Abstract
Background
Complex percutaneous coronary intervention (PCI) is increasingly performed in older adults (age ≥75 years) with stable ischemic heart disease. However, little is known about clinical outcomes.
Methods and Results
We derived a cohort of older adults undergoing elective PCI for stable ischemic heart disease across a large health system. We compared 12‐month event‐free survival (freedom from all‐cause death, nonfatal myocardial infarction, stroke, and major bleeding), all‐cause death, target lesion revascularization, and bleeding events for patients receiving complex versus noncomplex PCI and derived risk estimates with Cox regression models. We included 513 patients (mean age, 81±5 years). Patients receiving complex PCI versus noncomplex PCI did not significantly differ across a host of clinical characteristics including cardiovascular disease features, noncardiac comorbidities, guideline‐directed medical therapy use, and frailty. Patients receiving complex PCI versus noncomplex PCI experienced worse event‐free survival (80.4% versus 86.8%), which was not significant in adjusted analyses (hazard ratio [HR], 1.38 [95% CI, 0.88–2.16]). All‐cause death at 1 year for patients undergoing complex PCI was nearly double that seen for patients receiving noncomplex PCI (10.2% versus 5.9%), and the risk was significant in models adjusted for clinical characteristics (HR, 1.97 [95% CI, 1.02–3.79]). Target lesion revascularization risk was lower for patients receiving complex PCI (2.2% versus 3.5%, adjusted HR), but bleeding events were not statistically different between groups (25.3% versus 20.5%; P=0.19).
Conclusions
Complex PCI in older adults with stable ischemic heart disease was associated with lower risk of target lesion revascularization but higher all‐cause death compared with noncomplex PCI.
Keywords: complex percutaneous coronary intervention, coronary artery disease, older adults, revascularization
Subject Categories: Percutaneous Coronary Intervention, Catheter-Based Coronary and Valvular Interventions, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- EFS
event‐free survival
- SIHD
stable ischemic heart disease
- STICH
Surgical Treatment in Ischemic Heart Failure
- TLR
target lesion revascularization
Clinical Perspective.
What Is New?
In this detailed retrospective review of older adults receiving complex percutaneous coronary intervention for stable ischemic heart disease, we observed higher all‐cause death in the complex percutaneous coronary intervention group compared with the noncomplex PCI group after adjusting for clinical characteristics and percutaneous coronary intervention features.
What Are the Clinical Implications?
Further investigation of the safety of elective complex percutaneous coronary intervention in older adults is needed as these procedures become more common in the older adult population.
National surveys project exponential growth of the older adult population during the 21st century. 1 Cardiovascular disease is the leading cause of death in older adults (age ≥75 years) 2 and coronary artery disease (CAD) is a substantial contributor to morbidity and death for this age group. 2 , 3 Distinct from younger patients with CAD, older adults with CAD have additional unique medical considerations, such as frailty, multimorbidity, and increased periprocedural risks, which make CAD management more challenging. 4 Therefore, further investigation into the optimal management of CAD in older adults represents a pressing need.
While coronary artery revascularization for older patients with acute coronary syndrome is often indicated to improve clinical outcomes, revascularization for stable ischemic heart disease (SIHD) is primarily indicated for patients with persistent symptoms despite optimal medical therapy or in select cases where anatomy and clinical characteristics suggest survival benefit (ie, left main disease). 5 , 6 , 7 Older adults frequently have more complex coronary anatomy compared with younger patients with CAD; in addition, they are often deemed prohibitively high risk for surgical coronary artery bypass grafting (CABG) and have a higher risk aversion to CABG. 3 , 8 , 9 , 10 Complex percutaneous coronary intervention (PCI) for SIHD is thus becoming increasingly common in older adults. 8 , 11 Unfortunately, available data are limited to inform complex PCI in older adults, and a consistent definition of complex PCI is lacking. 12 A number of studies have evaluated the association between advanced chronologic age and increasing risk, reporting worse outcomes and higher rates of complications with PCI among older adults compared with younger populations 8 , 13 , 14 , 15 , 16 , 17 ; however, no studies have specifically looked at how the risk of complex PCI compares with that of noncomplex PCI within the older adult population to more precisely determine the specific risk conferred by the increasing complexity of the intervention. Given the paucity of complex PCI data in this population, coupled with the increasing prevalence in clinical practice, focused investigations of complex interventions in older adults are needed to elucidate whether the benefits in this at‐risk population outweigh the risks of intervention. 18
In this study of complex PCI in older adults, we examine death, major cardiovascular events, target lesion revascularization (TLR), and bleeding at 1 year in older adults undergoing complex versus noncomplex PCI for SIHD. We propose a definition of complex PCI based on procedures with inherent technical risk, such as bifurcation lesions and atherectomy, though we secondarily examine complex PCI based on stent and lesion length numeric cutoffs as has been done previously by other groups. 18 , 19 , 20 Finally, we compare bleeding events between patients who underwent complex and noncomplex PCI and derive risk estimates for each outcome in analyses adjusted for patient demographics and clinical characteristics.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Data Source
We collected data on all older adults (defined as aged ≥75 years) undergoing PCI from June 25, 2018, to March 30, 2021, at a large academic health system composed of 5 network hospitals. All variables were manually extracted from the electronic medical record by the study authors (J.H. and S.W.) with discrepancies resolved by the senior author (M.N.). Data were obtained from surrounding area hospitals that use the same electronic medical record system (Epic; Epic Systems, Verona, WI) to maximize capture of all outcomes via Care Everywhere. Outside records were specifically examined for cardiology outpatient notes and hospital admissions.
Study Population
Older adults (aged ≥75 years) undergoing elective PCI for SIHD during the study period were included. The following patients were excluded: (1) patients undergoing emergent or urgent PCI; and (2) patients lost to follow‐up (defined as complete loss of contact with the medical system before the end of the study period) within 1 year of the index revascularization (which includes the initial PCI and any subsequent staged PCI, if applicable; ie, the clock starts at the staged procedure, if performed). Patients undergoing elective PCI following myocardial infarction (MI), for example, as a staged procedure to emergent or urgent revascularization, were included.
Complex PCI Definition
We defined complex PCI as any PCI including a procedure or lesion that may carry inherently elevated risk of complications or PCI failure. As is typically done in the literature, we make a distinction between complex PCI (which is procedure‐focused) and high‐risk PCI (which is patient‐focused) and do not include heart failure or other high‐risk features in the definition that do not necessarily create technical complexity. 21 Procedure‐based criteria included multivessel PCI (≥3 vessels [including branches] or ≥2 vessels if including an unprotected left main coronary artery, or proximal left anterior descending artery), intervention on an unprotected left main coronary artery, saphenous vein graft, bifurcation (defined as at least 1 intervention [angioplasty or stenting] to each branch at a bifurcation; simple provisional wiring of the side branch did not qualify), or PCI involving atherectomy (including laser and lithotripsy). Lesion‐based criteria included intervention on a lesion with severe calcification (as judged by the operator) or a chronic total occlusion. Cases referred for CABG, as mentioned in cardiology notes, where the patient refused or was deemed ineligible for CABG were also considered complex PCI. Noncomplex PCI was defined as any procedure that did not meet the definition for complex PCI.
There is no uniform definition of complex PCI in the literature. 12 Some authors have used a definition of complex PCI that relies heavily on the number and length of the lesions and stents. 18 , 19 , 20 For comparability, we performed a sensitivity analysis using an alternative definition of complex PCI defined as multivessel PCI, ≥3 stents implanted, ≥3 lesions treated, total stent length >60 mm, total lesion length >30 mm, or bifurcation or chronic total occlusion intervention. 18 , 19 , 20
Study Covariates
We identified the following patient characteristics as possibly associated with complex PCI outcomes: (1) demographics (age, sex, race, marital status); (2) body mass index; (3) cardiovascular characteristics and risk factors (hypertension, hyperlipidemia, diabetes, smoking status [never, prior, or current smoker; smoking and marital status recorded as documented at time of data collection], prior MI, PCI, CABG, or cerebrovascular accident [including ischemic and hemorrhagic stroke and transient ischemic attack], history of heart failure [preserved, midrange, or reduced ejection fraction], peripheral artery disease, severe valvular disease, atrial fibrillation, or chronic kidney disease [with or without dialysis dependence]); (4) other comorbidities (chronic obstructive pulmonary disease, malignancy [excluding nonmelanoma skin cancer], cirrhosis, alcohol dependence, anemia, falls, gastrointestinal bleeding, depression); (5) complete guideline‐directed medical therapy PCI (defined as prescriptions on discharge for 1 of each of the following drugs [any drug within the classes listed was acceptable]: aspirin [or anticoagulation], P2Y12 inhibitor, high‐intensity statin, beta‐blocker, renin‐angiotensin system blocker [either angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor–neprilysin inhibitor]); and (6) frailty variables (specifically the home functioning variables used in the National Cardiovascular Data Registry: gait [0=independent, 1=walks with assistance, 2=wheelchair bound], cognition [0=normal, 1=mild impairment, 2=dementia], and dependence for activities or instrumental activities of daily living [0=independent, 1=partially dependent, 2=fully dependent]; scores in each of the 3 domains were added into a cumulative frailty index ranging from 0 to 6). 22
The following PCI‐related covariates were also extracted: (1) intravascular imaging (intravascular ultrasound or optical coherence tomography), (2) fractional flow reserve (including instantaneous wave‐free ratio; for lesions intervened upon), (3) brachytherapy, and (4) bivalirudin or glycoprotein IIb/IIIa inhibitor use.
Finally, the following physician and system covariates were extracted: (1) physician performing the PCI and (2) network‐member hospital where the PCI was performed. Physician operators were classified according to their complex PCI volume, specifically as either high‐ or low‐volume operators (high‐volume=in the top 10% of complex PCI operators by volume). PCI procedures captured in this data set were performed at only 3 of the 5 network‐member hospitals (designated as sites 1, 2, and 3).
Study Outcomes
The primary end point was event‐free survival (EFS) at 12 months following index revascularization, defined as freedom from all‐cause death, nonfatal MI, nonfatal stroke, and major bleeding (bleeding was defined according to the Bleeding Academic Research Consortium bleeding criteria 23 ; all PCI periprocedural and post‐PCI bleeding events were eligible; Bleeding Academic Research Consortium stage 3 or 5 was considered major bleeding 24 ). Major bleeding was included in the EFS composite, as older adults are at particularly high risk for major bleeds following PCI, which carry a profound clinical impact in this population. 24 Secondary outcomes included 12‐month all‐cause death, TLR (defined as intervention on the lesion treated during the index PCI within the subsequent 12 months), and all bleeding events (Bleeding Academic Research Consortium 1–3 and 5). Nonfatal MI, nonfatal stroke, and cardiovascular death (defined as death from any cardiac cause, including cardiac arrest) 25 were also described separately from the EFS composite. Patients without a clearly documented cause of death were not included in the cardiovascular death analysis. Cause of death was determined by physician review of all clinical documentation around the time of death for any information relevant to the cause of death. All outcomes were manually extracted from physician notes and other documentation within the medical record and did not rely on billing codes.
Statistical Analysis
Patients were divided into 2 groups: those receiving complex PCI and those receiving noncomplex PCI. We derived descriptive statistics for each study group. Patient characteristics between complex and noncomplex PCI groups were compared using the Mann–Whitney U test for numeric variables and the chi‐square test for categorical variables.
For the primary EFS end point, we developed Kaplan–Meier curves for both the complex and noncomplex PCI groups. We then constructed multivariable Cox proportional hazards regression models using forward variable selection with EFS as the dependent. Forward selection criteria were based on optimizing the model according to the Akaike information criterion. Candidate variables for the forward selection were any covariates identified by the authors as possible confounders of the association between EFS and complex PCI group. The candidate independent variables for the forward selection procedure included the exposure (PCI group), baseline characteristics (patient demographics, comorbidities, frailty scores, complete guideline‐directed medical therapy following PCI) and PCI features (use of any intravascular imaging, fractional flow reserve/instantaneous wave‐free ratio, bivalirudin, glycoprotein Iib/IIIa inhibitor, or brachytherapy as well as operator volume and hospital network site). The factor levels used in the model can be found where these variables are displayed in Tables 1 and 2. Risk estimates for all‐cause death were derived from the models for patients receiving complex PCI as compared with the reference noncomplex PCI group. We repeated these procedures for the other end points. Of note, forward selection was performed separately for each individual model.
Table 1.
Baseline Characteristics
| Total cohort | Noncomplex PCI | Complex PCI | ||
|---|---|---|---|---|
| N=513 | N=288 | N=225 | P value | |
| Sociodemographics | ||||
| Age, y | 0.42 | |||
| Median (interquartile range) | 81.0 (77.0–84.0) | 81.1 (4.46) | 80.0 (77.0–85.0) | |
| Sex, n (%) | 0.01 | |||
| Female | 142 (27.7) | 94 (32.6) | 48 (21.3) | |
| Race, n (%) | 0.39 | |||
| Non‐White | 19 (3.70) | 13 (4.5) | 6 (2.7) | |
| Marital status, n (%) | 0.01 | |||
| Married | 308 (60.0) | 159 (55.2) | 149 (66.2) | |
| Cardiovascular disease and risk factors | ||||
| Body mass index | 0.81 | |||
| Median (Interquartile range) | 28.0 (25.0–32.0) | 28.0 (25.0–32.0) | 28.0 (25.0–31.0) | |
| Smoking status, n (%) | 0.65 | |||
| Prior or current | 340 (66.3) | 188 (65.3) | 152 (67.6) | |
| Hypertension, n (%) | 0.72 | |||
| Yes | 451 (87.9) | 255 (88.5) | 196 (87.1) | |
| Hyperlipidemia, n (%) | 0.54 | |||
| Yes | 437 (86.7) | 244 (85.6) | 193 (88.1) | |
| Diabetes, n (%) | 0.87 | |||
| Yes | 175 (34.7) | 97 (34.0) | 78 (35.6) | |
| Chronic kidney disease, n (%) | 0.95 | |||
| Yes | 93 (18.1) | 53 (18.4) | 40 (17.8) | |
| Dialysis, n (%) | 0.48 | |||
| Yes | 8 (1.6) | 3 (1.0) | 5 (2.2) | |
| Prior myocardial infarction, n (%) | 0.13 | |||
| Yes | 102 (19.9) | 50 (17.4) | 52 (23.1) | |
| Prior PCI, n (%) | 0.39 | |||
| Yes | 192 (37.4) | 113 (39.2) | 79 (35.1) | |
| Prior coronary bypass surgery, n (%) | <0.001 | |||
| Yes | 108 (21.4) | 45 (15.8) | 63 (28.8) | |
| Prior cerebrovascular accident, n (%) | 0.54 | |||
| Yes | 68 (13.3) | 41 (14.2) | 27 (12.0) | |
| Peripheral artery disease, n (%) | 0.50 | |||
| Yes | 139 (27.1) | 74 (25.7) | 65 (28.9) | |
| Heart failure, n (%) | 0.09 | |||
| Preserved, midrange, or reduced | 148 (28.9) | 74 (25.7) | 74 (32.9) | |
| Severe valvular disease, n (%) | 0.33 | |||
| Yes | 107 (20.9) | 65 (22.6) | 42 (18.7) | |
| Atrial fibrillation, n (%) | 0.76 | |||
| Yes | 130 (25.3) | 75 (26.0) | 55 (24.4) | |
| Comorbidities | ||||
| Chronic obstructive lung disease, n (%) | 0.81 | |||
| Yes | 63 (12.3) | 34 (11.8) | 29 (12.9) | |
| Cirrhosis, n (%) | 0.37 | |||
| Yes | 2 (0.4) | 0 (0.0) | 2 (0.9) | |
| Malignancy, n (%) | 0.25 | |||
| Yes | 112 (21.8) | 57 (19.8) | 55 (24.4) | |
| Anemia, n (%) | 0.15 | |||
| Yes | 38 (7.4) | 26 (9.0) | 12 (5.3) | |
| Prior gastrointestinal bleed, n (%) | 0.43 | |||
| Yes | 31 (6.0) | 20 (6.9) | 11 (4.9) | |
| Alcohol use disorder, n (%) | 1.00 | |||
| Yes | 9 (1.8) | 5 (1.7) | 4 (1.8) | |
| Depression, n (%) | 0.55 | |||
| Yes | 37 (7.2) | 23 (8.0) | 14 (6.2) | |
| Geriatric comorbidities | ||||
| Dementia, n (%) | 0.24 | |||
| Yes | 5 (1.0) | 1 (0.3) | 4 (1.8) | |
| Frailty score, n (%) | 0.88 | |||
| 0 | 365 (71.2) | 204 (70.8) | 161 (71.6) | |
| 1 | 116 (22.6) | 67 (23.3) | 49 (21.8) | |
| 2–6 | 32 (6.2) | 17 (5.9) | 15 (6.7) | |
| Pharmacotherapy following PCI, n (%) | ||||
| Full guideline‐directed therapy | 0.15 | |||
| Yes | 133 (26.0) | 67 (23.3) | 66 (29.3) | |
| Aspirin | 0.49 | |||
| Yes | 425 (82.8) | 242 (84.0) | 183 (81.3) | |
| Anticoagulation | 1.00 | |||
| DOAC or warfarin | 126 (24.6) | 71 (24.7) | 55 (24.4) | |
| High‐intensity statin | 0.60 | |||
| Yes | 284 (55.4) | 156 (54.2) | 128 (56.9) | |
| Renin system blockade | 0.60 | |||
| Yes | 300 (58.5) | 165 (57.3) | 135 (60.0) | |
| Beta blocker | 0.56 | |||
| Yes | 373 (72.7) | 206 (71.5) | 167 (74.2) | |
| P2Y12 inhibitor | 0.06 | |||
| Yes | 490 (95.5) | 280 (97.2) | 210 (93.3) | |
| Dual antiplatelet therapy | 0.64 | |||
| Yes | 360 (70.2) | 205 (71.2) | 155 (68.9) | |
| Triple therapy | 0.30 | |||
| Yes | 55 (10.7) | 35 (12.2) | 20 (8.9) | |
DOAC indicates direct oral anticoagulant; and PCI, percutaneous coronary intervention.
Table 2.
PCI Characteristics
| Total cohort, n (%) | Noncomplex PCI, n (%) | Complex PCI, n (%) | ||
|---|---|---|---|---|
| N=504 | N=285 | N=219 | P value | |
| Indication | 0.47 | |||
| Symptomatic control | 335 (66.1) | 188 (65.7) | 147 (66.5) | |
| Asymptomatic, noninvasive Findings | 15 (3.0) | 9 (3.1) | 6 (2.7) | |
| Asymptomatic, before surgery | 1 (0.2) | 1 (0.4) | 0 (0.0) | |
| Before valvular intervention | 108 (21.3) | 66 (23.1) | 42 (19.0) | |
| Heart failure | 29 (5.7) | 12 (4.2) | 17 (7.7) | |
| Recent acute coronary syndrome | 19 (3.7) | 10 (3.5) | 9 (4.1) | |
| Complex PCI breakdown | ||||
| Unprotected left main | 18 (3.5) | … | 18 (8.0) | |
| Multivessel intervention | 71 (13.8) | … | 71 (31.6) | |
| Bifurcation intervention | 59 (11.5) | … | 59 (26.2) | |
| Saphenous vein graft intervention | 35 (6.8) | … | 35 (15.1) | |
| Atherectomy performed | 42 (8.2) | … | 42 (18.7) | |
| Severely calcified lesion | 101 (19.7) | … | 101 (44.9) | |
| Chronic total occlusion | 38 (7.4) | … | 38 (16.9) | |
| Declined for CABG | 5 (1.0) | … | 5 (2.2) | |
| Vessels | ||||
| Protected left main | 15 (2.9) | 5 (1.7) | 10 (4.4) | 0.12 |
| Ramus intermedius | 9 (1.8) | 4 (1.4) | 5 (2.2) | 0.71 |
| Left anterior descending | 253 (49.3) | 128 (44.4) | 125 (55.6) | 0.02 |
| Proximal left anterior descending | 135 (26.3) | 58 (20.1) | 77 (34.2) | <0.001 |
| Diagonal branch | 57 (11.1) | 19 (6.6) | 38 (16.9) | <0.001 |
| Left circumflex | 106 (20.6) | 48 (16.7) | 58 (25.8) | 0.02 |
| Obtuse marginal branch | 53 (10.3) | 25 (8.7) | 28 (12.4) | 0.21 |
| Right coronary | 156 (30.4) | 88 (30.6) | 68 (30.2) | 1.00 |
| Posterior descending | 15 (2.9) | 8 (2.8) | 7 (3.1) | 1.00 |
| Posterolateral | 6 (1.2) | 4 (1.4) | 2 (0.9) | 0.91 |
| Left internal mammary artery graft | 2 (0.4) | 2 (0.7) | 0 (0.0) | 0.59 |
| PCI strategies | ||||
| Fractional flow reserve | 0.04 | |||
| Yes | 77 (15.0) | 52 (18.1) | 25 (11.1) | |
| Any intravascular imaging | <0.001 | |||
| Yes | 125 (24.4) | 47 (16.3) | 78 (34.7) | |
| Brachytherapy | 0.23 | |||
| Yes | 7 (1.4) | 6 (2.1) | 1 (0.4) | |
| Periprocedural medications | ||||
| Bivalirudin | 0.06 | |||
| Yes | 31 (6.0) | 23 (8.0) | 8 (3.6) | |
| Glycoprotein IIb/IIIa Inhibitor | 0.31 | |||
| Yes | 13 (2.5) | 5 (1.7) | 8 (3.6) | |
| Vascular access | 0.06 | |||
| Radial artery | 323 (63.0) | 197 (68.4) | 126 (56.0) | |
| Femoral artery | 190 (37.0) | 91 (31.6) | 99 (44.0) | |
| Operator and hospital site | ||||
| High‐volume complex PCI operator | <0.001 | |||
| Yes | 150 (29.2) | 63 (21.9) | 87 (38.7) | |
| Hospital network site | 0.01 | |||
| Site 1 | 348 (67.8) | 186 (64.6) | 162 (72.0) | |
| Site 2 | 156 (30.4) | 93 (32.3) | 63 (28.0) | |
| Site 3 | 9 (1.8) | 9 (3.1) | 0 (0.0) | |
CABG indicates coronary artery bypass graft; and PCI, percutaneous coronary intervention.
We evaluated the association between the PCI group and the secondary outcome of any bleeding event within 1 year after PCI by constructing hierarchical binary logistic regression models with bleeding as the dependent variable and PCI group as the independent variable. Odds ratios were derived from the models. Anticoagulation, dual‐antiplatelet therapy, triple therapy (aspirin, P2Y12 inhibitor, and anticoagulation), and access location (femoral or radial) were included as additional covariates in the forward selection procedure in models with bleeding events as an outcome (either alone or as part of a composite). We repeated the above procedures in a sensitivity analysis using the alternative definition of complex PCI.
Scaled Schoenfeld residuals were computed for each unadjusted Cox model to test the proportional hazards assumption, which was supported in every model. Residual plots for select outcomes are displayed in Figure S1.
The level of statistical significance was set at 0.05. All analyses were 2‐sided and performed using R 4.0.3 (R Project for Statistical Computing, Vienna, Austria). The study was deemed exempt by the Yale University Institutional Review Board. Given the study's exempt status and retrospective nature, subjects were not contacted to obtain informed consent.
Results
During the study period, 574 older adults received elective PCI for SIHD. After excluding 61 patients lost to follow‐up, 513 patients (mean age, 81.3±4.6 years) were included in the final cohort, 56.1% (N=288) of whom underwent noncomplex PCI versus 43.9% (N=225) who underwent complex PCI. There was no difference in the indications for PCI between groups (P=0.5), with the most common indication being symptomatic control (66.1% of total cohort). A small minority of patients (3.7%; N=19) underwent elective PCI as a staged procedure to recent acute coronary syndrome intervention, which was not statistically different between groups (3.5 versus 4.1% for complex PCI; P=0.57), and 7.8% (N=40) underwent 2 elective PCI procedures as a staged revascularization. Nineteen patients (3.7%) had a failed or partially failed PCI in which ≥1 target lesions were not successfully revascularized, and only 7 (1.4%) patients received mechanical support during the procedure. There was a total of 39 operators performing PCI in older adults across the hospital network during the study period, only 4 of whom did not perform any complex PCI during the study period. Patients receiving complex versus noncomplex PCI were not statistically different across the vast majority of baseline (Table 1) and PCI characteristics (Table 2).
Event‐Free Survival
The EFS at 12 months for the entire cohort was 84.0% (95% CI, 80.9–87.2%). Patients receiving noncomplex versus complex PCI had higher EFS (86.8% versus 80.4%, respectively; Figure 1); however, there was no statistically significant association between PCI group and EFS after adjustment for patient demographics, clinical characteristics, including frailty and pharmacotherapy, and PCI features (hazard ratio [HR], 1.38 [95% CI, 0.88–2.16]; Table 3).
Figure 1. Kaplan–Meier curves for event‐free survival.
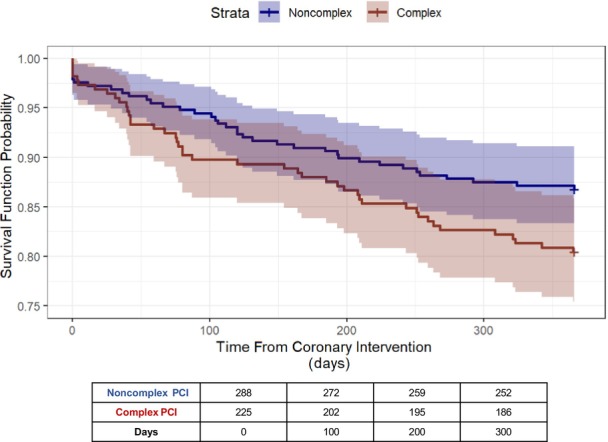
Figure displays the Kaplan–Meier survival curves (survival function probability vs time from coronary intervention in days) for the event‐free survival (freedom from all‐cause death, nonfatal myocardial infarction/stroke, and target lesion revascularization) at 12 months from index percutaneous coronary intervention (PCI). The blue curve represents the group receiving noncomplex PCI, and the red curve represents the group receiving complex PCI.
Table 3.
Regression Models
| Outcome | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Event‐free survival (events=82) | |||
| Unadjusted Cox regression | 1.53 | 0.99–2.36 | 0.06 |
| Adjusted Cox regression | 1.38 | 0.88–2.16 | 0.16 |
| Major events (unadjusted) | |||
| Myocardial infarction (events=7) | 0.96 | 0.21–4.28 | 0.96 |
| Stroke (events=14) | 1.28 | 0.45–3.64 | 0.65 |
| Cardiovascular death (events=22) | 1.09 | 0.47–2.53 | 0.84 |
| All‐cause death (N=40) | |||
| Unadjusted Cox regression | 1.78 | 0.95–3.33 | 0.07 |
| Adjusted Cox regression | 1.97 | 1.02–3.79 | 0.04 |
| Target lesion revascularization (events=15) | |||
| Unadjusted Cox regression | 0.64 | 0.22–1.87 | 0.41 |
| Adjusted Cox regression | 0.32 | 0.11–0.93 | 0.04 |
| Bleeding events (events=116) | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Unadjusted logistic regression | 1.32 | 0.87–2.00 | 0.19 |
| Adjusted logistic regression | 1.26 | 0.80–1.98 | 0.31 |
All displayed results represent the hazard or odds of the outcome at 12 months for complex PCI vs noncomplex PCI (reference). PCI indicates percutaneous coronary intervention.
- Event‐free survival: age, severe valvular disease, chronic kidney disease, peripheral artery disease, alcohol use disorder, chronic obstructive pulmonary disease, frailty score, full guideline‐directed medical therapy, triple therapy, dual‐antiplatelet therapy, anticoagulation
- All‐cause death: severe valvular disease, heart failure, dialysis, history of gastrointestinal bleeding, chronic obstructive pulmonary disease, glycoprotein IIb/IIIa inhibitor
- Target lesion revascularization: age, race, hyperlipidemia, heart failure, prior cerebrovascular accident, anemia, frailty score, intravascular imaging
- Bleeding events: age, hypertension, severe valvular disease, alcohol use disorder, chronic obstructive pulmonary disease, history of gastrointestinal bleeding, full guideline‐directed medical therapy, anticoagulation, bivalirudin, intravascular imaging
The 12‐month risk of nonfatal MI was 1.4% (95% CI, 0.4–2.4%) and of nonfatal stroke was 2.7% (1.3%–4.1%) for the entire cohort. There was no statistically significant difference between PCI groups in the risk of nonfatal MI or nonfatal stroke (Table 3).
All‐Cause Death
All‐cause death for the entire cohort was 7.8% (95% CI, 5.4%–10.1%) at 12 months following index PCI. Patients receiving complex PCI had higher all‐cause death at 12 months compared with patients receiving noncomplex PCI (10.2% versus 5.9%). We observed a higher risk of all‐cause death for patients undergoing complex PCI in adjusted regression models (HR, 1.97 [95% CI, 1.02–3.79; Table 3). Kaplan–Meier curves are displayed in Figure 2 and demonstrate early divergence between the 2 groups.
Figure 2. Kaplan–Meier curves for secondary outcomes.
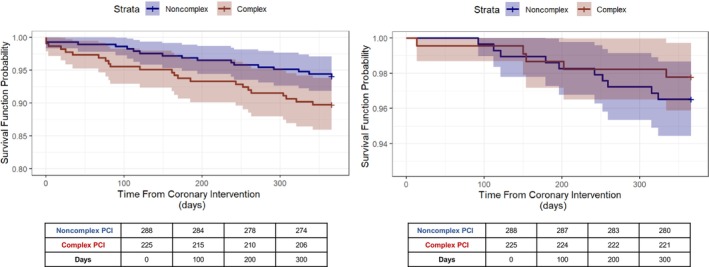
Figure displays the Kaplan–Meier survival curves (survival function probability vs time from coronary intervention in days) for all‐cause death (left) and target lesion revascularization (right) at 12 months from index percutaneous coronary intervention (PCI). At‐risk tables are shown below each respective graph. The blue curves represent the group receiving noncomplex PCI, and the red curves represent the group receiving complex PCI.
There was no significant difference in the risk of cardiovascular death between patients receiving complex versus noncomplex PCI (1.7% versus 1.8%, respectively; Table 3). Five patients did not have a clearly documented cause of death.
Target Lesion Revascularization
Overall TLR was low at 2.9% (95% CI, 1.5%–4.4%) and not statistically different between the complex PCI and noncomplex PCI groups in unadjusted models (2.2% versus 3.5%, respectively; unadjusted HR, 0.64 [95% CI, 0.22–1.87]; Figure 2). However, complex PCI patients had a lower 12‐month risk of TLR after adjusting for relevant clinical characteristics (HR, 0.32 [95% CI, 0.11–0.93]; Table 3).
Bleeding
Patients receiving complex versus noncomplex PCI had a higher proportion of bleeding events at 1 year (25.3% versus 20.5%, respectively; Table 4). Adjusted logistic regression models constructed using forward selection were better optimized when excluding the PCI group as an independent variable; however, when included as an additional covariate in the forward‐selected model, the PCI group was not sufficiently associated with bleeding events at 1 year (odds ratio, 1.26 [95% CI, 0.80–1.98]; Table 3).
Table 4.
Bleeding Events
| Total cohort, n (%) | Noncomplex PCI, n (%) | Complex PCI, n (%) | P value | |
|---|---|---|---|---|
| N=504 | N=285 | N=219 | ||
| Type of bleeding event | ||||
| All types | 116 (22.6) | 59 (20.5) | 57 (25.3) | 0.23 |
| PCI periprocedural bleeding or hematoma | 25 (4.9) | 12 (4.2) | 13 (5.8) | 0.53 |
| Gastrointestinal bleeding | 27 (5.3) | 13 (4.5) | 14 (6.2) | 0.51 |
| Epistaxis | 19 (3.7) | 10 (3.5) | 9 (4.0) | 0.94 |
| Hematuria | 18 (3.5) | 6 (2.1) | 12 (5.3) | 0.07 |
| Intracranial hemorrhage | 7 (1.4) | 4 (1.4) | 3 (1.3) | 1.00 |
| Dental procedure with excessive hemorrhage | 3 (0.6) | 3 (1.0) | 0 (0.0) | 0.34 |
| Traumatic laceration with excessive hemorrhage | 10 (1.9) | 7 (2.4) | 3 (1.3) | 0.57 |
| Spontaneous cutaneous hemorrhage | 4 (0.8) | 0 (0.0) | 4 (1.8) | 0.08 |
| Surgical site rebleeding or hematoma | 11 (2.1) | 7 (2.4) | 4 (1.8) | 0.84 |
| Hemoptysis | 1 (0.2) | 1 (0.3) | 0 (0.0) | 1.00 |
| Noncranial internal hemorrhage | 7 (1.4) | 6 (2.1) | 1 (0.2) | 0.23 |
| BARC staging* | 0.09 | |||
| 1 | 9 (1.8) | 3 (1.0) | 6 (2.7) | |
| 2 | 70 (13.6) | 38 (13.2) | 32 (14.2) | |
| 3a | 18 (3.5) | 6 (2.1) | 12 (5.3) | |
| 3b | 12 (2.3) | 8 (2.8) | 4 (1.8) | |
| 3c | 6 (1.2) | 4 (1.4) | 2 (0.9) | |
| 5 | 3 (0.6) | 0 (0.0) | 3 (0.6) | |
BARC indicates Bleeding Academic Research Consortium; and PCI percutaneous coronary intervention.
Highest BARC staging in patients with multiple bleeding episodes.
Alternative Complex PCI Definition
In secondary analyses that used an alternative definition of complex PCI, there was no difference in EFS between patients receiving complex versus noncomplex PCI (84.0% for both). The differences in the risk of all‐cause death or TLR did not reach statistical significance.
Discussion
In this investigation of complex PCI in older adults, we observed a 2‐fold risk of all‐cause death among older adults undergoing complex compared with noncomplex PCI even after adjusting for patient and PCI characteristics. Given the dramatic difference in death risk for older adults receiving complex PCI, we suggest that such interventions in this exceptionally vulnerable population should be approached with additional caution and that further investigations are needed to define causality.
Complex PCI is becoming increasingly common in older adults. 9 We observed a substantial number of older adults receiving complex PCI (almost half of the cohort). Our work demonstrates that complex PCI may be associated with reliable target vessel patency in older adults, given a lower risk of TLR as compared with patients receiving noncomplex PCI. Superior TLR for patients receiving complex PCI may be due to higher usage of PCI adjuncts among patients receiving complex interventions. For instance, use of atherectomy would classify an intervention as complex, by definition, but a lesion that may have benefited from atherectomy without receiving it may be classified as noncomplex. Ultimately, the absolute difference in TLR between complex versus noncomplex PCI was small (1%) and complex PCI was at least as effective as noncomplex interventions at maintaining target vessel patency. Importantly, TLR has been identified as an independent predictor of worse outcomes, including death. 26 Thus, the lower rates of TLR observed in the complex PCI groups certainly provide reassurance that older adults undergoing more technically complex procedures can experience excellent midterm revascularization outcomes.
This analysis also highlights potential risk in older adults undergoing complex PCI, with a signal for higher death in older adults receiving complex interventions. It is difficult to hypothesize the role of selection bias in these findings. One may expect older adults selected for complex, high‐risk procedures to have fewer comorbidities, or, alternatively, expect patients with more complex CAD to have more comorbidities and worse functional status. However, patients undergoing complex versus noncomplex PCI in this real‐world cohort did not significantly differ across any of a host of clinical characteristics. Although the observational study design cannot account for unmeasured confounders, it is nonetheless notable that the study groups were not statistically different across predictors of poor PCI outcomes in older adults, including frailty. 27 , 28 Therefore, the baseline characteristics between PCI groups suggest that simple differences in comorbid disease burden are unlikely to be the culprit. Furthermore, the risk of all‐cause death was actually amplified in adjusted analyses. Thus, we emphasize that the most notable observation from this analysis is the dramatic increase in adjusted death risk for older adults receiving complex PCI as compared with controls receiving noncomplex PCI. Five patients did not have a clearly documented cause of death. With an observational design, and without the benefit of adjudication as exists in prospective trials, 29 speculation about the root cause of the higher death risk is limited. The elevated risk of complex PCI, in the absence of other evidence, warrants, at a minimum, exceptional caution when electing older adults for complex PCI and should motivate further prospective investigations.
In contrast with the death signal observed in this older cohort, previous studies of complex PCI in younger populations have demonstrated mixed outcomes compared with noncomplex PCI. This includes some studies demonstrating no difference in all‐cause or cardiovascular death, 30 , 31 though an analysis from the Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents study of a younger patient population did observe higher 2‐year risk of major adverse cardiovascular events, MI, and stent thrombosis among individuals undergoing complex PCI. 30 This association was actually strongest among those with SIHD. Mohamed et al 18 identified a statistically significant death rate difference in a large, multicenter registry, though the absolute difference was relatively small (0.7%). Literature describing complex PCI outcomes in older adults are scarce. 8 An analysis of multinational registry data reported similar long‐term outcomes in older adults undergoing unprotected left main coronary artery PCI versus older adults undergoing CABG. 32 Another prior study described trends toward improved outcomes in English older adults over time despite increasing rates of complex interventions. 9 Two studies of chronic total occlusion interventions in older adults reported no difference in adjusted analyses of periprocedural complications in older adults versus younger patients. 15 , 33 Postprocedural outcomes were not a focus of these latter studies. Complex PCI studies in older patients with somewhat younger chronologic age than the cohort analyzed here, in the 65‐ to 75‐year range, are also uncommon. A recently published follow‐up to the SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) trial described similar death rates and quality of life between older adults undergoing PCI versus CABG. 11 Therefore, the risk of certain complex interventions may be similar to the risk of CABG for certain outcomes in older adults, though the broader long‐term risk of complex PCI in older adults remains unknown. It is worth mentioning that the survival benefit of CABG is less clear in older adults. A long follow‐up duration, namely 10 years, was necessary to observe a survival benefit in the STICH (Surgical Treatment in Ischemic Heart Failure) trial, which may substantially shift the risk–benefit calculus for patients in their 9th or 10th decades of life. 34 Nonetheless, while the benefits of surgical revascularization are potentially high in patients with SIHD being considered for CABG, given the possible survival benefit with certain surgical coronary anatomy, these benefits are less clear in the majority of patients with SIHD undergoing PCI for stable angina who do not have surgical anatomy. Complex PCI is often performed on the basis of anatomic considerations and for symptomatic or quality‐of‐life benefits in older populations, without a clear survival benefit from revascularization. In these cases, the inherent risk is more relevant, further underscoring the need for investigations to precisely quantify the short‐ and long‐term risks and benefits of complex PCI.
The lack of uniformity around defining complex PCI in the literature remains a barrier to future investigations. 12 Moreover, some studies blur the distinction between complex and high‐risk PCI. 21 We chose not to include numeric cutoffs (beyond multivessel PCI) in our primary definition of complex PCI, as such criteria only partially convey procedural complexity. For example, PCI with overlapping 38‐mm and 26‐mm stents deployed in the right coronary artery carries different technical risk from an unprotected left main trifurcation PCI or severely calcified chronic total occlusion PCI with atherectomy. Notably, stent length, specifically, no longer predicts worse outcomes in newer‐generation stents. 35 In our sensitivity analysis, using a similar definition to the definitions used in several complex PCI investigations, 18 , 19 , 20 we did not observe a statistically significant death rate difference, underscoring the need for a uniform, clinically relevant definition that effectively predicts interventions with higher associated risk.
Bleeding events are of primary concern in older adults receiving drug‐eluting stents. Many studies report higher bleeding risk in older adults on dual antiplatelet therapy. 8 The risk of bleeding was high in both groups, with almost one‐quarter of the total cohort experiencing a bleeding event within 1 year of index PCI. However, although the absolute difference was about 5%, we observed no statistically significant difference between patients receiving complex PCI versus noncomplex PCI. This may be considered clinically significant and may have reached statistical significance with a larger sample size.
The findings of this study should be viewed in light of the following limitations: as an observational study, we cannot infer causality, and unmeasured confounding may contribute to differences seen between treatment groups. We may be limited by sample size for some outcomes, although the number of patients is offset by the high level of granularity possible with a manual chart review that is not available with larger registry studies. Some patients were lost to follow‐up, though we did extract data from outside hospital records to limit the number of patients who were counted as lost to follow‐up and to ensure capture of all outcomes. The lack of definition around complex PCI limits comparison across other studies. This is balanced by our sensitivity analysis, which examines a previously used alternative complex PCI definition. Chart data extraction often involves some interpretation that may lead to imprecision, though we expect this limitation to be mitigated in this study as compared with studies without manual extraction or where nonphysician extractors record the data. Moreover, manual extraction by physicians is likely more accurate than the use of billing codes, which are often incorrect. 36 , 37 The study is limited to a single health system with multiple sites and so validation of these data across other centers will be needed. Finally, given the nature of the data set, we used the date of the health care encounter as a proxy for the date of the initial PCI procedure; however, these dates almost always coincide or fall within a few days of each other and we do not expect the difference to influence the study findings.
Conclusions
Older adults without substantial statistical differences in baseline characteristics undergoing elective complex versus noncomplex PCI for SIHD had lower TLR but higher risk of all‐cause death 12 months from the index intervention. Further investigation into the outcomes following complex PCI in older adults is needed.
Sources of Funding
None.
Disclosures
Dr Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging (P30‐AG021334) and mentored patient‐oriented research career development award from the National Heart, Lung, and Blood Institute (K23‐HL153771‐01). Dr Curtis has an institutional contract with the American College of Cardiology for his role as Chief Science Officer for the National Cardiovascular Data Registry and has equity interest in Medtronic. Dr Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation and salary support from the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award). The remaining authors have no disclosures to report.
Supporting information
Figure S1
This manuscript was sent to Jennifer Tremmel, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.029057
For Sources of Funding and Disclosures, see page 11.
See Editorial by Davies et al.
REFERENCES
- 1. Schneider EL. Aging in the third millennium. Science. 1999;283:796–797. doi: 10.1126/science.283.5403.796 [DOI] [PubMed] [Google Scholar]
- 2. Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70:1–114. [PubMed] [Google Scholar]
- 3. Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71:2015–2040. doi: 10.1016/j.jacc.2017.12.068 [DOI] [PubMed] [Google Scholar]
- 4. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen‐Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 5. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl‐Hofseth O, Ranhoff AH, Gullestad L, et al. Invasive versus conservative strategy in patients aged 80 years or older with non‐ST‐elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open‐label randomised controlled trial. Lancet. 2016;387:1057–1065. doi: 10.1016/S0140-6736(15)01166-6 [DOI] [PubMed] [Google Scholar]
- 6. Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142 [DOI] [PubMed] [Google Scholar]
- 7. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–3137. doi: 10.1161/CIR.0b013e3182776f83 [DOI] [PubMed] [Google Scholar]
- 8. Shanmugam VB, Harper R, Meredith I, Malaiapan Y, Psaltis PJ. An overview of PCI in the very elderly. J Geriatr Cardiol. 2015;12:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajani R, Lindblom M, Dixon G, Khawaja MZ, Hildick‐Smith D, Holmberg S, Belder A. Evolving trends in percutaneous coronary intervention. Br J Cardiol. 2011;18:73–76. [Google Scholar]
- 10. Nanna MG, Peterson ED, Wu A, Harding T, Galanos AN, Wruck L, Alexander KP. Age, knowledge, preferences, and risk tolerance for invasive cardiac care. Am Heart J. 2020;219:99–108. doi: 10.1016/j.ahj.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ono M, Serruys Patrick W, Hara H, Kawashima H, Gao C, Wang R, Takahashi K, O'Leary N, Wykrzykowska Joanna J, Sharif F, et al. 10‐Year follow‐up after revascularization in elderly patients with complex coronary artery disease. J Am Coll Cardiol. 2021;77:2761–2773. doi: 10.1016/j.jacc.2021.04.016 [DOI] [PubMed] [Google Scholar]
- 12. Wykrzykowska JJ, Kerkmeijer LSM. Complex PCI: time for a universal definition. EuroIntervention. 2020;16:536–537. doi: 10.4244/EIJV16I7A100 [DOI] [PubMed] [Google Scholar]
- 13. Batchelor WB, Anstrom KJ, Muhlbaier LH, Grosswald R, Weintraub WS, O'Neill WW, Peterson ED. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol. 2000;36:723–730. doi: 10.1016/S0735-1097(00)00777-4 [DOI] [PubMed] [Google Scholar]
- 14. Moscucci M, Fox KA, Cannon CP, Klein W, López‐Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815–1823. doi: 10.1016/S0195-668X(03)00485-8 [DOI] [PubMed] [Google Scholar]
- 15. Vemmou E, Alaswad K, Patel M, Mahmud E, Choi JW, Jaffer FA, Doing AH, Dattilo P, Karmpaliotis D, Krestyaninov O, et al. Chronic total occlusion percutaneous coronary intervention in octogenarians and nonagenarians. J Am Geriatr Soc. 2021;69:1560–1569. doi: 10.1111/jgs.17063 [DOI] [PubMed] [Google Scholar]
- 16. Numasawa Y, Inohara T, Ishii H, Yamaji K, Kohsaka S, Sawano M, Kodaira M, Uemura S, Kadota K, Amano T, et al. Comparison of outcomes after percutaneous coronary intervention in elderly patients, including 10628 nonagenarians: insights from a Japanese Nationwide Registry (J‐PCI Registry). J Am Heart Assoc. 2019;8:e011183. doi: 10.1161/JAHA.118.011017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vlaar PJ, Lennon RJ, Rihal CS, Singh M, Ting HH, Bresnahan JF, Holmes DR Jr. Drug‐eluting stents in octogenarians: early and intermediate outcome. Am Heart J. 2008;155:680–686. doi: 10.1016/j.ahj.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 18. Mohamed MO, Polad J, Hildick‐Smith D, Bizeau O, Baisebenov RK, Roffi M, Íñiguez‐Romo A, Chevalier B, von Birgelen C, Roguin A, et al. Impact of coronary lesion complexity in percutaneous coronary intervention: one‐year outcomes from the large, multicentre e‐Ultimaster registry. EuroIntervention. 2020;16:603–612. doi: 10.4244/EIJ-D-20-00361 [DOI] [PubMed] [Google Scholar]
- 19. Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong M‐K, Kim B‐K, Jang Y, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. 2016;68:1851–1864. doi: 10.1016/j.jacc.2016.07.760 [DOI] [PubMed] [Google Scholar]
- 20. Serruys PW, Takahashi K, Chichareon P, Kogame N, Tomaniak M, Modolo R, Chang CC, Komiyama H, Soliman O, Wykrzykowska JJ, et al. Impact of long‐term ticagrelor monotherapy following 1‐month dual antiplatelet therapy in patients who underwent complex percutaneous coronary intervention: insights from the Global Leaders trial. Eur Heart J. 2019;40:2595–2604. doi: 10.1093/eurheartj/ehz453 [DOI] [PubMed] [Google Scholar]
- 21. Bass TA. High‐risk percutaneous coronary interventions in modern day clinical practice: current concepts and challenges. Circ Cardiovasc Interv. 2015;8:e003405. doi: 10.1161/CIRCINTERVENTIONS.115.003405 [DOI] [PubMed] [Google Scholar]
- 22. Udell JA, Lu D, Bagai A, Dodson JA, Desai NR, Fonarow GC, Goyal A, Garratt KN, Lucas J, Weintraub WS, et al. Preexisting frailty and outcomes in older patients with acute myocardial infarction. Am Heart J. 2022;249:34–44. doi: 10.1016/j.ahj.2022.03.007 [DOI] [PubMed] [Google Scholar]
- 23. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 24. Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention: a consensus document from the Academic Research Consortium for High Bleeding Risk. Eur Heart J. 2019;40:2632–2653. doi: 10.1093/eurheartj/ehz372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, Solomon SD, Marler JR, Teerlink JR, Farb A, et al. 2017 Cardiovascular and stroke endpoint definitions for clinical trials. Circulation. 2018;137:961–972. doi: 10.1161/CIRCULATIONAHA.117.033502 [DOI] [PubMed] [Google Scholar]
- 26. Palmerini T, Riva DD, Biondi‐Zoccai G, Leon MB, Serruys PW, Smits PC, Cv B, Ben‐Yehuda O, Généreux P, Bruno AG, et al. Mortality following nonemergent, uncomplicated target lesion revascularization after percutaneous coronary intervention. J Am Coll Cardiol Intv. 2018;11:892–902. doi: 10.1016/j.jcin.2018.01.277 [DOI] [PubMed] [Google Scholar]
- 27. Wang P, Zhang S, Zhang K, Tian J. Frailty predicts poor prognosis of patients after percutaneous coronary intervention: a meta‐analysis of cohort studies. Front Med (Lausanne). 2021;8:696153. doi: 10.3389/fmed.2021.696153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;4:496–502. doi: 10.1161/CIRCOUTCOMES.111.961375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morrow DA, Wiviott SD. Classification of deaths in cardiovascular outcomes trials. Circulation. 2019;139:874–876. doi: 10.1161/CIRCULATIONAHA.118.038359 [DOI] [PubMed] [Google Scholar]
- 30. Généreux P, Giustino G, Redfors B, Palmerini T, Witzenbichler B, Weisz G, Stuckey TD, Maehara A, Mehran R, Kirtane AJ, et al. Impact of percutaneous coronary intervention extent, complexity and platelet reactivity on outcomes after drug‐eluting stent implantation. Int J Cardiol. 2018;268:61–67. doi: 10.1016/j.ijcard.2018.03.103 [DOI] [PubMed] [Google Scholar]
- 31. Endo H, Dohi T, Miyauchi K, Takahashi D, Funamizu T, Shitara J, Wada H, Doi S, Kato Y, Okai I, et al. Clinical impact of complex percutaneous coronary intervention in patients with coronary artery disease. Cardiovasc Interv Ther. 2020;35:234–241. doi: 10.1007/s12928-019-00608-7 [DOI] [PubMed] [Google Scholar]
- 32. Conrotto F, Scacciatella P, D'Ascenzo F, Chieffo A, Latib A, Park SJ, Kim YH, Onuma Y, Capranzano P, Jegere S, et al. Long‐term outcomes of percutaneous coronary interventions or coronary artery bypass grafting for left main coronary artery disease in octogenarians (from a Drug‐Eluting stent for LefT main Artery registry substudy). Am J Cardiol. 2014;113:2007–2012. doi: 10.1016/j.amjcard.2014.03.044 [DOI] [PubMed] [Google Scholar]
- 33. Blessing RRL, Ahoopai M, Geyer M, Brandt M, Zeiher AM, Vasa‐Nicotera M, Münzel T, Wenzel P, Gori T, Dimitriadis Z. Percutaneous coronary intervention for chronic total occlusion in octogenarians: a propensity score study. Sci Rep. 2022;12:3073. doi: 10.1038/s41598-022-06994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Velazquez EJ, Lee KL, Jones RH, Al‐Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yano H, Horinaka S, Ishimitsu T. Impact of everolimus‐eluting stent length on long‐term clinical outcomes of percutaneous coronary intervention. J Cardiol. 2018;71:444–451. doi: 10.1016/j.jjcc.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 36. Shah RU, Mukherjee R, Zhang Y, Jones AE, Springer J, Hackett I, Steinberg BA, Lloyd‐Jones DM, Chapman WW. Impact of different electronic cohort definitions to identify patients with atrial fibrillation from the electronic medical record. J Am Heart Assoc. 2020;9:e014527. doi: 10.1161/JAHA.119.014527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crabb BT, Lyons A, Bale M, Martin V, Berger B, Mann S, West WB Jr, Brown A, Peacock JB, Leung DT, et al. Comparison of International Classification of Diseases and Related Health Problems, Tenth Revision codes with electronic medical records among patients with symptoms of coronavirus disease 2019. JAMA Netw Open. 2020;3:e2017703. doi: 10.1001/jamanetworkopen.2020.17703 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


