Abstract
Objective:
To compare the clinical and patient-reported outcomes of minimal access and conventional nipple-sparing mastectomy (C-NSM). The secondary outcomes investigated included medical costs and oncological safety.
Background:
Minimal-access NSM has been increasingly applied in the treatment of patients with breast cancer. However, prospective multicenter trials comparing robotic-assisted NSM (R-NSM) versus C-NSM or endoscopic-assisted NSM (E-NSM) are lacking.
Methods:
A prospectively designed 3-arm multicenter, nonrandomized trial (NCT04037852) was conducted from October 1, 2019 to December 31, 2021, to compare R-NSM with C-NSM or E-NSM.
Results:
A total of 73 R-NSM, 74 C-NSM, and 84 E-NSM procedures were enrolled. The median wound length and operation time of C-NSM was (9 cm, 175 minutes), (4 cm, and 195 minutes) in R-NSM, and (4 cm and 222 minutes) in E-NSM. Complications were comparable among the groups. Better wound healing was observed in the minimal-access NSM group. The R-NSM procedure was 4000 and 2600 United States Dollars more expensive than C-NSM and E-NSM, respectively. Wound/scar and postoperative acute pain evaluation favored the use of minimal access NSM over C-NSM. Quality of life in terms of chronic breast/chest pain, mobility, and range of motion of the upper extremity showed no significant differences. The preliminary oncologic results showed no differences among the 3 groups.
Conclusions:
R-NSM or E-NSM is a safe alternative if compared with C-NSM in terms of perioperative morbidities, especially with better wound healing. The advantage of minimal access groups was higher wound-related satisfaction. Higher costs remain one of the major limiting factors in the widespread adoption of R-NSM.
Key Words: endoscopic-assisted nipple-sparing mastectomy, immediate prosthesis breast reconstruction, minimal access breast surgery, nipple-sparing mastectomy, robotic-assisted nipple-sparing mastectomy
Nipple-sparing mastectomy (NSM) has become one of the standard procedures for breast cancer treatment owing to improved cosmetic outcomes and quality of life (QoL)1 and acceptable oncologic safety.2,3 Novel NSM techniques, such as endoscopic-assisted NSM (E-NSM)4–7 or robotic-assisted NSM (R-NSM),8–10 have the advantage of an esthetic scarless mastectomy11 with improved patient satisfaction.
These12–14 techniques have raised concerns regarding oncological safety.15–18 Other critiques include prolonged operation time and higher costs.15–18 Furthermore, the advantages of minimal access (endoscopic or robotic-assisted) NSM over conventional NSM (C-NSM) have not been reported before.
Retrospective studies have compared E-NSM with C-NSM,12,19–21 R-NSM with C-NSM,22–27 and R-NSM with E-NSM.12,28 A prospective randomized clinical trial (RCT)29 directly compared the clinical results and patient-reported outcome measures (PROMs) of R-NSM with C-NSM. Despite current evidence, there remains a lack of prospective, multicenter trials to allow for direct comparison of different NSM approaches in a real-world setting.
To clarify the benefits and limitations of R-NSM versus C-NSM or E-NSM, a prospectively designed trial including three groups of patients was conducted (RCENSM-P, NCT04037852).30 The objectives of the current study were to assess clinical outcomes, medical costs, and PROMs, including esthetic results and QoL.
METHODS
Study Design
This study is a phase 3, open-label, multicenter, nonrandomized trial comparing C-NSM versus E-NSM or R-NSM in the surgical management of breast cancer. The study was designed by the principal investigator (H.W.L.) and conducted at 5 tertiary medical centers [Changhua Christian Hospital (CCH), Shin Kong Wu Ho-Su Memorial Hospital, China Medical University Hospital, National Cheng Kung University Hospital, and Taipei Medical University Hospital] in Taiwan. Data were collected by coinvestigators, and data managers ensured adherence to the protocol and accuracy of the data. All authors have contributed to the manuscript. The protocol was approved by the Institutional Review Board and ethics committee at the CCH (CCH IRB No.:170806 and 190414). The trial was registered at ClinicalTrials.gov (NCT04037852).30 Written informed consent was obtained for the use of the clinical records and pictures.
Patients Enrollment
Data of patients with operable breast cancer who underwent NSM from October 2019 to December 2021 were retrieved from a prospectively maintained database designated as RCENSM-P. Patients were offered 3 different NSM options. The final decision was made through a shared decision-making process between patients and treatment teams. In the current study, baseline demographics, comorbidities, breast size, degree of ptosis, and radiation history were recorded. Only patients who underwent immediate prosthesis breast reconstruction (IPBR) were selected to ensure homogeneity and reduce possible bias in esthetic results or QoL evaluations. Patients who had previously undergone breast-conserving surgery or radiation of the chest wall were excluded.
The current study was conducted in 5 tertiary medical centers in Taiwan, involving 12 breast surgeons [including 2 oncoplastic reconstructive breast surgeons (H.W.L. and F.T.F.C)], 4 plastic surgeons (including 1 dual-trained plastic surgeon, Y.L.K.), and 200 women with early breast cancer. The study design, patient allocation, and exclusion criteria in the current study are illustrated in Figure 1.
FIGURE 1.
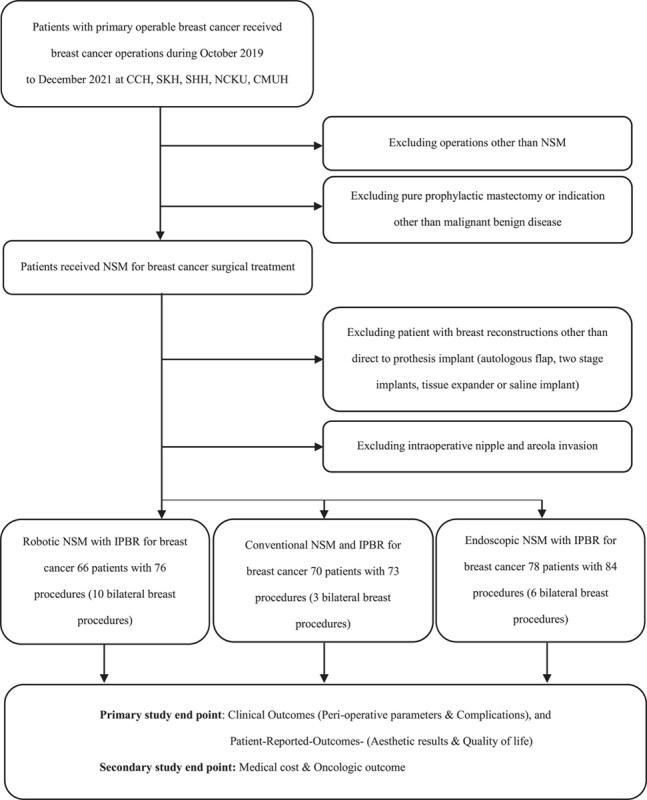
Flowchart of patients selected for R-NSM, C-NSM or E-NMS, and IPBR for surgical treatment of breast cancer. This is an open-label, nonrandomized, prospective, multicenter trial comparing 3 cohorts of patients (R-NSM vs C-NSM or E-NSM and IPBR).
Indications of Nipple-sparing Mastectomy
Patients selected for NSM should have no gross involvement in nipple areolar complex (NAC) through clinical examinations and imaging. In contrast, patients with nipple involvement during intraoperative frozen section were subjected to NAC excision, and skin-sparing mastectomy was performed instead.
Indications of Minimal Access Nipple-sparing Mastectomy
The inclusion criteria for R-NSM and E-NSM4,5,10,31 were early-stage breast cancer (carcinoma in situ, stage I–IIIA), tumor size <5 cm, no evidence of multiple lymph node metastases, and no evidence of nipple, skin, or chest wall invasion. Patients for whom minimal access NSM (R-NSM or E-NSM) was contraindicated included those with apparent NAC involvement, inflammatory breast cancer, breast cancer with chest wall or skin invasion, locally advanced breast cancer, and breast cancer with extensive axillary lymph node metastases (stage IIIB or later).
Nipple-sparing Mastectomy With Immediate Prosthesis Breast Reconstruction Technique
The surgical techniques of C-NSM, E-NSM, and R-NSM used in the current study have been described in previous studies4,5,10,31 and are illustrated in Figure 2. The IPBR performed in the current study was standardized across participating centers and placed in the subpectoral muscular pocket4,5,10,31 without the use of an acellular dermal matrix or mesh.
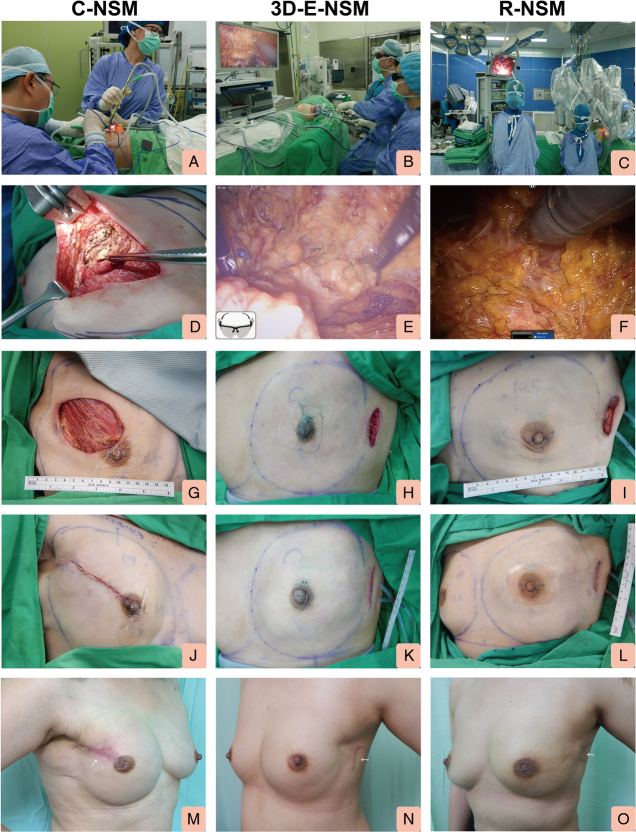
FIGURE 2.
Representative operative pictures showing C-NSM, E-NSM, and R-NSM with immediate prosthesis implant breast reconstruction. A, Photo showing surgeon performing C-NSM. B, Photo showing surgeon performing 3D (single port) E-NSM. C, Photo showing surgeon performing R-NSM. D, Representative view of the intraoperative finding of C-NSM. E, Representative view of the intraoperative finding of 3D E-NSM. F, Representative view of the intraoperative finding of R-NSM. G, Immediate postmastectomy view of C-NSM. H, Immediate postmastectomy view of 3D E-NSM. I, Immediate postmastectomy view of R-NSM. J, Photo showing an immediate postoperative view of patients receiving C-NSM and IPBR. K, Photo showing an immediate postoperative view of patients receiving 3D E-NSM and IPBR. L, Photo showing an immediate postoperative view of patients receiving R-NSM and IPBR. M, Photo showing postoperative 3 months right lateral view of the patient received C-NSM and IPBR. N, Photo showing postoperative 3 months left lateral view of the patient received 3D E-NSM and IPBR. O, Photo showing postoperative 3 months left lateral view of the patient received R-NSM and IPBR. 3D indicates 3-dimensional.
Clinical Outcomes of Various Nipple-sparing Mastectomy Techniques With Immediate Prosthesis Breast Reconstruction
Perioperative parameters, such as operation time, blood loss, complications, and hospital stay, were compared and analyzed. Early complications within 1 month after surgery were recorded. Complications were defined as individual events, such as delayed wound healing (extending beyond 2 weeks), ischemia/necrosis of NAC, seroma formation requiring repeat aspiration 1 month after the operation, skin flap blister formation, skin flap necrosis, hematoma formation, implant loss, or infections. Implant loss or removal within 3 months was considered a complication if it was ascertained to be related to the initial operation. The severity of complications was graded using the Clavien-Dindo (C-D) classification for severity evaluation.32
For oncological safety evaluation, the rates of positive surgical margin involvement, local regional recurrence, distant metastasis, disease-free survival, and overall survival were reported. Surgical margin involvement was defined as a tumor on ink33 for invasive carcinoma and a minimal 2 mm margin for pure ductal carcinoma in situ lesion. Margin involvement was considered a surrogate marker for oncological safety in this study. The incidence of recurrence or death due to breast cancer was ascertained at the most recent follow-up that ended on January 28, 2023.
Medical Cost Analysis of Robotic-assisted Nipple-sparing Mastectomy Versus Conventional Nipple-sparing Mastectomy or Endoscopic-assisted Nipple-sparing Mastectomy
The medical costs of the different NSM approaches were compared. The medical costs incurred for each procedure included the overall hospitalization costs. Information on surgical fees and expenses was obtained from participating institutions. The currency was expressed in the New Taiwan Dollar and the United States dollar (USD). An exchange rate of 31 New Taiwan Dollars/USDs was used for the purpose of this study.
Postoperative Pain Analysis
Postoperative pain was evaluated using a visual analog scale (VAS) ranging from 0 (no pain at all) to 10 (maximum pain experienced). The VAS pain scores were recorded at different time periods: 24 hours (day 1), 48 hours (day 2), 72 hours (day 3), and maximum pain scale experienced at any time during hospitalization.
Patient-reported Outcome Measures of Esthetic Results and Quality of Life
PROMs of esthetic results and QoL were compared among the 3 groups. Breast-Q,34 a validated questionnaire was adopted in the current trial for evaluation in the following domains: “satisfaction with breasts,” “psychosocial well-being,” “sexual well-being,” and “physical well-being.”
Wound-related Specific Questionnaire Used for Minimal Access Breast Surgery
A 10-question questionnaire is commonly used for PROM evaluation in minimal access breast surgery (MABS) studies.4,5,10 In this study, the same questionnaire was used to evaluate patient satisfaction with the various NSM and IPBR procedures.
Quality of Life Evaluation
QoL was evaluated by assessing postoperative pain and resumption of daily activities, with the range of motion, utility, and function of the upper extremity as surrogate indicators. The pain scores in the upper extremities and breast/chest were evaluated with a VAS ranging from 0 (minimal) to 10 (maximum). The range of motion of the shoulder and the utility and function of the upper extremity of the diseased side were also evaluated using self-reported questionnaires with 0 (minimal utility) to 10 (maximum range of motion).
Nipple and Skin Sensation Test
Patients who received various NSM procedures were followed up at the outpatient clinic and consent was taken by study nurses to conduct nipple and skin sensation tests. The sensation assessment was first evaluated on the normal breast by using finger touch to illustrate the feeling of normal touch sensation. Subsequently, finger touch on the operative side was conducted. Patients were then asked to score their sensation of the nipple or area of skin. Five measurement points were assessed and recorded as follows: nipple, superior breast skin, medial breast skin, inferior breast skin, and lateral breast skin. There were altogether 4 grades as follows: grade 0: no sensation at all, grade I: numbness, grade 2: fairly sensate, and grade 3: normal sensate. The sensation of the nipple and the 4 quadrants of the breast were recorded separately and compared among different surgical procedures.
These self-reported questionnaires were administered at a time interval of at least 6 months after the operation, when full healing had taken place, and without active complications.
Statistical Analyses
Differences in continuous variables were tested using the nonparametric Mann-Whitney U test and Kruskal-Wallis test and are reported as mean ± SD. The χ2 test was used for categorical comparisons of data when appropriate. A P significance was set at P <0.05, and all tests were 2-tailed. The operation time and blood loss were evaluated between 10% and 90%, excluding outliers, to prevent inaccurate interpretation of the data. All statistical analyses were performed using the R statistical package (version 4.1.3) by a statistic expert (Y.J.L.). To delineate the differences among subgroups, patients were compared among all 3 groups: conventional versus minimal access (E-NSM and R-NSM), C-NSM versus E-NSM, C-NSM versus R-NSM, and E-NSM versus R-NSM.
RESULTS
A total of 73 C-NSM, 84 E-NSM, and 76 R-NSM with IPBR procedures were included in the analyses (Fig. 1). The baseline patient and tumor characteristics showed no statistically significant differences (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E624). R-NSM had more bilateral procedures (26.3%, 20/76) than E-NSM (20.2%, 18/85) or C-NSM (8.2%, 6/73). In the R-NSM and E-NSM cohorts, there were no cases of conversion to C-NSM.
Perioperative Parameters of Various Nipple-sparing Mastectomy and Immediate Prosthesis Breast Reconstruction
The mean wound length of C-NSM, E-NSM, and R-NSM was 8.3 ± 2.4 cm (median 9), 4.2 ± 0.9 cm (median 4), and 4.1 ± 0.9 cm (median 4, P < 0.01; Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624, Fig. 2), respectively. The mean operation time for C-NSM and IPBR per procedure was 190.2 ± 64 minutes (median 175), 248.5 ± 87.3 minutes (median 222) in E-NSM, and 202.4 ± 42.3 minutes (median 195) in the R-NSM group (P< 0.01). The operation time in E-NSM and IPBR was much shorter in experienced hands (204.5 ± 44.5 (median 200) versus 355.7±71 minutes (median 361), P < 0.01; Supplemental Digital Content Table 1, http://links.lww.com/SLA/E624). Mean blood loss was 43.4 ± 57.2 mL (median 35) in the C-NSM group, 56.8 ± 53.5 mL (median 40) in the E-NSM group, and 33.8 ± 18.3 mL (median 30) in the R-NSM group (P < 0.01). No statistically significant differences were found in the mastectomy specimen weight or reconstruction implant volume among the 3 groups. The mean hospital stay was 5.1 ± 2 days (median, 4 days) in the C-NSM group, 5.8 ± 1.6 (median, 5 days) in the E-NSM group, and 5.2 ± 2.1 (median, 4 days) in the R-NSM group (P < 0.01; Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624).
Morbidities Assessment
In terms of morbidity, there were no differences in seroma or blister formation, skin flap necrosis, hematoma, infection, or implant loss (Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624). Delayed wound healing was higher in the C-NSM group (16.4%) than in the E-NSM (2.4%) and R-NSM (4%, P<0.01) groups. One (1.4%) patient in the C-NSM group experienced total necrosis of NAC, whereas no total NAC necrosis was noted in either the E-NSM or R-NSM groups. Complications were further subdivided by C-D classification, and there were 10 (13.8%) grade 3 complications in the C-NSM group, 4 (4.8%) in the E-NSM, and 2 (2.6%) in the R-NSM group (P = 0.02). We also analyzed the impact of different surgeons on the occurrence of complications, as shown in Supplemental Data (Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624).
Cost Analysis
The medical costs covered by national insurance, paid out-of-pocket by patients, and total costs were significantly different among the 3 types of NSM and IPBR, as summarized in Supplemental Table (Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624). R-NSM was significantly associated with higher medical costs than C-NSM or E-NSM, and the cost of E-NSM was higher than that of C-NSM (P < 0.01).
Postoperative Pain Analysis
The maximum VAS pain score during the first 3 days postoperatively was at a mean of 3.3 ± 1.3 in the C-NSM group, 2.8 ± 1.1 in E-NSM, and 2.9 ± 1.2 in R-NSM (P = 0.02) groups respectively. The VAS pain scores in the E-NSM and R-NSM groups on days 1 and 2 and the mean of 3 days postoperative period were all lower than those in the C-NSM group (Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624, Fig. 3).
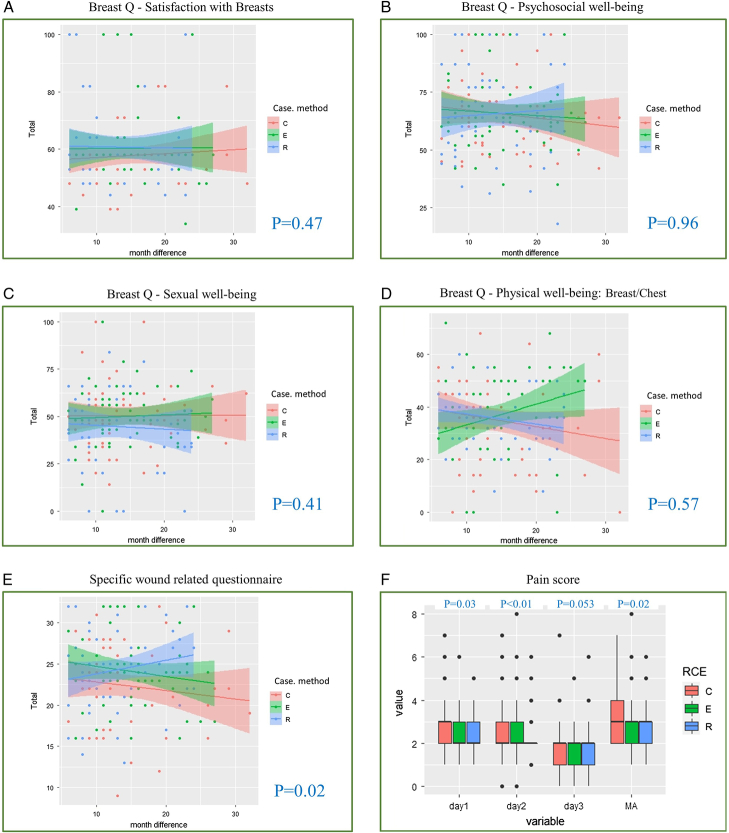
FIGURE 3.
PROMs of esthetic results and QoL for patients receiving C-NSM, E-NSM, or R-NSM. A, Breast-Q “satisfaction with breast” domain. There was no statistically significant difference in satisfaction with breasts in patients who received C-NSM, E-NSM, or R-NSM procedures (P = 0.47). B, Breast-Q “psychosocial well-being” domain. There was no statistically significant difference in psychosocial well-being in patients who received C-NSM, E-NSM, or R-NSM procedures (P = 0.96). C, Breast-Q “sexual well-being” domain. There was no statistically significant difference in sexual well-being in patients who received C-NSM, E-NSM, or R-NSM procedures (P = 0.41). D, Breast-Q “physical well-being: breast/chest” domain. There was no statistically significant difference in physical well-being in patients who received C-NSM, E-NSM, or R-NSM procedures (P = 0.57). E, Wound-specific questionnaire for patients receiving various NSM and IPBR. The overall satisfaction was higher in patients receiving E-NSM or R-NSM group than in C-NSM (P = 0.02). F, VAS for pain score among patients receiving R-NSM versus C-NSM or E-NSM. E-NSM and R-NSM were associated with decreased VAS pain scores for the maximum pain experienced, day 1 and day 2. No differences were observed among the groups after day 3.
Patient-reported Outcome Measures–Quality of Life Evaluation and NAC and Skin Sensation
The median duration of this PROM-QoL evaluation was 13 months postoperation and is summarized in Supplemental Table (Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624). The VAS pain scale of upper extremities was 1.3 ± 2.2 in the C-NSM group, 1.1 ± 1.6 in E-NSM, and 1.2 ± 1.8 in R-NSM (P = 0.06). Pain score in breast/chest was 1.5 ± 1.8 in the C-NSM group, 1.4 ± 1.6 in E-NSM, and 1.7 ± 1.7 in R-NSM (P = 0.46). The range of motion of the shoulder of the ipsilateral affected side was 6.9 ± 3.4 in the C-NSM group, 7.7 ± 2.9 in E-NSM, and 6.6 ± 3.3 in R-NSM (P = 0.2). The utility function of the upper extremity of the ipsilateral side was 7.6 ± 3.4 in the C-NSM group, 8.4 ± 2.4 in the E-NSM group, and 7.5 ± 2.9 in the R-NSM group (P = 0.13; Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624). The sensation of the nipple was higher in C-NSM (1.7 ± 1) group than 1.4±1.2 in the E-NSM group, and 1.7 ± 0.8 in the R-NSM group (P < 0.01).
Patient-reported Outcome Measures of Esthetic Results Breast-Q and Wound-specific Questionnaire for Minimal Access Breast Surgery
Sixty patients in each group completed the Breast-Q and the questionnaire used for MABS, which are summarized in Supplemental Table (Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624). The mean duration from postoperation to evaluation with Breast-Q was 14.3 ± 5.9 (median, 13 months). In Breast-Q “satisfaction with the breast” module, there were no statistically significant differences in score among C-NSM (59.5 ± 10.6), E-NSM (60.3 ± 13.8), or R-NSM (60.7 ± 14.4, P = 0.47). “Psychosocial well-being” scores were also not statistically different, which were 65.8 ± 17.2 in C-NSM, 65.8 ± 15.6 in E-NSM, and 65.5 ± 20.4 in R-NSM (P = 0.96). The “sexual well-being” scores were 48.3 ± 17.4 in the C-NSM group, 50 ± 17.1 in E-NSM, and 44.6 ± 21.9 in R-NSM (P = 0.41). The “physical well-being of breast/chest wall” scores were 35.8 ± 17 in the C-NSM group, 37 ± 16.8 in E-NSM, and 36 ± 11.2 in R-NSM (P = 0.57). The distribution of PROMs with 4 Breast-Q domains and time sequences is plotted in Figure 3.
In the MABS questionnaire, there were no differences in preoperative breast appearance satisfaction among the 3 groups of patients (P = 0.55). Postoperative satisfaction with scar appearance, length, and wound location consistently showed higher satisfaction scores with minimal access NSM than with C-NSM (Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624), but no differences were observed between E-NSM and R-NSM. Patients were asked if they could choose again to go for the same surgery if given a choice to do so, and 80% of C-NSM, 90% of E-NSM, and 83.3% of R-NSM patients would choose the same operation (P = 0.21). The overall score (summation of questions: 2–9) was higher in the MABS group [E-NSM (24.1 ± 4.5) or R-NSM (24.4±5)] than in the C-NSM group (23.6 ± 5.1, P = 0.02; Fig. 3; Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624); however, there was no statistical difference between E-NSM and R-NSM (P = 0.61). The skin incision types and locations of the R-NSM, C-NSM, and E-NSM are summarized and shown in Supplemental Figure (Supplemental Digital Content Fig. 1, http://links.lww.com/SLA/E624).
Oncologic Outcomes
The margin involvement rates were 1.5% (1/66) for C-NSM, 0% (0/79) for E-NSM, and 1.4% (1/72) for R-NSM (P = 0.56). The mean follow-up duration of these patients was 26.9 ± 7.8 (median, 27 months), which was too short to evaluate the long-term oncologic safety outcomes. Two (2.7%) local recurrence events were detected in the C-NSM group, one (1.2%) in the E-NSM, and one (1.3%) in the R-NSM (P = 0.72). Distant metastasis was found in one (1.2%) case in the E-NSM group whereas zero (0%) in the C-NSM or R-NSM group (P = 0.4). No mortality was reported until the last follow-up, which ended on January 28, 2023. Local recurrence, distant metastases, and overall survival rates are summarized in Supplemental Table (Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624).
DISCUSSION
In this study, to reflect a real-world setting, we collected and compared 73 C-NSM, 84 E-NSM, 76 R-NSM, and IPBR from 5 tertiary medical centers in Taiwan, involving 12 breast surgeons, 4 plastic surgeons, and 200 women with early breast cancer. Designed as a case-control comparison study, the age, tumor size, lymph node status, stage, breast size, and comorbidity of patients were similar among the 3 groups (Supplemental Digital Content Table 1, http://links.lww.com/SLA/E624).
A golden question that begs to be answered in R-NSM or E-NSM is whether these techniques result in lower complication rates compared with C-NSM. This study showed that there were no differences in complications, and the number of complications per case analyzed also showed no differences among the 3 groups (Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624). These results were comparable to those of reported case studies,12,23,24,35 meta-analyses,27 and RCT.29
In our study, a higher C-D classification grade 3 complication rate was noted in the C-NSM (13.7%) group than in the E-NSM (4.8%) and R-NSM (2.6%, P = 0.02, Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624) groups. These results might be due to confounding factors that were not accounted for, such as variations in techniques, patients, or surgeons’ experience. As shown in Supplemental Data (Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624), the performance of surgeons was quite different, which might be one of the contributing factors observed.
One observed complication that differed among the groups was delayed wound healing. The delayed wound healing rate was higher in the C-NSM group (16.4%) than in the E-NSM (2.4%) or R-NSM groups (4%, P < 0.01; Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624). Incisions of R-NSM or E-NSM were mainly placed peripherally at the axillary region, lateral chest wall, or anterior axillary line (Fig. 2; Supplemental Digital Content Fig. 1, http://links.lww.com/SLA/E624). Blood supply over these areas was better than if the incision was placed centrally (periareolar incision), as in some C-NSM cases.
Ischemia/necrosis of the NAC,3,7,36 which is the “Achilles tendon” of the NSM, remains an important concern. According to a meta-analysis,36 the risk of partial necrosis of NAC was 9.1% and that of total necrosis was up to 2%. In this study, only one (0.4%) total NAC necrosis was observed in the cohort of 231 NSM procedures (1/73 C-NSM, 0/84 E-NSM, and 0/74 R-NSM). This could be attributed to improvements in surgical techniques, better wound placement, decreased periareolar incision, appropriate patient selection, and better knowledge of risk factors affecting wound healing.
In terms of esthetic results derived from PROMs, Breast-Q evaluation was performed in 180 patients, ensuring at least 60 patients in each subgroup. The results of PROMs with Breast-Q in the domains tested were not significantly different among the groups (Fig. 3, Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624), indicating that NSM and IPBR achieved high satisfaction despite different surgical approaches. The wound-specific questionnaire (Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624) frequently used to assess wound satisfaction in MABS studies,4,5,10 the pre and postoperative breast appearance, size, symmetry, and NAC position were all comparable among the groups. These findings are compatible with the Breast-Q questionnaire, which reflects the reliability of the current study.
The wound length of E-NSM (4.2 ± 0.9 cm, median 4) and R-NSM (4.1 ± 0.9 cm, median 4) were significantly shorter than C-NSM (8.3 ± 2.4 cm, median 9, P < 0.01; Supplemental Digital Content Table 2, http://links.lww.com/SLA/E624, Fig. 2), and located at more inconspicuous extra-mammary positions. These benefits of minimal access NSMs were reflected by higher satisfaction with “scar appearance”, “wound length”, and “wound location” in the R-NSM and E-NSM groups than in the C-NSM group. However, no differences were observed between the two minimal access techniques (E-NSM and R-NSM) in these 3 aspects. The overall score of E-NSM (24.1 ± 4.5) or R-NSM (24.4 ± 5) was higher than C-NSM (23.6 ± 5.1, P = 0.02; Fig. 3; Supplemental Digital Content Table 4, http://links.lww.com/SLA/E624). These results highlight the advantages of scarless esthetic mastectomy.11
There was an improvement in the acute postoperative VAS pain score of E-NSM or R-NSM compared with the C-NSM group of patients on days 1 and 2, or the maximum pain experienced during hospitalization (Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624, Fig. 3). The QoL evaluation of long-term pain of the breast/chest wall and upper extremity or utility of the upper extremity and shoulder performed with a median of 13 months showed no differences among these 3 groups. In the nipple sensation test, C-NSM (1.7 ± 1) was associated with a higher score than E-NSM (1.5 ± 1.2) or R-NSM (1.6 ± 0.8, P < 0.01, Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624). Contrary to the finding of better NAC sensation in the R-NSM over the C-NSM group in a recently published RCT,29 the current study did not show improved skin or NAC sensation over C-NSM. It has to be noted, however, that the extent of axillary surgeries performed varies according to disease status and this might affect QoL interpretation in different subgroups.
In the current study, the overall medical costs of R-NSM were ~4000 USDs more than C-NSM and 2600 USDs more than E-NSM (Supplemental Digital Content Table 3, http://links.lww.com/SLA/E624). These findings were consistent with one study that reported an extra 1749 Euros for R-NSM compared with C-NSM.24 Increased costs from R-NSM have been one of the most important rate-limiting factors for its widespread adoption.15 From a cost-effectiveness point of view, E-NSM might be a more prudent choice than R-NSM as the minimal access procedure of choices as there were no significant differences in clinical outcomes and PROMs (esthetic evaluations and QoL).
The strength of this study lies in the fact that this is the first prospectively designed multicenter trial comparing R-NSM versus C-NSM or E-NSM and IPBR in the surgical management of breast cancer, with important outcome evaluations. Our study was limited by its small sample size and possible selection bias among the 3 methods in view of the nonrandomized nature of the study design. The heterogeneity of each surgical team (breast surgeons, oncoplastic reconstructive breast surgeons, dual-trained surgeons, and/or plastic surgeons) could also serve as one of the confounding factors affecting the outcomes of this study. However, this heterogeneity could serve to reflect real-world practice, and it is encouraging to know that important outcomes were not affected, further confirming the advantages and reproducibility of minimal-access NSM techniques. In addition to heterogeneity, the scars of the C-NSM group also varied widely, with some in less favorable locations, which might lead to higher complications or lower patient satisfaction. The extent of axillary surgeries varied according to disease status and might affect QoL interpretation.
From an oncological safety standpoint, the follow-up period was too short to establish long-term oncological safety. Preliminary outcomes showed comparable margin involvement, locoregional recurrence, or overall survival. The medical costs of these 3 approaches were rough estimations at best as a true “cost-effectiveness” analysis could not be realistically performed to show the actual benefit of either operation in terms of financial cost.
Despite these limitations, our study is the first to investigate patients with similar baseline characteristics undergoing 3 different surgical approaches. The results of this study are, therefore, meaningful as it adds to the current body of evidence in this field.
CONCLUSIONS
The results of this study showed that R-NSM or E-NSM was a safe alternative to C-NSM in terms of perioperative morbidities, with a trend towards better wound healing. The PROMs of esthetic results and QoL showed that all 3 approaches were equal. Higher medical costs remained a major limiting factor in R-NSM. A longer follow-up is needed to establish the long-term oncologic safety of R-NSM or E-NSM in the surgical treatment of breast cancer.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Yi-Ru Ke, Chin-Mei Tai, Yun-Ting Chang, Shu-Hsin Pai, and Ya-Ting Zhung for their assistance with this study.
Footnotes
This manuscript is not under review or considered for publication in another journal at the current status. However, a preprint was automatically posted during our last submission to The Lancet on August 16, 2022. (Preprint: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4191369).
This study was funded by the Ministry of Science and Technology of Taiwan, and the number of this funding was: MOST 111-2314-B-371-010-. Also, received research funding from Intuitive Surgery CRG09-08232019. This study was also sponsored by research funding provided by Changhua Christian Hospital 108-CCH-IRP-122, 109-CCH-IRP-093, 110-CCH-IRP-042, and 111-CCH-IRP-028.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.annalsofsurgery.com.
Contributor Information
Hung-Wen Lai, Email: hwlai650420@yahoo.com.tw;143809@cch.org.tw.
Dar-Ren Chen, Email: 115045@cch.org.tw.
Liang-Chih Liu, Email: dr0363@yahoo.com.tw.
Shou-Tung Chen, Email: 1886@cch.org.tw.
Yao-Lung Kuo, Email: ylkuo@mail.ncku.edu.tw.
Shih-Lung Lin, Email: 103280@cch.org.tw.
Yao-Chung Wu, Email: w89223@gmail.com.
Tsung-Chun Huang, Email: D21920@mail.cmuh.org.tw.
Chin-Sheng Hung, Email: hungcs@tmu.edu.tw.
Ying-Jen Lin, Email: 180681@cch.org.tw.
Hsin-Shun Tseng, Email: 91694@cch.org.tw.
Chi Wei Mok, Email: mok.chi.wei@singhealth.com.sg.
Fiona Tsui-Fen Cheng, Email: sgtw88@gmail.com.
REFERENCES
- 1. Bailey CR, Ogbuagu O, Baltodano PA, et al. Quality-of-Life Outcomes Improve with Nipple-Sparing Mastectomy and Breast Reconstruction. Plast Reconstr Surg. 2017;140:219–226. [DOI] [PubMed] [Google Scholar]
- 2. Cruz LDL, Moody AM, Tappy EE, et al. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systemic review. Ann Surg Oncol. 2015;22:3241–3249. [DOI] [PubMed] [Google Scholar]
- 3. Headon HL, Kasem A, Mokbel K. The oncologic safety of nipple-sparing mastectomy: a systemic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai HW, Lin SL, Chen ST, et al. Single-axillary-incision endoscopic-assisted hybrid technique for nipple-sparing mastectomy: technique, preliminary results, and patient-reported cosmetic outcome from preliminary 50 procedures. Ann Surg Oncol. 2018;25:1340–1349. [DOI] [PubMed] [Google Scholar]
- 5. Lai HW, Chen ST, Mok CW, et al. Single-port three-dimensional (3D) videoscope-assisted endoscopic nipple-sparing mastectomy in the management of breast cancer: technique, clinical outcomes, medical cost, learning curve, and patient-reported aesthetic results from 80 preliminary procedures. Ann Surg Oncol. 2021;28:7331–7344. [DOI] [PubMed] [Google Scholar]
- 6. Tukenmez M, Ozden BC, Agcaoglu O, et al. Videoendoscopic single-port nipple-sparing mastectomy and immediate reconstruction. J Laparoendosc Adv Surg Tech A. 2014;24:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daar DA, Abdou SA, Rosario L, et al. Is there a preferred incision location for nipple-sparing mastectomy? A systematic review and meta-analysis. Plast Reconstr Surg. 2019;143:906e–919e. [DOI] [PubMed] [Google Scholar]
- 8. Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: Feasibility and safety study. Breast. 2017;31:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarfati B, Struk S, Leymarie N, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. [DOI] [PubMed] [Google Scholar]
- 10. Lai HW, Chen ST, Lin SL, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2019;26:42–52. [DOI] [PubMed] [Google Scholar]
- 11. Yang JD, Lee JT, Lee JS, et al. Aesthetic scar-less mastectomy and breast reconstruction. J Breast Cancer. 2021;24:22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lai HW, Chen ST, Lin YJ, et al. Minimal access (endoscopic and robotic) breast surgery in the surgical treatment of early breast cancer- trend and clinical outcome from a single-surgeon experience over 10 years. Front Oncol. 2021;11:739144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryu JM, Kim JY, Choi HJ, et al. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: an initial experience of the Korea robot-endosopy minimal access breast surgery study group (KoREa-BSG). Ann Surg. 2022;275:985–991. [DOI] [PubMed] [Google Scholar]
- 14. Mok CW, Lai HW. Evolution of minimal access breast surgery. Gland Surg. 2019;8:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Margenthaler JA. Robotic mastectomy-program malfunction? JAMA Surg. 2020;155:461–462. [DOI] [PubMed] [Google Scholar]
- 16. Morrow M. Robotic mastectomy: the next major advance in breast cancer surgery? Br J Surg. 2021;108:233–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingram D. Is it time for breast cancer surgeons to embrace endoscopic-assisted mastectomy? ANZ J Surg. 2008;78:837–838. [DOI] [PubMed] [Google Scholar]
- 18. Hwang RF, Hunt KK. The emergence of robotic-assisted breast surgery: proceed with caution. Ann Surg. 2020;271:1013–1015. [DOI] [PubMed] [Google Scholar]
- 19. Lai HW, Chen ST, Liao CY, et al. Oncologic outcome of endoscopic assisted breast surgery compared with conventional approach in breast cancer: an analysis of 3426 primary operable breast cancer patients from single institute with and without propensity score matching. Ann Surg Oncol. 2021;28:7368–7380. [DOI] [PubMed] [Google Scholar]
- 20. Mok CW, Lai HW. Endoscopic-assisted surgery in the management of breast cancer: 20 years review of trend, techniques and outcomes. Breast. 2019;46:144–156. [DOI] [PubMed] [Google Scholar]
- 21. Lee HY, Changh YW, Yu DY, et al. Comparison of single incision endoscopic nipple-sparing mastectomy and conventional nipple-sparing mastectomy for breast cancer based on initial experience. J Breast Cancer. 2021;24:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lai HW, Chen ST, Mok CW, et al. Robotic versus conventional nipple sparing mastectomy and immediate gel implant breast reconstruction in the management of breast cancer—a case control comparison study with analysis of clinical outcome, medical cost, and patient-reported cosmetic results. J Plast Reconstr Aesthet Surg. 2020;73:1514–1525. [DOI] [PubMed] [Google Scholar]
- 23. Moon J, Lee J, Lee DW, et al. Postoperative pain assessment of robotic nipple-sparing mastectomy with immediate prepectoral prosthesis breast reconstruction: a comparison with conventional nipple-sparing mastectomy. Int J Med Sci. 2021;18:2409–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Houvenaeghel G, Barrou J, Jaufferet C, et al. Robotic versus conventional nipple-sparing mastectomy with immediate breast reconstruction. Front Oncol. 2021;11:637049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J, Park HS, Lee H, et al. Post-operative complications and nipple necrosis rates between conventional and robotic nipple-sparing mastectomy. Front Oncol. 2021;10:594388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang JJ, Chuang EY, Cheong DC, et al. Robotic-assisted nipple-sparing mastectomy followed by immediate microsurgical free flap reconstruction: feasibility and aesthetic results- Case series. Int J Surg. 2021;95:106143. [DOI] [PubMed] [Google Scholar]
- 27. Filipe MD, Bock E, Postma EL, et al. Robotic nipple-sparing mastectomy complication rate compared to traditional nipple-sparing mastectomy: a systematic review and meta-analysis. J Robot Surg. 2022;16:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lai HW, Chen ST, Tai CM, et al. Robotic versus endoscopic-assisted nipple-sparing mastectomy with immediate prosthesis breast reconstruction in the management of breast cancer: a case-control comparison study with analysis of clinical outcomes, learning curve, patient-reported aesthetic results, and medical cost. Ann Surg Oncol. 2020;27:2255–2268. [DOI] [PubMed] [Google Scholar]
- 29. Toesca A, Sangalli C, Maisonneuve P, et al. A randomized trial of robotic mastectomy versus open surgery in women with breast cancer or BRCA mutation. Ann Surg. 2022;276:11–19. [DOI] [PubMed] [Google Scholar]
- 30. NIH U.S. National Library of Medicine. ClinicalTrials.gov. Accessed September 13, 2021. https://clinicaltrials.gov/ct2/show/NCT04037852 [DOI] [PubMed]
- 31. Lai HW, Toesca A, Sarfati B, et al. Consensus statement on robotic mastectomy-expert panel from international endoscopic and robotic breast surgery symposium (IERBS) 2019. Ann Surg. 2020;271:1005–1012. [DOI] [PubMed] [Google Scholar]
- 32. Dindo D, Demartines N, Clavien P. Classification of surgical complications a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21:704–716. [DOI] [PubMed] [Google Scholar]
- 34. Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–353. [DOI] [PubMed] [Google Scholar]
- 35. Angarita FA, Castelo M, Englesakis M, et al. Robot-assisted nipple-sparing mastectomy: systematic review. Br J Surg. 2020;107:1580–1594. [DOI] [PubMed] [Google Scholar]
- 36. Piper M, Peled AW, Foster RD, et al. Total skin-sparing mastectomy: a systematic review of oncologic outcomes and postoperative complications. Ann Plast Surg. 2013;70:435–437. [DOI] [PubMed] [Google Scholar]