Abstract
Background
Dental caries (tooth decay) is a common disease that is preventable by reducing the dietary intake of free sugars and using topical sodium fluoride products. An antibacterial agent known as chlorhexidine may also help prevent caries. A number of over‐the‐counter and professionally administered chlorhexidine‐based preparations are available in a variety of formulations and in a range of strengths. Although previous reviews have concluded that some formulations of chlorhexidine may be effective in inhibiting the progression of established caries in children, there is currently a lack of evidence to either claim or refute a benefit for its use in preventing dental caries.
Objectives
To assess the effects of chlorhexidine‐containing oral products (toothpastes, mouthrinses, varnishes, gels, gums and sprays) on the prevention of dental caries in children and adolescents.
Search methods
We searched the Cochrane Oral Health Group Trials Register (25 February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12), MEDLINE via OVID (1946 to 25 February 2015), EMBASE via OVID (1980 to 25 February 2015) and CINAHL via EBSCO (1937 to 25 February 2015). We handsearched several journals placed no language restrictions on our search. After duplicate citations were removed, the electronic searches retrieved 1075 references to studies.
Selection criteria
We included parallel‐group, randomised controlled trials (RCTs) that compared the caries preventive effects of chlorhexidine gels, toothpastes, varnishes, mouthrinses, chewing gums or sprays with each other, placebo or no intervention in children and adolescents. We excluded trials with combined interventions of chlorhexidine and fluoride or comparisons between chlorhexidine and fluoride interventions.
Data collection and analysis
Two review authors independently extracted trial data and assessed risk of bias. We resolved disagreements by consensus. We contacted trial authors for clarification or additional study details when necessary. The number of included studies that were suitable for meta‐analysis was limited due to the clinical diversity of the included studies with respect to age, composition of intervention, and variation in outcome measures and follow‐up. Where we were unable to conduct meta‐analysis, we elected to present a narrative synthesis of the results.
Main results
We included eight RCTs that evaluated the effects of chlorhexidine varnishes (1%, 10% or 40% concentration) and chlorhexidine gel (0.12%) on the primary or permanent teeth, or both, of children from birth to 15 years of age at the start of the study. The studies randomised a total of 2876 participants, of whom 2276 (79%) were evaluated. We assessed six studies as being at high risk of bias overall and two studies as being at unclear risk of bias overall. Follow‐up assessment ranged from 6 to 36 months.
Six trials compared chlorhexidine varnish with placebo or no treatment. It was possible to pool the data from two trials in the permanent dentition (one study using 10% chlorhexidine and the other, 40%). This led to an increase in the DMFS increment in the varnish group of 0.53 (95% confidence interval (CI) ‐0.47 to 1.53; two trials, 690 participants; very low quality evidence). Only one trial (10% concentration chlorhexidine varnish) provided usable data for elevated mutans streptococci levels > 4 with RR 0.93 (95% CI 0.80 to 1.07, 496 participants; very low quality evidence). One trial measured adverse effects (for example, ulcers or tooth staining) and reported that there were none; another trial reported that no side effects of the treatment were noted. No trials reported on pain, quality of life, patient satisfaction or costs.
Two trials compared chlorhexidine gel (0.12% concentration) with no treatment in the primary dentition. The presence of new caries gave rise to a 95% confidence interval that was compatible with either an increase or a decrease in caries incidence (RR 1.00, 95% CI 0.36 to 2.77; 487 participants; very low quality evidence). Similarly, data for the effects of chlorhexidine gel on the prevalence of mutans streptococci were inconclusive (RR 1.26, 95% CI 0.95 to 1.66; two trials, 490 participants; very low quality evidence). Both trials measured adverse effects and did not observe any. Neither of these trials reported on the other secondary outcomes such as measures of pain, quality of life, patient satisfaction or direct and indirect costs of interventions.
Authors' conclusions
We found little evidence from the eight trials on varnishes and gels included in this review to either support or refute the assertion that chlorhexidine is more effective than placebo or no treatment in the prevention of caries or the reduction of mutans streptococci levels in children and adolescents. There were no trials on other products containing chlorhexidine such as sprays, toothpastes, chewing gums or mouthrinses. Further high quality research is required, in particular evaluating the effects on both the primary and permanent dentition and using other chlorhexidine‐containing oral products.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Cariostatic Agents; Cariostatic Agents/therapeutic use; Chlorhexidine; Chlorhexidine/therapeutic use; Dental Caries; Dental Caries/prevention & control; Gels; Mouthwashes; Mouthwashes/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Antiseptic treatment (chlorhexidine) to prevent tooth decay in children and young people
Review question
This review examined the effectiveness of varnishes and gels containing chlorhexidine in preventing tooth decay in children and young people.
Background
Tooth decay is a very common disease that over time destroys the tooth surface. It has been estimated to affect up to 80% of people in high‐income countries and, despite being preventable through oral hygiene and dietary measures and the use of agents such as fluoride that reduce risk of decay, it is likely to remain a problem, especially in low‐income countries. Tooth decay can result in pain and infection, and in young children may require treatment in hospital under a general anaesthetic. As well as causing anxiety and pain, this may mean the child or young person missing time at school and their parents or carers having to take time off work, possibly losing income and incurring extra costs. Prevention of tooth decay is simpler and possibly cheaper than waiting until it occurs and then requires extensive treatment.
Tooth decay is largely preventable, and a range of things may assist this: twice‐daily toothbrushing with a fluoride toothpaste, reducing both the amount of and number of times per day sugar is eaten, and drinking water that contains fluoride (bottled or tap, depending on where you live).
Tooth decay occurs when certain types of bacteria (germs) in the mouth, such as Streptococcus mutans, produce acids from the sugar we eat, which dissolve the hard enamel coating on our teeth. The chemical antiseptic treatment chlorhexidine is highly successful at destroying these bacteria and can be used safely at home in the form of a gel, spray, chewing gum, toothpaste or mouthrinse. Alternatively, chlorhexidine can be applied as a varnish to the surface of teeth by a dentist.
Study characteristics
The evidence in this review, carried out through the Cochrane Oral Health Group, is up‐to‐date at 25 February 2015. We found eight studies that were suitable to include in this review. The studies involved a total of 2876 children from birth to 15 years of age who were at moderate to high risk of tooth decay. Six of the studies looked at the effects of dental professionals applying different strengths of chlorhexidine varnishes to the baby teeth, permanent teeth or both types of teeth in children and adolescents. The other two studies looked at the effects of parents placing chlorhexidine gel on their children's baby teeth. There were no studies that examined other products containing chlorhexidine, such as sprays, toothpastes, chewing gums or mouthrinses.
Key results
The results did not provide evidence that chlorhexidine varnish or gel reduces tooth decay or reduces the bacteria that encourage tooth decay. The studies did not evaluate other outcomes such as pain, quality of life, patient satisfaction or direct and indirect costs of interventions. Four studies measured side effects and found none were observed.
Quality of the evidence
Due to the lack of suitable studies and concerns about possible bias in the included studies, the evidence is very low quality. As a result, we are not able to conclude whether or not chlorhexidine is effective in preventing tooth decay in children or adolescents, when compared to placebo (an inactive substitute for chlorhexidine) or no treatment. Future research on the use of chlorhexidine to prevent tooth decay is needed and should consider both primary and permanent teeth and should assess other chlorhexidine‐containing products that can be used at home, such as toothpastes or mouthrinses.
Summary of findings
Summary of findings for the main comparison. Summary of findings ‐ chlorhexidine varnish.
| Chlorhexidine varnish compared with placebo for the prevention of dental caries in children and adolescents | ||||||
|
Patient or population: children and adolescents Settings: school, nursery or dental clinic Intervention: chlorhexidine varnish Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Chlorhexidine | |||||
| Caries in the primary teeth (24‐months follow‐up) | Two trials with unit of analysis problem. Data imputations indicated no evidence to claim or refute a benefit | |||||
|
Caries in permanent dentition (DMFS) (30 to 36 months) Higher values indicate greater caries |
The mean increment in the control group was 5.821 | The increment in the intervention groups was 0.53 higher (1.53 higher to ‐0.47 lower) | ‐ | 690 (2) | ⊕⊝⊝⊝ very low2 | Chlorhexidine varnish concentration 10% varnish and 40% varnish. A further three trials provided some unusable data but indicted no evidence to claim or refute a benefit3 |
| Elevated mutans streptococci levels ≥4 with caries screen (6 to 36 months) | 620 per 10004 | 577 per 1000 (496 to 664) | RR 0.93 (0.80 to 1.07) | 496 (1) | ⊕⊝⊝⊝ very low5 | Chlorhexidine concentration 10% varnish Two other studies reported unusable data but indicated no evidence to claim or refute a benefit6 |
| Adverse events | One study reported no adverse events for ulcers or tooth staining. One study stated “side‐effects due to the CHX treatment were not noted" | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. In the two trials that assessed this outcome, the DMFS increment in the control groups was 5.25 and 6.39, mean 5.82. This value was considered to be a moderate caries increment.
2. We downgraded the quality of the evidence due to risk of bias (high and unclear risk of bias overall) and inconsistency.
3. Conclusions reflected in remaining 3 studies (high risk of bias) with 6‐ to 24‐months follow‐up and chlorhexidine concentrations of 1% and 10%, reporting caries outcomes in permanent dentition that could not be pooled in a meta‐analysis.
4. In the single trial that assessed this outcome as presence or absence of high mutans streptococci levels, the prevalence of high mutans streptococci in the placebo varnish group was 62%.
5. We downgraded the quality of the evidence due to risk of bias (unclear risk of bias overall), imprecision and inconsistency. Mutans streptococci outcomes were reported as mean mutans streptococci levels or presence or absence of high mutans streptococci.
6. Equivocal results reported in 2 other studies (high risk of bias) with 6‐ to 24‐months follow‐up and chlorhexidine concentrations of 1% and 10%, which could not be pooled in a meta‐analysis.
Summary of findings 2. Summary of findings ‐ chlorhexidine gel.
| Chlorhexidine gel compared with no treatment for prevention of caries in children and adolescents | ||||||
|
Patient or population: children and adolescents Settings: all Intervention: chlorhexidine gel Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Chlorhexidine | |||||
| Presence of new caries in the primary teeth (24‐months follow‐up) | 16 per 10001 |
16 per 1000 (6 to 45) |
RR 1.00 (0.36 to 2.77) | 487 (2) | ⊕⊝⊝⊝2 very low | Chlorhexidine concentration 0.12% gel |
| 70 per 1000 |
70 per 1000 (25 to 194) |
|||||
|
Mutans streptococci prevalence (24‐months follow‐up) |
180 per 10003 |
227 (171 to 299 |
RR 1.26 (0.95 to 1.66) | 490 (2) | ⊕⊝⊝⊝4 very low | Chlorhexidine concentration 0.12% gel |
| 466 per 10003 | 587 per 1000 (443 to 774) | |||||
| Adverse events (24‐months follow‐up) | Both studies reported there were no adverse events | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DMFS: decayed, missing and filled surfaces; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Two trials assessed this outcome; the reported risk of caries in the control group was 1.6% and 6.9%.
2. We downgraded the quality of the evidence due to risk of bias (we assessed both studies to be at high risk of bias overall), imprecision of the estimate due to low numbers of events in both the control and intervention groups, and indirectness (infants administered very low daily concentration).
3. Two trials assessed this outcome; the reported risk of mutans streptococci in the control group was 46.6% and 18.1%.
4. We downgraded the quality of the evidence due to risk of bias (we assessed both studies to be at high risk of bias overall), imprecision, and indirectness.
Background
Dental caries (tooth decay) is one of the most common diseases afflicting mankind and has been estimated to affect up to 80% of people in high‐income countries (Chadwick 2001). Dental caries is eminently preventable through a combination of oral hygiene and dietary measures and the use of anticariogenic agents, for example, water fluoridation or the use of fluoride‐containing toothpaste. Yet, notwithstanding an increased awareness of the array of preventive measures that are available, it is likely that dental caries will remain a common disease for the foreseeable future, with the prevalence being greatest in many of the low‐income countries (Yee 2002).
Description of the condition
Dental caries is a multifactorial disease in which the fermentation of food sugars by acidogenic bacteria, such as Streptococcus mutans (S. mutans), in the biofilm (dental plaque) causes localised demineralisation of tooth surfaces that can ultimately lead to cavity formation. Microbiological shifts within this biofilm can be triggered by such changes as an alteration in salivary flow or an increase in sugar consumption (Marsh 2006; Moynihan 2014; Selwitz 2007). The caries process progressively destroys and undermines tooth tissue, causing parts of the tooth to cavitate, which may eventually lead to cusp fracture under loading. Affected teeth can become painful when the lesion advances into the pulp (nerve) tissue, and if the infection passes further through the tooth, it can lead to a dental abscess. Dental caries can also have a negative impact on a child's health, particularly if the toothache is associated with a restricted dietary intake and the further possibility of impaired growth and reduced body weight (Sheiham 2006).
The prevalence and severity of caries are strongly associated with social deprivation, and as such is a concern of particular relevance to children (Sheiham 2005). In addition to causing human suffering, caries also result in a substantial financial burden that increases with recurrence of the disease process (Yee 2002). Emergency visits for dental treatment and hospitalisation can have a significant effect on a child's educational development, as well as the economy because of time lost from work by either parents or carers (Ratnayake 2005; Shepherd 1999).
Description of the intervention
Chlorhexidine gluconate is a cationic bis‐biguanide with a broad spectrum of antibacterial activity. It has been used as an antiplaque rinse for many years (Löe 1972), and its bacteriostatic and bactericidal effects on Streptococcus mutans are now well recognised (Matthijs 2002).
A number of over‐the‐counter and professionally administered chlorhexidine‐based preparations are available in a variety of formulations and a range of strengths. These include toothpastes (0.4%); mouthrinses in either alcohol‐based (ethanol) or non‐alcoholic formulations (0.12% and 0.2%); gels (1%) (Emilson 1994); thymol‐containing varnishes (1%, 10%, 20% and 35%) (Rodrigues 2008); chewing gums; and sprays (0.2%). Preparations can be administered by a dental healthcare professional (that is varnishes) or are self applied with or without supervision in the form of mouthrinses, gels and toothpastes and with wide variation in frequency of application or usage.
How the intervention might work
As dental caries is a plaque‐mediated disease, interventions based on chlorhexidine that have been shown to be effective in the growth suppression of plaque‐resident bacteria, in particular Streptococcus mutans, could form part of a strategy for the prevention of dental caries (Caufield 2001; Twetman 1998; Twetman 2004).
Although several mechanisms have been proposed, it is now widely accepted that the antimicrobial properties of chlorhexidine are directed principally at bacterial cell membrane disruption (Ribeiro 2007). In low concentrations, chlorhexidine affects the metabolic activity of bacteria and is bacteriostatic, while in high concentrations it acts as a bactericide by initiating irreversible precipitation of cellular content.
The effectiveness of any intervention may also be influenced by the nature of its formulation or mode of its application (Luoma 1992). Thus, whilst most of these formulations are likely to be equally capable of suppressing mutans streptococci on smooth enamel or proximal surfaces, the effects of chlorhexidine varnishes are likely to be longer lasting and therefore potentially more effective in pits and fissures than with the equivalent rinse or gel applications (Zhang 2007).
Why it is important to do this review
As dental caries affects an increasing number of children in many countries, its impact as a major public health issue should not be underestimated. Pain, distress, tooth loss and impaired function and growth are some of the most important sequelae.
Although laboratory studies have shown that chlorhexidine can result in reductions in the numbers of Streptococcus mutans bacteria (Järvinen 1993), dental caries is a complex process, involving other plaque species and salivary and dietary factors. Changes in the plaque ecosystem and recolonisation of tooth surfaces over time could influence the ability of chlorhexidine to prevent caries in vivo (Autio‐Gold 2008). Thus before any recommendations can be made, clinical, in‐vivo evidence is required on the effect of chlorhexidine on caries prevention.
Additionally, there is uncertainty about which formulation of oral product may provide the best mode of chlorhexidine delivery for caries prevention. Reports have claimed that chlorhexidine varnishes achieve the most persistent reduction in Streptococcus mutans, followed by gels and mouthrinses (Löe 1972), but there is still a degree of uncertainty on the comparative effectiveness of these agents (Ribeiro 2007).
Adverse effects associated with continuous usage of chlorhexidine preparations are well documented, the most common ones being temporary staining of teeth and discolouration of the tongue. Mucosal soreness and desquamation, bitter taste and temporary taste disturbances and an increase in calculus formation are also frequently reported. There are also increasing reports of immediate hypersensitivity to chlorhexidine, of the type that may result in life‐threatening anaphylaxis (Krishna 2014).
In view of the uncertainties surrounding the caries‐preventive and adverse effects of chlorhexidine‐based agents in children and adolescents, there was a need for an up‐to‐date systematic review.
Objectives
To assess the effects of chlorhexidine‐containing oral products (toothpastes, mouthrinses, varnishes, gels, gums and sprays) on the prevention of dental caries in children and adolescents.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the effects of chlorhexidine‐containing oral products with no treatment or placebo on dental caries, and comparing the effects of one chlorhexidine‐containing oral product with another. We revised our initial stipulation in the protocol that only studies reporting a treatment period of longer than one year were eligible for inclusion. Studies with a treatment period of less than one year were eligible for inclusion provided that administration of the intervention occurred at least once over that time period and that outcomes were measured at the end of the study period. To be included in the review, studies must have used explicit criteria for diagnosing dental caries, to include one or more of the following: standard visual and tactile examination with or without a supplementary radiograph or fibre‐optic transillumination.
We did not include RCTs of a split‐mouth design. The possibility of significant contamination with chlorhexidine of other sites cannot be ruled out (irrespective of the adhesiveness of the material to the tooth surface in the first hours after application).
Types of participants
Children and adolescents below the age of 18 years at the start of the study, with either mixed, permanent or primary dentition, irrespective of caries level or category of risk, socioeconomic status, health status or geographical location. We excluded studies involving participants undergoing fixed or removable orthodontic treatment.
Types of interventions
Chlorhexidine‐containing oral products such as gels, toothpastes, varnishes, mouthrinses, chewing gums and sprays compared to placebo or to no intervention (which can include routine dental care). Direct comparisons of different chlorhexidine interventions and comparisons of different concentrations of individual interventions and frequencies of application (single or multiple) were also eligible for inclusion. We excluded studies reporting only on combined interventions of chlorhexidine and fluoride, and/or comparisons between chlorhexidine and fluoride interventions.
Types of outcome measures
We have listed primary and secondary outcome measures below. These were not part of the inclusion criteria for studies in the review. We recorded the magnitude and variability of estimates of effect (for example, mean caries increment (standard deviation)).
Primary outcomes
Caries increment at the dentine level measured by change from baseline (or final measurement where caries increment was not reported) in the decayed, (missing) and filled surface/teeth (D(M)FS/T) index in all permanent teeth or molar teeth, and d(m)fs/t for primary teeth, erupted at the start and erupting over the course of the study (following Marinho 2004). Caries incidence could also be expressed as the number of children developing caries over the course of the study. The time point of interest was at final follow‐up examination.
Mutans streptococci bacteria, measured as a dichotomous outcome: either its presence or absence, or high or low levels.
Secondary outcomes
Secondary outcomes included measures of pain, quality of life or participant satisfaction. We also considered direct costs of interventions and any indirect costs related to materials and lost time from school or work as a result of attendance for treatment if reported. We noted any reported adverse effects related to any clinically diagnosed reactions to any of the interventions; those of specific interest included tooth staining/discolouration, soft tissue damage, hypersensitivity reactions, nausea and vomiting.
Search methods for identification of studies
Electronic searches
We developed detailed search strategies for each database we searched to identify studies to consider for this review. We based these on the search strategy developed for MEDLINE (Appendix 3), but revised appropriately for each database.
We searched the following databases:
Cochrane Oral Health Group Trials Register (searched 25 February 2015) (Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 12) (Appendix 2)
MEDLINE via OVID (1946 to 25 February 2015) (Appendix 3)
EMBASE via OVID (1980 to 25 February 2015) (Appendix 4)
CINAHL via EBSCO (1937 to 25 February 2015) (Appendix 5)
For the MEDLINE search, we ran the subject search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Section 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We searched the US National Institutes of Health Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform (ICTRP) http://apps.who.int/trialsearch/ (until 25 February 2015) for any registered or ongoing studies (see Appendix 6).
Searching other resources
We examined the reference lists of relevant articles and attempted to contact the investigators of included studies by e‐mail to ask for details of additional published and unpublished trials and any missing trial details. We handsearched the following journals recommended by the Cochrane Oral Health Group:
Caries Research (2003 to January 2014)
Community Dentistry and Oral Epidemiology (January 2014)
Journal of Dental Research (2003 to January 2014)
Journal of Dentistry for Children (2002 to January 2014)
We attempted to contact the manufacturers of several of the relevant chlorhexidine‐based products for information about any unpublished studies, but this proved unsuccessful. It appears that the manufacturers of some of these products are no longer actively promoting their use for the prevention of dental caries in children.
We placed no language restrictions on included studies and arranged to translate any studies that were not in the English language.
Data collection and analysis
Selection of studies
Two review authors independently assessed the abstracts of records retrieved from the searches. We obtained full copies of all relevant and potentially relevant studies, including those that appeared to meet the inclusion criteria and those for which there were insufficient data in the title and abstract to make a clear decision. The two review authors independently assessed the full‐text papers and resolved any disagreements on the eligibility of included studies through discussion and consensus, or if necessary by involving a third party. We excluded all records not meeting the eligibility requirements and noted the reasons for their exclusion in the Characteristics of excluded studies section of the review.
Data extraction and management
Two review authors independently and in duplicate extracted data. Disagreements were resolved by consulting with a third review author. We entered study details into the Characteristics of included studies table in RevMan 5 and collected outcome data using a piloted data extraction form designed for this review (RevMan 2014).
We extracted the following details of study reports:
Study characteristics: study design, country and setting, number of centres, recruitment period, funding source
Participants: inclusion and exclusion criteria, number randomised and evaluated in each trial
Intervention: (a) type and form of product; (b) concentration, dose and frequency; (c) duration of intervention and follow‐up
Comparator: (a) type and form of product (b) concentration, dose and frequency; (c) duration of intervention and follow‐up
Outcomes: primary and secondary outcomes (see Types of outcome measures) to include: diagnostic methods for caries assessment, measures of caries increment and any adverse effects
We entered study details into Characteristics of included studies tables and outcome data into additional tables or forest plots in Review Manager (RevMan) (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors carried out the 'Risk of bias' assessments independently and in duplicate by following the domain‐based evaluation described in Chapter 8 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We compared the assessments and discussed any inconsistencies, resolving them through consensus. We assessed each included study as at low risk of bias, unclear risk of bias or high risk of bias for the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting and other sources of bias.
For this systematic review we assessed risk of bias according to the following criteria:
Sequence generation: We will assess the use of a random number table, use of a computerised system, central randomisation, randomisation by an independent service using minimisation technique, random permuted block allocation as low risk of bias. If the paper merely states randomised or randomly allocated with no further information, we will assess this domain as unclear risk of bias.
Allocation concealment: We will assess centralised allocation including access by telephone call or fax or pharmacy‐controlled randomisation, sequentially numbered, sealed, opaque envelopes as low risk of bias. If allocation concealment is not stated, we will assess this domain as unclear risk of bias.
Blinding of participants and personnel: If blinding was not stated, we will assess this domain as unclear risk of bias.
Blinding of outcome assessment: If blinding was not stated, we will assess this domain as unclear risk of bias. Where studies were described as double blind, we assumed that both participants and outcome assessors were blinded.
Incomplete outcome data: We considered outcome data complete if all participants randomised were included in the analysis of the outcome(s). We assessed trials where 80% or more of those randomised were evaluated, where reasons for attrition or withdrawal were described for each group, and where both numbers and reasons were similar in each group, as being at low risk of bias due to incomplete outcome assessment. Where levels of attrition postrandomisation were greater than 20%, or reasons were not given for exclusions from each group, or where rates and reasons were different for each group, we assessed the risk of bias as unclear or high due to incomplete outcome data.
Selective outcome reporting: We assessed a trial as being at low risk of bias due to selective outcome reporting if the outcomes described in the methods section were systematically reported in the results section. Where outcomes were omitted or where the outcomes were not fully reported, we assessed this domain as high risk of bias.
Other bias: Sources of other potential bias included imbalance in potentially important prognostic factors between the groups at baseline (high risk of bias) or commercial funding of the trial (unclear risk of bias).
We categorised risk of bias in any included studies according to the following:
Low risk of bias (plausible bias unlikely to seriously alter the results).
Unclear risk of bias (plausible bias that raises some doubt about the results) if we assessed one or more domains as unclear.
High risk of bias (plausible bias that seriously weakens confidence in the results) if we assessed one or more domains as high risk of bias.
Measures of treatment effect
For the primary outcome of caries increment at the dentine level measured by change from baseline (or final value where caries increment was not reported) in the decayed, (missing) and filled surface/teeth (D(M)FS/T) in permanent teeth, and d(m)fs/t for primary teeth, the effect measure was the difference in means (standardised difference in means where the same outcome was measured using different scales). The same effect measure was used for levels of mutans streptococci expressed on a continuous scale.
For dichotomous data, or for continuous data that was reported as dichotomised data, the effect measure used was the risk ratio (RR). All effect measures were accompanied by 95% confidence intervals (CI).
Unit of analysis issues
Cluster‐randomised trials, that is groups of individuals randomised to intervention or control, were identified in the searches and were checked for unit‐of‐analysis errors based on the advice provided in Section 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where a unit‐of‐analysis error existed, and re‐analysis was not possible, we reported point estimates alone (no CIs or P values). Where re‐analysis was possible, we used intraclass correlation coefficient values (0, 0.05, 0.1, 0.2) to calculate the appropriate design effect and adjust the standard error of the effect estimate accordingly.
Dealing with missing data
Where possible, we attempted to contact authors of the included studies to obtain any missing trial details and data. We did not carry out imputations for missing data.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the similarity across the studies of the summary participant characteristics, the interventions and the outcomes as specified in the criteria for included studies section of this review. Clinical diversity between the studies meant that opportunities for pooling of the extracted data were limited. Where pooling was indicated, we assessed statistical heterogeneity using the Chi2 test and I2 statistic to quantify the percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (Higgins 2011).
Assessment of reporting biases
If we had identified a sufficient number of trials for inclusion in this review, we would have assessed publication bias according to the recommendations on testing for funnel plot asymmetry described in Section 10.4.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Egger 1997; Higgins 2011). If we had identified asymmetry, we would have assessed for other possible causes and explored these further in the discussion if appropriate.
Data synthesis
Where sufficiently homogeneous data to inform a clinically important question were available, we performed a quantitative meta‐analysis using RevMan (RevMan 2014). We used the fixed‐effect model to pool effect estimates where only a small number of studies were identified per comparison and heterogeneity was low. We calculated a pooled estimate of effect together with the corresponding 95% CI. In future updates where data are not limited, we will use a random‐effects model provided it is appropriate to pool the data (as assessed by clinical and statistical heterogeneity).
Subgroup analysis and investigation of heterogeneity
If we had included a sufficient number of studies in this review and identified statistical heterogeneity between the studies, we had planned to evaluate the caries preventive effects of the interventions for the following factors:
Caries risk at baseline
Modes of administration of chlorhexidine‐containing products (toothpastes, mouthrinses, varnishes, gels, gums and sprays)
Due to a lack of suitable data from the included studies we were unable to do a subgroup analysis, but we will consider carrying this out if further data are available from studies included in future updates of this review.
Sensitivity analysis
We did not carry out a sensitivity analysis in this review for the reasons mentioned previously. For future updates, if there are sufficient included studies, we plan to conduct sensitivity analyses to assess the robustness of our review results by repeating the analysis with the following adjustments: exclusion of studies with unclear or inadequate allocation concealment, unclear or inadequate blinding of outcomes assessment and unclear or inadequate completeness of follow‐up.
Summary of findings and assessing the quality of the evidence
We developed a 'Summary of findings' table for each comparison and for the main outcomes of this review following GRADE methods and using GRADEpro software (GRADE 2004). We assessed the quality of the body of evidence regarding the overall risk of bias of the included studies, the directness of the evidence, the inconsistency of the results, the precision of the estimates and the risk of publication bias. We categorised the quality of the body of evidence of each of the main outcomes for each comparison as high, moderate, low or very low.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Electronic searches retrieved 1762 records, which reduced to 1075 after de‐duplication. After examination of the titles and abstracts of these references, we discarded all of those that did not match our inclusion criteria and were clearly ineligible. We obtained full‐text copies of the remaining 45 studies for further evaluation. Of this number, we found eight studies (reported in 12 publications) eligible for inclusion and excluded 33 studies. Handsearching of journals and reference lists of review articles did not yield any additional articles. We searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) and the ClinicalTrials.gov databases up to February 2015 but identified no ongoing trials. We have illustrated the study flow in a PRISMA diagram, see Figure 1. Our electronic searches also retrieved citations for two studies that none of the previous systematic reviews had identified (Bretz 1997; Nordling 1999). Through e‐mail we were able to reach the principal investigator in Bretz 1997,who clarified some of the study details that were missing from this report. This investigator also provided us with an additional published version of the study, which we had not identified in any of our searches. The citation for Nordling 1999 consisted of a conference proceedings abstract; there were no further references to this study in the literature. We contacted the principal investigator, who indicated that the full study had never been published but provided us with an electronic copy of the complete report. As this was in the Swedish language, we arranged for the translation of the report prior to further assessment.
1.
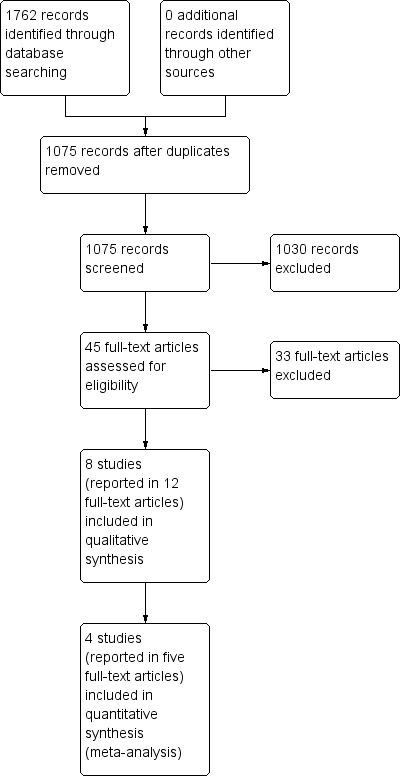
Results of searching for studies for inclusion in the review
Included studies
This review included eight RCTs (Baca 200X; Bretz 1997; De Soet 2002; Du 2006; Forgie 2000; Nordling 1999; Plonka 2013; Pukallus 2013), published between 1997 and 2013, that randomised 2876 children and provided data for 2276. We have provided details of the included studies in the Characteristics of included studies table. None of the included studies comprehensively addressed all of the primary and secondary outcomes specified in the protocol for this review.
Design
Six RCTs had a parallel design and randomisation at the individual level (Bretz 1997; De Soet 2002; Forgie 2000; Nordling 1999; Plonka 2013; Pukallus 2013). Two studies used randomisation at the cluster level (school class) with a parallel design (Baca 200X; Du 2006), justifying this as a mechanism to minimise contamination of the outcome between the intervention and comparator arms of the study. However, as these studies were analysed without taking into account the clustering of pupils within a class, it is likely that the precision of the effect estimate in these studies will be too narrow.
Setting
Three studies were principally carried out in school settings in Spain, China and Scotland (Baca 200X; Du 2006; Forgie 2000, respectively). The settings for the other trials were an orphanage in Rio de Janeiro in Brazil (Bretz 1997), a district polyclinic in Sweden (Nordling 1999), a youth dental care centre in Surinam, South America (De Soet 2002) and in the family home in Australia (Plonka 2013; Pukallus 2013).
The providers of treatment in the studies were either university dental hospital or dental care centre staff or parents, and the assessors of outcomes were the investigators and healthcare providers. The provider of treatment was unclear in Nordling 1999 and Bretz 1997. Treatment was delivered by a dentist in the Baca 200X and Du 2006 studies, by a dental nurse in De Soet 2002, a dental therapist or hygienist in Forgie 2000 and by a parent in Plonka 2013 and Pukallus 2013.
The shortest period of follow‐up was 6 months (Bretz 1997); the longest was 36 months (Forgie 2000). Four studies followed up participants for 24 months (Baca 200X; Du 2006; Nordling 1999; Plonka 2013; Pukallus 2013) and one study followed up participants for 30 months (De Soet 2002).
Characteristics of the participants
Children from birth to 15 years with a moderate to high risk for caries participated in the included studies. The total sample size comprised 2876 children and adolescents with a comparable gender distribution in each of the studies aside from Bretz 1997, in which only females participated. Four of the included studies evaluated caries in the permanent dentition only (Bretz 1997; De Soet 2002; Forgie 2000; Nordling 1999); one study evaluated caries in both the primary and permanent dentition (Baca 200X); and three studies evaluated caries in the primary dentition alone (Du 2006; Plonka 2013; Pukallus 2013).
Baseline caries levels varied in the included studies. Two studies in children up to two years started before the eruption of any teeth, therefore there were no baseline caries (Plonka 2013; Pukallus 2013). For the two studies with participants aged four to seven years, the baseline caries level was comparable: the mean decayed and filled surfaces (dfs) was 3.48 in the untreated group and 4.40 in the active intervention group (Baca 200X); baseline mean decayed, missing or filled molar surfaces (dmfs‐molar) was 2.6 in the placebo group and 2.8 in the active intervention group (Du 2006). Four studies included older children, ranging in age from 10 to 15 years. Where baseline caries was reported as decayed, missing and filled teeth/surfaces, participants were classified as "moderately caries active", with a mean decayed, missing or filled surfaces (DMFS) of 3.83 from clinical exam alone, at the D3 threshold (De Soet 2002), and "high caries risk", with a mean DMFS of 6.64 in the placebo varnish group and 7.26 in the active varnish group from clinical exam and bitewing radiographs, also at the D3 threshold (Forgie 2000). The baseline data were incompletely reported in the remaining two studies with older participants (Bretz 1997; Nordling 1999). The participants in two of the studies had access to continuing dental care either through the community dental services (Forgie 2000) or from the dental nurse at the dental care centre (De Soet 2002). However, whilst considerable dental treatment was provided in the first year for the participants in Forgie 2000, the investigators in De Soet 2002 indicated that not all children were able to access restorative treatment during the first year. The participants in Nordling 1999 received routine restorative treatment and prophylaxis. Although Bretz 1997 did not report the accessibility to ongoing dental care, the investigators indicated that not all of the participants had existing carious lesions restored during the study period. No organised oral healthcare programmes for preschool children were available in three studies (Du 2006; Plonka 2013; Pukallus 2013), and one further study reported that the children received no preventive treatment before or during the study period (Baca 200X).
Characteristics of the interventions
The active interventions in the trials consisted of concentrations (1%, 10%, 40%) of chlorhexidine varnish, each with a different application regimen, and one formulation of chlorhexidine gel at a concentration of 0.12%. Four studies used placebo varnish as a comparator; the other four studies used a comparator group of no treatment (Baca 200X; Bretz 1997; Plonka 2013; Pukallus 2013).
0.12% concentration gel
In the studies by Plonka 2013 and Pukallus 2013, parents of infants in the treatment group were instructed to apply a pea‐sized amount of the chlorhexidine gel onto a clean index finger and smear it onto the child's teeth after the evening toothbrushing with 0.304% fluoride toothpaste.
1% concentration varnish
In the study by Baca 200X, a thin coat of chlorhexidine varnish (1% chlorhexidine, 1% thymol) was professionally applied to all teeth during the first week and every 3 months until the end of the study at 24 months. In the study by Nordling 1999, six coatings of chlorhexidine varnish (1% chlorhexidine, 1% thymol) were professionally applied every four months over two years.
10% concentration varnish‐sealant
In the study by Bretz 1997, a 10% formulation of chlorhexidine varnish‐sealant was professionally applied once or twice (with an interval of a week between) at the start of the study and at the three‐month recall appointment (though frequency of application was varied according to S. mutans levels). In a later study of longer duration, the same intervention was professionally applied every week for a month and then at 3‐ and 6‐month intervals until the study's completion at 36 months (a maximum of 12 repeat applications) (Forgie 2000).
40% concentration varnish
In the study by De Soet 2002, chlorhexidine varnish was professionally applied as a 40% concentration every six months. In Du 2006, a chlorhexidine varnish of 40% chlorhexidine acetate in a sandarac resin was applied at the start of the study and every six months thereafter.
Oral instructions following professional application of the active interventions and placebo varied between studies. In Baca 200X, participants were discouraged from eating or drinking for three hours and were not allowed to brush their teeth for the first day or use dental floss for one week. In De Soet 2002, toothbrushing to remove the varnish was allowed after 10 minutes, whereas in Forgie 2000 participants were discouraged from any tooth cleaning for 24 hours and advised to refrain from flossing for 3 days. The participants in Nordling 1999 were instructed to avoid eating for three hours and to refrain from toothbrushing until the following day, whereas in Bretz 1997 participants were permitted to continue routine oral hygiene measures.
Characteristics of the outcome measures
All of the studies carried out clinical assessments of dental caries. Caries was measured using continuous measures as decayed and filled teeth/decayed and filled surfaces (DFT/DFS) on first permanent molars (Baca 200X), as decayed, missing and filled permanent teeth (DMFT) (Forgie 2000), as decayed, missing and filled primary molar surfaces (dmfs‐molar) (Du 2006) or as percentage of surfaces that were sound, decayed, restored or had white‐spot lesions (Bretz 1997). Plonka 2013 and Pukallus 2013 used dichotomous presence or absence of caries increment, as well as the mean number of carious teeth in each group. The only data provided in Nordling 1999 was that a "caries assessment" was carried out.
There was variability between the studies' assessment of dental caries, that is the time intervals between assessments, and whether these were solely clinical with a mirror and probe (Baca 200X; De Soet 2002; Du 2006; Pukallus 2013) or were supplemented by radiographs (Nordling 1999) or fibre‐optic transillumination and radiographs (Forgie 2000). Bretz 1997 did not provide the method of examination, and Plonka 2013 was not explicit about method.
Five studies also reported microbiological outcomes of mutans streptococci levels (Bretz 1997; De Soet 2002; Forgie 2000; Plonka 2013; Pukallus 2013). Salivary mutans streptococci levels were determined by classifying the number of colony forming units (cfu) per ml of saliva, in De Soet 2002, or per dip‐slide, in Bretz 1997, into categories ranging from 0 to greater than 105 cfu, in De Soet 2002, and 0 to greater than 106 cfu, in Bretz 1997. One study dichotomised the mutans streptococci levels as high or low, using a threshold of 250,000 cfu per ml of saliva (Forgie 2000), and two studies dichotomised the mutans streptococci outcome as presence or absence in saliva (Plonka 2013; Pukallus 2013).
Four studies reported that no adverse events were observed (De Soet 2002; Du 2006; Plonka 2013; Pukallus 2013); none of the other four studies reported that they had measured the occurrence of adverse events.
None of the included studies reported outcome measures of pain, quality of life, participant satisfaction or costs.
Excluded studies
We have listed all of the studies that were excluded from this review and the reasons for their exclusion in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We assessed six studies as at high risk of bias overall, resulting from a judgement of high risk of bias for at least one domain. We judged the remaining studies as at unclear risk of bias overall ( Du 2006 ; Forgie 2000). Concealment of the allocation sequence and blinding of outcome assessors are key domains in the assessment of risk of bias; none of the studies provided sufficient detail to enable the assessment of allocation concealment, and in five studies we assessed blinding of outcome assessors as low risk of bias.
For further details, see the 'Risk of bias' graph in Figure 2 and 'Risk of bias' summary in Figure 3.
2.
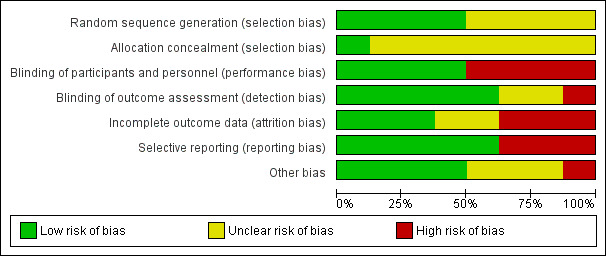
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
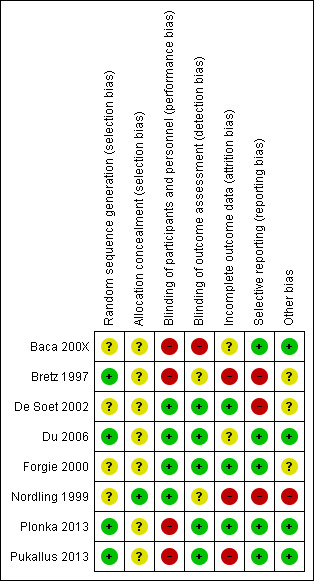
'Risk of bias' summary: review authors' judgements about each domain for each included study
Allocation
Method of randomisation
In four studies the method of randomisation to treatment or control groups was stated clearly: "randomly divided the children into two groups, based on the class they attended, by drawing a card from a bag" (Du 2006); by drawing colour‐coded sticks from an opaque bag (Plonka 2013; Pukallus 2013); or using a computer‐generated random number sequence (Bretz 1997, with additional details following request to authors). In the other four studies the methods used to randomise participants were not clearly described: the participants "were assigned randomly to an experimental group and a control group" (De Soet 2002); "randomised into one of four groups" with stratification prior to randomisation by gender, baseline caries experience, and history of eczema (Forgie 2000); "divided into two groups by randomisation, computer printouts with the children in birth order were used as input" (Nordling 1999 as translated); two classes in five schools were selected and the classes in each school were randomly allocated (Baca 200X). We judged the risk of bias as unclear in these four studies where was insufficient information reported about the method used to generate the randomisation sequence to permit judgement.
Allocation concealment
Due to inadequate reporting, indicated by uncertainty as to whether adequate measures had been taken to ensure that the investigators were unaware of the upcoming treatment or control assignment, we were not able to make a judgement of low or high risk of bias for this domain in seven of the studies (Baca 200X; Bretz 1997; De Soet 2002; Du 2006; Forgie 2000; Plonka 2013; Pukallus 2013). In the remaining study (Nordling 1999), the "code was locked in the department's safety deposit box," and thus we gave a judgement of low risk of bias for this domain.
Blinding
In one study (Baca 200X), neither the outcome assessor nor the participants were blinded, and so we judged the study to be at high risk of bias for both these domains. We judged three studies to be at low risk of bias for both domains (De Soet 2002; Du 2006; Forgie 2000), as they explicitly reported the blinding of participants using a placebo comparator and assessors were blinded to group allocation.
We judged Nordling 1999, a placebo‐controlled trial, as being at low risk of bias for blinding of participants, while we judged Bretz 1997, with a no‐treatment comparator, as at high risk of bias. Neither of these studies mentioned the blinding of outcome assessors, so we gave a judgement of unclear risk of bias for this domain. We assessed Plonka 2013 and Pukallus 2013 as at high risk of performance bias due to use of a no‐treatment comparator, but at low risk of detection bias, as clinical outcome assessment was blinded.
Incomplete outcome data
We assessed attrition bias as low where there was small loss to follow‐up, balanced across groups, in De Soet 2002; Forgie 2000; Plonka 2013, and unclear where loss to follow‐up was low for reasons not likely to be related to the outcome, but where there was insufficient supporting information on loss to attrition to enable a judgement to be made (Baca 200X; Du 2006). Where reported overall losses to follow‐up were large (Nordling 1999: 28% attrition over 24 months, described by the investigators as "unexpectedly large"; Bretz 1997: 26.5% over 6 months; Pukallus 2013: 40% over 24 months), we judged such studies to be at high risk of bias.
Selective reporting
Although the study protocols were unavailable for all of the included studies, based on information presented in the methods sections of each of the reports, we have concluded that the investigators appear to have reported on all of their stated objectives and fully reported the expected outcomes of relevance to this systematic review in five studies (Baca 200X; Du 2006; Forgie 2000; Plonka 2013; Pukallus 2013). We judged risk of bias from selective reporting as high where the expected outcome measure of mean DFT/DFS was either not reported (Bretz 1997 presented results as percentage of sound, decayed, restored surfaces and white‐spot lesions) or inadequately reported (Nordling 1999 reported no standard deviations for caries outcome; De Soet 2002 reported no numerical estimates of levels of mutans streptococci and variability, presenting this information in graphs only).
Other potential sources of bias
The investigators in two of the trials reported receiving funding from the manufacturers of one of the chlorhexidine varnishes ( De Soet 2002 ; Forgie 2000 ). Although no details were provided regarding the extent, if any, of the sponsors' involvement in the conduct of the trial or the analysis of data, we found no evidence suggesting this funding might bias the results, and so we judged these studies as unclear on this domain. Similarly, the principal investigator in Bretz 1997 confirmed in an e‐mail that "the study was supported by a grant from Somcana International Canada", but provided no further details. In Nordling 1999, the manufacturer supplied the active and placebo varnishes, but we found no evidence in the report to suggest that this might present a potential source of bias. However, there was substantial baseline imbalance in caries levels in this study, which we consequently judged as at high risk of bias. No other potential sources of bias were reported in the four remaining studies (Baca 200X; Du 2006; Plonka 2013; Pukallus 2013).
Effects of interventions
Chlorhexidine varnish compared with no treatment or placebo
Six studies, two at unclear risk of bias and four at high risk of bias, compared chlorhexidine varnish with no treatment or placebo, and evaluated 1786 children. Two studies involving 471 children reported on this comparison in the primary dentition (Baca 200X at high risk of bias, and Du 2006 at unclear risk of bias); five studies involving 1232 children reported effects on the permanent dentition (Baca 200X; Bretz 1997; De Soet 2002; Forgie 2000; Nordling 1999). Due to the wide variation in chlorhexidine concentration used in the studies and variation in outcome measures and length of follow‐up, we were unable to pool many of the studies.
Primary outcome: caries
Primary dentition
Data for the two studies reporting caries in the primary dentition are presented in Table 3. When we re‐analysed the cluster‐randomised studies using a range of intraclass correlation coefficients to take into account the clustering, we found no statistically significant difference in mean d(m)fs/t‐molar increment between the groups (Baca 200X; Du 2006).
1. Effects of intervention: Caries in primary dentition.
| Study ID | Intervention(s) and comparator | Outcome | Summary Intervention | Summary Comparator | Comment |
| Baca 200X | Cervitec (1% chlorhexidine, 1% thymol) varnish applied 4 x year (n = 86) No treatment (n = 95) |
Mean dft‐molar increment (24 months) Mean dfs‐molar increment (24 months) |
Varnish 0.97 (SD 1.45) Varnish 2.21 (SD 2.96) |
No treatment 0.94 (SD 1.50) No treatment 2.54 (SD 3.40) |
Authors report "..the incidence of caries lesions at 24 months showed no significant differences between the 2 groups... Among the children who were caries free at the onset of the study, those in the varnish group showed a significantly lower incidence of caries lesions in teeth (P < .05) and on surfaces (P < .05) of primary molars at 24 months compared with those in the control group. On the other hand, among the children with dft > 0 at baseline, there were no statistically significant differences between the varnish and control groups." Statistical analysis did not take into account the cluster randomisation by school class. Inflation of the standard error of the effect estimate through a design effect with ICCs of 0.05, 0.1 and 0.2 would lead to the same conclusions as those reported |
| Du 2006 | 40% w/w chlorhexidine acetate in a sandarac resin applied 2 x year (n = 155) Placebo alcohol solution of sandarac resin (n = 135) |
Mean dmfs‐molar increment (24 months) | Varnish 1.0 (SD 2.49) | Placebo 1.6 (SD 2.32) | Authors report "The mean caries increment of the primary molars was 1.0 dmfs‐molar in the test‐group children and 1.6 dmfs‐molar in the placebo group. The difference of 0.6 tooth surfaces...was statistically significant (p = 0.036)." Statistical analysis did not take into account the cluster randomisation by school class. Inflation of the standard error of the effect estimate through a design effect with ICCs of 0.05, 0.1 and 0.2 resulted in a non‐statistically significant result. ICC 0 MD ‐0.6 (95% CI ‐1.16 to ‐0.04) ICC 0.05 MD ‐0.6 (95% CI ‐1.71 to 0.51) ICC 0.1 MD ‐0.6 (95% CI ‐2.27 to 1.07) ICC 0.2 MD ‐0.6 (95% CI ‐3.38 to 2.18) |
| Pukallus 2013 | Curasept (0.12%) chlorhexidine gel applied 1 x daily (n = 61) No treatment (n = 58) |
Caries incidence (24 months) | Gel 3/61 | No treatment 4/58 | Authors report "These differences were not statistically significant (p = 0.7)." RR 0.71 (95% CI 0.17 to 3.05) |
| Plonka 2013 | Curasept (0.12%) chlorhexidine gel applied 1 x daily (n = 180) No treatment (n = 188) |
Caries incidence (24 months) | Gel 4/180 | No treatment 3/188 | Authors report "The differences were not statistically significant (p = 0.66)." RR 1.39 (95% CI 0.32 to 6.14) |
CI: confidence interval dfs‐molar: decayed and filled molar surfaces (primary) dft‐molar: decayed and filled molar teeth (primary) dmfs‐molar: decayed, missing and filled molar surfaces (primary) ICC: intraclass correlation coefficient MD: mean difference RR: risk ratio SD: standard deviation
Permanent dentition
Two studies evaluated the effects of 1% chlorhexidine with 1% thymol varnish. One study compared this to no treatment (Baca 200X), and one study compared this to placebo (Nordling 1999) (Table 4). Children ranged in age from 6 to 15 years, and the studies were carried out in an educational setting. We have presented the results from the 723 children analysed in Table 4. Both studies reported on caries increment. When we re‐analysed the cluster‐randomised study using a range of intraclass correlation coefficients to take into account the clustering (Baca 200X), we found no statistically significant difference in mean DFT/S‐molar (decayed or filled molar teeth or surfaces) increment between the chlorhexidine and no‐treatment groups. In the Nordling 1999 study, the mean DFS increment was slightly higher in the placebo group (1.9) than in the varnish group (1.8), but we were unable to analyse further due to the lack of standard deviations reported.
2. Effects of intervention: Caries in permanent dentition.
| Study ID | Intervention(s) and comparator | Outcome | Summary intervention | Summary comparator | Comment |
| Baca 200X | Cervitec (1% chlorhexidine, 1% thymol) varnish applied 4 x year (n = 86) No treatment (n = 95) |
Mean DFT increment (24 months) Mean DFS increment (24 months) |
Varnish 0.88 (SD 1.25) Varnish 0.95 (SD 1.38) |
No treatment 1.30 (SD 1.50) No treatment 1.85 (SD 2.27) |
Authors report "There was a significant difference in the increase in caries on surfaces at 24 months between the two groups.... However, when we consider the teeth (DFT index), although the increment was smaller in the varnish group (0.88) versus controls (1.30), the difference did not reach statistical significance." Statistical analysis did not take into account the cluster randomisation by school class. Inflation of the standard error of the effect estimate through a design effect with ICCs of 0.05, 0.1 and 0.2 resulted in a non‐statistically significant result for both outcomes. ICC 0 MD ‐0.42 (95% CI ‐0.82 to ‐0.02) ICC 0.05 MD ‐0.42 (95% CI ‐1.17 to 0.33) ICC 0.1 MD ‐0.42 (95% CI ‐1.51 to 0.67) ICC 0.2 MD ‐0.42 (95% CI ‐2.20 to 1.36) ICC 0 MD ‐0.9 (95% CI ‐1.45 to ‐0.35) ICC 0.05 MD ‐0.9 (95% CI ‐1.92 to 0.12) ICC 0.1 MD ‐0.9 (95% CI ‐2.40 to 0.60) ICC 0.2 MD ‐0.9 (95% CI ‐3.34 to 2.18) |
| Nordling 1999 | Cervitec (1% chlorhexidine, 1% thymol) varnish applied 3 x year (n = 271) Plain varnish (n = 271) | Mean DFS increment (24 months) | Varnish 1.8 (no SD reported) | Placebo 1.9 (no SD reported) | No re‐analysis possible due to incomplete reporting |
| Bretz 1997 | Chlorzoin 10% chlorhexidine applied after 3 months (n = 43) No treatment (n = 40) |
Mean % of sound surfaces (6 months) | Varnish 114.3 (SD 12.4) | No treatment 116.3 (SD 11.8) | Caries data presented as " dental decay parameters" i.e. % of sound, decayed, restored surfaces only. The trial reported that for all participants "...treatment groups were not predictors of dental decay parameters after 6 months" and for those participants treated as instructed at baseline examination "No significant differences on dental decay parameters were found between T and C groups at the 6 month examination". |
| Forgie 2000 | Chlorzoin 10% chlorhexidine applied 4 x year (n = 222) Placebo (n = 274) |
Mean DMFS increment (36 months) | Varnish 6.83 (SD 6.17) | Placebo 6.39 (SD 6.41) | Authors reported "...the clinical trial failed to reject the null hypothesis of no difference in caries‐reducing efficacy between active and placebo chlorhexidine varnishes." (MD 0.44, 95% CI ‐0.67 to 1.55) |
| De Soet 2002 | 40% chlorhexidine varnish (EC40®) applied 2 x year (n = 99) Placebo: neutral gel without chlorhexidine (n = 95) |
DMFS (30 months) | Varnish 6.16 (SD 10.01) | Placebo 5.25 (SD 6.03) | Authors reported "...no significant effect of CHX treatment on DMFS (p > 0.5)." (MD 0.91, 95% CI ‐1.40 to 3.22) |
CHX: chlorhexidine CI: confidence interval DFS: decayed and filled surfaces DFT: decayed and filled teeth DMFS: decayed, missing and filled surfaces ICC: intraclass correlation coefficient MD: mean difference SD: standard deviation
One study evaluated the effects of 40% chlorhexidine compared with placebo in children aged 13 to 14 years, in a dental clinic (De Soet 2002). We have presented the results from the 194 children analysed in Table 4. The study reported no statistically significant caries preventive effect of 40% chlorhexidine varnish compared to placebo.
Two studies evaluated the effects of 10% chlorhexidine compared with no treatment or placebo (Bretz 1997; Forgie 2000, respectively), in children aged 10 to 15 years in a residential and school setting. We have presented the caries results from the 579 children analysed in Table 4. The Bretz 1997 study presented the caries data as "dental decay parameters". The authors reported no statistically significant differences in dental decay at six‐month follow‐up. In the Forgie 2000 study, the mean difference in DMFS increment between the chlorhexidine varnish and placebo groups was 0.44 (95% CI ‐0.67 to 1.55). On this basis, we were unable to exclude the possibility that 10% chlorhexidine varnish has no caries preventive effect.
Pooling data from two of the studies with the caries increment outcome of DMFS at 30 (De Soet 2002) or 36 months (Forgie 2000) follow‐up indicated an imprecise result of no appreciable difference between the chlorhexidine and placebo groups (mean difference 0.53, 95% CI ‐0.47 to 1.53; 690 participants; Analysis 1.1).
1.1. Analysis.
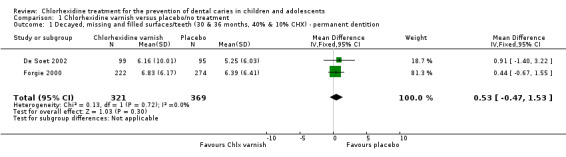
Comparison 1 Chlorhexidine varnish versus placebo/no treatment, Outcome 1 Decayed, missing and filled surfaces/teeth (30 & 36 months, 40% & 10% CHX) ‐ permanent dentition.
Primary outcome: mutans streptococci
Three studies reported on mutans streptococci (Bretz 1997; De Soet 2002; Forgie 2000; Table 5). A statistically significant difference in mutans streptococci levels was observed at 6 months in favour of chlorhexidine (Bretz 1997; Forgie 2000), but this finding was not replicated at longer follow‐up of 12, 24 and 36 months (Forgie 2000) (Analysis 1.2). De Soet 2002 also measured mutans streptococci, but did not fully report numerical estimates of mutans streptococci levels at the end of the study period and intermediate measurements, just "no significant differences between the two treatment groups" (Table 5).
3. Effects of intervention: Mutans streptococci in permanent dentition.
| Study ID | Intervention(s) and comparator | Outcome | Summary intervention | Summary comparator | Comment |
| Bretz 1997 | Chlorzoin 10% chlorhexidine applied after 3 months (n = 43) No treatment (n = 40) |
Mean MS (6 months) | Varnish 2.9 (SD 1.5) | No treatment 4.1 (SD 1.3) | Authors report "After 6 months the C group exhibited significantly higher levels of the mutans streptococci when compared to the T group." (MD ‐1.20, 95% CI ‐1.80 to ‐0.60) |
| Forgie 2000 | Chlorzoin 10% chlorhexidine applied 4 x year (n = 222) Placebo (n = 274) |
Number of participants with elevated MS levels Cariescreen® ≥ 4 (high) (36 months) | Varnish 129/224 | Placebo 169/272 | Authors report that Chlorzoin varnish "was not able to ensure continually low MS levels" following an initial benefit in favour of the active intervention observed at 3 and 6 months. This was not maintained at longer follow‐up at 12, 24 and 36 months. At 36 months there was no evidence of lower MS levels in the active varnish group (RR 0.93, 95% CI 0.80 to 1.07) |
| De Soet 2002 | 40% chlorhexidine varnish (EC40®) applied 2 x year (n = 99) Placebo: neutral gel without chlorhexidine (n = 95) |
MS (30 months) | Not reported | Not reported | Numerical estimates of MS levels at the end of the study and intermediate data collection points not presented. Authors report "...no significant differences between the two treatment groups on these microbiological measures (p < 0.2)" |
CI: confidence interval MD: mean difference MS: mutans streptococci RR: risk ratio
1.2. Analysis.
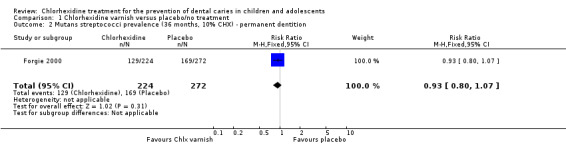
Comparison 1 Chlorhexidine varnish versus placebo/no treatment, Outcome 2 Mutans streptococci prevalence (36 months, 10% CHX) ‐ permanent dentition.
Secondary outcomes
No studies reported on pain, quality of life, patient satisfaction or costs. One study reported on adverse events: no adverse events such as ulceration or other mucosal lesions or tooth staining were observed during the course of the study (Du 2006). De Soet 2002 reported that “side‐effects due to the CHX treatment were not noted".
We judged the quality of the evidence for the outcomes of caries and mutans streptococci to be very low (Table 1).
Chlorhexidine gel compared with no treatment or placebo
Two studies, both at high risk of bias, compared chlorhexidine gel with no treatment (Plonka 2013; Pukallus 2013), randomising 490 children.
Primary outcome: caries
Primary dentition
Both studies reported the incidence of caries (Plonka 2013; Pukallus 2013), the pooled best estimate of effect being 1.00 (RR 1.00, 95% CI 0.36 to 2.77; Analysis 2.1) at 24 months. On the basis of these analyses, we were unable to exclude the possibility that chlorhexidine gel has no caries preventive effect.
2.1. Analysis.
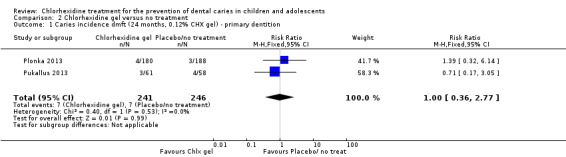
Comparison 2 Chlorhexidine gel versus no treatment, Outcome 1 Caries incidence dmft (24 months, 0.12% CHX gel) ‐ primary dentition.
Primary outcome: mutans streptococci
Both studies reported on the levels of mutans streptococci (Plonka 2013; Pukallus 2013) (Table 6). The pooled best estimate of effect was 1.26 (RR 1.26, 95% CI 0.95 to 1.66; Analysis 2.2) at 24 months. On the basis of this analysis, we were unable to exclude the possibility that chlorhexidine gel has no effect on the presence or absence of mutans streptococci.
4. Effects of intervention: Mutans streptococci in primary dentition.
| Study ID | Intervention(s) and comparator | Outcome | Summary Intervention | Summary Comparator | Comment |
| Pukallus 2013 | Curasept (0.12%) chlorhexidine gel applied 1 x daily (n = 61) No treatment (n = 58) |
MS prevalence (24 months) | Gel 28/61 | No treatment 27/58 | Authors report "Percentages of children with MS present were not significantly different between CHX and controls (P = 0.2)" RR 0.99 (95% CI 0.67 to 1.45) |
| Plonka 2013 | Curasept (0.12%) chlorhexidine gel applied 1 x daily (n = 180) No treatment (n = 188) |
MS prevalence (24 months) | Gel 49/183 | No treatment 34/188 | Authors report "No statistically significant differences were present, however, at later ages." P = 0.05 RR 1.48 (95% CI 1.01 to 2.18) |
CHX: chlorhexidine CI: confidence interval MS: mutans streptococci RR: risk ratio SD: standard deviation
2.2. Analysis.
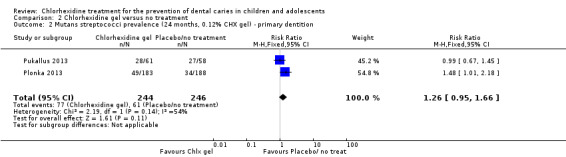
Comparison 2 Chlorhexidine gel versus no treatment, Outcome 2 Mutans streptococci prevalence (24 months, 0.12% CHX gel) ‐ primary dentition.
Secondary outcomes
Neither study reported on pain, quality of life, patient satisfaction or costs; however, both studies reported on adverse events: no adverse events such as ulceration or other mucosal lesions or tooth staining were observed during the course of the studies (Plonka 2013; Pukallus 2013).
We judged the quality of the evidence for the outcomes of caries and mutans streptococci to be very low (Table 1).
Discussion
Summary of main results
Eight studies met the inclusion criteria for this review, all evaluating the effects of chlorhexidine varnishes or gels on the primary or permanent teeth of children and adolescents. We assessed the quality of the body of evidence with reference to the risk of bias of the included studies, the directness of the evidence, the consistency of the results (heterogeneity), the precision of the effect estimates and the risk of publication bias (GRADE 2004). We have provided a summary of this quality assessment in the 'Summary of findings' tables, separately for chlorhexidine varnish (Table 1) and chlorhexidine gel (Table 2) dentitions. We assessed the body of evidence for both as very low quality.
We evaluated three separate concentrations (1%, 10%, and 40%) and formulations of chlorhexidine varnish in six studies, each with a different application regimen. We evaluated one concentration (0.12%) of chlorhexidine gel in two studies.
In the permanent dentition, pooled estimates were possible for the effects of 40% and 10% chlorhexidine varnish on caries increment and 10% chlorhexidine varnish on mutans streptococci levels. We judged the quality of this body of evidence to be very low. The 95% confidence limits for the pooled effect sizes were compatible with both an increase or a decrease in caries.
Pooled estimates from two studies were possible for the effects of 0.12% chlorhexidine gel in the primary dentition on caries incidence and mutans streptococci levels. We judged the quality of this body of evidence to be very low. The 95% confidence limits were compatible with both an increase or a decrease in both caries and mutans streptococci levels.
Due to variation in the measurement of outcomes and incomplete or inadequate reporting, we were unable to pool much of the data.
It was not possible to pool the data on the outcome 'caries' from the following comparisons:
40% chlorhexidine varnish against placebo in the primary dentition (Du 2006)
1% chlorhexidine and 1% thymol varnish against no treatment in both the primary and permanent dentitions (Baca 200X)
1% chlorhexidine and 1% thymol varnish against placebo in the permanent dentition (Nordling 1999)
10% chlorhexidine varnish against no treatment in the permanent dentition (Bretz 1997)
It was not possible to pool the data on the outcome 'mutans streptococci' from the following comparisons:
10% chlorhexidine varnish against no treatment in the permanent dentition (Bretz 1997)
40% chlorhexidine varnish against placebo in the permanent dentition (De Soet 2002)
When we carried out re‐analysis to take into account the effect of clustering in the cluster‐randomised trials (Baca 200X; Du 2006), we found uncertainty surrounding the effect estimates, with the 95% confidence intervals including both the possibility of benefit and harm at the longest follow‐up point of the study. Of the above five studies, we judged four to be at high risk of bias and one to be at unclear risk of bias.
We found some evidence from two of the studies that using chlorhexidine‐containing varnishes according to the manufacturers' recommended protocols resulted in early reductions in mutans streptococci levels at 3 and 6 months in the permanent dentition, but this effect was not sustained at longer periods of follow‐up at 12, 24 and 36 months. It should also be noted that mutans streptococci levels is a proxy outcome, and that it is unclear how reductions would translate into any effect on caries prevention, due to the effects of other cariogenic oral bacteria (for example Lactobacillus species), recolonisation from reservoirs or retention sites that were not affected by the chlorhexidine or the possibility of the development of resistance to chlorhexidine over time.
Four studies reported on adverse events (De Soet 2002; Du 2006; Plonka 2013; Pukallus 2013); none were recorded over a period of 24 or 30 months. No studies reported on the other secondary outcomes of pain, quality of life, patient satisfaction, or direct or indirect costs.
After evaluation of the available evidence, it has not been possible to either claim or refute a benefit of chlorhexidine varnishes or gels for the prevention of caries in the primary or permanent dentitions.
Overall completeness and applicability of evidence
We found no studies that assessed chlorhexidine‐based mouthrinses, toothpastes, chewing gums or sprays for the prevention of dental caries in children and adolescents. All available RCTs evaluated the effects of chlorhexidine varnishes or gels.
Due to the different formulations of chlorhexidine products used, the variation in measurement of outcomes, and the incomplete reporting of outcome data, it was not possible to pool the results of many of the studies that we included in this review. Individual studies were typically small in size and at high or unclear risk of bias.
Of particular concern is that four of the eight included studies did not report adverse events. Known adverse effects of chlorhexidine include staining of the teeth and tongue, mucosal soreness and desquamation, temporary taste disturbances, parotid gland swelling and hypersensitivity (including anaphylaxis) (BNF 2015; Krishna 2014). Although rare, there have been cases of death due to anaphylaxis associated with chlorhexidine, therefore it is extremely important that the body of evidence addresses both benefits and risks of any intervention. As no eligible studies addressed patient satisfaction, pain, quality of life, or direct or indirect costs of the intervention, the evidence is incomplete regarding these outcomes.
Only three studies gave an indication of the population exposure to fluoride. Two studies reported the level of fluoride in the water supply, and one study stated that the use of fluoride toothpaste in the area was uncommon. Lack of information on contextual factors limits the external validity of the evidence base.
Quality of the evidence
The evidence that we have evaluated does not permit us to draw any conclusions regarding the effects of chlorhexidine varnish or gel on the prevention of dental caries. Neither the pooled estimates nor the estimates from the individual studies produced statistically significant effect sizes. We judged the overall quality of the evidence to be very low, meaning that we are very uncertain about any estimates of effect (GRADE 2004).
Of the eight studies included in this review, we judged six to be at high risk of bias and two to be at unclear risk of bias. For the studies at high risk of bias, the most common issue was with blinding of participants and personnel (performance bias). Incomplete outcome data (attrition bias) and selective reporting (reporting bias) were the next most common reasons for judging studies to be at high risk of bias. For example, although the studies included sizeable numbers of children, five of the eight studies did not consistently and fully report losses to follow‐up, leading to the possibility of attrition bias. Furthermore, of the two studies at unclear risk of bias, unclear reporting of allocation concealment (selection bias) was common to both studies. In addition to this risk of bias, we downgraded the quality of the evidence due to imprecision (low numbers of events), inconsistency, and indirectness of the studies to the review question.
Potential biases in the review process
As two of the included studies (Bretz 1997; Nordling 1999), which were unpublished and retrieved in our comprehensive electronic searches, were not cited in any previous systematic reviews, the existence of other unpublished studies and the possibility of publication bias cannot be ruled out.
Agreements and disagreements with other studies or reviews
The most recent previous systematic review on the topic of chlorhexidine varnish for preventing dental caries in children and adolescents also concluded that the evidence “is inconclusive" (James 2010). This review included 12 trials with inclusion criteria that were different than this Cochrane review in that studies of a split‐mouth design or with fluoride therapies as a comparator were eligible for inclusion.
An earlier literature review (that is not a systematic review) on “the use of chlorhexidine for caries prevention” concluded that "chlorhexidine rinses should not be recommended for use in caries prevention due to a current lack of evidence” and that the evidence on the effectiveness of chlorhexidine gels and varnishes was "suggestive but incomplete" (Autio‐Gold 2008). As a literature review and not a systematic review of randomised clinical trials, the inclusion and exclusion criteria were not strictly defined, and the authors also considered studies using chlorhexidine‐fluoride combinations as the intervention.
A systematic review by Zhang 2006a of the caries‐inhibiting effect of chlorhexidine varnishes on the permanent dentition of children, adolescents and young adults included 10 studies, several of which we excluded from our Cochrane review because they used split‐mouth design (Bratthall 1995; Haukali 2003; Joharji 2001; Zhang 2006), or the participants included children receiving orthodontic treatment (Madlena 2000), or concomitant fluoride applications were made (Fennis‐le 1998). The authors “tentatively conclude[ed] that CHX varnish has a moderate caries inhibiting effect when applied every 3‐4 months”, but that this effect is diminished around “two years after the last application”.
Another (non‐systematic) review found 20 studies addressing caries outcomes in children and adolescents (and two studies investigating maternal chlorhexidine application to reduce caries in infants) (Twetman 2004). The authors included trials with the outcomes of root caries, white‐spot lesions and mother‐child transmission of S. mutans and intervention protocols utilising fluoride and split‐mouth designs. This review also found the evidence to be inconclusive for the use of “chlorhexidine varnishes for caries prevention in risk groups”.
A review by Anderson 2003 concluded that the "literature remains mixed on the success of chlorhexidine for the reduction of dental caries whilst its performance as an antimicrobial against streptococcus mutans is more consistent and favorable".
This Cochrane systematic review is thus in agreement with most of the previous reviews conducted on the effect of chlorhexidine products for the prevention of caries in children and adolescents, in that the evidence is incomplete and no conclusions can be drawn from the existing evidence base.
Authors' conclusions
Implications for practice.
There is little evidence from the eight studies included in this review to either support or refute the assertion that chlorhexidine is more effective than placebo or no treatment in the prevention of caries or the reduction of mutans streptococci levels in children and adolescents. Whilst not making specific clinical recommendations, several previous non‐Cochrane systematic reviews have reached not dissimilar conclusions about the lack of evidence of beneficial effects of chlorhexidine treatments on dental caries.
In line with this lack of high quality evidence, chlorhexidine‐containing products for the prevention of dental caries are not included in any of the following clinical guidelines: Prevention and Management of Dental Caries in Children, Scottish Dental Clinical Effectiveness Program (SCDEP 2014); Delivering better oral health: an evidence‐based toolkit for prevention, Public Health England (DBOH 2014); Guidelines on Prevention of Early Childhood Caries, European Academy of Paediatric Dentistry (EAPD 2008); and Guideline on Caries‐risk Assessment and Management for Infants, Children and Adolescents, American Academy of Pediatric Dentistry (AAPD 2013).
However, good evidence is available for the prevention of caries in children using topical fluorides in the form of varnish, toothpastes, gels and mouthrinses (Marinho 2004), and fissure sealants (Ahovuo‐Saloranta 2013). This is reflected in the advice of all of the above guidelines, which prioritise topical fluoride application, fissure sealants and sugar reduction as effective ways to prevent caries. This review does not provide any evidence that chlorhexidine could be an alternative.
Implications for research.
As we were unable to contact several authors of the included trials to clarify missing or incomplete study details, our review evaluated the quality of the trials as reported. Where trials fail to report clear steps taken to reduce bias, the reader cannot make a fully informed appraisal of the evidence. It is therefore important that any further trials be robust, well designed, and conducted and reported according to the Consolidated Standards of Reporting Trials statement (http://www.consortstatement.org/).
See Table 7 for further research recommendations based on the EPICOT format (Brown 2006).
5. Research recommendations based on a gap in the evidence on chlorhexidine treatment for the prevention of dental caries in children and adolescents.
| Core elements | Issues to consider | Status of research for this review |
| Evidence (E) | What is the current state of evidence? | A systematic review that identified eight RCTs matching the eligibility criteria, but were incompletely reported, had significant losses to follow‐up, and were assessed as at unclear or high risk of bias |
| Population (P) | Diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting |
EXCLUDED
|
| Intervention (I) | Type, frequency, dose, duration, prognostic factor | Chlorhexidine‐containing products:
Administration minimum once over a period of 1 year EXCLUDED
Compliance to be recorded |
| Comparison (C) | Type, frequency, dose, duration, prognostic factor |
EXCLUDED
Compliance to be recorded |
| Outcome (O) | Which clinical or patient‐related outcomes will the researcher need to measure, improve, influence or accomplish? Which methods of measurement should be used? | Dental caries (coronal)
Diagnosis (at the dentine level) clinically/radiographically confirmed.
Caries increment: change from baseline (D(M)FS/T, d(m)fs/t) index. MS reductions in levels Pain, quality of life or patient satisfaction outcomes measured on a validated scale |
| Time Stamp (T) | Date of literature search or recommendation | 14 January 2014 |
| Study Type | What is the most appropriate study design to address the proposed question? | RCT
Methods: Concealment of allocation sequence 'Clear' Blindness: Patients, carers, trialists, outcome assessors blind Setting for administration: home or dental hospital/clinic Setting for clinical outcome assessment: in dental hospital/clinic with appropriate length of follow‐up |
(D(M)FS/T: decayed, missing and filled surfaces or teeth (permanent) d(m)fs/t): decayed, missing and filled surfaces or teeth (primary) MS: mutans streptococci RCT: randomised controlled trial
Through the comprehensive literature search, we identified two studies where chlorhexidine was used in combination with fluoride or other active ingredients to prevent caries in children and adolescents (Hermida 2012; Sundell 2013). These studies did not fit the eligibility criteria for this review, but should be included in systematic reviews evaluating the caries preventive effects of different complex interventions in children and adolescents (a review on this topic has been registered with the Cochrane Oral Health Group).
Two of the trials showed some evidence of an mutans streptococci‐inhibiting effect at three and six months. If the hypothesis of reduced numbers of mutans streptococci in the oral environment leading to reductions in caries increments is correct, then further trials should consider application of chlorhexidine‐containing products at no less than three‐ or six‐month intervals. The review by Zhang also reported that any caries‐inhibiting effect seemed to be limited to studies utilising chlorhexidine applications of three to four months (Zhang 2006a).
Whilst on an individual basis progression rates of caries are variable, any future trial should ensure adequate length of follow‐up, considering that the rate of progression through the enamel in the permanent dentition is generally accepted to be faster in younger children than in adolescents, and in individuals with active caries.
Acknowledgements
The review authors would like to thank the Cochrane Oral Health Group and external referees Nicola Innes and Linda Slack‐Smith for their help in producing this systematic review. We would like acknowledge Ameera Radhi and Zbys Fedorowicz for their contributions to earlier drafts of the review.
Appendices
Appendix 1. Cochrane Oral Health Group Trials Register search strategy
From January 2014, searches of the Oral Health Group Trials Register were undertaken for this review using the Cochrane Register of Studies and the search strategy below:
1 ((caries or cavit* or carious or decay* or lesion* or deminerali* or reminerali*)) AND (INREGISTER) 2 ((chlorhexidine or chlorohex* or eludril* or corsodyl* or PerioChip or CHX or mk412a or "MK 412a" or MK‐412a or nolvasan or sebidin* tubulicid* or cervitec* or chlorzoin* or hibitane)) AND (INREGISTER) 3 (#1 and #2) AND (INREGISTER)
A previous search of the Oral Health Group Trials Register was undertaken in March 2010 using the Procite software and the search strategy below:
((caries or cavit* or carious or decay* or lesion* or deminerali* or reminerali*) AND (chlorhexidine or chlorohex* or eludril* or corsodyl* or PerioChip or CHX or mk412a or "MK 412a" or MK412a or nolvasan or sebidin* or tubulicid* or cervitec* or chlorzoin* or hibitane))
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Tooth Demineralization explode all trees #2 caries or carious #3 ((teeth near/5 cavit*) or (teeth near/5 caries) or (teeth near/5 carious) or (teeth near/5 decay*) or (teeth near/5 lesion*) or (teeth near/5 deminerali*) or (teeth near/5 reminerali*)) #4 ((tooth near/5 cavit*) or (tooth near/5 caries) or (tooth near/5 carious) or (tooth near/5 decay*) or (tooth near/5 lesion*) or (tooth near/5 deminerali*) or (tooth near/5 reminerali*)) #5 ((dental near/5 cavit*) or (dental near/5 caries) or (dental near/5 carious) or (dental near/5 decay*) or (dental near/5 lesion*) or (dental near/5 deminerali*) or (dental near/5 reminerali*)) #6 ((enamel near/5 cavit*) or (enamel near/5 caries) or (enamel near/5 carious) or (enamel near/5 decay*) or (enamel near/5 lesion*) or (enamel near/5 deminerali*) or (enamel near/5 reminerali*)) #7 ((dentin* near/5 cavit*) or (dentin* near/5 caries) or (dentin* near/5 carious) or (dentin* near/5 decay*) or (dentin* near/5 lesion*) or (dentin* near/5 deminerali*) or (dentin* near/5 reminerali*)) #8 ((root* near/5 cavit*) or (root* near/5 caries) or (root* near/5 carious) or (root* near/5 decay*) or (root* near/5 lesion*) or (root* near/5 deminerali*) or (root* near/5 reminerali*)) #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Chlorhexidine explode all trees #11 (chlorhexidine or chlorohex* or eludril* or corsodyl* or Periochip*) #12 (CHX):ti,ab,kw #13 (MK‐412a or "MK 412a" or MK412a) #14 (nolvasan* or sebidin* or tubulicid* or Cervitec* or Chlorzoin* or hibitane*) #15 (#10 OR #11 OR #12 OR #13 OR #14) #16 (#9 AND #15)
Appendix 3. MEDLINE (OVID) search strategy
exp TOOTH DEMINERALIZATION/
(caries or carious).mp.
(teeth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(tooth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(dental adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(enamel adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(dentin$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(root$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
or/1‐8
CHLORHEXIDINE/
(chlorhexidine or chlorohex$ or eludril$ or corsodyl$ or Periochip).mp.
CHX.ti,ab.
(MK‐412a or (MK adj 412a) or MK412a).mp.
(Nolvasan$ or Sebidin$ or Tubulicid$ or Cervitec$ or Chlorzoin$ or hibitane$).mp.
or/10‐14
9 and 15
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2009 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.0.2 [updated September 2009].
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. EMBASE via OVID search strategy
exp TOOTH DEMINERALIZATION/
(caries or carious).mp.
(teeth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(tooth adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(dental adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(enamel adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(dentin$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
(root$ adj5 (cavit$ or caries$ or carious or decay$ or lesion$ or deminerali$ or reminerali$)).mp.
or/1‐8
CHLORHEXIDINE/
(chlorhexidine or chlorohex$ or eludril$ or corsodyl$ or Periochip).mp.
CHX.ti,ab.
(MK‐412a or (MK adj 412a) or MK412a).mp.
(Nolvasan$ or Sebidin$ or Tubulicid$ or Cervitec$ or Chlorzoin$ or hibitane$).mp.
or/10‐14
9 and 15
The above search was linked to the Cochrane Oral Health Group search strategy for identifying randomized controlled trials in EMBASE via Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. CINAHL via EBSCO search strategy
S1 MH "Tooth demineralization+" S2 caries or carious S3 teeth N5 cavit* or teeth N5 caries or teeth n5 carious or teeth n5 decay* or teeth n5 lesion* or teeth n5 deminerali* or teeth n5 reminerali* S4 tooth N5 cavit* or tooth N5 caries or tooth n5 carious or tooth n5 decay* or tooth n5 lesion* or tooth n5 deminerali* or tooth n5 reminerali* S5 dental N5 cavit* or dental N5 caries or dental n5 carious or dental n5 decay* or dental n5 lesion* or dental n5 deminerali* or dental n5 reminerali* S6 enamel N5 cavit* or enamel N5 caries or enamel n5 carious or enamel n5 decay* or enamel n5 lesion* or enamel n5 deminerali* or enamel n5 reminerali* S7 dentin* N5 cavit* or dentin* N5 caries or dentin* n5 carious or dentin* n5 decay* or dentin* n5 lesion* or dentin* n5 deminerali* or dentin*n5 reminerali* S8 root* N5 cavit* or root* N5 caries or root* n5 carious or root* n5 decay* or root* n5 lesion* or root* n5 deminerali* or root* n5 reminerali* S9 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 S10 MH "Chlorhexidine" S11 chlorhexidine or chlorohex* or eludril* or corsodyl* or Periochip S12 TI CHX or AB CHX S13 mk‐412 or "mk 412" or mk412 S14 Nolvasan* or Sebidin* or Tubulicid* or Cervitec* or Chlorzoin* or hibitane* S15 S10 or S11 or S12 or S13 or S14 S16 S9 and S15
The above search was linked to the Cochrane Oral Health Group search strategy for identifying randomized controlled trials in CINAHL via EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. The US National Institutes of Health Trials Register (ClinicalTrials.gov) and the WHO Clinical Trials Registry Plaform search strategy
chlorhexidine and caries and children
Data and analyses
Comparison 1. Chlorhexidine varnish versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Decayed, missing and filled surfaces/teeth (30 & 36 months, 40% & 10% CHX) ‐ permanent dentition | 2 | 690 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐0.47, 1.53] |
| 2 Mutans streptococci prevalence (36 months, 10% CHX) ‐ permanent dentition | 1 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.07] |
Comparison 2. Chlorhexidine gel versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caries incidence dmft (24 months, 0.12% CHX gel) ‐ primary dentition | 2 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.36, 2.77] |
| 2 Mutans streptococci prevalence (24 months, 0.12% CHX gel) ‐ primary dentition | 2 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.95, 1.66] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baca 200X.
| Methods | Design: Parallel‐group cluster‐RCT Duration: 24 months Recruitment period: 1996‐1998 Administration setting: School Country: Spain Funding source: Supported by Consejeria de educacion y Ciencia and Fondo de Investigaciones del MSC, Spain Additional fluoride sources reported: Fluoride level in water 0.07 |
|
| Participants | Number of participants randomised: 229 (number randomised in each group unreported) Age: Mean 6.5 years (range 6 to 7 years) Sex: 50% male Baseline caries: Intervention DFT 0.25 (SD 0.67), Comparator DFT 0.17 (SD 0.54) Inclusion criteria: All children in the randomly selected classes were invited to participate Exclusion criteria: None reported Number of participants evaluated: 181 (Intervention 86, Comparator 95) Withdrawals/loss to follow‐up: Overall 48 (20.9%) loss to follow‐up, reasons given "moved schools or were withdrawn by parents". Losses not reported by group |
|
| Interventions | Total number of groups: 2 Intervention:
Comparator:
Any additional oral health provision: No preventive treatment before or during the study period. |
|
| Outcomes | Outcomes: Caries (DFT‐M/DFS‐M increment (first permanent molars with caries and fillings 2002), dft‐m/dfs‐m (primary molars 2004)) Time points: Assessment at baseline and 24 months Diagnostic criteria: WHO criteria using an explorer and a flat mirror by a single calibrated dentist (kappa 0.63, 20 children) Adverse events: Not reported |
|
| Notes | A cluster‐RCT, 5 schools randomly selected and 2 classes of the same school year were randomly allocated to either intervention or comparator trial arm. The statistical analysis did not take into account the clustering of children within school classes Sample size: No details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Five of the 21 primary schools...were selected at random" "Each school had two first‐year classes of 20–25 pupils, and one was randomly assigned for treatment with chlorhexidine varnish and the other to serve as controls" Comment: Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The control group received no treatment other than clinical examination" Comment: Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "A single dentist carried out all the examinations, which were not performed in a blinded fashion..." "A second dentist participated in the study only as an external observer...and was blinded to the status of the children examined" Comment: Unblinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow‐up (20.0%) due to moving schools or parental withdrawals, not clear in which groups Comment: Reasons for parental withdrawals could be related to trial arm but losses not reported by group. Insufficient information reported to make a judgement |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Groups comparable at baseline with respect to caries level Comment: No other potential sources of bias identified |
Bretz 1997.
| Methods | Design: Parallel‐group RCT Duration: 6 months Recruitment period: No start date recorded, but the earliest published report of this study was Bretz 1995 Administration setting: Orphanage Country: Brazil Funding source: Not reported Additional fluoride sources reported: Fluoride level in water not reported |
|
| Participants | Number of participants randomised: 113 (Intervention 58, Comparator 55) Age: Mean 12.1 years (range 1.3 years) Sex: Female only Baseline caries: Intervention % of decayed surfaces 0 (SD 0), Comparator % of decayed surfaces 0.04 (SD 0.3) Inclusion criteria: None reported Exclusion criteria: None reported Number of participants evaluated: 83 (Intervention 43, Comparator 40) Withdrawals/losses to follow‐up: Overall 30 (26.5%) loss to follow‐up, comparable across groups; reasons not given |
|
| Interventions | Total number of groups: 2 Existing carious lesions restored and prophylaxis given Intervention:
Comparator:
Any additional oral health provision: None reported |
|
| Outcomes | Outcomes: Caries scores (% sound surfaces, % decayed surfaces, % restored surfaces, % white‐spot lesions). MS salivary levels Time points: Baseline, 3 months, 6 months Diagnostic criteria: Caries criteria not stated, carried out by a single calibrated examiner (kappa not stated). MS salivary levels (Caritest®) were categorised according to scores ranging from 0 (non‐detected) to 6 (>106cfu/dip‐slide) Adverse events: Not reported |
|
| Notes | Many of the study details were incompletely reported, but we were able to obtain some additional information through email contact with the principal investigator. No data on sample size and power calculation Data presented as "dental decay parameters", i.e. % of sound, decayed, restored surfaces, were not amenable to meaningful transformation and were unusable for this review. Subgroup data reported excluding "some of the subjects who belonged to C (control) and T (treatment) groups still had retentive sites and open carious lesions at the 3‐month visit which should have been restored prior to the application of the chlorhexidine varnish‐sealant at baseline" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly allocated to control (C) and treatment (T) groups" Comment: Method of sequence generation not stated Clarification sought and obtained from principal investigator: "random allocation was performed by a computer random allocation program" Comment: Probably done |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | E‐mail communication with the principal investigator: "a different operator applied the varnish and was not involved in caries examinations" Comment: Personnel not blinded Comparator was no treatment, so blinding of participants and personnel not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated Comment: Insufficient information reported to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Subject attrition was minimal during the course of the study because subjects resided in the orphanage". Comment: Results presented for 83/113 children only (excluded 24 children, 26%) |
| Selective reporting (reporting bias) | High risk | Quote: "statistical analysis was performed in all subjects from C (control) and T (treatment) groups, and then on subjects who had their restorative needs met at baseline in C (n = 42) and T (n = 47) groups"
Comment: Analysis of caries data at 6 months not reported in format suitable for re‐analysis or meta‐analysis. No mean DFT/DFS caries scores reported Data for MS levels reported for subset of participants only |
| Other bias | Unclear risk | Chlorzoin intervention did not appear to have been delivered strictly according to the manufacturer's recommended protocol (a single application during 4 consecutive weeks)
The investigators confirmed by e‐mail that "the study was supported by a grant from Somcana International Canada". No further information was made available Groups comparable for caries levels at baseline |
De Soet 2002.
| Methods | Design: Parallel‐group RCT Duration: 30 months Recruitment period: No dates reported Administration setting: Center for Youth Dental Care in Paramaribo Country: Surinam, South America Funding source: Partially funded by a grant from Explore, Nijmegen, the Netherlands. Additional fluoride sources reported: Fluoride level in water not reported. Authors state that the use of fluoride toothpaste is not common in this region. No additional healthcare advice received |
|
| Participants | Number of participants randomised: 238 (Intervention 120, Comparator 118) Sex: 114 male, 124 female Age: Mean 13.2 years (SD 0.4) Baseline caries: Mean DMFS 3.83 (SD 5.35) "moderately caries‐active population" Inclusion criteria: Not reported Exclusion criteria: Children with orthodontic appliances Number of participants evaluated: 194 (Intervention 99, Comparator 95) Withdrawals/losses to follow‐up: 1 withdrawal due to untreated nursing caries (baseline DMFS = 35), overall 43 (18%) losses to follow‐up. No reasons reported, but losses comparable across groups |
|
| Interventions | Total number of groups: 2 At baseline, clinical exam and bitewings and all routine dental care completed. No additional professional cleaning of the teeth took place before the treatment with varnish Intervention:
Comparator:
Any additional oral health provision: No additional healthcare advice provided |
|
| Outcomes | Outcomes: Caries scores (DMFS). MS salivary levels Time points: Baseline, 12, 24 and 30 months Diagnostic criteria: Clinical examination (dental light on dried teeth, no probing) by a single calibrated blinded dentist (kappa not stated). Criteria (0 = no carious lesion; 1 = carious lesion restricted to enamel (no cavity); 2 = carious cavity restricted to enamel (only smooth surfaces); 3 = carious lesion in dentin) MS levels: Categorised to scores from log transformation of colony forming unit count (1 = not detected, 2 = 101 to 102 cfu/ml, 3 = 102 to 103 cfu/ml, 4 = 103 to 104 cfu/ml, 5 = 104 to 105 cfu/ml, 6 ≥ 105 cfu/ml) Adverse events: Reported |
|
| Notes | The study was government inspected by the Ministry of Health, Surinam. No details on sample size or power calculation were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were assigned randomly to an experimental group and a control group" Comment: Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients and investigator were blinded for the treatment in both groups. They did not know whether CHX was present in the varnish or in the placebo gel" Comment: Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients and investigator were blinded for the treatment in both groups. They did not know whether CHX was present in the varnish or in the placebo gel" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was withdrawn due to "untreated nursing caries". DMFS and MS data at 30 months available only for 194/238 (chlorhexidine group 99/120, placebo group 95/118). Incomplete outcome data (18%) balanced across groups, but reasons unreported |
| Selective reporting (reporting bias) | High risk | No numerical estimates for levels of MS at the end of study and intermediate time points reported; numerical estimates of caries levels reported at end of study only |
| Other bias | Unclear risk | The investigators declared: "this study was partially funded by a grant from Explore, Nijmegen...", who appears to be the manufacturer or distributor of the chlorhexidine varnish. The report did not indicate any other involvement of the funder in the study, and it appears to be free of other bias Groups comparable on baseline for caries levels |
Du 2006.
| Methods | Design: Parallel‐group cluster‐RCT Duration: 24 months Recruitment period: 1999 to 2001 Administration setting: Kindergarten Country: China Funding source: Ministry of Science and Technology, National Committee for Oral Health, People's Republic of China Additional fluoride sources reported: Fluoride level in water was 0.1 to 0.3 ppm. No organised oral healthcare programme available |
|
| Participants | Number of participants randomised: 334 (Intervention 175, Comparator 159) Sex: Ratio of boys to girls 1:1.04 Age: 4 to 5 years Baseline caries: Mean dmfs‐molar: Intervention 2.8, Comparator 2.6 Inclusion criteria: All children in the randomly selected kindergartens were invited to participate in the study Exclusion criteria: Not reported Number of participants evaluated: 290 (155 Intervention, 135 Comparator) Withdrawals/losses to follow‐up: Overall 44 (13%) lost to follow‐up: 31 moved school; 13 objected to the taste of the varnish and refused to be examined |
|
| Interventions | Total number of groups: 2 No professional cleaning of teeth was carried out prior to application Intervention:
Comparator:
Any additional oral health provision: None reported |
|
| Outcomes | Outcomes: Caries (dmfs‐molar) Time points: Baseline, 24 months Diagnostic criteria: WHO criteria using mouth mirrors and probes under natural daylight by 2 calibrated dentists (kappa > 0.90) Adverse events: Reported (soft tissues and teeth examined for any side effects, such as ulcers, other mucosal lesions, and tooth staining) |
|
| Notes | A cluster‐RCT. 4 kindergartens were randomly chosen and randomly allocated to groups based on the class they attended. 7 classes allocated to intervention, 7 classes to comparator. The statistical analysis did not take into account the clustering of children within schools Sample size: 150 participants per group required, based on the ability to detect a difference of 1 tooth surface in caries increment between the test and control group, at a 5% significance level and a power of 80%. However, this sample size did not take into account the effect of clustering, therefore the study was underpowered |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We randomly divided the children into two groups, based on the class they attended, by drawing a card from a bag." Coment: Random component specified |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The children, their parents, and the kindergarten staff were blinded as to the group assignment of the children". "The 2 varnishes were put into bottles that had the same appearance, to ensure blindness of the trial" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two calibrated dentists who did not know the group assignment of the children performed all clinical examinations". Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "44 children (13%) were lost to follow‐up, because some (n = 31) had moved to other kindergartens, and some (n = 13) objected to the taste of the varnish and refused to be examined." Comment: Reasons given for losses to follow‐up, but not clear in which groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Groups comparable for caries levels at baseline. No other potential sources of bias identified |
Forgie 2000.
| Methods | Design: Multicentre, parallel‐group RCT Duration: 36 months Recruitment period: 1994 to 1998 Administration setting: Multisite (30 secondary schools) and single‐centre (Dundee Dental Hospital and School) Country: Tayside, Scotland Funding source: Oralife Group, Canada Additional fluoride sources reported: Fluoride level in water not reported |
|
| Participants | Number of participants randomised: 1240 (stratified prior to randomisation by gender, baseline caries experience and history of eczema; n = 324, 324, 268, 324) Sex: 602 males, 638 females Age: 11 to 13 years Baseline caries: Mean DMFS 6.56 (SD 6.53) to 7.26 (SD 7.10) clinical plus bitewing radiographs "high caries risk" Inclusion criteria:
Exclusion criteria:
Number of participants evaluated: 987 (n = 248, 243, 222, 274) Withdrawals/losses to follow‐up: Group 1: observational control (n = 324); dropouts 76 (23%) Group 2: positive control (n = 324); dropouts 81 (25%) Group 3: active Chlorzoin varnish (n = 268); dropouts 46 (17%) Group 4: placebo varnish (n = 324); dropouts 50 (15%) |
|
| Interventions | Total number of groups: 4 Intervention:
Comparator:
Any additional oral health provision: Both groups "received comprehensive caries advice...and demonstrations in oral hygiene techniques" |
|
| Outcomes | Outcomes: Caries increments (DMFS) at D1 and D3 thresholds, MS levels (high or low) Time points: Caries: Baseline, 36 months. MS: Baseline, 3, 6, 12, 24, 36 months Diagnostic criteria: 5 annual exams (dental light/dried teeth/no probing) by a single calibrated, fully blinded dentist at the D1 (enamel and dentine caries) threshold (kappa between 0.79 and 0.86). The first and final examinations also included radiographs; intermediate and final examinations also included fibre‐optic transillumination Cariescreen® levels categorised as low < 3, or high > 4, with a threshold of approximately 250,000 cfu/ml of saliva Adverse events: Not reported |
|
| Notes | Participants' 'non‐compliance' was categorised as missing 1 or more varnish applications. Results at D1 level reported separately for compliant (per protocol) and non‐compliant (missing varnish applications) groups For the purpose of this review on the effectiveness of chlorhexidine on caries, the most appropriate comparator is placebo varnish, therefore we considered only groups 3 and 4 here Sample size: For 80% power to detect a 30% difference in caries increment, the number of children needed to complete the study was calculated at 237 children per group, which was achieved |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation: by gender, baseline caries experience, and history of eczema. No further details reported other than that "the children were randomized into one of four groups" and "the allocation of children to study groups was based on statistical random sampling". Comment: Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study staff and subjects were blind to the assignment of varnish group". "...the only difference between the active and placebo preparations being the inclusion of chlorhexidine" Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...the dental examiner was blind to all group allocations" Comment: Probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data for evaluation: Numbers lost to follow‐up/dropouts were accounted for by the investigators (active varnish 46/268 (17%), placebo varnish 50/324 (15%)). Although the reasons were not fully reported (child moved from region or left school and not contactable at home address), the losses were balanced across groups |
| Selective reporting (reporting bias) | Low risk | The stated objectives of the study appeared to match the fully reported outcomes |
| Other bias | Unclear risk | Quote: "Due to some subject loss between the first two examinations, there was a significant imbalance in initial caries experience of the subjects completing the trial, with those in the active varnish group having a higher initial caries experience" Comment: Baseline imbalance addressed through analysis of covariance taking initial baseline caries into account Levels of non‐compliance (missed varnish applications) with the study protocol were relatively high for the active varnish group 51/222 (23%) and placebo group 82/274 (30%) Comment: Study funded by Oralife Group Canada, a manufacturer of Chlorzoin, level of support unclear. Additional support from the Scottish Executive Health Department was unlikely to present any conflict of interest |
Nordling 1999.
| Methods | Design: Parallel‐group RCT Duration: 24 months Recruitment period: 1995 to 1998 Administration setting: Råslätts district polyclinic Country: Sweden Funding source: Not stated Additional fluoride sources reported: Fluoride level in water not stated. Organised oral healthcare programme not stated | |
| Participants | Number of participants randomised: 750 (number randomised in each group unreported) Sex: Not reported Age: 12 to 15 years Baseline caries: Not reported Inclusion criteria: Not reported Exclusion criteria: Not reported Number of participants evaluated: 542 (Intervention 271, Comparator 271) Withdrawals/losses to follow‐up: Overall 208 (28%). Reasons reported as failed to return (136), moved away (72) |
|
| Interventions | Total number of groups: 2 At baseline, clinical exam, caries assessment and long cone radiographs (no further details available). Routine restorative treatment including prophylaxis (no further details available) Intervention:
Comparator:
|
|
| Outcomes | Clinical exam, caries assessment and long cone radiographs (no further details available from the very limited information reported) Adverse events: Not reported |
|
| Notes | But "baseline differences between the groups were significant" No further references/citation to this study in the literature, the investigators were contacted by e‐mail (April 2010) Response from principal investigator Monica Nordling in May 2010. Report unpublished, full text (Swedish) received and translated Sample size: No details provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (as translated): "Children were divided into two groups by randomization...computer printouts with the children in birth order were used as input." Comment: Method used to generate random sequence unclear |
| Allocation concealment (selection bias) | Low risk | Quote (as translated): "The code was locked into the department's safety deposit box." Comment: Probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (as translated): "The manufacturer of Cervitec provided the intervention and placebo which were colour coded separately." Comment: Probably done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported Comment: Insufficient information reported to make a judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (as translated): "Losses were unexpectedly large which can be attributed to the population structure that exists within the clinic catchment area." Comment: Losses were high (overall 28%), and the reasons were listed |
| Selective reporting (reporting bias) | High risk | The stated objectives of the study appeared to match the listed outcomes. Outcomes incompletely reported (no SDs reported) |
| Other bias | High risk | Quote (as translated): "Baseline differences between the groups were significant." Comment: Baseline imbalance between the groups |
Plonka 2013.
| Methods | Design: Parallel‐group cluster‐RCT Duration: 24 months Recruitment period: Not reported Administration setting: Domicile Country: Australia Funding source: Dental Board of Queensland. Curaden Swiss donated Curasept. Colgate Oral Care, Australia donated toothbrushes and pastes Additional fluoride sources reported: Fluoride level in water not reported |
|
| Participants | Number of participants randomised: 622 (Group 1: 201, Group 2: 214, Group 3: 207) Sex: Not reported Age: 0 to 2 years Baseline caries: not applicable Inclusion criteria: None specifically reported: "All mothers of healthy children were approached..." Exclusion criteria: None reported Number of participants evaluated: 531 (Group 1: 171, Group 2: 183, Group 3: 188) Withdrawals/losses to follow‐up: Overall 80 (13%) losses to follow‐up Group 1: 10% CPP‐ACP paste, 30 (15%) Group 2: 0.12% chlorhexidine gel, 31 (15%) Group 3: No product, 19 (9%) |
|
| Interventions | Total number of groups: 3 Intervention:
Comparator:
Any additional oral health provision: All groups were instructed "twice daily tooth‐brushing using 0.304 percent fluoride toothpastes" and provided with free toothpastes and toothbrushes for the duration of the study. Oral health education provided to all mothers at each clinical examination |
|
| Outcomes | Outcomes: Caries incidence measured using dichotomous presence or absence of a decayed, missing and filled teeth (dmft) increment or not (D1 and D3 thresholds) at 24 months and mean number of carious teeth at 24 months. MS, proportion of participants positive at 24 months Time points: Caries: 6, 12, 18, 24 months. MS: 6, 12, 18, 24 months Diagnostic criteria: Not explicitly reported but examiners calibrated to standardised examination (intra and inter examiner kappa 0.94 and 0.86). Lap examination of child using head‐mounted lighting; teeth checked for cavitations and white‐spot lesions of early caries Adverse events: Reported |
|
| Notes | Sample size: Aimed for 200 participants per group at 24 months in order to have a 90% power for detecting a caries difference of 29% in the control group versus 15% in one of the treated groups (5% significance level and 20% attrition rate) Comment: Study funded by Dental Board of Queensland, toothpastes and gel supplied by industry |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "by each mother randomly picking a colour‐coded stick from an opaque bag (allocation ratio 1 : 1)" Comment: Random component specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to make a judgement. Not clear whether third party was involved in allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Intervention applied by mother. Comparator of no treatment, so blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...by examiners who were blinded to the treatment group" Comment: Clinical examination undertaken by 6 oral health therapists and 2 dentists. Assessment blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Acceptable levels of attrition over the course of the study, greatest in chlorhexidine and CPP‐ACP groups (15%), lowest in no‐treatment group (9%) |
| Selective reporting (reporting bias) | Low risk | The stated objectives of the study appeared to match the listed outcomes |
| Other bias | Low risk | Groups comparable at baseline |
Pukallus 2013.
| Methods | Design: Parallel‐group RCT Duration: 24 months Recruitment period: June 2007 to June 2008 Administration setting: Domicile Country: Australia Funding source: Dental Board of Queensland and the following Queensland Health Departments: Office of Health and Medical Research Fellowship, Health Practitioners Research Grant, and Metro South Health Service District, Oral Health Programme (Logan‐Beaudesert Division) Additional fluoride sources reported: Fluoride level in water not stated |
|
| Participants | Number of participants randomised: 199 (Intervention 110, Comparator 89) Sex: 102 males, 96 females Age: 0 to 2 years Baseline caries: not applicable Inclusion criteria: "healthy status and residence in the health service district of study" Exclusion criteria: None reported Number of participants evaluated: 119 (Intervention 61, Comparator 58) Withdrawals/losses to follow‐up: Intervention 49 (45%), Comparator 31 (35%). High loss to follow‐up but comparable across groups. The authors state, "The main reasons for drop‐out from both groups were inability to contact the mother, and her relocation beyond a reasonable distance from the research centre." |
|
| Interventions | Total number of groups: 2 Intervention:
Comparator:
Any additional oral health provision: All groups were instructed "twice daily tooth‐brushing using 0.304 percent fluoride toothpastes" as soon as the first tooth erupted. General oral health education including feeding and dietary advice was also given. Free toothbrushes, CHX pastes, and tubes of low‐dose fluoride dentifrice were mailed to the mothers after completion of the first telephone contact at 6 months and again at 12 and 18 months |
|
| Outcomes | Outcomes: Caries incidence measured using dichotomous presence or absence of a dmft increment or not (D1 and D3 thresholds) at 24 months and mean number of carious teeth at endpoint. MS, proportion of participants positive Time points: Caries: 24 months. MS: 24 months Diagnostic criteria: Clinical examination only. No indices reported Adverse events: Reported |
|
| Notes | Comment: Study funded by the Dental Board of Queensland and the following Queensland Health Departments: Office of Health and Medical Research Fellowship, Health Practitioners Research Grant, and Metro South Health Service District, Oral Health Programme (Logan‐Beaudesert Division). Curasept gel donated by Curaden Swiss; toothbrushes and pastes donated by Colgate Oral Care, Australia Sample size: To detect a difference in dmft of 5% in the treatment group and 22% in the control group with 80% power required 49 children per group. This was based on using a Chi2 test with a 2‐sided 5% significance level. This sample size was met |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation into the CHX or control groups was performed at recruitment by every mother selecting a colour‐coded stick out of an opaque bag (allocation ratio 1 : 1)" Comment: Method stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: Insufficient information to make a judgement. Not clear whether third party was involved in allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Comparator of no treatment so blinding was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: Clinical examination undertaken by 7 calibrated examiners "who were blinded to the treatment received" Comment: Assessment blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Substantial attrition over the course of the study (40%), comparable across groups |
| Selective reporting (reporting bias) | Low risk | The stated objectives of the study appeared to match the listed outcomes. There was no evidence of selective reporting of outcomes |
| Other bias | Low risk | Comment: Comparable groups at baseline |
cfu: colony‐forming unit CHX: chlorhexidine DFS: decayed and filled surfaces DFT: decayed and filled teeth DMFS: decayed, missing and filled surfaces dmfs‐molar: decayed, missing and filled molar surfaces DMFT: decayed, missing and filled teeth MS: mutans streptococci Non‐compliant: missed 1 or more varnish application RCT: randomised controlled trial SD: standard deviation WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Achong 1997 | No caries outcome reported, only bacterial counts |
| Araujo 2002 | Split‐mouth design |
| Axelsson 1976 | Concomitant fluoride administration |
| Axelsson 1987 | Prophylaxis available to all groups during the experimental period. Flouride varnish and additional chlorhexidine received by some members of each group |
| Bratthall 1995 | Split‐mouth design |
| Dolles 1980 | Concomitant fluoride administration |
| Emilson 1982 | Fluoride comparator |
| Ersin 2006 | Fluoride comparator |
| Ersin 2008 | Fluoride comparator. Only one of the interventions was considered eligible for this review, as the other comparisons were either not relevant or not considered 'routine dental care' |
| Fennis‐le 1998 | Concomitant fluoride administration |
| Gisselsson 1988 | Concomitant fluoride administration |
| Gisselsson 1994 | Concomitant fluoride administration |
| Gisselsson 2005 | Concomitant fluoride administration |
| Gokalp 2005 | Fluoride comparator |
| Haukali 2003 | Split‐mouth design |
| Hausen 2000 | Concomitant fluoride administration |
| Holst 2001 | Concomitant fluoride administration |
| Hoszek 1996 | Split‐mouth design |
| Hoszek 2005 | Split‐mouth design |
| Irmisch 2000 | Split‐mouth design |
| Joharji 2001 | Split‐mouth design |
| Leksell 2003 | Fluoride comparator |
| Lindquist 2010 | Concomitant fluoride administration |
| Neeraja 2008 | Not RCT |
| Petersson 1997 | Fluoride comparator |
| Petti 2006 | Not RCT |
| Plotzitza 2005 | Quote: "The allocation of children to groups CHX and C depended on the mothers voluntary participation in the 3‐month appointments for CHX applications". Non‐randomised study |
| Rodrigues 1999 | Split‐mouth design |
| Spets‐Happonen 1985 | Fluoride comparator |
| Spets‐Happonen 1991 | Concomitant fluoride administration |
| Splieth 2000 | Fluoride comparator |
| Zhang 2006 | Split‐mouth design |
| Zickert 1983 | Not RCT |
C: control CHX: chlorhexidine RCT: randomised controlled trial
Differences between protocol and review
Following discussions with the Editorial team at the Cochrane Oral Health Group, we agreed that we would exclude all studies using a split‐mouth design. We based this decision on the possibility that significant contamination of control sites with chlorhexidine could not be ruled out (irrespective of the adhesiveness of the material to the tooth surface in the first hours after application).
In view of the paucity of studies, we also lowered the threshold for study duration to permit inclusion of studies that were conducted over a period of less than one year, providing that administration of the intervention occurred at least once over that time period and that outcomes were measured at the end of the study period.
Contributions of authors
Tanya Walsh, Jeronimo M Oliveira‐Neto and Deborah Moore were responsible for:
organising the retrieval of papers
writing to authors of papers for additional information
screening search results
screening retrieved papers against inclusion criteria
appraising the quality of papers
data collection for the review
extracting data from papers
obtaining and screening data on unpublished studies
All review authors contributed to writing the review.
Sources of support
Internal sources
The University of Manchester, UK.
-
MAHSC, UK.
The Cochrane Oral Health Group is supported by the Manchester Academic Health Sciences Centre (MAHSC) and the NIHR Manchester Biomedical Research Centre.
External sources
-
National Institute for Health Research (NIHR), UK.
The NIHR is the largest single funder of the Cochrane Oral Health Group.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Group Global Alliance, Other.
The production of all our reviews is assisted by funding from our Global Alliance partners (http://ohg.cochrane.org/): British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and Royal College of Surgeons of Edinburgh, UK
Declarations of interest
None known. There were no financial conflicts of interest and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review.
New
References
References to studies included in this review
Baca 200X {published data only}
- Baca P, Junco P, Bravo M, Baca AP, Munoz MJ. Caries incidence in permanent first molars after discontinuation of a school‐based chlorhexidine‐thymol varnish program. Community Dentistry and Oral Epidemiology 2003;31(3):179‐83. [DOI] [PubMed] [Google Scholar]
- Baca P, Munoz MJ, Bravo M, Junco P, Baca AP. Effectiveness of chlorhexidine‐thymol varnish for caries reduction in permanent first molars of 6‐7‐year‐old children: 24‐month clinical trial. Community Dentistry and Oral Epidemiology 2002;30(5):363‐8. [DOI] [PubMed] [Google Scholar]
- Baca P, Muñoz MJ, Bravo M, Junco P, Baca AP. Effectiveness of chlorhexidine‐thymol varnish in preventing caries lesions in primary molars. Journal of Dentistry for Children 2004;71(1):61‐5. [PubMed] [Google Scholar]
Bretz 1997 {published data only}
- Bretz WA, Djahjah CA, Almeida RS, Villar do Valle E, Fonseca C, Valente I, et al. Effect of a chlorhexidine varnish on caries lesions. Oral Health 1995;85:29‐30. [PubMed] [Google Scholar]
- Bretz WA, do Valle EV, Almeida R, Djahjah C, Chen YM, Schork MA. Effects of a chlorhexidine varnish on the mutans streptococci and on dental caries. Biofilm Journal 1997;2(1):BF97002. [DOI: ] [Google Scholar]
De Soet 2002 {published data only}
- Soet JJ, Gruythuysen RJ, Bosch JA, Amerongen WE. The effect of 6‐monthly application of 40% chlorhexidine varnish on the microflora and dental caries incidence in a population of children in Surinam. Caries Research 2002;36(6):449‐55. [DOI] [PubMed] [Google Scholar]
Du 2006 {published data only}
- Du MQ, Tai BJ, Jiang H, Lo EC, Fan MW, Bian Z. A two‐year randomized clinical trial of chlorhexidine varnish on dental caries in Chinese preschool children. Journal of Dental Research 2006;85(6):557‐9. [DOI] [PubMed] [Google Scholar]
Forgie 2000 {published data only}
- Burnside G, Pine CM, Williamson PR. Modelling the bilateral symmetry of caries incidence. Caries Research 2008;42(4):291‐6. [DOI] [PubMed] [Google Scholar]
- Forgie AH, Paterson M, Pine CM, Pitts NB, Nugent ZJ. A randomised controlled trial of the caries‐preventive efficacy of a chlorhexidine‐containing varnish in high‐caries‐risk adolescents. Caries Research 2000;34(5):432‐9. [DOI] [PubMed] [Google Scholar]
Nordling 1999 {published data only (unpublished sought but not used)}
- Nordling M, Koch G, Hallonsten AL. Effect of a chlorhexidine varnish regiment on caries in children. International Journal of Paediatric Dentistry. 1999; Vol. 9:(Suppl 1):20 (Abstr 14.03) 17th Congress of the IAPD, London 2‐4 Sept 1999.
Plonka 2013 {published data only}
- Plonka KA, Pukallus ML, Holcombe TF, Barnett AG, Walsh LJ, Seow WK. Randomized controlled trial: a randomized controlled clinical trial comparing a remineralizing paste with an antibacterial gel to prevent early childhood caries. Pediatric Dentistry 2013;35(1):8E‐12. [PubMed] [Google Scholar]
Pukallus 2013 {published data only}
- Pukallus ML, Plonka KA, Barnett AG, Walsh LJ, Holcombe TF, Seow WK. A randomised, controlled clinical trial comparing chlorhexidine gel and low‐dose fluoride toothpaste to prevent early childhood caries. International Journal of Paediatric Dentistry 2013;23:216‐24. [DOI: 10.1111/j.1365-263X.2012.01248.x] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Achong 1997 {published data only}
- Achong RA, Briskie DM, Feigal RJ, Hildebrandt GH, Ignelzi MA, Loesche WJ. Effect of chlorhexidine varnish in caries active pediatric patients. Pediatric Dentistry 1997;19(2):152. [PubMed] [Google Scholar]
- Achong RA, Briskie DM, Hildebrandt GH, Feigal RJ, Loesche WJ. Effect of chlorhexidine varnish mouthguards on the levels of selected oral microorganisms in pediatric patients. Pediatric Dentistry 1999;21(3):169‐75. [PubMed] [Google Scholar]
Araujo 2002 {published data only}
- Araujo AM, Naspitz GM, Chelotti A, Cai S. Effect of Cervitec on mutans streptococci in plaque and on caries formation on occlusal fissures of erupting permanent molars. Caries Research 2002;36(5):373‐6. [DOI] [PubMed] [Google Scholar]
Axelsson 1976 {published data only}
- Axelsson P, Lindhe J, Waseby J. The effect of various plaque control measures on gingivitis and caries in schoolchildren. Community Dentistry and Oral Epidemiology 1976;4(6):232‐9. [DOI] [PubMed] [Google Scholar]
Axelsson 1987 {published data only}
- Axelsson P, Kristoffersson K, Karlsson R, Bratthall D. A 30‐month longitudinal study of the effects of some oral hygiene measures on Streptococcus mutans and approximal dental caries. Journal of Dental Research 1987;66(3):761‐5. [DOI] [PubMed] [Google Scholar]
Bratthall 1995 {published data only}
- Bratthall D, Serinirach R, Rapisuwon S, Kuratana M, Luangjarmekorn V, Luksila K, et al. A study into the prevention of fissure caries using an antimicrobial varnish. International Dental Journal 1995;45(4):245‐54. [PubMed] [Google Scholar]
Dolles 1980 {published data only}
- Dolles OK, Gjermo P. Caries increment and gingival status during 2 years' use of chlorhexidine‐ and fluoride‐containing dentifrices. Scandinavian Journal of Dental Research 1980;88(1):22‐7. [DOI] [PubMed] [Google Scholar]
Emilson 1982 {published data only}
- Emilson CG, Axelsson P, Kallenberg L. Effect of mechanical and chemical plaque control measures on oral microflora in schoolchildren. Community Dentistry and Oral Epidemiology 1982;10(3):111‐6. [DOI] [PubMed] [Google Scholar]
Ersin 2006 {published data only}
- Ersin NK, Oncag O. A comparison of the effectiveness of chlorhexidine varnish, fluoride varnish and fluoride gel in children with intellectual disability. Journal of Disability and Oral Health 2006;7:10‐16. [Google Scholar]
Ersin 2008 {published data only}
- Ersin NK, Eden E, Eronat N, Totu FI, Ates M. Effectiveness of 2‐year application of school‐based chlorhexidine varnish, sodium fluoride gel, and dental health education programs in high‐risk adolescents. Quintessence International 2008;39(2):e45‐51. [PubMed] [Google Scholar]
Fennis‐le 1998 {published data only}
- Fennis‐le YL, Verdonschot EH, Burgersdijk RC, Konig KG, 't Hof MA. Effect of 6‐monthly applications of chlorhexidine varnish on incidence of occlusal caries in permanent molars: a 3‐year study. Journal of Dentistry 1998;26(3):233‐8. [DOI] [PubMed] [Google Scholar]
Gisselsson 1988 {published data only}
- Gisselsson H, Birkhed D, Bjorn AL. Effect of professional flossing with chlorhexidine gel on approximal caries in 12‐ to 15‐year‐old schoolchildren. Caries Research 1988;22:187‐92. [DOI] [PubMed] [Google Scholar]
Gisselsson 1994 {published data only}
- Gisselsson H, Birkhed D, Bjorn AL. Effect of a 3‐year professional flossing program with chlorhexidine gel on approximal caries and cost of treatment in preschool children. Caries Research 1994;28(5):394‐9. [DOI] [PubMed] [Google Scholar]
Gisselsson 2005 {published data only}
- Gisselsson H, Emilson CG, Birkhed D, Bjorn AL. Approximal caries increment in two cohorts of schoolchildren after discontinuation of a professional flossing program with chlorhexidine gel. Caries Research 2005;39(5):350‐6. [DOI] [PubMed] [Google Scholar]
Gokalp 2005 {published data only}
- Gokalp S, Baseren M. Use of laser fluorescence in monitoring the durability and cariostatic effects of fluoride and chlorhexidine varnishes on occlusal caries: a clinical study. Quintessence International 2005;36:183‐9. [PubMed] [Google Scholar]
Haukali 2003 {published data only}
- Haukali G, Poulsen S. Effect of a varnish containing chlorhexidine and thymol (Cervitec) on approximal caries in 13‐ to 16‐year‐old schoolchildren in a low caries area. Caries Research 2003;37(3):185‐9. [DOI] [PubMed] [Google Scholar]
Hausen 2000 {published data only}
- Hausen H, Karkkainen S, Seppa L. Application of the high‐risk strategy to control dental caries. Community Dentistry and Oral Epidemiology 2000;28(1):26‐34. [DOI] [PubMed] [Google Scholar]
Holst 2001 {published data only}
- Holst A, Birkhed D, Jonasson B, Staaf G. Chlorhexidine gel, fluoride varnish and combination in caries active adolescents. Journal of Dental Research 2001, issue 4:1298 (Abs 230).
Hoszek 1996 {published data only}
- Hoszek A, Wretlind K, Ericson D. Chlorhexidine‐containing glass ionomer cement and fissure caries in Polish children, preliminary results. Swedish Dental Journal 1996;20(6):242 (Abstract 33rd annual congress of the Swedish Dental Society 14‐16 November 1996). [Google Scholar]
Hoszek 2005 {published data only}
- Hoszek A, Struzycka I, Jozefowicz A, Wojcieszek D, Wierzbicka M, Wretlind K, et al. Chlorhexidine‐containing glass ionomer cement. A clinical investigation on the fissure caries inhibiting effect in first permanent molars. Swedish Dental Journal 2005;29(3):89‐96. [PubMed] [Google Scholar]
Irmisch 2000 {published data only}
- Irmisch B, Perkuhn B. Prevention of fissure caries in primary dentition ‐ effect of 3‐monthly application of Cervitec: A 2‐year clinical study. Caries Research 2000;34(4):351 Abst 125. [Google Scholar]
Joharji 2001 {published data only}
- Joharji RA, Adenubi JO. Prevention of pit and fissure caries using an antimicrobial varnish ‐ Cervitec. Journal of Dental Research 2000;79: (Divisional Abstracts Issue):5 (Saudi Arabian Division) 1280 (Abs No 34). [Google Scholar]
- Joharji RA, Adenubi JO. Prevention of pit and fissure caries using an antimicrobial varnish: 9 month clinical evaluation. Journal of Dentistry 2001;29(4):247‐54. [DOI] [PubMed] [Google Scholar]
Leksell 2003 {published data only}
- Leksell E. Evaluation of three caries preventive methods: a 3‐year radiological study in Swedish urban school children with high caries risk. International Journal of Paediatric Dentistry 2003;13(Suppl 1):49. [Google Scholar]
Lindquist 2010 {published data only}
- Lindquist B, Gisselsson H, Wennerholm K. Effect of chlorhexidine gel on approximal caries increment in adolescents with high caries risk using professional flossing compared to individual trays. Swedish Dental Journal 2010;34(1):17‐25. [PubMed] [Google Scholar]
Neeraja 2008 {published data only}
- Neeraja R, Anantharaj A, Praveen P, Karthik V, Vinitha M. The effect of povidone‐iodine and chlorhexidine mouth rinses on plaque Streptococcus mutans count in 6‐ to 12‐year‐old school children: An in vivo study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2008;26(5):14‐8. [PubMed] [Google Scholar]
Petersson 1997 {published data only}
- Petersson L, Twetman S. Caries preventive effect of fluoride and chlorhexidine varnishes. Journal of Dental Research 1997;76:(Special Abstract Issue 1) 134 (Abs No 963). [Google Scholar]
Petti 2006 {published data only}
- Petti S, Hausen H. Caries‐preventive effect of chlorhexidine gel applications among high‐risk children. Caries Research 2006;40(6):514‐21. [DOI] [PubMed] [Google Scholar]
Plotzitza 2005 {published data only}
- Plotzitza B, Kneist S, Berger J, Hetzer G. Efficacy of chlorhexidine varnish applications in the prevention of early childhood caries. European Journal of Paediatric Dentistry 2005;6(3):149‐54. [PubMed] [Google Scholar]
Rodrigues 1999 {published data only}
- Rodrigues CR, Kabakura V, Miyamura A, Myaki S, Grande RH. Efficacy of cervitec on occlusal caries inhibition. Journal of Dental Research 1999;78(5):1002. [Google Scholar]
Spets‐Happonen 1985 {published data only}
- Spets‐Happonen S, Markkanen H, Pollanen L, Kauppinen T, Luoma H. Salivary Streptococcus mutans count and gingivitis in children after rinsing with a chlorhexidine‐fluoride solution with and without strontium. European Journal of Oral Sciences 1985;93(4):329‐35. [DOI] [PubMed] [Google Scholar]
Spets‐Happonen 1991 {published data only}
- Spets‐Happonen S, Luoma H, Forss H, Kentala J, Alaluusua S, Luoma AR, et al. Effects of a chlorhexidine‐fluoride‐strontium rinsing program on caries, gingivitis and some salivary bacteria among Finnish schoolchildren. Scandinavian Journal of Dental Research 1991;99(2):130‐8. [DOI] [PubMed] [Google Scholar]
Splieth 2000 {published data only}
- Splieth C, Steffen H, Rosin M, Welk A. Caries prevention with chlorhexidine‐thymol varnish in high risk schoolchildren. Community Dentistry and Oral Epidemiology 2000;28(6):419‐23. [DOI] [PubMed] [Google Scholar]
Zhang 2006 {published data only}
- Zhang Q, 't Hof MA, Truin GJ, Bronkhorst EM, Palenstein Helderman WH. Caries‐inhibiting effect of chlorhexidine varnish in pits and fissures. Journal of Dental Research 2006;85(5):469‐72. [DOI] [PubMed] [Google Scholar]
Zickert 1983 {published data only}
- Zickert I, Emilson CG, Krasse B. Correlation of level and duration of Streptococcus mutans infection with incidence of dental caries. Infection and Immunity 1983;39(2):982‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAPD 2013
- American Academy of Pediatric Dentistry. Caries‐risk assessment and management for infants, children and adolescents. American Academy of Pediatric Dentistry 2013.
Ahovuo‐Saloranta 2013
- Ahovuo‐Saloranta A, Helena Forss H, Walsh T, Hiiri A, Nordblad A, Mäkelä M, et al. Sealants for preventing dental decay in the permanent teeth. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD001830.pub4] [DOI] [PubMed] [Google Scholar]
Anderson 2003
- Anderson MH. A review of the efficacy of chlorhexidine on dental caries and the caries infection. Journal of the California Dental Association 2003;31(3):211‐4. [PubMed] [Google Scholar]
Autio‐Gold 2008
- Autio‐Gold J. The role of chlorhexidine in caries prevention. Operative Dentistry 2008;33(6):710‐6. [DOI] [PubMed] [Google Scholar]
BNF 2015
- British Medical Association and Royal Pharmaceutical Society. British National Formulary. Vol. Jan 2015, London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society, 2015. [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research questions. BMJ 2006;333(7572):804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caufield 2001
- Caufield PW, Dasanayake AP, Li Y. The antimicrobial approach to caries management. Journal of Dental Education 2001;65(10):1091‐5. [PubMed] [Google Scholar]
Chadwick 2001
- Chadwick BL, Dummer PMH, Dunstan F, Gilmour ASM, Jones RJ, Phillips CJ, et al. The longevity of dental restorations. A systematic review. NHS Centre for Reviews and Dissemination, University of York; 2001 Report No.: 19.
DBOH 2014
- Public Health England. Delivering better oral health: an evidence‐based toolkit for prevention. Public Health England 2014.
EAPD 2008
- European Academy of Paediatric Dentistry. Guidelines on prevention of early childhood caries. European Academy of Paediatric Dentistry 2008.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emilson 1994
- Emilson CG. Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. Journal of Dental Research 1994;73(3):682‐91. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(1490):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermida 2012
- Hermida ML, Alvarez L, Fabruccini A, Nor JE, Bretz. Effect of chlorhexidine vehicle in caries prevention in schoolchildren. Proceedings of the General Session of the International Association for Dental Research. International Association for Dental Research, 2012:Abstract no: 1146.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated September 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
James 2010
- James P, Parnell C, Whelton H. The caries‐preventive effect of chlorhexidine varnish in children and adolescents: a systematic review.. Caries Research 2010;44:333–40. [DOI] [PubMed] [Google Scholar]
Järvinen 1993
- Järvinen H, Tenovuo J, Huovinen P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrobial Agents and Chemotherapy 1993;37(5):1158‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2014
- Krishna MT, York M, Chin T, Gnanakumaran G, Heslegrave J, Derbridge C, et al. Multi‐centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: aetiology and diagnostic performance of acute serum tryptase. Clinical and Experimental Immunology 2014;178(2):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luoma 1992
- Luoma H. Chlorhexidine solutions, gels and varnishes in caries prevention. Proceedings of the Finnish Dental Society 1992;88(3‐4):147‐53. [PubMed] [Google Scholar]
Löe 1972
- Löe H, Fehr FR, Schiött CR. Inhibition of experimental caries by plaque prevention. The effect of chlorhexidine mouthrinses. Scandinavian Journal of Dental Research 1972;80:1‐9. [DOI] [PubMed] [Google Scholar]
Madlena 2000
- Madlena M, Vitalyos G, Marton S, Nagy G. Effect of chlorhexidine varnish on bacterial levels in plaque and saliva during orthodontic treatment. Journal of Clinical Dentistry 2000;11(2):42‐6. [PubMed] [Google Scholar]
Marinho 2004
- Marinho VC, Higgins JP, Logan S, Sheiham A. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002780.pub2] [DOI] [Google Scholar]
Marsh 2006
- Marsh PD. Dental plaque as a biofilm and a microbial community ‐ implications for health and disease. BMC Oral Health 2006;6 Suppl 1:S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthijs 2002
- Matthijs S, Adriaens PA. Chlorhexidine varnishes: a review. Journal of Clinical Periodontology 2002;29(1):1‐8. [DOI] [PubMed] [Google Scholar]
Moynihan 2014
- Moynihan PJ, Kelly SAM. Effect on caries of restricting sugars intake: systematic review to inform WHO guidelines. Journal of dental research 2014;93(1):8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratnayake 2005
- Ratnayake N, Ekanayake L. Prevalence and impact of oral pain in 8‐year‐old children in Sri Lanka. International Journal of Paediatric Dentistry 2005;15(2):105‐12. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribeiro 2007
- Ribeiro LGM, Hashizume LN, Maltz M. The effect of different formulations of chlorhexidine in reducing levels of mutans streptococci in the oral cavity: a systematic review of the literature. Journal of Dentistry 2007;35(5):359‐70. [DOI] [PubMed] [Google Scholar]
Rodrigues 2008
- Rodrigues CR, Marquezan M, Barroso LP, Grande RHM, Myaki SI, Kabakura V, et al. Effect of chlorhexidine‐thymol varnish on caries lesion development in first permanent molars. Journal of Clinical Dentistry 2008;19(1):18‐21. [PubMed] [Google Scholar]
SCDEP 2014
- Scottish Dental Clinical Effectiveness Program. Prevention and management of dental caries in children. http://www.sdcep.org.uk/index.aspx?o=2858 2014 (Accessed 16 March 2015).
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. The Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Sheiham 2005
- Sheiham A. Oral health, general health and quality of life. Bulletin of the World Health Organization 2005;83(9):644. [PMC free article] [PubMed] [Google Scholar]
Sheiham 2006
- Sheiham A. Dental caries affects body weight, growth and quality of life in pre‐school children. British Dental Journal 2006;201(10):625‐6. [DOI] [PubMed] [Google Scholar]
Shepherd 1999
- Shepherd MA, Nadanovsky P, Sheiham A. The prevalence and impact of dental pain in 8‐year‐old school children in Harrow, England. British Dental Journal 1999;187(1):38‐41. [DOI] [PubMed] [Google Scholar]
Sundell 2013
- Sundell AL, Ullbro C, Koch G. Evaluation of preventive programs in high caries active preschool children. Swedish Dental Journal 2013;37(1):23‐9. [PubMed] [Google Scholar]
Twetman 1998
- Twetman S, Petersson LG. Comparison of the efficacy of three different chlorhexidine preparations in decreasing the levels of mutans streptococci in saliva and interdental plaque. Caries Research 1998;32(2):113‐8. [DOI] [PubMed] [Google Scholar]
Twetman 2004
- Twetman S. Antimicrobials in future caries control? A review with special reference to chlorhexidine treatment. Caries Research 2004;38(3):223‐9. [DOI] [PubMed] [Google Scholar]
Yee 2002
- Yee R, Sheiham A. The burden of restorative dental treatment for children in Third World countries. International Dental Journal 2002;52(1):1‐9. [PubMed] [Google Scholar]
Zhang 2006a
- Zhang Q, Palenstein Helderman WH, van't Hof MA, Truin GJ. Chlorhexidine varnish for preventing dental caries in children, adolescents and young adults: a systematic review. European Journal of Oral Sciences 2006;114(6):449‐55. [DOI] [PubMed] [Google Scholar]
Zhang 2007
- Zhang Q, Mulder J, Truin GJ, Palenstein Helderman WH. Effect of 40% chlorhexidine varnish on mutans streptococci counts in pits and fissures of permanent first molars. Journal of Dentistry 2007;35(7):588‐92. [DOI] [PubMed] [Google Scholar]


