Abstract
Background and Objectives
Predicting neurodevelopmental outcome for neonates with hypoxic-ischemic encephalopathy (HIE) is important for clinical decision-making, care planning, and parent communication. We examined the relationship between EEG background and neurodevelopmental outcome among children enrolled in a trial of erythropoietin or placebo for neonates with HIE treated with therapeutic hypothermia.
Methods
Participants had EEG recorded throughout hypothermia. EEG background was classified as normal, discontinuous, or severely abnormal (defined as burst suppression, low voltage suppressed, or status epilepticus) at 5 1-hour epochs: onset of recording, 24, 36, 48, and 72 hours after birth. The predominant background pattern during the entire continuous video EEG monitoring recording was calculated using the arithmetic mean of the 5 EEG background ratings (normal = 0; discontinuous = 1; severely abnormal = 2) as follows: “predominantly normal” (mean = 0), “normal/discontinuous” (0 < mean<1), “predominantly discontinuous” (mean = 1), “discontinuous/severely abnormal” (1 < mean<2), or “predominantly severely abnormal” (mean = 2). Primary outcome was death or neurodevelopmental impairment (NDI) defined as cerebral palsy, Gross Motor Function Classification Score ≥1, or cognitive score <90 on Bayley Scales of Infant Toddler Development, third edition at age 2 years. Neurodevelopment was also categorized into a 5-level ordinal measure: no, mild, moderate, severe NDI, or death for secondary analysis. We used generalized linear regression models with robust standard errors to assess the relative risk of death or NDI by EEG background in both unadjusted and adjusted analyses controlling for the effects of treatment group, sex, HIE severity, and study recruitment site.
Results
Among 142 neonates, the predominant background EEG pattern was predominantly normal in 35 (25%), normal/discontinuous in 68 (48%), predominantly discontinuous in 11 (7.7%), discontinuous/severely abnormal in 16 (11%), and predominantly severely abnormal in 12 (8.5%). Increasing severity of background across monitoring epochs was associated with increasingly worse clinical outcomes. Children with severe EEG background abnormality at any time point (n = 36, 25%) were significantly more likely to die or have severe NDI at 2 years (adjusted relative risk: 7.95, 95% CI 3.49–18.12).
Discussion
EEG background is strongly associated with NDI at age 2 years. These results can be used to assist health care providers to plan follow-up care and counsel families for decision-making related to goals of care.
Introduction
Continuous video EEG monitoring (cEEG) provides important information about prognosis in neonates with hypoxic-ischemic encephalopathy (HIE). In the setting of acute neonatal encephalopathies such as HIE, severely abnormal EEG background and high seizure burden are associated with the extent of brain injury and later neurodevelopmental impairment (NDI).1-9
The High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) multicenter, randomized trial evaluated erythropoietin (Epo) vs placebo for neuroprotection in neonates with moderate/severe HIE who were treated with therapeutic hypothermia. Although small trials of Epo for neuroprotection in HIE had suggested lower death and disability among treated children, the HEAL trial did not show a difference between treatment groups in death or disability at age 2 years.10 The primary study did not assess the association between cEEG background and treatment group or outcome.
To examine the relationship between cEEG background and 2-year neurodevelopmental outcome among neonates with HIE who were treated with hypothermia and Epo or placebo, we examined the subset of children enrolled in the HEAL trial who were evaluated with cEEG throughout cooling and rewarming.11 We hypothesized that cEEG background would be associated with neurodevelopmental outcome and that the predictive value of cEEG would differ between Epo and placebo groups.
Methods
Study Design
This was an ancillary study of the HEAL trial, a multicenter, randomized trial of Epo compared with placebo for neuroprotection in newborns with moderate or severe neonatal encephalopathy due to HIE who were treated with hypothermia therapy (NCT02811263).11 Epo or placebo was administered on 5 days: within 24 hours of birth (day 1) and on days 2, 3, 4, and 7. The parent trial had strict inclusion and exclusion criteria, qualifying Sarnat examinations and outcome measures performed by certified examiners, and high rate of follow-up as detailed by Wu et al. and outlined below.10,12
Inclusion and Exclusion Criteria
Parent trial eligibility criteria were (1) gestational age 36 weeks or older at birth; (2) perinatal depression marked by Apgar score <5 at 10 minutes, more than 10 minutes of cardiorespiratory resuscitation, and pH < 7.00 or base deficit ≥15 mmol/L (cord or infant arterial or venous gas obtained  60 minutes after birth; 3) moderate or severe encephalopathy, which was defined as ≥3 of 6 modified Sarnat criteria present at 1–6 hours of age; and 4) therapeutic hypothermia started by 6 hours of age. Exclusions were birthweight <1,800 grams, head circumference <30 cm, genetic or congenital condition known to affect neurodevelopment, hematocrit >65.0%, bedside team considering transition to palliative care, encephalopathy due to an event occurring after birth, guardian with diminished capacity to provide informed consent, or not likely to be followed due to unstable social situation.10
60 minutes after birth; 3) moderate or severe encephalopathy, which was defined as ≥3 of 6 modified Sarnat criteria present at 1–6 hours of age; and 4) therapeutic hypothermia started by 6 hours of age. Exclusions were birthweight <1,800 grams, head circumference <30 cm, genetic or congenital condition known to affect neurodevelopment, hematocrit >65.0%, bedside team considering transition to palliative care, encephalopathy due to an event occurring after birth, guardian with diminished capacity to provide informed consent, or not likely to be followed due to unstable social situation.10
Seven of the 17 HEAL sites complete cEEG during cooling as per American Clinical Neurophysiology Society guidelines13 as part of routine clinical care and provided EEGs for centralized review and inclusion in this ancillary study. Additional inclusion criteria for the ancillary study were (1) cEEG recorded without interruption during cooling or until removal for transition to palliative care and (2) EEG quality satisfactory for neurophysiologist evaluation. Participants were excluded from the current analysis a priori if they were diagnosed with a congenital or genetic condition known to affect neurodevelopment.
Measurements
Clinical demographics from the mother and child were drawn from the medical record. Severity of encephalopathy was determined by a modified Sarnat evaluation. If equal numbers of moderate and severe elements were present, the severity was determined based on the level of consciousness.12
EEG Acquisition and Interpretation
Continuous EEG was acquired using no fewer than of 8 cerebral electrodes using 10–20 electrode placement modified for the neonate. Clinical identifiers were removed from recordings, which were stripped of video. Two board-certified, pediatric clinical neurophysiologists with expertise in neonatal neurophysiology (A.L.N. and C.J.W.) reviewed each EEG recording. Files were reviewed using Persyst software; neurophysiologists were able to format montages as needed for interpretation. Neurophysiologists were not aware of the treatment allocation, the clinical interpretation, or the neurodevelopmental outcome. The neurophysiologists each reviewed the files independently. Disagreements were resolved by consensus.
To determine EEG background, an artifact-free period of 60 minutes was selected at 5 epochs: onset of recording, 24, 36, 48, and 72 hours after birth. Background classification was based on the predominant pattern within the epoch and followed American Clinical Neurophysiology Society (ACNS) classification of neonatal EEG background for term neonates ≥36 weeks gestation, which has good inter-rater agreement for EEG background categorization:14 normal—uninterrupted electrical activity with <2 seconds of voltage attenuation <25 μV pp (includes the tracé alternant pattern of quiet sleep); excessively discontinuous—abnormally discontinuous tracings with bursts that contain normal patterns separated by prolonged (>6 seconds) voltage depressed (<25 μV) interburst intervals; severely abnormal consisting of any of the following: (1) burst suppression (invariant, abnormally composed EEG bursts separated by prolonged interburst intervals with voltages <5 μV pp), (2) low voltage suppressed (baseline voltage is < 10 μV), or (3) status epilepticus (half or more of the recording hour shows seizures); or cannot determine because artifact precludes interpretation or EEG not available during the period.15 Inter-rater agreement of EEG background ratings was calculated using the weights of 0.5 for disagreements one rating category apart.
We developed the “predominant cEEG background pattern” score to capture and summarize data over the entire cEEG recording. The arithmetic mean of the EEG background ratings (normal = 0, discontinuous = 1, severely abnormal = 2) over the 5 epochs was categorized as follows: “predominantly normal” (mean = 0), “normal discontinuous” (0 < mean <1), “predominantly discontinuous” (mean = 1), “discontinuous/severely abnormal” (1 < mean <2), or “predominantly severely abnormal” (mean = 2).
Seizures were defined as a sudden, abnormal EEG activity defined by a recurrent and evolving pattern with at least 2 μV peak-to-peak voltage and a minimum duration of 10 seconds.15 Status epilepticus was defined as seizures encompassing ≥50% of any 1-hour epoch of recording.15
Outcome Measures
All neurologic and Bayley Scales of Infant Toddler Development, third edition (BSID-III), examiners were centrally trained and certified on an annual basis. Cognitive outcome was based on the BSID-III cognitive subscale. Motor outcome was defined by the presence of cerebral palsy (CP) as determined by a validated standardized neurologic examination16 and a modified Gross Motor Function Classification System (GMFCS) score. A GMFCS score was determined for all participants regardless of the presence of CP to describe the degree of functional disability.17 Owing to the extenuating circumstances of the COVID-19 pandemic, we extended the primary end point assessment window from the planned 22–26 months to 22–36 months of age.
The primary outcome was death or severe NDI at age 2 years. Severe NDI was defined as BSID-III cognitive score <70, quadriparesis with GMFCS ≥1 hemiparesis/diparesis, or no CP with GMFCS ≥3.
As a secondary outcome, neurodevelopment was evaluated as a 5-level ordinal measure also determined at age 2 years: no NDI, mild NDI, moderate NDI, severe NDI, or death. The severity of NDI was defined as the worst result for either the cognitive or motor outcome. Cognitive outcome was assessed using the BSID-III cognitive score and categorized as normal (≥90), mild (85–89), moderate (70–84), or severe (<70). For motor outcome, hemiparesis or diparesis with GMFCS <1 or no CP with GMFCS = 1 was considered mild. Quadriparesis with GMFCS <1, hemiparesis/diparesis with GMFCS  2, or no CP with GMFCS = 2 was considered moderate.
2, or no CP with GMFCS = 2 was considered moderate.
Analysis
We tabulated cEEG background score (normal = 0, excessively discontinuous = 1, severely abnormal = 2) at each cEEG epoch by the treatment group and used a chi-squared statistic to test for differences in background distribution between Epo and placebo treatment groups. Sankey diagrams18 were used to illustrate the change in background over time between treatment groups. The cEEG background at 24 hours was considered the primary measure as it has been shown predictive in neonates treated with hypothermia.3,19 Other cEEG epochs were considered exploratory. Chi-squared tests (for categorical variables) and 2-sample t tests (for continuous variables) were used to describe differences between clinical and demographic characteristics between infants with a severely abnormal background at any point in time compared with those who never had a severely abnormal background.
The distribution of the secondary ordinal outcome was tabulated against the predominant background pattern using a stacked bar chart and compared using the Fisher exact test. We used (log-link) logistic regression models with robust standard errors to assess the relative risk of death or severe NDI by cEEG characteristic in both unadjusted and adjusted analyses (adjusting for treatment group, sex, HIE severity, and study recruitment site). Specific cEEG background characteristics evaluated were severely abnormal background initially or at 24, 36, 48, or 72 hours; at any time; and whether status epilepticus was observed. The presence of any seizures or maximum hourly seizure burden >13 minutes per hour (previously shown to be associated with adverse outcome)20 were also evaluated in separate models. Finally, we also assessed the association of longitudinal “trajectory” in cEEG background with the relative risk of death or severe NDI by including the derived slope in cEEG background measurements over time into the logistic regression model. The derived slope was entered into the model as a separate covariate and in an interaction term with treatment.
We performed a sensitivity analysis to adjust for morphine exposure during the entire EEG period to examine whether the association between cEEG characteristics and outcome was confounded by concurrent exposure to morphine.
All analyses were conducted using R software version 4.0.2.21
Power and Sample Size
Statistical power and sample size of 150 cEEG recordings were determined a priori for the primary study goal of demonstrating reduction in overall seizure burden due to treatment with Epo.11
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board at each center. Newborns were evaluated only after obtaining written informed consent by a parent or legal guardian (NCT02811263).
Data Availability
HEAL Trial data sharing plan is available elsewhere.22
Results
Of 500 neonates randomized into HEAL from January 25, 2017, to October 9, 2019, 235 (47%) children were enrolled at a HEAL-EEG site. One hundred eighty-five of 235 (79%) cEEG recordings were evaluated for background and seizures, to reach the desired sample size of 150 studies with suitable quality for research interpretation (35 were excluded due to low quality, 13 who received Epo and 22 who received placebo; eFigure 1, links.lww.com/WNL/D164). Twenty-three cEEGs were not evaluated due to appropriate high-quality recordings to reach the prespecified sample size. An additional 8 children were excluded due to diagnosis of a genetic (n = 5) or congenital (n = 2) condition or both (n = 1) known to affect neurodevelopment, leaving 142 neonates in this cohort. The maternal, pregnancy, delivery, infant, and monitoring characteristics at baseline were similar between the full HEAL cohort compared with the 142 children included in this study11 nor for the 79 who received Epo compared with the 63 who received placebo.
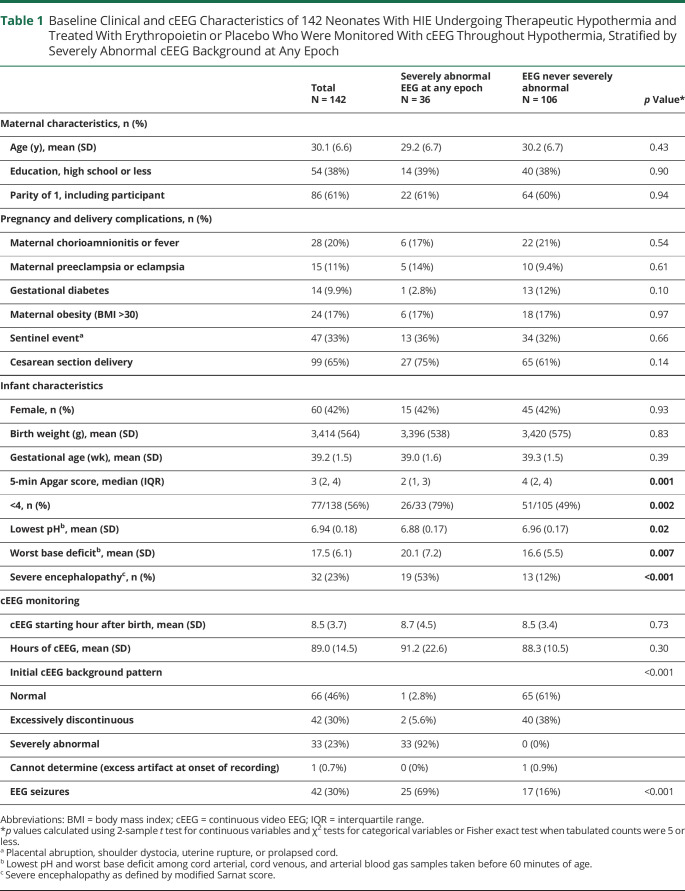
Using the 5-level cEEG background scoring, the predominant EEG background was normal in 35 (25%), normal/discontinuous in 68 (48%), discontinuous in 11 (7.7%), discontinuous/severely abnormal in 16 (11%), and severely abnormal in 12 (8.5%). Inter-rater agreement of EEG background ratings between the 2 readers was substantial (weighted Cohen kappa = 0.74). The clinical characteristics of children with a severe cEEG background abnormality at any of the 5 epochs (n = 36, 25%) and no severely abnormal cEEG background (n = 106, 75%) are presented in Table 1. Neonates with severely abnormal cEEG at any time were more likely to have lower Apgar scores, worse pH and base deficit, and severe clinical encephalopathy. There was no difference in the timing of cEEG initiation after birth or total duration of cEEG recording time between groups.
Table 1.
Baseline Clinical and cEEG Characteristics of 142 Neonates With HIE Undergoing Therapeutic Hypothermia and Treated With Erythropoietin or Placebo Who Were Monitored With cEEG Throughout Hypothermia, Stratified by Severely Abnormal cEEG Background at Any Epoch
| Total N = 142 |
Severely abnormal EEG at any epoch N = 36 |
EEG never severely abnormal N = 106 |
p Value* | |
| Maternal characteristics, n (%) | ||||
| Age (y), mean (SD) | 30.1 (6.6) | 29.2 (6.7) | 30.2 (6.7) | 0.43 |
| Education, high school or less | 54 (38%) | 14 (39%) | 40 (38%) | 0.90 |
| Parity of 1, including participant | 86 (61%) | 22 (61%) | 64 (60%) | 0.94 |
| Pregnancy and delivery complications, n (%) | ||||
| Maternal chorioamnionitis or fever | 28 (20%) | 6 (17%) | 22 (21%) | 0.54 |
| Maternal preeclampsia or eclampsia | 15 (11%) | 5 (14%) | 10 (9.4%) | 0.61 |
| Gestational diabetes | 14 (9.9%) | 1 (2.8%) | 13 (12%) | 0.10 |
| Maternal obesity (BMI >30) | 24 (17%) | 6 (17%) | 18 (17%) | 0.97 |
| Sentinel eventa | 47 (33%) | 13 (36%) | 34 (32%) | 0.66 |
| Cesarean section delivery | 99 (65%) | 27 (75%) | 65 (61%) | 0.14 |
| Infant characteristics | ||||
| Female, n (%) | 60 (42%) | 15 (42%) | 45 (42%) | 0.93 |
| Birth weight (g), mean (SD) | 3,414 (564) | 3,396 (538) | 3,420 (575) | 0.83 |
| Gestational age (wk), mean (SD) | 39.2 (1.5) | 39.0 (1.6) | 39.3 (1.5) | 0.39 |
| 5-min Apgar score, median (IQR) | 3 (2, 4) | 2 (1, 3) | 4 (2, 4) | 0.001 |
| <4, n (%) | 77/138 (56%) | 26/33 (79%) | 51/105 (49%) | 0.002 |
| Lowest pHb, mean (SD) | 6.94 (0.18) | 6.88 (0.17) | 6.96 (0.17) | 0.02 |
| Worst base deficitb, mean (SD) | 17.5 (6.1) | 20.1 (7.2) | 16.6 (5.5) | 0.007 |
| Severe encephalopathyc, n (%) | 32 (23%) | 19 (53%) | 13 (12%) | <0.001 |
| cEEG monitoring | ||||
| cEEG starting hour after birth, mean (SD) | 8.5 (3.7) | 8.7 (4.5) | 8.5 (3.4) | 0.73 |
| Hours of cEEG, mean (SD) | 89.0 (14.5) | 91.2 (22.6) | 88.3 (10.5) | 0.30 |
| Initial cEEG background pattern | <0.001 | |||
| Normal | 66 (46%) | 1 (2.8%) | 65 (61%) | |
| Excessively discontinuous | 42 (30%) | 2 (5.6%) | 40 (38%) | |
| Severely abnormal | 33 (23%) | 33 (92%) | 0 (0%) | |
| Cannot determine (excess artifact at onset of recording) | 1 (0.7%) | 0 (0%) | 1 (0.9%) | |
| EEG seizures | 42 (30%) | 25 (69%) | 17 (16%) | <0.001 |
Abbreviations: BMI = body mass index; cEEG = continuous video EEG; IQR = interquartile range.
*p values calculated using 2-sample t test for continuous variables and χ2 tests for categorical variables or Fisher exact test when tabulated counts were 5 or less.
Placental abruption, shoulder dystocia, uterine rupture, or prolapsed cord.
Lowest pH and worst base deficit among cord arterial, cord venous, and arterial blood gas samples taken before 60 minutes of age.
Severe encephalopathy as defined by modified Sarnat score.
Follow-up was at median 25.1 months (interquartile range [IQR] 24.0–27.2): 82/142 (58%) of children were normal, 19/142 (13%) had mild NDI, 10/142 (7.0%) had moderate NDI, 11/142 (7.7%) had severe NDI, and 20/142 (14%) died.
EEG Background With Epo vs Placebo
There was no significant difference in cEEG background between neonates in the Epo group compared with neonates in the placebo group (eTable, links.lww.com/WNL/D166).
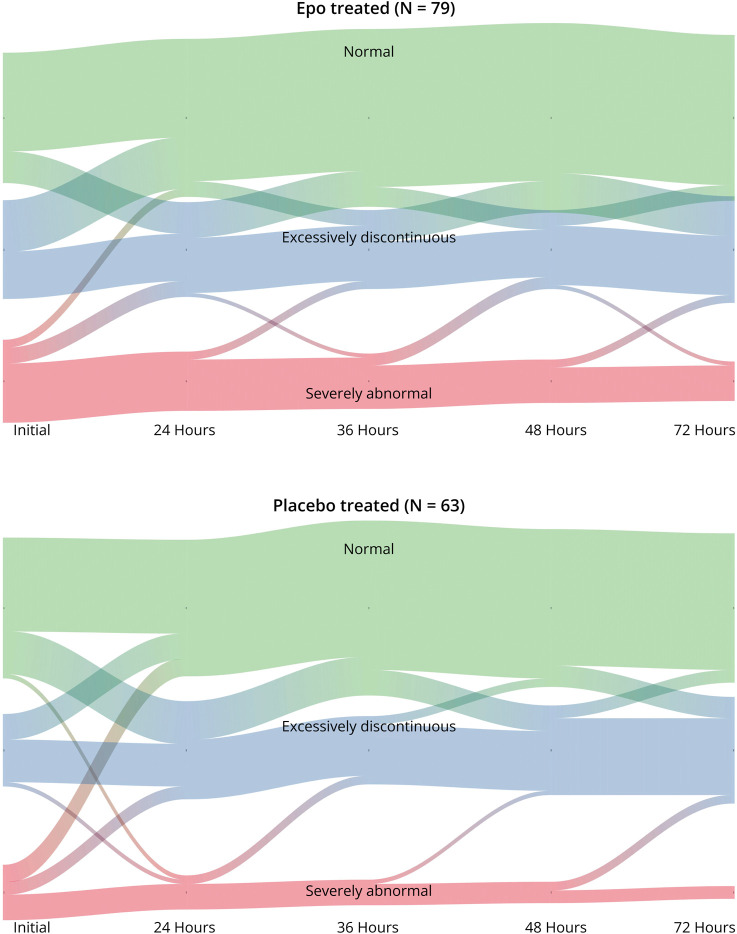
EEG background tended to improve (i.e., recover) over the course of therapeutic hypothermia (Figure 1). There was no difference in EEG recovery between the Epo and placebo groups.
Figure 1. River Plot Showing Relative Frequency of Neonates With Normal, Discontinuous, or Severely Abnormal EEG Over Time by Treatment Group for 142 Neonates With Hypoxic-Ischemic Encephalopathy (HIE) Treated With Erythropoietin or Placebo Who Were Monitored With Continuous Video EEG During Hypothermia.
Association Between EEG Background and Neurodevelopmental Outcome at Age 2 Years
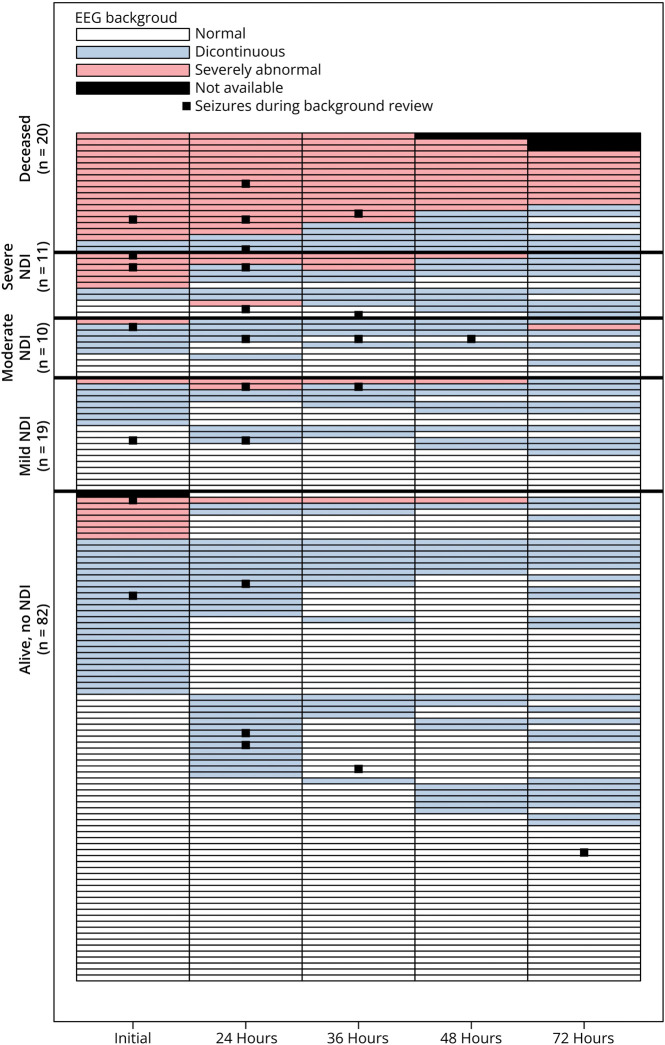
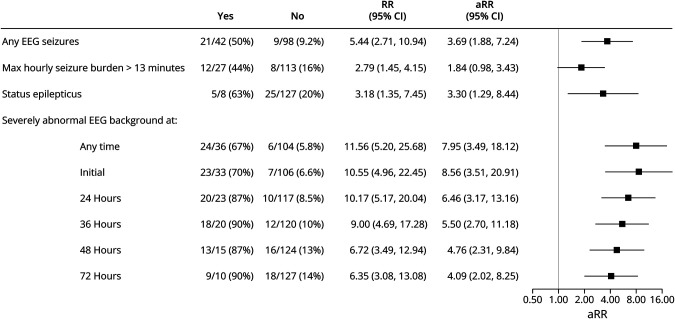
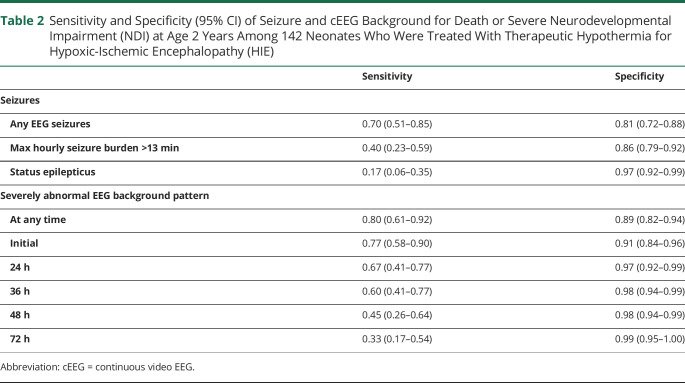
Figure 2 shows the EEG background at each epoch for each participant stratified by neurodevelopmental outcome at age 2 years. Figure 3 presents the unadjusted and adjusted relative risk of death or severe NDI by cEEG characteristic. The presence of EEG seizures, maximal hourly seizure burden >13 minutes, status epilepticus, or severely abnormal background at any time were all associated with increased risk of death or severe NDI. Among the 23 children with severely abnormal cEEG background at 24 hours, 20 (87%) had any NDI or death as compared with 10/117 (8.5%) of children without a severely abnormal cEEG background at 24 hours (adjusted relative risk 6.46 95% CI 3.17–13.16). Sensitivity and specificity of high seizure burden, status epilepticus, and severely abnormal cEEG at each time point are presented in Table 2.
Figure 2. Swimmer Plot of EEG Background and Seizures Over Time for 142 Neonates With Hypoxic-Ischemic Encephalopathy (HIE) Who Were Monitored With Continuous Video EEG During Hypothermia, Stratified by 2-Year Outcome.
Each row represents the EEG background pattern over time for 1 study participant.
Figure 3. Unadjusted Relative Risk (RR) and Adjusted Relative Risk (aRR) of Death or Severe NDI by EEG Characteristic for 142 Neonates With Hypoxic-Ischemic Encephalopathy (HIE) Who Were Monitored With Continuous Video EEG During Hypothermia.
Table 2.
Sensitivity and Specificity (95% CI) of Seizure and cEEG Background for Death or Severe Neurodevelopmental Impairment (NDI) at Age 2 Years Among 142 Neonates Who Were Treated With Therapeutic Hypothermia for Hypoxic-Ischemic Encephalopathy (HIE)
| Sensitivity | Specificity | |
| Seizures | ||
| Any EEG seizures | 0.70 (0.51–0.85) | 0.81 (0.72–0.88) |
| Max hourly seizure burden >13 min | 0.40 (0.23–0.59) | 0.86 (0.79–0.92) |
| Status epilepticus | 0.17 (0.06–0.35) | 0.97 (0.92–0.99) |
| Severely abnormal EEG background pattern | ||
| At any time | 0.80 (0.61–0.92) | 0.89 (0.82–0.94) |
| Initial | 0.77 (0.58–0.90) | 0.91 (0.84–0.96) |
| 24 h | 0.67 (0.41–0.77) | 0.97 (0.92–0.99) |
| 36 h | 0.60 (0.41–0.77) | 0.98 (0.94–0.99) |
| 48 h | 0.45 (0.26–0.64) | 0.98 (0.94–0.99) |
| 72 h | 0.33 (0.17–0.54) | 0.99 (0.95–1.00) |
Abbreviation: cEEG = continuous video EEG.
Predominantly Normal EEG Background
Among the 35 children with predominantly normal cEEG throughout hypothermia, 27 (77%) were normal, 6 (17%) had mild, and 2 (5.7%) had moderate NDI. None of these children died or were diagnosed with CP or severe NDI. The average cognitive score on the Bayley-III for children with predominantly normal EEG background was 97.6 (SD 12.9, eFigure 2, links.lww.com/WNL/D165).
Normal/Discontinuous, Predominantly Discontinuous, and Discontinuous/Severe EEG Background
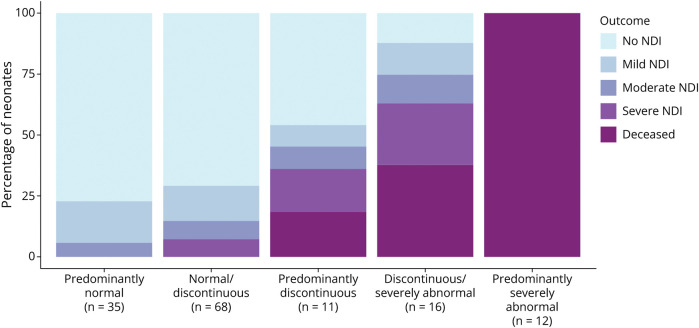
Children with a cEEG background categorized as normal/discontinuous, predominantly discontinuous, or discontinuous/severe had increasing risk of moderate and severe NDI or death (Figure 4). Among the 68 children with normal/discontinuous cEEG, 48 (71%) were normal, 10 (15%) were diagnosed with mild NDI, 5 (7.4%) with moderate NDI, and 5 (7.4%) with severe NDI. Four of 66 (6%) children evaluated at age 2 years were diagnosed with CP (1/66, 2% with GMFCS ≥III), and none died. The average cognitive score on the Bayley-III for children with normal/discontinuous neuromonitoring was 93.5 (SD 15.2).
Figure 4. Two-Year Neurodevelopmental Outcome by Predominant Background Pattern for 142 Neonates With Hypoxic-Ischemic Encephalopathy (HIE) Treated With Erythropoietin or Placebo Who Were Monitored With Continuous Video EEG During Hypothermia.
Among the 11 children with predominantly discontinuous cEEG, 5 (45%) were normal, 1 (9.1%) was diagnosed with mild NDI, 1 (9.1%) with moderate NDI, 2 (18%) with severe NDI, and 2 (18%) died. Among survivors, 1/9 (11%) children were diagnosed with CP (1/9, 11% with GMFCS ≥III). The average cognitive score on the Bayley-III for children with predominantly discontinuous cEEG background was 86.7 (SD 19.7).
Among the 16 children with discontinuous/severely abnormal EEG, 6 (38%) were normal, 2 (13%) were diagnosed with mild NDI, 2 (13%) with moderate NDI, 4 (25%) with severe NDI, and 6 (38%) died. Four of 10 (40%) survivors were diagnosed with CP (1/10, 10% with GMFCS ≥III). The average cognitive score on the Bayley-III for children with discontinuous/severely abnormal EEG background was 76.5 (SD 14.7).
Predominantly Severely Abnormal EEG Background
All 12 children with severely abnormal EEG background at all 5 time points died at a median age of 5 days (IQR 4–7.5).
Figure 4 presents the relationship between predominant cEEG background and the 5-level neurodevelopmental outcome. Increasing background severity across monitoring epochs was associated with increasingly worse clinical outcome (p < 0.001).
Sensitivity Analysis
After adjusting for morphine administration during the cEEG, the association between EEG background and outcome remained significant with minimal change in effect size.
Discussion
In this large, multicenter, prospective cohort of neonates with HIE who were treated with therapeutic hypothermia, EEG background acquired and assessed according to ACNS criteria and a novel, 5-level scoring system was strongly associated with neurodevelopmental outcome. EEG background trajectory was not different between the Epo and placebo study groups. Among children with predominantly normal EEG (i.e., normal EEG background at all epochs), <25% had NDI and none died, whereas among neonates with severely abnormal EEG background throughout the recording, all died. These results are in keeping with previous studies showing a robust association between EEG background and neurodevelopmental outcome1-9 and further refine this association to facilitate clinical decision-making, care planning, and parent communication.
This study supports existing literature showing an important association between neurophysiology monitoring and developmental outcome. There are 3 key novel aspects of our study. First, this study was nested within the randomized, controlled HEAL trial, which had strict inclusion and exclusion criteria, and qualifying and follow-up examinations were performed by a certified examiner. Second, each center in this study monitored newborns undergoing therapeutic hypothermia with gold standard continuous EEG according to ACNS criteria,13 2 neurophysiologists with expertise in neonatal neurophysiology reviewed each recording, and the EEG background was classified according to standardized ACNS terminology.15 The ACNS EEG background classification has good inter-rater agreement in previous studies14 and in this study. The ACNS terminology guidelines were initially proposed to unify practices around neonatal EEG interpretation to enhance reproducibility of research and clinical interpretation. We now present the largest study of neonates undergoing therapeutic hypothermia that has applied the rigorous, standardized classification in an analysis of how EEG background is associated with outcome. Finally, we used the standardized ACNS terminology to develop a novel, 5-level EEG background classification that considers both the degree and duration of background abnormality over the first 3 days after birth. Using ACNS terminology and our simple scoring system, the neurophysiologist and bedside clinician can now incorporate this novel tool into prognostic discussions with the family.
Although cEEG is recommended for neonates with HIE,13 this practice has not been uniformly adopted. Our findings support the utility of neurophysiologic monitoring throughout therapeutic hypothermia, given the important added prognostic information of cEEG background over time. Sensitivity and specificity of severely abnormal EEG background for death or severe NDI were very high at all time points, including within the first 24 hours after birth, suggesting that providers may initiate discussions about prognosis on the first day of therapeutic hypothermia. For centers with limited resources, recording cEEG for the first 24 hours after birth can provide some prognostic information. However, in rare cases, the EEG is severely abnormal at the onset of recording and improves rapidly; these children may have a favorable outcome. The small size of the subgroup of newborns with rapid EEG recovery limits our ability to use clinical data to investigate in detail which infants are most likely demonstrate rapid EEG recovery, and so recording longer than 24 hours remains of value. Previous studies have shown simplified neurophysiology monitoring such as amplitude-integrated EEG (aEEG)—which is increasingly used at centers that do not have capacity for continuous video EEG—is also associated with neurodevelopment.23-25 Whether aEEG can provide similarly robust prognostic information deserves further study.
We hypothesized that EEG recovery would be different among children who were treated with Epo vs placebo, like what was observed after the introduction of therapeutic hypothermia.19 However, the EEG background trajectory was similar between study groups, a finding that mirrors the results of the HEAL trial, which showed no difference in death or NDI between study groups.10
Although we present a large, multicenter cohort with centralized EEG interpretation by 2 pediatric neurophysiologists with extensive experience in neonatal EEG, our study has limitations. First, whether EEG reporting by a less experienced neurophysiologist would yield as robust an association is not certain. However, the high kappa for inter-rater reliability supports that the results are likely to be generalizable. Similarly, the standardized classification of background using ACNS terminology has previously been shown to have good inter-rater agreement.14 Further studies examining the relationship between expert centralized review and local clinical interpretations would further inform generalizability. Second, we examined the EEG by qualitative expert background interpretation at key epochs during therapeutic hypothermia. Whether quantitative methods of EEG analysis would provide equal or better predictive value is not known. Third, although we excluded infants who were identified as having a congenital or genetic condition known to affect neurodevelopment, there may be children who were diagnosed with these after the final follow-up and therefore included in the analysis. This is important because children with congenital or genetic conditions may have normal cEEG background and adverse neurodevelopment. Third, particularly for those infants with a persistently severely abnormal background, death may reflect redirection to palliative care, which may have been influenced by the EEG findings. While the neurophysiologists who interpreted the EEGs for this study were blinded to clinical course, the bedside clinicians were able to make decisions based on local interpretation of the cEEG. Fourth, EEG was applied at a median of 8.5 hours after birth, which is after the onset of cooling, and so we were unable to assess the effect of cooling on EEG background. Finally, of our study design, in which we reviewed 60 minutes of EEG background per epoch, for children with status epilepticus throughout the review epoch, we classified the results as “severely abnormal” as when an interictal background pattern could not be discerned.
cEEG background is strongly associated with neurodevelopment at age 2 years in neonates with HIE treated with therapeutic hypothermia. Children with normal cEEG background throughout therapeutic hypothermia are very likely to have normal neurodevelopment at age 2 years. The more severely abnormal the cEEG background and the more prolonged the abnormality, the higher the risk of NDI and death; children with a severely abnormal EEG throughout cooling were all deceased. Severely abnormal EEG background at any time point, including within the first 24 hours after birth, is an important risk factor for NDI. These results can be used to help health care providers personalize resources for follow-up care and counsel families for decision-making related to goals of care. Validation of our novel predominant cEEG background score as a prognostic tool in other neonatal cohorts will be important to confirm its utility in clinical practice. Further studies examining the added value of cEEG after accounting for clinical and MRI features are needed as combining neurophysiology measures with clinical and neuroimaging findings may provide the best prognostic accuracy, particularly for children with intermediate results on our novel predominant EEG background classification.24
Glossary
- ACNS
American Clinical Neurophysiology Society
- aEEG
amplitude-integrated EEG
- BSID-III
Bayley Scales of Infant Toddler Development, third edition
- cEEG
continuous video EEG monitoring
- CP
cerebral palsy
- Epo
erythropoietin
- GMFCS
Gross Motor Function Classification System
- IQR
interquartile range
- HEAL
High-Dose Erythropoietin for Asphyxia and Encephalopathy
- HIE
hypoxic-ischemic encephalopathy
- NDI
neurodevelopmental impairment
Appendix. Authors
| Name | Location | Contribution |
| Hannah C. Glass, MDCM, MAS | Departments of Neurology and Epidemiology & Biostatistics, and Weill Institute for Neuroscience, University of California, San Francisco | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Adam L. Numis, MD | Department of Neurology and Weill Institute for Neuroscience, University of California San Francisco, San Francisco, CA and Department of Pediatrics, UCSF Benioff Children's Hospital, University of California San Francisco, San Francisco, CA | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Bryan A. Comstock, MS | Department Biostatistics, University of Washington, Seattle, WA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Fernando F. Gonzalez, MD | Department of Pediatrics, UCSF Benioff Children's Hospital, University of California San Francisco, San Francisco, CA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Ulrike Mietzsch, MD | Department of Pediatrics, Division of Neonatology, University of Washington School of Medicine, Seattle Children's Hospital, Seattle, WA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sonia Lomeli Bonifacio, MD | Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University, Palo Alto, CA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Shavonne Massey, MD, MSCE | Departments of Neurology and Pediatrics, Children's Hospital of Philadelphia and Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Cameron Thomas, MD, MS | Department of Pediatrics, University of Cincinnati and Division of Neurology, Cincinnati Children' Hospital Medical Center, Cincinnati, OH | Drafting/revision of the manuscript for content, including medical writing for content |
| Niranjana Natarajan, MD | Department of Neurology, University of Washington School of Medicine, Seattle, WA | Drafting/revision of the manuscript for content, including medical writing for content |
| Dennis E. Mayock, MD | Department of Pediatrics, Division of Neonatology, University of Washington School of Medicine, Seattle Children's Hospital, Seattle, WA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Gregory M. Sokol, MD | Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Krisa P. Van Meurs, MD | Department of Pediatrics, Division of Neonatal and Developmental Medicine, Stanford University, Palo Alto, CA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Kaashif A. Ahmad, MD | Pediatrix Neonatology of San Antonio | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Nathalie Maitre, MD, PhD | Department of Pediatrics, and Emory + Children's Pediatric Institute, Emory University, Atlanta GA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Patrick J. Heagerty, MD | Department Biostatistics, University of Washington, Seattle, WA | Study concept or design; analysis or interpretation of data |
| Sandra E. Juul, MD, PhD | Department of Pediatrics, Division of Neonatology, University of Washington School of Medicine, Seattle Children's Hospital, Seattle, WA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Yvonne W. Wu, MD, MPH | Department of Neurology and Weill Institute for Neuroscience, University of California San Francisco, San Francisco, CA and Department of Pediatrics, UCSF Benioff Children's Hospital, University of California San Francisco, San Francisco, CA | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Courtney J. Wusthoff, MD | Department of Neurology, Stanford University, Palo Alto, CA | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
Study Funding
The study was funded by NIH/NINDS R01NS104322, U01NS092764, and U01NS092553. Adam L. Numis, MD, received grant support during the study period from NINDS K23NS105918.
Disclosure
K.A. Ahmad, S.L. Bonifacio, B.A. Comstock report no disclosures; H.C. Glass is funded by NIH R01NS104322, R01NS111166, R01NS124051, and her spouse holds shares in Elemeno Health; F.F. Gonzalez, P.J. Heagerty, S.E. Juul, N. Maitre, S.L. Massey, D.E. Mayock, U. Mietzsch, N. Natarajan, A.L. Numis, G.M. Sokol, C. Thomas, K.P. Van Meurs, Y.W. Wu report no disclosures; C.J. Wusthoff reports: disclosures: Scientific Advisory Boards (1) PRA Health Sciences- Data And Safety Monitoring (2) ICON- Data And Safety Monitoring Editorial Boards (1) Journal of Clinical Neurophysiology, Associate Editor, 2020–2022; editorial board 2022-current (2) Neurology, Associate Editor, 2022-current Research Support, Government Entities (1) NIH/NINDS, 1K02NS102598, PI, 2017–2020 (2) PCORI 1507–31187, study neurophysiologist, 2016–2020 (3) NIH/NINDS, 1R01NS104322-01A1, co-investigator, 2018–2022 (4) NIH/NINDS, 1R01NS111166-01A1, co-investigator, 2020–2024 Research Support, Foundations and Societies (1) Thrasher Research Fund 2020-2023. Go to Neurology.org/N for full disclosures.
References
- 1.Holmes GL, Lombroso CT. Prognostic value of background patterns in the neonatal EEG. J Clin Neurophysiol. 1993;10(3):323-352. doi: 10.1097/00004691-199307000-00008 [DOI] [PubMed] [Google Scholar]
- 2.Hellstrom-Westas L, Rosen I, Svenningsen NW. Predictive value of early continuous amplitude integrated EEG recordings on outcome after severe birth asphyxia in full term infants. Arch Dis Child Fetal Neonatal Ed. 1995;72(1):F34-F38. doi: 10.1136/fn.72.1.f34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology. 2011;76(6):556-562. doi: 10.1212/WNL.0b013e31820af91a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourel-Ponchel E, Querne L, Flamein F, Ghostine-Ramadan G, Wallois F, Lamblin MD. The prognostic value of neonatal conventional-EEG monitoring in hypoxic-ischemic encephalopathy during therapeutic hypothermia. Dev Med Child Neurol. 2023;65(1):58-66. doi: 10.1111/dmcn.15302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Wispelaere LA, Ouwehand S, Olsthoorn M, et al. Electroencephalography and brain magnetic resonance imaging in asphyxia comparing cooled and non-cooled infants. Eur J Paediatr Neurol. 2019;23(1):181-190. doi: 10.1016/j.ejpn.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 6.Han Y, Fu N, Chen W, et al. Prognostic value of electroencephalography in hypothermia-treated neonates with hypoxic-ischemic encephalopathy: a meta-analysis. Pediatr Neurol. 2019;93:3-10. doi: 10.1016/j.pediatrneurol.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 7.van Laerhoven H, de Haan TR, Offringa M, Post B, van der Lee JH. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: a systematic review. Pediatrics. 2013;131(1):88-98. doi: 10.1542/peds.2012-1297 [DOI] [PubMed] [Google Scholar]
- 8.Weeke LC, Boylan GB, Pressler RM, et al. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol. 2016;20(6):855-864. doi: 10.1016/j.ejpn.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 9.Polat M, Simşek A, Tansuğ N, et al. Prediction of neurodevelopmental outcome in term neonates with hypoxic-ischemic encephalopathy. Eur J Paediatr Neurol. 2013;17(3):288-293. doi: 10.1016/j.ejpn.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Wu YW, Comstock BA, Gonzalez FF, et al. Trial of erythropoietin for hypoxic-ischemic encephalopathy in newborns. N Engl J Med. 2022;387(2):148-159. doi: 10.1056/NEJMoa2119660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass HC, Wusthoff CJ, Comstock BA, et al. Risk of seizures in neonates with hypoxic-ischemic encephalopathy receiving hypothermia plus erythropoietin or placebo. Pediatr Res. 2023;94(1):252-259. doi: 10.1038/s41390-022-02398-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juul SE, Comstock BA, Heagerty PJ, et al. High-dose erythropoietin for asphyxia and encephalopathy (HEAL): a randomized controlled trial–background, aims, and study protocol. Neonatology. 2018;113(4):331-338. doi: 10.1159/000486820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society's guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28(6):611-617. doi: 10.1097/WNP.0b013e31823e96d7 [DOI] [PubMed] [Google Scholar]
- 14.Wusthoff CJ, Sullivan J, Glass HC, et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia. 2017;58(3):429-435. doi: 10.1111/epi.13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30(2):161-173. doi: 10.1097/WNP.0b013e3182872b24 [DOI] [PubMed] [Google Scholar]
- 16.Kuban KC, Allred EN, O'Shea M, Paneth N, Pagano M, Leviton A, ELGAN Study Cerebral Palsy-Algorithm Group. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153(4):466-472. doi: 10.1016/j.jpeds.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214-223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M. The sankey diagram in energy and material flow management. Part II: methodology and current applications. J Ind Ecol. 2008;12(2):173-185. doi: 10.1111/j.1530-9290.2008.00015.x [DOI] [Google Scholar]
- 19.Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126(1):e131-e139. doi: 10.1542/peds.2009-2938 [DOI] [PubMed] [Google Scholar]
- 20.Kharoshankaya L, Stevenson NJ, Livingstone V, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic-ischemic encephalopathy. Dev Med Child Neurol. 2016;58(12):1242-1248. doi: 10.1111/dmcn.13215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Team R Core. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2022. [Google Scholar]
- 22.Wu YW, Monsell SE, Glass HC, et al. How well does neonatal neuroimaging correlate with neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy? Pediatr Res. 2023;94(3):1018-1025. doi: 10.1038/s41390-023-02510-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancora G, Maranella E, Grandi S, et al. Early predictors of short term neurodevelopmental outcome in asphyxiated cooled infants. A combined brain amplitude integrated electroencephalography and near infrared spectroscopy study. Brain Dev. 2013;35(1):26-31. doi: 10.1016/j.braindev.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Steiner M, Urlesberger B, Giordano V, et al. Outcome prediction in neonatal hypoxic-ischaemic encephalopathy using neurophysiology and neuroimaging. Neonatology. 2022;119(4):483-493. doi: 10.1159/000524751 [DOI] [PubMed] [Google Scholar]
- 25.Weeke LC, Vilan A, Toet MC, van Haastert IC, de Vries LS, Groenendaal F. A comparison of the thompson encephalopathy score and amplitude-integrated electroencephalography in infants with perinatal asphyxia and therapeutic hypothermia. Neonatology. 2017;112(1):24-29. doi: 10.1159/000455819 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HEAL Trial data sharing plan is available elsewhere.22