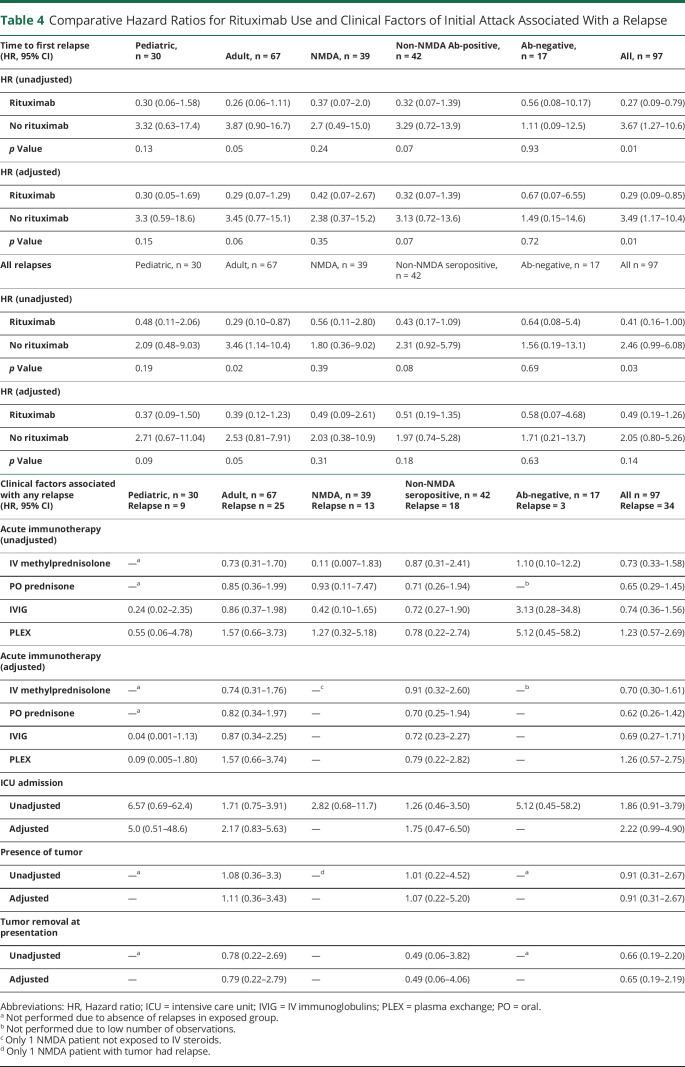
Table 4.
Comparative Hazard Ratios for Rituximab Use and Clinical Factors of Initial Attack Associated With a Relapse
| Time to first relapse (HR, 95% CI) | Pediatric, n = 30 | Adult, n = 67 | NMDA, n = 39 | Non-NMDA Ab-positive, n = 42 | Ab-negative, n = 17 | All, n = 97 |
| HR (unadjusted) | ||||||
| Rituximab | 0.30 (0.06–1.58) | 0.26 (0.06–1.11) | 0.37 (0.07–2.0) | 0.32 (0.07–1.39) | 0.56 (0.08–10.17) | 0.27 (0.09–0.79) |
| No rituximab | 3.32 (0.63–17.4) | 3.87 (0.90–16.7) | 2.7 (0.49–15.0) | 3.29 (0.72–13.9) | 1.11 (0.09–12.5) | 3.67 (1.27–10.6) |
| p Value | 0.13 | 0.05 | 0.24 | 0.07 | 0.93 | 0.01 |
| HR (adjusted) | ||||||
| Rituximab | 0.30 (0.05–1.69) | 0.29 (0.07–1.29) | 0.42 (0.07–2.67) | 0.32 (0.07–1.39) | 0.67 (0.07–6.55) | 0.29 (0.09–0.85) |
| No rituximab | 3.3 (0.59–18.6) | 3.45 (0.77–15.1) | 2.38 (0.37–15.2) | 3.13 (0.72–13.6) | 1.49 (0.15–14.6) | 3.49 (1.17–10.4) |
| p Value | 0.15 | 0.06 | 0.35 | 0.07 | 0.72 | 0.01 |
| All relapses | Pediatric, n = 30 | Adult, n = 67 | NMDA, n = 39 | Non-NMDA seropositive, n = 42 | Ab-negative, n = 17 | All n = 97 |
| HR (unadjusted) | ||||||
| Rituximab | 0.48 (0.11–2.06) | 0.29 (0.10–0.87) | 0.56 (0.11–2.80) | 0.43 (0.17–1.09) | 0.64 (0.08–5.4) | 0.41 (0.16–1.00) |
| No rituximab | 2.09 (0.48–9.03) | 3.46 (1.14–10.4) | 1.80 (0.36–9.02) | 2.31 (0.92–5.79) | 1.56 (0.19–13.1) | 2.46 (0.99–6.08) |
| p Value | 0.19 | 0.02 | 0.39 | 0.08 | 0.69 | 0.03 |
| HR (adjusted) | ||||||
| Rituximab | 0.37 (0.09–1.50) | 0.39 (0.12–1.23) | 0.49 (0.09–2.61) | 0.51 (0.19–1.35) | 0.58 (0.07–4.68) | 0.49 (0.19–1.26) |
| No rituximab | 2.71 (0.67–11.04) | 2.53 (0.81–7.91) | 2.03 (0.38–10.9) | 1.97 (0.74–5.28) | 1.71 (0.21–13.7) | 2.05 (0.80–5.26) |
| p Value | 0.09 | 0.05 | 0.31 | 0.18 | 0.63 | 0.14 |
| Clinical factors associated with any relapse (HR, 95% CI) | Pediatric, n = 30 Relapse n = 9 |
Adult, n = 67 Relapse n = 25 |
NMDA, n = 39 Relapse n = 13 |
Non-NMDA seropositive, n = 42 Relapse = 18 |
Ab-negative, n = 17 Relapse = 3 |
All n = 97 Relapse = 34 |
| Acute immunotherapy (unadjusted) | ||||||
| IV methylprednisolone | —a | 0.73 (0.31–1.70) | 0.11 (0.007–1.83) | 0.87 (0.31–2.41) | 1.10 (0.10–12.2) | 0.73 (0.33–1.58) |
| PO prednisone | —a | 0.85 (0.36–1.99) | 0.93 (0.11–7.47) | 0.71 (0.26–1.94) | —b | 0.65 (0.29–1.45) |
| IVIG | 0.24 (0.02–2.35) | 0.86 (0.37–1.98) | 0.42 (0.10–1.65) | 0.72 (0.27–1.90) | 3.13 (0.28–34.8) | 0.74 (0.36–1.56) |
| PLEX | 0.55 (0.06–4.78) | 1.57 (0.66–3.73) | 1.27 (0.32–5.18) | 0.78 (0.22–2.74) | 5.12 (0.45–58.2) | 1.23 (0.57–2.69) |
| Acute immunotherapy (adjusted) | ||||||
| IV methylprednisolone | —a | 0.74 (0.31–1.76) | —c | 0.91 (0.32–2.60) | —b | 0.70 (0.30–1.61) |
| PO prednisone | —a | 0.82 (0.34–1.97) | — | 0.70 (0.25–1.94) | — | 0.62 (0.26–1.42) |
| IVIG | 0.04 (0.001–1.13) | 0.87 (0.34–2.25) | — | 0.72 (0.23–2.27) | — | 0.69 (0.27–1.71) |
| PLEX | 0.09 (0.005–1.80) | 1.57 (0.66–3.74) | — | 0.79 (0.22–2.82) | — | 1.26 (0.57–2.75) |
| ICU admission | ||||||
| Unadjusted | 6.57 (0.69–62.4) | 1.71 (0.75–3.91) | 2.82 (0.68–11.7) | 1.26 (0.46–3.50) | 5.12 (0.45–58.2) | 1.86 (0.91–3.79) |
| Adjusted | 5.0 (0.51–48.6) | 2.17 (0.83–5.63) | — | 1.75 (0.47–6.50) | — | 2.22 (0.99–4.90) |
| Presence of tumor | ||||||
| Unadjusted | —a | 1.08 (0.36–3.3) | —d | 1.01 (0.22–4.52) | —a | 0.91 (0.31–2.67) |
| Adjusted | — | 1.11 (0.36–3.43) | — | 1.07 (0.22–5.20) | — | 0.91 (0.31–2.67) |
| Tumor removal at presentation | ||||||
| Unadjusted | —a | 0.78 (0.22–2.69) | — | 0.49 (0.06–3.82) | —a | 0.66 (0.19–2.20) |
| Adjusted | — | 0.79 (0.22–2.79) | — | 0.49 (0.06–4.06) | — | 0.65 (0.19–2.19) |
Abbreviations: HR, Hazard ratio; ICU = intensive care unit; IVIG = IV immunoglobulins; PLEX = plasma exchange; PO = oral.
Not performed due to absence of relapses in exposed group.
Not performed due to low number of observations.
Only 1 NMDA patient not exposed to IV steroids.
Only 1 NMDA patient with tumor had relapse.