Abstract
Background
The association of the American Heart Association's updated cardiovascular health score, the Life's Essential 8 (LE8), with cardiovascular disease (CVD) and death is not described in the FHS (Framingham Heart Study).
Methods and Results
We evaluated Framingham Offspring participants at examinations 2 and 6 (n=2888 and 1667; and mean age, 44 and 57 years, respectively), free of CVD with information on LE8 components. Using age‐sex–adjusted Cox models, we related LE8 and its change (examination 2 to examination 6) with CVD and death risk and compared associations with those of the Life's Simple 7 score. Mean LE8 score at examination 2 was 67 points (minimum, 26 points; maximum, 100 points). At both examinations, participants were reclassified to a different cardiovascular health status, depending on which method (LE8 versus Life's Simple 7) was used (60% of participants in ideal Life's Simple 7 status were in intermediate LE8 category). On follow‐up after examination 2 (median, 30 and 33 years for CVD and death, respectively), we observed 966 CVD events, and 1195 participants died. Participants having LE8≥68 (sample median) were at lower CVD and death risk compared with those with LE8<68 (examination 2: CVD hazard ratio [HR], 0.47 [95% CI, 0.41–0.54]; death HR, 0.55 [95% CI, 0.49–0.62]; all P<0.001). Participants maintaining low LE8 scores during life course were at highest CVD and death risk (CVD: HRs ranging from 1.8 to 2.3; P<0.001; death HR, 1.45 [95% CI, 1.13–1.85]; P=0.003 versus high‐high group).
Conclusions
Further studies are warranted to elucidate whether the LE8 score is a better marker of CVD and death risk, compared with Life's Simple 7 score.
Keywords: American Heart Association, cardiovascular disease, ideal cardiovascular health, Life's Essential 8
Subject Categories: Epidemiology
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CVH
cardiovascular health
- FHS
Framingham Heart Study
- LE8
Life's Essential 8
- LS7
Life's Simple 7
Clinical Perspective.
What Is New?
Using the American Heart Association's updated cardiovascular health score (ie, Life's Essential 8) results in a different categorization of participants compared with that using Life's Simple 7 score.
What Are the Clinical Implications?
Maintaining a high Life's Essential 8 score throughout the life course results in a lower risk of cardiovascular disease and death compared with other groups.
Further studies should evaluate whether the Life's Essential 8 score is a better marker of cardiovascular disease and death risk, compared with the Life's Simple 7 score.
The American Heart Association's (AHA's) Life's Simple 7 (LS7) score 1 has been well studied over the years, with numerous studies demonstrating its inverse association with multiple disease outcomes (cardiovascular disease [CVD], cardiometabolic disease, cancer, and death) and emphasizing the value of maintaining a good LS7 score throughout the life course. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 However, because of the significant scientific knowledge gained since 2010 on sleep and its beneficial impact on overall health, the AHA created an updated tool for assessing cardiovascular health (CVH), titled Life's Essential 8 (LE8). 15 LE8 is an incremental version of LS7 that includes an additional metric (sleep health) and a novel 100‐point scoring system for each of its 8 component factors. Furthermore, the new scoring system can be used at both the individual and the population level. The LE8 construct is relatively new, only a limited number of studies have investigated its association with disease outcomes, and fewer still have compared the relative prognostic utility of LE8 over the preceding LS7 score. A study using data from the National Health and Nutrition Examination Survey described the prevalence of CVH categories in the US population using the LE8 score. 16 In addition, Shetty et al compared the LS7 and LE8 scores in young adults, using National Health and Nutrition Examination Survey data, revealing a discrepancy in the CVH classification between the 2 scores, with nearly half of those classified as having ideal CVH by the LS7 score being reclassified as having intermediate or poor CVH using the LE8 score. 17 Moreover, recent studies have established an inverse relation between LE8 scores and the risk of major adverse cardiac events, 18 early vascular aging in individuals with obesity, 19 and metabolic fatty liver disease, 20 , 21 as well as CVD and all‐cause mortality. 22 , 23
We aimed to describe the prevalence of LE8 categories in individuals from the Framingham Offspring Cohort, during their second and sixth examination cycles, and to examine the potential incremental utility of sleep above and beyond that of the LS7 score. We hypothesized that the LE8 score is inversely related to the risk of CVD and all‐cause mortality, that the addition of sleep will offer additional incremental information on risk of CVD and death compared with LS7 alone, and that maintaining a high LE8 score across midlife is related to a lower risk of disease.
METHODS
All FHS (Framingham Heart Study) cohort data and materials have been made publicly available at the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repositories Information Coordinating Center at https://biolincc.nhlbi.nih.gov/studies/fhs/.
Study Samples
The selection criteria and design of the FHS have been well documented. The present investigation included participants from the Framingham Offspring Cohort who attended their second (1979–1982) and sixth (1995–1998) examination cycles, with available data on LE8 score, and who were free of clinical CVD at both examinations. These examination cycles were chosen for their relatively wide range of available data, facilitating consistent and accurate classification of LE8 scores. FHS attendees underwent routine physical and clinical examinations at each visit. See Figure 1 for details on study sample derivation. All participants provided written informed consent, and the protocol for this study was approved by the institutional review board of Boston University Medical Center.
Figure 1. Sample derivation.
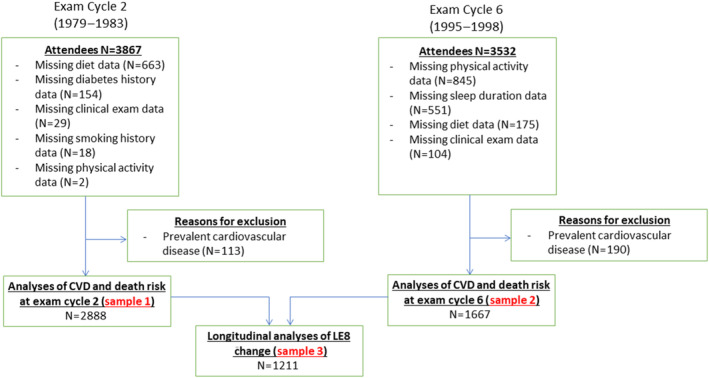
CVD indicates cardiovascular disease; Exam, examination; and LE8, Life's Essential 8.
LS7 and LE8 Scores
We have comprehensively defined the LS7 score using FHS data at multiple examinations and have reported the association of LS7 with different disease outcomes (Table S1). 2 , 6 , 12 , 24 , 25 , 26 For the present investigation, we defined LE8 using the guidelines reported by Lloyd‐Jones et al. 15 Specifically, the LE8 score is calculated on a 0 to 100 scale, where a participant is assigned a composite score based on his/her adherence to each of the 8 healthy lifestyle components: diet, physical activity, smoking habits, body mass index, cholesterol, fasting blood glucose, blood pressure, and sleep duration (Table S2). Sleep was defined as self‐reported average hours of sleep per night (Table S2). The final LE8 score is the average value of the points assigned to each of the 8 components. Four components (body mass index, diet, cholesterol, and blood pressure) match their definitions from the original LS7 score guidelines.
Physical activity at examination cycle 6 was calculated using the Paffenbarger formula to estimate the kilocalorie expenditure of each participant per week based on the number of times engaged in intense physical exercise, the number of flights of stairs climbed, and the number of city blocks walked per week. We classified participants into quantiles of physical activity by using kilocalorie expenditure and then used those quantiles to assign point values per AHA guidelines.
Because we did not have available data on time since quitting smoking at examination cycle 6, we used the “currently smoking” variable and the dates of the examination cycles to estimate the time since quitting smoking. For example, if a participant was known to be smoking at an examination dated 10 years earlier but was no longer smoking at a follow‐up examination 5 years earlier, we averaged the time difference between the 2 examinations with available information and estimated 7.5 years as the time since quitting. Once these estimates of time since quitting smoking were calculated, we classified participants into LE8 smoking categories based on the AHA guidelines.
Outcomes
Outcomes of interest included CVD (defined as incident coronary heart disease, stroke, heart failure, and peripheral arterial disease) and all‐cause mortality. A panel consisting of 3 experienced physicians reviewed all available medical records to adjudicate suspected CVD events using standardized FHS criteria. 27
Statistical Analysis
Cross‐Classification of Participants Using LS7 and LE8 Scores
We created binary indicator variables in both examination cycles 2 and 6 where participants were classified as having an LE8 score greater than/equal to (versus less than) the sample median at the respective examination cycle. Next, we used these categories to classify participants who were consistently healthy (ie, above the median LE8 score at both examination cycles 2 and 6; referent group) and labeled this group with a “high‐high” LE8 score, with the other groupings being “high‐low,” “low‐high,” and “low‐low” LE8 score.
In addition, we created 3‐level categories for LS7 and LE8 scores, assigning participants to the “ideal,” “intermediate,” or “poor” categories, separately for LS7 and LE8, based on their respective definitions. The LE8 status was defined as ideal if LE8≥80, intermediate if LE8 was 50 to 79, and poor if LE8<50. 16 The LS7 categories were defined as ideal (10–14), intermediate (5–9), and poor (0–4). 1
Associations of LS7 and LE8 With CVD and Death
We used Cox proportional hazards regression models to evaluate the association of LS7 and LE8 (both as a continuous and as a binary variable, separate model for each) with incidence of CVD and death (separate models for each outcome), adjusting for age and sex, after verifying that the proportional hazards assumptions were met. We evaluated these associations at 2 time points first to examine the LE8 score's prognostic value in older and younger populations, and second to evaluate change in cardiovascular health and its association with long‐term risk of CVD and death. We further explored the incremental utility of sleep, beyond that of the established LS7 score, by including the sleep variable in a Cox regression model with LS7, age, and sex. We evaluated effect modification of the association between sleep and risk of CVD and death by sex. We also used Fine‐Gray models to account for the competing risk of death when modeling time to CVD as the outcome. In addition, we evaluated the associations between the binary LE8 variables and CVD and death, also estimating Fine‐Gray models for CVD incidence. We also assessed relations of LE7 and LE8 with risk of CVD and death using a categorical variable (ideal, intermediate, and poor) for the CVH score (Tables S3 and S4) as well as further adjusting models for the change in CVH score between examinations 2 and 6 (Table S5). Finally, we used Kaplan‐Meier plots to depict the relation between LE8 groups and risk of CVD and death (Figure S1).
All P values were based on 2‐sided tests, with a value of <0.05 considered the threshold for statistical significance.
RESULTS
Table 1 displays the baseline characteristics of our study sample and the sex‐specific distributions of all components of the LE8 score, at the second and sixth Framingham Offspring Study examination cycles.
Table 1.
Baseline Characteristics and LE8 Component Distributions, by Sex
| Variable | Examination cycle 2 (1979–1982) | Examination cycle 6 (1995–1998) | ||
|---|---|---|---|---|
| Women (n=1497) | Men (n=1391) | Women (n=869) | Men (n=798) | |
| Age, y | 44±10 | 44±10 | 57±9 | 57±9 |
| LS7 | 9.2±2.1 | 8.6±2.1 | 8.6±2.3 | 7.9±2.1 |
| LE8 | 70±13 | 64±12 | 68±14 | 64±12 |
| PA component score, n (%) | ||||
| 100 | 156 (10) | 272 (20) | 111 (13) | 128 (16) |
| 90 | 145 (10) | 269 (19) | 127 (15) | 133 (17) |
| 80 | 211 (14) | 204 (15) | 112 (13) | 134 (17) |
| 60 | 233 (16) | 176 (13) | 126 (14) | 109 (14) |
| 40 | 258 (17) | 134 (10) | 126 (14) | 124 (16) |
| 20 | 260 (17) | 161 (12) | 132 (15) | 100 (13) |
| 0 | 234 (16) | 175 (13) | 135 (16) | 70 (9) |
| Mean±SD | 56±35 | 57±34 | ||
| BMI score, n (%) | ||||
| 100 | 999 (67) | 488 (35) | 350 (40) | 163 (20) |
| 70 | 341 (23) | 695 (50) | 311 (36) | 391 (49) |
| 30 | 97 (6) | 180 (13) | 131 (15) | 179 (22) |
| 15 | 42 (3) | 26 (2) | 45 (5) | 50 (6) |
| 0 | 18 (1) | 2 (<1) | 32 (4) | 15 (2) |
| Mean±SD | 80±25 | 67±30 | ||
| BP score, n (%) | ||||
| 100 | 800 (53) | 414 (30) | 317 (36) | 188 (24) |
| 80 | 10 (1) | 4 (<1) | 31 (4) | 35 (4) |
| 75 | 139 (9) | 151 (11) | 99 (11) | 92 (12) |
| 55 | 13 (1) | 6 (<1) | 20 (2) | 23 (3) |
| 50 | 313 (21) | 464 (33) | 160 (18) | 190 (24) |
| 30 | 42 (3) | 44 (3) | 48 (6) | 61 (8) |
| 25 | 98 (7) | 183 (13) | 82 (9) | 88 (11) |
| 5 | 43 (3) | 58 (4) | 58 (7) | 70 (9) |
| 0 | 39 (3) | 67 (5) | 54 (6) | 51 (6) |
| Mean±SD | 67±32 | 59±34 | ||
| Diabetes score, n (%) | ||||
| 100 | 1122 (75) | 760 (55) | 616 (71) | 402 (50) |
| 60 | 354 (24) | 580 (42) | 198 (23) | 329 (41) |
| 30 | 21 (1) | 51 (4) | 55 (6) | 67 (8) |
| Mean±SD | 85±21 | 82±23 | ||
| Cholesterol score, n (%) | ||||
| 100 | 565 (38) | 276 (20) | 246 (28) | 149 (19) |
| 80 | 0 | 0 | 10 (1) | 20 (3) |
| 60 | 414 (28) | 423 (30) | 249 (29) | 218 (27) |
| 40 | 309 (21) | 382 (27) | 195 (22) | 249 (31) |
| 20 | 131 (9) | 206 (15) | 107 (12) | 120 (15) |
| 0 | 78 (5) | 104 (7) | 62 (7) | 42 (5) |
| Mean±SD | 58±31 | 55±30 | ||
| Smoking score, n (%) | ||||
| 100 | 580 (39) | 435 (31) | 439 (51) | 424 (53) |
| 80 | 0 | 0 | 56 (6) | 48 (6) |
| 75 | 288 (19) | 396 (28) | 196 (23) | 166 (21) |
| 55 | 0 | 0 | 26 (3) | 32 (4) |
| 50 | 63 (4) | 65 (5) | 29 (3) | 23 (3) |
| 30 | 0 | 0 | 6 (1) | 6 (1) |
| 25 | 27 (2) | 15 (1) | 11 (1) | 3 (<1) |
| 5 | 0 | 0 | 1 (<1) | 0 |
| 0 | 539 (36) | 480 (35) | 105 (12) | 96 (12) |
| Mean±SD | 55±43 | 77±33 | ||
| Sleep score, n (%) | ||||
| 100 | 1122 (75) | 1044 (75) | 626 (72) | 551 (69) |
| 90 | 122 (8) | 50 (4) | 67 (8) | 52 (7) |
| 70 | 178 (12) | 227 (16) | 105 (12) | 147 (18) |
| 40 | 62 (4) | 62 (4) | 59 (7) | 40 (5) |
| 20 | 10 (1) | 7 (1) | 10 (1) | 6 (1) |
| 0 | 3 (<1) | 1 (<1) | 2 (<1) | 2 (<1) |
| Mean±SD | 92±17 | 90±19 | ||
| Diet (DASH) score, n (%) | ||||
| 100 | 91 (6) | 36 (3) | 59 (7) | 30 (4) |
| 80 | 410 (27) | 260 (19) | 250 (29) | 138 (17) |
| 50 | 362 (24) | 302 (22) | 253 (29) | 213 (27) |
| 25 | 341 (23) | 346 (25) | 185 (21) | 230 (29) |
| 0 | 293 (20) | 447 (32) | 122 (14) | 187 (23) |
| Mean±SD | 40±32 | 44±30 | ||
Values are mean±SD unless otherwise indicated. Component definitions are shown in Table S1. BMI indicates body mass index; BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; PA, physical activity; LE8, Life's Essential 8; and LS7, Life's Simple 7.
When we cross‐classified participants using binary LE8 scores at examination cycles 2 and 6, we observed that on follow‐up, only 40% started and remained in the high LE8 category, and 31% of participants did not manage to improve their low LE8 status (Table 2). When we included LS7 in the cross‐classification, of the 1197 participants classified with an ideal LS7 score (examination 2), 60% were classified as intermediate using the LE8 score at examination 2. Of the 1624 participants classified as intermediate (examination 2), 86% were classified in the same status, and 13% were classified as poor using the LE8 score at examination 2. When using examination cycle 6 data, we observed that among the 516 participants classified with an ideal LS7 score, almost half were classified as intermediate using the LE8 score; none of these participants were classified as having a poor LE8 score. Of the 1068 participants in the intermediate LS7 category, most remained intermediate when categorized by LE8 score, whereas 13% were reclassified as poor, and 2% were upwardly reclassified as ideal using the LE8 score (Table 3).
Table 2.
Cross‐Classification Using Serial LE8 Score Measures as Binary Variables
| Examination 2 LE8 score | Examination 6 LE8 score | |
|---|---|---|
| High (n=632 [52%]) | Low (n=579 [48%]) | |
| High (n=697 [58%]) | 488 (40) | 209 (17) |
| Low (n=514 [42%]) | 144 (12) | 370 (31) |
Data are given as number (percentage). High, above median LE8 score; low, below median LE8 score. Median examination 2 LE8 score=69, and median examination 6 LE8 score=66. LE8 indicates Life's Essential 8.
Table 3.
Cross‐Classification Using LS7 and LE8 Score 3‐Level Variables
| Examination cycle 2 (1979–1982) | Examination cycle 6 (1995–1998) | ||||||
|---|---|---|---|---|---|---|---|
| LS7 score | LE8 score | LS7 score | LE8 score | ||||
| Ideal (n=493 [17%]) | Intermediate (n=2120 [73%]) | Poor (n=275 [10%]) | Ideal (n=286 [17%]) | Intermediate (n=1174 [71%]) | Poor (n=207 [12%]) | ||
| Ideal (n=1197 [41%]) | 478 (40) | 718 (60) | 1 (<1) | Ideal (n=516 [31%]) | 268 (52) | 248 (48) | 0 |
| Intermediate (n=1624 [56%]) | 15 (1) | 1395 (86) | 214 (13) | Intermediate (n=1068 [64%]) | 18 (2) | 909 (85) | 141 (13) |
| Poor (n=67 [2%]) | 0 | 7 (10) | 60 (90) | Poor (n=83 [5%]) | 0 | 17 (20) | 66 (80) |
Data are given as number (percentage). LE8 indicates Life's Essential 8; and LS7, Life's Simple 7.
Two factors driving the significant amount of participants who were reclassified from ideal CVH (using LS7) to intermediate or lower (using LE8) were the updated definitions of the smoking and diet components in the LE8 score. For smoking, the LS7 assigns an ideal score for participants who have quit smoking at least 12 months before the index examination. However, the LE8 score is more stringent and classifies any history of smoking into the intermediate CVH category. For the diet component, the LE8 score used the Dietary Approaches to Stop Hypertension diet scoring metric, which is different than the LS7 diet scoring metric and is also scored at the population level using quantiles rather than at the individual level.
A third factor driving the difference in classification is the continuous 100‐point nature of LE8 versus the categorical nature of LS7. We observed that among the 719 participants who were reclassified from LS7 ideal to LE8 intermediate or poor, the average LE8 score was 74 (just 6 points from the ideal cutoff of 80 points). As the LE8 score is an average of the 8 component scores, participants who have mostly ideal scores in the lower ideal range (close to 80) can be pushed into an overall intermediate LE8 classification by 1 or 2 low‐scoring intermediate components. The LS7 score requires at least 5 separate components (total LS7 score of ≤9) to be intermediate to result in an overall intermediate LS7 score and is therefore more resistant to individual intermediate‐scoring components.
Associations of LE8 With CVD and Death
Using examination cycle 2 as the baseline (median follow‐up of 30 years for CVD and 33 years for death), we observed 966 CVD events on follow‐up (41% women), and 1195 participants died (44% women). Using examination cycle 6 as the baseline (median follow‐up of 18 years for CVD and 20 years for death), we observed 406 CVD events on follow‐up (43% women), and 485 participants died (43% women). LE8 (both as continuous and binary) was inversely associated with the risk of CVD and death, adjusting for age and sex at both examination cycles 2 and 6. The associations with the risk of CVD persisted when death was modeled as a competing risk (Tables 4 and 5). More importantly, when adding the sleep variable to a model including LS7, age, and sex, we observed that impaired sleep was significantly associated with a higher risk of CVD and death when using examination cycle 2 as the baseline (CVD: hazard ratio [HR], 0.993 [95% CI, 0.990–0.996]; P<0.001; death HR, 0.996 [95% CI, 0.993–0.999]; P=0.018); however, the change in the C‐statistic was minimal (Tables 4 and 5). We did not observe an association between sleep and the risk of CVD and death when using examination cycle 6 as the baseline (data not shown). Effect modification of the association between sleep and risk of CVD and death by sex was not present.
Table 4.
Associations of LS7 and LE8 With Incidence of CVD and All‐Cause Mortality, Examination Cycle 2 (1979–1982) as Baseline
| Outcome | CVD | C statistic | Mortality | C statistic | |||
|---|---|---|---|---|---|---|---|
| No. events/No. at risk | 966/2888 (33) | ||||||
| Model adjustments | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Continuous variables* | |||||||
| LS7 | Age, sex | 0.68 (0.64–0.73) | <0.001 | 0.74 | 0.72 (0.68–0.76) | <0.001 | 0.78 |
| LE8 | Age, sex | 0.64 (0.60–0.68) | <0.001 | 0.75 | 0.70 (0.66–0.74) | <0.001 | 0.78 |
| LS7 | Age, sex, sleep | 0.68 (0.64–0.73) | <0.001 | 0.75 | 0.72 (0.68–0.76) | <0.001 | 0.78 |
| LS7 | Age, sex, death competing risk | 0.71 (0.68–0.75) | <0.001 | 0.75 | … | … | … |
| LE8 | Age, sex, death competing risk | 0.68 (0.65–0.72) | <0.001 | 0.75 | … | … | … |
| Binary variables | |||||||
| Greater than median LS7* | Age, sex | 0.53 (0.47–0.61) | <0.001 | 0.74 | 0.58 (0.52–0.65) | <0.001 | 0.78 |
| Greater than median LE8* | Age, sex | 0.47 (0.41–0.54) | <0.001 | 0.74 | 0.55 (0.49–0.62) | <0.001 | 0.78 |
| Greater than median LS7* | Age, sex, sleep | 0.54 (0.47–0.61) | <0.001 | 0.74 | 0.58 (0.52–0.65) | <0.001 | 0.78 |
| Greater than median LS7* | Age, sex, death competing risk | 0.56 (0.51–0.62) | <0.001 | 0.74 | … | … | … |
| Greater than median LE8* | Age, sex, death competing risk | 0.53 (0.47–0.59) | <0.001 | 0.75 | … | … | … |
HRs are reported per 1‐SD increase in continuous LS7 and LE8 score. CVD indicates cardiovascular disease; HR, hazard ratio; LE8, Life's Essential 8; and LS7, Life's Simple 7.
Median examination 2 LS7 score=9; median examination 2 LE8 score=68.
Table 5.
Associations of LS7 and LE8 With Incidence of CVD and All‐Cause Mortality, Examination Cycle 6 (1995–1998) as Baseline
| Outcome | CVD | C statistic | Mortality | C statistic | |||
|---|---|---|---|---|---|---|---|
| No. events/No. at risk | 406/1667 (24) | ||||||
| Model adjustments | HR (95% CI) | P value | HR (95% CI) | P value | |||
| Continuous variables | |||||||
| LS7 | Age, sex | 0.79 (0.71–0.88) | <0.001 | 0.70 | 0.86 (0.79–0.95) | 0.002 | 0.77 |
| LE8 | Age, sex | 0.73 (0.66–0.81) | <0.001 | 0.71 | 0.80 (0.73–0.89) | <0.001 | 0.77 |
| LS7 | Age, sex, sleep | 0.79 (0.71–0.88) | <0.001 | 0.70 | 0.86 (0.79–0.95) | 0.002 | 0.77 |
| LS7 | Age, sex, death competing risk | 0.85 (0.78–0.92) | <0.001 | 0.72 | … | … | … |
| LE8 | Age, sex, death competing risk | 0.80 (0.74–0.86) | <0.001 | 0.73 | … | … | … |
| Binary variables | |||||||
| Greater than median LS7* | Age, sex | 0.71 (0.58–0.87) | 0.001 | 0.70 | 0.82 (0.68–0.98) | 0.03 | 0.77 |
| Greater than median LE8* | Age, sex | 0.66 (0.54–0.80) | <0.001 | 0.70 | 0.77 (0.64–0.92) | 0.004 | 0.77 |
| Greater than median LS7* | Age, sex, sleep | 0.71 (0.58–0.87) | 0.001 | 0.70 | 0.82 (0.68–0.99) | 0.03 | 0.77 |
| Greater than median LS7* | Age, sex, death competing risk | 0.78 (0.67–0.91) | 0.002 | 0.72 | … | … | … |
| Greater than median LE8* | Age, sex, death competing risk | 0.74 (0.64–0.87) | <0.001 | 0.72 | … | … | … |
HRs are reported per 1‐SD increase in continuous LS7 and LE8 score. CVD indicates cardiovascular disease; HR, hazard ratio; LE8, Life's Essential 8; and LS7, Life's Simple 7.
Median examination 6 LS7 score=8; median examination 6 LE8 score=66.
When evaluating the association of LE8 change over time with CVD and death, participants in the high‐high LE8 group were at the lowest risk of CVD, compared with all the other groups (Table 6 and Figure 2); all associations remained statistically significant when accounting for the competing risk of death. When modeling time to death, participants in the low‐low LE8 group were at higher risk compared with those in the high‐high LE8 group. More importantly, we did not observe a higher risk of death for those in the high‐low or low‐high LE8 groups, compared with the referent high‐high LE8 group (Table 6 and Figure 2).
Table 6.
Associations Between Serial LE8 Groups and Incidence of CVD and Death After Examination Cycle 6
| CVD incidence | No. events/No. at risk | HR (95% CI) | P value | HR (95% CI)* | P value* | No. events/No. at risk† | HR (95% CI)† | P value† |
|---|---|---|---|---|---|---|---|---|
| CVD | All‐cause mortality | |||||||
| LE8 score groups | Fine‐gray models | |||||||
| High‐high | 73/488 (15) | Referent | … | Referent | … | 112/488 (23) | Referent | … |
| High‐low | 57/209 (27) | 1.84 (1.30–2.60) | 0.001 | 1.79 (1.27–2.52) | 0.001 | 60/209 (29) | 1.15 (0.84–1.57) | 0.40 |
| Low‐high | 47/144 (33) | 2.31 (1.59–3.34) | <0.001 | 2.14 (1.47–3.11) | <0.001 | 40/144 (28) | 1.16 (0.80–1.67) | 0.43 |
| Low‐low | 134/370 (36) | 2.34 (1.75–3.13) | <0.001 | 2.23 (1.67–2.99) | <0.001 | 152/370 (41) | 1.45 (1.13–1.85) | 0.003 |
High, above median LE8 score; low, below median LE8 score. Median examination 2 LE8 score=69, and median examination 6 LE8 score=66. CVD indicates cardiovascular disease; HR, hazard ratio; and LE8, Life's Essential 8.
Fine‐Gray models with death as a competing risk.
Mortality models.
Figure 2. Kaplan‐Meier plot for change in LE8 vs death (A) and CVD (B).
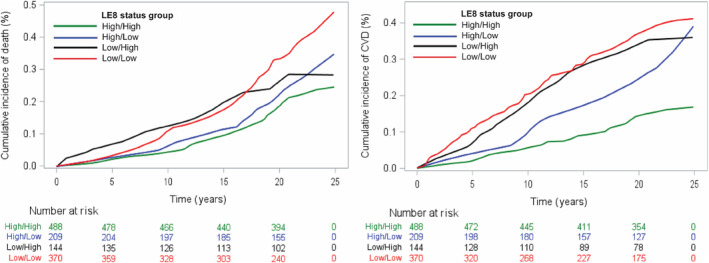
High, above median LE8 score; low, below median LE8 score. Median examination 2 LE8 score=69, and median examination 6 LE8 score=66. CVD indicates cardiovascular disease; and LE8, Life's Essential 8.
DISCUSSION
Principal Findings
The principal findings of our study were 3‐fold: (1) the LE8 score led to substantial changes in CVH classification compared with the LS7 score, mainly in individuals who were previously classified as having an ideal CVH using the LS7 score; (2) LE8 was inversely associated with the risk of CVD and mortality on long‐term follow‐up; and (3) maintaining a high LE8 score across examinations in midlife was associated with a lower risk of both CVD and death.
Comparison With the Published Literature
The LE8 score uses a new, 100‐point scoring system for each component, with the final score calculated as the unweighted mean of all 8 metrics. This modification was implemented to enhance the sensitivity of the score to variations in each metric, which could not be easily captured using the 3‐point scoring system of the LS7 score, thus increasing the informativeness of each individual's CVH score. Results from our investigation indicate that this modification in calculation of the CVH score has led to substantial changes in CVH status between the LS7 and LE8 scores, particularly among individuals who were previously classified as having optimal CVH using the LS7 score. Shetty et al also showed similar results among young adults in the United States, with 47% of individuals classified as having an ideal CVH score by LS7 being reclassified as having an intermediate or poor CVH score by LE8. 17 Notably, reclassification mainly affected individuals with an ideal LS7 CVH score in both studies, providing opportunities for preventive interventions in individuals previously thought to have optimal CVH.
Multiple studies over the years have demonstrated the benefits of a high LS7 score and the risks of multiple health outcomes associated with lower LS7 scores. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 However, the predictive value of the novel LE8 score has yet to be extensively investigated. A study using data from the UK Biobank reported that individuals whose LE8 scores were in the lowest quartile had a significantly higher risk of experiencing major adverse cardiac events compared with those whose scores were in the highest quartile (HR, 2.07 [95% CI, 1.99–2.16]). 18 Similarly, our study revealed that the LE8 score of individuals was inversely associated with the risk of CVD and mortality.
Although having an ideal CVH lowers the risk of CVD, maintaining it over the years may be crucial. This notion was established by numerous studies assessing the effect of longitudinal changes in an individual's LS7. 2 , 14 , 24 , 28 To our knowledge, our investigation is the first one to assess the value of maintaining a high LE8 score over time, by evaluating changes in the LE8 scores between examination cycles 2 (1979–1982) and 6 (1995–1998) of the Framingham Offspring Study participants. We observed that having an LE8 score below the median results in a higher risk of CVD regardless of whether individuals had an above median score in the past or attained one in the future. In addition, individuals with LE8 scores below the median at both examination cycles had a higher risk of death compared with individuals with LE8 scores above the median at both examination cycles.
Sleep has been demonstrated to be a crucial aspect of CVH, with both sleep duration and the presence of sleep disorders being linked to the risk of diabetes, hypertension, coronary artery disease, and stroke. 29 In our investigation, the sleep metric score was relatively high compared with other metrics, with a mean value of 92 (SD, 17) at examination cycle 2 and a mean value of 90 (SD, 19) at examination cycle 6. Adding the sleep component to a model with the LS7 score did not result in significant changes in the C‐statistic for models evaluating CVD and death. The demographic features of our study sample (predominantly White race and middle class) may explain the high sleep scores reported, as sleep duration is associated with both socioeconomic status and race and ethnicity. 30 However, another study that evaluated National Health and Nutrition Examination Survey data did not report any disparities in self‐reported sleep scores across socioeconomic groups. 16
It has been reported that sleep duration Is associated with all of the other 7 components of the LE8 score. 31 , 32 , 33 , 34 , 35 , 36 , 37 This observation may suggest that including sleep duration as an additional component in the score may not improve its ability to predict CVD and mortality when used in conjunction with the other LS7 metrics. However, a study of 1920 adults in the MESA (Multi‐Ethnic Study of Atherosclerosis) Sleep study reported that incorporating sleep duration as an eighth component of the LS7 score could enhance its prognostic information for incidence of CVD. 38 The MESA Sleep study used objectively measured sleep duration, whereas previous studies, including ours, relied on self‐reported sleep data.
The diet metric of the LS7 score, as noted by Lloyd‐Jones et al, had a narrow focus on the intake of only 5 dietary components, making it challenging for individuals to achieve an ideal score even when following the guidance of a clinician. 15 This was evidenced by the AHA's “Heart Disease and Stroke Statistics” report, which revealed that only 0.2% of individuals aged >20 years in the United States achieved an ideal score for diet in the LS7 score. 39 To address this limitation, the diet metric of the LE8 score was developed to more accurately reflect the overall healthiness of an individual's diet, rather than being restricted to the 5 nutrients that were the focus of the LS7 score. However, we observed that the diet component remains the lowest‐scoring LE8 metric among individuals, with only a small fraction achieving an ideal score for this LE8 metric at both examination cycles.
Strengths and Limitations
The strengths of the current investigation include the large community‐based sample, the availability of longitudinal information on all 8 components of the LE8 score, and a long follow‐up period. Several limitations merit consideration. The sample population consisted primarily of middle‐aged individuals of European descent with a high prevalence of hypertension, which may limit the generalizability of the findings to other age groups and ethnicities with varying risk factor distributions. Furthermore, at examination cycle 2, we defined the physical activity component using a different standard, because 92% of participants self‐reported ≥150 minutes of moderate‐to‐intense physical activity per week, with the remaining 8% reporting no physical activity; therefore, we used the Physical Activity Index, which we then used to classify participants into quantiles, and then used these quantiles to assign point values according to AHA guidelines.
Similarly, we were not able to strictly adhere to AHA guidelines when calculating the smoking exposure component for LE8 CVH scores. AHA guidelines classify former smoking in individuals who have quit 1 to 4 years before in the 50‐point category and former smoking in individuals with <1 year since quitting into the 25‐point category. However, data at examination cycle 2 include “years since quitting smoking” in discrete year values, and, therefore, we could not classify any participants into the 25‐point category. We adjusted our scoring system to assign 50 points to participants who had quit 2 to 4 years before an examination and 25 points to those who have quit for 1 year preceding an FHS examination. In addition, as the examination cycle 2 questionnaire did not include secondhand smoking status, we had to omit the 20‐point penalty for secondhand smoke exposure included in the AHA guidelines (Table S1).
Last, we did not have available data on hemoglobin A1c at either examination cycle 2 or examination cycle 6; therefore, we assigned all participants with diabetes a score of 30 points.
CONCLUSIONS
In our community‐based sample, we observed inverse associations between the LE8 score and the risk of CVD and death. Of importance, participants who maintained their high LE8 score during the life course were at the lowest risk of CVD, compared with other groups, and those who maintained a low LE8 score were at higher risk of death compared with those who maintained a high LE8 score during the life course. Further studies are warranted to elucidate whether the LE8 score is a better marker of CVD and death risk, compared with the LS7 score.
Sources of Funding
This work is supported by contracts NO1‐HC‐25195, HHSN268201500001I, and 75N92019D00031 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr Mitchell is the owner of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. He also serves as a consultant to and receives grants and honoraria from Novartis, Merck, Bayer, Servier, Philips, and deCODE genetics. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S5
Figure S1
This article was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030764
For Sources of Funding and Disclosures, see page 10.
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 2. Corlin L, Short MI, Vasan RS, Xanthakis V. Association of the duration of ideal cardiovascular health through adulthood with cardiometabolic outcomes and mortality in the Framingham Offspring Study. JAMA Cardiol. 2020;5:549–556. doi: 10.1001/jamacardio.2020.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. doi: 10.1001/jama.2012.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li N, Bian L, Wu J, Jia Q, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. doi: 10.1161/STROKEAHA.113.678839 [DOI] [PubMed] [Google Scholar]
- 5. Lachman S, Peters RJ, Lentjes MA, Mulligan AA, Luben RN, Wareham NJ, Khaw KT, Boekholdt SM. Ideal cardiovascular health and risk of cardiovascular events in the EPIC‐Norfolk Prospective Population Study. Eur J Prev Cardiol. 2016;23:986–994. doi: 10.1177/2047487315602015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nayor M, Enserro DM, Vasan RS, Xanthakis V. Cardiovascular health status and incidence of heart failure in the Framingham Offspring Study. Circ Heart Fail. 2016;9:e002416. doi: 10.1161/CIRCHEARTFAILURE.115.002416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health during adult life and cardiovascular structure and function among the elderly. Circulation. 2015;132:1979–1989. doi: 10.1161/CIRCULATIONAHA.115.017882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaye B, Mustafic H, Laurent S, Perier MC, Thomas F, Guibout C, Tafflet M, Pannier B, Boutouyrie P, Jouven X, et al. Ideal cardiovascular health and subclinical markers of carotid structure and function: the Paris Prospective Study III. Arterioscler Thromb Vasc Biol. 2016;36:2115–2124. doi: 10.1161/ATVBAHA.116.307920 [DOI] [PubMed] [Google Scholar]
- 9. Robbins JM, Petrone AB, Carr JJ, Pankow JS, Hunt SC, Heiss G, Arnett DK, Ellison RC, Gaziano JM, Djoussé L. Association of ideal cardiovascular health and calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Am Heart J. 2015;169:371–378. doi: 10.1016/j.ahj.2014.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bundy JD, Ning H, Zhong VW, Paluch AE, Lloyd‐Jones DM, Wilkins JT, Allen NB. Cardiovascular health score and lifetime risk of cardiovascular disease: the cardiovascular lifetime risk pooling project. Circ Cardiovasc Qual Outcomes. 2020;13:e006450. doi: 10.1161/CIRCOUTCOMES.119.006450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen NB, Krefman AE, Labarthe D, Greenland P, Juonala M, Kähönen M, Lehtimäki T, Day RS, Bazzano LA, Van Horn LV, et al. Cardiovascular health trajectories from childhood through middle age and their association with subclinical atherosclerosis. JAMA Cardiol. 2020;5:557–566. doi: 10.1001/jamacardio.2020.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. doi: 10.1161/CIRCULATIONAHA.114.009273 [DOI] [PubMed] [Google Scholar]
- 13. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan Study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laitinen TT, Pahkala K, Magnussen CG, Oikonen M, Viikari JS, Sabin MA, Daniels SR, Heinonen OJ, Taittonen L, Hartiala O, et al. Lifetime measures of ideal cardiovascular health and their association with subclinical atherosclerosis: the cardiovascular risk in young Finns Study. Int J Cardiol. 2015;185:186–191. doi: 10.1016/j.ijcard.2015.03.051 [DOI] [PubMed] [Google Scholar]
- 15. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's Essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's New “Life's Essential 8” metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/CIRCULATIONAHA.122.060911 [DOI] [PubMed] [Google Scholar]
- 17. Shetty NS, Parcha V, Patel N, Yadav I, Basetty C, Li C, Pandey A, Kalra R, Li P, Arora G, et al. AHA Life's Essential 8 and ideal cardiovascular health among young adults. Am J Prev Cardiol. 2023;13:100452. doi: 10.1016/j.ajpc.2022.100452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petermann‐Rocha F, Deo S, Celis‐Morales C, Ho FK, Bahuguna P, McAllister D, Sattar N, Pell JP. An opportunity for prevention: associations between the Life's Essential 8 score and cardiovascular incidence using prospective Data from UK biobank. Curr Probl Cardiol. 2022;48:101540. doi: 10.1016/j.cpcardiol.2022.101540 [DOI] [PubMed] [Google Scholar]
- 19. Cunha MR, Mattos S, Klein M, Neves MF. Early vascular aging in obese individuals with low cardiovascular health. High Blood Press Cardiovasc Prev. 2022;30:45–54. doi: 10.1007/s40292-022-00555-0 [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Wang A, Zhang R, Cheng S, Pang Y. Life's Essential 8 and MAFLD in the United States. J Hepatol. 2022;78:e61–e63. doi: 10.1016/j.jhep.2022.10.014 [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Yi J, Guo X, Ren X. Associations between Life's Essential 8 and non‐alcoholic fatty liver disease among US adults. J Transl Med. 2022;20:616. doi: 10.1186/s12967-022-03839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun J, Li Y, Zhao M, Yu X, Zhang C, Magnussen CG, Xi B. Association of the American Heart Association's new “Life's Essential 8” with all‐cause and cardiovascular disease‐specific mortality: prospective cohort study. BMC Med. 2023;21:116. doi: 10.1186/s12916-023-02824-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isiozor NM, Kunutsor SK, Voutilainen A, Laukkanen JA. Life's Essential 8 and the risk of cardiovascular disease death and all‐cause mortality in Finnish men. Eur J Prev Cardiol. 2023;30:658–667. doi: 10.1093/eurjpc/zwad040 [DOI] [PubMed] [Google Scholar]
- 24. Enserro DM, Vasan RS, Xanthakis V. Twenty‐year trends in the American Heart Association cardiovascular health score and impact on subclinical and clinical cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008741. doi: 10.1161/JAHA.118.008741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peloso GM, Beiser AS, Satizabal CL, Xanthakis V, Vasan RS, Pase MP, Destefano AL, Seshadri S. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology. 2020;95:e1341–e1350. doi: 10.1212/WNL.0000000000010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bourdillon MT, Gaye B, Song RJ, Vasan RS, Xanthakis V. Notable paradoxical phenomena in associations between cardiovascular health score, subclinical and clinical cardiovascular disease in the community: the Framingham Heart Study. PLoS ONE. 2022;17:e0267267. doi: 10.1371/journal.pone.0267267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kannel W, Wolf P, Garrison R. The Framingham Heart Study, Section 34: An Epidemiological Investigation of Cardiovascular Disease: Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements: 30‐Year Follow‐Up. National Institutes of Health; Publication No 87‐2703; 1987. [Google Scholar]
- 28. Yang X, Wang A, Liu X, An S, Chen S, Wang Y, Wang Y, Wu S. Positive changes in ideal CVH metrics reduce the incidence of stroke. Sci Rep. 2016;6:19673. doi: 10.1038/srep19673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. St‐Onge M‐P, Grandner MA, Brown D, Conroy MB, Jean‐Louis G, Coons M, Bhatt DL. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37:601–611. doi: 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Godos J, Grosso G, Castellano S, Galvano F, Caraci F, Ferri R. Association between diet and sleep quality: a systematic review. Sleep Med Rev. 2021;57:101430. doi: 10.1016/j.smrv.2021.101430 [DOI] [PubMed] [Google Scholar]
- 32. De Nys L, Anderson K, Ofosu EF, Ryde GC, Connelly J, Whittaker AC. The effects of physical activity on cortisol and sleep: a systematic review and meta‐analysis. Psychoneuroendocrinology. 2022;143:105843. doi: 10.1016/j.psyneuen.2022.105843 [DOI] [PubMed] [Google Scholar]
- 33. Cohrs S, Rodenbeck A, Riemann D, Szagun B, Jaehne A, Brinkmeyer J, Gründer G, Wienker T, Diaz‐Lacava A, Mobascher A, et al. Impaired sleep quality and sleep duration in smokers‐results from the German Multicenter Study on nicotine dependence. Addict Biol. 2014;19:486–496. doi: 10.1111/j.1369-1600.2012.00487.x [DOI] [PubMed] [Google Scholar]
- 34. Wu Y, Zhai L, Zhang D. Sleep duration and obesity among adults: a meta‐analysis of prospective studies. Sleep Med. 2014;15:1456–1462. doi: 10.1016/j.sleep.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 35. Kaneita Y, Uchiyama M, Yoshiike N, Ohida T. Associations of usual sleep duration with serum lipid and lipoprotein levels. Sleep. 2008;31:645–652. doi: 10.1093/sleep/31.5.645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaput JP, Després JP, Bouchard C, Tremblay A. Association of sleep duration with type 2 diabetes and impaired glucose tolerance. Diabetologia. 2007;50:2298–2304. doi: 10.1007/s00125-007-0786-x [DOI] [PubMed] [Google Scholar]
- 37. Lo K, Woo B, Wong M, Tam W. Subjective sleep quality, blood pressure, and hypertension: a meta‐analysis. J Clin Hypertens (Greenwich, Conn). 2018;20:592–605. doi: 10.1111/jch.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Makarem N, Castro‐Diehl C, St‐Onge MP, Redline S, Shea S, Lloyd‐Jones D, Ning H, Aggarwal B. Redefining cardiovascular health to include sleep: prospective associations with cardiovascular disease in the MESA Sleep Study. J Am Heart Assoc. 2022;11:e025252. doi: 10.1161/JAHA.122.025252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figure S1


