In the article by Paul J. Wang et al, “Randomized Clinical Trial to Evaluate an Atrial Fibrillation Stroke Prevention Shared Decision‐Making Pathway,” which published online February 7, 2023 (J Am Heart Assoc. 023;11:e028562. DOI: 10.1161/JAHA.122.028562) and was included in the February 7, 2023 issue of the journal, corrections were needed.
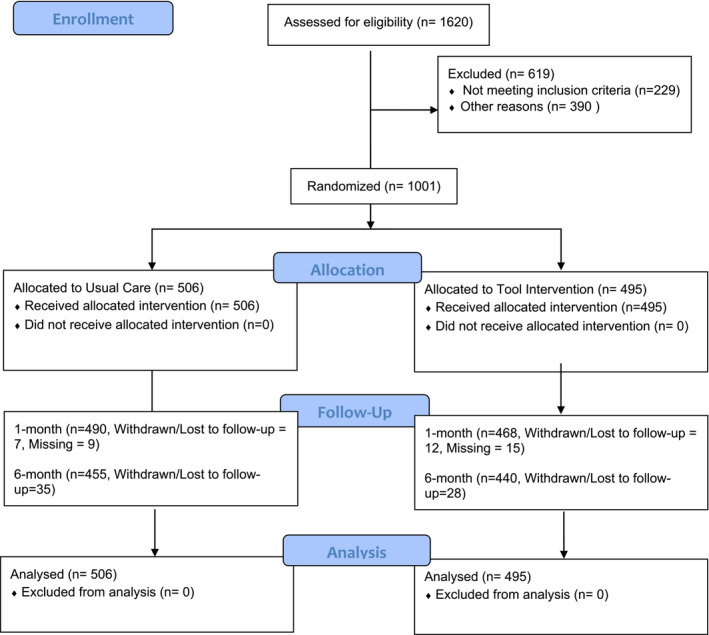
In Figure 2, the number of patients included in the 6‐month follow‐up for the tool allocation intervention was incorrectly reported as 439, and the number reported as withdrawn/lost to follow‐up was incorrectly reported as 29. However, one initially reported death was determined to have occurred after a participant had completed the study. This figure has been corrected.
Figure 2. Consolidated standards of reporting trials diagram.
In Table S2, in the tool intervention arm, the number of patients who experienced any adverse events (AEs) was incorrectly reported as 47, the number of patients who experienced serious AEs was incorrectly reported as 31, the number of serious AEs that were unexpected and not related was incorrectly reported as 36, and the number of deaths was incorrectly reported as 8. These errors have been corrected.
Table S2.
Clinical Outcomes and Adverse Events (AEs)
| Usual care (N=506) | Tool intervention (N=495) | P value | |
|---|---|---|---|
| Patients experienced any AEs, n (%) | 58 (11.5%) | 46 (9.3%) | 0.30 |
| Patients experienced serious AEs, n (%) | 44 (8.7%) | 30 (6.0%) | 0.12 |
| Number of serious AEs, n | 65 | 41 | |
| Not‐related and expected, n | 21 | 6 | |
| Not‐related and unexpected, n | 44 | 35 | |
| Number of deaths* | 11 | 7 | 0.48 |
The authors regret the errors.
The online version of the article has been updated and is available here: https://www.ahajournals.org/doi/10.1161/JAHA.122.028562.
*All deaths are unrelated and unexpected.


