Abstract
Background
Postoperative atrial fibrillation (POAF) is the most frequent complication of cardiac surgery. Despite clinical and economic implications, ample variability in POAF assessment method and definition exist across studies. We performed a study‐level meta‐analysis to evaluate the influence of POAF assessment method and definition on its incidence and association with clinical outcomes.
Methods and Results
A systematic literature search was conducted to identify studies comparing the outcomes of patients with and without POAF after cardiac surgery that also reported POAF assessment method. The primary outcome was POAF incidence. The secondary outcomes were in‐hospital mortality, stroke, intensive care unit length of stay, and postoperative length of stay. Fifty‐nine studies totaling 197 774 patients were included. POAF cumulative incidence was 26% (range: 7.3%–53.1%). There were no differences in POAF incidence among assessment methods (27%, [range: 7.3%–53.1%] for continuous telemetry, 27% [range: 7.9%–50%] for telemetry plus daily ECG, and 19% [range: 7.8%–42.4%] for daily ECG only; P>0.05 for all comparisons). No differences in in‐hospital mortality, stroke, intensive care unit length of stay, and postoperative length of stay were found between assessment methods. No differences in POAF incidence or any other outcomes were found between POAF definitions. Continuous telemetry and telemetry plus daily ECG were associated with higher POAF incidence compared with daily ECG in studies including only patients undergoing isolated coronary artery bypass grafting.
Conclusions
POAF incidence after cardiac surgery remains high, and detection rates are variable among studies. POAF incidence and its association with adverse outcomes are not influenced by the assessment method and definition used, except in patients undergoing isolated coronary artery bypass grafting.
Keywords: assessment method, cardiac surgery, definition, incidence, postoperative atrial fibrillation
Subject Categories: Electrocardiology (ECG)
Nonstandard Abbreviations and Acronyms
- POAF
postoperative atrial fibrillation
Clinical Perspective.
What Is New?
In this study‐level meta‐analysis, we found no difference in incidence of postoperative atrial fibrillation by assessment method, whether telemetry only, telemetry plus ECG, or ECG only were used, and there was also no difference in the incidence of postoperative atrial fibrillation irrespective of the definition used.
What Are the Clinical Implications?
There was no difference among assessment methods in incidence of postoperative atrial fibrillation or the association of postoperative atrial fibrillation with adverse clinical outcomes.
Postoperative atrial fibrillation (POAF) is the most frequent complication of cardiac surgery, with an incidence ranging from 15% to 40%. 1 Often regarded as a transient event triggered by inflammation after surgery, POAF has been associated with worse clinical outcomes (including mortality, stroke, and heart failure) 2 , 3 and increased cost of care. 4 , 5 Despite the proven success of medical 6 and surgical 7 strategies for POAF prevention, a high proportion of patients remains affected, resulting in efforts to elucidate the pathophysiologic mechanisms of, and risk factors for, POAF as potential targets for intervention.
The term POAF generally refers to new‐onset atrial fibrillation during the postoperative hospitalization period. 1 , 8 , 9 However, no consensus definition for POAF has been established by professional societies, 10 , 11 leading to marked heterogeneity in POAF definitions across studies. Some groups have reported POAF episodes regardless of duration or need for treatment, 12 , 13 others have used duration‐based POAF definitions (with arbitrarily defined cutoffs to report the arrhythmia ranging from 30 seconds to 60 minutes), 14 , 15 and others have reported only those POAF episodes that required treatment or intervention. 16 , 17 In addition, there is variability in the POAF assessment methods employed across studies, ranging from monitoring with continuous telemetry during the whole postoperative hospitalization to the use of daily ECG only. The heterogeneity in POAF assessment method and definition opens questions on the interpretation and generalizability of published studies.
We performed a systematic review and meta‐analysis to evaluate the incidence of POAF and its association with clinical outcomes according to the POAF assessment method and definition used in individual studies.
METHODS
The present review was registered in the National Institute for Health Research International Registry of Systematic Reviews (CRD42023399670). The article is compliant with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guideline. 18 Institutional review board approval was waived. Informed consent was not required.
Search Strategy
A comprehensive literature search was performed by a medical librarian (M.D.) to identify studies that compared outcomes of patients who developed POAF versus patients who did not after cardiac surgery. Searches were run on July 6, 2022 and updated on May 31, 2023 in the following databases: Ovid MEDLINE (ALL; 1946 to present), Ovid EMBASE (1974 to present), and The Cochrane Library (Wiley; 1992 to present). The complete search strategy for Ovid MEDLINE is available in Table S1.
Study Selection and Data Extraction
After deduplication, records were screened by 2 independent reviewers (L.H. and R.P.O.) using Microsoft Excel version 16.73. Any discrepancies were adjudicated by the senior author (M.G.). Titles and abstracts were reviewed against predefined inclusion and exclusion criteria. Studies were considered for inclusion if they compared outcomes of patients who developed POAF after noncongenital cardiac surgery versus patients who did not. Animal studies, abstracts, case reports, commentaries, editorials, expert opinions, conference presentations, and studies that did not report POAF assessment method were excluded. The full text of the selected articles was pulled for a second round of eligibility screening. The reference lists were also reviewed for relevant studies not captured by the original search. The methodological quality of the included studies was assessed by 2 reviewers (L.H. and R.P.O.) in 3 domains: (1) cohort selection and comparability, (2) reporting of POAF assessment method and POAF incidence, and (3) reporting of POAF definition. This assessment was based on the Newcastle‐Ottawa Scale. Details of POAF definition in each study are provided in Table S2.
Two investigators (T.C. and R.P.O.) independently performed data extraction, and the accuracy was verified by the senior author (M.G.). The variables included were study characteristics (publication year, institution, country of origin, study period, type of surgery, sample size, POAF definition, POAF incidence, and POAF assessment method), patients' demographic characteristics (age, sex, left ventricular ejection fraction, hypertension, diabetes, chronic obstructive pulmonary disease, prior cerebrovascular accident, prior myocardial infarction, preoperative use of beta blockers, prior cardiac surgery, and chronic kidney disease), in‐hospital mortality, stroke, intensive care unit (ICU) length of stay (LOS) and postoperative LOS.
Outcomes
The primary outcome was POAF incidence according to the POAF assessment method used. Three different assessment methods were identified: (1) continuous telemetry until hospital discharge, (2) continuous telemetry during ICU stay followed by daily ECG while patients were in the regular ward, and (3) daily ECG only.
The secondary outcomes were in‐hospital mortality, stroke, ICU LOS, and postoperative LOS according to the POAF assessment method used.
Secondary Analyses
In the secondary analyses, the primary and secondary outcomes were analyzed based on the definition of POAF used in individual studies: (1) intervention‐based definition, which included only POAF episodes requiring treatment, and (2) nonintervention‐based definition, which included POAF regardless of episode duration or need for treatment. Details of the assigned POAF definition category in each study are provided in Table 1.
Table 1.
Characteristics of the Included Studies
| Study, year | Institution or trial name | Country | Study period | Type of surgery | Total number of patients | Incidence of POAF | Assessment method | POAF definition category (duration, if applicable) |
|---|---|---|---|---|---|---|---|---|
| Creswell, 1993 21 | Washington University Medical Center | United States | 1986–1991 | Multiple | 3983 | 34.6% | Telemetry | Not reported |
| Aranki, 1996 16 | Brigham and Women's Hospital | United States | 1993–1994 | CABG | 570 | 33.2% | Telemetry | Intervention‐based |
| Stamou, 2000 17 | Washington Hospital Center | United States | 1987–1999 | CABG | 969 | 21.3% | Telemetry | Intervention‐based |
| Tamis, 2000 22 | St. Luke's Hospital | United States | 1992–1994 | CABG | 216 | 25.5% | Telemetry+ECG | Nonintervention‐based (≥30 min) |
| Hakala, 2002 23 | Kuopio University Hospital | Finland | 1992–1996 | CABG | 3676 | 31% | ECG | Not reported |
| Silva, 2004 24 | Institute of Cardiology of Rio Grande/Fundacion Universitaria de Cardiologia | Brazil | 2002 | Multiple | 158 | 28.5% | Telemetry+ECG | Nonintervention‐based (≥15 min) |
| Villareal, 2004 12 | Texas Heart Institute | United States | 1993–1999 | CABG | 6475 | 15.4% | ECG | Nonintervention‐based (any duration) |
| Kalavrouziotis, 2007 25 | Maritime Heart Center | Canada | 1995–2003 | CABG±AVR | 7347 | 27.9% | Telemetry+ECG | Intervention‐based |
| Mariscalco, 2007 26 | Umea University Hospital | Sweden | 1994–2004 | Multiple | 8434 | 25.6% | Telemetry+ECG | Nonintervention‐based (≥15 min) |
| Nisanoglu, 2007 13 | Turgut Ozal Medical Center | Turkey | 2001–2005 | CABG | 426 | 21.4% | Telemetry+ECG | Nonintervention‐based (any duration) |
| Mariscalco, 2008 27 | Varese University Hospital/Monizo Cardiology Center | Italy | 2000–2005 | CABG | 1832 | 31.1% | Telemetry | Nonintervention‐based (≥15 min) |
| Ahlsson, 2010 28 | Örebo University Hospital | Sweden | 1999–2000 | CABG | 571 | 28.9% | Telemetry+ECG | Nonintervention‐based (≥1 min) |
| Bramer, 2010 29 | Catharina Hospital | Netherlands | 2003–2007 | CABG | 5098 | 22% | Telemetry+ECG | Nonintervention‐based (≥30 min) |
| Shirzad, 2010 30 | Tehran Heart Center | Iran | 2002–2008 | Multiple | 15 580 | 7.2% | Telemetry | Nonintervention‐based (≥5 min) |
| Attaran, 2011 31 | Liverpool Heart and Chest Hospital | United Kingdom | 1998–2009 | Multiple | 17 379 | 28.7% | ECG | Nonintervention‐based (any duration) |
| Bramer, 2011 32 | Catharina Hospital | Netherlands | 2003–2010 | Mitral valve repair/replacement ± CABG±tricuspid valve repair/replacement | 856 | 42.2% | Telemetry+ECG | Nonintervention‐based (≥30 min) |
| Girerd, 2012 33 | Laval Hospital | Canada | 2000–2007 | CABG | 6728 | 27.8% | Telemetry+ECG | Intervention‐based |
| Helgadottir, 2012 34 | Landspitali Hospital | Iceland | 2002–2006 | CABG or AVR | 744 | 43.8% | Telemetry+ECG | Nonintervention‐based (≥5 min) |
| Saxena, 2012 35 | Australasian Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database | Australia | 2001–2009 | CABG | 19 947 | 27.8% | Telemetry+ECG | Intervention‐based |
| Horwich, 2013 36 | Queen Elizabeth II Health Sciences Centre | Canada | 1995–2009 | CABG | 8058 | 27.5% | Telemetry | Intervention‐based |
| O'neal, 2013 37 | East Carolina Heart Institute | United States | 1992–2011 | CABG | 13 165 | 22.1% | Telemetry | Intervention‐based |
| Saxena, 2013 38 | Australasian Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database | Australia | 2001–2009 | AVR | 2065 | 35.1% | Telemetry+ECG | Intervention‐based |
| Ivanovic, 2014 39 | Clinical Center of Serbia | Serbia | 2006–2009 | CABG | 460 | 22.4% | Telemetry+ECG | Nonintervention‐based (≥15 min) |
| Philip, 2014 40 | Cleveland Clinic | United States | 1993–2005 | CABG | 5135 | 29% | Telemetry+ECG | Nonintervention‐based (≥2 min) |
| Pivatto, 2014 41 | Cardiology Institute/University Foundation of Cardiology | Brazil | 2000–2011 | AVR | 348 | 32.8% | Telemetry+ECG | Nonintervention‐based (any duration) |
| Weidinger, 2014 42 | University of Maryland | Austria and United States | 2001–2010 | CABG | 384 | 15.4% | Telemetry | Intervention‐based |
| Junior, 2015 43 | Santa Isabel Hospital | Brazil | 2011–2013 | CABG | 230 | 16.1% | ECG | Not reported |
| Melduni, 2015 15 | Mayo Clinic | United States | 2000–2005 | Multiple | 603 | 37.5% | Telemetry | Nonintervention‐based (≥30 s) |
| Tsai, 2015 44 | Tri‐Service General Hospital, National Defense Medical Center | Taiwan | 2009–2012 | CABG | 266 | 47.4% | Telemetry+ECG | Intervention‐based |
| Tulla, 2015 45 | Kuopio University Hospital | Finland | 2000–2010 | CABG | 276 | 50% | Telemetry+ECG | Nonintervention‐based (≥5 min) |
| Omer, 2016 46 | Michael E. DeBakey VA Medical Center | United States | 2006–2013 | CABG | 1248 | 17.2% | Telemetry | Not reported |
| Sahin, 2016 47 | Kolan International Hospital | Turkey | 2008–2012 | CABG | 149 | 36.9% | Telemetry+ECG | Not reported |
| Ismail, 2017 48 | King Faisal Specialist Hospital and Research Center | Saudi Arabia | 2013–2015 | CABG | 252 | 33.3% | Telemetry | Intervention‐based |
| Lee, 2017 49 | Severance Cardiovascular Hospital | Korea | 2005–2011 | CABG | 1664 | 24.8% | Telemetry+ECG | Nonintervention‐based (any duration) |
| Park, 2017 50 | Gachon University Gil Medical Center | Korea | 1999–2010 | Multiple | 938 | 22.1 | Telemetry+ECG | Nonintervention‐based (≥1 min) |
| Swinkels, 2017 14 | St. Antonius Hospital, Academic Medical Center | Netherlands | 1990–1994 | AVR±CABG | 569 | 42.4% | ECG | Nonintervention‐based (>120 min) |
| Kosmidou, 2018 51 | Evaluation of XIENCE versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization trial | International | 2010–2014 | CABG | 893 | 18% | Telemetry+ECG | Nonintervention‐based (≥30 s) |
| Schwann, 2018 52 | University of Toledo | United States | 1994–2012 | CABG±carotid reconstruction | 8807 | 22.6% | Telemetry | Intervention‐based |
| Almassi, 2019 5 | Randomized On/Off Bypass Trial | United States | 2002–2008 | CABG | 2103 | 26.2% | Telemetry | Nonintervention‐based (≥30 min) |
| Carter‐storch, 2019 53 | Odense University Hospital | Denmark | 2014–2016 | AVR±CABG | 96 | 53.1% | Telemetry | Nonintervention‐based (any duration) |
| Filardo, 2019 54 | Baylor University Medical Center, The Heart Hospital at Baylor Plano, Emory University, Washington University | United States | 2002–2010 | CABG | 9203 | 31.5% | Telemetry | Nonintervention‐based (any duration) |
| Hernández‐leiva, 2019 4 | Institute of Cardiology | Colombia | Not reported | Multiple | 44 | 50% | Telemetry | Nonintervention‐based (≥30 s) |
| Kato, 2019 55 | Sakakibara Heart Institute, Kishiwada Tokushukai Hospital, Lobe City Medical Center General Hospital, The Cardiovascular Institute, Fukuyama Cardiovascular Hospital, St. Luke's International Hospital, Kitano Hospital, Shizuoka Medical Center, Higashi Takarazuku Satoh Hospital, Sakakibara Heart Institute of Okayama | Japan | 2015–2016 | Multiple | 302 | 21.9% | Telemetry | Nonintervention‐based (≥5 min) |
| Benedetto, 2020 3 | Arterial Revascularization Trial | International | 2004–2007 | CABG | 3023 | 24.3% | Telemetry+ECG | Nonintervention‐based (≥30 s) |
| Cole, 2020 56 | Liverpool Heart and Chest Hospital | United Kingdom | 2013–2018 | Multiple | 5588 | 24.8% | Telemetry+ECG | Not reported |
| Fragao, 2020 57 | University Of Porto | Portugal | 2014–2015 | AVR | 379 | 42.2% | Telemetry+ECG | Nonintervention‐based (≥30 s) |
| Krishna, 2020 58 | Kasturba Medical College | India | 2015 | CABG | 99 | 20.2% | ECG | Intervention‐based |
| Malhotra, 2020 59 | Sanjay Gandhi Postgraduate Institute of Medical Sciences | India | 2018–2019 | CABG | 263 | 9.1% | ECG | Not reported |
| Thoren, 2020 60 | Uppsala University Hospital | Sweden | 1996–2012 | CABG | 7145 | 30.6% | Telemetry+ECG | Intervention‐based |
| Fan, 2021 61 | Peking University People's Hospital | China | 2012–2015 | CABG | 165 | 15.2% | Telemetry | Nonintervention‐based (≥30 s) |
| Gaudino, 2021 7 | Weill Cornell Medicine‐New York Presbyterian Hospital | United States | 2017–2021 | Multiple | 420 | 24.5% | Telemetry | Nonintervention‐based (≥30 s) |
| Hsu, 2021 62 | National Taiwan University Hospital | Taiwan | 2007–2017 | Multiple | 6267 | 32.2% | Telemetry+ECG | Nonintervention‐based (≥30 s) |
| Lee, 2021 63 | Sejong General Hospital | Korea | 2015–2017 | CABG | 507 | 18.5% | Telemetry | Not reported |
| Omar, 2021 64 | Cairo University Hospitals | Egypt | 2019–2020 | CABG | 1000 | 7.8% | ECG | Nonintervention‐based (≥30 s) |
| Wang, 2021 65 | National University Heart Centre Singapore, National Heart Centre Singapore | Singapore | 2008–2012 | Multiple | 2740 | 20.9% | Telemetry+ECG | Nonintervention‐based (≥60 min) |
| Zhao, 2021 66 | Fuwai Hospital | China | 2012–2019 | Total arch repair | 1271 | 32.3% | Telemetry | Nonintervention‐based (≥5 min) |
| Musa, 2022 67 | Institute Jantung Negana | Malaysia | 2019–2021 | CABG±valve | 242 | 36.4% | Telemetry | Nonintervention‐based (≥30 s) |
| Oraii, 2022 68 | Tehran Heart Center | Iran | 2012–2016 | CABG | 9310 | 12.9% | Telemetry+ECG | Nonintervention‐based (≥30 s) |
| Potdar, 2022 69 | Rabindranath Tagore International Institute of Cardiac Sciences | India | 2018 | CABG | 1108 | 7.9% | Telemetry+ECG | Intervention‐based |
AVR indicates aortic valve replacement; CABG, coronary artery bypass grafting; and POAF, postoperative atrial fibrillation.
Subgroup and Additional Analyses
A subgroup analysis for the primary outcome was performed in studies including only patients undergoing isolated coronary artery bypass grafting (CABG). An additional analysis looking at the trend in POAF incidence over the study period was also performed.
Statistical Analysis
Categorical variables were extracted as numbers and continuous variables were extracted as mean and SD.
For each assessment method, the incidence of POAF across studies was pooled as an overall proportion (overall number of events/total number of patients) using an inverse variance method, which takes into account the weight of each study relative to the study sample size. Both common and random effects estimates were reported. Ninety‐five percent CIs were estimated using the Clopper–Pearson interval.
Subsequently, pooled proportions were compared between the different types of monitoring using a standard test for heterogeneity across the subgroup results, as previously described. 19 Similarly, categorical outcomes were compared by assessment methods.
The proportion of POAF across monitoring methods was also compared using chi‐square test among the subgroups.
Trend in the postoperative incidence of POAF during the study years was investigated using the locally estimated scatterplot smoothing.
For continuous outcomes (ICU and postoperative LOS), the LOS across studies was pooled as an overall mean using an inverse variance method and subsequently compared using a test for subgroup differences.
Statistical heterogeneity was assessed with I 2, which describes the percentage of the variability in the effect estimates due to heterogeneity rather than sampling error. Low, moderate, and high heterogeneity were defined as I 2 < 25%, 25% to 50%, and >50%, respectively. 20 Tau‐squared using DerSimonian–Laird model was used to estimate the between‐study variance.
Funnel plot and Egger's test were used to assess for publication bias graphically and quantitatively.
Univariable, random‐effects meta‐regression was used to explore the association between POAF incidence and the rigor of the assessment method used. Compared with the main analysis, which is based on pairwise comparisons, the meta‐regression uses the monitoring methods as an ordinal, 3‐level variable, where continuous telemetry until hospital discharge is considered more rigorous than telemetry during ICU stay followed by daily ECG in the regular ward, which is considered more rigorous than daily ECG only. Moreover, logistic regression with robust SE was performed for the association of POAF and categorical variables after reproducing the individual level data.
Statistical analyses were performed in R version 4.0.3 (R Foundation for Statistical Computing) using the package meta.
Data Availability
Data collected for the study will be made available by the corresponding author upon reasonable request after publication.
RESULTS
Study and Patient Characteristics
Among the 8974 identified (6212 screened) articles, a total of 59 studies published between 1993 and 2022 were included in the present analysis. 3 , 4 , 5 , 7 , 12 , 13 , 14 , 15 , 16 , 17 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow diagram outlining the study selection process and the checklist are detailed in Figure S1 and Table S3, respectively. All studies were considered of high or good methodological quality for the purpose of this meta‐analysis (Table S4). Eighteen studies (30.5%) were from Asia, 16 (27.1%) from North America, 15 (25.4%) from Europe, 4 (6.8%) from South America, 3 (5.1%) from multiple regions, 2 (3.4%) from Oceania, and 1 (1.7%) from Africa. Thirty‐six studies (61%) included patients undergoing isolated CABG, 20 (33.9%) included more than 1 cardiac surgical procedure, 2 (3.4%) included patients undergoing isolated aortic valve replacement, and 1 (1.7%) included patients undergoing total arch repair (Table 1).
A total of 197 774 patients were included in the pooled analysis. The number of patients in each study ranged from 44 to 19 947 with a median sample size of 969 (interquartile range: 364–5362). The cumulative incidence of POAF was 26% (range: 7.3%–53.1%; Figure S2). Twenty‐one (35.6%) studies reported POAF incidence based on continuous telemetry until hospital discharge, 30 (50.9%) based on telemetry during ICU stay followed by daily ECG in the regular floor, and 8 (13.6%) based on daily ECG only. Fifteen (25.4%) studies had an intervention‐based POAF definition, 36 (61%) a nonintervention‐based definition, and 8 (13.6%) did not report POAF definition (Table 1).
The mean age range was 53.7 to 77.4 years in patients with POAF, and 48.0 to 76.5 years in patients without POAF. Female patients ranged from 0.9% to 49.1% in the POAF group and 1.1% to 43.6% in the non‐POAF group. The mean left ventricular ejection fraction range was 43.2% to 65.6% in patients with POAF and 44.4% to 64.0% in patients without POAF. The prevalence of hypertension ranged from 35.1% to 95.3% in patients with POAF and 19.7% to 97.0% in patients without POAF. The prevalence of diabetes ranged from 3.2% to 80.0% in patients with POAF, and 3.4% to 60.4% in patients without POAF. The prevalence of chronic obstructive pulmonary disease ranged from 0% to 41.8% in patients with POAF and 0.8% to 36.7% in patients without POAF. Preoperative use of beta blockers ranged from 26.2% to 91.7% in patients with POAF and 24% to 95% in patients without POAF. Demographic data of the patient population in each study are summarized in Table S5.
Meta‐Analysis
Primary Outcome
The cumulative incidence of POAF was 26% (range: 7.3%–53.1%). POAF incidence in the group that used continuous telemetry until hospital discharge was 27% (range: 7.3%–53.1%), compared with 27% (range: 7.9%–50%) for the telemetry plus daily ECG group and 19% (range: 7.8%–42.4%) for the group that only used daily ECG. No difference in POAF incidence was found between any of the assessment methods (continuous telemetry versus telemetry plus daily ECG: P=0.89; continuous telemetry versus daily ECG only: P=0.12; telemetry plus daily ECG versus daily ECG only: P=0.09; Table 2; Figure 1A through D and 2; Table S6).
Table 2.
Summary of Primary and Secondary Outcomes Based on Assessment Method
| Primary outcome | ||||
|---|---|---|---|---|
| Outcome | Comparison group | Pooled estimates (range) | P value | Tau‐squared |
| Postoperative atrial fibrillation incidence | Telemetry vs telemetry+ECG | 27% (7.3%–53.1%) vs 27% (7.9%–50%) | 0.89 | 0.30 vs 0.25 |
| Telemetry vs ECG only | 27% (7.3%–53.1%) vs 19% (7.8%–42.4%) | 0.12 | 0.30 vs 0.48 | |
| Telemetry+ECG vs ECG only | 27% (7.9%–50%) vs 19% (7.8%–42.4%) | 0.09 | 0.26 vs 0.48 | |
| Secondary outcomes | ||||
|---|---|---|---|---|
| Outcome | Comparison group | Pooled estimates (95% CI) | P value | |
| Mortality | Telemetry vs telemetry+ECG | 4% (3%–5%) vs 3% (2–4%) | 0.29 | 0.24 vs 0.68 |
| Telemetry vs ECG only | 4% (3%–5%) vs 2% (1–4%) | 0.16 | 0.24 vs 0.76 | |
| Telemetry+ECG vs ECG only | 3% (2%–4%) vs 2% (1–4%) | 0.47 | 0.68 vs 0.76 | |
| Stroke | Telemetry vs telemetry+ECG | 3% (2%–4%) vs 2% (2–3%) | 0.30 | 0.13 vs 0.73 |
| Telemetry vs ECG only | 3% (2%–4%) vs 2% (1–4%) | 0.32 | 0.13 vs 0.17 | |
| Telemetry+ECG vs ECG only | 2% (2%–3%) vs 2% (1–4%) | 0.88 | 0.17 vs 0.73 | |
| Intensive care unit LOS | Telemetry vs telemetry+ECG | 3.7 d (2.1–5.2) vs 3.2 d (2.3–4.1) | 0.63 | 3.56 vs 1.50 |
| Telemetry vs ECG only | 3.7 d (2.1–5.2) vs 3.0 d (1.8–4.3) | 0.55 | 3.56 vs 1.92 | |
| Telemetry+ECG vs ECG only | 3.2 d (2.3–4.1) vs 3.0 d (1.8–4.3) | 0.82 | 1.92 vs 1.50 | |
| Postoperative LOS | Telemetry vs telemetry+ECG | 13.6 d (9.1–18.1) vs 11.0 d (9.4–12.6) | 0.29 | 56.50 vs 12.43 |
| Telemetry vs ECG only | 13.6 d (9.1–18.1) vs 10.1 d (7.7–12.6) | 0.18 | 56.50 vs 8.75 | |
| Telemetry+ECG vs ECG only | 11.0 d (9.4–12.6) vs 10.1 d (7.7–12.6) | 0.56 | 12.43 vs 8.75 | |
LOS indicates length of stay.
Figure 1. Comparison of postoperative atrial fibrillation incidence by assessment method.
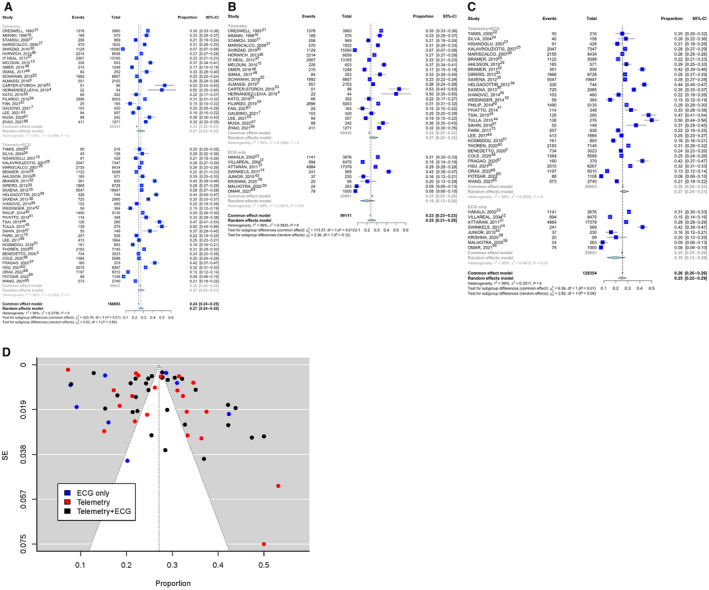
A, Telemetry vs telemetry plus ECG group, P=0.89. B, Telemetry vs ECG only, P=0.12. C, Telemetry plus ECG vs ECG only, P=0.09. D, Grouped funnel plot based on the monitoring approaches with untransformed proportion and standard error (as a measure of precision).
Figure 2. Summary figure of the main results.
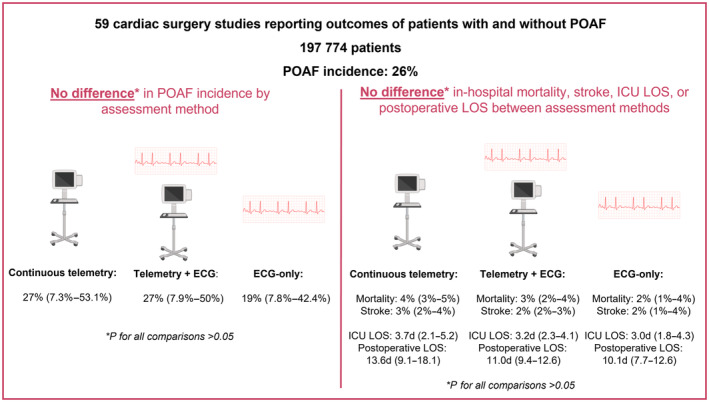
ICU indicates intensive care unit; LOS, length of stay; and POAF, postoperative atrial fibrillation.
Secondary Outcomes
No differences in in‐hospital mortality, stroke, ICU LOS, and postoperative LOS were found between the 3 POAF assessment methods. (Table 2; Figure 2, Tables S7 and S8; Figures S2 through S6).
Secondary Analyses
No difference in POAF incidence was found between intervention‐ and nonintervention‐based POAF definitions (26% [range: 7.9%–47.4%] versus 27% [range: 7.3%–53.1%], respectively; P=0.67). No differences in in‐hospital mortality, stroke, ICU LOS, and postoperative LOS were found between both definition categories (Table 3; Table S9; Figures S7 through S11).
Table 3.
Summary of Secondary Analyses Based on Postoperative Atrial Fibrillation Definition
| Outcome | Intervention‐based definition (95% CI, unless noted otherwise) | Nonintervention‐based definition (95% CI, unless noted otherwise) | P value |
|---|---|---|---|
| Postoperative atrial fibrillation incidence | 26% (range 7.9%–47.4%) | 27% (range: 7.3%–53.1%) | 0.67 |
| Mortality | 2% (1%–3%) | 2% (1%–2%) | 0.69 |
| Stroke | 1% (1%–2%) | 2% (1%–2%) | 0.43 |
| Intensive care unit LOS | 2.7 d (1.2–4.2) | 2.3 d (1.7–2.8) | 0.57 |
| Postoperative LOS | 9.7 d (7.1–12.4) | 9.5 d (8–10.9) | 0.86 |
LOS indicates length of stay.
There was no evidence of publication bias (Egger's intercept test P=0.86; Figure S12).
Subgroup Analysis
In CABG studies significant differences in POAF incidence between continuous telemetry and daily ECG (25% [range: 15.2%–33.3%] versus 15% [range: 7.8%–31%], respectively; P=0.02), and between telemetry plus daily ECG compared with daily ECG only (26% [range: 7.9–50%] versus 15% [range: 7.8%–31%], respectively; P=0.02) were found (Table 4; Figure S13A through C).
Table 4.
Summary of the Subgroup Analyses in Studies That Included Only Patients Undergoing Isolated Coronary Artery Bypass Grafting According to Postoperative Atrial Fibrillation Assessment Method
| Outcome | Comparison group | Pooled estimates (range) | P value |
|---|---|---|---|
| Postoperative atrial fibrillation incidence | Telemetry vs telemetry+ECG | 25% (15.2%–33.3%) vs 26% (7.9%–50%) | 0.81 |
| Telemetry vs ECG only | 25% (15.2%–33.3%) vs 15% (7.8%–31%) | 0.02 | |
| Telemetry+ECG vs ECG only | 26% (7.9%–50%) vs 15% (7.8–31%) | 0.02 |
Trend in POAF Incidence
There was no significant change in POAF incidence during the study period (P for trend=0.54; Figure 3).
Figure 3. Trends in postoperative atrial fibrillation incidence over the study period.
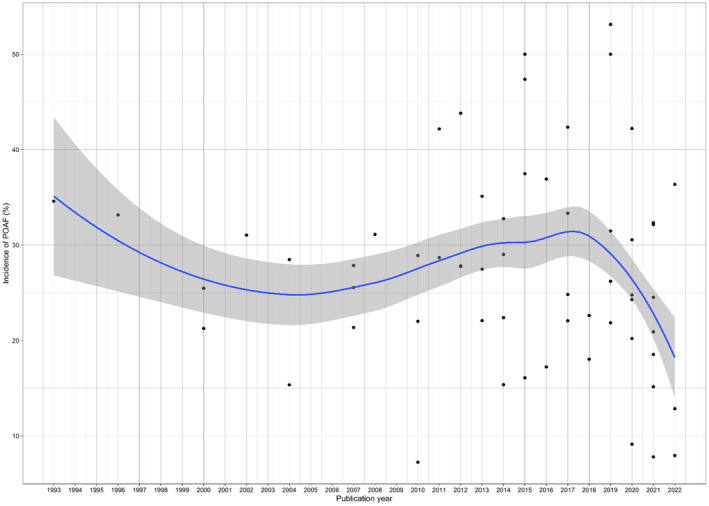
P for trend=0.54. POAF indicates postoperative atrial fibrillation.
Meta‐Regression
No association between POAF incidence and increasing rigor of the assessment method was found when all cardiac surgeries were considered (beta coefficient 0.17 [95% CI, −0.04 to 0.39, P=0.10]); however, an association was found in studies including only patients undergoing isolated CABG where an increase in the rigor of the POAF assessment method was associated with higher POAF detection rates (beta coefficient 0.27 [95% CI, 0.04–0.50], P=0.02; Tables S10 and S11).
DISCUSSION
In the present meta‐analysis of 59 studies, we found no significant difference in the incidence of POAF after cardiac surgery or its association with adverse outcomes based on the POAF definition or assessment method used; however, in studies including only patients undergoing isolated CABG, continuous telemetry in the ICU and telemetry in the ICU plus daily ECG in the regular ward were associated with higher POAF incidence compared with daily ECG only.
Prior evidence has suggested that continuous telemetry during the complete postoperative hospitalization is associated with higher POAF incidence compared with other assessment methods 70 and this finding was seen also in studies in patients who had noncardiac surgery. 71 Our results did not support this finding when all cardiac surgeries were considered; however, this was the case when considering studies that included only patients undergoing isolated CABG. In this subgroup, the use of telemetry at any point postoperatively (either throughout the postoperative stay or only in the ICU) was associated with higher POAF incidence compared with daily ECG use only. The lack of difference in POAF incidence between complete stay telemetry and ICU‐only telemetry could be explained by the characteristics of POAF, in that over 70% of POAF episodes occur within 72 hours of surgery 72 and the mean ICU LOS in both groups was approximately 72 hours, suggesting the preponderance of POAF is captured by in‐ICU telemetry.
The lack of difference in POAF incidence between intervention‐ and nonintervention‐based POAF definitions could be explained by heterogeneity in the individual study definitions of what is considered treatment of POAF. For example, one study counted POAF episodes only if the arrhythmia required either medical or electrical cardioversion, 33 whereas others limited the description of the definition to any POAF episode requiring treatment 48 , 52 without further elaboration. The range of possible interventions in the last setting includes rate control treatment with beta blockers (received by virtually all patients with POAF in the absence of contraindications), to anticoagulation (with variability in treatment recommendations from different professional societies 11 , 73 , 74 ) and cardioversion (reserved for patients with hemodynamic instability or resistance/contraindications to medical treatment).
Methodological and Clinical Implications
Our findings have methodological implications for the selection of POAF detection methods in future studies. The main finding is that in patients who had cardiac surgery POAF incidence and its association with adverse outcomes are not influenced by the assessment method and definition used. However, in studies that included only patients undergoing isolated CABG, the increasing sensitivity of the POAF assessment method was associated with higher POAF detection rates. While the reason for this difference is unclear, it is possible that the less invasive nature of isolated CABG compared with other cardiac surgeries results in less inflammation and shorter POAF episodes (lasting <24 hours), 1 making POAF less likely to be captured by daily ECG. It is also possible that the shorter duration of ICU stay after CABG may have played a role in the reported difference.
From a clinical standpoint, although POAF rates are not affected by the assessment methods, it must be noted that continuous telemetry monitoring outside of the ICU may allow detection of other clinically relevant arrhythmias and is consistent with recommendations from professional societies. 75
Limitations
This study must be interpreted considering its limitations. Although our systematic review identified the best available evidence comparing outcomes of patients with and without POAF after cardiac surgery, POAF assessment methods were nonrandomized in all studies, creating the possibility for biases and confounding. Additionally, not all studies reported the secondary outcomes of interest, decreasing the power of some comparisons. Moreover, heterogeneity was high for all the outcomes, and it is also possible that studies that used daily ECG only for POAF assessment come from low‐resource centers or are using data from an older era.
CONCLUSIONS
POAF incidence remains high after cardiac surgery, although detection rates exhibit variability among different studies. No differences in POAF incidence, in‐hospital mortality, stroke, ICU LOS, and postoperative LOS were found between assessment methods and POAF definitions. POAF incidence was higher in studies that included only patients undergoing isolated CABG that used telemetry for POAF assessment (regardless of whether it was during the whole postoperative stay or only in the ICU) compared with daily ECG only.
Sources of Funding
None.
Disclosures
Giuseppe Biondi‐Zoccai has consulted for Amarin, Balmed, Cardionovum, Crannmedical, Endocore Lab, Eukon, Guidotti, Innovheart, Meditrial, Microport, Opsens Medical, Terumo, and Translumina, outside the present work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S11
Figures S1–S13
This article was sent to Luciano A. Sposato, MD, MBA, FRCPC, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030907
For Sources of Funding and Disclosures, see page 12.
References
- 1. Dobrev D, Aguilar M, Heijman J, Guichard J‐B, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. doi: 10.1038/s41569-019-0166-5 [DOI] [PubMed] [Google Scholar]
- 2. Caldonazo T, Kirov H, Rahouma M, Robinson NB, Demetres M, Gaudino M, Doenst T, Dobrev D, Borger MA, Kiehntopf M, et al. Atrial fibrillation after cardiac surgery: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg. 2021;1:94–103.e24. [DOI] [PubMed] [Google Scholar]
- 3. Benedetto U, Gaudino MF, Dimagli A, Gerry S, Gray A, Lees B, Flather M, Taggart DP, Westaby S, Cook J, et al. Postoperative atrial fibrillation and long‐term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. 2020;142:1320–1329. doi: 10.1161/CIRCULATIONAHA.120.046940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernández‐Leiva E, Alvarado P, Dennis RJ. Postoperative atrial fibrillation: evaluation of its economic impact on the costs of cardiac surgery. Braz J Cardiovasc Surg. 2019;34:34. doi: 10.21470/1678-9741-2018-0218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Almassi GH, Hawkins RB, Bishawi M, Shroyer AL, Hattler B, Quin JA, Collins JF, Bakaeen FG, Ebrahimi R, Grover FL, et al. New‐onset postoperative atrial fibrillation impact on 5‐year clinical outcomes and costs. J Thorac Cardiovasc Surg. 2021;161:1803–1810.e3. doi: 10.1016/j.jtcvs.2019.10.150 [DOI] [PubMed] [Google Scholar]
- 6. Arsenault KA, Yusuf AM, Crystal E, Healey JS, Morillo CA, Nair GM, Whitlock RP. Interventions for preventing post‐operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst Rev. 2013;2013:CD003611. doi: 10.1002/14651858.CD003611.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaudino M, Sanna T, Ballman KV, Robinson NB, Hameed I, Audisio K, Rahouma M, Di Franco A, Soletti GJ, Lau C, et al. Posterior left pericardiotomy for the prevention of atrial fibrillation after cardiac surgery: an adaptive, single‐centre, single‐blind, randomised, controlled trial. Lancet. 2021;398:2075–2083. doi: 10.1016/S0140-6736(21)02490-9 [DOI] [PubMed] [Google Scholar]
- 8. Gaudino M, Di Franco A, Rong LQ, Piccini J, Mack M. Postoperative atrial fibrillation: from mechanisms to treatment. Eur Heart J. 2023;44:1020–1039. doi: 10.1093/eurheartj/ehad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaudino M, Di Franco A, Rong LQ, Cao D, Pivato CA, Soletti GJ, Chadow D, Cancelli G, Perezgrovas Olaria R, Gillinov M, et al. Pericardial effusion provoking atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2022;79:2529–2539. doi: 10.1016/j.jacc.2022.04.029 [DOI] [PubMed] [Google Scholar]
- 10. Frendl G, Sodickson AC, Chung MK, Waldo AL, Gersh BJ, Tisdale JE, Calkins H, Aranki S, Kaneko T, Cassivi S, et al. 2014 AATS guidelines for the prevention and management of perioperative atrial fibrillation and flutter for thoracic surgical procedures. J Thorac Cardiovasc Surg. 2014;148:e153–e193. doi: 10.1016/j.jtcvs.2014.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 12. Villareal RP, Hariharan R, Liu BC, Kar B, Lee V‐V, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 13. Nisanoglu V, Erdil N, Aldemir M, Ozgur B, Berat Cihan H, Yologlu S, Battaloglu B. Atrial fibrillation after coronary artery bypass grafting in elderly patients: incidence and risk factor analysis. Thorac Cardiovas Surg. 2007;55:32–38. doi: 10.1055/s-2006-924711 [DOI] [PubMed] [Google Scholar]
- 14. Swinkels BM, de Mol BA, Kelder JC, Vermeulen FE, ten Berg JM. New‐onset postoperative atrial fibrillation after aortic valve replacement: effect on long‐term survival. J Thorac Cardiovasc Surg. 2017;154:492–498. doi: 10.1016/j.jtcvs.2017.02.052 [DOI] [PubMed] [Google Scholar]
- 15. Melduni RM, Schaff HV, Bailey KR, Cha SS, Ammash NM, Seward JB, Gersh BJ. Implications of new‐onset atrial fibrillation after cardiac surgery on long‐term prognosis: a community‐based study. Am Heart J. 2015;170:659–668. doi: 10.1016/j.ahj.2015.06.015 [DOI] [PubMed] [Google Scholar]
- 16. Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, Collins JJ, Cohn LH, Burstin HR. Predictors of atrial fibrillation after coronary artery surgery: current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.CIR.94.3.390 [DOI] [PubMed] [Google Scholar]
- 17. Stamou SC, Dangas G, Hill PC, Pfister AJ, Dullum MKC, Boyce SW, Bafi AS, Garcia JM, Corso PJ. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64–67. doi: 10.1016/S0002-9149(00)00829-8 [DOI] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;2021:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Borenstein M, Higgins JPT. Meta‐analysis and subgroups. Prev Sci. 2013;14:134–143. doi: 10.1007/s11121-013-0377-7 [DOI] [PubMed] [Google Scholar]
- 20. Higgins JPT. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. doi: 10.1016/0003-4975(93)90894-N [DOI] [PubMed] [Google Scholar]
- 22. Tamis JE, Steinberg JS. Atrial fibrillation independently prolongs hospital stay after coronary artery bypass surgery. Clin Cardiol. 2000;23:155–159. doi: 10.1002/clc.4960230305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hakala T, Pitkänen O, Hippeläinen M. Feasibility of predicting the risk of atrial fibrillation after coronary artery bypass surgery with logistic regression model. Scand J Surg. 2002;91:339–344. doi: 10.1177/145749690209100406 [DOI] [PubMed] [Google Scholar]
- 24. Silva RGD, Lima GGD, Laranjeira A, Costa ARD, Pereira E, Rodrigues R. Fatores de risco e morbimortalidade associados à fibrilação atrial no pós‐operatório de cirurgia cardíaca. Arq Bras Cardiol. 2004;83:99–104. doi: 10.1590/S0066-782X2004001400002 [DOI] [Google Scholar]
- 25. Kalavrouziotis D, Buth KJ, Ali IS. The impact of new‐onset atrial fibrillation on In‐hospital mortality following cardiac surgery. Chest. 2007;131:833–839. doi: 10.1378/chest.06-0735 [DOI] [PubMed] [Google Scholar]
- 26. Mariscalco G, Engström KG. Atrial fibrillation after cardiac surgery: risk factors and their temporal relationship in prophylactic drug strategy decision. Int J Cardiol. 2008;129:354–362. doi: 10.1016/j.ijcard.2007.07.123 [DOI] [PubMed] [Google Scholar]
- 27. Mariscalco G, Klersy C, Zanobini M, Banach M, Ferrarese S, Borsani P, Cantore C, Biglioli P, Sala A. Atrial fibrillation after isolated coronary surgery affects late survival. Circulation. 2008;118:1612–1618. doi: 10.1161/CIRCULATIONAHA.108.777789 [DOI] [PubMed] [Google Scholar]
- 28. Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardio‐Thorac Surg. 2010;37:1353–1359. doi: 10.1016/j.ejcts.2009.12.033 [DOI] [PubMed] [Google Scholar]
- 29. Bramer S, Van Straten AHM, Soliman Hamad MA, Berreklouw E, Martens EJ, Maessen JG. The impact of new‐onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. Ann Thorac Surg. 2010;90:443–449. doi: 10.1016/j.athoracsur.2010.03.083 [DOI] [PubMed] [Google Scholar]
- 30. Shirzad M, Karimi A, Tazik M, Aramin H, Ahmadi SH, Davoodi S, Marzban M. Determinants of postoperative atrial fibrillation and associated resource utilization in cardiac surgery. Rev Esp Cardiol. 2010;63:1054–1060. doi: 10.1016/S1885-5857(10)70209-7 [DOI] [PubMed] [Google Scholar]
- 31. Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM. Atrial fibrillation postcardiac surgery: a common but a morbid complication. Interact Cardiovasc Thorac Surg. 2011;12:772–777. doi: 10.1510/icvts.2010.243782 [DOI] [PubMed] [Google Scholar]
- 32. Bramer S, Van Straten AHM, Soliman Hamad MA, Van Den Broek KC, Maessen JG, Berreklouw E. New‐onset postoperative atrial fibrillation predicts late mortality after mitral valve surgery. Ann Thorac Surg. 2011;92:2091–2096. doi: 10.1016/j.athoracsur.2011.06.079 [DOI] [PubMed] [Google Scholar]
- 33. Girerd N, Pibarot P, Daleau P, Voisine P, O'Hara G, Després J‐P, Mathieu P. Statins reduce short‐ and long‐term mortality associated with postoperative atrial fibrillation after coronary artery bypass grafting: impact of postoperative atrial fibrillation and statin therapy on survival. Clin Cardiol. 2012;35:430–436. doi: 10.1002/clc.21008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Helgadottir S, Sigurdsson MI, Ingvarsdottir IL, Arnar DO, Gudbjartsson T. Atrial fibrillation following cardiac surgery: risk analysis and long‐term survival. J Cardiothorac Surg. 2012;7:87. doi: 10.1186/1749-8090-7-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109:219–225. doi: 10.1016/j.amjcard.2011.08.033 [DOI] [PubMed] [Google Scholar]
- 36. Horwich P, Buth KJ, Légaré J‐F. New onset postoperative atrial fibrillation is associated with a long‐term risk for stroke and death following cardiac surgery. J Cardiac Surg. 2013;28:8–13. doi: 10.1111/jocs.12033 [DOI] [PubMed] [Google Scholar]
- 37. O'Neal WT, Efird JT, Davies SW, O'Neal JB, Anderson CA, Ferguson TB, Chitwood WR, Kypson AP. The impact of postoperative atrial fibrillation and race on long‐term survival after coronary artery bypass grafting. J Cardiac Surg. 2013;28:484–491. doi: 10.1111/jocs.12178 [DOI] [PubMed] [Google Scholar]
- 38. Saxena A, Shi WY, Bappayya S, Dinh DT, Smith JA, Reid CM, Shardey GC, Newcomb AE. Postoperative atrial fibrillation after isolated aortic valve replacement: a cause for concern? Ann Thorac Surg. 2013;95:133–140. doi: 10.1016/j.athoracsur.2012.08.077 [DOI] [PubMed] [Google Scholar]
- 39. Ivanovic B, Tadic M, Bradic Z, Zivkovic N, Stanisavljevic D, Celic V. The influence of the metabolic syndrome on atrial fibrillation occurrence and outcome after coronary bypass surgery: a 3‐year follow‐up study. Thorac Cardiovasc Surg. 2014;62:561–568. doi: 10.1055/s-0034-1372349 [DOI] [PubMed] [Google Scholar]
- 40. Philip F, Becker M, Galla J, Blacksone E, Kapadia SR. Transient post‐operative atrial fibrillation predicts short and long term adverse events following CABG. Cardiovasc Diagn Ther. 2014;4:365–372. doi: 10.3978/j.issn.2223-3652.2014.09.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pivatto Júnior F, Teixeira Filho GF, Sant'anna JR, Py PM, Prates PR, Nesralla IA, Kalil RA. Advanced age and incidence of atrial fibrillation in the postoperative period of aortic valve replacement. Rev Bras Cirurgia Cardiovasc. 2014;29:45–50. doi: 10.5935/1678-9741.20140010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weidinger F, Schachner T, Bonaros N, Hofauer B, Lehr EJ, Vesely M, Zimrin D, Bonatti J. Predictors and consequences of postoperative atrial fibrillation following robotic totally endoscopic coronary bypass surgery. Eur J Cardio‐Thorac Surg. 2014;45:318–322. doi: 10.1093/ejcts/ezt282 [DOI] [PubMed] [Google Scholar]
- 43. Bohatch Júnior MS, Matkovski PD, Giovanni FJD, Fenili R, Varella EL, Dietrich A. Incidence of postoperative atrial fibrillation in patients undergoing on‐pump and off‐pump coronary artery bypass grafting. Rev Bras Cirurgia Cardiovasc. 2015;30:316–324. doi: 10.5935/1678-9741.20150040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsai YT, Lai C, Loh SH, Lin CY, Lin YC, Lee CY, Ke HY, Tsai CS. Assessment of the risk factors and outcomes for postoperative atrial fibrillation patients undergoing isolated coronary artery bypass grafting. Acta Cardiol Sinica. 2015;31:436–443. doi: 10.6515/acs20150609a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tulla H, Hippeläinen M, Turpeinen A, Pitkänen O, Hartikainen J. New‐onset atrial fibrillation at discharge in patients after coronary artery bypass surgery: short‐ and long‐term morbidity and mortality. Eur J Cardio‐Thorac Surg. 2015;48:747–752. doi: 10.1093/ejcts/ezu526 [DOI] [PubMed] [Google Scholar]
- 46. Omer S, Cornwell LD, Bakshi A, Rachlin E, Preventza O, Rosengart TK, Coselli JS, LeMaire SA, Petersen NJ, Pattakos G, et al. Incidence, predictors, and impact of postoperative atrial fibrillation after coronary artery bypass grafting in military veterans. Tex Heart Inst J. 2016;43:397–403. doi: 10.14503/THIJ-15-5532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sahin M. Incidence and predisposing factors of atrial fibrillation after coronary artery bypass surgery. J Clin Anal Med. 2016;7. doi: 10.4328/JCAM.3839 [DOI] [Google Scholar]
- 48. Ismail MF, El‐mahrouk AF, Hamouda TH, Radwan H, Haneef A, Jamjoom AA. Factors influencing postoperative atrial fibrillation in patients undergoing on‐pump coronary artery bypass grafting, single center experience. J Cardiothorac Surg. 2017;12:40. doi: 10.1186/s13019-017-0609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee S‐H, Lee H, Park J‐K, Uhm J‐S, Kim J‐Y, Pak H‐N, Lee M‐H, Yoon H‐G, Joung B. Gender difference in the long‐term clinical implications of new‐onset atrial fibrillation after coronary artery bypass grafting. Yonsei Med J. 2017;58:1119–1127. doi: 10.3349/ymj.2017.58.6.1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park YM, Cha MS, Park C‐H, Choi CH, Jeon YB, Kang WC, Choi IS, Park KY. Newly developed post‐operative atrial fibrillation is associated with an increased risk of late recurrence of atrial fibrillation in patients who underwent open heart surgery: long‐term follow up. Cardiol J. 2017;24:633–641. doi: 10.5603/CJ.a2017.0073 [DOI] [PubMed] [Google Scholar]
- 51. Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ, Puskas JD, Kandzari DE, Taggart DP, Morice M‐C, Buszman PE, et al. New‐onset atrial fibrillation after PCI or CABG for left main disease. J Am Coll Cardiol. 2018;71:739–748. doi: 10.1016/j.jacc.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 52. Schwann TA, Al‐Shaar L, Engoren MC, Bonnell MR, Goodwin M, Schwann AN, Habib RH. Effect of new‐onset atrial fibrillation on cause‐specific late mortality after coronary artery bypass grafting surgery. Eur J Cardio‐Thorac Surg. 2018;54:294–301. doi: 10.1093/ejcts/ezy028 [DOI] [PubMed] [Google Scholar]
- 53. Carter‐Storch R, Dahl JS, Christensen NL, Pecini R, Søndergård EV, Øvrehus KA, Møller JE. Postoperative atrial fibrillation after aortic valve replacement is a risk factor for long‐term atrial fibrillation. Interact Cardiovasc Thorac Surg. 2019;29:378–385. doi: 10.1093/icvts/ivz094 [DOI] [PubMed] [Google Scholar]
- 54. Filardo G, Ailawadi G, Pollock BD, Da Graca B, Phan TK, Thourani V, Damiano RJ. Postoperative atrial fibrillation: sex‐specific characteristics and effect on survival. J Thorac Cardiovasc Surg. 2020;159:1419–1425.e1. doi: 10.1016/j.jtcvs.2019.04.097 [DOI] [PubMed] [Google Scholar]
- 55. Kato M, Saitoh M, Kawamura T, Iwata K, Sakurada K, Okamura D, Tahara M, Yuguchi S, Kamisaka K, Oura K, et al. Postoperative atrial fibrillation is associated with delayed early rehabilitation after heart valve surgery: a multicenter study. Phys Ther Res. 2019;22:1–8. doi: 10.1298/ptr.E9957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cole OM, Tosif S, Shaw M, Lip GYH. Acute kidney injury and postoperative atrial fibrillation In patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34:1783–1790. doi: 10.1053/j.jvca.2019.12.048 [DOI] [PubMed] [Google Scholar]
- 57. Fragão‐Marques M, Mancio J, Oliveira J, Falcão‐Pires I, Leite‐Moreira A. Gender differences in predictors and long‐term mortality of new‐onset postoperative atrial fibrillation following isolated aortic valve replacement surgery. Ann Thorac Cardiovasc Surg. 2020;26:342–351. doi: 10.5761/atcs.oa.19-00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krishna V, Patil N, Nileshwar A. Prospective evaluation of the utility of CHA2DS2‐VASc score in the prediction of postoperative atrial fibrillation after off‐pump coronary artery bypass surgery—an observational study. Ann Cardiac Anaesth. 2020;23:122. doi: 10.4103/aca.ACA_161_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Malhotra P, Pande S, Mahindru S, Thukral A, Kotwal A, Gupta R, Tewari P, Agarwal S. Postoperative atrial fibrillation in coronary artery bypass grafting herald poor outcome. Ann Cardiac Anaesth. 2021;24:464. doi: 10.4103/aca.ACA_30_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thorén E, Wernroth M‐L, Christersson C, Grinnemo K‐H, Jidéus L, Ståhle E. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long‐term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol. 2020;109:1232–1242. doi: 10.1007/s00392-020-01614-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fan G, Liu J, Dong S, Chen Y. Postoperative atrial fibrillation after minimally invasive direct coronary artery bypass: a single‐center, 5‐year follow‐up study: POAF after MIDCAB. Heart Surg Forum. 2021;24:E456–E460. doi: 10.1532/hsf.3621 [DOI] [PubMed] [Google Scholar]
- 62. Hsu J‐C, Huang C‐Y, Chuang S‐L, Yu H‐Y, Chen Y‐S, Wang C‐H, Lin L‐Y. Long term outcome of postoperative atrial fibrillation after cardiac surgery—a propensity score‐matched cohort analysis. Front Cardiovasc Med. 2021;8:650147. doi: 10.3389/fcvm.2021.650147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee H, Kim HJ, Yoo JS, Kim DJ, Yeom SY, Cho KR. Early pharmacologic conversion of atrial fibrillation after off‐pump coronary artery bypass grafting. J Thorac Dis. 2021;13:4072–4082. doi: 10.21037/jtd-21-466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Omar A, Elshihy EM, Singer M, Zarif D, Dawoud O. Perioperative risk factors predisposing to atrial fibrillation after CABG surgery. Heart Surg Forum. 2021;24:E402–E406. doi: 10.1532/hsf.3759 [DOI] [PubMed] [Google Scholar]
- 65. Wang KKP, Liu W, Chew STH, Ti LK, Shen L. New‐onset atrial fibrillation after cardiac surgery is a significant risk factor for long‐term stroke: an eight‐year prospective cohort study. J Cardiothorac Vasc Anesth. 2021;35:3559–3564. doi: 10.1053/j.jvca.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 66. Zhao M, Woodward M, Vaartjes I, Millett ERC, Klipstein‐Grobusch K, Hyun K, Carcel C, Peters SAE. Sex differences in cardiovascular medication prescription in primary care: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e014742. doi: 10.1161/JAHA.119.014742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Musa AF, Dillon J, Taib MEM, Yunus AM, Sanusi AR, Nordin MN, Smith JA. Incidence and outcomes of postoperative atrial fibrillation after coronary artery bypass grafting of a randomized controlled trial: a blinded end‐of‐cycle analysis. Rev Cardiovasc Med. 2022;23:122. doi: 10.31083/j.rcm2304122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Oraii A, Masoudkabir F, Pashang M, Jalali A, Sadeghian S, Mortazavi SH, Ghorbanpour Landy M, Pourhosseini H, Salarifar M, Mansourian S, et al. Effect of postoperative atrial fibrillation on early and mid‐term outcomes of coronary artery bypass graft surgery. Eur J Cardiothorac Surg. 2022;62:ezac264. doi: 10.1093/ejcts/ezac264 [DOI] [PubMed] [Google Scholar]
- 69. Potdar SP, Shales S, Baviskar M, Sharma M, Kapoor L, Narayan P. Incidence, predictors, and outcome for post‐operative atrial fibrillation in Indian patients undergoing off‐pump coronary artery bypass grafting—a prospective observational study. Indian J Thorac Cardiovasc Surg. 2022;38:366–374. doi: 10.1007/s12055-022-01358-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maesen B, Nijs J, Maessen J, Allessie M, Schotten U. Post‐operative atrial fibrillation: a maze of mechanisms. Europace. 2012;14:159–174. doi: 10.1093/europace/eur208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McIntyre WF, Belley‐Côté EP, Vadakken ME, Rai AS, Lengyel AP, Rochwerg B, Bhatnagar AK, Deif B, Um KJ, Spence J, et al. High‐sensitivity estimate of the incidence of new‐onset atrial fibrillation in critically ill patients. Crit Care Explor. 2021;3:e0311. doi: 10.1097/CCE.0000000000000311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Perezgrovas‐Olaria R, Chadow D, Lau C, Rahouma M, Soletti GJ, Cancelli G, Harik L, Dimagli A, Rong LQ, Gillinov M, et al. Characteristics of postoperative atrial fibrillation and the effect of posterior pericardiotomy. Ann Thorac Surg. 2023;116:615–622. doi: 10.1016/j.athoracsur.2022.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, Cox JL, Dorian P, Gladstone DJ, Healey JS, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. 2020;36:1847–1948. doi: 10.1016/j.cjca.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 74. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sandau KE, Funk M, Auerbach A, Barsness GW, Blum K, Cvach M, Lampert R, May JL, McDaniel GM, Perez MV, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation. 2017;136:e273–e344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S11
Figures S1–S13
Data Availability Statement
Data collected for the study will be made available by the corresponding author upon reasonable request after publication.


