Abstract
Background
We performed a meta‐analysis of reconstructed time‐to‐event data from randomized controlled trials (RCTs) and propensity‐score matched (PSM) studies comparing transcatheter versus surgical aortic valve replacement (TAVR versus SAVR) to evaluate midterm outcomes in patients considered low risk for SAVR.
Methods and Results
Study‐level meta‐analysis of reconstructed time‐to‐event data from Kaplan–Meier curves of RCTs and PSM studies published by December 31, 2022 was conducted. Eight studies (3 RCTs, 5 PSM studies) met our eligibility criteria and included 5444 patients; 2639 patients underwent TAVR, and 2805 patients underwent SAVR. TAVR showed a higher risk of all‐cause mortality at 8 years of follow‐up (hazard ratio [HR] 1.22, [95% CI, 1.03–1.43], P=0.018). Up to 2 years of follow‐up, TAVR was not inferior to SAVR (HR, 1.08 [95% CI, 0.89–1.31], P=0.448); however, we observed a statistically significant difference after 2 years with higher mortality with TAVR (HR, 1.51 [95% CI, 1.14–2.00]; P=0.004). This difference was driven by PSM studies; our sensitivity analysis showed a statistically significant difference between TAVR and SAVR when we included only PSM studies (HR, 1.41 [95% CI, 1.16–1.72], P=0.001) but no statistically significant difference when we included only RCTs (HR, 0.89 [95% CI, 0.69–1.16], P=0.398).
Conclusions
In comparison with TAVR, SAVR appeared to be associated with improved survival beyond 2 years in low‐risk patients. However, the survival benefit of SAVR was observed only in PSM studies and not in RCTs. The addition of data from ongoing RCTs as well as longer follow‐up in previous RCTs will help to confirm if there is a difference in mid‐ and long‐term survival between TAVR versus SAVR in the low‐risk population.
Keywords: cardiac surgical procedures, cardiovascular surgical procedures, heart valve diseases, heart valve prosthesis implantation, meta‐analysis, transcatheter aortic valve replacement
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Cardiovascular Surgery
Nonstandard Abbreviations and Acronyms
- PSM
propensity score matching
- RMST
restricted mean survival time
- SAVR
surgical aortic valve replacement
- TAVR
transcatheter aortic valve replacement
Clinical Perspective.
What Is New?
In the scenario of transcatheter versus surgical aortic valve replacement in patients deemed low risk for surgical aortic valve replacement, surgery is associated with improved survival after 2 years when we consider randomized controlled trials and propensity‐score matched studies together.
Considering randomized controlled trials and propensity‐score matched studies separately, the benefit of surgical aortic valve replacement over transcatheter aortic valve replacement is apparent in the latter but not in the former.
What Are the Clinical Implications?
Because there remain some discrepancies between real‐world and randomized controlled trial‐generated data, heart teams should not adopt transcatheter aortic valve replacement indiscriminately in low‐risk patients but rather make decisions on a case‐by‐case basis.
Several randomized controlled trials (RCTs) have been conducted in the past decade to compare the outcomes of transcatheter aortic valve replacement (TAVR) versus surgical aortic valve replacement (SAVR) in patients with severe aortic stenosis (AS). Recently, Barili et al 1 showed that the mortality rates in RCTs of TAVR versus SAVR in patients with severe aortic stenosis (AS) are affected by treatments with a time‐varying effect. In a second study, Barili et al 2 showed that TAVR for the treatment of native valves shows a strong protective effect in the short term, which is no longer present after 1 year; however, it becomes a risk factor for all‐cause mortality after 24 months and for rehospitalization after 6 months. Although these studies were well conducted, Barili et al 1 , 2 included in their studies patients at high, intermediate, and low risk and pooled them together, which may have led to some confounding bias due to the differences in the risk profiles across the studies.
When it comes specifically to the population at low risk for SAVR, controversy exists as whether the available data justify further TAVR expansion to this group, which represents up to 75% of all patients with AS. 3 Therefore, we performed a meta‐analysis with reconstructed time‐to‐event data of RCTs and propensity score‐matched (PSM) studies to compare overall survival at the longest follow‐up after TAVR and SAVR in low‐risk symptomatic patients with severe AS.
Methods
Eligibility Criteria, Databases, and Search Strategy
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐analyses reporting guideline. 4 All data support the findings of this study are available from the corresponding author upon reasonable request. Using the Population, Interventions, Comparison, Outcome, and Study design strategy, studies were included if the following criteria were fulfilled:
The population comprised patients with severe AS.
There was an intervention group undergoing TAVR.
There was a second intervention group undergoing SAVR.
Outcomes studied included overall survival and/or all‐cause death (with Kaplan–Meier curves).
The study design was RCT or PSM.
The following sources were searched for articles meeting our inclusion criteria and published by December 31, 2022: PubMed/MEDLINE, EMBASE, SciELO, LILACS, CENTRAL/CCTR (Cochrane Controlled Trials Register), Google Scholar, and the reference lists of relevant articles. The search strategy used for each database is fully expanded in Tables S1–S6. Exclusion criteria included studies with overlapping samples, studies without Kaplan–Meier curves for analysis of survival, and studies considered non‐RCTs and non‐PSM studies.
The following steps were taken for study selection: (1) identification of titles of records through database search; (2) removal of duplicates; (3) screening and selection of abstracts; (4) assessment for eligibility through full‐text articles; and (5) final inclusion in study. Studies were selected by 2 independent reviewers. When there was disagreement, a third reviewer made the decision to include or exclude the study. Ethical approval was not applicable for this study, as it consisted of a systematic review and meta‐analysis. There were no language restrictions.
Assessment of Risk of Bias
The Cochrane tools Risk of Bias in randomized trials 2 5 and Risk of Bias in Non‐Randomized Studies of Interventions 6 were systematically used to assess included studies for risk of bias. Two independent reviewers assessed risk for bias. When there was a disagreement, a third reviewer checked the data and made the final decision.
Statistical Analysis
Individual patient data derived from published Kaplan–Meier graphs from all included studies were reconstructed using the “curve approach.” 7 We used the 2‐stage approach as described by Liu et al 8 based on the R package “IPDfromKM” (version 1.2.3.0). In the first stage, raw data coordinates (time, survival probability) were extracted from each subgroup in each of the respective Kaplan–Meier curves. In the second stage, the data coordinates were processed based on the raw data coordinates from the first stage in conjunction with the numbers at risk at given time points, and individual patient data (time‐to‐event or time‐to‐last‐follow‐up for each individual patient) were reconstructed. To construct the final study data set, reconstructed individual patient data from individual studies were merged. Overall survival at follow‐up in patients at low risk were visually assessed using Kaplan–Meier estimates. Hazard ratios (HRs) with 95% CIs for the difference between both subgroups were calculated using a Cox frailty regression model. Between‐study heterogeneity was assessed by the inclusion of a γ frailty term, where individual studies modeled as a random effect using random intercepts. A likelihood ratio test was used to test the significance of this γ frailty term. A robust variance estimator was used to account for violations of the assumption of homoscedasticity (or homogeneity of variances, which is an assumption of equal or similar variances in different groups being compared). An HR >1 indicated a higher risk of mortality in patients underwent TAVR in comparison with SAVR. The proportional hazards assumption of each Cox model was checked with the Grambsch‐Therneau test and diagnostic plots based on Schoenfeld residuals. 9 The landmark analysis was performed based on either visual or statistical violation of proportionality of the hazards. Flexible parametric survival models (Royston‐Parmar models or generalized survival models) with B‐splines were calculated to provide HRs with 95% CIs as a measure of association between intervention and mortality, while allowing for a time‐varying effect. 10 Additionally, we modeled the difference in restricted mean survival times (RMSTs) over time using the R package “survRM2” (version 1.0–4). 11
Finally, we explored the outcomes according to type of study (RCTs versus PSM studies) and possible modulating effects of covariates (such as age, female sex, diabetes, coronary artery disease, risk score, permanent pacemaker implantation, prosthesis‐patient mismatch, paravalvular leakage) on mortality risk with meta‐regression analyses. All analyses were completed with R Statistical Software (version 4.2.1, Foundation for Statistical Computing, Vienna, Austria).
Results
Study Selection and Characteristics
After excluding duplicates and noneligible studies, 8 studies 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 met our eligibility criteria (Figures S1–S6). Three studies were RCTs 13 , 15 , 16 and 5 studies were PSM 12 , 14 , 17 , 18 , 19 (Table S2). A total of 5444 patients were included (2639 patients underwent TAVR, and 2805 patients underwent SAVR). Patients' characteristics are shown in Table S3. Mean age in all the studies were over 70 years, ranging from 73.3 to 80.1 years. Female patients were well represented in the studies. Transfemoral access was the most frequently used approach for TAVR. Six studies 13 , 14 , 15 , 16 , 17 , 18 reported the mean score for the perioperative procedural risk of mortality using the Society of Thoracic Surgeons Predicted Risk of Mortality score at 30 days, ranging from 1.9 to 3.0 points. In addition, 2 studies 12 , 19 reported the mean score for the logistic European System for Cardiac Operative Risk Evaluation II, ranging from 2.0 to 2.6 points. Figure S2 shows the qualitative assessment of the studies with the tools Risk of Bias in Randomized Trials 2 and Risk of Bias in Non‐Randomized Studies of Interventions.
Analysis of Overall Survival
Figure 1 depicts the pooled overall survival combining data from the 8 included studies. The overall survival in the TAVR arm was 48.4% (95% CI, 41.6–56.2), and overall survival in the SAVR arm was 57.2% (95% CI, 49.8–65.6). Our pooled survival analysis revealed that patients who underwent TAVR had a significantly higher risk of mortality compared with patients who underwent SAVR (HR, 1.22 [95% CI, 1.03–1.43], P=0.018). There was significant statistical heterogeneity between studies (likelihood ratio test, P<0.001; Table S4).
Figure 1. Main analysis of overall survival up to 8 years, including all studies.
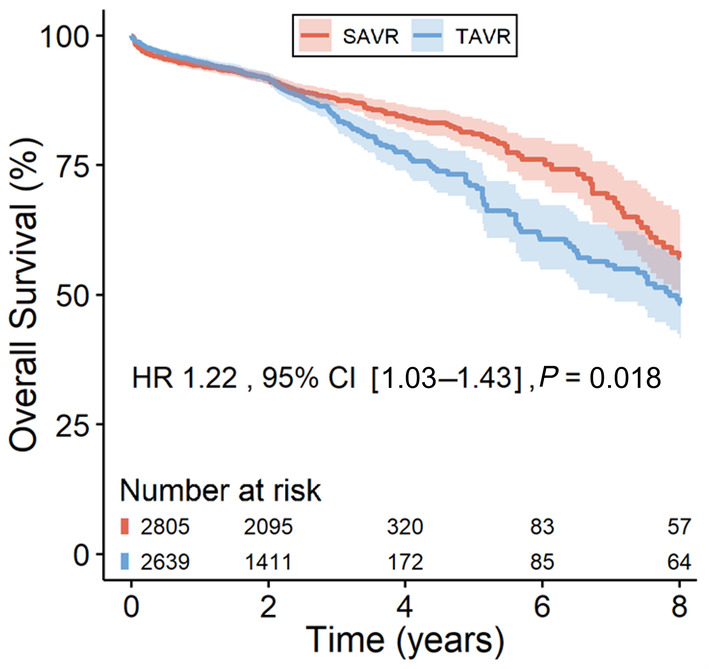
The solid lines represent the estimates, and the surrounding bands are 95% CI. HR indicates hazard ratio; SAVR, surgical aortic valve replacement; and TAVR, transcatheter aortic valve replacement.
There was visual evidence of violation of the proportional hazards assumption in the Kaplan–Meier curve (Figure 1 around the 2‐year time point), and in the plot of Schoenfeld residuals against time (Figure S3), whereas the Grambsch‐Therneau test for time‐invariant effect was not statistically significant (P=0.053; Figure S3). Figure 2 presents the analysis of time‐varying HRs for mortality based on flexible parametric survival models with B‐splines. This revealed a statistically significant decreased risk of mortality with TAVR (HR <1) in the postprocedural period (up to 3.2 months) followed by a short period of similar risk between 3.2 months and 11 months. However, thereafter, a reversal starts at the 11‐month mark, indicating a higher risk associated with TAVR during the remaining follow‐up up to 8 years.
Figure 2. Analysis of time‐varying hazard ratios for mortality based on flexible parametric survival models with B‐splines.
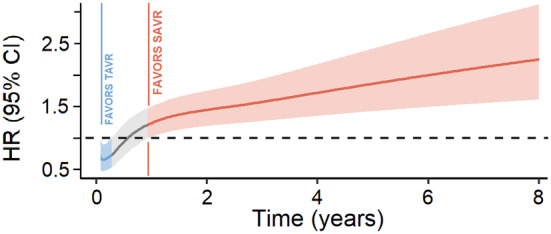
HR indicates hazard ratio; SAVR, surgical aortic valve replacement; and TAVR, transcatheter aortic valve replacement.
Figure 3 shows the difference in the mean event‐free survival time (eg, difference in RMST) over the entire follow‐up. The relatively stable trend of the curve up to 4 years shows that the early benefit due to reduced mortality risk in the TAVR arm influences the RMST up to 4.13 years. In the first year following intervention, the treatment difference in RMST is statistically significant. A greater number of events in the TAVR arm takes place thereafter reducing its benefit, as apparent in the downwards trend. At 8 years follow‐up after the primary intervention, the treatment difference in RMST is statistically significant, resulting in a benefit of 187.2 days (95% CI, 85.1–289.2, P<0.001) in the SAVR arm.
Figure 3. Restricted mean survival time over the entire follow‐up.
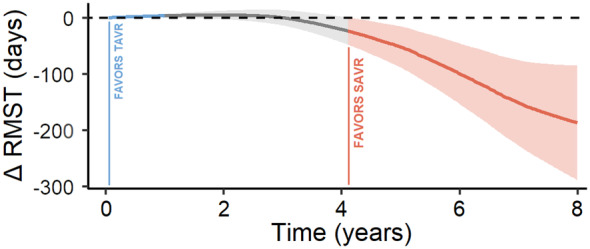
RMST indicates restricted mean survival time; SAVR, surgical aortic valve replacement; and TAVR, transcatheter aortic valve replacement.
Landmark Analysis
Landmark analysis was performed, designating 2 years as the landmark time point (Figure 4) as we can visualize the curves crossing at this timepoint in Figure 1. In the first 2 years after intervention, the risk of mortality was similar in both groups (HR, 1.08 [95% CI, 0.89–1.31], P=0.448). Beyond 2 years, the landmark analysis showed an increased risk of mortality associated with TAVR in comparison with SAVR (HR, 1.51 [95% CI, 1.14–2.00], P=0.004).
Figure 4. Landmark analysis.
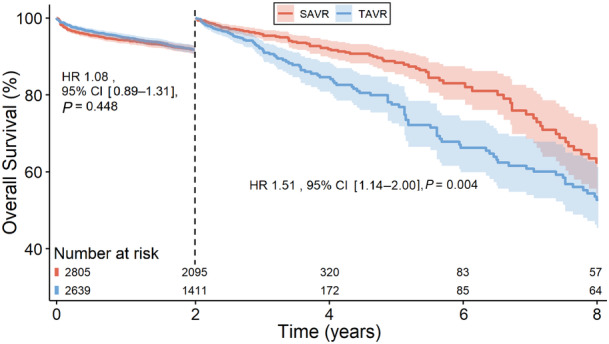
HR indicates hazard ratio; SAVR, surgical aortic valve replacement; and TAVR, transcatheter aortic valve replacement.
Sensitivity Analysis
We investigated the overall survival according to type of study (RCT versus PSM) and found out that there was no statistically significant difference between TAVR and SAVR when we included only RCTs (Figure 5A shows risk of mortality for TAVR in comparison with SAVR, HR, 0.89 [95% CI, 0.69–1.16], P=0.398). However, a statistically significant difference was observed when we included only PSM studies (Figure 5B shows risk of mortality for TAVR in comparison with SAVR, HR, 1.41 [95% CI, 1.16–1.72], P=0.001). The plot of Schoenfeld residuals against time and results from the Grambsch‐Therneau test are presented in Figure S4.
Figure 5. Sensitivity analysis.
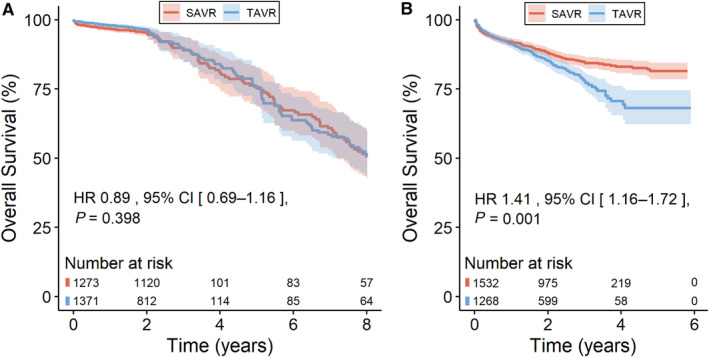
A, Randomized controlled trials. B, Propensity‐score matched studies. HR indicates hazard ratio; SAVR, surgical aortic valve replacement; and TAVR, transcatheter aortic valve replacement.
Meta‐Regression Analysis
Our meta‐regression analyses (Table S5) were unable to identify significant modulating effects of covariates (such as age, sex, diabetes, coronary artery disease, risk score, postprocedural permanent pacemaker implantation, prosthesis‐patient mismatch, and paravalvular leakage) on the outcomes. These covariates can be found in Table S3 and Table S6.
Discussion
Summary of Evidence
To the best of our knowledge, this is the largest pooled meta‐analysis of reconstructed time‐to‐event data comparing TAVR and SAVR in low‐risk patients. The main finding of this analysis was that survival is similar with SAVR versus TAVR for the first 2 years but appears to be better with SAVR beyond 2‐year follow‐up. However, the fact that this time‐varying difference in survival between SAVR and TAVR was mostly observed in PSM studies and not in RCTs precludes any definitive conclusion.
Comments
The main findings of this study are that the difference in overall survival between TAVR and SAVR and may be minimal or absent in the short term (<2 years) but may favor SAVR in the mid‐ and long‐term follow‐up. However, the fact that this survival benefit of SAVR versus TAVR beyond 2‐year follow‐up was observed only in PSM studies and not in RCTs precludes definitive conclusion regarding the superiority of SAVR for midterm survival.
Considering that the feasibility and immediate safety of TAVR are well reported in RCTs, 13 , 15 , 16 the fast development and broader indication of TAVR in younger and lower‐risk patients have led to closer attention to longer follow‐ups beyond the immediate postprocedural period. 20 Although immediate and short‐term outcomes such as shorter length of hospital stay and lower rates of readmission in the first months is a proof of the better outcomes of TAVR within the first months due to the less invasive nature of this procedure, 21 later outcomes such as valve thrombosis, structural valve deterioration, and coronary events may alter the initial early survival benefit of TAVR.
Although our meta‐regression analyses failed to show any modulating effects of the rates of permanent pacemaker implantation, prosthesis‐patient mismatch, and paravalvular leakage on the outcomes, these complications may likely have had an impact on the long‐term outcomes. Recent meta‐analyses based on reconstructed time‐to‐event data 22 , 23 , 24 showed the negative impact of permanent pacemaker implantation, prosthesis‐patient mismatch, and paravalvular leakage on later all‐cause death, heart‐failure‐related rehospitalization and cardiovascular death after TAVR. As shown in Table S2, TAVR is associated with substantially higher rates of postprocedural permanent pacemaker implantation and paravalvular leakage, whereas SAVR presents higher rates of prosthesis‐patient mismatch (the latter being associated with worse survival in both TAVR 22 and SAVR 25 ).
How Does Our Meta‐Analysis Differ From Previous Meta‐Analyses?
Recently, Ahmad et al 26 published a meta‐analysis comparing TAVR to SAVR with a pragmatic risk classification partitioning lower‐risk and higher‐risk patients. The authors included exclusively RCTs and concluded that, in lower‐risk patients, there was an early mortality reduction with TAVR but no differences after follow‐up in comparison with SAVR. Although this study was well conducted, there were several limitations that merit highlighting:
The study population, that is, “lower‐risk patients,” was a mix of low‐ and intermediate‐risk patients, considering that the authors included in their analysis the SURTAVI 27 (Safety and Efficacy Study of the Medtronic CoreValve System in the Treatment of Severe, Symptomatic Aortic Stenosis in Intermediate Risk Subjects Who Need Aortic Valve Replacement) and UK‐TAVI 28 (UK Transcatheter Aortic Valve Implantation) trials, whose populations are substantially composed of intermediate‐risk patients;
The pooled Kaplan–Meier curves included only a 5‐year follow‐up, whereas 8‐year follow‐up data of the NOTION (Nordic Aortic Valve Intervention) trial had already been published;
There was no inclusion of high‐quality real‐word studies and large registries to compare with the results of the RCTs.
In our meta‐analysis we included both RCTs and PSM studies and analyzed them separately. There was an important difference in the results obtained in the RCTs versus the PSM studies. The PSM studies have the advantage of better reflecting the real‐world population and practice, whereas the RCTs generally include highly selected patients. To this effect, Alperi et al 29 reported that in the real‐world setting close to one‐half of the low‐risk patients with severe AS exhibited at least 1 major exclusion criterion from the major TAVR versus SAVR RCTs. On the other hand, the PSM studies are not able to achieve the level of matching that a RCT would do. In particular, the PSM process is incomplete and is not able to match the TAVR and SAVR cohorts for some key risk factors that are not measured in the initial studies, one of the most important being the frailty index. Hence, it is likely that, despite PSM, the TAVR cohorts might still present with a higher residual risk profile compared with the SAVR matched cohorts.
It is also important to remember that not everything we observe in the RCTs are also observed in the real world and we should be constantly aware of this aspect. Furthermore, RCT is not a synonym for the absence of biases. In this sense, the International Evidence Grading Research Initiative Targeting Transparency and Quality recently published a systematic review with meta‐analysis 30 showing that the RCT design did not protect from biases other than nonrandom allocation in the pivotal studies exploring the subject of TAVR versus SAVR. The authors found systematic imbalances in the proportion of deviations from random assigned treatment, loss to follow‐up, and receipt of additional procedures and additional myocardial revascularization, which may have posed a serious threat to internal validity due to high risk of performance bias and attrition bias.
Limitations
All‐cause mortality was the sole midterm outcome reported in all studies with Kaplan–Meier curves, whereas other outcomes (such as cardiovascular death, valvular‐related death, rehospitalization, stroke, myocardial infarction, reintervention for structural valve deterioration) that might affect quality of life and life expectancy were not available in all the studies for us to be able to carry out more thorough and comprehensive analyses. The original studies included early‐ and new‐generation devices together, whereas also mixing balloon‐expandable and self‐expandable. Our findings should be validated also in RCTs with newer transcatheter heart valves and surgical devices and techniques (such as stentless, sutureless, rapid‐deployment valves; sternal‐sparing SAVR) that might reveal improved outcomes for both approaches.
The field of TAVR in low‐risk patients is still in its infancy. Therefore, long‐term comparative follow‐up data at 10 years (or more) are not available. Most studies included in our meta‐analysis described follow‐up durations only up to 2 to 8 years, which does not allow us to draw any conclusions on long‐term outcomes or prosthesis durability. We should also draw some attention to the important drop in the number of patients at risk after 2 years.
Some studies attempted to minimize the impact of some confounders using propensity score matching. This method, although useful to reduce the selection and treatment biases, does not consider all baseline factors that may differ between TAVR versus SAVR and may have not achieved a perfectly well‐balanced comparison of these treatment strategies. For example, if we look closely at the baseline characteristics of some PSM studies included in our analyses, we still can identify some imbalances after the matching process:
AVALON (Aortic Valve Replacement in Elective Patients From Aortic Valve Multicenter) registry 12 : the SAVR group had more severe aortic regurgitation in the baseline characteristics (17.2% versus 8.8%; P<0.001);
Vilalta et al 14 : the SAVR group had more coronary artery disease than the TAVR group (27.5% versus 19.9%; P=0.098);
FinnValve registry 17 : the SAVR group had more planned concomitant percutaneous coronary intervention or coronary artery bypass graft surgery than the TAVR group (16.1% versus 2.0%; P<0.001);
Schaefer et al 18 : the TAVR group had more patients with New York Heart Association class III/IV than the SAVR group (67.9% versus 56.9%; P=0.090).
Hence, more RCTs (with longer follow‐up) are warranted to build more balanced groups for comparison.
Conclusions
This meta‐analysis shows that in low‐risk patients with severe AS, survival is similar between TAVR versus SAVR during the first 2 years but may be better with SAVR beyond the 2‐year follow‐up. However, the survival benefit of SAVR was observed only in PSM studies and not in RCTs, which precludes any definitive conclusion with regard to any survival superiority with SAVR versus TAVR in this population. The addition of data from ongoing RCTs, as well as longer follow‐up in previous RCTs, will help to confirm if there is a difference in mid‐ and long‐term survival between TAVR versus SAVR in the low‐risk population.
Sources of Funding
None.
Disclosures
Ibrahim Sultan receives institutional research support from Abbott, Artivion, Boston Scientific, Edwards, Medtronic, and Terumo Aortic. Philippe Pibarot has echocardiography Core Laboratory contracts with Edwards Lifesciences, for which he receives no direct compensation. Marie‐Annick Clavel has a computed tomography Core Laboratory contract with Edwards Lifesciences, for which she receives no direct compensation and received a research grant from Medtronic. Danny Chu is proctor and consultant for the Japanese Organization for Medical Device Development, Inc. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S6
Figures S1–S4
This article was sent to Amgad Mentias, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030012
For Sources of Funding and Disclosures, see page 7.
See Editorial by Imran and Saad.
This work was presented as an Abstract at the American Heart Association Scientific Sessions, November 11–13, 2023 in Philadelphia, PA.
REFERENCES
- 1. Barili F, Freemantle N, Pilozzi Casado A, Rinaldi M, Folliguet T, Musumeci F, Gerosa G, Parolari A. Mortality in trials on transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta‐analysis of Kaplan‐Meier‐derived individual patient data. Eur J Cardiothorac Surg. 2020;58:221–229. doi: 10.1093/ejcts/ezaa087 [DOI] [PubMed] [Google Scholar]
- 2. Barili F, Freemantle N, Musumeci F, Martin B, Anselmi A, Rinaldi M, Kaul S, Rodriguez‐Roda J, Di Mauro M, Folliguet T, et al. Five‐year outcomes in trials comparing transcatheter aortic valve implantation versus surgical aortic valve replacement: a pooled meta‐analysis of reconstructed time‐to‐event data. Eur J Cardiothorac Surg. 2022;61:977–987. doi: 10.1093/ejcts/ezab516 [DOI] [PubMed] [Google Scholar]
- 3. Thourani VH, Suri RM, Gunter RL, Sheng S, O'Brien SM, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Bavaria JE, et al. Contemporary real‐world outcomes of surgical aortic valve replacement in 141,905 low‐risk, intermediate‐risk, and high‐risk patients. Ann Thorac Surg. 2015;99:55–61. doi: 10.1016/j.athoracsur.2014.06.050 [DOI] [PubMed] [Google Scholar]
- 4. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng H‐Y, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 6. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei Y, Royston P. Reconstructing time‐to‐event data from published Kaplan‐Meier curves. Stata J. 2017;17:786–802. doi: 10.1177/1536867X1801700402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan‐Meier survival curves. BMC Med Res Methodol. 2021;21:111. doi: 10.1186/s12874-021-01308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 10. Royston P, Parmar MK. Flexible parametric proportional‐hazards and proportional‐odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203 [DOI] [PubMed] [Google Scholar]
- 11. Royston P, Parmar MKB. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time‐to‐event outcome. BMC Med Res Methodol. 2013;13:152. doi: 10.1186/1471-2288-13-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kowalówka AR, Kowalewski M, Wańha W, Kołodziejczak M, Mariani S, Li T, Pasierski M, Łoś A, Stefaniak S, Malinowski M, et al. Surgical and transcatheter aortic valve replacement for severe aortic stenosis in low‐risk elective patients: analysis of the aortic valve replacement in elective patients from the aortic valve multicenter registry [published online October 28, 2022]. J Thorac Cardiovasc Surg 2022. doi: 10.1016/j.jtcvs.2022.10.026 [DOI] [PubMed] [Google Scholar]
- 13. Forrest JK, Deeb GM, Yakubov SJ, Rovin JD, Mumtaz M, Gada H, O'Hair D, Bajwa T, Sorajja P, Heiser JC, et al. 2‐year outcomes after transcatheter versus surgical aortic valve replacement in low‐risk patients. J Am Coll Cardiol. 2022;79:882–896. doi: 10.1016/j.jacc.2021.11.062 [DOI] [PubMed] [Google Scholar]
- 14. Vilalta V, Alperi A, Cediel G, Mohammadi S, Fernández‐Nofrerias E, Kalvrouziotis D, Delarochellière R, Paradis JM, González‐Lopera M, Fadeuilhe E, et al. Midterm outcomes following sutureless and transcatheter aortic valve replacement in low‐risk patients with aortic stenosis. Circ Cardiovasc Interv. 2021;14:e011120. doi: 10.1161/CIRCINTERVENTIONS.121.011120 [DOI] [PubMed] [Google Scholar]
- 15. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, Alu MC, Madhavan MV, Chau KH, Russo M, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. 2021;77:1149–1161. doi: 10.1016/j.jacc.2020.12.052 [DOI] [PubMed] [Google Scholar]
- 16. Jørgensen TH, Thyregod HGH, Ihlemann N, Nissen H, Petursson P, Kjeldsen BJ, Steinbrüchel DA, Olsen PS, Søndergaard L. Eight‐year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. surgical aortic valve replacement. Eur Heart J. 2021;42:2912–2919. doi: 10.1093/eurheartj/ehab375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virtanen MPO, Eskola M, Jalava MP, Husso A, Laakso T, Niemelä M, Ahvenvaara T, Tauriainen T, Maaranen P, Kinnunen EM, et al. Comparison of outcomes after transcatheter aortic valve replacement vs surgical aortic valve replacement among patients with aortic stenosis at low operative risk. JAMA Network Open. 2019;2:e195742. doi: 10.1001/jamanetworkopen.2019.5742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schaefer A, Schofer N, Goßling A, Seiffert M, Schirmer J, Deuschl F, Schneeberger Y, Voigtländer L, Detter C, Schaefer U, et al. Transcatheter aortic valve implantation versus surgical aortic valve replacement in low‐risk patients: a propensity score‐matched analysis. Eur J Cardiothorac Surg. 2019;56:1131–1139. doi: 10.1093/ejcts/ezz245 [DOI] [PubMed] [Google Scholar]
- 19. Rosato S, Santini F, Barbanti M, Biancari F, D'Errigo P, Onorati F, Tamburino C, Ranucci M, Covello RD, Santoro G, et al. Transcatheter aortic valve implantation compared with surgical aortic valve replacement in low‐risk patients. Circ Cardiovasc Interv. 2016;9:e003326. doi: 10.1161/CIRCINTERVENTIONS.115.003326 [DOI] [PubMed] [Google Scholar]
- 20. Sá MP, Erten O, Ramlawi B. Transcatheter aortic valve implantation in elderly patients with aortic valve stenosis: the role of frailty, malnutrition, and sarcopenia. J Am Heart Assoc. 2022;11:e027705. doi: 10.1161/JAHA.122.027705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. Transcatheter aortic‐valve replacement with a balloon‐expandable valve in low‐risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 22. Sá MP, Jacquemyn X, Van den Eynde J, Tasoudis P, Dokollari A, Torregrossa G, Sicouri S, Clavel MA, Pibarot P, Ramlawi B. Impact of prosthesis‐patient mismatch after transcatheter aortic valve replacement: meta‐analysis of Kaplan‐Meier‐derived individual patient data. JACC Cardiovasc Imaging. 2023;16:298–310. doi: 10.1016/j.jcmg.2022.07.013 [DOI] [PubMed] [Google Scholar]
- 23. Sá MP, Jacquemyn X, Van den Eynde J, Tasoudis P, Erten O, Sicouri S, Macedo FY, Pasala T, Kaple R, Weymann A, et al. Impact of paravalvular leak on outcomes after transcatheter aortic valve implantation: meta‐analysis of Kaplan‐Meier‐derived individual patient data. Structural Heart. 2023;7:100118. doi: 10.1016/j.shj.2022.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sá MP, Jacquemyn X, Sun T, Van den Eynde J, Tasoudis P, Erten O, Sicouri S, Torregrossa G, Clavel MS, Pibarot P, et al. Late outcomes of permanent pacemaker implantation after TAVR: meta‐analysis of reconstructed time‐to‐event data. J Soc Cardiovasc Angiog Interv. 2022;1:100434. doi: 10.1016/j.jscai.2022.100434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sá MPBO, de Carvalho MMB, Sobral Filho DC, Cavalcanti LRP, Rayol SDC, Diniz RGS, Menezes AM, Clavel MA, Pibarot P, Lima RC. Surgical aortic valve replacement and patient‐prosthesis mismatch: a meta‐analysis of 108 182 patients. Eur J Cardiothorac Surg. 2019;56:44–54. doi: 10.1093/ejcts/ezy466 [DOI] [PubMed] [Google Scholar]
- 26. Ahmad Y, Howard JP, Arnold AD, Madhavan MV, Cook CM, Alu M, Mack MJ, Reardon MJ, Thourani VH, Kapadia S, et al. Transcatheter versus surgical aortic valve replacement in lower‐risk and higher‐risk patients: a meta‐analysis of randomized trials. Eur Heart J. 2023;44:836–852. doi: 10.1093/eurheartj/ehac642 [DOI] [PubMed] [Google Scholar]
- 27. Van Mieghem NM, Deeb GM, Søndergaard L, Grube E, Windecker S, Gada H, Mumtaz M, Olsen PS, Heiser JC, Merhi W, et al. Self‐expanding transcatheter vs surgical aortic valve replacement in intermediate‐risk patients: 5‐year outcomes of the SURTAVI randomized clinical trial. JAMA Cardiol. 2022;7:1000–1008. doi: 10.1001/jamacardio.2022.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. UK TAVI Trial Investigators ; Toff WD, Hildick‐Smith D, Kovac J, Mullen MJ, Wendler O, Mansouri A, Rombach I, Abrams KR, Conroy SP, et al. Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all‐cause mortality in patients with aortic stenosis: a randomized clinical trial. JAMA. 2022;327:1875–1887. doi: 10.1001/jama.2022.5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alperi A, Voisine P, Kalavrouziotis D, Dumont E, Dagenais F, Perron J, Silva I, Bernardi F, Mohammadi S, Rodés‐Cabau J. Aortic valve replacement in low‐risk patients with severe aortic stenosis outside randomized trials. J Am Coll Cardiol. 2021;77:111–123. doi: 10.1016/j.jacc.2020.10.056 [DOI] [PubMed] [Google Scholar]
- 30. Barili F, Brophy JM, Ronco D, Myers PO, Uva MS, Almeida RMS, Marin‐Cuartas M, Anselmi A, Tomasi J, Verhoye JP, et al. International Evidence Grading Research Initiative Targeting Transparency and Quality (INTEGRITTY). Risk of bias in randomized clinical trials comparing transcatheter and surgical aortic valve replacement: a systematic review and meta‐analysis. JAMA Netw Open. 2023;6:e2249321. doi: 10.1001/jamanetworkopen.2022.49321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S4


