Abstract
Background
Marijuana leaf vaporizers, which heat plant material and sublimate Δ‐9‐tetrahydrocannabinol without combustion, are popular alternatives to smoking cannabis that are generally perceived to be less harmful. We have shown that smoke from tobacco and marijuana, as well as aerosol from e‐cigarettes and heated tobacco products, impair vascular endothelial function in rats measured as arterial flow‐mediated dilation (FMD).
Methods and Results
We exposed 8 rats per group to aerosol generated by 2 vaporizer systems (Volcano and handheld Yocan) using marijuana with varying Δ‐9‐tetrahydrocannabinol levels, in a single pulsatile exposure session of 2 s/min over 5 minutes, and measured changes in FMD. To model secondhand exposure, we exposed rats for 1 minute to diluted aerosol approximating release of uninhaled Volcano aerosol into typical residential rooms. Exposure to aerosol from marijuana with and without cannabinoids impaired FMD by ≈50%. FMD was similarly impaired by aerosols from Yocan (237 °C), and from Volcano at both its standard temperature (185 °C) and the minimum sublimation temperature of Δ‐9‐tetrahydrocannabinol (157 °C), although the low‐temperature aerosol condition did not effectively deliver Δ‐9‐tetrahydrocannabinol to the circulation. Modeled secondhand exposure based on diluted Volcano aerosol also impaired FMD. FMD was not affected in rats exposed to clean air or water vapor passed through the Volcano system.
Conclusions
Acute direct exposure and modeled secondhand exposure to marijuana leaf vaporizer aerosol, regardless of cannabinoid concentration or aerosol generation temperature, impair endothelial function in rats comparably to marijuana smoke. Our findings indicate that use of leaf vaporizers is unlikely to reduce the vascular risk burden of smoking marijuana.
Keywords: aerosol, cannabis, endothelial function, marijuana, vaporizer
Subject Categories: Animal Models of Human Disease, Endothelium/Vascular Type/Nitric Oxide, Physiology, Vascular Biology
Nonstandard Abbreviations and Acronyms
- FMD
flow‐mediated dilation
- THC
Δ‐9‐tetrahydrocannabinol
Research Perspective.
What Is New?
Brief exposure to undiluted aerosol from marijuana loose leaf vaporizers impairs endothelial function in rats comparably to marijuana smoke, regardless of the presence or absence of cannabinoids in the aerosol.
Aerosol that has been diluted to mimic secondhand exposure also impairs endothelial function.
What Question Should Be Addressed Next?
Because our findings suggest that use of leaf vaporizers is unlikely to reduce vascular risk burden of smoking, future study of effects of vaporizer use in humans is warranted.
The prevalence of marijuana use has increased in the US adult population, and perception of harm from marijuana smoking has decreased. 1 , 2 , 3 The growing number of states that have legalized medicinal and recreational marijuana has led to a rise in its prevalence and has spurred an expansion of the marijuana industry. 4 , 5 Marijuana use is associated with adverse cardiovascular and cerebrovascular health consequences including myocardial infarction 6 and stroke 7 in humans.
Marijuana loose leaf vaporizers, sometimes called dry herb vaporizers, are popular for medicinal and recreational use because they are presumed to be less harmful than smoking. 8 , 9 Vaporizer devices heat ground leaf or bud material to a temperature below the combustion threshold and release lower levels of organic compounds compared with smoke. 10 Abrams et al 11 reported that people who used marijuana leaf vaporizers to obtain similar plasma Δ‐9‐tetrahydrocannabinol (THC) levels as marijuana smokers nonetheless produced less expired CO. However, the cardiovascular effects of exposure to marijuana aerosol have not been well studied in humans.
A variety of vaporizers are currently available in the United States. Two popular brands are Volcano (Storz‐Bickel, Tuttlingen, Germany) and Yocan (Yocan Technology Co. Ltd, Guangdong, China). Although these 2 types of devices both vaporize marijuana leaves and eliminate combustion, they are slightly different in mechanism of aerosol production. Volcano, a desktop balloon vaporization system, collects aerosols in an 8‐L bag (called a balloon although it is not elastic) by blowing hot air through a small heating chamber containing marijuana leaves. Subsequently, the user inhales the contents of the balloon, and in some circumstances can share the same balloon with other users. Yocan, on the other hand, is a handheld, battery‐powered device that contains a small heating chamber where the marijuana leaves are placed and heated, delivering aerosol directly to the mouth. The device is activated each time the user inhales from it. Both types of vaporizers can also be used to aerosolize liquid forms of THC, such as THC oil and alcohol solutions of dronabinol. This is a fundamentally different process more analogous to nicotine e‐cigarettes, and the aerosols resulting from the THC liquids and from minimally processed plant material may be different in particle size and chemical composition. This study focused solely on the aerosols generated from minimally processed marijuana plant material, at temperatures below the combustion point.
Arterial flow‐mediated dilation (FMD), defined as the percent by which the arteries vasodilate in response to an increase in blood flow, is a validated approach for measurement of endothelial function. 12 , 13 , 14 In humans, brachial artery FMD is a well‐established clinical prognostic indicator of endothelial function that correlates with endothelium‐dependent vasodilation of the coronary arteries 15 and other measures of cardiovascular health. 12 , 14 , 16 , 17 Chronic active smoking and both chronic and acute exposure to secondhand tobacco smoke impair FMD in humans. 18 , 19 , 20 , 21 Acute and chronic use of e‐cigarettes and heated tobacco products have also been reported to impair FMD in humans, 22 , 23 , 24 , 25 although not all studies have detected this effect (eg, Haptonstall et al 26 ). Although a single impairment of FMD by smoke is transient and has not been demonstrated to lead to adverse health outcomes per se, Celermajer et al's work 19 indicated that repeated exposures to secondhand smoke ultimately led to chronic endothelial dysfunction.
Chronic exposure of rabbits to tobacco smoke, and both chronic and acute exposure of mice to e‐cigarette aerosol, impair endothelium‐dependent vascular relaxation in several isolated vessel models. 27 , 28 , 29 We previously have developed and established an in vivo rat model for FMD measurement that is similar procedurally and mechanistically to clinical FMD measurement. 30 We have shown that a single acute exposure to sidestream smoke (smoke from the smoldering tip) of tobacco cigarettes, little cigars, and marijuana cigarettes impairs FMD in rats, despite endothelium‐independent vasodilation caused by nitroglycerin remaining intact. 31 , 32 , 33 We also have shown that exposure to aerosols from heated tobacco products, such as IQOS and e‐cigarettes such as JUUL impairs vascular function to the same extent as mainstream smoke (smoke inhaled through the cigarette) from combusted tobacco products. 34 , 35 , 36 In this study, we show that a single acute exposure to aerosol and modeled secondhand aerosol from marijuana leaf vaporizers impairs endothelial function in rats comparably to marijuana smoke exposure.
Methods
M.L.S. had full access to all of the data in the study and takes responsibility for their integrity and the data analysis. All of the data are publicly available at https://doi.org/10.5061/dryad.4xgxd25gv.
Animals
We used male and female Sprague–Dawley rats from Charles River Laboratory (n=8 per group) at 9 to 11 weeks of age, with body weights of 250 to 350 g for males and 200 to 250 g for females. The initial experiments were performed in female rats to match the conditions of our earlier marijuana smoke studies, 33 and we subsequently started using both sexes to comply with National Institutes of Health guidance (the Results section lists experiments in a logical order for presentation, rather than chronological order, so sex distribution is specified for each experiment). Rats were anesthetized with ketamine/xylazine by intraperitoneal injection. Starting doses were ketamine (100 mg/kg)/xylazine (5 mg/kg). Body temperature of rats was maintained by placing a heating pad underneath to avoid inconsistent vasodilation from anesthesia‐induced hypothermia. Animals were monitored throughout the surgery and FMD measurements for changes in respiration to ensure adequate anesthesia. Intramuscular ketamine/xylazine injections were given as one‐third to one‐half doses as needed. Experiments were terminal. All procedures were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
FMD Measurement
FMD was measured in anesthetized rats with the same technique we used in prior publications. 30 Briefly, a 1‐cm incision was made to expose the iliac artery on the right side, then an arterial occluder consisting of 5–0 Prolene filament was positioned under the artery and passed through 15‐cm polyethylene 90 tubing to enable transient occlusion of the arterial flow. After equilibration and capturing the baseline diameter (arterial diameter before occlusion of flow), the blood flow was transiently occluded for 5 minutes followed by reperfusion. Serial 2‐dimensional images of the femoral artery diameter and corresponding Doppler blood flow images were captured every 30 seconds for 3 minutes and then every minute for a total of 5 minutes, using a 35‐MHz ultrasound transducer (Vevo660, VisualSonics, and subsequently Vevo 3100LT, VisualSonics, Toronto, Canada). Diameter measurements were performed offline using an automated analyzer system (Brachial Analyzer 5 and 6; Medical Imaging Applications, Coralville, IA). 30 All diameter images were taken in diastole. FMD was calculated as percent change: [peak diameterpostischemia−diameterbaseline]/diameterbaseline×100. FMD was measured pre‐exposure and again either 10 or 30 minutes after the end of exposure, depending on the experiment.
Marijuana and Associated Security
Acquisition and possession of marijuana was approved by the Drug Enforcement Agency, Food and Drug Administration, Research Advisory Panel of California, and the University of California, San Francisco Office of Environmental Health and Safety. All marijuana in this study was from sinsemilla (seedless) plants with stems removed, and consisted of buds, bracts, small leaves, and leaf fragments, and was grown without any pesticides at the University of Mississippi. Marijuana at 4.2% THC and placebo marijuana from which cannabinoids had been removed via chemical extraction (0.01% THC) were supplied as prerolled cigarettes weighing ≈0.9 g by RTI International (Research Triangle Park, NC), contracted through the National Institute on Drug Abuse Drug Supply Program. Upon receipt of the marijuana cigarettes, they were individually weighed, wrapped in plastic wrap, and stored in airtight containers at −20 °C. Marijuana at 9.6% THC was supplied in bulk by RTI, and for mainstream/sidestream smoke conditions, we rolled cigarettes by machine with the same dimensions as standard tobacco cigarettes to fit in our cigarette smoking machine without further modification. For all vaporizer conditions, 9.6% THC marijuana was sampled from the provided bulk material, and 4.2% and placebo marijuana was removed from the prerolled cigarettes; all marijuana samples were then ground in grinders purchased with the Volcano device, with different grinders used for materials with different THC content to avoid cross‐contamination. To meet the requirements of the Drug Enforcement Agency, the marijuana was stored in a chest freezer, bolted to the floor, with high‐security padlock and a code‐deactivated open‐door alarm that communicated with the University of California Police Department. The freezer was in a controlled‐access room outfitted with a solid door with high‐security lock and hinge pins that were nonremovable from the outside.
Before each experiment, marijuana cigarettes to be used for smoke generation were humidified overnight at room temperature by placing them in airtight container over saturated sodium chloride solution as per instruction by RTI, in a locked desk drawer. Marijuana for use in vaporizers was not humidified.
Vaporizer Devices
For producing aerosol from ground marijuana, we used a digital Volcano Medic balloon‐based device (Storz & Bickel GmbH, Tuttlingen, Germany) and a handheld Yocan Explore vaporizing device (purchased from a local smoke shop in San Francisco, CA). Different balloons and loading chambers were used for different exposure conditions to avoid cross‐contamination of products. For 1 experiment, the Volcano balloon was replaced by an inert 8.1‐L Tedlar bag with septum valve (catalog number 1092319; Fisher Scientific, Waltham, MA).
Exposure
Rats were exposed to marijuana aerosols generated at different temperatures and from marijuana of different percentages of THC (details are provided for each experiment in the Results section). To model active use of a marijuana vaporizer, exposure sessions lasted 5 minutes and consisted of 5‐second pulses of aerosol exposure, delivered either once every 30 seconds or once every 60 seconds. During these pulsatile vaporizer aerosol exposures, the rats were removed from the nose cones to inhale clean air between the 5‐second exposures. To model secondhand exposure to marijuana vaporizer aerosol, exposures lasted 1 minute and were constant. We used 1‐minute, constant exposure to sidestream marijuana smoke as a positive control, because we have already shown that this exposure impairs FMD. Each experiment included a negative control group that was exposed to room air passed through an empty heating chamber into a clean Volcano balloon. An instant read thermometer with metal probe (Polder Products, Oxford, CT) was used to confirm no significant difference between the temperatures of air passing through empty heating chambers versus those that contained marijuana (Table S1). The researcher who measured FMD was blinded to the exposure condition (nose‐only exposure prevented the rats from smelling like marijuana).
To generate aerosol from the Volcano, 0.25 g of marijuana was ground and placed in the heating chamber, the device was set to the desired temperature, and activated to pass hot air through the marijuana and fill an 8‐L balloon supplied with the device with marijuana aerosol. The balloon was connected via an included 1‐way valve (Easy Valve; Storz & Bickel) to a Gram Universal Vaping machine (Version 5; Gram Research, Oakland, CA). For pulsatile exposure to aerosol from the Yocan, the device was directly connected to the vaping machine. To model active use of the vaporizers, the vaping machine pulled 35 mL of the aerosol from the Volcano balloon or the Yocan device using an automatic 3‐way syringe pump and pushed it to the nose cone.
We used constant exposure to sidestream smoke from marijuana as a positive control for impairment of FMD by inhalational exposure. 33 To generate sidestream smoke, we used a modified cigarette smoking system as described previously for tobacco smoke experiments. 31 The system collects sidestream smoke from the burning tip of the tobacco/marijuana cigarette in a 21‐L plexiglass exposure chamber as a ventilator pump simulates human puffing. Smoke was generated from marijuana cigarettes using conditions similar to tobacco cigarette smoking International Organization for Standardization standard 3308:2012, 35‐mL puff volume over 2 seconds once per minute. Smoke was collected into the chamber, the cigarette was extinguished, and excess smoke was vented from the chamber to obtain the desired starting concentration of ≈600 μg/m3 respirable suspended particles (RSP; ie, PM2.5), a realistic concentration of particles in secondhand tobacco smoke approximating those in smoky restaurants that we have typically used for other studies. 31 , 32 , 33 Air was mixed with the smoke using a small fan. A Sidepak AM510 personal aerosol monitor (TSI, Shoreview, MN) with 2.5‐μm impactor was used to monitor the RSP concentration in the exposure chamber; sampled air was exhausted back into the chamber. The nose and mouth of the rat was inserted through a gasket in the chamber wall to breathe in the smoky air.
For modeled secondhand exposure to aerosol, rats were exposed to 1 minute of diluted aerosol approximating release of a partial Volcano balloon into a typical residential room as described for that experiment. Dilutions were accomplished by filling a Volcano balloon with aerosol as described for undiluted exposures, removing the desired volume of aerosol from the balloon with a syringe, and injecting the contents of the syringe into the 21‐L exposure chamber through a port located 8 cm above the rat exposure port. The rat was placed in the nose cone immediately after the aerosol was injected. The Sidepak aerosol monitor was not used during the secondhand exposure experiments. For dilute Yocan aerosol exposure, aerosol was pulled from the syringe directly from the nose cone outlet of the Gram system.
Particle Measurements
Because the particle concentrations for some of the vaporizer exposures were low, we performed a separate experiment to measure the Volcano vaporizer particle concentrations using a portable Dusttrak aerosol particle monitor (model 8532; TSI Inc, Shoreview, MN). Animals were not exposed during these experiments. The Dusttrak was calibrated and zeroed according to the manufacturer's instructions and connected to the exposure chamber with tubing inserted through the nosecone fitting. The Dusttrak sampling rate was 3.0 L/min and it exhausted into the fume hood. Dilute Volcano aerosol was generated and injected as above. Sidestream smoke was generated as above and measured after venting to achieve a starting level of 600 μg/m3. The test of each condition lasted 10 minutes, and the tests were performed in series. The exposure chamber was cleaned after the marijuana smoke condition, during the room air measurement, before the empty chamber air was measured again. We used the particle concentration data from the first minute of measurement for analysis because particle sorption to surfaces and the 3.0 L/min air flow rate of the Dusttrak led to a rapid attenuation of the particle concentration in the exposure chamber.
Determination of Serum THC Levels
Blood was collected ≈20 minutes by cardiac puncture after the end of exposure while rats were still anesthetized from the terminal FMD measurement procedure, and serum was isolated by standard methods. Samples were sent to the University of California, San Francisco Clinical Pharmacology Laboratory at Zuckerberg San Francisco General Hospital for analysis of THC. Free THC was determined by liquid chromatography tandem mass spectrometry (Thermo Quantiva with heated electro spray ion source in positive ion mode) on a pentane extract of serum. Briefly, following the addition of d3‐THC internal standard, 0.4 mL of serum was deproteinized by the addition 1 mL of acetonitrile. The supernatant was diluted with 1 mL of H2O and extracted with 5 mL of pentane. The pentane was nitrogen evaporated and reconstituted in 200 μL of methanol. A Waters 3×100 1.6‐μm ultra‐performance liquid chromatography column and a methanol formic acid gradient were used for the chromatographic separation of the THC. Calibration was done using air‐exposed rat serum.
Statistical Analysis
Assuming normal distribution of outcomes, at a significance level of 0.05, the sample size of 8 rats per group provided 80% power to detect an effect size of 1.51 (ie, a group difference of 1.5×SD) with independent t test, and an effect size of 1.27 (before and after difference of 1.27×SD) with paired t test. Mean and SD were used to summarize FMD and other measures pre‐ and post‐exposure in each experiment. The primary analysis used paired t tests for each exposure condition, comparing means of pre‐ and post‐exposure values within each group, to determine if FMD was impaired. Normality testing with the Shapiro‐Wilk test on the FMD data for all groups at each time point confirmed that all but 3 sets of values were normally distributed. For those that were not, the Wilcoxon signed rank test was used to compare the means of pre‐ and post‐exposure FMD in these 3 groups. In 2 instances, rats that were deemed to be outliers based on extremely different FMD values from the rest of the group were not omitted, and the results are reported with and without the outliers.
To determine if the extent of impairment significantly differed between exposure conditions, the FMD impairment under each exposure condition was also calculated for each rat as [(pre‐exposure FMD – post‐exposure FMD)/pre‐exposure FMD]×100, the means of percent FMD reduction were calculated for each exposure condition, and the differences in percent FMD reduction between groups were assessed by 1‐way ANOVA and adjusted for multiple comparisons using the Šidák method or assessed by the Kruskal‐Wallis test when normality was not shown. All the analyses were done with Stata 13.1.
Results
Marijuana Aerosol Impaired FMD Similar to Mainstream or Sidestream Marijuana Smoke
Female rats (8 per group) were exposed to pulsatile (2 seconds every 30 seconds for 5 minutes) aerosol from marijuana with a range of THC contents (9.6%, 4.2%, and <0.01% THC), pulsatile mainstream marijuana smoke (9.6% THC), 1 minute constant sidestream marijuana smoke (9.6% THC, 600 μg/m3 RSP), and clean air from the Volcano vaporizer balloon.
As shown in Figure 1, FMD was impaired by all of the smoke and aerosol exposure conditions. Volcano aerosol from 9.6% THC marijuana reduced FMD by 48% (P=0.026) from an average of 9.5±2.7% pre‐exposure to an average of 4.9±2.3% post‐exposure. Aerosol from 4.2% THC marijuana reduced FMD by 46% (P=0.004), from 10.0±2.6% to 5.4±2.2%. Aerosol from <0.01% THC marijuana reduced FMD by 36% (P=0.047), from 8.1±1.5% pre‐exposure versus 5.2±3.0% post‐exposure, if we omitted 1 outlier. If the outlier was included, the reduction in FMD was not statistically significant (see asterisks in Figure 1). Mainstream smoke from 9.6% THC marijuana reduced FMD by 32% (P=0.016), from 11.8±2.7% to 8.0±2.3%. Sidestream smoke from 9.6% THC marijuana reduced FMD by 56% (P=0.017), from 10.5±2.5% to 4.6±3.7%. No significant impairment of FMD was seen in the air group (8.9±2.4% pre‐exposure versus 7.2±2.3% post‐exposure, P=0.063 with nonparametric test). There was not a significant difference between the percent reduction of the nonair groups.
Figure 1. Impairment of FMD by pulsatile exposure (5 seconds, 2 times per minute over 5 minutes) to Volcano aerosol from marijuana with varying THC concentrations, as well as pulsatile mainstream and constant sidestream marijuana smoke.
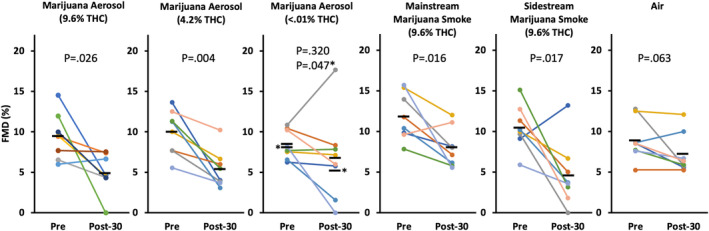
The impairment of FMD occurred regardless of THC concentration and was similar to the impairment by mainstream and sidestream smoke; no impairment was observed in the air negative control group. Each colored line represents an individual rat; black bars show group means. Post‐30=30 minutes after final exposure. *P value and means with outlier removed. P values are from paired t tests except for the air group, for which Shapiro‐Wilk was used due to nonnormal distribution. FMD indicates flow‐mediated dilation; and THC, Δ‐9‐tetrahydrocannabinol.
Marijuana Aerosol From Yocan at 237 °C and Volcano at 185 °C and 157 °C Impaired FMD
To determine if there are vaporizing temperatures that can successfully deliver THC to the circulation without impairing FMD, and to confirm that there were not device‐specific factors responsible for the impairment (such as the Volcano balloon), we exposed rats (now 5 seconds every 60 seconds for 5 minutes) to aerosol from 9.6% THC marijuana generated from the Volcano vaporizer at its typical heating temperature of 185 °C, and at 157 °C (the minimum sublimation temperature of THC), as well as the handheld Yocan vaporizer at its standard temperature of 237 °C. Sidestream marijuana smoke for 1 minute (9.6% THC, 600 μg/m3 RSP) and clean air through the Volcano were included as controls. Then we measured THC metabolites in their urine. For this experiment, we used 4 female and 4 male rats per group (see Animals section in Methods).
As shown in Figure 2, FMD was impaired by pulsatile exposure to aerosol generated from the Volcano and Yocan vaporizer devices irrespective of temperature. Aerosol from the Volcano at 185 °C reduced FMD by 49% (P<0.001), from 10.7±3.0% to 5.4±1.5%. Aerosol from the Volcano at 157 °C reduced FMD by 37% (P=0.002), from 10.3±3.1% to 6.5±2.1%. Aerosol from the Yocan at 237 °C reduced FMD by 47% (P=0.001), from 14.9±4.6% to 7.9±2.6%. As expected, FMD was also impaired by sidestream marijuana smoke (35% reduction, P=0.042, from 11.5±3.1% to 7.5±4.7%), but not by air (P=0.688 with nonparametric test, from 9.7±2.9% to 9.3±2.3%). There was not a significant difference between the percent reduction of the nonair groups.
Figure 2. Impairment of FMD by pulsatile exposures (2 seconds, 1 time per minute over 5 minutes) to Volcano and Yocan (handheld) marijuana aerosol generated at different temperatures.
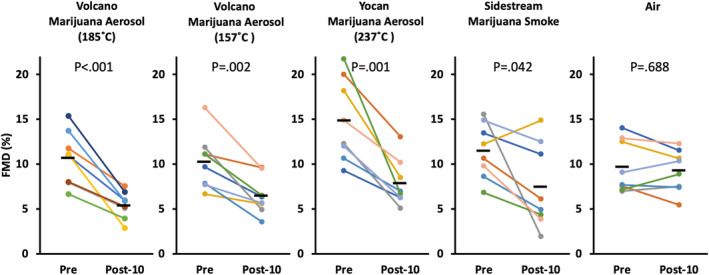
Lowering the device temperature to a level that did not efficiently deliver THC (see the Table) did not prevent the impairment of FMD. Each colored line represents an individual rat; black bars show group means. Post‐10=10 minutes after final exposure. P values are from paired t tests except for the air group, for which the Shapiro‐Wilk test was used due to nonnormal distribution. See Figure S1 for results broken out by sex, revealing no evidence of a sex‐specific effect. FMD indicates flow‐mediated dilation; and THC, Δ‐9‐tetrahydrocannabinol.
Notably, THC was efficiently delivered to the bloodstream by each vaporizer at its standard temperature, but not by the Volcano at 157 °C (7 out of 8 rats had THC values below or at the limit of quantitation), indicating that there was not a temperature that was useful for THC administration that did not also impair FMD (Table). Similarly, THC was not efficiently delivered by 1 minute of sidestream smoke exposure, despite impairing FMD.
Table 1.
Serum THC Levels (ng/mL) From Rats in Figure 2
| Rat no. | Volcano 185 °C | Volcano 157 °C | Yocan 237 °C | Sidestream smoke | Air |
|---|---|---|---|---|---|
| 1 | 0.48 | BLQ | 1.32 | 0.36 | BLQ |
| 2 | 0.52 | BLQ | 0.86 | BLQ | BLQ |
| 3 | BLQ | BLQ | 0.74 | BLQ | BLQ |
| 4 | 0.29 | 0.59 | 0.54 | BLQ | BLQ |
| 5 | 0.45 | 0.20 | 1.49 | BLQ | BLQ |
| 6 | 0.52 | BLQ | 3.75 | BLQ | BLQ |
| 7 | 0.22 | BLQ | 2.63 | BLQ | BLQ |
| 8 | 0.37 | BLQ | 1.07 | BLQ | BLQ |
| Mean | 0.37 | BLQ | 1.55 | BLQ | BLQ |
| SD | 0.14 | N/A | 1.03 | N/A | N/A |
LOQ is 0.2 ng/mL. The BLQ value for rat 3 in the 185 °C group was imputed to be LOQ/2=0.1 for purposes of calculation of the mean and SD for that group. BLQ indicates below limit of quantitation; LOQ, limit of quantitation; N/A, not applicable; and THC, Δ‐9‐tetrahydrocannabinol.
Exposure to Modeled Secondhand Aerosol From Volcano at Realistic Doses Impaired FMD
To examine potential effects of secondhand exposure to vaporizer aerosol, we roughly modeled a scenario in which unused aerosol was released from the balloon by collecting Volcano aerosol in the balloon and diluting a known volume into our 21‐L sidestream smoke exposure chamber. The groups consisted of 5 minutes of 1 time per min pulsatile exposure to undiluted Volcano aerosol (as described for Figure 2) as a positive control for FMD impairment, constant 1‐minute exposure to secondhand aerosol from the Volcano at ≈4666‐fold dilution (4.5 mL diluted to 21 L), and a clean air negative control. The dilution was roughly the equivalent of releasing 4 L of Volcano aerosol (one‐half of a balloon) into a 66‐sq ft bedroom, assuming 10‐ft ceiling height. This experiment used female rats (having been performed before the experiment shown in Figure 2); marijuana was 9.6% THC.
As shown in Figure 3, FMD was impaired by all of the aerosol conditions regardless of dilution. In the undiluted aerosol positive control, FMD was reduced by 44% (P<0.001), from 11.6±2.9% to 6.5±3.9%. FMD was also reduced by constant 1‐minute exposure to modeled secondhand marijuana aerosol at 4666‐fold dilution (47% reduction, P=0.005, from 12.7±2.7% to 6.7±2.6% with 1 outlier omitted; see asterisks in Figure 3). No significant impairment of FMD was observed in the air group (P=0.47, from 10.5±2.7% to 9.4±4.9%).
Figure 3. Impairment of FMD by exposure to 4.5 mL of Volcano marijuana aerosol diluted to 21 L.
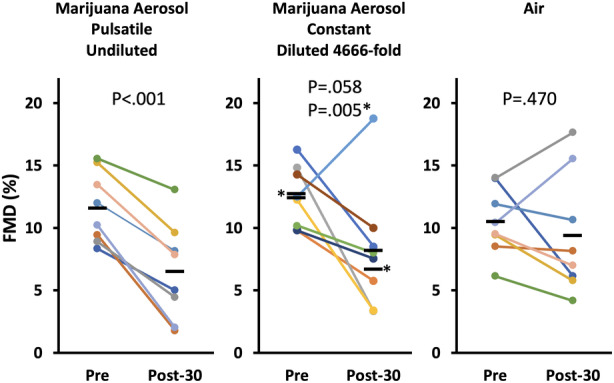
FMD was impaired similarly by concentrated and diluted aerosol, suggesting a threshold effect. Each colored line represents an individual rat; black bars show group means. Post‐30=30 minutes after final exposure. *P value and means with outlier removed. FMD indicates flow‐mediated dilation.
We were surprised to observe that such a low concentration of marijuana aerosol significantly impaired FMD, so we measured the concentration of particles using a DustTrak particle monitor in 4 separate experiments to confirm that it was within detectable levels. The Dusttrak monitor is from the same manufacturer as the Sidepak monitor used for standard calibrations; however, it has an enhanced sensor that can quantify a wider range of particle concentrations. The average mass of particles ≤2.5 μm in diameter in room air was 1.4±1.3 μg/m3. Air in the empty chamber was 0.74±0.84 μg/m3. The injection of 4.5 mL of Volcano aerosol into the exposure chamber raised the particle concentration to 52.7±12.1 μg/m3 (mean of the peak levels detected over the first minute in each of the 4 experiments; see paragraph about limitations in the Discussion section). The 1‐minute average particle concentrations of the undiluted Volcano aerosol were 37 749 μg/m3 when injected into the chamber, and 88 329 μg/m3 when measured directly from the Volcano balloon. The particle concentration of the sidestream cannabis smoke was 600 μg/m3.
Because FMD was impaired by such low concentrations of marijuana aerosol, we conducted an additional series of control exposures to determine if the effect could have been actually caused by factors other than the marijuana, such as reactions of aerosol or water vapor with the balloon polymer. For this experiment, rats received constant exposure for 1 minute to 42 000‐fold dilution (0.5 mL in the 21‐L chamber) of 9.6% THC marijuana aerosol generated as follows: Yocan (which does not use a balloon), Volcano with a standard supplied balloon, Volcano with an inert Tedlar bag instead of the balloon, Volcano with no balloon (piping aerosol from the heating chamber directly to the nose cone), water vapor passed through a supplied Volcano balloon, and clean air passed through a supplied Volcano balloon.
As shown in Figure 4, FMD was impaired by highly diluted aerosol generated from all of the conditions in which marijuana was present in the heating chamber, and by none of the conditions in which marijuana was absent. Diluted Yocan aerosol reduced FMD by 53% (P=0.0006; from 9.7±2.6% to 4.6±1.3%). Diluted aerosol from Volcano with standard balloon reduced FMD by 55% (P=0.012 with non‐parametric test; from 7.7±1.8% to 3.5±0.72%), from Volcano with inert Tedlar bag reduced FMD by 48% (P=0.001; from 8.6±2.6% to 4.5±2.3%), and from Volcano with no balloon reduced FMD by 43% (P=0.002; from 9.4±2.5% to 5.4±2.3%). No significant impairment of FMD was observed in rats exposed to water vapor collected in a normal Volcano balloon (P=0.61; 7.0±2.0% to 7.7±3.9%), or to air (P=0.38; 9.0±2.1% to 8.5±2.7%). There was not a significant difference between the percent reduction of the aerosol and smoke groups. See Figure S1 for results broken out by sex, revealing no evidence of a sex‐specific effect.
Figure 4. Impairment of FMD by modeled secondhand exposure to marijuana aerosol from both types of vaporizers controlling for balloon material and effects of water vapor.
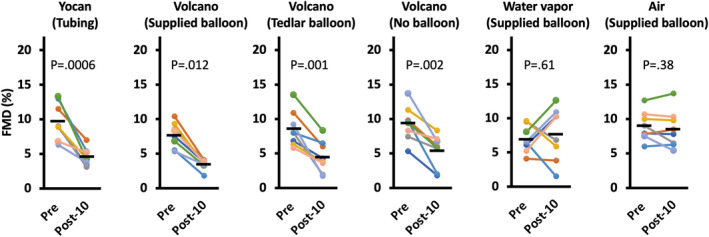
This series of controls for the impairment of FMD by highly diluted aerosol confirmed that the effect was not an artifact of reactions of aerosol or water vapor with balloon material. Each colored line represents an individual rat; black bars show group means. Post‐10=10 minutes after exposure. P values are from paired t tests except for the Volcano with supplied balloon group, for which the Shapiro‐Wilk test was used due to nonnormal distribution. FMD indicates flow‐mediated dilation.
Discussion
With an increasing number of states legalizing marijuana for recreational and medical use, the prevalence of its use has increased in recent years. 37 There is growing evidence that marijuana use is associated with cardiovascular disease, 6 , 7 , 38 , 39 although retrospective studies frequently do not specify whether use referred to smoking, vaporizing, or vaping/ingesting THC, which underscores the importance of understanding how adverse cardiovascular effects vary with different modes of exposure. The most common route of administration for medical marijuana is through inhalation of marijuana smoke via pipes, bongs, or rolled joints/blunts, and through inhalation of plant‐ or liquid‐derived aerosols from vaporization devices. 40 Notably, the term vaporizing cannabis is colloquially used to refer to the use of vaporizers both for plant material and for liquid THC, with the latter being analogous to a nicotine e‐cigarette and producing aerosol that is fundamentally different from that formed by leaf sublimation products. However, we and others have already shown that FMD is impaired in humans and rats by aerosols both from heated tobacco products (essentially tobacco leaf vaporizers) and from e‐cigarettes, 22 , 23 , 24 , 25 , 34 , 35 , 36 so there was a precedent to expect that marijuana leaf vaporizer aerosol also has this effect. Nonetheless, because cannabis leaf vaporizers use temperatures that are substantially lower than heated tobacco products (185–237 °C versus 350 °C), there was a possibility that marijuana leaf aerosol would exert less of an effect than heated tobacco product aerosol. Our findings that marijuana leaf vaporizer aerosol impairs endothelial function in rats, comparably to marijuana smoke and to emissions from various tobacco products, indicate that the identity of the plant and the temperature of vaporization do not determine whether the aerosol impairs FMD. This is consistent with our recently reported findings that acute impairment of FMD is caused by a wide range of disparate inhaled materials that appears to result from airway irritation. 41
We have taken pains to approximate real‐world vaporizer use conditions by using nose cone exposure rather than whole body exposure. Several rodent studies in the past have used whole‐body exposure chambers to deliver aerosolized THC liquid, 42 , 43 , 44 , 45 and were thus not as relevant to the real‐world pulsatile exposure to aerosols containing plant sublimation products that we have modeled here. Farra et al 46 have performed mouse studies using nose‐only pulsatile exposure to cannabis plant material vaporized using a Volcano to study neurological and behavioral effects. Use of nose cones enables truly pulsatile exposure of rats to aerosol that is undiluted during each pulse, and replaced completely by room air in between pulses by removing the rats from the nose cones, thus mimicking the interval between each aerosol inhalation that marijuana users take. Avoiding the use of whole‐body chambers also avoids potential ocular exposure or continued intake from self‐grooming after the exposure period.
Our experiment that tested effects of vaporizer aerosol generated at different temperatures was an attempt to find a condition that could fulfill the intended purpose of the vaporizer use (self‐administration of THC) without the consequence of FMD impairment. However, we observed conditions in which THC was not effectively delivered but FMD was still impaired, not the other way around. Another interesting observation from that experiment was that of the 2 conditions that did deliver detectable THC to the blood, the handheld Yocan at 237 °C (1.55±1.03 ng/mL) was more effective than the Volcano at 185 °C (0.37±0.14 ng/mL). It is unclear whether this difference resulted from the different temperatures or the direct connection of the Yocan versus the balloon intermediate step of the Volcano. For comparison, plasma THC levels in humans were reported to be roughly 10 ng/mL when sampled 10 minutes after a session of Volcano use involving inhalation of 3 complete balloons of aerosol, 47 which is a more extreme exposure regimen than the 5 inhalations in 5 minutes experienced by the rats in this study. Notably, 1 minute of sidestream smoke at a realistic concentration of 600 μg/m3 RSP did not lead to detectable serum THC levels, which is consistent with the difficulty in achieving a secondhand contact high, 48 although FMD was still substantially impaired.
Indoor marijuana vaporizing can create high particle concentrations hazardous to humans and rivaling those seen in extreme air pollution events. 49 Therefore, secondhand exposure to marijuana vaporizer aerosol is a potentially serious hazard in homes or for workers in cannabis lounges that is not well understood. Unlike combustible tobacco and marijuana cigarettes, which produce sidestream smoke from the burning tip even when not being actively puffed, liquid vaping devices and leaf vaporizers primarily produce secondhand emissions by users exhaling the inhaled aerosol. This is difficult to model, due to complexities of filtration and absorption of droplets in mouth and the airway. However, the Volcano can produce secondhand aerosol via a different process, the expulsion of unused balloon contents into the room. The Volcano balloons contain ≈8 L of aerosol, and not every user consumes the entire contents every time. We thus focused on simulating this scenario as a more controllable and tractable model. Surprisingly, we observed that FMD was impaired in rats exposed to extremely low levels of aerosols comparable to the expected dilution of partial Volcano bags into residential rooms, to roughly the same extent as the impairment from concentrated aerosols. Our particle mass measurements were done under the exact same conditions, with the aerosol injected 8 cm above the port where the rats were exposed, and the data were averaged over the same time: 1 minute. With these high dilutions, the particle concentrations declined rapidly and may not have been detectable if we had located the sampling port a greater distance from the injection port. The FMD impairment, which was confirmed through meticulous and comprehensive controls, is again consistent with our report that FMD impairment from inhalational exposures occurs within 1 minute and can result from airway irritation in a process involving the vagus nerve. 41 These results should raise awareness that secondhand exposure to vaporizer aerosols may be not without risk, and it is worthwhile to consider if someone uses vaporizers for medicinal or recreational purposes where other people are present.
Limitations
Although our model has advantages over some of the prior models explained above, it has limitations. First, the FMD measurement technique required that the animals were anesthetized, which may influence FMD. However, all control animals were similarly anesthetized, and FMD impairment from smoke and aerosol exposure has also been observed in conscious humans.
These results cannot be generalized to marijuana vaporizers using cannabidiol oil/THC‐containing liquids. Moreover, the THC content and postharvest handling of the research marijuana (eg, drying regimen) used in this study differs from that of the marijuana available to the public. As such, our results may not be completely reflective of the extent of endothelial dysfunction after the use of marijuana in the real world. However, we have reported at the 2022 American Heart Association Scientific Sessions 50 that impairment of endothelial function by marijuana smoke and vaporizer aerosol is not influenced by cannabinoid profile or drying regimen.
Although evidence indicates that repeated exposures to secondhand tobacco smoke leads to chronic endothelial dysfunction, 19 the direct clinical implications of impaired FMD by acute exposure to vaporizer aerosol are uncertain. Cardiovascular measurements in human studies of vaporizer use would be worthwhile.
Of note, our modeling of secondhand exposure by diluting small volumes of concentrated aerosol directly into a large chamber of air likely does not result in immediate even distribution; there are swirls and tendrils of more concentrated material. Consistent with this concern, our individual particle measurement runs were highly variable, and fluctuated quite a bit during the measurement period (measured 4 times over 1 minute). This highlights how unreliable the dilution is, regardless of technique, due to stochastic nature of how particles behave during the dilution process. Even putting the rat's nose into the space after the concentrated aerosol has been pushed in from the syringe does not prevent the nose from encountering unpredictable and uncharacterized hyperlocal levels transiently that may be much higher than the intended target concentration. Therefore, results from our secondhand exposure model should be interpreted qualitatively; that is, FMD can be impaired by highly diluted levels of aerosol, but we cannot conclude that the specific level that we measured as ≈50 μg/m3 impairs FMD.
In conclusion, exposure of rats to marijuana leaf vaporizer aerosol impairs endothelial function, regardless of the marijuana cannabinoid level, temperature, and the device used. Modeled secondhand exposure to dilute marijuana aerosol impairs endothelial function comparably to marijuana smoke. It is highly likely that vaporizing marijuana plant material does avoid some of the adverse effects of smoking it, based on the highly complex chemical and physical properties of smoke. However, our findings suggest that aerosols from marijuana leaf vaporizers are not harmless and can result in adverse vascular effects similar to those of smoking it, both to the user and potentially to bystanders.
Sources of Funding
Research reported in this publication was supported by grants 25IR‐0030 and 28IR‐0049 from the California Tobacco‐Related Disease Research Program, and a generous donation from the Elfenworks Foundation in memory of Deb O'Keefe. Laboratory resources for analytical chemistry at University of California, San Francisco were supported in part by National Institutes of Health grant P30 DA012393.
Disclosures
None.
Supporting information
Table S1
Figure S1
Acknowledgments
The authors thank the National Drug Supply Program of the National Institute of Drug Abuse for providing research marijuana, Drs Glantz and Ling for helpful discussions about this study, and Dr Wang for helpful discussions and for preparing the publicly accessible data set.
This article was sent to Daniel Edmundowicz, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032969
For Sources of Funding and Disclosures, see page 10.
References
- 1. Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry. 2015;72:1235–1242. doi: 10.1001/jamapsychiatry.2015.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Compton WM, Han B, Jones CM, Blanco C, Hughes A. Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross‐sectional surveys. Lancet Psychiatry. 2016;3:954–964. doi: 10.1016/S2215-0366(16)30208-5 [DOI] [PubMed] [Google Scholar]
- 3. Chambers J, Keyhani S, Ling PM, Hoggatt KJ, Hasin D, Nguyen N, Woods A, Ryder A, Cohen BE. Perceptions of safety of daily cannabis vs tobacco smoking and secondhand smoke exposure, 2017–2021. JAMA Netw Open. 2023;6:e2328691. doi: 10.1001/jamanetworkopen.2023.28691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haffajee RL, MacCoun RJ, Mello MM. Behind schedule–reconciling federal and state marijuana policy. N Engl J Med. 2018;379:501–504. doi: 10.1056/NEJMp1804408 [DOI] [PubMed] [Google Scholar]
- 5. Barry RA, Glantz S. A public health framework for legalized retail marijuana based on the US experience: avoiding a new tobacco industry. PLoS Med. 2016;13:e1002131. doi: 10.1371/journal.pmed.1002131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladha KS, Mistry N, Wijeysundera DN, Clarke H, Verma S, Hare GMT, Mazer CD. Recent cannabis use and myocardial infarction in young adults: a cross‐sectional study. CMAJ. 2021;193:E1377–e1384. doi: 10.1503/cmaj.202392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18‐44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke. 2020;51:308–310. doi: 10.1161/STROKEAHA.119.027828 [DOI] [PubMed] [Google Scholar]
- 8. Varlet V, Concha‐Lozano N, Berthet A, Plateel G, Favrat B, De Cesare M, Lauer E, Augsburger M, Thomas A, Giroud C. Drug vaping applied to cannabis: is "Cannavaping" a therapeutic alternative to marijuana? Sci Rep. 2016;6:25599. doi: 10.1038/srep25599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giroud C, de Cesare M, Berthet A, Varlet V, Concha‐Lozano N, Favrat B. E‐cigarettes: a review of new trends in cannabis use. Int J Environ Res Public Health. 2015;12:9988–10008. doi: 10.3390/ijerph120809988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gieringer DH. Cannabis “vaporization” a promising strategy for smoke harm reduction. J Cannabis Therap. 2001;1:153–170. doi: 10.1300/J175v01n03_10 [DOI] [Google Scholar]
- 11. Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther. 2007;82:572–578. doi: 10.1038/sj.clpt.6100200 [DOI] [PubMed] [Google Scholar]
- 12. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-F [DOI] [PubMed] [Google Scholar]
- 13. Pyke KE, Tschakovsky ME. The relationship between shear stress and flow‐mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568:357–369. doi: 10.1113/jphysiol.2005.089755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4 [DOI] [PubMed] [Google Scholar]
- 16. Nabel EG, Selwyn AP, Ganz P. Large coronary arteries in humans are responsive to changing blood flow: an endothelium‐dependent mechanism that fails in patients with atherosclerosis. J Am Coll Cardiol. 1990;16:349–356. doi: 10.1016/0735-1097(90)90584-C [DOI] [PubMed] [Google Scholar]
- 17. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/S0735-1097(03)00994-X [DOI] [PubMed] [Google Scholar]
- 18. Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose‐related and potentially reversible impairment of endothelium‐dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.CIR.88.5.2149 [DOI] [PubMed] [Google Scholar]
- 19. Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium‐dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303 [DOI] [PubMed] [Google Scholar]
- 20. Kato T, Inoue T, Morooka T, Yoshimoto N, Node K. Short‐term passive smoking causes endothelial dysfunction via oxidative stress in nonsmokers. Can J Physiol Pharmacol. 2006;84:523–529. doi: 10.1139/y06-030 [DOI] [PubMed] [Google Scholar]
- 21. Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040 [DOI] [PubMed] [Google Scholar]
- 22. Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AG, De Falco E, Chimenti I, et al. Acute impact of tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest. 2016;150:606–612. doi: 10.1016/j.chest.2016.04.012 [DOI] [PubMed] [Google Scholar]
- 23. Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019;293:97–106. doi: 10.1148/radiol.2019190562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biondi‐Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, et al. Acute effects of heat‐not‐burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome‐Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR–VAPES) 2 randomized trial. J Am Heart Assoc. 2019;8:e010455. doi: 10.1161/JAHA.118.010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohammadi L, Han DD, Xu F, Huang A, Derakhshandeh R, Rao P, Whitlatch A, Cheng J, Keith RJ, Hamburg NM, et al. Chronic E‐cigarette use impairs endothelial function on the physiological and cellular levels. Arterioscler Thromb Vasc Biol. 2022;42:1333–1350. doi: 10.1161/ATVBAHA.121.317749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haptonstall KP, Choroomi Y, Moheimani R, Nguyen K, Tran E, Lakhani K, Ruedisueli I, Gornbein J, Middlekauff HR. Differential effects of tobacco cigarettes and electronic cigarettes on endothelial function in healthy young people. Am J Physiol Heart Circ Physiol. 2020;319:H547–h556. doi: 10.1152/ajpheart.00307.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hutchison SJ, Sievers RE, Zhu BQ, Sun YP, Stewart DJ, Parmley WW, Chatterjee K. Secondhand tobacco smoke impairs rabbit pulmonary artery endothelium‐dependent relaxation. Chest. 2001;120:2004–2012. doi: 10.1378/chest.120.6.2004 [DOI] [PubMed] [Google Scholar]
- 28. Olfert IM, DeVallance E, Hoskinson H, Branyan KW, Clayton S, Pitzer CR, Sullivan DP, Breit MJ, Wu Z, Klinkhachorn P, et al. Chronic exposure to electronic cigarettes results in impaired cardiovascular function in mice. J Appl Physiol (1985). 2018;124:573–582. doi: 10.1152/japplphysiol.00713.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jin L, Lynch J, Richardson A, Lorkiewicz P, Srivastava S, Theis W, Shirk G, Hand A, Bhatnagar A, Srivastava S, et al. Electronic cigarette solvents, pulmonary irritation, and endothelial dysfunction: role of acetaldehyde and formaldehyde. Am J Physiol Heart Circ Physiol. 2021;320:H1510–H1525. doi: 10.1152/ajpheart.00878.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heiss C, Sievers RE, Amabile N, Momma TY, Chen Q, Natarajan S, Yeghiazarians Y, Springer ML. In vivo measurement of flow‐mediated vasodilation in living rats using high‐resolution ultrasound. Am J Physiol Heart Circ Physiol. 2008;294:H1086–H1093. doi: 10.1152/ajpheart.00811.2007 [DOI] [PubMed] [Google Scholar]
- 31. Pinnamaneni K, Sievers RE, Sharma R, Selchau AM, Gutierrez G, Nordsieck EJ, Su R, An S, Chen Q, Wang X, et al. Brief exposure to secondhand smoke reversibly impairs endothelial vasodilatory function. Nicotine Tob Res. 2014;16:584–590. doi: 10.1093/ntr/ntt189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Wang X, Narayan S, Glantz SA, Schick SF, Springer ML. Impairment of endothelial function by little cigar secondhand smoke. Tob Regul Sci. 2016;2:56–63. doi: 10.18001/TRS.2.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, Danforth OM, Pinnamaneni K, Rodriguez HJ, Luu E, et al. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc. 2016;5:e003858. doi: 10.1161/JAHA.116.003858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nabavizadeh P, Liu J, Havel CM, Ibrahim S, Derakhshandeh R, Jacob Iii P, Springer ML. Vascular endothelial function is impaired by aerosol from a single IQOS HeatStick to the same extent as by cigarette smoke. Tob Control. 2018;27:s13–s19. doi: 10.1136/tobaccocontrol-2018-054325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao P, Liu J, Springer ML. JUUL and combusted cigarettes comparably impair endothelial function. Tob Regul Sci. 2020;6:30–37. doi: 10.18001/TRS.6.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rao P, Han DD, Tan K, Mohammadi L, Derakhshandeh R, Navabzadeh M, Goyal N, Springer ML. Comparable impairment of vascular endothelial function by a wide range of electronic nicotine delivery devices. Nicotine Tob Res. 2022;24:1055–1062. doi: 10.1093/ntr/ntac019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cerdá M, Mauro C, Hamilton A, Levy NS, Santaella‐Tenorio J, Hasin D, Wall MM, Keyes KM, Martins SS. Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry. 2020;77:165–171. doi: 10.1001/jamapsychiatry.2019.3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abouk R, Adams S. Examining the relationship between medical cannabis laws and cardiovascular deaths in the US. Int J Drug Policy. 2018;53:1–7. doi: 10.1016/j.drugpo.2017.11.022 [DOI] [PubMed] [Google Scholar]
- 39. Page RL II, Allen LA, Kloner RA, Carriker CR, Martel C, Morris AA, Piano MR, Rana JS, Saucedo JF. Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2020;142:e131–e152. doi: 10.1161/CIR.0000000000000883 [DOI] [PubMed] [Google Scholar]
- 40. Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross‐sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1:131–138. doi: 10.1089/can.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nabavizadeh P, Liu J, Rao P, Ibrahim S, Han DD, Derakhshandeh R, Qiu H, Wang X, Glantz SA, Schick SF, et al. Impairment of endothelial function by cigarette smoke is not caused by a specific smoke constituent, but by vagal input from the airway. Arterioscler Thromb Vasc Biol. 2022;42:1324–1332. doi: 10.1161/ATVBAHA.122.318051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freels TG, Baxter‐Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, Petrie GN, Yu Z, Clowers BH, Cuttler C, et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug‐seeking behavior in rats. J Neurosci. 2020;40:1897–1908. doi: 10.1523/JNEUROSCI.2416-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javadi‐Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA. Effects of Δ9‐THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology. 2018;235:2541–2557. doi: 10.1007/s00213-018-4946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spencer S, Neuhofer D, Chioma VC, Garcia‐Keller C, Schwartz DJ, Allen N, Scofield MD, Ortiz‐Ithier T, Kalivas PW. A model of Δ(9)‐tetrahydrocannabinol self‐administration and reinstatement that alters synaptic plasticity in nucleus accumbens. Biol Psychiatry. 2018;84:601–610. doi: 10.1016/j.biopsych.2018.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manwell LA, Mallet PE. Comparative effects of pulmonary and parenteral Δ9‐tetrahydrocannabinol exposure on extinction of opiate‐induced conditioned aversion in rats. Psychopharmacology. 2015;232:1655–1665. doi: 10.1007/s00213-014-3798-5 [DOI] [PubMed] [Google Scholar]
- 46. Farra YM, Eden MJ, Coleman JR, Kulkarni P, Ferris CF, Oakes JM, Bellini C. Acute neuroradiological, behavioral, and physiological effects of nose‐only exposure to vaporized cannabis in C57BL/6 mice. Inhal Toxicol. 2020;32:200–217. doi: 10.1080/08958378.2020.1767237 [DOI] [PubMed] [Google Scholar]
- 47. Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol. 2019;43:233–258. doi: 10.1093/jat/bky104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R. Non‐smoker exposure to secondhand cannabis smoke II: effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug Alcohol Depend. 2015;151:194–202. doi: 10.1016/j.drugalcdep.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murphy MB, Huang AS, Schick SF. PM2.5 concentrations in a cannabis store with on‐site consumption. Environ Health Perspect. 2021;129:067701. doi: 10.1289/EHP8689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rao P, Goyal N, Derakhshandeh R, Havel C, Jacob P III, Springer ML. Abstract 10022: marijuana smoke or vaporizer aerosol impairs endothelial function regardless of drying regimen or cannabinoid profile. Circulation. 2022;146:A10022. doi: 10.1161/circ.146.suppl_1.10022 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


