Abstract
The integration of natural language processing (NLP) tools into neurology workflows has the potential to significantly enhance clinical care. However, it is important to address the limitations and risks associated with integrating this new technology. Recent advances in transformer-based NLP algorithms (e.g., GPT, BERT) could augment neurology clinical care by summarizing patient health information, suggesting care options, and assisting research involving large datasets. However, these NLP platforms have potential risks including fabricated facts and data security and substantial barriers for implementation. Although these risks and barriers need to be considered, the benefits for providers, patients, and communities are substantial. With these systems achieving greater functionality and the pace of medical need increasing, integrating these tools into clinical care may prove not only beneficial but necessary. Further investigation is needed to design implementation strategies, mitigate risks, and overcome barriers.
Introduction
Evidence-based medicine places data at the heart of decision-making processes. In modern practice, neurologists take in details of the patient's history, findings from examinations (e.g., muscle weakness, cognitive impairment), and results of ancillary tests (e.g., EEG, MRI). They then use all these data points in combination with their training, medical literature, and experience to formulate a diagnosis and care plan. These processes mandate a uniquely human connection between the physician and patient. Despite this connection, there are multiple places where the overall clinical workflow can be improved by technology, especially in the setting of data analysis and processing.
Recent advances in artificial intelligence (AI) such as those seen in Open-AI's ChatGPT have generated tremendous interest for enhancing industries from marketing to education.1 Similarly, health care and more specifically, neurologic care should investigate the benefits and risks this and similar systems might provide. These tools might not only transform patient data for the benefit of both provider and patient, but they may present avenues for removing barriers that have built up in modern practice. In addition, these tools can allow for rapid and continual learning from large datasets, which makes them ideal for identifying complex symptoms in charts and investigating treatment efficacy.
With increasing levels of physician burnout, constrained access to neurologic care, and growing demand for neurology services, it is vital to explore ways of improving provider and patient experiences with neurology clinical care.2,3 We discuss transformer-based natural language processing (NLP) models including understanding of their technology, identifying potential uses in neurology and neurosciences, highlighting challenges and limitations, recommendations of AI utilization and implementation, risks and barriers of AI, and the testing and validation needed for implementation into neurology health care models.
Recent Advancements: Technology and Terminology
There are various tiers by which technology in health care is taxonomized. Technology is “assistive” when it can detect clinically relevant data without analysis and generally requires the physician to finalize interpretation. “Augmentative” technology is when the machine analyzes or quantifies data in a clinically meaningful way but still requires interpretation by a physician or other qualified health care professional. And last, any machine that interprets data and independently generates clinically meaningful conclusions without physician involvement can be considered as “autonomous.”4
To understand NLP and their functionality, it is important to understand their basic technology and terminology including machine learning, neural networks, and transformers (see eAppendix 1, links.lww.com/WNL/D179).
Limitations and Barriers to NLP in Neurologic Care
Before discussing the potential benefits these technologies pose for neurologic care, it is important to understand the challenges, limitations, and barriers that exist across various use cases. Understanding these limitations is crucial to ensure the safe and effective deployment of these technologies.
Patient Safety
Augmenting neurology clinical practice comes with risks to patient safety, confidentiality, and equity. NLP models are essentially large language models (LLMs), which means they use context to predict the next word and then generate whole sentences or paragraphs.5 One weakness of LLMs is that they can combine plausible words to yield confabulation.
For neurology care, this inaccuracy can affect crucial medical information and treatment plans. In Figure 1A, we show an example whereby ChatGPT recommends valproic acid for a 25-year-old woman with juvenile myoclonic epilepsy (JME), who is planning for pregnancy. Although valproic acid is typically the drug of choice for patients with JME, it may not be considered for a 25-year-old woman in her reproductive years. Clinical experience suggests that lamotrigine, which is also used in JME and has considerably less side effects than valproic acid, especially in pregnant women, would be better suited. This response generated did not consider the potential teratogenic effects of valproic acid in this age group.
Figure 1. Limitations of ChatGPT in Neurology Clinical Practice.
Prompt conveys standard neurology clinical inquiry. ChatGPT Response is generated by ChatGPT.18
Furthermore, NLP tools are limited in how clinical suggestions relate to the patient. As shown in Figure 1B, ChatGPT will often return vague or generalized responses when it does not possess enough information to make a definitive suggestion. In this case, various treatments have been cited in the literature as alternatives to epilepsy surgery, and yet options such as psychotherapies may be impractical for certain patients.
Until this limitation across multiple NLP models, including ChatGPT, can be resolved and rigorously validated, utilization of “over-the-counter” open-AI language model advises a cautionary approach.
Data Quality, Variability, and Security
Another limitation is quality and variability of the data. Inaccurate or incomplete data can affect the performance of machine learning models and algorithms. Data sources may vary in terms of format, structure, and quality, posing challenges for standardization efforts, documentation efficiency tools, and analysis techniques. Ensuring data accuracy, consistency, and representativeness remains a challenge in all use cases.
Incorporating this technology into clinical practice brings with it an innate risk for data security. To incorporate models into health care, these systems will need to be rigorously tested before any patient data is used for training and testing. There are risks surrounding privately owned closed-source nonmedical LLMs (ChatGPT, BERT, etc) because information ingested by these LLMs could violate data privacy laws or result in imprudent or unauthorized disclosure of confidential information. The standard licensing terms of these private platforms could include granting intellectual property rights in data submitted to the vendor making available the Generative AI, which would conflict with internal health system policies. It is therefore imperative that algorithms operate in end-to-end encryption and Health Insurance Portability and Accountability Act (HIPAA)–compliant systems.
Discrimination and Bias
Unlike humans, AI language models do not have personal opinions, beliefs, or biases. However, it is possible for models to generate biases that are present in the training data. This usually stems from biased language, stereotypes, or discriminatory attitudes that humans consciously or unconsciously imposed on the training dataset, which is then reflected in the model's output.6 It is therefore imperative that models use diverse datasets and team's employee programmers from different perspectives, cultures, sexes, races, and ethnicities. It is the responsibility of developers, researchers, and organizations to be mindful of these issues and regularly evaluate them for biases. Future research would be needed prioritizing ethical considerations throughout the design, development, and deployment of advanced technologies. The design of this research should be focused on ensuring patient privacy, data security, and compliance with regulations through the implementation of robust privacy protocols, anonymization techniques, and secure data storage.
In addition, AI systems can only see outcomes as probabilities and trends. The interpretation of these findings is left to health care professionals. A major risk with incorporating these tools into health care is how it might lead to over medicalization or increased health disparities. It will come down to medical teams and governing bodies to scrutinize any AI medical findings before making guidance decisions. Furthermore, it is important that care teams and governing bodies remain well versed in how these models operate and their innate limitations. They must remain cognizant of the narrow utility of these tools and ensure they do not expand beyond their designated purpose or replace medical sense.
Training the “AI-NLP Team Member”
It is worth mentioning the effect of having an “AI-NLP Team Member.” There would essentially need to be a neurology residency for AI-NLP technology, and the efforts to train the assistant need to be balanced against the benefits of having such assistance. For very specialized task, there must be continued “training or retraining” of the models that would be required including updating them, revalidating and retesting as performed with any other software tool. Similar to the validation of other software's, there will need to be a specialized field of knowledge for the model. Nonetheless, continuing medical education for AI-NLP tools will have to be formalized and validated. The concept is similar to various proposals to add human Advanced Practice Providers to clinical workflow. Qualitative studies assessing the feasibility of training these “AI-NLP Team Members” will be needed to successfully implement them in neurology health care practices.
Barriers for Implementation
Several barriers are to be considered even before implementation of NLP models in Neurology clinics and departments. From a technological standpoint, NLP tools will need to overcome cost, time, and skill set needed to build, test, validate, and update current AI algorithms. For example, ChatGPT is a massive model with more than 175 billion parameters, which requires an enormous corpus for pretraining.5,7 Because the information is stored within the whole model, it would be expensive and time-consuming to frequently update it. Unlike webpages or databases where information can be quickly changed, these complex models need to be fully retrained and fine-tuned. Some companies have started charging for premium access (higher limits, faster performance) to meet these financial constraints and finance future updates.8 In addition, the timescale for retraining these models might work for well-established neurology guidelines and medical facts, but they would quickly become outdated with new publications and treatment innovations. Last, even dialog-capable systems, such as ChatGPT, will require machine learning experts and prompt engineers to maintain and update clinical tools.
Additional Limitations and Barriers
Additional limitations and barriers to NLP models in neurologic practice include factors of generalizability and external validation, cost and resource implications, the human-machine collaboration, and factors of interpretability and explainability (eAppendix 1, links.lww.com/WNL/D179).
Recommendations for AI-NLP Implementation and Utilization in Neurologic Care
Several recommendations can be made to optimize the implementation and utilization of advanced technologies in neurology clinical practice. By implementing these recommendations, a neurology clinical practice can leverage the potential of advanced NLP tools while addressing the limitations and barriers discussed earlier. These steps will contribute to safer, more effective, and ethically responsible deployment of emerging and new solutions, ultimately improving patient outcomes and enhancing the overall quality of neurology care.
Training and Education
To implement and use AI-NLP models into clinical practice, it is important to provide a comprehensive training and education program for clinicians to effectively use and understand the functionalities and limitations of these models. This includes front-end speech and ambient clinical intelligence (ACI). Offering continuous professional development opportunities is important to keep clinicians updated with the latest advancements and ensure they can leverage these technologies optimally.
Testing, Validation, and External Review
Testing and validation plays a crucial role in the development and deployment of language and machine learning (LLM) models, ensuring their accuracy, reliability, and suitability for real-world applications.
One key component to the validation of LLM is dataset selection, where careful consideration is given to choosing diverse and representative datasets that accurately reflect the target population and encompass various linguistic nuances and challenges.9
Benchmarking against existing models is another critical component of testing and validation. Comparing the performance of LLMs against state-of-the-art existing models is a way to achieve improvements and provide added value. This step helps establish the effectiveness and superiority of models, boosting their credibility and reliability in practical applications. Similar benchmarks have been investigated in medical imaging datasets.10
In addition, the establishment of robust evaluation metrics is imperative. These metrics serve as objective measures to assess the performance of LLMs, allowing for quantitative comparisons and meaningful interpretations. For implementation into clinical practice, it is prudent to carefully define and select appropriate evaluation metrics and take into account factors such as precision, recall, accuracy, and F1 score, among others, to ensure a comprehensive framework.
Moreover, the involvement of expert clinicians in the validation process is crucial. Their domain expertise and clinical insights are invaluable for evaluating the practical utility and effectiveness of LLMs in real-world health care settings. Collaborating with expert clinicians allows for critical feedback, validation of the clinical relevance and accuracy of the models, and identification for areas of improvement.
Incorporating external validation is a vital step in testing and validation process. By collaborating with external partners and leveraging independent datasets, one can assess the performance, generalizability, and real-world applicability of LLMs.11 Rigorous testing and validation protocols help ensure that the models are fit for their intended purpose and mitigate the risks associated with their usage beyond their intended scope.
Ethical and Legal Considerations (Focus on NLP)
Implementing AI-NLP models requires careful consideration of ethical and legal factors. Ensuring patient privacy, data security, and compliance with regulations such as HIPAA and General Data Protection Regulation is essential. In addition, addressing potential biases in algorithms, maintaining transparency in data usage, and obtaining informed consent from patients are critical ethical considerations. Obtaining US Food and Drug Administration (FDA) clearance of AI-NLP medical devices is also essential before mass production and utilization into health care systems. No AI-NLP model has been approved by the FDA for usage in clinical practice to date.12
Recommendations for implementation include the prioritization of ethical considerations throughout the design, development, and deployment of advanced technologies. Implementing robust privacy protocols, anonymization techniques, and secure data storage can ensure patient privacy, data security, and compliance with regulations. Steps to mitigate biases and ensure fairness in algorithms too are essential to prevent disparities in health care delivery. Previous models have been offered to show the incorporation of AI into the legal standard of care for a clinical situation.13
Additional Recommendations for Implementation
Additional recommendations for NLP implementation within neurology clinical practice include collaboration with all stakeholders, having standardization and interoperability and considering ways for continuous improvement and iterative development for these models (eAppendix 1, links.lww.com/WNL/D179).
Applications of NLP in Neurology Clinical Practice
The integration of NLP tools in neurology clinical practice holds significant promise for enhancing patient care and advancing research.14,15 These tools have the potential to streamline the analysis of clinical notes, other electronic health records, and medical literature, allowing neurologists to efficiently extract relevant information and gain deeper insights into patient conditions.16 NLP can aid in automating the extraction of patient demographics, medical history, symptoms, and diagnostic information from textual data, enabling improved clinical decision-making and personalized treatment approaches.17 Furthermore, NLP tools can assist in the identification and monitoring of disease progression, detecting patterns in large-scale datasets, predicting treatment outcomes, and identifying adverse effects.18 In addition, NLP can support the creation of neurology-specific ontologies and standardized vocabularies, facilitating better information exchange and interoperability across different health care systems and research institutions.19 Applications of NLP models can also be expanded to inpatient and outpatient operations and be suitable in the creation of new data streams (eAppendix 1, links.lww.com/WNL/D179). The below discussion entails incorporations of NLP tools in clinical practice, which if entertained in future neurology health care systems would most certainly require validation through rigorous prospective and randomized studies.
Chart Review and Documentation
To demonstrate how NLP models could potentially affect neurologic care, we provide the following simulated case as an example. Consider a 64-year-old man with new-onset generalized tonic-clonic seizures, who presented a week ago to an emergency department (ED). The patient is now being seen in an outpatient neurology clinic after receiving MRI and CT imaging. During the appointment, the provider will need to evaluate risk factors, address the recent ED visit, review imaging results, assess neurologic status, and create an assessment and personalized care plan.
Before the neurologist even starts this new patient consultation, they will need to familiarize themselves with information taken from the ED visit and the imaging results. Typically, this will include a detailed chart review, but in a busy clinic, an NLP tool might be able to augment this process while ensuring the provider reviews important items and concerns. The “History Bot” in Figure 2 shows how the notes for a hypothetical patient might be summarized using an AI-based NLP model. In this case, we had used open-AI.
Figure 2. “History Bot” Summarizes Chart for Provider.
Prompt conveys standard neurology clinical inquiry. Entry created by neurologist to emulate a real patient note. ChatGPT Response is generated by ChatGPT.18
Once the neurologist is ready, they would then start the consultation and document the patient's history of present illness including medical history, medications, and social history. This is another area where NLP can enhance the visit: advancements in medical documentation to improve clinician-patient experiences. Cutting-edge technologies such as front-end speech and AI-driven ACI have further accelerated the documentation process.20 Clinicians can now dictate patient encounters directly into electronic medical records using voice commands, significantly improving efficiency. ACI captures patient encounters through mobile applications and automatically converts them into comprehensive medical records, enhancing accuracy and streamlining the workflow. These advancements, in conjunction with conversational and ambient AI, hold potential for further automation and simplification of clinical tasks, paving the way for a future where health care is driven by innovative technologies.
An example of this is the NLP-enabled speech-to-text systems such as Nuance's Dragon Ambient eXperience and 3M's M*Modal Fluency Direct; this consultation can be directly transcribed into the electronic health record.20 In March and April 2023, partnerships were announced between Nuance and Microsoft21 HCA Healthcare and Augmedix22 and Amazon and 3M23 for the development of such clinical documentation systems.
Of note, these speech-to-text systems are not without drawbacks. One apparent one is the prone to error. There is a need to balance the ease of generating the summary with the necessity to double-check it. Just as with human scribes, it may take longer to edit a file created by AI than to gather and document the information oneself. Further studies comparing independent documentation, human scribes, and NLP-enabled speech-to-text systems will be needed before AI can be considered to be completely autonomous.
Assessment and Care Plans
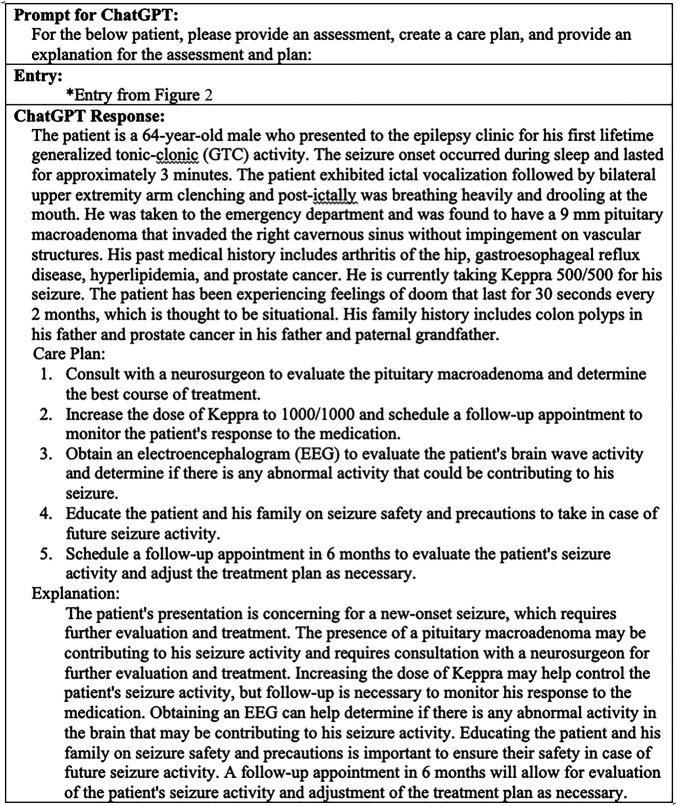
NLP tools may also help providers formulate thorough assessments and suggest care plans. Unlike current internet medical services, which search through restrictive symptom lists for potential diagnoses, NLP tools could potentially search through a patient's entire chart to identify key aspects of their history and test results. Furthermore, by implementing these tools in neurology clinics, they could enhance a trained physician's ability to problem solve complex cases and formulate differential diagnoses in much the same way a medical team might collaborate. As shown in Figure 3, an “AI Team Member,” using ChatGPT for demonstration purposes, could use NLP to identify key aspects of our patient's chart and provide customized recommendations. Furthermore, these care options come from the vast corpus of medical knowledge that the model is trained on. By increasing and updating the dataset used to train these models, these AI Team Members could improve their suggestions and provide alternative and cutting-edge treatment options.
Figure 3. “AI Team Member” Gives Assessment and Care Options.
Prompt conveys standard neurology clinical inquiry. Entry is same as seen in Figure 2 entry. ChatGPT Response is generated by ChatGPT.18
Once a care plan is chosen, the “AI Team Member” could also help identify medication conflicts, potential side effects, or more cost-effective care options. BERT-based systems have recently been developed to identify adverse drug reactions using current data.24 Furthermore, by giving these tools access to current drug prices and insurance information, they could provide patient-specific treatment costs (eAppendix 1, links.lww.com/WNL/D179). These tools could also be programmed with information specific to the health system and local community including community resources and support groups and financial assistance plans.
Medical Education
Patients and caregivers might also benefit from diagnostic and treatment-related information. By providing a well-trained and validated AI chat service to communities, health care systems can ensure patients get their questions answered quickly and accurately.25 Easy-to-understand health information would give patients greater autonomy and confidence in their care. For now, some AI-NLP models, especially ChatGPT, include a disclaimer for clinical questions that reminds patients to speak with a licensed health care provider.5 However, a reviewed and validated tool designed and tested by a hospital can direct patients to their own care team for future questions.26 These tools can also attract new patients to a health system/practice.
In addition, per the 21st Century Cures Act, which restricts information blocking, patients now have unprecedented and immediate access to their medical records. While there are myriad benefits to this transparency, instances of misunderstandings, confusion, and anxiety are bound to continue, and AI-NLP tools could help with understanding and even simplifying medical terminology and connect patients and their families to verified resources.
Conclusion
As we advance further into the 21st century, we believe AI-NLP models have the potential to overlap with health care systems. Several questions are yet to be answered however: “Can AI-NLP be autonomous?” and “When will neurology health care systems be ready to consider AI-NLP as an integral part of the team?” The answer to these questions would require prospective and randomized studies for validation and additional studies needed on how to train and assess the performance of these AI tools, similar to how medical students partake in the United States Medical Licensing Examination or residents/fellows must meet annual Accreditation Council for Graduate Medical Education milestones.
Using AI-NLP tools in clinical practice could help neurology clinics addresses the increasing demand for neurology care.27 As this technology continues to improve, doubts about its efficacy will only erode further. However, the benefits of integrating this technology for patients, providers, and communities must be weighed against inherent risks. Governing bodies and vendors should begin to design testing strategies and safe points of integration for these tools. Meanwhile, neurologists and clinical staff should work with programming teams to design workflows and implementation strategies. But most importantly, patients and communities need to be placed front and center. Their data security, care, and trust should be all parties' top priority. By starting this process now, we not only equip neurologists with the tools they need to deliver the highest quality care but allow for the creation of safe and effective solutions.
Glossary
- ACI
ambient clinical intelligence
- AI
artificial intelligence
- ED
emergency department
- FDA
US Food and Drug Administration
- HIPAA
Health Insurance Portability and Accountability Act
- JME
juvenile myoclonic epilepsy
- LLM
large language model
- NLP
natural language processing
Appendix. Authors
| Name | Location | Contribution |
| Wendong Ge, PhD | Department of Neurology, Massachusetts General Hospital, Boston | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Hunter J. Rice, BS, CRC | Department of Neurology, Massachusetts General Hospital, Boston | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Irfan S. Sheikh, MD | Department of Neurology, Massachusetts General Hospital, Boston | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| M. Brandon Westover, MD, PhD | Department of Neurology, Beth Israel Deaconess Medical Center, Boston, MA | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Allison L. Weathers, MD | Information Technology Division, Cleveland Clinic, OH | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
| Lyell K. Jones, MD | Department of Neurology, Mayo Clinic, Rochester, MN | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Lidia Moura, MD, PhD, MPH | Department of Neurology, Massachusetts General Hospital; Department of Neurology, Harvard Medical School, Boston, MA | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
This work was supported by the NIH (1R01AG073410-01).
Disclosure
W. Ge, H.J. Rice, and I.S. Sheikh report no conflicts of interest. M.B. Westover is a co-founder and holds equity in Beacon Biosignals, and receives royalties for authoring Pocket Neurology from Wolters Kluwer and Atlas of Intensive Care Quantitative EEG by Demos Medical. M.B. Westover was supported by grants from the NIH (R01NS102190, R01NS102574, R01NS107291, RF1AG064312, RF1NS120947, R01AG073410, R01HL161253, R01NS126282, R01AG073598), and NSF (2014431). A.L. Weathers has a noncompensated relationship as the chair of the Epic Neurology Specialty Steering Board (Epic Systems) and has received personal compensation in the range of $5000 to $9000 for serving as a CME question writer and interviewer for the American Academy of Neurology. She reports no conflicts of interest. L.K. Jones has received publishing royalties from a publication relating to health care, has noncompensated relationships as a member of the board of directors of the Mayo Clinic Accountable Care Organization and the American Academy of Neurology Institute, and has received personal compensation in the range of $10,000 to $49,999 for serving as an editor, associate editor, or editorial advisory board member for the American Academy of Neurology. L. Moura receives research support from the CDC (U48DP006377) and the NIH (NIA 1 R01 AG073410-01, NIA 1 R01 AG062282, NIA 2 P01 AG032952-11). She also serves as consultant to the Epilepsy Foundation. She reports no conflicts of interest. Go to Neurology.org/N for full disclosures.
References
- 1.Villasenor J. How ChatGPT Can Improve Education, Not Threaten It. Scientific American; 2023. [Google Scholar]
- 2.Weathers AL. Chipping away at neurologist burnout, one refill request at a time. Neurol Clin Pract. 2016;6(5):379-380. doi: 10.1212/CPJ.0000000000000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates SW. Physician stress and burnout. Am J Med. 2020;133(2):160-164. doi: 10.1016/j.amjmed.2019.08.034 [DOI] [PubMed] [Google Scholar]
- 4.Frank RA, Jarrin R, Pritzker J, et al. Developing current procedural terminology codes that describe the work performed by machines. NPJ Digit Med. 2022;5(1):177. doi: 10.1038/s41746-022-00723-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.OpenAI. GPT-4 Technical Report. 2023. [Google Scholar]
- 6.Gianfrancesco MA, Tamang S, Yazdany J, Schmajuk G. Potential biases in machine learning algorithms using electronic health record data. JAMA Intern Med. 2018;178(11):1544-1547. doi: 10.1001/jamainternmed.2018.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radford A, Narasimhan K, Salimans T, Sutskever I. Improving Language Understanding by Generative Pre-Training. 2018. https://s3-us-west-2.amazonaws.com/openai-assets/research-covers/language-unsupervised/language_understanding_paper.pdf [Google Scholar]
- 8.Wiggers K. OpenAI Begins Piloting ChatGPT Professional, a Premium Version of Its Viral Chatbot. TechCrunch; 2023. [Google Scholar]
- 9.Norori N, Hu Q, Aellen FM, Faraci FD, Tzovara A. Addressing bias in big data and AI for health care: a call for open science. Patterns (NY). 2021;2(10):100347. doi: 10.1016/j.patter.2021.100347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajpurkar P, Lungren MP. The current and future state of AI interpretation of medical images. N Engl J Med. 2023;388(21):1981-1990. doi: 10.1056/NEJMra2301725 [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Sharma B, Thompson HM, et al. External validation of a machine learning classifier to identify unhealthy alcohol use in hospitalized patients. Addiction. 2022;117(4):925-933. doi: 10.1111/add.15730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US FDA. Artificial Intelligence and Machine Learning (AI/ML): Enabled Medical Devices. 2022. Accessed July 15, 2023. fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices. [Google Scholar]
- 13.Price WN II, Gerke S, Cohen IG. Potential liability for physicians using artificial intelligence. JAMA. 2019;322(18):1765-1766. doi: 10.1001/jama.2019.15064 [DOI] [PubMed] [Google Scholar]
- 14.Xie K, Gallagher RS, Conrad EC, et al. Extracting seizure frequency from epilepsy clinic notes: a machine reading approach to natural language processing. J Am Med Inform Assoc. 2022;29(5):873-881. doi: 10.1093/jamia/ocac018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi T, Magdamo CG, Noori A, et al. NeuraHealth: an automated screening pipeline to detect undiagnosed cognitive impairment in electronic health records with deep learning and natural language processing. 2022. ArxIV. Preprint posted online June 20, 2022. https://arxiv.org/pdf/2202.00478.pdf. [Google Scholar]
- 16.Harkema H, Chapman WW, Saul M, Dellon ES, Schoen RE, Mehrotra A. Developing a natural language processing application for measuring the quality of colonoscopy procedures. J Am Med Inform Asscoc. 2011;18(suppl 1):i150-i156. doi: 10.1136/amiajnl-2011-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berge GT, Granmo OC, Tveit TO, Munkvold BE, Ruthjersen AL, Sharma J. Machine learning-driven clinical decision support system for concept-based searching: a field trial in a Norwegian hospital. BMC Med Inform Decis Mak. 2023;23(1):5. doi: 10.1186/s12911-023-02101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norori N, Hu Q, Aellen FM, Faraci FD, Tzovara A. Addressing bias in big data and AI for health care: a call for open science. Patterns (NY). 2021;2(10):100347. doi: 10.1016/j.patter.2021.100347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azizi S, Hier DB, Wunsch Ii DC. Enhanced neurologic concept recognition using a named entity recognition model based on transformers. Front Digit Health. 2022;4:1065581. doi: 10.3389/fdgth.2022.1065581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambient Clinical Intelligence | Automatically Document Care | Nuance. Nuance Communications. 2023. [Google Scholar]
- 21.Keating C. Nuance and Microsoft Announce the First Fully AI-Automated Clinical Documentation Application for Healthcare. Nuance Communications; 2023. [Google Scholar]
- 22.Chesler M. Augmedix Announces Partnership with HCA Healthcare to Accelerate the Development of AI-enabled Ambient Documentation. GlobeNewswire Newsroom. April 18, 2023. [Google Scholar]
- 23.Source 3M. 3M Health Information Systems collaborates with AWS to accelerate AI innovation in clinical documentation. PR Newswire Cision. April 18, 2023. Accessed October 28, 2023. https://www.prnewswire.com/news-releases/3m-health-information-systems-collaborates-with-aws-to-accelerate-ai-innovation-in-clinical-documentation-301799571.html. [Google Scholar]
- 24.Martenot V, Masdeu V, Cupe J, et al. LiSA: an assisted literature search pipeline for detecting serious adverse drug events with deep learning. BMC Med Inform Decis Mak. 2022;22(1):338. doi: 10.1186/s12911-022-02085-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu L, Sanders L, Li K, Chow JCL. Chatbot for health care and oncology applications using artificial intelligence and machine learning: systematic review. JMIR Cancer. 2021;7(4):e27850. doi: 10.2196/27850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieces Predict. Accessed July 15, 2023. piecestech.com.
- 27.DeBusk A, Subramanian PS, Scannell Bryan M, Moster ML, Calvert PC, Frohman LP. Mismatch in supply and demand for neuro-ophthalmic care. J Neuroophthalmol. 2022;42(1):62-67. doi: 10.1097/WNO.0000000000001214 [DOI] [PubMed] [Google Scholar]