Abstract
Background
One‐time assessment of the Society for Cardiovascular Angiography and Interventions (SCAI) shock classification robustly predicts mortality in the cardiac intensive care unit (CICU). We sought to determine whether serial SCAI shock classification could improve risk stratification.
Methods and Results
Unique admissions to a single academic level 1 CICU from 2015 to 2018 were included in this retrospective cohort study. Electronic health record data were used to assign the SCAI shock stage during 4‐hour blocks of the first 24 hours of CICU admission. Shock was defined as hypoperfusion (SCAI shock stage C, D, or E). In‐hospital death was evaluated using logistic regression. Among 2918 unique CICU patients, 1537 (52.7%) met criteria for shock during ≥1 block, and 266 (9.1%) died in the hospital. The SCAI shock stage on admission was: A, 37.6%; B, 31.5%; C, 25.9%; D, 1.8%; and E, 3.3%. Patients who met SCAI criteria for shock on admission (first 4 hours) and those with worsening SCAI shock stage after admission were at higher risk for in‐hospital death. Each higher admission (adjusted odds ratio, 1.36 [95% CI, 1.18–1.56]; area under the receiver operating characteristic curve, 0.70), maximum (adjusted odds ratio, 1.59 [95% CI, 1.37–1.85]; area under the receiver operating characteristic curve, 0.73) and mean (adjusted odds ratio, 2.42 [95% CI, 1.99–2.95]; area under the receiver operating characteristic curve, 0.78) SCAI shock stage was incrementally associated with a higher in‐hospital mortality rate. Discrimination was highest for the mean SCAI shock stage (P<0.05). Each additional 4‐hour block meeting SCAI criteria for shock predicted a higher mortality rate (adjusted odds ratio, 1.15 [95% CI, 1.07–1.24]).
Conclusions
Dynamic assessment of shock using serial SCAI shock classification assignment can improve mortality risk stratification in CICU patients by quantifying the magnitude and duration of shock.
Keywords: cardiogenic, heart failure, mortality, myocardial infarction, shock, cardiac intensive care unit
Subject Categories: Cardiopulmonary Resuscitation and Emergency Cardiac Care
Nonstandard Abbreviations and Acronyms
- AKI
acute kidney injury
- CICU
cardiac intensive care unit
- CS
cardiogenic shock
- SCAI
Society for Cardiovascular Angiography and Interventions
Clinical Perspective.
What Is New?
Assigning the Society for Cardiovascular Angiography and Interventions shock classification every 4 hours during the first day on cardiac intensive care unit admission improved discrimination for in‐hospital death by defining the average Society for Cardiovascular Angiography and Interventions shock stage and duration of shock.
What Are the Clinical Implications?
Dynamic evaluation of the presence and severity of shock improves risk stratification in the cardiac intensive care unit.
Cardiogenic shock (CS) is common among cardiac intensive care unit (CICU) patients, accounting for substantial morbidity and death. 1 , 2 , 3 , 4 The severity of CS can be graded using the Society for Cardiovascular Angiography and Interventions (SCAI) shock classification, a 5‐stage system ranging from patients at risk of CS (SCAI shock stage A) to those with refractory CS (SCAI shock stage E); according to the SCAI shock classification, the presence of hypoperfusion due to circulatory failure defines shock (ie, SCAI shock stages C and higher). 5 , 6 Numerous studies have demonstrated a clear incremental association between the SCAI shock classification and in‐hospital death in patients with or at risk for CS, particularly for patients meeting the shock criteria. 6 , 7 , 8
Most published studies demonstrating the prognostic value of the SCAI shock classification have assigned the SCAI shock stage either on admission or analyzed the worst value during hospitalization. 9 , 10 , 11 , 12 , 13 Given the rapidly changing condition of patients with CS over time, it has been proposed that determination of the SCAI shock stage serially could refine classification and improve prognostication. 6 , 7 , 8 Early prognostication is likely to be valuable insofar as the explicit purpose of the SCAI shock classification is to facilitate clinical decision making based on the severity of shock (eg, regarding the need for escalation of support or transfer to a higher level of care), and dynamic risk stratification could assist in these decisions. 5
Several analyses have assigned the SCAI shock classification on admission and again after 24 hours in patients with CS, showing incremental prognostic value of reassessing shock severity. 14 , 15 , 16 Another analysis in a larger cohort of patients with CS examined the performance of the SCAI shock classification on admission and the worst value during hospitalization. 17 However, no study has evaluated the association between temporal changes in SCAI stages during the first 24 hours of CICU admission and death. Accordingly, we sought to evaluate whether frequent reassessment of the SCAI shock classification could improve prognostication by assigning the SCAI shock stage every 4 hours during the first 24 hours of admission in a mixed CICU cohort.
Methods
Study Population
This retrospective observational cohort study was approved by the Institutional Review Board of Mayo Clinic Rochester as posing minimal risk to patients and was performed under a waiver of informed consent for patients who had provided Minnesota Research authorization. To create a cohort of unique CICU patients admitted to the Mayo Clinic Rochester CICU from 2015 to 2018 with available data, we crossed 2 existing data sets and analyzed the resulting cohort (Figure S1). The first data set was a well‐described cohort, including data from the first CICU admission for unique CICU patients admitted from 2007 to 2018, excluding readmissions. 1 , 4 The second data set included all consecutive patient admissions to the Mayo Clinic Rochester CICU from 2015 to 2018. 18 The first CICU cohort primarily included a 1‐time assessment of clinical data at the time of CICU admission; the second cohort was generated using a different methodology with higher‐frequency clinical data during the first 24 hours of CICU admission to overcome this limitation. 1 , 18 We combined these data sets to identify unique CICU patients, excluding readmissions and patients without any available vital signs or laboratory data to determine the SCAI shock classification.
Data Sources
Demographic, clinical, laboratory, treatment, and outcome data were extracted electronically from the medical record, including admission laboratory values, defined as the value recorded closest to CICU admission. 1 Time‐stamped data were extracted from Mayo Clinic databases, including the maximum, minimum, and mean values of vital signs recorded every 15 minutes. The first 24 hours of each CICU admission was divided into 4‐hour blocks, and data recorded during each 4‐hour block was assigned according to the time stamp. 18 Maximum vasoactive drug doses were used to calculate the Vasoactive‐Inotropic Score and norepinephrine equivalent dose during the first 2 hours and the second 2 hours of each CICU block. 19 , 20 , 21 The Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation III/IV scores, and Charlson Comorbidity Index were calculated automatically using validated electronic algorithms. 1 , 22 , 23 , 24 Admission diagnoses were determined on the basis of all International Classification of Diseases, Tenth Revision (ICD‐10) diagnosis codes documented within 1 day of CICU admission; these were not mutually exclusive, and the primary admission diagnoses could not be identified. 24
SCAI Shock Classification
The most extreme clinical, laboratory, and vital sign values from each block were used to determine the presence of hemodynamic instability, hypoperfusion (including the presence of acute kidney injury [AKI]), deterioration, and refractory shock (Table S1). 18 These constructs were used to assign the SCAI shock classification within that block for patients who remained in the CICU for any part of that block, using mutually exclusive categories of escalating shock severity from A to E; patients not meeting criteria for a higher SCAI shock stage were assigned to stage A. 5 , 6 , 7 Missing variables to determine the SCAI shock stage were assumed normal, and prior values were not carried forward except for creatinine (to identify AKI) and rising lactate (to identify deterioration). 18 SCAI shock stages were compared across six 4‐hour blocks constituting the first 24 hours of ICU admission. The maximum, minimum, and mean SCAI shock stage values from all available 4‐hour blocks during the first 24 hours were determined by treating SCAI shock stage as a continuous variable. The admission SCAI shock stage was defined as the SCAI shock stage during the first 4‐hour block. Patients were classified into improving, unchanged, or worsening SCAI shock stages hierarchically based first on the maximum and then the minimum SCAI shock stage compared with the admission SCAI shock stage; patients with maximum SCAI shock stage higher than the admission SCAI shock stage were classified as worsening even if they also met criteria for improving. Shock during each block was defined as hypoperfusion (ie, SCAI shock stage C, D, or E), and patients who met SCAI criteria for shock during the first CICU block were classified as having admission shock. 18 Patients without admission shock (ie, in SCAI shock stage A or B on admission) who developed shock (ie, met criteria for SCAI shock stage C, D, or E) during a subsequent block were classified as having delayed shock. Patients who left the CICU during a previous block were classified as ICU discharges or ICU deaths.
Statistical Analysis
The primary outcome was all‐cause in‐hospital death determined by electronic health record review; early death was death within 24 hours of CICU admission. Summary statistics included median (interquartile range) for continuous variables, with groups compared using the Kruskal–Wallis rank‐sum test, and number (percentage) for categorical variables, with groups compared using the Pearson chi‐square test. Odds ratio (OR) and 95% CI values were estimated using logistic regression before and after multivariable adjustment. Discrimination for in‐hospital death was evaluated using the area under the receiver operating characteristic curve (AUC, C‐statistic) values from logistic regression, and AUC values were compared using De Long's test. Multivariable models were adjusted for the following variables selected a priori: age, sex, Charlson Comorbidity Index, Acute Physiology and Chronic Health Evaluation IV predicted mortality rate, and admission diagnosis of cardiac arrest; these variables together yielded an AUC value of 0.84 for in‐hospital mortality. The SCAI shock stage was treated as a continuous variable (ie, 1–5) to demonstrate incremental associations per SCAI shock stage (including the admission, maximum, minimum, and mean), as per our prior analysis. 9 To assess the incremental performance of serial SCAI shock classification assignment, data from each block were added to the logistic regression model sequentially, with the last data carried forward for patients who left the CICU during an earlier block. Statistical analysis was performed using BlueSky version 7.4 (BlueSky LLC, Chicago, IL). The authors declare that all supporting data are available within the manuscript and its Supplemental Material.
Results
Population Demographics
Between 3568 unique CICU patient admissions and 3381 unique CICU patients, we identified 2918 unique CICU patients with available clinical data who comprised the final study population (Figure S1). The median age of the population was 70 (60–80) years, and 39.4% were females; 92.8% were White individuals. Admission diagnoses (Table 1) included acute coronary syndromes (41.6%), heart failure (57.8%), and cardiac arrest (11.6%).
Table 1.
Comparison of Patients With and Without Shock During the First 24 h, Including Those With Admission (Prevalent) Shock Versus Late (Incident) Shock
| Total (N=2918) | No shock (N=1381) | Any shock (N=1537) | Late shock (N=633) | Admission shock (N=904) | P value (any shock vs no shock) | P value (late shock vs admission shock) | P value (late shock vs no shock) | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, y | 70.0 (60.0–80.0) | 69.0 (58.0–79.0) | 71.0 (61.0–81.0) | 72.0 (60.0–82.0) | 71.0 (61.0–80.0) | <0.001 | 0.733 | <0.001 |
| Female sex | 1150 (39.4) | 511 (37.0) | 639 (41.6) | 275 (43.4) | 364 (40.3) | 0.012 | 0.213 | 0.006 |
| White race | 2707 (92.8) | 1284 (93.0) | 1423 (92.6%) | 585 (92.4) | 838 (92.7) | 0.682 | 0.835 | 0.652 |
| CCI | 2.0 (0.0–4.0) | 1.0 (0.0–3.0) | 2.0 (0.0–4.0) | 2.0 (0.0, 4.0) | 2.0 (0.0–4.0) | <0.001 | 0.986 | <0.001 |
| Admit source | 0.004 | 0.299 | 0.146 | |||||
| Catheterization laboratory | 1107 (38.5) | 525 (39.1) | 582 (37.9) | 239 (37.8) | 343 (38.0) | |||
| Direct admit | 763 (26.5) | 361 (26.9) | 402 (26.2) | 158 (25.0) | 244 (27.1) | |||
| ED | 481 (16.7) | 233 (17.3) | 248 (16.2) | 106 (16.8) | 142 (15.7) | |||
| Floor | 432 (15.0) | 200 (14.9) | 232 (15.1) | 105 (16.6) | 127 (14.1) | |||
| ICU | 42 (1.5) | 13 (1.0) | 29 (1.9) | 13 (2.1) | 16 (1.8) | |||
| OR/PACU | 53 (1.8) | 12 (0.9) | 41 (2.7) | 11 (1.7) | 30 (3.3) | |||
| Admission diagnoses and comorbidities | ||||||||
| Cardiac arrest | 338 (11.6) | 92 (6.7) | 246 (16.0) | 55 (8.7) | 191 (21.2) | <0.001 | <0.001 | <0.001 |
| Any shock | 566 (19.5) | 114 (8.3) | 452 (29.5) | 95 (15.1) | 357 (39.6) | <0.001 | <0.001 | 0.105 |
| CS | 450 (15.5) | 93 (6.8) | 357 (23.3) | 70 (11.1) | 287 (31.8) | <0.001 | <0.001 | <0.001 |
| Sepsis | 202 (6.9) | 40 (2.9) | 162 (10.6) | 41 (6.5) | 121 (13.4) | <0.001 | <0.001 | <0.001 |
| Respiratory failure | 977 (33.6) | 344 (25.0) | 633 (41.3) | 210 (33.3) | 423 (46.9) | <0.001 | <0.001 | <0.001 |
| ACS | 1209 (41.6) | 588 (42.7) | 621 (40.5) | 252 (39.9) | 369 (40.9) | 0.224 | 0.703 | 0.238 |
| HF | 1681 (57.8) | 709 (51.5) | 972 (63.4) | 382 (60.5) | 590 (65.4) | <0.001 | 0.051 | <0.001 |
| CKD | 652 (22.5) | 275 (20.1) | 377 (24.7) | 147 (23.3) | 230 (25.7) | 0.003 | 0.290 | 0.106 |
| Prior dialysis | 89 (3.0) | 25 (1.8) | 64 (4.2) | 25 (3.9) | 39 (4.3) | <0.001 | 0.725 | 0.004 |
| Severity of illness | ||||||||
| SOFA Score | 2.0 (1.0–5.0) | 2.0 (1.0–3.0) | 4.0 (2.0–7.0) | 3.0 (1.0–5.0) | 5.0 (2.0–8.0) | <0.001 | <0.001 | <0.001 |
| APACHE III score | 56.0 (43.0–70.0) | 50.0 (37.0–61.0) | 63.0 (49.0–79.0) | 58.0 (45.0–71.0) | 67.0 (53.0–85.0) | <0.001 | <0.001 | <0.001 |
| APACHE IV predicted mortality rate, % | 9.4 (4.2–20.6) | 6.5 (3.0–13.4) | 13.0 (5.8–29.1) | 10.4 (4.6–21.0) | 15.8 (6.8–38.4) | <0.001 | <0.001 | <0.001 |
| LVEF, % | 51.0 (36.0–60.0) | 53.0 (40.0–61.0) | 50.0 (33.0–60.0) | 52.0 (37.0–61.0) | 48.0 (30.0–60.0) | <0.001 | 0.002 | 0.533 |
| Maximum SCAI shock stage | <0.001 | <0.001 | <0.001 | |||||
| A | 548 (18.8) | 548 (39.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| B | 833 (28.5) | 833 (60.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| C | 1269 (43.5) | 0 (0.0) | 1269 (82.6) | 603 (95.3) | 666 (73.7) | |||
| D | 115 (3.9) | 0 (0.0) | 115 (7.5) | 17 (2.7) | 98 (10.8) | |||
| E | 153 (5.2) | 0 (0.0) | 153 (10.0) | 13 (2.1) | 140 (15.5) | |||
| Critical care therapies and procedures | ||||||||
| IMV | 470 (16.1) | 85 (6.2) | 385 (25.0) | 90 (14.2) | 295 (32.6) | <0.001 | <0.001 | <0.001 |
| NIPPV | 483 (16.6) | 203 (14.7) | 280 (18.2) | 116 (18.3) | 164 (18.1) | 0.011 | 0.927 | 0.039 |
| CRRT | 47 (1.6) | 7 (0.5) | 40 (2.6) | 12 (1.9) | 28 (3.1) | <0.001 | 0.145 | 0.003 |
| Dialysis | 96 (3.3) | 21 (1.5) | 75 (4.9) | 27 (4.3) | 48 (5.3) | <0.001 | 0.350 | <0.001 |
| Vasopressors | 638 (21.9) | 66 (4.8) | 572 (37.2) | 105 (16.6) | 467 (51.7) | <0.001 | <0.001 | <0.001 |
| Inotropes | 231 (7.9) | 77 (5.6) | 154 (10.0) | 48 (7.6) | 106 (11.7) | <0.001 | 0.008 | 0.083 |
| Vasoactives | 745 (25.5) | 131 (9.5) | 614 (39.9) | 128 (20.2) | 486 (53.8) | <0.001 | <0.001 | <0.001 |
| IABP | 197 (6.8) | 28 (2.0) | 169 (11.0) | 27 (4.3) | 142 (15.7) | <0.001 | <0.001 | 0.004 |
| Impella/ECMO | 56 (1.9) | 16 (1.2) | 40 (2.6) | 12 (1.9) | 28 (3.1) | 0.005 | 0.145 | 0.257 |
| PAC | 451 (15.5) | 167 (12.1) | 284 (18.5) | 88 (13.9) | 196 (21.7) | <0.001 | <0.001 | 0.190 |
| Catheterization | 1565 (53.6) | 750 (54.3) | 815 (53.0) | 334 (52.8) | 481 (53.2) | 0.488 | 0.864 | 0.519 |
| PCI | 818 (28.0) | 428 (31.0) | 390 (25.4) | 170 (26.9) | 220 (24.3) | <0.001 | 0.264 | 0.059 |
| Transfusion | 212 (7.3) | 45 (3.3) | 167 (10.9) | 63 (10.0) | 104 (11.5) | <0.001 | 0.336 | <0.001 |
| Outcomes | ||||||||
| CICU LOS, d | 1.6 (0.9–2.5) | 1.2 (0.8–2.0) | 1.8 (1.0–2.9) | 1.8 (1.1–2.8) | 1.8 (1.0–3.0) | <0.001 | 0.617 | <0.001 |
| CICU LOS <1 d | 881 (30.2) | 519 (37.6) | 362 (23.6) | 133 (21.0) | 229 (25.3) | <0.001 | 0.049 | <0.001 |
| Hospital LOS, d | 4.7 (2.6–8.1) | 3.8 (2.2–7.0) | 5.4 (3.0–9.3) | 5.0 (2.9–9.0) | 5.8 (3.0–9.3) | <0.001 | 0.333 | <0.001 |
| CICU mortality | 157 (5.4) | 20 (1.4) | 137 (8.9) | 27 (4.3) | 110 (12.2) | <0.001 | <0.001 | <0.001 |
| Early death | 73 (2.5) | 15 (1.1) | 58 (3.8) | 5 (0.8) | 53 (5.9) | <0.001 | <0.001 | <0.001 |
| In‐hospital mortality | 266 (9.1) | 48 (3.5) | 218 (14.2) | 57 (9.0) | 161 (17.8) | <0.001 | <0.001 | <0.001 |
Categorical variables were reported as numbers (percentages), and continuous variables were reported as medians (interquartile ranges). ACS indicates acute coronary syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; CCI, Charlson Comorbidity Index; CICU, cardiac intensive care unit; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; ED, emergency department; HF, heart failure; IABP, intra‐aortic balloon pump; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay; LVEF, left ventricular ejection fraction; NIPPV, noninvasive positive pressure ventilation; OR, operating room; PAC, pulmonary artery catheter; PACU, postanesthesia care unit; PCI, percutaneous coronary intervention; SCAI, Society for Cardiovascular Angiography and Interventions; and SOFA, Sequential Organ Failure Assessment.
Prevalence of Shock
A total of 1537 (52.7%) patients met the SCAI‐based criteria for shock (ie, SCAI shock stage C or greater) during any CICU block during the first 24 hours, including 904 (31.0%) with admission shock and 633 (21.7%) with delayed shock. Among patients meeting criteria for shock, 463 (61.3% with available data) had an elevated lactate level, 503 (32.7%) required vasopressors, 115 (7.5%) required mechanical circulatory support, and 1018 (66.2%) met criteria for AKI. Among these 4 markers of hypoperfusion, 1025 (66.7%) had 1, 341 (22.2%) had 2, 116 (7.5%) had 3, and 11 (0.7%) had all 4. Overall, 1269 (82.6%) patients meeting SCAI‐based criteria for shock had a maximum SCAI shock stage of C. Among the 2014 (69.0%) patients without admission shock, 31.4% developed incident delayed shock, including 334 (30.5%) of those who were in SCAI shock stage A and 299 (32.6%) of those who were in SCAI shock stage B on admission; >90% of patients with delayed shock met criteria for SCAI shock stage C. Significant differences were observed between patients with and without shock and those with admission or delayed shock (Table 1); patients with admission shock had higher illness severity including greater shock severity (SCAI shock stage D/E in 26.3% versus 4.7%). The number of hypoperfusion criteria among patients with shock increased with SCAI shock stage (Figure S2). The prevalence of shock generally decreased over time (Figure 1).
Figure 1. Sankey diagram demonstrating the evolution of shock (defined using the SCAI shock classification) during each 4‐hour CICU block.
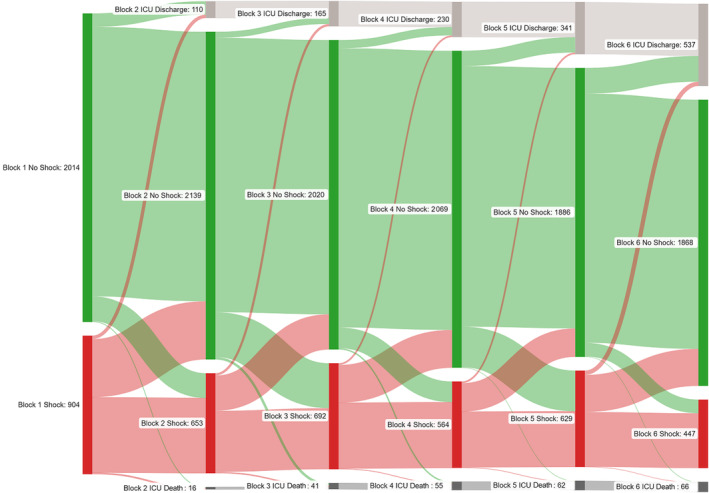
Patients who left the CICU during a prior block were classified as CICU discharge or CICU deaths during subsequent blocks. Figure generated using SankeyMATIC (www.sankeymatic.com). CICU indicates cardiac intensive care unit; ICU, intensive care unit; and SCAI, Society for Cardiovascular Angiography and Interventions.
SCAI Shock Classification
The distribution of admission SCAI shock stages was: A, 37.6%; B, 31.5%; C, 25.9%; D, 1.8%; and E, 3.3% (Figure S1). The maximum SCAI shock stages distribution was: A, 18.8%; B, 28.5%; C, 43.5%; D, 3.9%; and E, 5.2%. Compared with the admission SCAI shock stage, 945 (32.4%) patients had an increase of at least 1 SCAI shock stage (worsening), and 1075 (36.8%) had a decrease by at least 1 SCAI shock stage without meeting criteria for worsening (improving); the remaining 898 (30.8%) patients had an unchanged SCAI shock stage over the first 24 hours. Significant differences were observed between patients with improving, unchanged, and worsening SCAI shock stages (Table 2).
Table 2.
Comparison of Patients With Improving, Unchanged, and Worsening SCAI Shock Stage After Admission
| Improving (N=1075) | Unchanged (N=898) | Worsening (N=945) | P value (all groups) | P value (unchanged vs worsening) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 70.0 (60.0–80.0) | 69.0 (58.0–79.0) | 71.0 (60.0–81.0) | 0.008 | 0.003 |
| Female sex | 423 (39.3) | 335 (37.3) | 392 (41.5) | 0.186 | 0.067 |
| White | 1009 (93.9) | 820 (91.3) | 878 (92.9) | 0.092 | 0.203 |
| CCI | 2.0 (0.0–4.0) | 1.0 (0.0–3.8) | 2.0 (0.0–4.0) | 0.017 | 0.005 |
| Admit source | 0.013 | 0.255 | |||
| Catheterization laboratory | 457 (42.5) | 300 (34.9) | 350 (37.1) | ||
| Direct admit | 252 (23.4) | 260 (30.3) | 251 (26.6) | ||
| ED | 173 (16.1) | 149 (17.3) | 159 (16.8) | ||
| Floor | 152 (14.1) | 128 (14.9) | 152 (16.1) | ||
| ICU | 18 (1.7) | 7 (0.8) | 17 (1.8) | ||
| OR/PACU | 23 (2.1) | 15 (1.7) | 15 (1.6) | ||
| Admission diagnoses and comorbidities | |||||
| Cardiac arrest | 158 (14.8) | 77 (8.6) | 103 (10.9) | <0.001 | 0.092 |
| Any shock | 251 (23.4) | 142 (15.8) | 173 (18.4) | <0.001 | 0.161 |
| CS | 202 (18.9) | 114 (12.7) | 134 (14.2) | <0.001 | 0.346 |
| Sepsis | 77 (7.2) | 44 (4.9) | 81 (8.6) | 0.007 | 0.002 |
| Respiratory failure | 375 (35.0) | 273 (30.5) | 329 (34.9) | 0.060 | 0.042 |
| ACS | 464 (43.3) | 382 (42.6) | 363 (38.5) | 0.069 | 0.074 |
| HF | 624 (58.3) | 498 (55.6) | 559 (59.3) | 0.244 | 0.103 |
| CKD | 258 (24.3) | 170 (19.1) | 224 (23.8) | 0.013 | 0.014 |
| Prior dialysis | 33 (3.1) | 21 (2.3) | 35 (3.7) | 0.234 | 0.088 |
| Severity of illness | |||||
| SOFA score | 3.0 (1.0–5.0) | 2.0 (1.0–3.2) | 3.0 (1.0–5.0) | <0.001 | <0.001 |
| Apache III score | 58.0 (44.0–73.5) | 51.0 (39.0–63.8) | 58.0 (45.0–74.0) | <0.001 | <0.001 |
| APACHE IV predicted mortality, % | 10.8 (4.6–23.2) | 7.1 (3.3–14.5) | 10.8 (4.7–23.2) | <0.001 | <0.001 |
| LVEF, % | 50.0 (35.0–60.0) | 52.0 (37.0–60.0) | 52.0 (36.0–61.0) | 0.188 | 0.898 |
| Critical care therapies and procedures | |||||
| IMV | 207 (19.3) | 95 (10.6) | 168 (17.8) | <0.001 | <0.001 |
| NIPPV | 179 (16.7) | 130 (14.5) | 174 (18.4) | 0.075 | 0.023 |
| CRRT | 13 (1.2) | 11 (1.2) | 23 (2.4) | 0.050 | 0.054 |
| Dialysis | 28 (2.6) | 26 (2.9) | 42 (4.4) | 0.050 | 0.078 |
| Vasopressors | 316 (29.4) | 113 (12.6) | 209 (22.1) | <0.001 | <0.001 |
| Inotropes | 95 (8.8) | 48 (5.3) | 88 (9.3) | 0.003 | 0.001 |
| Vasoactives | 371 (34.5) | 137 (15.3) | 237 (25.1) | <0.001 | <0.001 |
| IABP | 57 (5.3) | 86 (9.6) | 54 (5.7) | <0.001 | 0.002 |
| Impella/ECMO | 20 (1.9) | 18 (2.0) | 18 (1.9) | 0.973 | 0.877 |
| PAC | 163 (15.2) | 144 (16.0) | 144 (15.2) | 0.845 | 0.637 |
| Catheterization | 587 (54.6) | 487 (54.2) | 491 (52.0) | 0.448 | 0.328 |
| PCI | 317 (29.5) | 257 (28.6) | 244 (25.8) | 0.167 | 0.177 |
| Transfusion | 76 (7.1) | 52 (5.8) | 84 (8.9) | 0.036 | 0.011 |
| Outcomes | |||||
| CICU LOS, d | 1.7 (1.0–2.6) | 1.1 (0.6–2.0) | 1.8 (1.0–2.8) | <0.001 | <0.001 |
| CICU LOS <1 d | 289 (26.9) | 385 (42.9) | 207 (21.9) | <0.001 | <0.001 |
| Hospital LOS, d | 4.7 (2.8–8.0) | 4.1 (2.2–7.9) | 4.9 (2.8–8.8) | <0.001 | <0.001 |
| CICU death | 61 (5.7) | 40 (4.5) | 56 (5.9) | 0.325 | 0.155 |
| Early death | 28 (2.6) | 31 (3.5) | 14 (1.5) | 0.025 | 0.006 |
| In‐hospital death | 97 (9.0) | 72 (8.0) | 97 (10.3) | 0.244 | 0.095 |
Categorical variables were reported as numbers (percentages), and continuous variables were reported as medians (interquartile ranges). ACS indicates acute coronary syndrome; APACHE, Acute Physiology and Chronic Health Evaluation; CCI, Charlson Comorbidity Index; CICU, cardiac intensive care unit; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; CS, cardiogenic shock; ECMO, extracorporeal membrane oxygenation; ED, emergency department; HF, heart failure; IABP, intra‐aortic balloon pump; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay; LVEF, left ventricular ejection fraction; NIPPV, noninvasive positive pressure ventilation; OR, operating room; PAC, pulmonary artery catheter; PACU, postanesthesia care unit; PCI, percutaneous coronary intervention; SCAI, Society for Cardiovascular Angiography and Interventions; and SOFA, Sequential Organ Failure Assessment.
Unadjusted In‐Hospital Mortality Rate and Shock
Two hundred sixty‐six (9.1%) patients died during hospitalization, including 157 (5.4%) deaths during the CICU stay. Compared with patients without shock, patients with shock during the first 24 hours were at higher risk of in‐hospital death, with an incrementally higher mortality rate with admission shock versus delayed shock overall and in acute coronary syndrome, heart failure, and patients with cardiac arrest (Figure 2). Each additional marker of hypoperfusion was associated with an incrementally higher mortality rate (Figure S3). Each additional block in which shock was present was incrementally associated with a higher in‐hospital mortality rate, both overall and across admission diagnoses (Figure 3). When patients were stratified on the basis of whether they left the CICU (died or discharged) before block 6, both the unadjusted OR and AUC values increased: left CICU before block 6 (unadjusted OR, 1.70 per block with shock [95% CI, 1.42–2.03]; AUC, 0.72) versus remained in CICU through block 6 (unadjusted OR, 1.57 per block with shock [95% CI, 1.46–1.69]; AUC, 0.76). Discrimination for in‐hospital death based on the presence of shock improved with each subsequent ICU block, with the AUC (C‐statistic) increasing from 0.66 in block 1 to 0.75 in block 6 (Figure S4). The cumulative sensitivity of shock for in‐hospital death increased from 60.5% in block 1 to 82.0% in block 6 (Figure S5).
Figure 2. In‐hospital death according to the presence of admission shock and delayed shock during the first 24 hours of the CICU stay, overall and according to admission diagnosis.
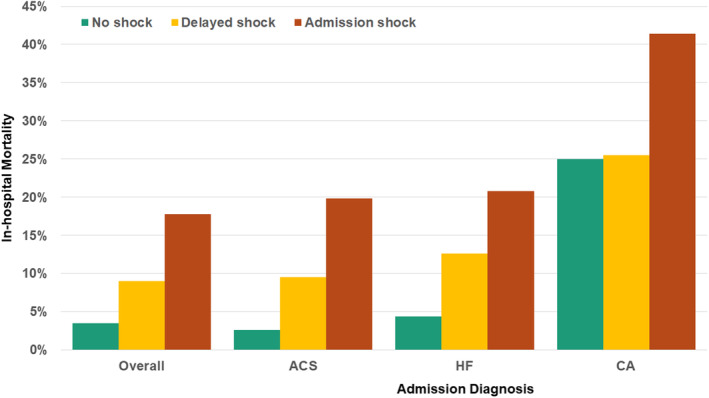
ACS indicates acute coronary syndrome; CA, cardiac arrest; CICU cardiac intensive care unit; and HF, heart failure.
Figure 3. In‐hospital death according to the number of 4‐hour CICU blocks during which shock was present on the basis of the SCAI shock classification in the first 24 hours, overall and according to admission diagnosis.
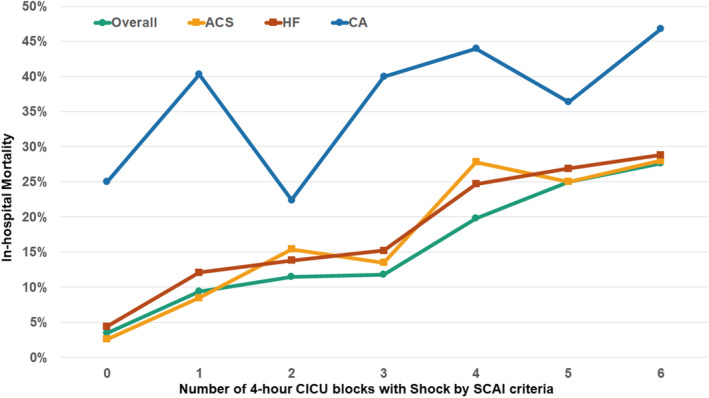
ACS indicates acute coronary syndrome; CA, cardiac arrest; CICU, cardiac intensive care unit; HF, heart failure; and SCAI, Society for Cardiovascular Angiography and Interventions.
In‐Hospital Death and SCAI Shock Classification, Unadjusted Analyses
In‐hospital death increased with a higher admission, maximum, minimum, and mean SCAI shock stage, including patients with acute coronary syndrome, heart failure, and cardiac arrest (Figure 4). A mortality gradient was observed according to whether the patient had an improving, unchanged, or worsening SCAI shock stage after admission (Figure 5). Cumulative discrimination for in‐hospital death using the SCAI shock classification improved with each subsequent ICU block, with the AUC (C‐statistic) increasing from 0.70 in block 1 to 0.78 in block 6 (Figure S4). The AUC (C‐statistic) for in‐hospital death was moderately higher for the maximum SCAI shock stage than the admission SCAI shock stage, both overall (Table 3) and in each admission diagnosis (Table S2). Furthermore, the mean SCAI shock stage had even better discrimination (P<0.001 versus admission and P=0.04 versus maximum).
Figure 4. In‐hospital death according to the admission, maximum, minimum, and mean SCAI shock stage overall and in each admission diagnosis group.
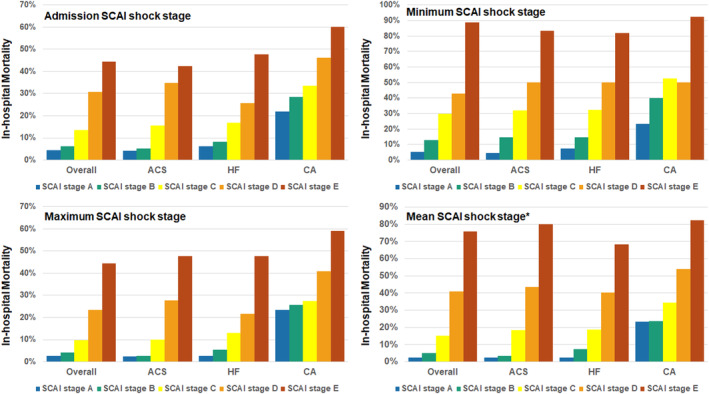
*Note that the mean SCAI shock stage was rounded up to the next highest stage. ACS indicates acute coronary syndrome; CA, cardiac arrest; HF, heart failure; and SCAI, Society for Cardiovascular Angiography and Interventions.
Figure 5. In‐hospital death according to the admission SCAI shock stage and the presence of an improving, unchanged, or worsening SCAI shock stage.
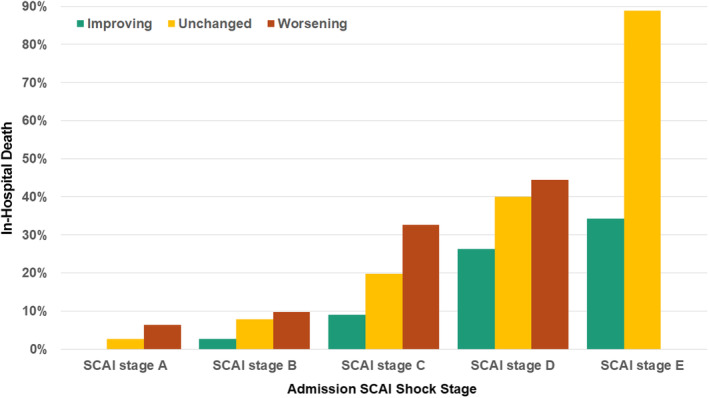
Note that patients initially in SCAI shock stage A could not improve, and patients initially in SCAI shock stage E could not worsen. SCAI indicates Society for Cardiovascular Angiography and Interventions.
Table 3.
Logistic Regression Models for Prediction of In‐Hospital Death in the Overall Population, Including Unit OR Values With 95% CIs and AUC (C‐statistic)
| Variable | Univariable analyses | Multivariable analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR | Lower 95% CI | Upper 95% CI | P value | AUC | Adjusted OR | Lower 95% CI | Upper 95% CI | P value | AUC | |
| Any shock | 4.590 | 3.328 | 6.331 | <0.001 | 0.661 | 2.107 | 1.476 | 3.010 | <0.001 | 0.849 |
| Number of blocks with shock | 1.441 | 1.360 | 1.527 | <0.001 | 0.715 | 1.152 | 1.070 | 1.241 | <0.001 | 0.850 |
| Admission SCAI shock stage | 2.101 | 1.873 | 2.357 | <0.001 | 0.698 | 1.356 | 1.181 | 1.558 | <0.001 | 0.846 |
| Maximum SCAI shock stage | 2.543 | 2.237 | 2.891 | <0.001 | 0.731 | 1.589 | 1.366 | 1.850 | <0.001 | 0.851 |
| Minimum SCAI shock stage | 2.845 | 2.459 | 3.291 | <0.001 | 0.694 | 1.973 | 1.656 | 2.351 | <0.001 | 0.858 |
| Mean SCAI shock stage | 3.974 | 3.363 | 4.696 | <0.001 | 0.776 | 2.424 | 1.991 | 2.951 | <0.001 | 0.866 |
Multivariable models are adjusted for age, sex, Charlson Comorbidity Index, APACHE IV predicted mortality, and admission diagnosis of cardiac arrest. The SCAI Shock Classification was analyzed as a continuous variable to generate the unit OR value per each higher SCAI shock stage. APACHE indicates Acute Physiology and Chronic Health Evaluation; AUC, area under the receiver operating characteristic curve; OR, odds ratio; and SCAI, Society for Cardiovascular Angiography and Interventions.
Adjusted In‐Hospital Mortality Rate
After adjustment, patients who met SCAI criteria for shock at any time during the first 24 hours were at higher risk of in‐hospital death, with an incremental association between the number of CICU blocks with shock and a progressively higher mortality rate (Table 3). After further adjusting for whether patients remained in the CICU through block 6, the strength of this association increased (adjusted OR, 1.27 per each block with shock [95% CI, 1.17–1.38]; AUC, 0.86). The adjusted in‐hospital mortality rate did not differ for patients with admission versus delayed shock. The admission, maximum, minimum, and mean SCAI shock stage all remained strongly associated with an adjusted in‐hospital mortality rate (Table 3). Patients with either unchanged or worsening SCAI shock stage had a higher mortality rate versus those with improving SCAI shock stage, particularly when adjusted for the admission SCAI shock stage.
Discussion
In this cohort of nearly 3000 unique CICU patients with or at risk for CS, we observed that serial assignment of the SCAI shock classification at 4‐hour intervals during the first 24 hours of the CICU stay provided robust predictive value for in‐hospital death. The presence of worsening shock, defined by a rising SCAI shock stage, was associated with a higher mortality rate in patients with and without shock on CICU admission. Importantly, the number of CICU blocks during which SCAI criteria for shock were met was incrementally associated with a higher mortality rate. Although the admission, maximum, and minimum SCAI shock stage had good discrimination for in‐hospital death, the average (mean) shock severity was most strongly predictive. Collectively, our analysis has demonstrated that both the magnitude and duration of shock (ie, the AUC) are important predictors of death in CICU patients. These findings show the promise of dynamic shock severity assessment using the SCAI shock classification to describe the shock trajectory, as could be facilitated using an automated electronic health record–based algorithm.
Prior analyses examining serial assignment of the SCAI shock classification have generally involved single‐center or multicenter cohorts of 166 to 300 patients with CS with the assessment of shock severity on admission and after 24 hours. 14 , 15 , 16 These studies have consistently demonstrated incremental associations between admission and 24‐hour SCAI shock stage with in‐hospital death. Notably, the shock trajectory was particularly salient, and those patients whose SCAI shock stage increased by even a single stage (or failed to decrease) were at markedly higher risk. Indeed, while those who met the criteria for SCAI shock stage E (refractory shock) on admission were at the highest risk, subsequent death varied substantially according to the 24‐hour SCAI shock stage. These studies generally used physician assignment of the SCAI shock stage, differing substantially from our analysis using strict criteria based on physiological data. Nonetheless, our analysis replicates these findings in a broader CICU population, showing a marked gradient of in‐hospital death according to the admission and minimum/maximum 24‐hour SCAI shock stage. These important studies clearly demonstrated the incremental prognostic value of reassessing the SCAI shock classification over time to evaluate a patient's response to therapy, with nonimproving or worsening shock associated with poor outcomes.
The analysis from the Cardiogenic Shock Working Group included a large multicenter cohort of 3455 patients with CS, with SCAI shock stage assigned on the basis of physiological data on admission and then again using the worst values during hospitalization. 17 As shown in smaller studies, the admission and worst SCAI shock stage were incrementally associated with in‐hospital death, particularly for patients meeting SCAI shock stage D or E criteria. We confirmed and expanded on their findings using a similar set of variables to assign the SCAI Shock Stage using a different algorithm based on, but distinct from, our prior work. 9 , 20 Importantly, while we found the maximum SCAI shock stage to generally outperform the admission SCAI shock stage, the mean SCAI shock stage performed the best. Therefore, while it has been convincingly demonstrated that reassessing the SCAI shock classification after admission is important for refining prognostication, we believe that frequent calculation may prove superior to a single‐time reevaluation using a longer time window by facilitating more rapid recognition of changes in shock trajectory.
Compared with these important previous studies, our study adds several novel findings. First, frequent assessment of the presence and severity of shock during the first 24 hours of CICU admission using objective criteria is feasible and can improve mortality prediction. More than 80% of patients who died in the hospital met our SCAI criteria for shock during the first 24 hours of CICU admission, although most patients who met criteria for shock did survive hospitalization. Second, the maximum, minimum, and especially mean shock severity are superior to a 1‐time assessment on admission. This phenomenon has been demonstrated in prior analyses using serial assignment of the Sequential Organ Failure Assessment score as an overall assessment of illness severity. 22 , 25 Third, both the severity and duration of shock are important prognostic variables that can be evaluated using the SCAI shock classification. This is one of the first analyses to describe the incidence and prevalence of shock longitudinally in CICU patients using the SCAI shock classification definition, finding that discrimination for in‐hospital death based on the SCAI shock stage increased over time. Although the incremental increase in the multivariable adjusted AUC value with serial SCAI shock classification evaluation was modest, the increase in univariable AUC with the mean SCAI shock stage was more substantial (0.78 versus 0.70), and this univariable AUC approached that of the Acute Physiology and Chronic Health Evaluation IV itself (univariable AUC, 0.81). Most patients with shock (including those meeting criteria for SCAI shock stage D/E) on admission improved subsequently, and those who did not were at particularly high risk of death. Fourth, the shock trajectory is important for prognostication, and patients with either worsening or unchanged shock severity after admission had worse outcomes. Interestingly, patients with delayed shock had lower illness severity and mortality rate than those with shock on admission, similar to our observation with early versus delayed vasoactive drug use. 20 Patients with delayed shock primarily met criteria for hypoperfusion on the basis of the presence of AKI, which appeared to carry a more favorable prognosis than lactic acidosis or vasopressor dependence (which were more common in admission shock). While oliguria can be an important marker of hypoperfusion, AKI in the CICU may not always represent shock. 6 , 9 , 26 , 27 However, we have used a similar definition of AKI in other SCAI shock classification analyses. 9 , 18 , 27 The inclusion of AKI in our definition of shock may explain the substantially higher prevalence of shock identified in this study compared with prior analyses using different criteria, perhaps due to inclusion of some patients with noncardiogenic shock. 1 , 2 , 3 , 4 , 9 , 24 While our use of objective criteria to define hypoperfusion on the basis of electronic health record data could have increased sensitivity for milder forms of shock, it remains likely that some patients with nonhemodynamic AKI could have been misclassified as shock. The comparatively low mortality risk in patients meeting our definition of shock (>80% of whom were SCAI shock stage C) could result from detection of early, mild, or transient shock states or inclusion of patients without shock with AKI.
This analysis sets the stage for potentially implementing an intermittently or continuously active electronic health record algorithm that can assign the SCAI shock stage in real time to improve risk stratification. 7 , 8 Such an approach could serve as an early warning system for identifying patients with new‐onset, impending, or worsening shock, recognizing that the diagnosis of shock requires clinician input to exclude other forms of organ injury. Importantly, development of an electronic health record–based algorithm to assign the SCAI shock classification in real time would require consideration of important issues related to data quality, including treatment of missing or erroneous values, when to carry prior data points forward, what specific cutoffs to use for continuous variables, and whether multiple criteria should be met to define hypoperfusion when assigning shock.
For the future care of patients with and at risk for shock, it will be essential to systematically assess clinical markers of hemodynamic instability and hypoperfusion and to reliably apply a structured classification system such as the SCAI shock classification. This approach will facilitate timely recognition of patients with early or impending shock, enable appropriate interventions tailored to the degree of shock severity, and allow consistent treatment of patients according to best clinical practice standards. Most importantly, frequent serial reassessment of shock severity at periodic intervals (eg, every 4 hours as in our analysis) can provide a better understanding of the dynamic evolution of shock trajectory and response to therapy, which can refine prognostic assessment and facilitate intensification of therapy for patients who are not responding well to current treatments. This analysis demonstrates the feasibility of such an approach, which could potentially be streamlined using an electronic health record algorithm that can integrate numerous clinical data points in a continuous manner to permit rapid identification and staging of shock. This strategy could be coupled with decision‐support tools, which could enable dependable implementation of stage‐specific evaluation and management strategies to allow individualized treatment concordant with clinical guidelines.
Limitations
This study carries the same limitation as all single‐center retrospective cohort analyses. Perhaps the most important limitation is the potential for missing data to have impacted the accuracy of the SCAI shock classification assignment and its subsequent association with death. Only data obtained or recorded during each 4‐hour CICU block was used to assign the SCAI shock stage during that block, and variables recorded even a short time before or after were not incorporated (with limited exceptions). 18 The presence of missing data likely resulted in the misclassification of some patients, primarily by lowering the SCAI shock stage and resulting in failure to recognize shock if data used to define hypoperfusion were unavailable; it is likely that sicker patients had more frequent laboratory assessment. In addition, we selected 4‐hour CICU blocks a priori, and a different reassessment interval could have performed differently. 18 We did not summate data to calculate the maximum SCAI shock stage as in some prior studies and instead reported the maximum of the individual SCAI shock stages recorded in any of the 6 blocks. 17 The SCAI shock classification we used for this analysis differs somewhat from that used in our prior studies in CICU patients and used cutoffs that were defined a priori without specifically using data‐driven cutoffs. 9 , 18 , 20 While we believe this analysis validates our new approach to the SCAI shock classification, the performance of this SCAI shock classification system was not compared with our prior constructs. In addition, we evaluated only the first 24 hours of the CICU stay, limiting our ability to comment on the late development of CS, which has been associated with worse outcomes. 9 , 18 To simplify the analysis and demonstrate clear results that would be easier to understand at the bedside, we performed standard logistic regression for prediction of in‐hospital death instead of using a more complex method such as analysis of survival using shock as a time‐varying covariate or the use of generalized estimating equations to assess outcome in each block as a function of shock severity in that block individually. This is relevant considering that in each block some patients died or were discharged from the CICU and therefore did not have data for later blocks, which could have affected the observed performance of serial assessment of shock. This was evident for analyses regarding the number of blocks with shock, which appeared to have been influenced by this confounding factor. Finally, this analysis cannot determine the causes of new, worsening, or improving shock, and it is imperative to identify effective therapies that can be employed for high‐risk patients with refractory, nonrecovering, or deteriorating shock.
Conclusions
In CICU patients with or at risk for CS, the magnitude and duration of shock, as determined objectively using frequent serial assignment of the SCAI shock classification, are strongly associated with in‐hospital death. Dynamic shock severity evaluation using this approach is feasible and improves prediction over a 1‐time assessment or simple consideration of the maximum severity alone. The study provides proof of concept that a strategy of real‐time monitoring for new or worsening shock leveraging the electronic health record could be effective for risk stratification. Future research is needed to prospectively confirm our findings, to determine whether our SCAI shock classification algorithm can be improved, to understand the optimal reassessment interval for the SCAI shock classification, and to identify treatments that can be linked to the recognition of new or worsening shock.
Source of Funding
No direct funding was involved in the conduct of this research. Dr Jentzer is supported in part by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. This publication was supported by grant number UL1 TR002377 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Supporting information
Tables S1–S2
Figures S1–S5
This work was presented at AHA Scientific Sessions, November 11–13, 2023, in Philadelphia, PA.
This manuscript was sent to Julie K. Freed, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032748
For Sources of Funding and Disclosures, see page 12.
References
- 1. Jentzer JC, van Diepen S, Barsness GW, Katz JN, Wiley BM, Bennett CE, Mankad SV, Sinak LJ, Best PJ, Herrmann J, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J. 2019;215:12–19. doi: 10.1016/j.ahj.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 2. Berg DD, Bohula EA, van Diepen S, Katz JN, Alviar CL, Baird‐Zars VM, Barnett CF, Barsness GW, Burke JA, Cremer PC, et al. Epidemiology of shock in contemporary cardiac intensive care units. Circ Cardiovasc Qual Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohula EA, Katz JN, van Diepen S, Alviar CL, Baird‐Zars VM, Park JG, Barnett CF, Bhattal G, Barsness GW, Burke JA, et al. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the Critical Care Cardiology Trials Network prospective North American multicenter registry of cardiac critical illness. JAMA Cardiol. 2019;4:928–935. doi: 10.1001/jamacardio.2019.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jentzer JC, Ahmed AM, Vallabhajosyula S, Burstein B, Tabi M, Barsness GW, Murphy JG, Best PJ, Bell MR. Shock in the cardiac intensive care unit: changes in epidemiology and prognosis over time. Am Heart J. 2021;232:94–104. doi: 10.1016/j.ahj.2020.10.054 [DOI] [PubMed] [Google Scholar]
- 5. Baran DA, Grines CL, Bailey S, Burkhoff D, Hall SA, Henry TD, Hollenberg SM, Kapur NK, O'Neill W, Ornato JP, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94:29–37. doi: 10.1002/ccd.28329 [DOI] [PubMed] [Google Scholar]
- 6. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, Grines CL, Diercks DB, Hall S, Kapur NK, et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies: this statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022;79:933–946. doi: 10.1016/j.jacc.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 7. Jentzer JC, Rayfield C, Soussi S, Berg D, Kennedy JN, Sinha SS, Brant E, Mebazaa A, Billia F, Kapur NK, et al. Advances in the staging and phenotyping of cardiogenic shock part 1: clinical context. JACC Adv. 2022;1:1–14. doi: 10.1016/j.jacadv.2022.100120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hill KL, Rustin MA, Asche MA, Bennett CE, Patel PC, Jentzer JC. Cardiogenic shock classification and associated mortality risk. Mayo Clin Proc. 2023;98:771–783. doi: 10.1016/j.mayocp.2022.12.007 [DOI] [PubMed] [Google Scholar]
- 9. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, Naidu SS, Baran DA. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74:2117–2128. doi: 10.1016/j.jacc.2019.07.077 [DOI] [PubMed] [Google Scholar]
- 10. Jentzer JC, Baran DA, Bohman JK, van Diepen S, Radosevich M, Yalamuri S, Rycus P, Drakos SG, Tonna JE. Cardiogenic shock severity and mortality in patients receiving venoarterial extracorporeal membrane oxygenator support. Eur Heart J Acute Cardiovasc Care. 2022;11:891–903. doi: 10.1093/ehjacc/zuac119 [DOI] [PubMed] [Google Scholar]
- 11. Thayer KL, Zweck E, Ayouty M, Garan AR, Hernandez‐Montfort J, Mahr C, Morine KJ, Newman S, Jorde L, Haywood JL, et al. Invasive hemodynamic assessment and classification of in‐hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13:e007099. doi: 10.1161/CIRCHEARTFAILURE.120.007099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schrage B, Dabboura S, Yan I, Hilal R, Neumann JT, Sorensen NA, Gossling A, Becher PM, Grahn H, Wagner T, et al. Application of the SCAI classification in a cohort of patients with cardiogenic shock. Catheter Cardiovasc Interv. 2020;96:E213–E219. doi: 10.1002/ccd.28707 [DOI] [PubMed] [Google Scholar]
- 13. Lawler PR, Berg DD, Park JG, Katz JN, Baird‐Zars VM, Barsness GW, Bohula EA, Carnicelli AP, Chaudhry SP, Jentzer JC, et al. The range of cardiogenic shock survival by clinical stage: data from the Critical Care Cardiology Trials Network registry. Crit Care Med. 2021;49:1293–1302. doi: 10.1097/CCM.0000000000004948 [DOI] [PubMed] [Google Scholar]
- 14. Baran DA, Long A, Badiye AP, Stelling K. Prospective validation of the SCAI shock classification: single center analysis. Catheter Cardiovasc Interv. 2020;96:1339–1347. doi: 10.1002/ccd.29319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hanson ID, Tagami T, Mando R, Kara Balla A, Dixon SR, Timmis S, Almany S, Naidu SS, Baran D, Lemor A, et al. SCAI shock classification in acute myocardial infarction: insights from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv. 2020;96:1137–1142. doi: 10.1002/ccd.29139 [DOI] [PubMed] [Google Scholar]
- 16. Morici N, Frea S, Bertaina M, Sacco A, Corrada E, Dini CS, Briani M, Tedeschi M, Saia F, Colombo C, et al. SCAI stage reclassification at 24 h predicts outcome of cardiogenic shock: insights from the Altshock‐2 registry. Catheter Cardiovasc Interv. 2023;101:22–32. doi: 10.1002/ccd.30484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez‐Montfort J, Zhang Y, Li B, Baca P, Dieng F, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol. 2022;80:185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 18. Jentzer JC, Senghavi D, Patel PC, Bhattacharyya A, Van Diepen S, Herasevich V, Gajic O, Kashani K. Shock severity classification and mortality in adults with cardiac, medical, surgical and neurological critical illness. Mayo Clin Proc. doi: 10.1016/j.mayocp.2023.08.007 [DOI] [PubMed] [Google Scholar]
- 19. Jentzer JC, Wiley B, Bennett C, Murphree DH, Keegan MT, Kashani KB, Bell MR, Barsness GW. Temporal trends and clinical outcomes associated with vasopressor and inotrope use in the cardiac intensive care unit. Shock. 2020;53:452–459. doi: 10.1097/SHK.0000000000001390 [DOI] [PubMed] [Google Scholar]
- 20. Jentzer JC, Patel PC, Van Diepen S, Morrow DA, Barsness GW, Kashani KB. Changes in vasoactive drug requirements and mortality in cardiac intensive care unit patients. Shock. 2023;59:864–870. doi: 10.1097/SHK.0000000000002123 [DOI] [PubMed] [Google Scholar]
- 21. Burstein B, Vallabhajosyula S, Ternus B, Murphree D, Barsness GW, Kashani K, Jentzer JC. Outcomes associated with norepinephrine use among cardiac intensive care unit patients with severe shock. Shock. 2021;56:522–528. doi: 10.1097/SHK.0000000000001767 [DOI] [PubMed] [Google Scholar]
- 22. Jentzer JC, Bennett C, Wiley BM, Murphree DH, Keegan MT, Gajic O, Wright RS, Barsness GW. Predictive value of the sequential organ failure assessment score for mortality in a contemporary cardiac intensive care unit population. J Am Heart Assoc. 2018;7:e008169. doi: 10.1161/JAHA.117.008169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett CE, Wright RS, Jentzer J, Gajic O, Murphree DH, Murphy JG, Mankad SV, Wiley BM, Bell MR, Barsness GW. Severity of illness assessment with application of the APACHE IV predicted mortality and outcome trends analysis in an academic cardiac intensive care unit. J Crit Care. 2019;50:242–246. doi: 10.1016/j.jcrc.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jentzer JC, van Diepen S, Murphree DH, Ismail AS, Keegan MT, Morrow DA, Barsness GW, Anavekar NS. Admission diagnosis and mortality risk prediction in a contemporary cardiac intensive care unit population. Am Heart J. 2020;224:57–64. doi: 10.1016/j.ahj.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 25. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 26. Jentzer JC, Breen T, Sidhu M, Barsness GW, Kashani K. Epidemiology and outcomes of acute kidney injury in cardiac intensive care unit patients. J Crit Care. 2020;60:127–134. doi: 10.1016/j.jcrc.2020.07.031 [DOI] [PubMed] [Google Scholar]
- 27. Padkins M, Breen T, Van Diepen S, Barsness G, Kashani K, Jentzer JC. Incidence and outcomes of acute kidney injury stratified by cardiogenic shock severity. Catheter Cardiovasc Interv. 2021;98:330–340. doi: 10.1002/ccd.29692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S5


