Abstract
Background
Mortality from cardiovascular diseases in Asian populations is considerable. Menopause is a risk‐enhancing factor for cardiovascular disease, but it is unclear whether menopause is an independent risk factor for cardiovascular disease and mortality in Asian women.
Methods and Results
A total of 1 159 405 postmenopausal women, who had participated in the health examinations of the Korean National Health Insurance Service in 2009, were analyzed, and their reproductive histories were taken. A multivariable Cox proportional hazard model assessed the hazard ratios (HRs) of myocardial infarction (MI), ischemic stroke, and all‐cause mortality, according to the history of premature menopause and age at menopause. After an average 10‐year follow‐up, there were 31 606, 45 052, and 77 680 new cases of MI, ischemic stroke, and all‐cause mortality, respectively. The women with premature menopause exhibited increased risks of MI (HR, 1.40 [95% CI, 1.31–1.50]), ischemic stroke (HR, 1.24 [95% CI, 1.17–1.31]), and all‐cause mortality (HR, 1.19 [95% CI, 1.14–1.24]) when compared with women with menopause aged ≥50 years. The highest risk was evident with menopause between the ages of 30 and 34 years (HR for MI, 1.52 [95% CI, 1.30–1.78]; HR for ischemic stroke, 1.29 [95% CI, 1.12–1.48]; HR for all‐cause mortality, 1.33 [95% CI, 1.20–1.47]) when compared with women with menopause aged ≥50 years.
Conclusions
Earlier age at menopause was associated with increased risks for MI, ischemic stroke, and all‐cause mortality. Future guidelines and risk assessment tools should consider menopause as an independent risk factor of cardiovascular disease in Korean women.
Keywords: all‐cause mortality, cardiovascular disease, early menopause, Korean, premature menopause
Subject Categories: Epidemiology
Nonstandard Abbreviation and Acronym
- NHIS
National Health Insurance Service
Clinical Perspective.
What Is New?
Both premature and early menopause were associated with significantly increased risks for myocardial infarction, ischemic stroke, and all‐cause mortality compared with women undergoing normal menopause.
The earlier menopause occurred, the higher the risks of cardiovascular disease and mortality, which increased in an inverse dose‐response pattern.
What Are the Clinical Implications?
Earlier age at menopause is a predictor for myocardial infarction, ischemic stroke, and all‐cause mortality in healthy Korean women.
Menopause should be considered as an independent risk factor for cardiovascular disease in future risk assessment and major guidelines.
Cardiovascular disease (CVD) is the leading cause of mortality globally. 1 Among all the worldwide deaths attributed to CVD in 2019, Asia accounted for nearly 60%. 2 Ever since the hypothesis on the relationship between menopause and CVD risk was introduced 20 years ago, 3 studies have accumulated to highlight the importance of menopause transition as the period when CVD risk accelerates. 4 Menopause is defined as the cessation of the menstrual cycle for >12 months from the last menstruation. When menstruation ceases before the age of 40 years, it is defined as premature menopause, 5 and when menstrual termination occurs between the ages of 40 and 44 years, it is termed early menopause. 6 Nowadays, women from both Western and Eastern countries undergo menopause at an average age of 51 to 52 years, 7 , 8 but the prevalence of premature menopause differs according to race or ethnicity. 9 Korean women experience a higher prevalence of premature and early menopause than North American women. 8 , 10 , 11 Considering the large Asian population and high CVD burden in this population, it is important to study the public health risks associated with earlier age at menopause with respect to race.
Many American and Europeans studies have emphasized the associations between premature menopause, with higher risks of premature death, CVD, and strokes. A multinational study from Zhu et al (N=301 438; pooled data from 15 observational studies) demonstrated that premature and early menopause escalated the risk of nonfatal CVD before the age of 60 years by factors of 1.88 and 1.4, respectively. 12 UK Biobank study from Honigberg et al (N=144 260; 7‐year follow‐up) suggested that natural or surgical premature menopause was significantly associated with 1.36 and 1.87 times higher risk for CVD, respectively. 13 Furthermore, US population cohort study from Malek et al (N=30 239; 7.1‐year follow‐up) reported that premature or early menopause increased the risk of all‐cause mortality in women who had ever undergone hormone replacement therapy (HRT) by a factor of 1.31. 14 A pan‐European case‐cohort study from Dam et al (N=15 402; 11‐year follow‐up) prospectively analyzed how earlier age at menopause in European women increased the risk of higher coronary heart disease. 15 Yet, menopause is not regarded as an independent risk factor for primary CVD in major guidelines and risk assessment calculations.
The 2019 Primary Prevention of Cardiovascular Disease Guidelines defined premature menopause as a risk‐enhancing factor for individuals in the borderline (5% to <7.5%) and intermediate (≥7.5% to <20%) CVD risk categories. 16 Furthermore, the recent statement from the American Heart Association has highlighted the role of menopause as an independent risk factor for CVD. 4 However, more robust data are warranted to impact clinical decisions on when to initiate statin and other CVD preventive treatments in women with earlier age at menopause, especially in Asian populations. Furthermore, physicians often use the pooled cohort equations to evaluate the atherosclerotic CVD risk of patients, but the current equations derived the data from community‐dwelling White and Black individuals in the United States. 17 Hence, recent cohort data on CVD risk factors in Asian populations were limited.
Asian populations in general have lower CVD risk than other racial groups, and Koreans have a relatively low CVD mortality rate among Asian subgroups. 2 Korean women would make an ideal reference sample for studying the relationship between earlier age at menopause and the risk of CVD and mortality among Asian populations. Therefore, we aim to study the association between age at menopause with risks of CVD and all‐cause mortality in community‐dwelling postmenopausal Korean women and to evaluate menopause as an independent risk factor for primary CVD.
METHODS
Data
This present study used the database of the National Health Insurance System (NHIS) as the primary source. Researchers with a research proposal and an Institutional Review Board approval letter may request the data at (http://nhiss.nhis.or.kr). On reasonable request, some of the data will be available from the authors with the permission of the NHIS committee. The NHIS of the National Health Insurance Corporation in South Korea collects medical information from ≈50 million citizens, and National Health Insurance Corporation is the single insurer under the South Korean government. The NHIS database contains the demographic and medical information of the citizens from birth to death, such as residence, age, sex, medical use/transaction information, claims and deduction data, the insurers' payment coverage, International Classification of Diseases, Tenth Revision (ICD‐10), codes, treatments, and health screening data. The NHIS database contains ≈97.0% of the health insurance claims of the Korean people. The database has been thoroughly described in previous studies. 18 , 19 The NHIS recommends that all insured Korean people aged >40 years participate in the health checkup every 2 years. On the other hand, the annual checkup is recommended for the employee subscribers who are aged >20 years. The NHIS health checkup includes anthropometric measurements; hearing and visual acuity checks; laboratory tests; and family, medical, surgical, and social history. Moreover, the NHIS manages the qualification and training of examiners and hospitals. The hospitals may then participate in the health checkups after receiving certification by the NHIS.
National Cancer Screening Program is a part of the NHIS health screening program. Within the questionnaire of National Cancer Screening Program, there was a separate section dedicated only to women asking a specific question about the current status of menstruation. Three answer choices are provided, “I still have regular periods,” “I had undergone a hysterectomy,” or “I am menopaused.” If she answered “I am menopaused,” then there was an additional question asking to report the age at menopause. The survey additionally queried about the age at menarche and, if there was any, the duration of HRT (0, <2, 2–5, and ≥5 years).
Patient and Public Involvement
The current study used the data from the NHIS database, especially of the women who had participated in the health screenings in 2009. The year 2009 was chosen because that is when more comprehensive screening items were introduced to the health checkup programs, which include the personal history related to smoking, alcohol, and physical activity, the waist circumference, and lipid panel. 20 No additional participants were enrolled for the study. This is retrospective research using the NHIS data, and the operating regulations of NHIS were strictly obeyed during the analyses. According to the research guidelines and ethics of Institutional Review Board and NHIS, no individual consent was necessary for the study. The Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines were followed. 21
Study Participants
Of the participants who had undergone the health screenings in 2009, those who were women and aged >30 years were initially pooled from the NHIS database (n=3 291 974). The women who did not answer the question related to the age at menarche or answered “never experienced menarche” (n=107 394), those with a history of hysterectomy (n=205 537), and those with menarche before 5 or after 30 years of age plus those reported to have experienced menopause before 30 or after 60 years of age (n=177 563) were excluded. The participants with missing data in the general health screening questionnaire (123 629), those with missing data in the National Cancer Screening Program questions (216 808), the women who have not experienced menopause at the checkup (n=1 164 430), and those with a history of myocardial infarction (MI) or ischemic stroke before the health checkup (n=137 208) were also excluded from the analysis. Finally, a total of 1 159 405 postmenopausal women (premature menopausal women=19 999; and without premature menopause=1 139 406) were included in the study (Figure 1 and Table 1). The women were followed up until December 31, 2019. This study was approved by the Institutional Review Board of Korea University Anam Hospital (No. 2020AN0112), and permission was granted to use the NHIS health checkup data. Deidentified and anonymized data were used for the analyses, and informed consent was waived.
Figure 1. Flowchart of the study population selection.
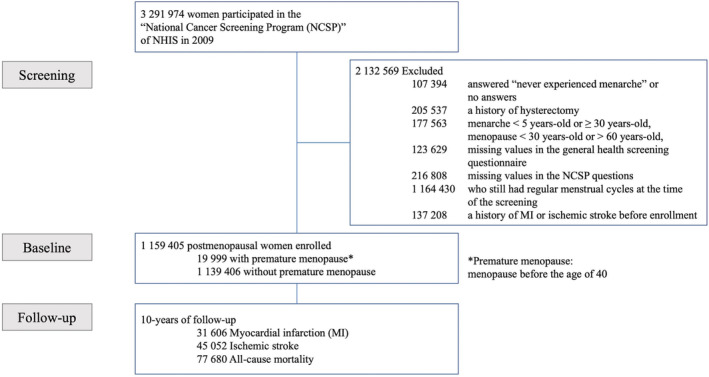
MI indicates myocardial infarction; NCSP, National Cancer Screening Program; and NHIS, National Health Insurance Service.
Table 1.
General Characteristics of the Study Participants
| Characteristic | Premature menopause | |||
|---|---|---|---|---|
| No | Yes | P value | ASMD | |
| No. | 1 139 406 | 19 999 | ||
| Age, y | 61.15±8.25 | 63.08±10.48 | <0.01 | 0.20 |
| 30–39 | 0 (0) | 114 (0.57) | <0.01 | 0.11 |
| 40–49 | 40 933 (3.59) | 2379 (11.9) | 0.31 | |
| 50–59 | 485 430 (42.6) | 4006 (20.03) | 0.50 | |
| 60–69 | 405 102 (35.55) | 7099 (35.5) | 0.00 | |
| 70–79 | 182 814 (16.04) | 5704 (28.52) | 0.30 | |
| ≥80 | 25 127 (2.21) | 697 (3.49) | 0.08 | |
| Height, cm | 153.62±5.69 | 152.39±6 | <0.01 | 0.21 |
| Weight, kg | 57±8.24 | 56.27±8.96 | <0.01 | 0.08 |
| BMI, kg/m2 | 24.13±3.13 | 24.19±3.39 | 0.01 | 0.02 |
| Obesity, yes | 416 280 (36.53) | 7681 (38.41) | <0.01 | 0.04 |
| Waist circumference, cm | 79.83±8.24 | 80.65±8.77 | <0.01 | 0.10 |
| Abdominal obesity, yes | 308 102 (27.04) | 6346 (31.73) | <0.01 | 0.10 |
| Fasting blood glucose, mg/dL | 99.28±23.69 | 100.25±25.86 | <0.01 | 0.04 |
| SBP, mm Hg | 125.34±16.11 | 126.35±16.81 | <0.01 | 0.06 |
| DBP, mm Hg | 76.82±10.15 | 77.34±10.34 | <0.01 | 0.05 |
| Total cholesterol, mg/dL | 208.49±38.54 | 206.74±39.18 | <0.01 | 0.05 |
| HDL‐C, mg/dL | 57.73±33.2 | 56.55±32.01 | <0.01 | 0.04 |
| LDL‐C, mg/dL | 126.05±38.16 | 123.75±37.55 | <0.01 | 0.06 |
| Triglyceride, mg/dL | 132.3±78.37 | 139.6±82.44 | <0.01 | 0.09 |
| Creatinine, mg/dL | 0.87±0.85 | 0.88±0.81 | 0.62 | 0.00 |
| eGFR | 82.4±28.12 | 81.82±27.53 | <0.01 | 0.02 |
| CKD, yes | 125 499 (11.01) | 2815 (14.08) | <0.01 | 0.09 |
| Smoking, yes | <0.01 | |||
| None | 1 097 620 (96.33) | 18 846 (94.23) | 0.10 | |
| Ex‐smoker | 11 708 (1.03) | 296 (1.48) | 0.04 | |
| Current smoker | 30 078 (2.64) | 857 (4.29) | 0.09 | |
| Ever smoking, yes | 41 786 (3.67) | 1153 (5.77) | <0.01 | 0.10 |
| Alcohol drinking | <0.01 | |||
| None | 995 245 (87.35) | 17 546 (87.73) | 0.01 | |
| Mild | 138 314 (12.14) | 2318 (11.59) | 0.02 | |
| Heavy | 5847 (0.51) | 135 (0.68) | 0.02 | |
| Alcohol drinking, yes | 144 161 (12.65) | 2453 (12.27) | 0.10 | 0.01 |
| Regular exercise, yes | 212 351 (18.64) | 3137 (15.69) | <0.01 | 0.08 |
| Income (quartile 1; lowest quartile) | 248 527 (21.81) | 4193 (20.97) | <0.01 | 0.02 |
| Diabetes, yes | 135 544 (11.9) | 2916 (14.58) | <0.01 | 0.08 |
| Hypertension, yes | 490 538 (43.05) | 9390 (46.95) | <0.01 | 0.08 |
| Dyslipidemia, yes | 356 143 (31.26) | 6112 (30.56) | 0.04 | 0.02 |
| Outcome event, yes | ||||
| Myocardial infarction | 30 721 (2.7) | 885 (4.43) | <0.01 | |
| Ischemic stroke | 43 866 (3.85) | 1186 (5.93) | <0.01 | |
| All‐cause mortality | 75 479 (6.62) | 2201 (11.01) | <0.01 | |
| Follow‐up duration, y | ||||
| Myocardial infarction | 10.08±1.48 | 9.9±1.86 | <0.01 | |
| Ischemic stroke | 10.02±1.61 | 9.82±2 | <0.01 | |
| All‐cause mortality | 10.18±1.28 | 10.06±1.61 | <0.01 | |
Data are given as mean±SD or number (percentage). P value was obtained by t test for continuous variables and χ2 test for categorical variables. ASMD indicates absolute standardized mean difference; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; and SBP, systolic blood pressure.
Anthropometric Measurements
Anthropometric measurements, such as weight (kg), height (cm), waist circumference (cm), systolic blood pressure (mm Hg), and diastolic blood pressure (mm Hg) were measured by trained examiners. Body mass index was calculated by dividing the weight by square of the height and was categorized by the definition of obesity as body mass index ≥25 kg/m2, according to the World Health Organization recommendations for Asian populations. 22 Abdominal obesity in women was defined as waist circumference ≥85 cm.
Biochemical Measurements
The NHIS health checkup includes the laboratory tests, such as fasting blood glucose (mg/dL), total cholesterol (mg/dL), triglycerides (mg/dL), high‐density lipoprotein cholesterol (mg/dL), low‐density lipoprotein cholesterol (mg/dL), aspartate aminotransferase (IU/L), alanine aminotransferase (IU/L), γ‐glutamyl transferase (IU/L), serum creatinine levels (mg/dL), and urine analyses. The Korean Association of Laboratory Quality Control warrants the quality of the laboratory tests, and the hospitals performing the health checkups were certified by the NHIS.
Definitions of Premature Menopause and Early Menopause
On the basis of the self‐reported age at menopause on the questionnaire, premature menopause was defined as having experienced menopause before the age of 40 years, and early menopause was defined as having experienced menopause between the ages of 40 and 44 years, in accordance to the 2019 Primary Prevention of Cardiovascular Disease Guidelines. 16 Table 2 provides an overview of the reproductive history of the study population.
Table 2.
Reproductive History of the Participants
| Variable | Premature menopause | ||
|---|---|---|---|
| No | Yes | P value | |
| No. | 1 139 406 | 19 999 | |
| Age at menopause, y | 50.22±3.55 | 36.75±2.59 | <0.01 |
| Lifetime duration of menstruation, y | 33.79±3.96 | 19.91±3.31 | <0.01 |
| Lifetime duration of menstruation, y | <0.01 | ||
| <30 | 139 331 (12.23) | 19 987 (99.94) | |
| 30–34 | 484 365 (42.51) | 12 (0.06) | |
| 35–39 | 444 468 (39.01) | 0 (0) | |
| ≥40 | 71 242 (6.25) | 0 (0) | |
| HRT, yes | 183 459 (16.1) | 3697 (18.49) | <0.01 |
| HRT duration | <0.01 | ||
| No | 955 947 (83.9) | 16 302 (81.51) | |
| <2 y | 107 473 (9.43) | 1623 (8.12) | |
| 2–5 y | 43 781 (3.84) | 843 (4.22) | |
| ≥5 y | 32 205 (2.83) | 1231 (6.16) | |
Data are given as mean±SD or number (percentage). P value was obtained by t test for continuous variables and χ2 test for categorical variables. HRT indicates hormone replacement therapy.
Primary End Points and Definitions
The primary end points were newly diagnosed MI, ischemic stroke, or all‐cause mortality. MI was defined as the recording of ICD‐10 codes I21 or I22 during hospitalization or these codes having been recorded at least 2 times. Ischemic stroke was defined as the recording of ICD‐10 codes I63 or I64 during hospitalization with claims for brain magnetic resonance imaging or brain computed tomography. All‐cause mortality was counted between January 1, 2009 and December 31, 2019 for each participant from the NHIS database. In women with a history of premature menopause, the average follow‐up durations were 9.9±1.86 years for MI, 9.82±2 years for ischemic stroke, and 10.06±1.61 years for all‐cause mortality.
Definitions of Chronic Diseases
Type 2 diabetes was defined as a fasting blood glucose level ≥126 mg/dL or at least 1 claim per year for the prescription of hypoglycemic drugs under ICD‐10 codes E11 to E14. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or at least 1 claim per year for antihypertensive medication prescription under ICD‐10 codes I10 to I13 and I15. Dyslipidemia was defined by total cholesterol ≥240 mg/dL or at least 1 claim per year for the prescription of antidyslipidemic agents under ICD‐10 code E78. Chronic kidney disease was defined as estimated glomerular filtration rate <60 mL/min per 1.73 m2.
General Health Behavior and Economic Variables
Participants were categorized as nonsmokers, ex‐smokers, and current smokers. Ever smoking was defined as being an ex‐smoker or current smoker. Alcohol drinking was categorized into none, mild (alcohol drinking <30 g/day in men and <20 g/day in women), and heavy (alcohol drinking ≥30 g/day in men and ≥20 g/day in women). Regular exercise was defined as moderate exercise ≥5 days or vigorous exercise ≥3 days in a week. Income level was divided by quartile as follows: quartile 1 (the lowest), quartile 2, quartile 3, and quartile 4 (the highest). Quartile 1 was defined as receiving medical aid and income in the low 25th percentile.
Statistical Analysis
The general characteristics of participants were analyzed by t test for continuous variables and χ2 test for categorical variables and are expressed as means±SDs for continuous variables and percentages for categorical variables between participants with and without premature menopause. The hazard ratios (HRs) and 95% CIs for MI, ischemic stroke, and all‐cause mortality were obtained by using multivariable Cox proportional hazard models with no adjustment in model 1 after adjusting for age in model 2; age, ever smoking, alcohol drinking, regular exercise, and income (quartile 1) in model 3; and covariates of model 3 and body mass index, type 2 diabetes, hypertension, dyslipidemia, chronic kidney disease, and HRT in model 4 (Tables 3 and 4). The incidence rate per 1000 person‐year and HRs for MI, ischemic stroke, and all‐cause mortality were analyzed in women with premature menopause or early menopause compared with menopause at the age of ≥50 years (Table 3). The incidence rates and HRs for MI, ischemic stroke, and all‐cause mortality, and P for trend, were calculated among 5 menopausal age groups (30–34, 35–39, 40–44, 45–49, and ≥50 years) compared with menopause at ≥50 years (Table 4).
Table 3.
Incidence Rates and HRs of MI, Ischemic Stroke, and All‐Cause Mortality in the Women With PM or EM
| Age at menopause, y | No. of women | Events, n | Duration (person‐years) | Incidence rate | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | Model 4, HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| MI | ||||||||
| 30–39 (PM) | 19 999 | 885 | 198 064.75 | 4.47 | 1.75 (1.63–1.87) | 1.42 (1.33–1.53) | 1.40 (1.31–1.50) | 1.40 (1.31–1.50) |
| 40–44 (EM) | 66 279 | 2370 | 658 875.37 | 3.60 | 1.41 (1.35–1.47) | 1.19 (1.14–1.25) | 1.18 (1.13–1.23) | 1.18 (1.13–1.24) |
| 45–49 | 317 609 | 8825 | 3 200 098 | 2.76 | 1.08 (1.05–1.10) | 1.1 (1.07–1.13) | 1.10 (1.07–1.13) | 1.10 (1.08–1.13) |
| ≥50 | 755 518 | 19 526 | 7 627 023.7 | 2.56 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Ischemic stroke | ||||||||
| 30–39 (PM) | 19 999 | 1186 | 196 296.47 | 6.04 | 1.64 (1.54–1.73) | 1.26 (1.19–1.33) | 1.24 (1.17–1.32) | 1.24 (1.17–1.31) |
| 40–44 (EM) | 66 279 | 3580 | 653 283.96 | 5.48 | 1.48 (1.43–1.54) | 1.18 (1.14–1.23) | 1.18 (1.14–1.22) | 1.18 (1.14–1.22) |
| 45–49 | 317 609 | 12 226 | 3 181 666.08 | 3.84 | 1.04 (1.02–1.06) | 1.05 (1.03–1.07) | 1.05 (1.03–1.07) | 1.06 (1.03–1.08) |
| ≥50 | 755 518 | 28 060 | 7 582 102.8 | 3.70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| All‐cause mortality | ||||||||
| 30–39 (PM) | 19 999 | 2201 | 201 131.2 | 10.94 | 1.79 (1.71–1.86) | 1.22 (1.17–1.27) | 1.20 (1.15–1.25) | 1.19 (1.14–1.24) |
| 40–44 (EM) | 66 279 | 6715 | 667 247.51 | 10.06 | 1.64 (1.60–1.69) | 1.16 (1.13–1.19) | 1.14 (1.11–1.17) | 1.13 (1.11–1.16) |
| 45–49 | 317 609 | 21 660 | 3 231 556.47 | 6.70 | 1.09 (1.08–1.11) | 1.06 (1.05–1.08) | 1.06 (1.04–1.08) | 1.06 (1.04–1.08) |
| ≥50 | 755 518 | 47 104 | 7 695 544.15 | 6.12 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
Incidence rate in 1000 person‐years. The HRs for MI, ischemic stroke, and all‐cause mortality were calculated using the women with menopause at ≥50 years as a reference. The HRs and 95% CIs were obtained by using multivariable Cox proportional hazard models. Each model was adjusted for the following Model 1: none. Model 2: age. Model 3: age, ever smoking, alcohol drinking, regular exercise, and income (quartile 1; lowest quartile). Model 4: age, ever smoking, alcohol drinking, regular exercise, income (quartile 1), body mass index, type 2 diabetes, hypertension, dyslipidemia, estimated glomerular filtration rate <60 mL/min per 1.73 m2, and hormone replacement therapy. PM: age at menopause is between 30 and 39 years. EM: age at menopause is between 40 and 44 years. EM indicates early menopause; HR, hazard ratio; MI, myocardial infarction; and PM, premature menopause.
Table 4.
Incidence Rates and the HRs of MI, Ischemic Stroke, and All‐Cause Mortality According Age at Menopause
| Menopause age group, y | No. | Events, n | Duration(person‐years) | Incidence rate | Model 1, HR (95% CI) | Model 2, HR (95% CI) | Model 3, HR (95% CI) | Model 4, HR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| MI | ||||||||
| 30–34 | 3436 | 157 | 34 019.72 | 4.62 | 1.81 (1.54–2.11) | 1.57 (1.34–1.84) | 1.53 (1.31–1.79) | 1.52 (1.30–1.78) |
| 35–39 | 16 563 | 728 | 164 045.03 | 4.44 | 1.74 (1.61–1.87) | 1.40 (1.30–1.51) | 1.38 (1.28–1.48) | 1.38 (1.28–1.48) |
| 40–44 | 66 279 | 2370 | 658 875.37 | 3.60 | 1.41 (1.35–1.47) | 1.19 (1.14–1.25) | 1.18 (1.13–1.23) | 1.18 (1.13–1.24) |
| 45–49 | 317 609 | 8825 | 3 200 098 | 2.76 | 1.08 (1.05–1.10) | 1.1 (1.07–1.13) | 1.10 (1.07–1.13) | 1.10 (1.08–1.13) |
| ≥50 | 755 518 | 19 526 | 7 627 023.7 | 2.56 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| P value for trend | … | … | … | … | <0.01 | <0.01 | <0.01 | <0.01 |
| Ischemic stroke | ||||||||
| 30–34 | 3436 | 197 | 33 745.53 | 5.84 | 1.58 (1.37–1.82) | 1.32 (1.14–1.51) | 1.30 (1.13–1.49) | 1.29 (1.12–1.48) |
| 35–39 | 16 563 | 989 | 162 550.94 | 6.08 | 1.65 (1.55–.75) | 1.25 (1.17–1.33) | 1.23 (1.16–1.31) | 1.23 (1.15–1.31) |
| 40–44 | 66 279 | 3580 | 653 283.96 | 5.48 | 1.48 (1.43–1.54) | 1.18 (1.14–1.23) | 1.18 (1.14–1.22) | 1.18 (1.14–1.22) |
| 45–49 | 317 609 | 12 226 | 3 181 666.08 | 3.84 | 1.04 (1.02–1.06) | 1.05 (1.03–1.07) | 1.05 (1.03–1.07) | 1.06 (1.03– 1.08) |
| ≥50 | 755 518 | 28 060 | 7 582 102.8 | 3.70 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| P value for trend | … | … | … | … | <0.01 | <0.01 | <0.01 | <0.01 |
| All‐cause mortality | ||||||||
| 30–34 | 3436 | 383 | 34 587.29 | 11.07 | 1.81 (1.64–2.00) | 1.37 (1.24–1.51) | 1.34 (1.21–1.48) | 1.33 (1.20–1.47) |
| 35–39 | 16 563 | 1818 | 166 543.9 | 10.92 | 1.78 (1.70–1.87) | 1.19 (1.14–1.25) | 1.17 (1.12–1.23) | 1.17 (1.11–1.22) |
| 40–44 | 66 279 | 6715 | 667 247.51 | 10.06 | 1.64 (1.60–1.69) | 1.16 (1.13–1.19) | 1.14 (1.11–1.17) | 1.13 (1.11–1.16) |
| 45–49 | 317 609 | 21 660 | 3 231 556.47 | 6.70 | 1.09 (1.08–1.11) | 1.06 (1.05–1.08) | 1.06 (1.04–1.08) | 1.06 (1.04–1.08) |
| ≥50 | 755 518 | 47 104 | 7 695 544.15 | 6.12 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| P value for trend | … | … | … | … | <0.01 | <0.01 | <0.01 | <0.01 |
Incidence rate in 1000 person‐years. The HRs for MI, ischemic stroke, all‐cause mortality, and P values for trend were calculated using the women with menopause at ≥50 years as a reference. The HRs and 95% CIs were obtained by using multivariable Cox proportional hazard models. Each model was adjusted for the following. Model 1: none. Model 2: age. Model 3: age, ever smoking, alcohol drinking, income (quartile 1; lowest quartile), and regular exercise. Model 4: age, ever smoking, alcohol drinking, income (quartile 1), regular exercise, body mass index, type 2 diabetes, hypertension, dyslipidemia, estimated glomerular filtration rate <60 mL/min per 1.73 m2, and hormone replacement therapy. HR indicates hazard ratio; and MI, myocardial infarction.
The risks of MI, ischemic stroke, and all‐cause mortality according to age at menopause were analyzed using the Kaplan–Meier method (Figures 2 and 3). The cumulative hazard for MI, ischemic stroke, and all‐cause mortality for up to 10 years was obtained according to menopause status (Figure 2) and age at menopause (Figure 3). Subgroup analysis was also performed for the following markers: aged ≥65 years, obesity, type 2 diabetes, hypertension, dyslipidemia, and HRT duration; and the HRs of MI, ischemic stroke, and all‐cause mortality according to age at menopause were obtained after adjusting for all covariates (Table 5). All statistical analyses were calculated using SAS, version 9.3 (SAS Institute, Cary, NC), and all 2‐tailed P<0.05 values were considered statistically significant.
Figure 2. Kaplan–Meier curves of cumulative hazard for myocardial infarction (MI), ischemic stroke, and all‐cause mortality up to 10 years according to history of premature menopause (PM) or early menopause (EM).
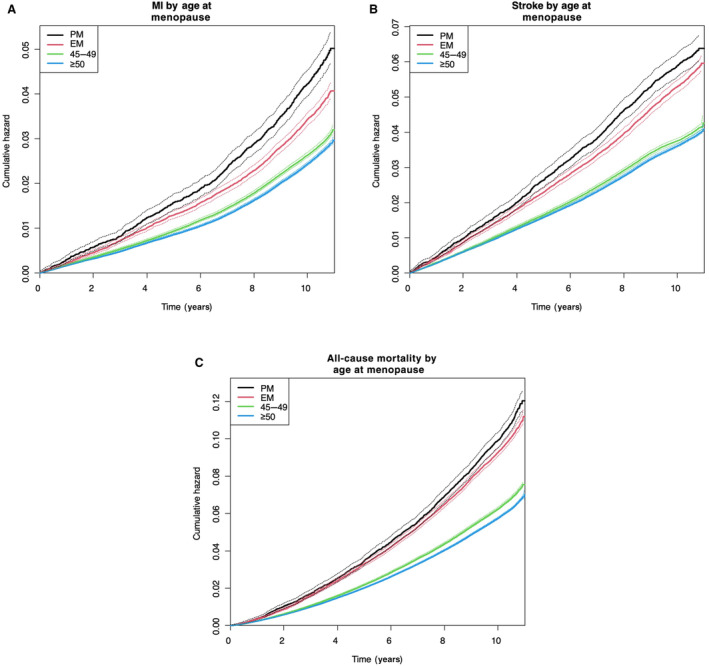
History of PM was associated with increased risk of MI (A), ischemic stroke (B), and all‐cause mortality (C) during the follow‐up period compared with menopause at ≥50 years (P<0.0001). PM: menopause at <40 years; EM: menopause between 40 and 44 years.
Figure 3. Kaplan–Meier curves of cumulative hazard for myocardial infarction (MI), ischemic stroke, and all‐cause mortality up to 10 years according to age at menopause.
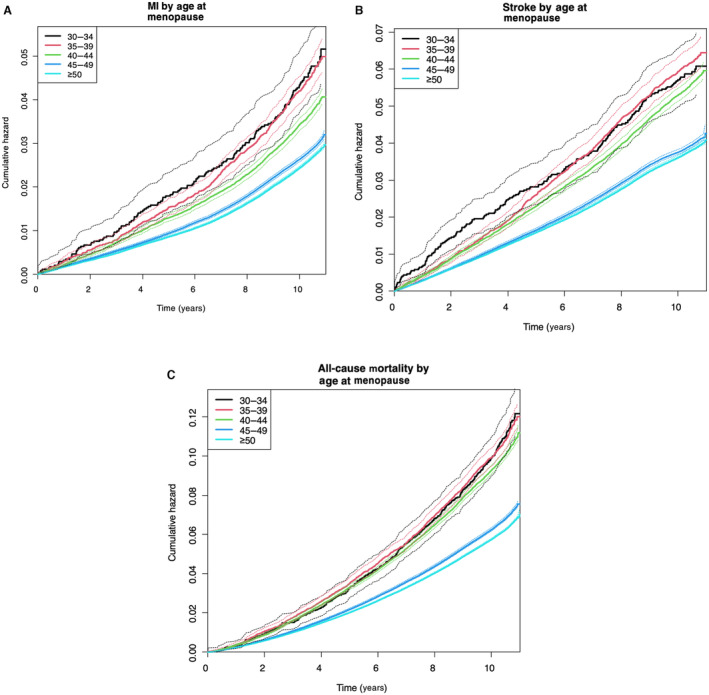
Earlier age at menopause was associated with increased risk of MI (A), ischemic stroke (B), and all‐cause mortality (C) during the follow‐up period compared with menopause at ≥50 years (P<0.0001).
Table 5.
Risk of MI, Ischemic Stroke, and All‐Cause Mortality in Women With a History of Premature Menopause, Early Menopause, or Menopause Between the Ages of 45 and 49 Years Compared With Normal Menopause Within Each Subgroup
| Subgroup | MI | Ischemic stroke | All‐cause mortality | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30–39 y | 40–44 y | 45–49 y | ≥50 y | P value for interaction | 30–39 y | 40–44 y | 45–49 y | ≥50 y | P value for interaction | 30–39 y | 40–44 y | 45–49 y | ≥50 | P value for interaction | ||
| Age, y | <65 | 1.53 (1.34–1.74) | 1.25 (1.16–1.36) | 1.13 (1.09–1.18) | 1 (Reference) | 0.05 | 1.35 (1.19–1.54) | 1.32 (1.22–1.41) | 1.08 (1.04–1.12) | 1 (Reference) | <0.01 | 1.59 (1.43–1.77) | 1.47 (1.38–1.57) | 1.17 (1.13–1.21) | 1 (Reference) | <0.01 |
| ≥65 | 1.34 (1.24–1.45) | 1.15 (1.09–1.21) | 1.08 (1.04–1.12) | 1 (Reference) | 1.18 (1.11–1.26) | 1.12 (1.08–1.17) | 1.03 (1.01–1.06) | 1 (Reference) | 1.13 (1.08–1.19) | 1.08 (1.05–1.11) | 1.03 (1.01–1.05) | 1 (Reference) | ||||
| Obese | No | 1.37 (1.25–1.50) | 1.24 (1.17–1.31) | 1.11 (1.07–1.14) | 1 (Reference) | 0.07 | 1.25 (1.16–1.34) | 1.19 (1.14–1.25) | 1.05 (1.02–1.07) | 1 (Reference) | 0.45 | 1.2 (1.14–1.26) | 1.16 (1.12–1.20) | 1.067 (1.05–1.09) | 1 (Reference) | 0.10 |
| Yes | 1.44 (1.30–1.59) | 1.11 (1.03–1.19) | 1.10 (1.06–1.15) | 1 (Reference) | 1.23 (1.12–1.34) | 1.15 (1.09–1.22) | 1.07 (1.04–1.11) | 1 (Reference) | 1.17 (1.09–1.27) | 1.09 (1.04–1.14) | 1.04 (1.02–1.07) | 1 (Reference) | ||||
| Diabetes | No | 1.37 (1.27–1.48) | 1.20 (1.14–1.26) | 1.13 (1.09–1.16) | 1 (Reference) | 0.01 | 1.26 (1.18–1.34) | 1.21 (1.16–1.25) | 1.06 (1.03–1.08) | 1 (Reference) | 0.07 | 1.20 (1.14–1.26) | 1.13 (1.10–1.17) | 1.07 (1.05–1.09) | 1 (Reference) | 0.27 |
| Yes | 1.50 (1.30–1.70) | 1.13 (1.03–1.24) | 1.03 (0.97–1.09) | 1 (Reference) | 1.18 (1.04–1.33) | 1.08 (1.01–1.17) | 1.05 (1.01–1.10) | 1 (Reference) | 1.18 (1.08–1.29) | 1.14 (1.08–1.20) | 1.03 (0.99–1.06) | 1 (Reference) | ||||
| Hypertension | No | 1.45 (1.30–1.61) | 1.19 (1.11–1.28) | 1.10 (1.06–1.15) | 1 (Reference) | 0.88 | 1.28 (1.16–1.42) | 1.25 (1.18–1.33) | 1.05 (1.01–1.09) | 1 (Reference) | 0.05 | 1.16 (1.08–1.25) | 1.15 (1.11–1.20) | 1.08 (1.05–1.11) | 1 (Reference) | 0.16 |
| Yes | 1.37 (1.26–1.49) | 1.18 (1.12–1.24) | 1.11 (1.07–1.14) | 1 (Reference) | 1.22 (1.13–1.31) | 1.14 (1.09–1.19) | 1.06 (1.03–1.09) | 1 (Reference) | 1.21 (1.15–1.27) | 1.12 (1.09–1.16) | 1.05 (1.03–1.07) | 1 (Reference) | ||||
| Dyslipidemia | No | 1.37 (1.25–1.49) | 1.19 (1.13–1.26) | 1.12 (1.08–1.15) | 1 (Reference) | 0.44 | 1.23 (1.15–1.32) | 1.16 (1.11–1.21) | 1.03 (1.00–1.06) | 1 (Reference) | 0.02 | 1.18 (1.12–1.24) | 1.12 (1.09–1.15) | 1.06 (1.04–1.08) | 1 (Reference) | 0.26 |
| Yes | 1.46 (1.31–1.63) | 1.17 (1.09–1.26) | 1.08 (1.04–1.13) | 1 (Reference) | 1.25 (1.13–1.39) | 1.22 (1.14–1.29) | 1.11 (1.07–1.15) | 1 (Reference) | 1.21 (1.12–1.31) | 1.17 (1.12–1.23) | 1.05 (1.02–1.08) | 1 (Reference) | ||||
| HRT | None | 1.38 (1.28–1.48) | 1.17 (1.12–1.22) | 1.10 (1.08–1.13) | 1 (Reference) | 0.50 | 1.23 (1.16–1.31) | 1.18 (1.14–1.22) | 1.06 (1.04–1.08) | 1 (Reference) | 0.32 | 1.18 (1.13–1.24) | 1.13 (1.10–1.16) | 1.06 (1.04–1.08) | 1 (Reference) | 0.12 |
| <2 y | 1.57 (1.17–2.11) | 1.21 (1.01–1.44) | 1.09 (0.99–1.20) | 1 (Reference) | 1.30 (0.97–1.73) | 1.16 (0.99–1.37) | 0.97 (0.89–1.06) | 1 (Reference) | 1.52 (1.19–1.93) | 1.21 (1.05–1.40) | 1.06 (0.97–1.14) | 1 (Reference) | ||||
| 2–5 y | 1.78 (1.17–2.70) | 1.56 (1.21–2.01) | 1.11 (0.95–1.29) | 1 (Reference) | 1.84 (1.27–2.67) | 1.24 (0.96–1.60) | 1.16 (1.02–1.33) | 1 (Reference) | 1.31 (0.89–1.94) | 1.47 (1.19–1.81) | 1.02 (0.90–1.16) | 1 (Reference) | ||||
| ≥5 y | 1.51 (1.03–2.22) | 1.39 (1.07–1.82) | 1.16 (0.99–1.37) | 1 (Reference) | 1.12 (0.76–1.67) | 1.06 (0.81–1.39) | 1.03 (0.89–1.20) | 1 (Reference) | 1.16 (0.81–1.64) | 1.31 (1.05–1.64) | 1.07 (0.94–1.22) | 1 (Reference) | ||||
Subgroup analyses were performed using the data of model 4 in Table 3. Adjusted for age, ever smoking, alcohol drinking, income (quartile 1; lowest quartile), regular exercise, body mass index, type 2 diabetes, hypertension, dyslipidemia, estimated glomerular filtration rate <60 mL/min per 1.73 m2, and HRT. The hazard ratios and 95% CIs were obtained by using multivariable Cox proportional hazard models. HRT indicates hormone replacement therapy; and MI, myocardial infarction.
RESULTS
Baseline Characteristics
Figure 1 described the flowchart of the study population selection, including the primary outcomes during the 10‐year follow‐up duration. Table 1 showed the general characteristics of the participants with or without a history of premature menopause. From a total of 1 159 405 postmenopausal women, 19 999 (1.72%) women had a history menopause before the age of 40 years. Women with premature menopause were older (average age, 63.1 years) and more likely to have higher values of waist circumference, systolic blood pressure, diastolic blood pressure, fasting glucose, and triglyceride when compared with women without premature menopause (all P<0.01). In addition, the women with premature menopause had a higher percentage of chronic kidney disease, ever smoking, obesity, abdominal obesity, type 2 diabetes, and hypertension, but a lower percentage of alcohol drinking and regular exercise, compared with the women without premature menopause (all P<0.01). Table 2 summarized the reproductive history of the study population based on the answers from the questionnaire. In the study population, the average age at menopause was 36.8 years with premature menopause and 50.2 years without premature menopause (Table 2).
Risks of MI, Ischemic Stroke, and All‐Cause Mortality in Premature Menopause and Early Menopause
Table 3 showed how premature menopause and early menopause were associated with higher risks of MI, ischemic stroke, and all‐cause mortality compared with normal menopause. The risks for MI, ischemic stroke, and all‐cause mortality were all increased in premature menopause (HRs [95% CIs] were 1.40 [1.31–1.50], 1.24 [1.17–1.31], and 1.19 [1.14–1.24], respectively) when compared with women with menopause at ≥50 years after adjusting for all covariates (model 4; Table 3). The women with early menopause, defined by age at menopause between 40 and 44 years, demonstrated the increased risk of MI, ischemic strokes, and all‐cause mortality compared with women who experienced menopause at ≥50 years (HRs [95% CIs] were 1.18 [1.13–1.24], 1.18 [1.14–1.22], and 1.13 [1.11–1.16], respectively) (model 4; Table 3).
Table 4 demonstrated the risks of MI, ischemic stroke, and all‐cause mortality according to age at menopause in 5 different groups. More important, the women with a history of menopause between 30 and 34 years showed the highest risk of MI, ischemic strokes, and all‐cause mortality compared with women who experienced menopause at ≥50 years (HRs [95% CIs] were 1.52 [1.30–1.78], 1.29 [1.12–1.48], and 1.33 [1.20–1.47], respectively). The women who experienced menopause between the ages of 35 and 39 years exhibited the second‐most increased risk of MI, ischemic strokes, and all‐cause mortality compared with women who experienced menopause at ≥50 years (HRs [95% CIs] were 1.38 [1.28–1.48], 1.23 [1.15–1.31], and 1.17 [1.11–1.22], respectively).
Figure 2 demonstrated the higher cumulative hazard of MI, ischemic stroke, and all‐cause mortality in the women with premature menopause and early menopause compared with the women with menopause at ≥50 years. Of note, the women with menopause between the ages of 45 and 49 years also displayed higher risks of MI, ischemic stroke, and all‐cause mortality compared with normal menopause (Figure 2). Figure 3 showed a detailed Kaplan–Meier curve of the cumulative hazard for MI, ischemic stroke, and all‐cause mortality according to age at menopause in 5 different groups.
Because a large number of women were excluded (Figure 1), additional analyses were conducted to compare any differences in the general characteristics and primary outcomes in both the enrolled and excluded women (Tables S1 and S2). The baseline characteristics of the excluded women did not significantly differ from the enrolled women (all absolute standardized mean difference, <0.1) (Table S1). Furthermore, there were no significant differences in the risks of MI, ischemic strokes, or all‐cause mortality between the enrolled and excluded women, even after adjusting for age (Table S2).
Inverse Dose‐Response Relationship Between Age at Menopause With CVD and All‐Cause Mortality
The earlier the menopause occurred, the higher the HRs of MI, ischemic stroke, and all‐cause mortality were throughout the menopausal age groups when compared with the women who experienced menopause at ≥50 years (Table 4; model 4). The relationship remained consistent even after adjusting for all covariates (P for trend <0.01). The highest HRs of MI, ischemic stroke, and all‐cause mortality were found in the women who experienced menopause between the ages of 30 to 34 years (HRs [95% CIs] were 1.52 [1.30–1.78], 1.29 [1.12–1.48], and 1.33 [1.20–1.47], respectively).
Subgroup Analysis
Table 5 described the subgroup analysis of the women with history of premature menopause, early menopause, menopause between 45 and 49 years, or menopause at ≥50 years. In the subgroup analysis according to old age, <65‐year‐old women with premature menopause, early menopause, or menopause between 45 and 49 years were associated with significantly higher risks of MI, ischemic stroke, and all‐cause mortality when compared <65‐year‐old women with menopause at ≥50 years. The respective risks in ≥65‐year‐old women were also slightly elevated. Irrespective of the age at menopause, women with dyslipidemia were associated with a higher risk of ischemic stroke compared with those without dyslipidemia. In the women with premature menopause, those with diabetes were associated with a higher risk of MI than those without. However, such association attenuated in early menopause and menopause between 45 and 49 years. In the women with premature or early menopause, those who did not have hypertension were associated with greater risks of ischemic stroke than in those with hypertension. The women with premature menopause who had undergone HRT, regardless of the treatment duration, were not associated with significant risk of MI, strokes, and all‐cause mortality.
DISCUSSION
In the large cohort of postmenopausal Korean women, premature menopause was associated with 1.4 times the risk of MI, 1.24 times the risk of ischemic stroke, and 1.19 times the risk of all‐cause mortality when compared with the women with menopause at ≥50 years. As the age at menopause became younger, the HRs for MI, ischemic stroke, and all‐cause mortality were increased in an inverse dose‐response manner (P for trend <0.01). The women with menopause between the ages of 30 and 34 years were associated with 1.52 times the risk of MI, 1.29 times the risk of ischemic stroke, and 1.33 times the risk of all‐cause mortality compared with the women with menopause at ≥50 years, and such associations persisted even after adjusting for conventional cardiovascular risk factors.
The current study has many strengths. It is the first large‐scale population study to suggest the association between age at menopause with the risks of MI, ischemic strokes, and all‐cause mortality in Korean women. The number of events of MI, stroke, and all‐cause mortality in premature menopause were 885, 1186, and 2201, respectively, and in early menopause, the number of events were 2370, 3580, and 6715, respectively. Second, we have adjusted for the various CVD covariates during the analyses. Third, we have successfully demonstrated an inverse dose‐response relationship between age at menopause and risks of MI, ischemic strokes, and mortality, and the trend was true as premature or early menopausal women became older, at least up until 10 years. Previous population research studies were either outdated 23 or have suggested a mixed view on the association between CVD and mortality risks with premature or early menopause. 12 , 13 , 14 , 23 , 24 , 25 , 26
This study extends beyond past studies. Past reports are inconsistent on the association between earlier age at menopause and CVD and mortality with respect to race. Malek et al analyzed a cohort consisting of 40% Black women but found meaningful association between earlier age at menopause and all‐cause mortality only in White women. 14 Association between earlier age at menopause and stroke was not found in that study, 14 but our study observed this association in Korean women (Tables 3 and 4). The study from Honigberg et al reported the association between earlier age at menopause with CVD in only White women, and no mortality data were present, 13 whereas our study presented the mortality risk associated with earlier age at menopause in Korean women (Tables 3 and 4). Zhu et al analyzed a diverse set of races and ethnicities, including Asian women, but the total numbers of Asian women with premature or early menopause were rather small (n=131 and 510, respectively) and perhaps inadequate to represent Asian population. 12 But our data included a huge number of Korean women with premature and early menopause (n=19 999 and 66 279, respectively). Korean people experience lower CVD mortality than other Asian subgroups; 2 therefore, it can be inferred that the CVD burden may be much higher in other Asian countries with a limited access to health care.
The present study concurs well with the past research studies. 12 , 13 , 15 , 24 Honigberg et al demonstrated how the risks of coronary artery disease and stroke increased with earlier age at menopause, showing that the earlier the age at menopause, the higher the risk of CVD was. 13 Such findings were displayed in the current study as well. Zhu et al reported that earlier age at menopause had an increased risk of first nonfatal CVD event before the age of 60 years, but not after the age of 70 years. 12 This is in line with our subgroup analysis. Among <65‐year‐old women, those with premature menopause, early menopause, or menopause between the ages 45 and 49 years displayed significantly higher risks of the primary outcomes compared with those with menopause at ≥50 years. Honigberg et al found the association between earlier age at menopause and increased risk of CVD in White women, 13 but we were able to find the association between earlier age at menopause with higher risks of CVD and all‐cause mortality in Korean women. Dam et al prospectively analyzed the European women and found the inverse dose‐response relationship between earlier age at menopause and coronary heart disease. 15 Our data reported the similar inverse dose‐response pattern in Korean women, and we were able to report the increased risk of stroke and all‐cause mortality in Korean women.
The findings in this study will improve the understanding of the association between CVD and mortality related to earlier age at menopause in Asian women and guide further research on the underlying mechanism behind the association. The current data provide the evidence for updates in the guideline on the primary prevention of CVD with respect to risk assessment and statin initiation in women with early menopause (menopause between the ages of 40 and 44 years), at least in Korean women.
Earlier age at menopause presents a great therapeutic window period where the primary prevention of CVD can be initiated. The FHS (Framingham Heart Study) has suggested the bidirectionality of the associations between menopause and CVD risk, where earlier age at menopause may be interpreted as an alarming signal of poor underlying premenopausal cardiovascular health. 27 Although menopause could have occurred because of the baseline CVD risk, in the current study, we have adjusted for common CVD risk factors, and the analyses consistently displayed the association between earlier age at menopause with increased risks of MI, stroke, and all‐cause mortality during the 10‐year follow‐up. Therefore earlier age at menopause, both premature and early menopause, in Korean women is an independent CVD risk factor. The study's results indicate that the initiation of statins should be considered beyond the current guideline, which identifies premature menopause only as a risk‐enhancing factor, 16 in Korean women with premature menopause (aged <40 years), early menopause (aged <45 years), and menopause before the age of 49 years.
Unfortunately, there is limited research on the use of statin in Korean women with premature menopause, early menopause, or menopause before the age of 49 years. However, there is a report on the use of statin for dyslipidemia in young women. According to Korean Dyslipidemia Guideline 2018, the treatment rates for dyslipidemia in individuals aged 30 to 49 years were found to be 24.7% for men and 18.2% for women. 28 In general, men are more likely to be prescribed statin than women are, and younger women are particularly undertreated. 29 This may be because women in their childbearing age generally have a lower CVD risk than men, but it may also be attributable to the current guidelines not recognizing menopause as an independent risk factor for CVD. Considering the higher CVD risk in women with premature menopause, early menopause, or menopause before the age of 49 years, clinicians should pay sufficient attention to lowering the vascular risks in the women.
This study presents meaningful data to clinicians and policy makers. The 2019 Primary Prevention of Cardiovascular Disease Guidelines acknowledged premature menopause as a risk‐enhancing factor. The data supporting the association between menopause and CVD risk in White and Black women exist. 12 , 13 , 14 , 30 The studies on age at menopause and incident CVD and mortality were not consistent. Recent population cohort data on the subject were limited in the Asian population. Because Korean women received regular health screenings, we considered the women a good candidate to study the risks of CVD and mortality associated with age at menopause. The association between earlier age at menopause and risks of CVD and mortality was clearly demonstrated in Korean women, who have a relatively low CVD mortality risk among Asian subgroups. Therefore, menopause is an independent risk factor for CVD and mortality, at least in Korean women.
Many researchers have proposed the underlying mechanisms behind the association between menopause and CVD. As women get older, the level of estrogen in the body subsequently decreases and hypertension and CVD are more likely to be observed. 31 Therefore, earlier age at menopause will inevitably offer less protection against CVD risk. 12 Endogenous estrogen confers a protective effect on the cardiovascular system via maintaining vasodilatory status. 14 Changes in the levels of androgens and sex hormone–binding globulins were associated with increased risk of CVD in postmenopausal women. 32 Postmenopausal state is associated with increased oxidative stress and cytokines, and perhaps it plays a role in osteogenesis of valvular cells. 33 , 34 , 35 Yet, more data are needed to understand the mechanism between premature menopause and aortic stenosis. Incident atrial fibrillation and venous thromboembolism were significantly associated with earlier menopause in past studies. 13 , 36 However, it has been suggested that the general risk factors of CVD, such as hypertension, diabetes, obesity, smoking, high cholesterol, and family history of CVD if present in early life, may contribute to both premature or early menopause and subsequent cardiovascular events. 30 But for this precise reason, we have adjusted the data for various covariates, including body mass index, diabetes, hypertension, dyslipidemia, smoking, and chronic kidney disease.
There are limitations in this study. First, surgical menopause was excluded so the CVD risks related to those women were not represented in the study. However if surgical menopause was included in the present study, then the association between age at menopause and risks of CVD and mortality could have been stronger. The risk of CVD in surgical menopause compared with natural menopause has been reported to be higher. 13 Second, we used self‐reported age at menopause, and recall bias is possible. The average age of the women who responded as having experienced premature menopause was 63.08 years. However, the use of self‐reported age at menopause for research purposes has been validated in the past. 37 , 38 Furthermore, future research will be restricted if menopause is categorized as a binary variable. Third, serum hormone levels of the participants were not available to validate the menopausal status. Fourth, the regimen and dosing information of HRT were not available. But in the current study, HRT had no meaningful effect on the association between earlier age at menopause and CVD and all‐cause mortality, which is in line with past research. 12 , 30 , 39 Fifth, the current research only included Korean population, so it is difficult to generalize the findings to other races and ethnicities. But considering how South Korea has one of the lowest CVD mortality rates among Asian countries 2 and most Korean people receive health screenings, including serum lipid profile, such as triglycerides, high‐density lipoprotein cholesterol, and low‐density lipoprotein cholesterol, 20 earlier age at menopause may confer higher risks of CVD and mortality in other Asian countries with limited access to health care. Sixth, healthy user effect is a possibility. However the current study aimed to find primary CVD risk factors in asymptomatic women without established CVD, which is in line with the American College of Cardiology/American Heart Association guideline on the primary prevention of CVD. 16 Seventh, the associations between premature menopause with CVD and mortality could have been underestimated because the follow‐up began at the time of health examination rather than at the time of menopause and the women who experienced MI or ischemic stroke before the examination were excluded. Eighth, hemorrhagic stroke was not included in the analysis. Under the operational definition of NHIS database, ischemic stroke can be accurately measured. But on the other hand, hemorrhagic stroke is difficult to identify from the database. 40 In addition, the present study serves as evidence supporting the use of statin in women with premature menopause. Therefore, we sought to explore the association between premature menopause and the risk of atherosclerotic CVD. Ninth, there were statistical differences in the baseline characteristics of the participants in Table 1, so the HRs were adjusted for the clinically meaningful variables with multiple regression analyses (Tables 3 and 4). In addition, we also calculated absolute standardized mean differences in the participants, which demonstrated that the differences in distribution were small (Table 1). Furthermore, because of the possibility of unmeasured confounding in Table 1, the E‐values were calculated to assess the effects that the unmeasured confounders may have on the outcomes. The E‐values indicated that it was less likely to affect the outcome in the present study (Table S3). Tenth, the statistical analysis in the present study was done excluding the missing data rather than performing imputation because the data were missing completely at random. 41 At most, the percentage of the missing data was 13.6%. Moreover, among the excluded and included women, the baseline characteristics and the risks of MI, ischemic stroke, and all‐cause mortality did not differ significantly (Tables S1 and S2). Finally, given the current retrospective design, causality cannot be drawn. Although bidirectionality between menopause with CVD and mortality has been mentioned in the past, the current study has successfully adjusted for the conventional CVD risk factors; and the association between earlier age at menopause and CVD and mortality in Korean women persisted over a 10‐year period. Future prospective studies and research on the mechanisms beneath the observed association are warranted.
CONCLUSIONS
In the present cohort study of postmenopausal Korean women, age at natural menopause was associated with the risks of MI, stroke, and all‐cause mortality in an inverse dose‐response manner over a 10‐year period. The earlier menopause occurred, the higher the risks of CVD and mortality were. Just as in North American and European women, elevated risks of CVD and mortality were linked with age at menopause in Korean women. To provide optimal CVD risk assessment and to reduce the public health care burden for Asian women, menopause should be considered as an independent risk factor for CVD in future risk assessment and major guidelines.
Sources of Funding
This study was supported by a Korea University grant (K1824401).
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
We would like to thank the Korean National Health Insurance Corporation and all the participants of the study and health checkup.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030117
For Sources of Funding and Disclosures, see page 14.
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Contributor Information
Kyungdo Han, Email: hkd@ssu.ac.kr.
Yang‐Hyun Kim, Email: 9754031@korea.ac.kr.
References
- 1. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao D. Epidemiological features of cardiovascular disease in Asia. J Am Coll Cardiol: Asia. 2021;1:1–13. doi: 10.1016/j.jacasi.2021.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447 [DOI] [PubMed] [Google Scholar]
- 4. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506–e532. doi: 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 5. Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3 [DOI] [PubMed] [Google Scholar]
- 6. Santoro N. Mechanisms of premature ovarian failure. Ann Endocrinol (Paris). 2003;64:87–92. [PubMed] [Google Scholar]
- 7. Zhu D, Chung HF, Pandeya N, Dobson AJ, Kuh D, Crawford SL, Gold EB, Avis NE, Giles GG, Bruinsma F, et al. Body mass index and age at natural menopause: an international pooled analysis of 11 prospective studies. Eur J Epidemiol. 2018;33:699–710. doi: 10.1007/s10654-018-0367-y [DOI] [PubMed] [Google Scholar]
- 8. Choe SA, Sung J. Trends of premature and early menopause: a comparative study of the US National Health and Nutrition Examination Survey and the Korea National Health and Nutrition Examination Survey. J Korean Med Sci. 2020;35:e97. doi: 10.3346/jkms.2020.35.e97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta‐analyses of studies across six continents. Int J Epidemiol. 2014;43:1542–1562. doi: 10.1093/ije/dyu094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park CY, Lim JY, Park HY. Age at natural menopause in Koreans: secular trends and influences thereon. Menopause. 2018;25:423–429. doi: 10.1097/Gme.0000000000001019 [DOI] [PubMed] [Google Scholar]
- 11. Shifren JL, Gass ML; NAMS Recommendations for Clinical Care of Midlife Women Working Group . The North American Menopause Society recommendations for clinical care of midlife women. Menopause. 2014;21:1038–1062. doi: 10.1097/GME.0000000000000319 [DOI] [PubMed] [Google Scholar]
- 12. Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4:e553–e564. doi: 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL, Scott NS, Natarajan P. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019;322:2411–2421. doi: 10.1001/jama.2019.19191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malek AM, Vladutiu CJ, Meyer ML, Cushman M, Newman R, Lisabeth LD, Kleindorfer D, Lakkur S, Howard VJ. The association of age at menopause and all‐cause and cause‐specific mortality by race, postmenopausal hormone use, and smoking status. Prev Med Rep. 2019;15:100955. doi: 10.1016/j.pmedr.2019.100955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dam V, van der Schouw YT, Onland‐Moret NC, Groenwold RHH, Peters SAE, Burgess S, Wood AM, Chirlaque MD, Moons KGM, Oliver‐Williams C, et al. Association of menopausal characteristics and risk of coronary heart disease: a pan‐European case‐cohort analysis. Int J Epidemiol. 2019;48:1275–1285. doi: 10.1093/ije/dyz016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muntner P, Colantonio LD, Cushman M, Goff DC Jr, Howard G, Howard VJ, Kissela B, Levitan EB, Lloyd‐Jones DM, Safford MM. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311:1406–1415. doi: 10.1001/jama.2014.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, Park JY, Lee KU, Ko KS, Lee BW. Background and data configuration process of a nationwide population‐based study using the Korean national health insurance system. Diabetes Metab J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YH, Han K, Son JW, Lee SS, Oh SW, Kwon HS, Shin SA, Kim YY, Lee WY, Yoo SJ. Data analytic process of a nationwide population‐based study on obesity using the National Health Information Database presented by the National Health Insurance Service 2006‐2015. J Obes Metab Syndr. 2017;26:23–27. doi: 10.7570/jomes.2017.26.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin DW, Cho J, Park JH, Cho BL. National General Health Screening Program in Korea: history, current status, and future direction a scoping review. Precis Future Med. 2022;6:9–31. doi: 10.23838/pfm.2021.00135 [DOI] [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; the STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 22. WHO/IASO/IOTF . The Asia‐Pacific Perspective: Redefining Obesity and its Treatment. Health Communications Australia Pty Limited; 2000. Accessed September 19, 2023. http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf [Google Scholar]
- 23. Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–510. doi: 10.1097/gme.0b013e3181c7dd41 [DOI] [PubMed] [Google Scholar]
- 24. Muka T, Oliver‐Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all‐cause mortality: a systematic review and meta‐analysis. JAMA Cardiol. 2016;1:767–776. doi: 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 25. Gong DD, Sun J, Zhou YJ, Zou C, Fan Y. Early age at natural menopause and risk of cardiovascular and all‐cause mortality: a meta‐analysis of prospective observational studies. Int J Cardiol. 2016;203:115–119. doi: 10.1016/j.ijcard.2015.10.092 [DOI] [PubMed] [Google Scholar]
- 26. Yang L, Lin L, Kartsonaki C, Guo Y, Chen Y, Bian Z, Xie K, Jin D, Li L, Lv J, et al. Menopause characteristics, total reproductive years, and risk of cardiovascular disease among Chinese women. Circ Cardiovasc Qual Outcomes. 2017;10:e004235. doi: 10.1161/CIRCOUTCOMES.117.004235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Helen S, KMvA K, van der Schouw YT, van der Tweel I, Peeters PHM, Wilson PWF, Pearson PL, Grobbee DE. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976–1983. doi: 10.1016/j.jacc.2005.12.066 [DOI] [PubMed] [Google Scholar]
- 28. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, Song Y, Lim JH, Kim HJ, Choi S, et al. 2018 guidelines for the management of dyslipidemia in Korea. Korean J Intern Med. 2019;34:1171. doi: 10.3904/kjim.2019.188.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al‐Kindi SG, DeCicco A, Longenecker CT, Dalton J, Simon DI, Zidar DA. Rate of statin prescription in younger patients with severe dyslipidemia. JAMA Cardiol. 2017;2:451–452. doi: 10.1001/jamacardio.2016.5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: the Multi‐Ethnic Study of Atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46:28–49. doi: 10.1016/s0008-6363(00)00005-5 [DOI] [PubMed] [Google Scholar]
- 32. Rexrode KM, Manson JE, Lee IM, Ridker PM, Sluss PM, Cook NR, Buring JE. Sex hormone levels and risk of cardiovascular events in postmenopausal women. Circulation. 2003;108:1688–1693. doi: 10.1161/01.CIR.0000091114.36254.F3 [DOI] [PubMed] [Google Scholar]
- 33. Paik JK, Kim JY, Kim OY, Lee Y, Jeong TS, Sweeney G, Jang Y, Lee JH. Circulating and PBMC Lp‐PLA2 associate differently with oxidative stress and subclinical inflammation in nonobese women (menopausal status). PLoS One. 2012;7:e29675. doi: 10.1371/journal.pone.0029675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena‐Silva R, Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol. 2008;52:843–850. doi: 10.1016/j.jacc.2008.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Branchetti E, Sainger R, Poggio P, Grau JB, Patterson‐Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, et al. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol. 2013;33:e66–e74. doi: 10.1161/ATVBAHA.112.300177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Canonico M, Plu‐Bureau G, O'Sullivan MJ, Stefanick ML, Cochrane B, Scarabin PY, Manson JE. Age at menopause, reproductive history, and venous thromboembolism risk among postmenopausal women: the Women's Health Initiative hormone therapy clinical trials. Menopause. 2014;21:214–220. doi: 10.1097/GME.0b013e31829752e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clavel‐Chapelon F, Dormoy‐Mortier N. A validation study on status and age of natural menopause reported in the E3N cohort. Maturitas. 1998;29:99–103. doi: 10.1016/s0378-5122(98)00020-6 [DOI] [PubMed] [Google Scholar]
- 38. Colditz GA, Stampfer MJ, Willett WC, Stason WB, Rosner B, Hennekens CH, Speizer FE. Reproducibility and validity of self‐reported menopausal status in a prospective cohort study. Am J Epidemiol. 1987;126:319–325. doi: 10.1093/aje/126.2.319 [DOI] [PubMed] [Google Scholar]
- 39. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–534. doi: 10.1056/NEJMoa030808 [DOI] [PubMed] [Google Scholar]
- 40. Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—a practical guide with flowcharts. BMC Med Res Methodol. 2017;17:162. doi: 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


