Abstract
Background
Whether statin use can reduce the risk of heart failure (HF) remains controversial. The present study evaluates the association between statin use and HF in patients with atrial fibrillation.
Methods and Results
Patients with newly diagnosed atrial fibrillation from 2010 to 2018 were included. An inverse probability of treatment weighting was used to balance baseline covariates between statin users (n=23 239) and statin nonusers (n=29 251). The primary outcome was incident HF. Cox proportional hazard models with competing risk regression were used to evaluate the risk of HF between statin users and nonusers. The median age of the cohort was 74.7 years, and 47.3% were women. Over a median follow‐up of 5.1 years, incident HF occurred in 3673 (15.8%) statin users and 5595 (19.1%) statin nonusers. Statin use was associated with a 19% lower risk of HF (adjusted subdistribution hazard ratio, 0.81 [95% CI, 0.78–0.85]). Restricted to the statin users, duration of statin use was measured during follow‐up; compared with short‐term use (3 months to <2 years), there was a stepwise reduction in the risk of incident HF among those with 2 to <4 years of statin use (subdistribution hazard ratio, 0.86 [95% CI, 0.84–0.88]), 4 to <6 years of statin use (subdistribution hazard ratio, 0.74 [95% CI, 0.72–0.76]), and ≥6 years of statin use (subdistribution hazard ratio, 0.71 [95% CI, 0.69–0.74]). Subgroup analysis showed consistent reductions in the risk of HF with statin use.
Conclusions
Statin use was associated with a decreased risk of incident HF in a duration‐dependent manner among patients with atrial fibrillation.
Keywords: atrial fibrillation, heart failure, low‐density lipoprotein cholesterol, population‐based cohort study, statins
Subject Categories: Atrial Fibrillation, Big Data and Data Standards, Heart Failure
Nonstandard Abbreviations and Acronyms
- GIB
gastrointestinal bleeding
- IPTW
inverse probability of treatment weighting
Clinical Perspective.
What Is New?
Statin therapy is associated with reduced risk of heart failure (HF), HF‐related death, and all‐cause mortality among patients with atrial fibrillation.
This inverse association is independent of low‐density lipoprotein cholesterol levels and consistent across all subgroups.
The association between statin use and lower risk of HF is duration dependent.
What Are the Clinical Implications?
Our study provides evidence on the relationship between statin use and HF in patients with atrial fibrillation through thorough consideration of potential confounding and biases achieved by propensity score analytics.
These findings reveal low‐density lipoprotein cholesterol control as a potential target and provide a crucial rationale for exploring lipid‐lowering therapies in preventing HF in patients with atrial fibrillation.
The association between statin use and lower all‐cause mortality shown in our data, consistent with other observations, may reflect an overall beneficial impact on adverse cardiovascular outcomes.
The aging population, alongside the growing prevalence of chronic diseases such as hypertension and diabetes, is driving the epidemic of atrial fibrillation (AF) globally. 1 Heart failure (HF) is a significant complication of AF, regardless of preexisting cardiovascular comorbidities, 2 , 3 with ≈3 times the risk compared with those without AF. 4 , 5 , 6 , 7 Indeed, HF is becoming a leading cause of hospitalization and nonfatal cardiovascular events in patients with AF, 8 which worsens prognosis in patients with AF. 9 , 10 , 11 Thus, there is a pressing need for preventive strategies to mitigate the burden of HF in patients with AF.
Statin use may reduce the risk of HF rehospitalization and improve survival in patients with preexisting HF and coronary artery disease (CAD). 12 , 13 Two randomized studies, however, failed to show a protective effect of statins against HF. 14 , 15 Although the role of statins in preventing HF remains controversial, 12 , 13 , 14 , 15 1 of the proposed mechanisms by which statins may reduce the development of HF is through preventing CAD and myocardial infarction (via lowering low‐density lipoprotein cholesterol [LDL‐C]). 16 The pleiotropic effects of statins, including anti‐inflammatory, antioxidative, 17 and antithrombotic properties, 18 may play a role in reducing HF. 19 These mechanisms may modulate the shared pathologic milieu between HF and AF, 20 , 21 which places statin use as a potential preventive strategy to alleviate the HF burden in the population with AF.
Using a territory‐wide population with well‐validated and prospectively updated data, the present study sought to evaluate the association between statin use and the risk of incident HF in the population with AF.
Methods
For legal reasons, study data cannot be made available to other researchers.
This retrospective cohort study was conducted with data retrieved from the Clinical Data Analysis Reporting System (CDARS), a territory‐wide database developed by the Hong Kong Hospital Authority, the sole provider of public health care in Hong Kong. CDARS documents the clinical records of 87% to 94% of inpatient and outpatient services of the local population of 7.5 million. Diagnoses were determined using the International Classification of Diseases, Ninth Revision (ICD‐9), and previous validation exercises have shown a high degree of coding accuracy. 22 , 23 , 24 The study has been approved by the institutional review board of the University of Hong Kong and the West Cluster of the Hong Kong Hospital Authority. Informed consent was waived because of the anonymized nature of the data.
Study Population
We included all patients, aged ≥18 years, with newly diagnosed AF from January 1, 2010, to December 31, 2018, using the ICD‐9 code 427.31. 25 Newly diagnosed AF was defined as patients without a previous diagnosis of AF at any time in CDARS. We used a look‐back period until January 1, 1993. The index date was defined as the date of the first diagnosis of AF. We excluded patients with rheumatic heart disease, or previous valve surgery at the time of AF diagnosis, as well as those who died within 90 days after the index date. We also excluded patients who had diagnoses/procedures closely related to transient AF (myocarditis, pericarditis, pulmonary embolism, and cardiac surgery) within 90 days before the index date. To allow the evaluation of incident HF as our primary outcome, we excluded patients with prior HF history or HF within 90 days after the index date. The study flowchart is shown in Figure S1.
Baseline Information
For each patient, we collected baseline information, including age at index date, sex, smoking, CHA2DS2‐VASc (congestive HF, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65–74 years, and sex category [female]) score, AF duration (from the date of first AF diagnosis to the follow‐up end date), and comorbidities (hypertension, diabetes, ischemic stroke, transient ischemic attack, CAD, chronic kidney disease, peripheral vascular diseases, venous thromboembolism, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, ankylosing spondylitis, liver cirrhosis, anemia, cancer, gastrointestinal bleeding [GIB], dyslipidemia, and obesity) at any time before the index date. We also collected medication history (baseline use of non–vitamin K antagonist oral anticoagulant, warfarin, aspirin, angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker [ACEI/ARB], β‐blocker, and statin) within 1 year after the index date, which was defined as >30 days of consecutive use. 26 Procedures (AF ablation and cardioversion) within 1 year after the index date were also collected. Detailed ICD‐9 codes are summarized in Table S1.
Exposure Definition
Our study adopted an intention‐to‐treat design, where exposure to statin therapy was defined as ≥90 days of consecutive statin use beginning within the first year of the index date. 22 Patients who used statins for <90 days within the first year after the index date were excluded (n=1026). Statins available during the study period include simvastatin, atorvastatin, and rosuvastatin. Consequently, 23 239 statin users and 29 251 statin nonusers were identified (Figure S1). To assess duration response, statin use was modeled as a time‐varying exposure. We summed the duration of all filled prescriptions (in days) and updated these data at each yearly interval of follow‐up. For each statin user, we confirmed that the number of tablets dispensed in each visit is congruent with the period to the next statin prescription, with refilling at each visit (when the patient has presumably taken all tablets dispensed from the last). Moreover, patients who have not been prescribed statins for 30 days consecutively are considered to have discontinued statin therapy.
Outcome
The primary outcome was incident heart failure (ICD‐9 codes 402, 404, 425, and 428) after the diagnosis of AF. 22 Secondary outcomes were HF‐related death and all‐cause mortality. HF‐related death was defined as patients with HF as the primary death cause. Follow‐up was started at 1 year after the index date. Patients were followed up until the occurrence of HF, death, or the study end date on October 31, 2021, whichever was sooner.
Data Validation
Because patient information is anonymous in CDARS, we validated 200 patients with diagnosis of HF using the electronic medical record system at our center (Queen Mary Hospital), of whom 197 (98.5%) had a confirmed diagnosis. This high degree of accuracy of using ICD‐9 diagnosis codes in CDARS has been extensively validated. 22 , 23 , 24
Statistical Analysis
Continuous variables were presented as means with SD, and categorical variables were presented as counts with proportion. Differences between groups were compared using the t test for continuous variables and the χ2 test for categorical variables. Incidence rates were calculated as the number of events per 100 person‐years of follow‐up.
To avoid bias attributable to treatment selection caused by a lack of randomization, a propensity score approach was used. All aforementioned covariates were logistically regressed to the probability of receiving treatment. An inverse probability of treatment weighting (IPTW) was used to generate a pseudopopulation in which individuals were assigned weights that corresponded to the inverse of their probability of receiving treatment given observed covariates. 27 Covariates were considered balanced between statin users and nonusers if the standardized mean difference was ≤0.10.
Cox proportional hazards models were used to evaluate the association between statin use and the risk of HF. Fine‐Gray regression was applied to account for competing risks, with all‐cause mortality defined as the competing event. To evaluate the duration‐dependent association between statin use and study outcomes, we further entered the use of statins during follow‐up into the Fine‐Gray model as a time‐varying variable to minimize the risk of immortal time bias.
To evaluate the associations between LDL‐C levels and HF risk, the time‐weighted mean of LDL‐C was calculated on the basis of LDL‐C levels measured at least 3 months following statin initiation for statin users and LDL‐C levels measured after the index date for statin nonusers until study end point. 22 Restricted cubic splines were used to characterize the association between LDL‐C (as a continuous variable) and incident HF. Patients were subsequently categorized into 3 groups based on the time‐weighted mean of LDL‐C: <1.8, 1.8 to 2.6, and >2.6 mmol/L. Subgroup analysis was performed according to age (<75 and ≥75 years), sex, hypertension, diabetes, CAD, smoking, LDL‐C levels (<1.8, 1.8–2.6, and >2.6 mmol/L), and use of anticoagulants, aspirin, ACEI/ARB, and β‐blockers. Tests for interaction were also conducted.
We conducted various sensitivity analyses to assess the robustness of our study findings as follows: (1) conventional Cox regression analyses without competing risk or IPTW were performed to allow comparison with previous studies 12 , 21 ; (2) adopting an alternative 1:1 propensity score–matched design; (3) excluding patients with a history of statin use (n=19 369); and (4) analyses of the association between statin use and incident GIB (excluding those who with previous GIB history) as a falsification end point. The E‐value, which provides information on the strength of unmeasured confounders required to invalidate results, was also reported. 28 A 2‐tailed P<0.05 was considered statistically significant. Data management was performed using R, version 3.2.2 (R Foundation).
Results
We identified 52 490 newly diagnosed patients with AF (mean [SD] age, 74.7 [12.2] years; 47.3% women) between 2010 and 2018, of whom 29 251 were statin nonusers and 23 239 were statin users. Over a median follow‐up of 5.06 (interquartile range, 5.01–5.10) years, incident HF occurred in 9268 (17.7%) patients: 3673 (15.8%) and 5595 (19.1%) in statin users and statin nonusers, respectively (Table S2). The proportions with diabetes, hypertension, ischemic stroke, transient ischemic attack, CAD, chronic kidney disease, peripheral vascular disease, dyslipidemia, obesity, and baseline medications, including non–vitamin K antagonist oral anticoagulant, warfarin, aspirin, β‐blockers, and ACEI/ARB were consistently higher in statin users compared with statin nonusers (Table 1). Baseline characteristics were well balanced after matching on IPTW (Table S3).
Table 1.
Baseline Characteristics
| Characteristic | Overall | Statin nonusers | Statin users | Standardized mean difference before IPTW | Standardized mean difference after IPTW |
|---|---|---|---|---|---|
| (N=52 490) | (n=29 251) | (n=23 239) | |||
| Age, mean (SD), y | 74.74 (12.21) | 74.77 (13.25) | 74.71 (10.75) | 0.005 | 0.005 |
| Female sex, N (%) | 24 833 (47.3) | 13 956 (47.7) | 10 877 (46.8) | 0.018 | 0.002 |
| Smoking, N (%) | 3650 (7.0) | 2304 (7.9) | 1346 (5.8) | 0.083 | 0.004 |
| CHA2DS2‐VASc score, mean (SD) | 2.82 (1.62) | 2.58 (1.53) | 3.12 (1.67) | 0.333 | 0.006 |
| AF duration, mean (SD), y | 4.70 (3.12) | 4.75 (3.27) | 4.65 (2.92) | 0.033 | <0.001 |
| Medical conditions, N (%) | |||||
| Diabetes | 21 551 (41.1) | 9861 (33.7) | 11 690 (50.3) | 0.341 | 0.007 |
| Hypertension | 13 529 (25.8) | 6298 (21.5) | 7231 (31.1) | 0.219 | 0.004 |
| Ischemic stroke | 6052 (11.5) | 1957 (6.7) | 4095 (17.6) | 0.339 | 0.004 |
| TIA | 1215 (2.3) | 395 (1.4) | 820 (3.5) | 0.142 | 0.005 |
| CAD | 6858 (13.1) | 2393 (8.2) | 4465 (19.2) | 0.325 | 0.022 |
| CKD | 1908 (3.6) | 873 (3.0) | 1035 (4.5) | 0.078 | 0.004 |
| PVD | 1944 (3.7) | 722 (2.5) | 1222 (5.3) | 0.145 | 0.003 |
| VTE | 470 (0.9) | 255 (0.9) | 215 (0.9) | 0.006 | <0.001 |
| Autoimmune diseases* | 7188 (13.7) | 4092 (14.0) | 3096 (13.3) | 0.015 | 0.003 |
| Liver cirrhosis | 318 (0.6) | 246 (0.8) | 72 (0.3) | 0.070 | <0.001 |
| Anemia | 4718 (9.0) | 2801 (9.6) | 1917 (8.3) | 0.047 | 0.004 |
| Cancer | 8054 (15.4) | 4589 (15.7) | 3465 (14.9) | 0.022 | 0.001 |
| GIB | 5763 (11.0) | 3360 (11.5) | 2403 (10.3) | 0.037 | 0.003 |
| Dyslipidemia | 4656 (8.9) | 1263 (4.3) | 3393 (14.6) | 0.357 | 0.018 |
| Obesity | 407 (0.8) | 150 (0.5) | 257 (1.1) | 0.066 | 0.002 |
| Medication use, N (%) | |||||
| NOAC | 22 198 (42.3) | 10 276 (35.1) | 11 922 (51.3) | 0.331 | 0.006 |
| Warfarin | 11 047 (21.0) | 5451 (18.6) | 5596 (24.1) | 0.133 | 0.006 |
| Aspirin | 23 039 (43.9) | 9933 (34.0) | 13 106 (56.4) | 0.463 | 0.003 |
| β‐Blockers | 15 807 (30.1) | 7879 (26.9) | 7928 (34.1) | 0.156 | 0.002 |
| ACEI/ARB | 24 137 (46.0) | 10 656 (36.4) | 13 481 (58.0) | 0.443 | 0.004 |
| Procedure, N (%) | |||||
| Ablation | 307 (0.6) | 185 (0.6) | 122 (0.5) | 0.014 | <0.001 |
| Cardioversion | 204 (0.4) | 129 (0.4) | 75 (0.3) | 0.019 | 0.001 |
A variable is considered balanced between users and nonusers where the standardized mean difference is ≤0.1. ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker; AF, atrial fibrillation; CAD, coronary artery disease; CHA2DS2‐VASc, (congestive HF, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65–74 years, and sex category [female]) score; CKD, chronic kidney disease; GIB, gastrointestinal bleeding; IPTW, inverse probability of treatment weighting; NOAC, non–vitamin K antagonist oral anticoagulant; PVD, peripheral vascular disease; TIA, transient ischemic attack; and VTE, venous thromboembolism.
Autoimmune diseases include rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, and ankylosing spondylitis.
Use of Statin and HF
The incidence rate of HF in the entire study population was 3.75 per 100 person‐years, whereas the rates in statin users and statin nonusers were 3.40 and 4.03, respectively. The cumulative incidence curves for statin users versus nonusers are shown in Figure 1. After IPTW, statin users had a 19% lower risk of incident HF compared with the nonusers (subdistribution hazard ratio [SHR], 0.81 [95% CI, 0.78–0.85]; P<0.001) (Table 2). The incidence rate of HF‐related death was 0.66 per 100 person‐years, corresponding to 0.61 and 0.69 in statin users and statin nonusers, respectively (Table S2). Statin users had a 16% lower risk of HF‐related death compared with nonusers (SHR, 0.84 [95% CI, 0.74–0.94]; P<0.001) after multivariable adjustment (Table 2).
Figure 1. Cumulative incidence of heart failure between statin users and nonusers.
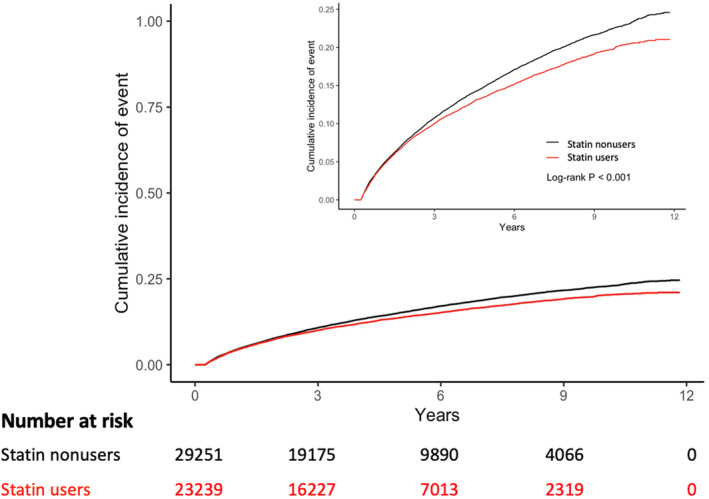
We calculated the P value using log‐rank test for equality of the cumulative functions between each exposure group after inverse probability of treatment weighting, accounting for competing risks of all‐cause mortality. The inset shows the same data on an expanded y axis.
Table 2.
Effect of Statin Use on the Risk of Incident HF and HF‐Related Death
| Variable | Events, N (%) | Incident rate | Unadjusted SHR (96% CI) P value | Adjusted SHR (96% CI) P value* |
|---|---|---|---|---|
| Incident HF | ||||
| Statin nonusers (N=29 251) | 5595 (19.1) | 4.03 | 1.00 (Reference) | 1.00 (Reference) |
| Statin users (N=23 239)† | 3673 (15.8) | 3.40 |
0.84 (0.81–0.87) <0.001 |
0.81 (0.78–0.85) <0.001 |
| HF‐related death | ||||
| Statin nonusers (N=29 251) | 959 (3.3) | 0.69 | 1.00 (Reference) | 1.00 (Reference) |
| Statin users (N=23 239) | 661 (2.8) | 0.61 |
0.85 (0.76–0.95) 0.003 |
0.84 (0.74–0.94) <0.001 |
HF indicates heart failure; and SHR, subdistribution hazard ratio.
A multivariable‐adjusted model further accounted for the following prognostic covariates: age at index date, sex, smoking, CHA2DS2‐VASc score (congestive HF, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65–74 years, and sex category [female]) score, atrial fibrillation duration, comorbidities, including diabetes, hypertension, ischemic stroke, transient ischemic attack, coronary artery disease, chronic kidney disease, peripheral vascular disease, venous thromboembolism, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, ankylosing spondylitis, liver cirrhosis, anemia, cancer, gastrointestinal bleeding, dyslipidemia, and obesity, and baseline use of non–vitamin K antagonist oral anticoagulant, warfarin, aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, ablation, and cardioversion.
Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date. Hazard estimates were obtained with the use of a proportional subdistribution hazards regression model fit to the inverse probability of treatment weighted cohort that accounted for competing risk; the model was conditioned on age at index date.
LDL‐C Levels and HF
Among the entire cohort, 48 558 (92.5%) patients had time‐weighted mean of LDL‐C, in which 9424 (19.4%) were categorized as LDL‐C <1.8 mmol/L, 19 045 (39.2%) as LDL‐C 1.8 to 2.6 mmol/L, and 20 089 (41.4%) as LDL‐C >2.6 mmol/L. When LDL‐C was treated as a continuous variable, every 1‐mmol/L increase in LDL‐C was associated with a 7% increased risk of incident HF (SHR, 1.07 [95% CI, 1.05–1.09]; P<0.001); and the restricted cubic splines analysis showed a similar pattern (Figure S2). Compared with an LDL‐C <1.8 mmol/L, LDL‐C 1.8 to 2.6 mmol/L had a similar risk of HF (SHR, 1.03 [95% CI, 0.98–1.08]; P=0.15), whereas LDL‐C >2.6 mmol/L had an 11% increased risk of HF (SHR, 1.11 [95% CI, 1.07–1.17]; P<0.001) (Table S4). Notably, across all strata of LDL‐C levels, the crude incidence rates of HF were consistently lower in statin users compared with statin nonusers (LDL‐C <1.8 mmol/L: 3.77 versus 4.80; LDL‐C 1.8–2.6 mmol/L: 3.05 versus 4.38; and LDL‐C >2.6 mmol/L: 3.34 versus 3.69; all P<0.001).
The reduced risk of HF in statin users across LDL‐C groups remained consistent following multivariate adjustment (LDL‐C <1.8 mmol/L: SHR, 0.66 [95% CI, 0.60–0.71]; LDL‐C 1.8–2.6 mmol/L: SHR, 0.73 [95% CI, 0.69–0.77]; and LDL‐C >2.6 mmol/L: SHR, 0.85 [95% CI, 0.79–0.90]; all P<0.001) (Figure 2).
Figure 2. Multivariable stratified analysis of the association between statin use and risk of incident heart failure.
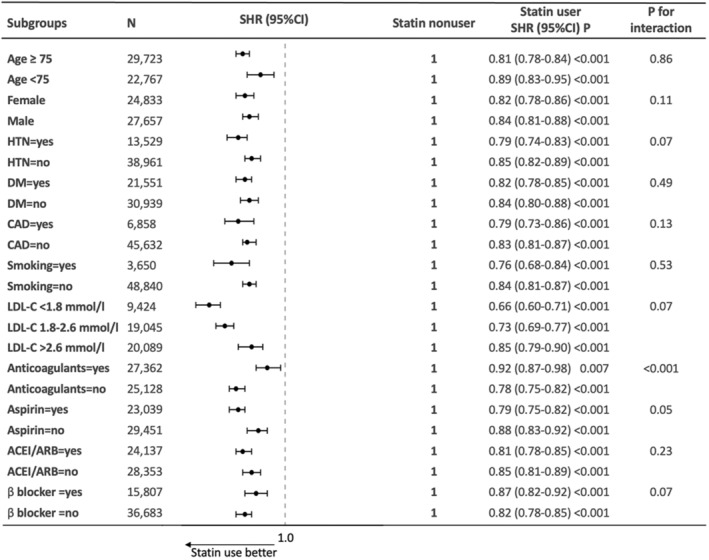
ACEI/ARB indicates angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker; CAD, coronary artery disease; DM, diabetes; HTN, hypertension; LDL‐C, low‐density lipoprotein cholesterol; and SHR, subdistribution hazard ratio.
Duration of Statin Use
To evaluate the duration‐dependent relationship, we modeled statin use as a time‐varying exposure. Among 23 239 statin users, 1972 (8.5%) patients discontinued statin use before the outcome, death, or study end. The incidence rate of HF declined with an increasing duration of statin use (Table 3). The duration of statin use was calculated during the follow‐up period; compared with short‐term statin use (3 months to <2 years), there was a stepwise reduction in risk of incident HF among those with 2 to <4 years of statin use (SHR, 0.86 [95% CI, 0.84–0.88]; P<0.001), 4 to <6 years of statin use (SHR, 0.74 [95% CI, 0.72–0.76]; P<0.001), and ≥6 years of statin use (SHR, 0.71 [95% CI, 0.69–0.74]; P<0.001).
Table 3.
Effect of Duration of Statin Use on the Risk of Incident HF and HF‐Related Death Among Statin Users
| Duration of statin use | Incident rate of 100 person‐years | SHR (95% CI) P value | ||
|---|---|---|---|---|
| Total/events | Unadjusted | Adjusted* | ||
| HF | ||||
| 3 mo to <2 y | 23 239/315 | 4.79 | 1.00 (Reference) | 1.00 (Reference) |
| 2 to <4 y | 19 870/393 | 4.69 | 0.84 (0.83–0.86) <0.001 | 0.86 (0.84–0.88) <0.001 |
| 4 to <6 y | 17 445/516 | 3.76 | 0.72 (0.70–0.74) <0.001 | 0.74 (0.72–0.76) <0.001 |
| ≥6 y | 14 168/2449 | 3.07 | 0.70 (0.67–0.73) <0.001 | 0.71 (0.69–0.74) <0.001 |
| HF‐related death | ||||
| 3 mo to <2 y | 23 239/75 | 0.61 | 1.00 (Reference) | 1.00 (Reference) |
| 2 to <4 y | 19 870/83 | 0.62 | 1.09 (1.07–1.13) <0.001 | 1.06 (1.03–1.09) <0.001 |
| 4 to <6 y | 17 445/111 | 0.48 | 0.84 (0.81–0.87) <0.001 | 0.85 (0.82–0.87) <0.001 |
| ≥6 y | 14 168/392 | 0.44 | 0.76 (0.73–0.79) <0.001 | 0.77 (0.74–0.80) <0.001 |
Statin user was defined by filled prescription for at least 90 consecutive days of statin use after the index date. The cumulative duration of statin use was modeled as a time‐varying exposure. Hazard estimates were obtained with the use of a proportional subdistribution hazards regression model fit to the inverse probability of treatment weighted cohort that accounted for competing risk; the model was conditioned on age at index date. HF indicates heart failure; and SHR, subdistribution hazard ratio.
A multivariable‐adjusted model further accounted for the following prognostic covariates: age at index date, sex, smoking, CHA2DS2‐VASc score (congestive HF, hypertension, age ≥75 years [doubled], diabetes, stroke [doubled], vascular disease, age 65–74 years, and sex category [female]) score, atrial fibrillation duration, comorbidities, including diabetes, hypertension, ischemic stroke, transient ischemic attack, coronary artery disease, chronic kidney disease, peripheral vascular disease, venous thromboembolism, rheumatoid arthritis, systemic sclerosis, systemic lupus erythematosus, ankylosing spondylitis, liver cirrhosis, anemia, cancer, gastrointestinal bleeding, dyslipidemia, and obesity, and baseline use of non–vitamin K antagonist oral anticoagulant, warfarin, aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, ablation, and cardioversion.
A similar duration‐dependent relationship was also found in the association between statin use and HF‐related death. Compared with short‐term statin use (3 months to <2 years), 4 to <6 years of statin use and ≥6 years of statin use were associated with a 15% (SHR, 0.85 [95% CI, 0.82–0.87]; P<0.001) and a 23% reduction (SHR, 0.77 [95% CI, 0.74–0.80]; P<0.001) in HF‐related death, respectively.
Use of Statin and Mortality
The incidence rates of all‐cause mortality per 100 person‐years of follow‐up were 9.89 and 8.83 in statin nonusers and statin users, respectively (Table S2). Cox regression analysis showed that statin users had a lower risk of all‐cause mortality (hazard ratio [HR], 0.85 [95% CI, 0.82–0.87]; P<0.001) compared with statin nonusers (Table S5).
Subgroup Analysis
Results of subgroup analyses are shown in Figure 2. Compared with nonusers, statin users were associated with a lower risk of incident HF across all subgroups, including age, sex, hypertension, diabetes, CAD, smoking, and baseline use of anticoagulants, aspirin, ACEI/ARB, and β‐blockers. There was no interaction for all subgroups, except for those categorized by baseline status of anticoagulant treatment (P interaction<0.001), where the benefit of statin use appeared to be greater in the subgroup of patients without anticoagulant use compared with those with anticoagulant use.
Sensitivity Analyses
To allow comparisons with previous studies, we also conducted competing risk without propensity score matching (adjusted SHR, 0.74 [95% CI, 0.70–0.77]; P<0.001) and conventional multivariable Cox regression without considering competing risk (HR, 0.75 [95% CI, 0.71–0.78]; P<0.001) (Table S6), which showed consistent results with our primary analysis. When 1:1 propensity score matching was used (Table S7) instead of IPTW, the multivariate‐adjusted SHR after accounting for competing risk was 0.75 (95% CI, 0.71–0.79; P<0.001) (Table S6).
In addition, we identified 3870 new statin users who started statin treatment after the index date by excluding patients with baseline statin use. A 1:1 propensity score matching approach was used to create a cohort to compare the risk of HF between statin new users and statin nonusers; after accounting for competing risk, the multivariable‐adjusted SHR for incident HF was 0.70 (95% CI, 0.64–0.77; P<0.001) and the multivariable‐adjusted SHR for HF‐related death was 0.75 (95% CI, 0.68–0.82; P<0.001) for statin new users (Table S8). The E‐value was 1.6 for the upper limit of the CI, suggesting that for an unmeasured confounder to render the results statistically insignificant, it would need to be strongly associated with both statin use and HF (>60% difference in prevalence among users and nonusers and an HR >1.6/<0.6 on HF).
As a falsification analysis, we used GIB as a negative control, of which 10 220 patients with GIB diagnosis were identified. After excluding 6274 patients with previous GIB history before the index date, 3946 patients with GIB were included. The association between statin use and GIB was insignificant after multivariable adjustment (SHR, 1.01 [95% CI, 0.94–1.08]; P=0.77) (Table S9).
Discussion
This territory‐wide cohort study of newly diagnosed patients with AF who started statin treatment within 1 year demonstrated that statin use was independently associated with a 19% reduced risk of HF, a 16% reduced risk of HF‐related death, and a 15% reduced risk of all‐cause mortality. Second, there was a significant duration‐dependent association between statin use and the risk of HF and HF‐related death. This inverse association was consistent across subgroups of age, sex, comorbidities, anticoagulation use, and LDL‐C levels.
HF, surpassing stroke and thromboembolism, 29 has emerged as the major complication of AF, afflicting 11% to 34% of the population. 30 Its substantial individual and societal burden 31 is underlined by the increased mortality and rehospitalization risk among patients with AF who have HF, 8 with HF after AF (as in our study) carrying the highest risk of all‐cause mortality (compared with concurrent AF and HF or HF before AF). 32 Our study, using a large contemporary population with AF, corroborates with previous studies to highlight the profoundly high HF and related mortality risk in patients with AF. These observations highlight the need to develop effective strategies to prevent HF to improve outcome in newly diagnosed patients with AF.
Although the precise mechanisms involved in HF development in patients with AF are not well understood, increased LDL‐C levels, comorbidities (hypertension, diabetes, and CAD), thromboembolic effects, cardiac remodeling and dysfunction, neurohumoral activation, inflammation, and oxidative stress are likely to contribute to HF pathogenesis. 21 Notably, accumulating evidence illustrates that epicardial fat, which is closely linked to LDL‐C, is associated with HF 33 as well as AF progression, emphasizing the potential role of dyslipidemia in mediating the inextricable link between HF and AF. 34 , 35 Supporting this notion, our study, which includes a large cohort of newly diagnosed patients with AF who started statin treatment within 1 year following diagnosis of AF, demonstrated that an elevated time‐weighted LDL‐C level >2.6 mmol/L (versus <1.8 mmol/L) had an 11% increased risk of incident HF. These findings unveil LDL‐C control as a potential target and provide a crucial rationale for exploring lipid‐lowering therapies in preventing HF in patients with AF. Correspondingly, prior evidence has demonstrated that LDL‐C reduction was associated with a 13% lower risk of major cardiovascular events in patients with HF 36 and that statins could reduce the risk of new‐onset HF or HF hospitalization. 18 , 37 Our study, extending previous literature, provides evidence on the relationship between statin use and HF in patients with AF through thorough consideration of potential sources of confounding and biases achieved by propensity score analytics.
Although one may be inclined to perceive LDL‐C control as the sole mechanism for statins' benefit, our study demonstrates that statin use is associated with reduced HF incidence across all LDL‐C strata, pointing to the potential pleiotropic effect of statin use in reducing HF burden in patients with AF. Previous fundamental research has revealed several pleiotropic effects, including anti‐inflammatory and antioxidant effects, 17 endothelial function improvement, 38 cardiac remodeling, 39 and autonomic nervous system modulation, that may reduce HF incidence. 40 Clinical data have further solidified these mechanisms in reducing the risk of HF. 41 , 42 , 43 A substudy of the Controlled rosuvastatin multinational study in heart failure (CORONA) trial demonstrated that inflammatory biomarkers, including high‐sensitivity CRP (C‐reactive protein), that could be reduced by statin were associated with lower risk of cardiovascular mortality and HF rehospitalization in patients with chronic HF. 44 The consistent risk reduction among patients with and without CAD further confirms that statins provided additional protective mechanisms against HF beyond its ability to attenuate atherosclerotic burden. 12 The potential of statins to reduce tachycardia‐induced structural remodeling 45 and chamber‐specific selectivity to minimize atrial fibrosis through the stain reductase pathway 46 may provide complementary mechanisms to reduce HF specifically in patients with AF. Indeed, the favorable association between statins and study outcomes in all LDL‐C subgroups further justified the pleiotropic benefit in ameliorating the burden of HF by statin use in patients with AF. Nevertheless, there is a trend of higher risk of HF in those with LDL‐C control of <1.8 mmol/L in our study (including both patients with and without statin use), and this observation is consistent with prior studies that indicated that LDL‐C <1.8 mmol/L was associated with a higher risk of cardiovascular mortality. 47 , 48 The potential adverse association with low LDL‐C in relation to HF, perhaps related to malnutrition status in particular to those at risk of HF, 49 deserves verification by future prospective studies.
Despite the apparent effect of statins to protect HF in prior studies19,50 and the present study, 2 randomized studies (CORONA and Gruppo Italiano per lo Studio della Sopravvivenza nell'Insufficienza Cardiaca‐Heart Failure) failed to demonstrate a reduction of major adverse outcome in subjects with New York Heart Association class II to IV; in addition, presence of AF did not affect the neutral effects of statins on HF. 14 , 51 However, a post hoc analysis of the CORONA trial did show that rosuvastatin led to fewer HF hospitalizations when repeated events were included. 15 Nonetheless, only ≈20% of patients enrolled in these 2 trials had underlying AF, and the failure to include important confounding factors, such as anticoagulation and AF ablation, indicated that the neutral effect of statins to protect from HF cannot be extended to our study population. In both trials, ≈30% died within the study trial, of which adverse events should be adjusted with competing risk for mortality, yet were not considered. Furthermore, like all randomized studies, strict inclusion criteria and a restricted number of participants may not be able to mimic real‐life treatment situations. These constraints were well addressed by our study design, which includes the use of a territory‐wide, well‐validated electronic health care database (CDARS) with records of all diagnoses, laboratory results, and details of drug dispenses, allowing the collection of the relevant information required to preclude selection and recall biases. The validity of the current result is further enhanced by extensive adjustments for medications/procedures, such as ACEI/ARB and AF ablation, which may modify the risk of HF. The application of IPTW to an unselected population with AF with detailed clinical and medication history also provided convincing evidence on the potential benefits of statins in preventing HF and HF‐related mortality. In addition, using a time‐varying model, we demonstrated a duration‐dependent reduction of HF by statin use, suggestive of a potential causal relationship. The association between statins and lower risk of all‐cause mortality, as shown in our data, consistent with other observations, 52 , 53 may reflect an overall beneficial impact on adverse cardiovascular outcomes. Pragmatic randomized controlled trials in patients with AF are needed to confirm the role of statins in preventing incident HF.
Limitations
The current study did not distinguish AF types (paroxysmal, persistent, and permanent) and treatment strategies (rhythm and rate control). Echocardiographic data were not available in CDARS; thus, the differential impact of left ventricular filling pressure/function could not be evaluated in the present study. Previous studies have indicated similar rates of adverse events across different left ventricular systolic grades in patients with AF 54 ; however, the impact of statin use (different types and intensity) on different HF subtypes (HF with preserved ejection fraction and HF with reduced eject fraction) within the context of AF remains unclear, and future studies are needed to investigate these associations. The present study assessed the association between HF and statin use in patients who started statin treatment within 1 year following diagnosis of AF. As such, our findings cannot be generalized to those patients who initiate statins >1 year after newly diagnosed AF. Residual confounders remain possible despite propensity score analytics; however, such confounders need to be of great significance to invalidate the present results based on our reported E‐value. Finally, the study was conducted in an Asian cohort, and further studies in other racial groups may be required to confirm our observations.
Conclusions
In this large population‐based cohort of patients with AF, statin use was significantly associated with a reduced risk of HF incidence and HF‐related mortality, independent of LDL‐C levels. The negative association was duration dependent and consistent across all subgroups.
Sources of Funding
This work was supported by Sanming Project of Medicine in Shenzhen, China (No. SZSM201911020) and Hong Kong University‐Shen Zhen Hospital Fund for Shenzhen Key Medical Discipline (No. SZXK2020081).
Disclosures
None.
Supporting information
Tables S1–S9
Figures S1–S2
Acknowledgments
Author contributions: All authors critically reviewed and contributed to the intellectual content of the manuscript. Kai‐Hang Yiu, Gregory Y.H. Lip and Jia‐Yi Huang were involved with the conception of the study. Initial data preparation was done by Jia‐Yi Huang. Statistical analyses were undertaken by Jia‐Yi Huang, supported by Yi‐Kei Tse, Hang‐Long Li, Si‐Yeung Yu, Mei‐Zhen Wu, Qing‐Wen Ren, with inputs from Cong Chen, Chun‐Ting Zhao, Ming‐Ya Liu, Denise Hung, Yap‐Hang Chen and Ka‐Lam Leung. Yi‐Kei Tse and Jia‐Yi Huang drafted several versions of the manuscript. Xin‐Li Li, Hung‐Fat Tse, Gregory Y.H. Lip and Kai‐Hang Yiu provided the clinical expertise. All authors have read and approved the final version of the manuscript.
This article was sent to Luciano A. Sposato, MD, MBA, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032378
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, McManus DD, Ko D, Weng LC, Lunetta KL, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham heart study. BMJ. 2018;361:k1453. doi: 10.1136/bmj.k1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njolstad I, Vartiainen E, Sans S, Pasterkamp G, Hughes M, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in Europe). Circulation. 2017;136:1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna W, Seward JB, Iwasaka T, Tsang TS. Incidence and mortality risk of congestive heart failure in atrial fibrillation patients: a community‐based study over two decades. Eur Heart J. 2006;27:936–941. doi: 10.1093/eurheartj/ehi694 [DOI] [PubMed] [Google Scholar]
- 5. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schnabel RB, Rienstra M, Sullivan LM, Sun JX, Moser CB, Levy D, Pencina MJ, Fontes JD, Magnani JW, McManus DD, et al. Risk assessment for incident heart failure in individuals with atrial fibrillation. Eur J Heart Fail. 2013;15:843–849. doi: 10.1093/eurjhf/hft041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chamberlain AM, Gersh BJ, Alonso A, Kopecky SL, Killian JM, Weston SA, Roger VL. No decline in the risk of heart failure after incident atrial fibrillation: a community study assessing trends overall and by ejection fraction. Heart Rhythm. 2017;14:791–798. doi: 10.1016/j.hrthm.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, Curtis LH, Heckbert SR. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. 2014;35:250–256. doi: 10.1093/eurheartj/eht483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade atrial fibrillation study. Eur J Heart Fail. 2013;15:415–424. doi: 10.1093/eurjhf/hft004 [DOI] [PubMed] [Google Scholar]
- 10. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017;5:44–52. doi: 10.1016/j.jchf.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 11. Kartas A, Samaras A, Akrivos E, Vrana E, Papazoglou AS, Moysidis DV, Papanastasiou A, Baroutidou A, Botis M, Liampas E, et al. Tauhe association of heart failure across left ventricular ejection fraction with mortality in atrial fibrillation. ESC Heart Fail. 2021;8:3189–3197. doi: 10.1002/ehf2.13440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non‐ischemic heart failure. J Am Coll Cardiol. 2004;43:642–648. doi: 10.1016/j.jacc.2003.07.049 [DOI] [PubMed] [Google Scholar]
- 13. Preiss D, Campbell RT, Murray HM, Ford I, Packard CJ, Sattar N, Rahimi K, Colhoun HM, Waters DD, LaRosa JC, et al. The effect of statin therapy on heart failure events: a collaborative meta‐analysis of unpublished data from major randomized trials. Eur Heart J. 2015;36:1536–1546. doi: 10.1093/eurheartj/ehv072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2008;372:1231–1239. doi: 10.1016/S0140-6736(08)61240-4 [DOI] [PubMed] [Google Scholar]
- 15. Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201 [DOI] [PubMed] [Google Scholar]
- 16. Kumar A, Cannon CP. The role of statins in the prevention of heart failure after acute coronary syndrome. Heart Fail Clin. 2008;4:129–139. doi: 10.1016/j.hfc.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 17. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, Liu Y, Hoffmann U, Bauer DC, Newman AB, et al. Inflammatory markers and incident heart failure risk in older adults: the health ABC (health, aging, and body composition) study. J Am Coll Cardiol. 2010;55:2129–2137. doi: 10.1016/j.jacc.2009.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Udell JA, Ray JG. Primary and secondary prevention of heart failure with statins. Expert Rev Cardiovasc Ther. 2006;4:917–926. doi: 10.1586/14779072.4.6.917 [DOI] [PubMed] [Google Scholar]
- 19. Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112:357–363. doi: 10.1161/CIRCULATIONAHA.104.519876 [DOI] [PubMed] [Google Scholar]
- 20. Sanchez‐Quinones J, Marin F, Roldan V, Lip GY. The impact of statin use on atrial fibrillation. QJM. 2008;101:845–861. doi: 10.1093/qjmed/hcn101 [DOI] [PubMed] [Google Scholar]
- 21. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community‐based study. Circulation. 2013;128:1085–1093. doi: 10.1161/CIRCULATIONAHA.113.001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren QW, Yu SY, Teng TK, Li X, Cheung KS, Wu MZ, Li HL, Wong PF, Tse HF, Lam CSP, et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J. 2021;42:3049–3059. doi: 10.1093/eurheartj/ehab325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau WC, Chan EW, Cheung CL, Sing CW, Man KK, Lip GY, Siu CW, Lam JK, Lee AC, Wong IC. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. 2017;317:1151–1158. doi: 10.1001/jama.2017.1363 [DOI] [PubMed] [Google Scholar]
- 24. Li HL, Tromp J, Teramoto K, Tse YK, Yu SY, Lam LY, Li KY, Wu MZ, Ren QW, Wong PF, et al. Temporal trends and patterns of infective endocarditis in a Chinese population: a territory‐wide study in Hong Kong (2002‐2019). Lancet Reg Health West Pac. 2022;22:100417. doi: 10.1016/j.lanwpc.2022.100417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kezerle L, Tsadok MA, Akriv A, Senderey AB, Bachrach A, Leventer‐Roberts M, Haim M. Pre‐diabetes increases stroke risk in patients with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2021;77:875–884. doi: 10.1016/j.jacc.2020.12.030 [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Perez LE, Boye KS, Rosilio M, Jung H, Heitmann E, Norrbacka K, Federici MO, Gentilella R, Guerci B, Giorgino F, et al. The real‐world observational prospective study of health outcomes with dulaglutide and liraglutide in type 2 diabetes patients (TROPHIES): design and baseline characteristics. Diabetes Ther. 2021;12:1929–1946. doi: 10.1007/s13300-021-01076-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau WCY, Cheung CL, Man KKC, Chan EW, Sing CW, Lip GYH, Siu CW, Lam JKY, Lee ACH, Wong ICK. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population‐based cohort study. Ann Intern Med. 2020;173:1–9. doi: 10.7326/M19-3671 [DOI] [PubMed] [Google Scholar]
- 28. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 29. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, Commerford P, Jansky P, Avezum A, Sigamani A, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161–1169. doi: 10.1016/S0140-6736(16)30968-0 [DOI] [PubMed] [Google Scholar]
- 31. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201 [DOI] [PubMed] [Google Scholar]
- 32. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 33. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail. 2018;20:1559–1566. doi: 10.1002/ejhf.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jian B, Li Z, Wang J, Zhang C. Correlation analysis between heart rate variability, epicardial fat thickness, visfatin and AF recurrence post radiofrequency ablation. BMC Cardiovasc Disord. 2022;22:65. doi: 10.1186/s12872-022-02496-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaihov‐Teper O, Ram E, Ballan N, Brzezinski RY, Naftali‐Shani N, Masoud R, Ziv T, Lewis N, Schary Y, Levin‐Kotler LP, et al. Extracellular vesicles from epicardial fat facilitate atrial fibrillation. Circulation. 2021;143:2475–2493. doi: 10.1161/CIRCULATIONAHA.120.052009 [DOI] [PubMed] [Google Scholar]
- 36. Wang N, Fulcher J, Abeysuriya N, Park L, Kumar S, Di Tanna GL, Wilcox I, Keech A, Rodgers A, Lal S. Intensive LDL cholesterol‐lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta‐analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020;8:36–49. doi: 10.1016/S2213-8587(19)30388-2 [DOI] [PubMed] [Google Scholar]
- 37. Schubert J, Lindahl B, Melhus H, Renlund H, Leosdottir M, Yari A, Ueda P, James S, Reading SR, Dluzniewski PJ, et al. Low‐density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021;42:243–252. doi: 10.1093/eurheartj/ehaa1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ferrier KE, Muhlmann MH, Baguet JP, Cameron JD, Jennings GL, Dart AM, Kingwell BA. Intensive cholesterol reduction lowers blood pressure and large artery stiffness in isolated systolic hypertension. J Am Coll Cardiol. 2002;39:1020–1025. doi: 10.1016/S0735-1097(02)01717-5 [DOI] [PubMed] [Google Scholar]
- 39. Hayashidani S, Tsutsui H, Shiomi T, Suematsu N, Kinugawa S, Ide T, Wen J, Takeshita A. Fluvastatin, a 3‐hydroxy‐3‐methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–873. doi: 10.1161/hc0702.104164 [DOI] [PubMed] [Google Scholar]
- 40. Strehlow K, Wassmann S, Bohm M, Nickenig G. Angiotensin AT1 receptor over‐expression in hypercholesterolaemia. Ann Med. 2000;32:386–389. doi: 10.3109/07853890008995944 [DOI] [PubMed] [Google Scholar]
- 41. Nymo SH, Hulthe J, Ueland T, McMurray J, Wikstrand J, Askevold ET, Yndestad A, Gullestad L, Aukrust P. Inflammatory cytokines in chronic heart failure: interleukin‐8 is associated with adverse outcome. Results from CORONA. Eur J Heart Fail. 2014;16:68–75. doi: 10.1093/eurjhf/hft125 [DOI] [PubMed] [Google Scholar]
- 42. Chaar D, Dumont B, Vulesevic B, Neagoe PE, Rakel A, Sirois MG, White M. Neutrophils pro‐inflammatory and anti‐inflammatory cytokine release in patients with heart failure and reduced ejection fraction. ESC Heart Fail. 2021;8:3855–3864. doi: 10.1002/ehf2.13539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
- 44. McMurray JJ, Kjekshus J, Gullestad L, Dunselman P, Hjalmarson A, Wedel H, Lindberg M, Waagstein F, Grande P, Hradec J, et al. Effects of statin therapy according to plasma high‐sensitivity C‐reactive protein concentration in the controlled rosuvastatin multinational trial in heart failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–2196. doi: 10.1161/CIRCULATIONAHA.109.849117 [DOI] [PubMed] [Google Scholar]
- 45. Yeh YH, Kuo CT, Chang GJ, Chen YH, Lai YJ, Cheng ML, Chen WJ. Rosuvastatin suppresses atrial tachycardia‐induced cellular remodeling via Akt/Nrf2/heme oxygenase‐1 pathway. J Mol Cell Cardiol. 2015;82:84–92. doi: 10.1016/j.yjmcc.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 46. Rizvi F, DeFranco A, Siddiqui R, Negmadjanov U, Emelyanova L, Holmuhamedov A, Ross G, Shi Y, Holmuhamedov E, Kress D, et al. Chamber‐specific differences in human cardiac fibroblast proliferation and responsiveness toward simvastatin. Am J Physiol Cell Physiol. 2016;311:C330–C339. doi: 10.1152/ajpcell.00056.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu HH, Zhang M, Chen RZ, Zhou JY, Qian J, Dou KF, Yan HB, Li JJ. Low‐density lipoprotein cholesterol in oldest old with acute myocardial infarction: is lower the better? Age Ageing. 2022;51:afac202. doi: 10.1093/ageing/afac202 [DOI] [PubMed] [Google Scholar]
- 48. Cheng Q, Liu XC, Chen CL, Huang YQ, Feng YQ, Chen JY. The U‐shaped association of non‐high‐density lipoprotein cholesterol levels with all‐cause and cardiovascular mortality among patients with hypertension. Front Cardiovasc Med. 2021;8:707701. doi: 10.3389/fcvm.2021.707701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Czapla M, Juarez‐Vela R, Lokiec K, Karniej P. The association between nutritional status and in‐hospital mortality among patients with heart failure‐a result of the retrospective nutritional status heart study 2 (NSHS2). Nutrients. 2021;13:1669. doi: 10.3390/nu13051669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krum H, Latini R, Maggioni AP, Anand I, Masson S, Carretta E, Ingrilli F, Pettinati G, Glazer R, Tognoni G, et al. Statins and symptomatic chronic systolic heart failure: a post‐hoc analysis of 5010 patients enrolled in Val‐HeFT. Int J Cardiol. 2007;119:48–53. doi: 10.1016/j.ijcard.2006.07.106 [DOI] [PubMed] [Google Scholar]
- 51. Rogers JK, Jhund PS, Perez AC, Bohm M, Cleland JG, Gullestad L, Kjekshus J, van Veldhuisen DJ, Wikstrand J, Wedel H, et al. Effect of rosuvastatin on repeat heart failure hospitalizations: the CORONA trial (controlled rosuvastatin multinational trial in heart failure). JACC Heart Fail. 2014;2:289–297. doi: 10.1016/j.jchf.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 52. Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali‐Serdoz L, Petrescu L, Darabantiu D, Crijns HJ, Kirchhof P, Vardas P, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow‐up of the EURObservational research Programme‐atrial fibrillation general registry pilot phase (EORP‐AF pilot registry). Eur Heart J. 2014;35:3365–3376. doi: 10.1093/eurheartj/ehu374 [DOI] [PubMed] [Google Scholar]
- 53. Pastori D, Baratta F, Di Rocco A, Farcomeni A, Del Ben M, Angelico F, Violi F, Pignatelli P, Lip GYH. Statin use and mortality in atrial fibrillation: a systematic review and meta‐analysis of 100,287 patients. Pharmacol Res. 2021:Epub ahead of print;165:105418. doi: 10.1016/j.phrs.2021.105418 [DOI] [PubMed] [Google Scholar]
- 54. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid‐range, and reduced ejection fraction. J Am Coll Cardiol. 2017;5:565–574. doi: 10.1016/j.jchf.2017.05.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S2


