Abstract
Broad-spectrum antimicrobials are needed to effectively treat patients infected in the event of a pandemic or intentional release of a pathogen prior to confirmation of the pathogen's identity. Engineered cationic antimicrobial peptides (eCAPs) display activity against a number of bacterial pathogens including multi-drug-resistant strains. Two lead eCAPs, WLBU2 and WR12, were compared with human cathelicidin (LL-37) against three highly pathogenic bacteria: Francisella tularensis, Yersinia pestis and Burkholderia pseudomallei. Both WLBU2 and WR12 demonstrated bactericidal activity greater than that of LL-37, particularly against F. tularensis and Y. pestis. Only WLBU2 had bactericidal activity against B. pseudomallei. WLBU2, WR12 and LL-37 were all able to inhibit the growth of the three bacteria in vitro. Because these bacteria can be facultative intracellular pathogens, preferentially infecting macrophages and dendritic cells, we evaluated the activity of WLBU2 against F. tularensis in an ex vivo infection model with J774 cells, a mouse macrophage cell line. In that model WLBU2 was able to achieve greater than 50 % killing of F. tularensis at a concentration of 12.5 μM. These data show the therapeutic potential of eCAPs, particularly WLBU2, as a broad-spectrum antimicrobial for treating highly pathogenic bacterial infections.
Abbreviations:
CAP, cationic antimicrobial peptide; MDR, multi-drug resistant; LPS, lipopolysaccharide..
Introduction
Biodefence agents are pathogenic organisms or toxins that pose a severe threat to public health and can be used against humans, animals or plants for terrorist purposes. These pathogens include the Gram-negative bacteria Francisella tularensis, Burkholderia pseudomallei and Yersinia pestis. Due to their ability to cause fatal disease when inhaled, the use or even threatened use of such organisms can produce widespread social disruption. Many of the viruses and bacteria considered to be potential biological weapons have very similar initial prodromes with ‘influenza-like’ symptoms. A delay in diagnosis can be a critical determinant in whether the patient will survive the infection. While diagnostics are an area of intense focus, there would currently be a considerable delay before initiation of treatment. A priority area of research is the development of broad-spectrum countermeasures that could be utilized prior to confirmation of the diagnosis to rapidly reduce pathogen load and improve the odds of saving the patient.
Cationic antimicrobial peptides (CAPs) are amphipathic peptides of 12–50 aa with a net positive charge. They are ubiquitous peptides naturally found in all living species and are known to be active components of the innate immunity against infectious pathogens, including viruses and bacteria (Hancock & Chapple, 1999). In response to infectious pathogens, CAPs can be released from macrophages, neutrophil granules, or in secretions from epithelial cells found in the skin or mucosal tissues. CAPs can act against a wide range of targets including Gram-positive and Gram-negative bacteria, fungi and parasites; the essential component in these diverse targets is a negatively charged plasma membrane (Boman, 1995). Therefore, normal eukaryotic cells have a relatively high resistance due to the preference of CAPs for negatively charged membranes. The mechanism by which CAPs function is not fully understood, although studies have implicated the electrostatic interaction between the peptides and the lipid molecules on the bacterial membrane. When compared with other antibiotics, CAPs are able to kill bacteria rapidly, within 30 to 180 s, limiting the bacterium's ability to develop resistance against these peptides (Ntwasa, 2012). Therefore, CAPs are considered a good candidate for use against multi-drug resistant (MDR) bacteria.
Although natural CAPs have a wide spectrum of action against pathogens, their activity strongly depends on the environment in which they are acting. The optimal potency of each peptide is only achieved in specific environmental conditions and readily lost when subjected to a different setting (Goldman et al., 1997). Therefore, to be able to utilize their antimicrobial activity to the fullest, we have designed de novo-synthesized engineered CAPs.
Studies of the lentivirus lytic peptide 1 (LLP1) domain of HIV-1 gp41 demonstrated that it had remarkable antimicrobial properties (Miller et al., 1993). That work demonstrated that an α-helical amphipathic CAP, using arginine residues on the polar side and valine residues on the non-polar side, greatly improved the potency and selectivity of LLP1. Further studies of the influence of length and helical structure on activity led to development of a series of rationally engineered peptides designated WLBU (Tencza et al., 1999). WLBU peptides are composed of arginine in the hydrophilic face and valine and tryptophan residues in the hydrophobic face arranged to form idealized amphipathic helices. Moreover, WLBU studies demonstrated that tryptophan substitution significantly increased antimicrobial activity in environments such as serum and blood. Detailed studies demonstrated that WLBU2, a 24-mer, had the minimum length required for optimal activity against a wide range of bacteria including methicillin-resistant Staphylococcus aureus (MRSA), and MDR strains of Klebsiella pneumoniae and Acinetobacter baumannii.
To further minimize length in optimally active eCAPs, a second series of peptides (the WR series) were developed with only arginine in the hydrophilic face and tryptophan in the hydrophobic face (Deslouches et al., 2005b). In vitro studies demonstrated that WR12, consisting of only 12 amino acids, had better activity against bacteria than longer peptides in the WR series and at least comparable to WLBU2 (Deslouches et al., 2005a). These data suggested that WLBU2 and WR12 could serve as a broad-spectrum countermeasure against a wide range of bacteria including those that represent potential biological weapons.
In this study we compared the potency of WLBU2 and WR12 with that of the natural peptide LL37 using in vitro and ex vivo evaluations against F. tularensis, Y. pestis and B. pseudomallei. The results demonstrated that de novo synthesized peptides outperformed LL37 against all three pathogens.
Methods
Biosafety
All experiments using F. tularensis, B. pseudomallei or Y. pestis were performed in the biosafety level 3+ Regional Biocontainment Laboratory (RBL) at the University of Pittsburgh. Powered air purifying respirators were worn for respiratory protection, and all work was conducted in a class II biosafety cabinet using Vesphene IIse (diluted 1 : 128; Steris) as a disinfectant. All three bacteria are tier 1 select agents and required Department of Justice approval, an approved security risk assessment with the Centers for Disease Control's Division of Select Agents & Toxins, and a Tier 1 suitability assessment for access to the pathogens.
Bacteria
All of the bacteria used in these studies were obtained from the Biodefence and Emerging Infections Research Resources Repository, Manassas, VA, USA. All bacteria were passaged a single time in culture and frozen at − 80 °C prior to use in these studies. For all three bacteria, a similar process was followed for preparation of the bacteria to use in these experiments. Bacteria were grown first on solid media prior to an overnight culture in 25 ml liquid broth media using a baffled, vented 125 ml polycarbonate Erlenmeyer flask incubated in a bacterial shaker set at 200 r.p.m. Francisella tularensis SchuS4 strain was grown on cysteine heart agar (CHA Becton Dickinson, La Jolla, CA) for 2 days at 37 °C prior to culture in brain heart infusion broth (BHI; Becton Dickinson, La Jolla, CA) for 17–19 h at 37 °C (Faith et al., 2012). Burkholderia pseudomallei strain 1026b was grown on Luria–Bertani (LB; Becton Dickinson, La Jolla, CA) Agar for 1 day at 37 °C and then cultured overnight in BHI for 11 h at 37 °C. Yersinia pestis strain CO92 was grown on tryptic soy agar (TSA; Becton Dickinson, La Jolla, CA) for 2 days at 30 °C and then cultured in heart infusion broth (Becton Dickinson, La Jolla, CA) for 24–27 h at 37 °C.
Peptides
Peptides were synthesized using standard Fmoc (9-fluorenylmethoxycarbonyl chloride) synthesis protocols (Genscript). Synthetic peptides were characterized and purified by reverse-phase HPLC on Vydac C18 or C4 columns (The Separations Group). The identification of each was established by mass spectrometry (Electrospray Quatro II triple quadruple mass spectrometer; Micromass). Peptide concentrations were determined using a quantitative ninhydrin assay. Details of the peptides and their sequences are in Deslouches et al., 2005b.
Bacterial killing assay
Bacteria cultured overnight were diluted to a concentration of 1 × 106 c.f.u. ml− 1 in phosphate buffer saline or potassium phosphate buffer and 100 μl was placed per well in the first column of a 96-well plate. Twofold dilutions of peptides (concentration of 1.5 μM to 100 μM) were added to the bacteria, and the plate was incubated for 60 min at 37 °C. Serial dilutions were plated on agar plates to determine surviving bacteria. Colonies were counted following an appropriate incubation time (24 h for B. pseudomallei; 48–72 h for F. tularensis and Y. pestis) to determine the reduction in bacterial count.
Growth inhibition assay
Bacterial suspensions (concentration of 1 × 106 c.f.u. ml− 1) in growth media were incubated with twofold dilutions of peptides (concentration of 1.5 μM to 100 μM) in a 96-well plate for 24 to 48 h at 37 °C. After incubation, the optical density of each well was measured at 600 nm using a spectrophotometer (FluoStar Omega, BMG LabTech). Per cent inhibition was calculated from the difference in optical density measured.
Ex vivo infection assay
For ex vivo infection with F. tularensis, a standardized assay using a mouse macrophage cell line (J774) was used. J774 cells were seeded in Dulbecco's Modification of Eagle's Medium (DMEM; Cellgro) supplemented with 10 % fetal bovine serum at 37 °C on 12-well cell culture plates at a density of 3 × 107 cells per well. Seeded plates were incubated overnight at 37 °C, 5 % CO2, 95 % humidity. Cells were infected with F. tularensis SCHU S4 at an m.o.i. of 1 : 100 and incubated further for 2 h. Cells were washed three times with 2 ml PBS containing 50 μg gentamicin ml− 1 followed by addition of 1 ml medium with 50 μg gentamicin ml− 1 to each well and incubated for 1 h. Subsequently, cells were washed again three times with PBS and different concentrations of WLBU2 (3 μM to 25 μM) were added to each well and incubated for another 1 h. J774 cells were then lysed using 0.02 % SDS and 100 μl of the lysate was further diluted in PBS and plated on CHA. Colonies were counted to determine bacterial count reduction with WLBU2.
Statistical analysis
Analysis was performed using percentages. Determination of significant differences in bacterial killing or inhibition between different peptides was conducted using a two-way ANOVA in Graphpad Prism 6. A P ≤ 0.001 was considered to be statistically significant.
Results
Direct killing of F. tularensis, Y. pestis and B. pseudomallei by eCAPs
As an initial evaluation of the test peptides, we evaluated the direct bactericidal activity of the engineered peptides against the target bacteria, as compared with the natural LL37 peptide. Both WLBU2 and WR12 outperformed LL37 against F. tularensis in bacterial killing (Fig. 1a). WLBU2 killed 50 % of F. tularensis at ∼2 μM while WR12 required 50 μM to achieve the same effect; LL37 only achieved 2.5 % killing of F. tularensis at 100 μM. Two-way ANOVA revealed statistically significant differences between WLBU2 and LL37 at concentrations ≥ 1.5 μM and between WR12 and LL37 at concentrations ≥ 25 μM (P < 0.0001). WLBU2 had significantly better antimicrobial activity against F. tularensis than WR12 at concentrations ≤ 50 μM (P < 0.0001). At 25 μM, WLBU2 achieved 98 % killing compared with only 31 % killing for WR12 and less than 1 % killing for LL37 (Table 1).
Fig. 1.
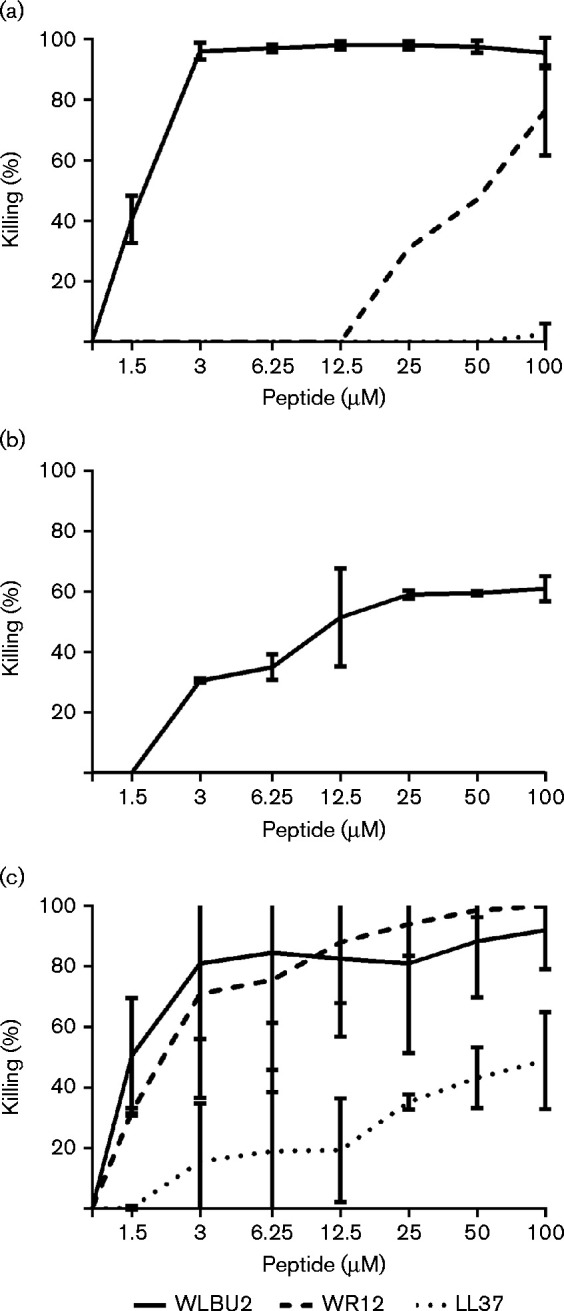
Dose-dependent killing of F. tularensis, B. pseudomallei and Y. pestis by WLBU2, WR12 and LL37. Bacterial cultures (1–2 × 106 c.f.u. ml− 1) were incubated for 1 h with twofold dilutions of the peptides diluted in PBS. Graphs show percentage reduction in bacterial count (y-axis) upon treatment plotted as a function of peptide concentration (x-axis) for F. tularensis (a), B. pseudomallei (b) and Y. pestis (c). The data shown are the mean of three independent experimental trials; error bars are the sd.
Table 1.
Bactericidal activity of peptides at 25 μM
| Bacterium | LL37 | WLBU2 | WR12 |
|---|---|---|---|
| F. tularensis | 0* | 98 | 31 |
| B. pseudomallei | 0 | 59 | 0 |
| Y. pestis | 35 | 81 | 94 |
Mean per cent killing compared with control cultures, n = 3 repetitions.
Initial results suggested that none of the three antimicrobial peptides tested was able to kill B. pseudomallei (data not shown). Another group has reported killing of B. pseudomallei with LL37 in the presence of potassium phosphate buffer rather than PBS (Kanthawong et al., 2009, 2012). Even with potassium phosphate buffer, however, we did not observe reduced bacterial counts of B. pseudomallei with either LL37 or WR12, while WLBU2 achieved at best 61 % killing at 100 μM (Fig. 1b). Statistical analysis indicated that the antimicrobial activity for WLBU2 against B. pseudomallei was significant compared with both WR12 and LL37. At 25 μM, WLBU2 achieved 59 % killing, while with WR12 and LL37 no detectable killing of B. pseudomallei was observed (Table 1).
Both WLBU2 and WR12 were significantly more effective at killing Y. pestis than was LL37. WLBU2 and WR12 produced a 50 % reduction in bacterial count at a peptide concentration of 1.5–2 μM, while LL37 required 100 μM to achieve 50 % killing (Fig. 1c). Statistical analysis revealed a significant difference between WLBU2 and LL37 bactericidal activity at concentrations ≥ 3 μM and between WR12 and LL37 at concentrations ≥ 1.5 μM (P < 0.0001). No significant difference between WLBU2 and WR12 was found against Y. pestis. At 25 μM, WLBU2 achieved 81 % killing, while WR12 achieved 94 % killing; in contrast, LL37 achieved only 35 % killing against Y. pestis at 25 μM (Table 1).
Growth inhibition of F. tularensis, Y. pestis and B. pseudomallei by eCAPs
While the preceding assays evaluated the direct killing activity of the test peptides in PBS, we next evaluated the ability of each peptide to inhibit bacterial growth in nutrient media. WLBU2 displayed the highest level of growth inhibition activity against F. tularensis with >90 % inhibition at 25 μM (Fig. 2a). WR12 had moderate inhibitory activity at concentrations ≥ 12.5 μM, but only achieved 80 % inhibition at the highest concentration tested (100 μM). LL37 was only slightly less capable than WLBU2 at inhibiting the growth of F. tularensis. By two-way ANOVA the results for WLBU2 and LL37 were not significantly different from one another. Both LL37 and WLBU2 were significantly better than WR12 at inhibiting the growth of F. tularensis at concentrations greater than 1.5 μM. At 25 μM, WLBU2 inhibited growth of F. tularensis by 92 % while LL37 displayed 87 % inhibition and WR12 achieved 65 % inhibition (Table 2).
Fig. 2.
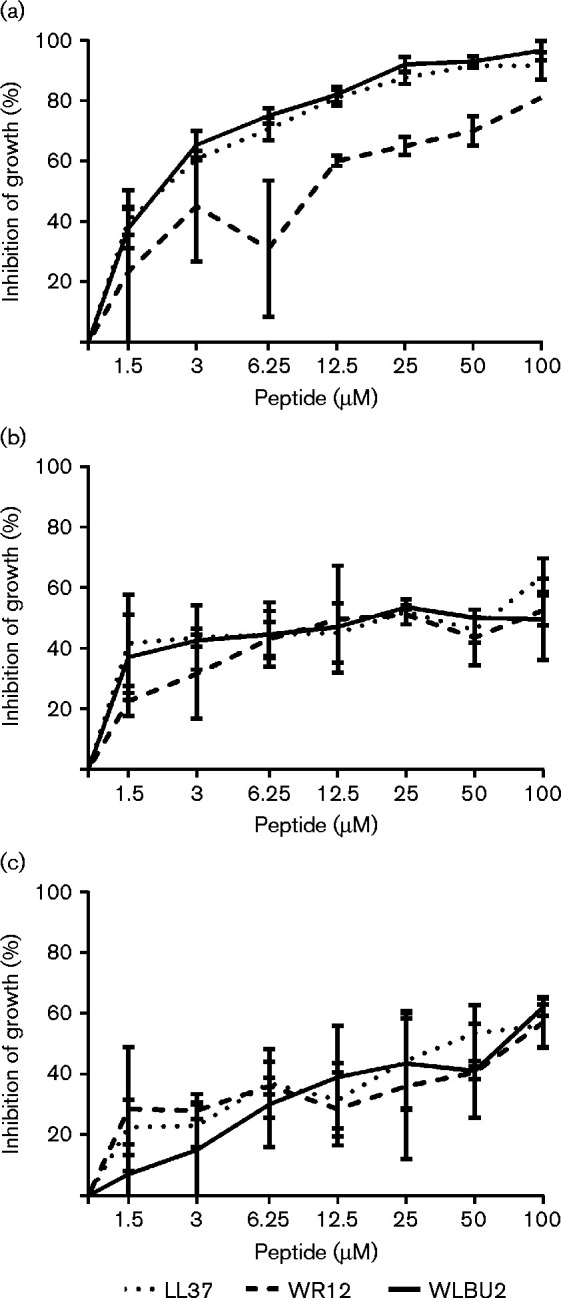
Growth inhibition of F. tularensis, Y. pestis and B. pseudomallei by WLBU2, WR12 and LL37. Bacterial cultures (1–2 × 106 c.f.u. ml− 1) were cultured overnight in BHI with twofold dilutions of the peptide. Graphs show percentage inhibition in bacterial growth (y-axis) upon treatment plotted as a function of peptide concentration (x-axis) for F. tularensis (a), B. pseudomallei (b) and Y. pestis (c). The data shown are the mean of three independent experimental trials; error bars are the sd.
Table 2.
Growth inhibition by peptides at 25 μM
| Bacterium | LL37 | WLBU2 | WR12 |
|---|---|---|---|
| F. tularensis | 87* | 92 | 65 |
| B. pseudomallei | 52 | 54 | 51 |
| Y. pestis | 45 | 44 | 36 |
Averaged result, per cent inhibition compared with control cultures, n = 3 repetitions.
With B. pseudomallei, both WLBU2 and LL37 were able to achieve ∼40 % growth inhibition at 1.5 μM; growth inhibition by WR12 was lower at 1.5 μM but not significantly. Growth inhibition largely remained flat at concentrations >1.5 μM, achieving between 50 and 60 % inhibition at 100 μM, the highest concentration tested (Fig. 2b). At 25 μM, WLBU2 inhibited growth of B. pseudomallei by 54 % while LL37 produced 52 % inhibition and WR12 achieved 51 % inhibition (Table 2). Statistical analysis indicated no significant difference in activity between the three peptides at any of the peptide concentrations tested.
Similar to the B. pseudomallei results, all three peptides were able to inhibit the growth of Y. pestis, but only inhibited growth 50–60 % at the highest concentration tested (Fig. 2c). At 25 μM, WLBU2 inhibited growth of Y. pestis by 44 % while LL37 produced 45 % inhibition and WR12 achieved 36 % inhibition (Table 2). There were no significant differences between the three peptides for inhibition of Y. pestis at any of the peptide concentrations tested.
WLBU2 can kill F. tularensis inside infected macrophages
In vitro tests proved that of the three peptides, WLBU2 had the highest level of antimicrobial activity against F. tularensis. It is well established that F. tularensis is a facultative intracellular bacterium that can infect a wide variety of cell types, although it is thought to preferentially infect macrophages and dendritic cells. It was therefore important to demonstrate whether antimicrobial peptides can kill F. tularensis inside infected eukaryotic cells. To determine the intracellular antimicrobial effect of WLBU2, we used a well-established ex vivo infection model wherein a mouse macrophage cell line (J774A cells) is infected with SCHU S4. SCHU S4 readily infects J774A cells and replicates in the cytosol over 24–72 h in the presence of gentamicin that kills any extracellular bacteria.
To determine the intracellular antimicrobial effect of WLBU2, ex vivo bacterial clearance of SCHU S4 from infected macrophage cells (J774A.1) was assessed using concentrations ranging from 3 μM to 25 μM. Fig. 3 illustrates the dose-dependent killing of intracellular F. tularensis by WLBU2. WLBU2 demonstrated a high antimicrobial efficacy against intracellular bacteria with only 20 % bacteria remaining after 1 h of incubation with 12.5 μM and 10 % at 25 μM. Thus, these data demonstrate the ability of WLBU2 to efficiently penetrate the mammalian cell membrane and effectively inactivate intracellular bacteria.
Fig. 3.
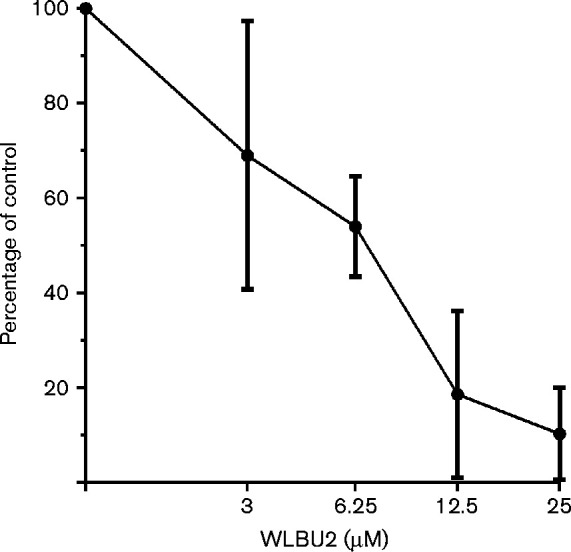
WLBU2 kills F. tularensis inside infected macrophages. F. tularensis-infected J774A cells (m.o.i. 1 : 100) were incubated for 1 h with twofold dilutions of WLBU2. The percentage of bacteria remaining (y-axis) upon treatment is plotted as a function of peptide concentration (x-axis). The data shown are the mean of three independent experimental trials; error bars are the sd.
Discussion
The results of the current study reveal for the first time to our knowledge the markedly increased efficacy of eCAPs against key biodefence bacterial pathogens compared with a reference natural antimicrobial peptide, LL37. The two eCAPs demonstrated superior bactericidal activity against F. tularensis and Y. pestis when compared in parallel with a natural antimicrobial peptide (LL37), thus demonstrating the improvements derived from the rational design of peptide structure. WLBU2 proved to be the best candidate against F. tularensis with a single dose of 3 μM peptide able to achieve a 90 % reduction in bacterial count and >90 % killing at 25 μM. Both WLBU2 and WR12 achieved similar results against Y. pestis, although only WR12 at 50 μM was able to attain >90 % killing. B. pseudomallei provided a greater challenge with only moderate susceptibility to WLBU2. Nevertheless, WLBU2 antimicrobial effectiveness against B. pseudomallei surpassed that of LL37 in our hands. Finally, WLBU2 achieved 80–90 % activity against intracellular F. tularensis in an ex vivo infection model. These data demonstrate the utility of eCAPs against these three bacteria, all of which cause endemic infectious diseases and are considered potential bioweapon threats.
The generally poor activity of WLBU2, WR12 and LL37 against B. pseudomallei was not expected. Results published by another group have demonstrated bactericidal activity for LL37 and derivatives against B. pseudomallei (Kanthawong et al., 2009, 2012). One potential difference was the choice of buffer for the bactericidal activity as we typically employ PBS, while the prior results with LL37 used potassium phosphate buffer lacking NaCl that can suppress CAP antibacterial activity of CAPs. Using potassium phosphate buffer in our assay did result in increased killing with WLBU2, but did not improve LL37 or WR12 killing. We did note that we used a higher starting concentration of bacteria (106 c.f.u. ml− 1) compared with the 105 c.f.u. ml− 1 used in the previously published study (Kanthawong et al., 2009, 2012). The strains of B. pseudomallei and choice of culture medium were also different, which may contribute to the different results obtained in the two studies. It has been noted with F. tularensis and B. pseudomallei that culture conditions alter gene and protein expression, which can impact virulence and aerosolization (Chantratita et al., 2007; Hazlett et al., 2008; Faith et al., 2012).
The mechanism of action of eCAPs is still under investigation, but the general assumption is that their antimicrobial action stems from their ability to attach and disrupt the outer bacterial membrane leading to depolarization, metabolite leakage, and ultimately death of the bacteria (Brogden, 2005). The variability of surface-exposed structures on the outer membrane and capsule of the three pathogens in this study may explain the difference in the antimicrobial activity of the eCAPs. Several studies have verified the influence of certain antigens found on the surface of Y. pestis and F. tularensis in increasing their susceptibility to certain CAPs. For example, Galván et al. (2008) demonstrated that the susceptibility of Y. pestis to CAPs such as cathelicidin (LL37) is mediated by the capsular antigen fraction 1. Also, Vonkavaara et al. (2013) illustrated the importance of surface lipid A and Kdo core for interactions of F. tularensis with CAPs. On the other hand, Madhongsa et al. (2013) demonstrated that the inhibitory activity of peptides such as polymyxin B for B. pseudomallei is several magnitudes higher than that against Pseudomonas aeruginosa or Escherichia coli due to the type of lipopolysaccharide (LPS) moiety on its outer membrane. Further, B. pseudomallei resists treatment by forming micro-colonies using its glycol-calyx polysaccharide capsule and creating a protective environment against antimicrobial peptides (Leelarasamee, 1998). This might explain the moderate antimicrobial effect of WLBU2 and failure of WR12 against B. pseudomallei.
Since LPS form a major part of the outer membrane of the bacteria, variation in LPS structure might contribute to the variation in bacterial susceptibility to antimicrobial peptides (Burtnick & Woods, 1999). Additional complexity on bacterial surfaces is generated by production of capsules which can be composed of polysaccharides or polypeptides. It is worth noting that Y. pestis, the most sensitive of the three bacteria to eCAPs in the studies reported here, has a polypeptide capsule while both F. tularensis and B. pseudomallei have polysaccharide capsules. Bacterial capsule is known to hinder complement activation and phagocytosis of B. pseudomallei (Reckseidler-Zenteno et al., 2005). Wikraiphat et al. (2009) further showed that capsule mutants of B. pseudomallei survived poorly in macrophages and were more susceptible to antimicrobial killing by histatin and lactoferrin compared with the WT bacterial strain. Also, Hayden et al. (2012) suggested that genetic changes that occur in subpopulations of B. pseudomallei during acute infections and post-treatment are not found in the 1026b bacterial strain, which could contribute to the variance seen in the antimicrobial activity of the peptides.
F. tularensis is an intracellular organism that invades and replicates intracellularly in macrophages (Steiner et al., 2014). Accordingly, for the peptides to be effective in vivo, they would have to exert antimicrobial activity on intracellular bacteria with minimal effect on the macrophages. WLBU2 demonstrated a high antimicrobial efficacy against intracellular bacteria with an 80 % reduction in bacteria after treatment with 12.5 μM for 1 h and 90 % reduction at 25 μM. These results suggest that WLBU2 has the potential to effectively treat in vivo infections by F. tularensis and other intracellular bacteria, thus increasing the activity spectrum previously reported for this lead eCAP peptide.
In this study, we demonstrated the potential of WLBU2 as a broad-spectrum antimicrobial agent against F. tularensis and Y. pestis. The results were less conclusive for B. pseudomallei although this may be a result of the assays or culture media employed. The results suggest that in a mass casualty setting, eCAPs might be useful in at least slowing the infection until a diagnosis can be made and appropriate treatment initiated. Further research of WLBU2, WR12 and other eCAPs against these bacteria is warranted, particularly with animal studies to evaluate the in vivo safety, pharmacology and efficacy of eCAPs as an antimicrobial treatment.
Acknowledgements
These studies were supported in part by funds from the Center for Vaccine Research of the University of Pittsburgh. Dr Ronald Montelaro and Dr Jonathan Steckbeck hold stock in Peptilogics. Dr Montelaro serves on an advisory board for Peptilogics, and Dr Steckbeck has a management role at Peptilogics. Although a financial conflict of interest was identified based on the authors’ relationship with Peptilogics, the research findings included in this publication may not necessarily be related to the interests of Peptilogics.
References
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Brogden K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Burtnick M. N., Woods D. E. Isolation of polymyxin B-susceptible mutants of Burkholderia pseudomallei and molecular characterization of genetic loci involved in polymyxin B resistance. Antimicrob Agents Chemother. 1999;43:2648–2656. doi: 10.1128/aac.43.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantratita N., Wuthiekanun V., Boonbumrung K., Tiyawisutsri R., Vesaratchavest M., Limmathurotsakul D., Chierakul W., Wongratanacheewin S., Pukritiyakamee S., other authors Biological relevance of colony morphology and phenotypic switching by Burkholderia pseudomallei . J Bacteriol. 2007;189:807–817. doi: 10.1128/JB.01258-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches B., Islam K., Craigo J. K., Paranjape S. M., Montelaro R. C., Mietzner T. A. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob Agents Chemother. 2005a;49:3208–3216. doi: 10.1128/AAC.49.8.3208-3216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslouches B., Phadke S. M., Lazarevic V., Cascio M., Islam K., Montelaro R. C., Mietzner T. A. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob Agents Chemother. 2005b;49:316–322. doi: 10.1128/AAC.49.1.316-322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith S. A., Smith L. P., Swatland A. S., Reed D. S. Growth conditions and environmental factors impact aerosolization but not virulence of Francisella tularensis infection in mice. Front Cell Infect Microbiol. 2012;2:126. doi: 10.3389/fcimb.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván E. M., Lasaro M. A., Schifferli D. M. Capsular antigen fraction 1 and Pla modulate the susceptibility of Yersinia pestis to pulmonary antimicrobial peptides such as cathelicidin. Infect Immun. 2008;76:1456–1464. doi: 10.1128/IAI.01197-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M. J., Anderson G. M., Stolzenberg E. D., Kari U. P., Zasloff M., Wilson J. M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/S0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Chapple D. S. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden H. S., Lim R., Brittnacher M. J., Sims E. H., Ramage E. R., Fong C., Wu Z., Crist E., Chang J., other authors Evolution of Burkholderia pseudomallei in recurrent melioidosis. PLoS One. 2012;7:e36507. doi: 10.1371/journal.pone.0036507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett K. R., Caldon S. D., McArthur D. G., Cirillo K. A., Kirimanjeswara G. S., Magguilli M. L., Malik M., Shah A., Broderick S., other authors Adaptation of Francisella tularensis to the mammalian environment is governed by cues which can be mimicked in vitro. Infect Immun. 2008;76:4479–4488. doi: 10.1128/IAI.00610-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthawong S., Nazmi K., Wongratanacheewin S., Bolscher J. G., Wuthiekanun V., Taweechaisupapong S. In vitro susceptibility of Burkholderia pseudomallei to antimicrobial peptides. Int J Antimicrob Agents. 2009;34:309–314. doi: 10.1016/j.ijantimicag.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Kanthawong S., Bolscher J. G., Veerman E. C., van Marle J., de Soet H. J., Nazmi K., Wongratanacheewin S., Taweechaisupapong S. Antimicrobial and antibiofilm activity of LL-37 and its truncated variants against Burkholderia pseudomallei . Int J Antimicrob Agents. 2012;39:39–44. doi: 10.1016/j.ijantimicag.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Leelarasamee A. Burkholderia pseudomallei: the unbeatable foe? Southeast Asian J Trop Med Public Health. 1998;29:410–415. [PubMed] [Google Scholar]
- Madhongsa K., Pasan S., Phophetleb O., Nasompag S., Thammasirirak S., Daduang S., Taweechaisupapong S., Lomize A. L., Patramanon R. Antimicrobial action of the cyclic peptide bactenecin on Burkholderia pseudomallei correlates with efficient membrane permeabilization. PLoS Negl Trop Dis. 2013;7:e2267. doi: 10.1371/journal.pntd.0002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Cloyd M. W., Liebmann J., Rinaldo C. R., Jr., Islam K. R., Wang S. Z., Mietzner T. A., Montelaro R. C. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology. 1993;196:89–100. doi: 10.1006/viro.1993.1457. [DOI] [PubMed] [Google Scholar]
- Ntwasa M. Cationic peptide interactions with biological macromolecules. In: Abdelmohsen K., editor. Binding Proteins. Rijeka: InTech; 2012. pp. 139–160. Edited by. [Google Scholar]
- Reckseidler-Zenteno S. L., DeVinney R., Woods D. E. The capsular polysaccharide of Burkholderia pseudomallei contributes to survival in serum by reducing complement factor C3b deposition. Infect Immun. 2005;73:1106–1115. doi: 10.1128/IAI.73.2.1106-1115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner D. J., Furuya Y., Metzger D. W. Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect Drug Resist. 2014;7:239–251. doi: 10.2147/IDR.S53700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencza S. B., Creighton D. J., Yuan T., Vogel H. J., Montelaro R. C., Mietzner T. A. Lentivirus-derived antimicrobial peptides: increased potency by sequence engineering and dimerization. J Antimicrob Chemother. 1999;44:33–41. doi: 10.1093/jac/44.1.33. [DOI] [PubMed] [Google Scholar]
- Vonkavaara M., Pavel S. T., Hölzl K., Nordfelth R., Sjöstedt A., Stöven S. Francisella is sensitive to insect antimicrobial peptides. J Innate Immun. 2013;5:50–59. doi: 10.1159/000342468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikraiphat C., Charoensap J., Utaisincharoen P., Wongratanacheewin S., Taweechaisupapong S., Woods D. E., Bolscher J. G., Sirisinha S. Comparative in vivo and in vitro analyses of putative virulence factors of Burkholderia pseudomallei using lipopolysaccharide, capsule and flagellin mutants. FEMS Immunol Med Microbiol. 2009;56:253–259. doi: 10.1111/j.1574-695X.2009.00574.x. [DOI] [PubMed] [Google Scholar]


