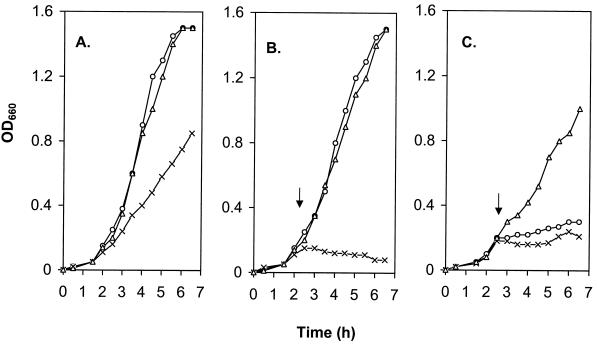
Cells were generally more sensitive to the effects of the organic solvents in liquid assays than in the plate assays. Consistent with its lower log Pow value, p-xylene (log Pow of 3.1) was more toxic than n-hexane (log Pow of 3.9) and, thus, wild-type P. aeruginosa PAO1 was unable to grow in the presence of p-xylene at 1% (vol/vol) (Fig. 1C) or even 0.5% (vol/vol) (data not shown), although it grew well in hexane at 2% (vol/vol) (Fig. 1B). In contrast, nalB mutant strain OCR1 grew well and the ΔmexAB-oprM mutant K1119 failed to grow at all in the presence of either solvent at these concentrations (Fig. 1B and C). Thus, solvent tolerance in liquid medium, as on solid medium, was enhanced by the presence of MexAB-OprM and was compromised by its absence. The failure to observe any differences in tolerance to hexane between PAO1 and its nalB derivative OCR1 (Fig. 1B), despite the qualitative differences seen on solid medium in the EOP experiments described above, probably reflected the levels of hexane used in the liquid-medium assays (2% [vol/vol]). Still, increasing the hexane level to 10% (vol/vol) in these assays also failed to discriminate between these strains, both of which grew quite well at this solvent concentration (data not shown). It seems likely, therefore, that the intrinsic levels of MexAB-OprM are more than sufficient to provide substantial tolerance to hexane, and only at much higher levels of hexane would differences between PAO1 and OCR1 be seen. Certainly, the differences on solid medium described above were observed when undiluted (i.e., 100%) hexane was used. Similarly, although PAO1 demonstrated tolerance to xylene on a solid medium, consistent with the expression of MexAB-OprM in this strain, neither PAO1 nor its mexAB-oprM deletion mutant K1119 grew in LB broth containing 0.5 to 1% (vol/vol) xylene. Indeed, we were unable to define a concentration of xylene which could discriminate between the wild-type strain and the MexAB-OprM− mutant in LB broth. These data highlight differences between the discriminating powers of the two assays and likely reflect unknown differences in the manner in which solvents interact with cells growing statically on solid surfaces versus shaken in liquid medium. Nonetheless, the obviously increased sensitivity of the mexAB-oprM deletion strains to organic solvents is consistent with the notion that MexAB-OprM plays a significant role in intrinsic organic solvent tolerance in P. aeruginosa, just as it plays a major role in intrinsic multidrug resistance in this organism (17–20, 31). Interestingly, the growth rate of the mexA-mexB-oprM deletion mutant was reduced relative to that of the other two strains, even in the absence of solvent (Fig. 1A), although unlike solvent-containing cultures, solvent-free cultures of this mutant still elicited growth. It is likely, then, that some important cellular process (independent of multidrug and solvent efflux) is compromised as a result of the loss of MexAB-OprM in this organism.
FIG. 1.
Influence of the MexA-MexB-OprM efflux system on the growth of P. aeruginosa in the presence of organic solvents. P. aeruginosa PAO1 (○), K1119 (ΔmexA-mexB-oprM) (×), and OCR1 (nalB) (▵) were grown in LB broth at 37°C to the early exponential phase, at which time (arrow) no solvent (A), n-hexane at 2% (vol/vol) (B), or p-xylene at 1% (vol/vol) (C) was added and growth determined by monitoring OD660.
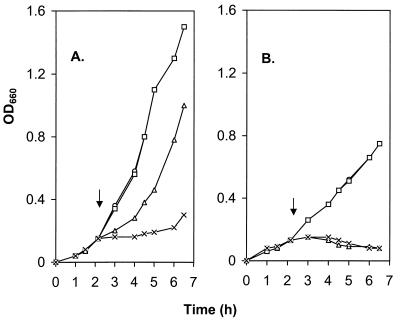
The MexAB-OprM system functions as an energy-dependent exporter. Thus, any contribution of this efflux system to organic solvent tolerance (presumably via efflux) should be similarly energy dependent. To assess this, then, the influence of the protonophore CCCP, which was previously shown to compromise MexAB-OprM-mediated multidrug resistance and export (19), on the solvent tolerance of MexAB-OprM+ strain PAO1 was assessed. Although PAO1 grew well in liquid medium in the presence of 0.2% (vol/vol) p-xylene (Fig. 2A), exposure of the cells to a concentration of CCCP (20 μM) which itself failed to adversely affect growth (Fig. 2A) almost completely abrogated the growth of this strain in the presence of 0.2% (vol/vol) p-xylene. Indeed, the effect of CCCP addition on the growth of PAO1 in the presence of xylene was comparable to the effect of deleting the mexAB-oprM-encoded efflux system (Fig. 2B), consistent with the idea that CCCP compromises MexAB-OprM activity and, thus, its contribution to solvent tolerance. Taken together, these results suggest that the MexAB-OprM efflux system exports organic solvents, as well as antibiotics.
FIG. 2.
Effect of CCCP on the organic solvent tolerance of P. aeruginosa PAO1 (A) and K1119 (ΔmexA-mexB-oprM) (B). Cells were grown in LB broth at 37°C to the early exponential phase, at which time (arrow) CCCP at 20 μM (□), p-xylene at 0.2% (vol/vol) (▵), or CCCP at 20 μM and p-xylene at 0.2% (vol/vol) (×) were added and growth was determined by monitoring OD660. Control cultures (○, overlapped with the CCCP group [□]) received no supplementation.
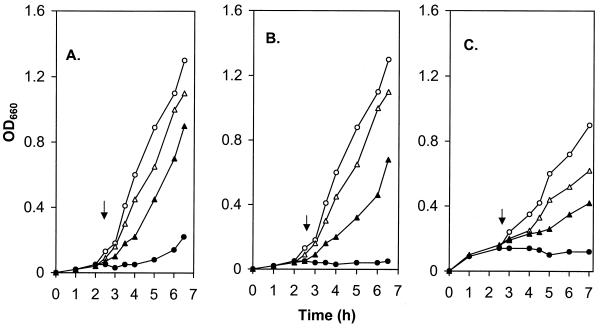
The MexCD-OprJ and MexEF-OprN efflux systems are not expressed in wild-type cells, at least under standard laboratory conditions and in rich media (16, 29). To determine, therefore, if these systems could similarly contribute to organic solvent tolerance, strains hyperexpressing these efflux systems had to be examined. To overcome the contribution of MexAB-OprM to organic solvent tolerance, MexCD-OprJ and MexEF-OprN hyperexpression was selected in strains lacking MexAB-OprM. Strain K1121 lacks solvent tolerance as a result of the absence of MexAB-OprM, and an nfxB derivative of this strain (K1131) failed to demonstrate tolerance to organic solvents (on solid media overlaid with solvent) at levels seen for MexAB-OprM+ strain ML5087, despite the hyperexpression of MexCD-OprJ (Table 2). Still, K1131 grew substantially better than K1121 in LB broth supplemented with either 1% n-hexane (Fig. 3A) or 0.1% p-xylene (Fig. 3B), indicating that MexCD-OprJ hyperexpression did provide some measure of tolerance to these solvents. This was, however, markedly less than the 10% n-hexane or 1% p-xylene tolerance level seen in MexAB-OprM-hyperproducing strains. MexAB-OprM- and MexCD-OprJ-deficient strain K1115 was also sensitive to organic solvents in the solid-medium assay and remained so, despite the hyperexpression of MexEF-OprN (in K1117) (Table 2). Strain K1117 did, however, grow markedly better than K1115 in LB broth supplemented with a very modest 0.08% p-xylene (Fig. 3C), indicating that MexEF-OprN could contribute to a low-level tolerance to this solvent. No difference in tolerance to hexane at any concentration could be discerned between K1115 and K1117 (data not shown). Thus, all of the three known efflux systems in P. aeruginosa can contribute to organic solvent tolerance, although MexAB-OprM is by far the superior system for providing solvent tolerance. Given the known roles of these systems in drug export and the demonstration here that organic solvent tolerance could be compromised by an energy inhibitor, it is likely that these systems influence the solvent tolerance of P. aeruginosa by exporting the solvents out of the cell. Moreover, differences in tolerance levels afforded by each of the efflux systems likely reflect differences in the efficiency with which they accommodate the various organic solvents, reminiscent of differences in the abilities of these systems to accommodate the various antibiotics which are known to be substrates for these pumps (16, 19, 29, 30).
FIG. 3.
Influence of the MexC-MexD-OprJ (A and B) and MexE-MexF-OprN (C) efflux systems on the growth of P. aeruginosa in the presence of organic solvents. (A and B) P. aeruginosa K1121 (MexCD-OprJ−) (○, •) and K1131 (MexCD-OprJ+) (▵, ▴) were grown in LB broth at 37°C to the early exponential phase, at which time (arrow) n-hexane (1% [vol/vol]; A, solid symbols) or p-xylene (0.1% [vol/vol]; B, solid symbols) was added and growth was determined by monitoring OD660. (C) P. aeruginosa K1115 (MexEF-OprN−) (○, •) and K1117 (MexEF-OprN+) (▵, ▴) were grown in LB broth at 37°C to the early exponential phase, at which time (arrow) p-xylene (0.08% [vol/vol]; solid symbols) was added and growth was determined by monitoring OD660. Growth of solvent-free cultures is represented by the open symbols in panels A, B, and C.
Mechanistically, it is unclear how the P. aeruginosa multidrug efflux systems and similar efflux systems such as AcrAB-To1C, accommodate organic solvents. Antibiotics, being generally amphipathic molecules, are predicted to partition into the inner (most antibiotics) or outer (β-lactams) leaflet of the cytoplasmic membrane, from whence they are accessed by these efflux systems (25). Given that organic solvents are known to dissolve in lipid membranes and that their lethal effect likely involves compromising of the cytoplasmic membrane function, it is conceivable that these efflux systems also access organic solvents from within the bilayer as well. Still, it is unclear if the rate at which these solvents could be removed from the bilayer would be sufficient to ameliorate their toxic effects, and thus, the possibility that they are accessed prior to their dissolution in the cytoplasmic membrane cannot be ruled out. Nonetheless, these results appear to extend the known substrates for MexAB-OprM to organic solvents and once again serve to highlight the incredibly broad specificity exhibited by this efflux system, which accommodates most classes of antibiotics, as well as dyes, detergents, and, now, organic solvents.
Acknowledgments
This research was supported by an operating grant from the Canadian Cystic Fibrosis Foundation to K.P. X.-Z.L. acknowledges the support of the Canadian Cystic Fibrosis Foundation in the form of a studentship. K.P. is an NSERC University Research Fellow.
REFERENCES
- 1.Alekshun M N, Levy S B. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/aac.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aono R, Aibe K, Inoue A, Horikoshi K. Preparation of organic solvent-tolerant mutants of Escherichia coli. Agric Biol Chem. 1991;55:1935–1938. [Google Scholar]
- 3.Aono R, Ito M, Inoue A, Horikoshi K. Isolation of novel toluene-tolerant strain of Pseudomonas aeruginosa. Biosci Biotechnol Biochem. 1992;56:145–146. [Google Scholar]
- 4.Aono R, Kobayashi M, Nakajima H, Kobayashi H. A close correlation between improvement of organic solvent tolerance levels and alteration of resistance toward low levels of multiple antibiotics in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:213–218. doi: 10.1271/bbb.59.213. [DOI] [PubMed] [Google Scholar]
- 5.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein To1C, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asako H, Nakajima H, Kobayashi K, Kobayashi M, Aono R. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl Environ Microbiol. 1997;63:1428–1433. doi: 10.1128/aem.63.4.1428-1433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corwin H, Anderson S M. The effect of intramolecular hydrophobic bonding on partition coefficients. J Org Chem. 1967;32:2583–2586. [Google Scholar]
- 8.Fralick J A. Evidence that To1C is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda H, Hosaka M, Hirai K, Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990;34:1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotoh N, Tsujimoto H, Poole K, Yamagishi J-I, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heipieper H, Webber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 12.Hirai K, Suzue S, Irikura T, Iyobe S, Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987;31:582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hohnadel D, Haas D, Meyer J-M. Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1986;36:195–199. [Google Scholar]
- 14.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentration of toluene. Nature (London) 1989;338:264–265. [Google Scholar]
- 15.Isken S, de Bont J A M. Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 17.Li X-Z, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob Agents Chemother. 1994;38:1732–1741. doi: 10.1128/aac.38.8.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X-Z, Ma D, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to β-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X-Z, Zhang L, Srikumar R, Poole K. β-Lactamase inhibitors are substrates of the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:399–403. doi: 10.1128/aac.42.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda N, Ohya S. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1992;36:1847–1851. doi: 10.1128/aac.36.9.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima H, Kobayashi H, Aono R, Horikoshi K. Effective isolation and identification of toluene-tolerant Pseudomonas strains. Biosci Biotechnol Biochem. 1992;56:1872–1873. [Google Scholar]
- 23.Nakajima H, Kobayashi K, Kobayashi M, Asako H, Aono R. Overexpression of the robA gene increases organic solvent tolerance and multiple antibiotic and heavy metal ion resistance in Escherichia coli. Appl Environ Microbiol. 1995;61:2302–2307. doi: 10.1128/aem.61.6.2302-2307.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima H, Kobayashi M, Negishi T, Aono R. soxRS gene increased the level of organic solvent tolerance in Escherichia coli. Biosci Biotechnol Biochem. 1995;59:1323–1325. doi: 10.1271/bbb.59.1323. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido H. Multidrug efflux pumps in gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikaido H, Okusu H, Ma D, Li X-Z. Multidrug efflux pumps make a major contribution to drug resistance in pseudomonads. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: ASM Press; 1996. pp. 353–362. [Google Scholar]
- 27.Okii M, Iyobe S, Mitsuhashi S. Mapping of the gene specifying aminoglycoside 3′-phosphotransferase II on the Pseudomonas aeruginosa chromosome. J Bacteriol. 1983;155:643–649. doi: 10.1128/jb.155.2.643-649.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okusu H, Ma D, Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 30.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 31.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in a multidrug-sensitive Escherichia coli strain. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srikumar R, Li X-Z, Poole K. The inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao, Q., X.-Z. Li, R. Srikumar, and K. Poole. Contribution of the outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Submitted for publication. [DOI] [PMC free article] [PubMed]