Abstract
Background
Albuminuria is a known marker of mortality risk. Whether the association between albuminuria and mortality differs by demographic and comorbidity factors remains unclear. Therefore, we sought to determine whether albuminuria is differentially associated with mortality.
Methods and Results
This study included 49 640 participants from the National Health and Nutrition Examination Survey (1999–2018). All‐cause mortality through 2019 was linked from the National Death Index. Multivariable‐adjusted Poisson regression models were used to determine whether levels of urine albumin‐to‐creatinine ratio (ACR) were associated with mortality. Models were adjusted for demographic, socioeconomic, behavioral, and clinical factors. Mean age in the population was 46 years, with 51.3% female, and 30.3% with an ACR ≥10 mg/g. Over a median follow‐up of 9.5 years, 6813 deaths occurred. Compared with ACR <10, ACR ≥300 was associated with increased risk of mortality by 132% overall (95% CI, 2.01–2.68), 124% among men (95% CI, 1.84–2.73), 158% among women (95% CI, 2.14–3.11), 130% among non‐Hispanic White adults (95% CI: 1.89–2.79), 135% among non‐Hispanic Black adults (95% CI, 1.82–3.04), and 114% among Hispanic adults (95% CI, 1.55–2.94). Compared with ACR <10, ACR ≥300 was associated with increased risk of mortality by 148% among individuals with neither hypertension nor hypercholesterolemia (95% CI, 1.69–3.64), 128% among individuals with hypertension alone (95% CI, 1.86–2.79), and 166% among individuals with both hypertension and hypercholesterolemia (95% CI, 2.18–3.26).
Conclusions
We found strong associations between albuminuria and mortality risk, even at mildly increased levels of albuminuria. Associations persisted across categories of sex, race or ethnicity, and comorbid conditions, with subtle differences.
Keywords: albumin‐creatinine ratio, albuminuria, mortality
Subject Categories: Epidemiology
Nonstandard Abbreviations and Acronyms
- ACR
albumin‐to‐creatinine ratio
- IDR
incidence density ratio
- NDI
National Death Index
- NHANES
National Health and Nutrition Examination Survey
- PY
person‐years
Clinical Perspective.
What Is New?
From a population study of ≈50 000 US adults with a median follow‐up of 9.5 years, albuminuria was significantly associated with an elevated all‐cause mortality risk, with subtle yet distinct associations that persisted across categories of sex, race or ethnicity, and the presence of hypertension and hypercholesterolemia.
What Are the Clinical Implications
Regardless of severity, albuminuria is associated with an increased risk of all‐cause mortality across a broad range of subgroups by sex, race or ethnicity, and comorbidity status.
Albuminuria should be considered for broad inclusion in risk assessment tools in the clinical setting, especially in the setting of other cardiovascular risk factors.
Albuminuria is a strong predictor of cardiovascular and all‐cause mortality, both in the general population and in cohorts at high risk for cardiovascular disease (CVD). 1 , 2 , 3 , 4 In fact, there appears to be a continuous relationship between the urine albumin‐to‐creatinine ratio (ACR) and all‐cause and cardiovascular mortality, with increased risk apparent at levels of albuminuria even below 30 mg/g, 1 , 2 , 5 , 6 , 7 , 8 which has long been a commonly accepted threshold for “abnormal albuminuria” used in clinical practice. 9 However, the association of albuminuria with mortality across categories of sex, race or ethnicity, and comorbidities remains understudied in the general US population. 7 , 8 , 10 , 11 , 12 Moreover, although screening for albuminuria is recommended in high‐risk patients, such as those with diabetes and hypertension, 13 , 14 , 15 , 16 there is no consensus with regard to the benefit of albuminuria screening among the general US population and across subgroups of sex, race or ethnicity, and specific combinations of CVD‐related comorbidities. Given the current state of population health in the United States, with its increasing levels of diversity and high burden of comorbidities, this represents a critical area for further study in a diverse, nationally representative US cohort with recent data across all categories of albuminuria.
Our study aims were to determine whether the association between albuminuria and mortality differs according to demographic factors (sex and race or ethnicity) or the presence of CVD‐related comorbidities (hypertension and hypercholesterolemia). To do so, we leveraged data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2018 linked to the National Death Index (NDI) with up to 20 years of mortality follow‐up data.
Methods
All data have been made publicly available by the National Center for Health Statistics and can be accessed at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Study Sample
NHANES is a nationally representative survey assessing the health and nutritional status of noninstitutionalized US adults and children. 17 Since 1999, this cross‐sectional survey has been conducted annually in 2‐year cycles. Surveys include household interviews in questionnaire form and physical examinations conducted in mobile examination centers. Data for this study were sourced from 10 NHANES cycles (1999–2018) that included measured urine albumin and creatinine used to define albuminuria. In order to be included in the analytical data set, participants were required to have complete information for serum and urine measures of albumin and creatinine, blood pressure (BP), and a fasting blood draw to measure cholesterol. The final analytical data set included a total of 49 640 adults aged ≥18 years who also had mortality data updated through December 2019 (described in detail subsequently and shown in Figure S1). NHANES protocols were approved by the National Center for Health Statistics internal review board and informed consent was obtained before study initiation for all participants. 18
Albuminuria
From spot urine samples, urine albumin (mg/L) was measured using the solid‐phase fluorescent immunoassay method. 19 Urine creatinine (mg/dL) was measured by the Jaffe rate reaction using the Beckman Synchron CX3 Clinical Analyzer through the 2005 to 2006 data cycle and the enzymatic method using the Roche/Hitachi Modular P Chemistry Analyzer from the 2007 to 2008 cycle onward. 20 The ratio of urinary albumin to creatinine was calculated and reported in milligrams per gram (mg/g). Albuminuria was subsequently categorized into 4 levels according to the Kidney Disease: Improving Global Outcomes guideline 21 : (1) normal (ACR <10 mg/g), (2) mildly increased (≥10 and <30), (3) moderately increased (≥30 and <300), or (4) severely increased (≥300 mg/g).
Mortality
The National Center for Health Statistics has made NDI data publicly available for linkage to US‐based population surveys such as NHANES. 22 NDI data are matched with survey participants through social security numbers, names, birthdates, and other personal identifiable information. NHANES data were merged with deidentified NDI data with death information ascertained through December 31, 2019. Cause‐specific mortality was classified by primary cause of death according to International Classification of Diseases, Tenth Revision (ICD‐10) codes, including diseases of the heart, malignant neoplasms, accidents, cerebrovascular diseases, Alzheimer's disease, diabetes, and all other causes.
Comorbidities
We classified comorbidities with the following mutually exclusive categories: (1) neither hypertension nor hypercholesterolemia, (2) hypercholesterolemia without hypertension, (3) hypertension without hypercholesterolemia, or (4) hypertension and hypercholesterolemia. Hypercholesterolemia was defined as total cholesterol ≥240 mg/dL (measured enzymatically from fasting blood samples) or self‐reported use of lipid‐lowering medication. Based on the Seventh Report of the Joint National Committee, 23 hypertension was defined as measured systolic BP ≥140 mm Hg, measured diastolic BP ≥90 mm Hg, or self‐reported use of antihypertensive medication. In a supplemental analysis, hypertension was defined according to the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline (discussed subsequently). 24
Other Variables
Self‐reported demographic characteristics included age, sex, race or ethnicity, education level (<high school, high school graduate, some college, or college graduate or higher), annual household income (<$55 000 versus $55000+), marital status (yes/no), and insurance status (yes/no). Race or ethnicity was divided into four categories: non‐Hispanic White, non‐Hispanic Black, Hispanic, and non‐Hispanic other (including Asian, Native American, Pacific Islander, and multiracial). Self‐reported health behaviors included current smoking, consumption of ≥12 alcoholic beverages per year, self‐rated health, and any moderate or vigorous physical activity in the past 30 days, all reported via standardized questionnaire.
Body mass index was calculated as measured weight (kg)/height (m2). Diabetes was defined as (1) self‐report of diabetes or (2) diabetes medication use or (3) measured fasting plasma glucose ≥126 mg/dL. History of CVD was self‐reported. We calculated the estimated glomerular filtration rate (eGFR) using serum creatinine values collected during the medical examination. Serum creatinine (mg/dL) was measured by the Jaffe rate method using the Beckman Coulter UniCel DxC 880 Synchron Clinical System, and eGFR was calculated from serum creatinine, age, and sex using the 2021 Chronic Kidney Disease Epidemiology Collaboration creatinine equation. 25 Due to a high proportion of missing data for certain variables (not all questions were asked of all participants), a category for missing data was created for each of the following: education, annual household income, marital status, insurance status, smoking, alcohol consumption in the past year, self‐rated health, and history of CVD. Proportions of missing data are reported in Table 1.
Table 1.
Characteristics of US Adults, the National Health and Nutrition Examination Survey, 1999 to 2018
| Stratified by comorbidities | |||||
|---|---|---|---|---|---|
| Overall N=49 640 | Neither (n=23 983) | Hypercholesterolemia (n=8820) | Hypertension (n=7171) | Both (n=9666) | |
| Characteristics | Mean or % (95% CI) | Mean or % (95% CI) | Mean or % (95% CI) | Mean or % (95% CI) | Mean or % (95% CI) |
| Age group, y* | |||||
| 18–34 | 30.1 (29.2–31.1) | 50.1 (48.9–51.4) | 17.9 (16.8–19.0) | 8.4 (7.5–9.5) | 2.7 (2.1–3.3) |
| 35–54 | 37.9 (37.1–38.7) | 38.0 (36.8–39.1) | 48.1 (46.6–49.6) | 37.2 (35.5–38.9) | 26.5 (25.1–28.0) |
| 55–64 | 15.0 (14.5–15.6) | 7.2 (6.7–7.7) | 19.3 (18.2–20.5) | 21.9 (20.6–23.3) | 27.7 (26.4–29.0) |
| 65+ | 16.9 (16.3–17.6) | 4.7 (4.3–5.2) | 14.7 (13.8–15.7) | 32.5 (31.1–34.0) | 43.1 (41.7–44.5) |
| Female sex* | 51.3 (50.9–51.8) | 52.4 (51.7–53.0) | 48.5 (47.2–49.7) | 50.5 (48.9–52.0) | 52.2 (51.0–53.5) |
| Race or ethnicity* | |||||
| Non‐Hispanic White | 68.6 (66.5–70.6) | 64.8 (62.7–67.0) | 73.8 (71.8–75.6) | 68.3 (65.6–70.8) | 73.6 (71.3–75.9) |
| Non‐Hispanic Black | 10.6 (9.5–11.7) | 10.7 (9.7–11.9) | 6.1 (5.4–6.8) | 15.6 (13.8–17.6) | 11.5 (10.1–13.0) |
| Hispanic | 14.1 (12.6–15.7) | 17.1 (15.4–18.9) | 13.5 (12.0–15.0) | 10.3 (8.8–12.0) | 8.9 (7.5–10.4) |
| Non‐Hispanic other | 6.8 (6.2–7.4) | 7.3 (6.7–8.0) | 6.7 (6.0–7.5) | 5.9 (5.1–6.7) | 6.0 (5.2–6.9) |
| Education level* | |||||
| <High school | 17.6 (16.7–18.5) | 17.2 (16.3–18.1) | 14.5 (13.4–15.6) | 21.2 (19.9–22.6) | 19.6 (18.3–21.0) |
| High school graduate | 24.3 (23.6–25.1) | 23.5 (22.5–24.6) | 22.9 (21.6–24.2) | 26.9 (25.4–28.3) | 26.5 (25.1–27.9) |
| Some college | 30.9 (30.2–31.7) | 31.8 (30.7–32.9) | 29.8 (28.4–31.2) | 29.9 (28.4–31.4) | 30.4 (29.0–31.8) |
| College graduate + | 27.1 (25.7–28.5) | 27.4 (25.8–29.1) | 32.8 (30.8–34.9) | 22.0 (20.4–23.6) | 23.4 (21.7–25.3) |
| Annual household income* | |||||
| <$55 000 | 47.9 (46.4–49.4) | 47.1 (45.5–48.7) | 42.2 (40.0–44.4) | 53.2 (51.1–55.2) | 53.0 (51.0–55.0) |
| $55 000 + | 44.4 (42.9–45.9) | 45.0 (43.5–46.6) | 50.5 (48.4–52.6) | 39.1 (37.2–41.1) | 39.4 (37.4–41.4) |
| Married* | 54.3 (53.2–55.4) | 48.1 (46.8–49.5) | 62.8 (61.2–64.3) | 57.7 (55.9–59.4) | 60.0 (58.5–61.5) |
| Insured* | 82.1 (81.2–82.9) | 76.2 (75.0–77.3) | 85.0 (83.8–86.2) | 86.8 (85.5–87.9) | 92.0 (91.2–92.8) |
| At least 12 alcoholic drinks in past year* | 67.4 (66.2–68.5) | 67.4 (66.0–68.7) | 72.3 (70.6–74.0) | 63.9 (62.1–65.7) | 64.1 (62.5–65.7) |
| Current smoker* | 20.7 (20.0–21.5) | 22.6 (21.7–23.6) | 22.2 (20.9–23.5) | 18.7 (17.5–20.0) | 15.2 (14.3–16.1) |
| Fair or poor health* | 16.8 (16.2–17.5) | 11.4 (10.8–12.0) | 16.1 (14.9–17.3) | 23.9 (22.4–25.4) | 28.1 (26.7–29.6) |
| Physical activity* | |||||
| Moderate | 28.3 (27.6–29.1) | 25.0 (24.1–25.9) | 31.4 (30.0–32.7) | 30.4 (28.8–32.0) | 33.1 (31.7–34.5) |
| Vigorous | 30.2 (29.1–31.3) | 38.8 (37.6–40.1) | 29.5 (27.9–31.2) | 18.5 (16.9–20.1) | 14.7 (13.6–15.8) |
| Body mass index, kg/m2 * | 28.6 (28.5–28.8) | 27.4 (27.2–27.5) | 28.5 (28.4–28.7) | 30.8 (30.6–31.1) | 30.8 (30.6–31.0) |
| Systolic blood pressure, mm Hg* | 122.3 (122.0–122.7) | 114.7 (114.4–114.9) | 117.9 (117.5–118.2) | 139.4 (138.8–140.1) | 137.1 (136.5–137.7) |
| Fasting plasma glucose, mg/dL* | 104.8 (104.2–105.4) | 97.8 (97.3–98.3) | 106.0 (104.8–107.1) | 111.4 (109.8–113.0) | 118.6 (116.9–120.3) |
| Diabetes* | 10.4 (10.0–10.8) | 3.3 (3.0–3.6) | 9.8 (9.0–10.7) | 15.6 (14.4–16.8) | 27.5 (26.4–28.6) |
| Total cholesterol*, mg/dL | 195.6 (194.8–196.3) | 181.0 (180.3–181.6) | 226.0 (224.4–227.6) | 188.4 (187.5–189.4) | 207.7 (206.1–209.3) |
| Estimated glomerular filtration rate, mL/min* | 96.2 (95.8–96.6) | 104.1 (103.7–104.6) | 95.1 (94.5–95.7) | 86.6 (86.0–87.3) | 81.7 (81.1–82.3) |
| History of cardiovascular disease* | 8.1 (7.7–8.5) | 2.2 (2.0–2.4) | 7.4 (6.7–8.2) | 12.0 (11.1–12.9) | 22.9 (21.6–24.1) |
A missing data category was created for missing/refused/do not know responses. The following proportions of the variables were coded as missing in the overall sample: education level: 0.1%; income: 7.7%; marital status: 3.0%; insurance status: 0.4%; alcohol consumption: 7.9%; smoking: 2.4%; health status: 0.1%; and history of CVD: 3.6%.
Indicates characteristic differs significantly (P<0.05) by comorbidity (chi‐square tests for categorical variables and ANOVAs for means).
Statistical Analysis
Demographic, behavioral, and clinical characteristics were described overall and according to the presence of comorbidities (hypertension and hypercholesterolemia). We used chi‐square tests and ANOVAs to test for significant differences across groups. Next, we estimated the age‐standardized prevalence of albuminuria overall and according to demographic characteristics and the presence of comorbidities. We estimated age‐adjusted incidence rates of mortality (per 1000 person‐years [PY]) according to ACR category, overall and by demographic characteristics and by comorbidities. We then used a series of multivariable‐adjusted Poisson regression models to determine the incidence density ratio (IDR) for the association between ACR category with mortality overall, with follow‐up time included as an offset in all models. Model 1 adjusted for demographic characteristics (age, race or ethnicity, and sex) and follow‐up time. Model 2 additionally adjusted for socioeconomic factors (education, income, marital status, and insurance status). Model 3 additionally adjusted for behavioral factors (smoking, alcohol consumption, physical activity, self‐rated health, and body mass index). Model 4 additionally adjusted for clinical factors (diabetes, history of CVD, hypertension, hypercholesterolemia, and eGFR). We used multiplicative interaction terms to test for effect modification between ACR category with demographic characteristics (sex and race or ethnicity) and comorbidities (hypertension and hypercholesterolemia). We considered an alpha value of 0.10 significant for interactions. Finally, we graphed nonparametric adjusted (Model 4) survival curves showing the association between ACR category and years to death for each comorbidity category.
Supplementary Analyses
We conducted several supplemental analyses. First, we replicated our main analyses using the 2017 American College of Cardiology/American Heart Association hypertension definition. 24 We also estimated the association between albuminuria category and cause‐specific mortality. Additionally, we estimated the association between albuminuria category and all‐cause mortality stratified by other comorbidities (diabetes, obesity, CVD) and eGFR category: <60, 60 to <90, or ≥90 mL/min per 1.73 m2). Finally, to provide statistical significance for between sexes and comorbid conditions, we estimated the association between albuminuria categories with all‐cause mortality, with the following reference categories: men with ACR <10 mg/g and in a separate model, people without hypertension or hypercholesterolemia and ACR <10 mg/g. All analyses accounted for the complex survey design of the NHANES sample and were weighted using a combined 20‐year mobile examination center sample weight. Analyses were conducted using SUDAAN v11.0.3 (Research Triangle Institute, Research Triangle Park, NC).
Results
The population was mostly young: 30.1% were age 18 to 34 years, 37.9% were 35 to 54 years, 15% were 55 to 64 years, and 16.9% were ≥65 years (Table 1). Approximately 51% were women, 68.6% were non‐Hispanic White, 10.6% were non‐Hispanic Black, 14.1% were Hispanic, and 6.8% were non‐Hispanic other. Mean body mass index was 28.6 kg/m2, mean systolic BP was 122.3 mm Hg, mean fasting glucose was 104.8 mg/dL, and mean total cholesterol was 195.6 mg/dL. Individuals with hypertension or hypercholesterolemia were more likely to be older, non‐Hispanic White, lower income, and married; have health insurance; report fair or poor health; have higher body mass index, systolic BP, fasting plasma glucose, and total cholesterol; and were more likely to have diabetes or a history of CVD (all P values <0.05).
Distribution of Albuminuria
The age‐standardized prevalence of ACR <10, 10 to 30, 30 to 300, or ≥300 mg/g was 69.7% (95% CI, 69.0%–70.3%), 20.8% (95% CI, 20.4%–21.3%), 8.2% (95% CI, 7.8%–8.5%), and 1.3% (95% CI, 1.2%–1.5%), respectively (Figure 1). Men were more likely to have normal ACR compared with women, and non‐Hispanic White adults were more likely to have normal ACR compared with all other racial or ethnic groups (all P values <0.05). Likewise, adults with hypercholesterolemia alone or with neither comorbidity were more likely to have normal ACR compared with adults with hypertension alone or with both comorbidities (P<0.05).
Figure 1. Age‐adjusted prevalence of albuminuria by sex, race or ethnicity, and comorbidities among US adults, the National Health and Nutrition Examination Survey, 1999 to 2018.
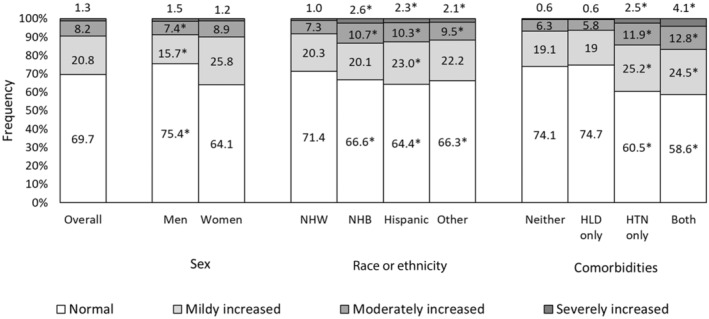
*Chi‐square P value indicates statistically different at <0.05 for sex (ref: women), race or ethnicity (ref: NHW), and comorbidities (ref: none). HLD indicates hypercholesterolemia; HTN, hypertension; NHB, Non‐Hispanic Black; and NHW, Non‐Hispanic White.
Age‐Adjusted Associations Between Albuminuria and Mortality
After an average follow‐up of 9.9 years, the age‐adjusted mortality rate per 1000 PY was 12.6 (95% CI, 12.0–13.2) and increased across categories of albuminuria (ACR <10: 9.0 per 1000 PY [95% CI, 8.4–9.5]; ACR 10–30 mg/g: 13.8 per 1000 PY [95% CI, 12.9–14.7]; ACR 30–300 mg/g: 22.5 per 1000 PY [95% CI, 21.1–24.0]; ACR ≥300 mg/g: 39.5 per 1000 PY [95% CI, 34.7–44.9]; P for trend <0.01) (Figure S2). Figure 2 displays higher age‐adjusted mortality rates with greater ACR (P<0.05) across all strata of sex, race or ethnicity, and comorbid conditions.
Figure 2. Age‐adjusted mortality rate according to category of albuminuria by sex, race or ethnicity, and comorbidities among US adults, 1999 to 2019.
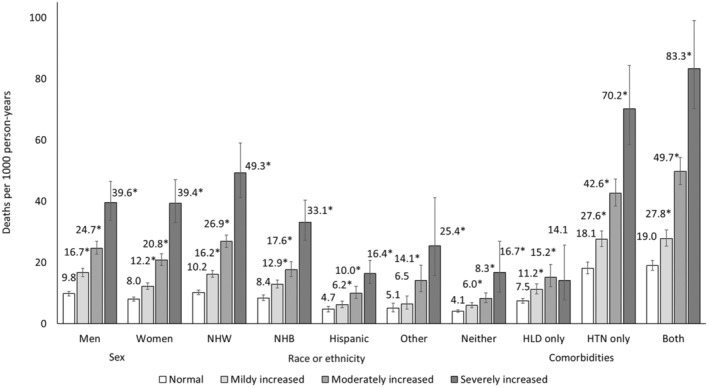
*Chi‐square P value statistically significant at <0.05 for sex, race or ethnicity, and comorbidities. P values for trends were statistically significant (<0.05) for all sex, race or ethnicity, and comorbidity strata. HLD indicates hypercholesterolemia; HTN, hypertension; NHB, non‐Hispanic Black; and NHW, non‐Hispanic White.
Multivariable‐Adjusted Associations Between Albuminuria and Mortality by Sex and Race or Ethnicity
There was a graded association between albuminuria and mortality (Table 2). For example, from fully adjusted Poisson model 4, compared with ACR <10 mg/g, risk of all‐cause mortality was greater by 38% (IDR, 1.38 [95% CI, 1.29–1.48]) with ACR 10 to 30 mg/g, 85% (IDR, 1.85 [95% CI, 1.70–2.01]) with ACR 30 to 300 mg/g, and 132% (IDR, 2.32 [95% CI, 2.01–2.68]) with ACR ≥300 mg/g. ACR was associated with increased risk of mortality for all sex and race or ethnicity groups, though with subtle, yet significant differences (P for interactions <0.10 for sex). For example, in fully adjusted models, compared with normal albuminuria (ACR <10 mg/g), ACR ≥300 mg/g was associated with a 124% increased mortality risk among men (IDR, 2.24 [95% CI, 1.84–2.73]) and 158% among women (IDR, 2.58 [95% CI, 2.14–3.11]). Across race or ethnicity groups, effect size differences were evident in minimally adjusted models but not in fully adjusted models (Model 4).
Table 2.
Associations of ACR Categories With All‐Cause Mortality Overall and According to Sex and Race or Ethnicity Among US Adults, 1999 to 2019
| n | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| IDR (95% CI) | IDR (95% CI) | IDR (95% CI) | IDR (95% CI) | ||
| All ACR, mg/g | |||||
| <10 (ref) | 32 414 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 11 183 | 1.55* (1.45–1.67) | 1.52* (1.41–1.63) | 1.44* (1.34–1.54) | 1.38* (1.29–1.48) |
| 30–<300 | 5042 | 2.55* (2.36–2.75) | 2.30* (2.12–2.49) | 2.06* (1.90–2.23) | 1.85* (1.70–2.01) |
| ≥300 | 1001 | 4.54* (3.95–5.23) | 3.76* (3.28–4.31) | 3.11* (2.70–3.57) | 2.32* (2.01–2.68) |
| Men ACR, mg/g | |||||
| <10 (ref) | 17 015 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 4261 | 1.72* (1.55–1.90) | 1.56* (1.41–1.72) | 1.48* (1.33–1.64) | 1.43* (1.30–1.59) |
| 30–<300 | 2339 | 2.55* (2.30–2.83) | 2.28* (2.05–2.54) | 2.03* (1.83–2.26) | 1.83* (1.63–2.05) |
| ≥300 | 560 | 4.17* (3.49–4.98) | 3.49* (2.94–4.14) | 2.81* (2.34–3.39) | 2.24* (1.84–2.73) |
| Women ACR, mg/g | |||||
| <10 (ref) | 15 399 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 6922 | 1.54* (1.38–1.72) | 1.49* (1.34–1.65) | 1.40* (1.27–1.54) | 1.33* (1.21–1.47) |
| 30–<300 | 2703 | 2.64* (2.35–2.97) | 2.33* (2.05–2.64) | 2.08* (1.84–2.35) | 1.89* (1.67–2.13) |
| ≥300 | 441 | 5.07* (4.20–6.14) | 4.33* (3.56–5.26) | 3.74* (3.11–4.49) | 2.58* (2.14–3.11) |
| Non‐Hispanic White ACR, mg/g | |||||
| <10 (ref) | 14 201 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 4959 | 1.65* (1.51–1.80) | 1.54* (1.41–1.67) | 1.45* (1.33–1.58) | 1.39* (1.28–1.52) |
| 30–<300 | 2150 | 2.67* (2.42–2.95) | 2.36* (2.13–2.62) | 2.08* (1.88–2.30) | 1.89* (1.71–2.09) |
| ≥300 | 326 | 4.71* (3.86–5.74) | 3.81* (3.16–4.60) | 3.02* (2.49–3.66) | 2.30* (1.89–2.79) |
| Non‐Hispanic Black ACR, mg/g | |||||
| <10 (ref) | 6685 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 2062 | 1.59* (1.37–1.84) | 1.51* (1.30–1.75) | 1.42* (1.22–1.64) | 1.35* (1.16–1.57) |
| 30–<300 | 1129 | 2.10* (1.78–2.47) | 1.93* (1.63–2.28) | 1.72* (1.45–2.04) | 1.55* (1.30–1.85) |
| ≥300 | 268 | 3.83* (3.01–4.88) | 3.60* (2.79–4.65) | 3.20* (2.49–4.11) | 2.35* (1.82–3.04) |
| Hispanic ACR, mg/g | |||||
| <10 (ref) | 8562 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 3152 | 1.40* (1.19–1.66) | 1.40 (1.19–1.66) | 1.33* (1.09–1.63) | 1.27* (1.02–1.57) |
| 30–<300 | 1355 | 2.18* (1.81–2.62) | 2.12* (1.76–2.54) | 1.97* (1.60–2.43) | 1.68* (1.36–2.07) |
| ≥300 | 315 | 3.47* (2.67–4.52) | 3.41* (2.54–4.57) | 3.21* (2.33–4.42) | 2.14* (1.55–2.94) |
| Non‐Hispanic other ACR, mg/g | |||||
| <10 (ref) | 2966 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 1010 | 1.33 (0.89–2.00) | 1.28 (0.84–1.96) | 1.19 (0.77–1.84) | 1.26 (0.82–1.93) |
| 30–<300 | 408 | 2.88* (1.87–4.44) | 2.45* (1.57–3.82) | 2.29* (1.46–3.61) | 2.00* (1.25–3.20) |
| ≥300 | 92 | 5.02* (2.73–9.24) | 4.16* (2.24–7.75) | 3.56* (1.96–6.48) | 2.51* (1.18–5.35) |
Indicates estimate is significant (P<0.05).
ACR indicates albumin‐to‐creatinine ratio; and IDR, incidence rate ratio. Model 1 is adjusted for age group, race or ethnicity (except for race‐stratified), and sex (except for sex‐stratified). Model 2 is additionally adjusted for Model 1 + education, income, marital status, and insurance status. Model 3 is additionally adjusted for Model 2 + smoking, alcohol consumption, physical activity, self‐rated health, and body mass index. Model 4 is additionally adjusted for Model 3 + diabetes, history of cardiovascular disease, comorbidities and estimated glomerular filtration rate.
Multivariable‐Adjusted Associations Between Albuminuria and Mortality by the Presence of Comorbidities
ACR was associated with increased mortality risk across all comorbidity categories, again with subtle, yet significant differences (P for interactions <0.10). For example, compared with normal albuminuria, ACR ≥300 mg/g was associated with a 148% increased mortality risk among those with neither comorbidity (IDR, 2.48 [95% CI, 1.69–3.64]), 128% among those with hypertension alone (IDR, 2.28 [95% CI, 1.86–2.79]), and 166% among those with both hypertension and hypercholesterolemia (IDR, 2.66 [95% CI, 2.18–3.26]), Table 3. Fully adjusted survival curves displaying these associations are shown in Figure 3.
Table 3.
Associations of ACR Categories With All‐Cause Mortality According to Comorbidities Among US Adults, 1999 to 2019
| n | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| IDR (95% CI) | IDR (95% CI) | IDR (95% CI) | IDR (95% CI) | ||
| Neither comorbidity ACR, mg/g | |||||
| <10 | 17 928 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 4462 | 1.60* (1.37–1.88) | 1.53* (1.31–1.80) | 1.46* (1.24–1.72) | 1.44* (1.22–1.69) |
| 30–<300 | 1455 | 2.13* (1.72–2.64) | 1.85* (1.49–2.31) | 1.59* (1.27–1.99) | 1.44* (1.14–1.82) |
| ≥300 | 138 | 4.20* (2.52–6.99) | 3.98* (2.60–6.08) | 2.83* (1.78–4.50) | 2.48* (1.69–3.64) |
| Hypercholesteremia only ACR, mg/g | |||||
| <10 | 6302 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 1828 | 1.59* (1.32–1.92) | 1.52* (1.25–1.83) | 1.43* (1.19–1.72) | 1.39* (1.14–1.69) |
| 30–<300 | 610 | 2.09* (1.63–2.67) | 1.95* (1.52–2.50) | 1.71* (1.31–2.21) | 1.60* (1.22–2.11) |
| ≥300 | 80 | 2.09* (1.12–3.91) | 1.74 (0.92–3.30) | 1.47 (0.78–2.78) | 1.26 (0.68–2.33) |
| Hypertension only ACR, mg/g | |||||
| <10 | 3616 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 2053 | 1.58* (1.38–1.81) | 1.49* (1.30–1.72) | 1.38* (1.19–1.59) | 1.37* (1.19–1.58) |
| 30–<300 | 1215 | 2.39* (2.07–2.76) | 2.19* (1.89–2.53) | 1.98* (1.72–2.26) | 1.84* (1.60–2.12) |
| ≥300 | 287 | 3.93* (3.19–4.85) | 3.19* (2.56–3.96) | 2.88* (2.34–3.54) | 2.28* (1.86–2.79) |
| Both comorbidities ACR, mg/g | |||||
| <10 | 4568 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10–<30 | 2840 | 1.51* (1.35–1.69) | 1.42* (1.28–1.58) | 1.38* (1.24–1.54) | 1.37* (1.22–1.53) |
| 30–<300 | 1762 | 2.73* (2.42–3.09) | 2.44* (2.17–2.75) | 2.25* (2.00–2.53) | 2.12* (1.89–2.38) |
| ≥300 | 496 | 4.50* (3.68–5.51) | 4.00* (3.30–4.86) | 3.39* (2.77–4.14) | 2.66* (2.18–3.26) |
ACR indicates albumin‐to‐creatinine ratio; and IDR, incidence density ratio. Model 1 is adjusted for age group, race or ethnicity, and sex. Model 2 is additionally adjusted for Model 1 + education, income, marital status, and insurance status. Model 3 is additionally adjusted for Model 2 + smoking, alcohol consumption, physical activity, self‐rated health, and body mass index. Model 4 is additionally adjusted for Model 3 + diabetes, history of cardiovascular disease, and estimated glomerular filtration rate.
Indicates estimate is significant (P<0.05).
Figure 3. Adjusted survival curves showing the associations between categories of ACR and all‐cause mortality according to comorbidities among US adults, 1999 to 2019.
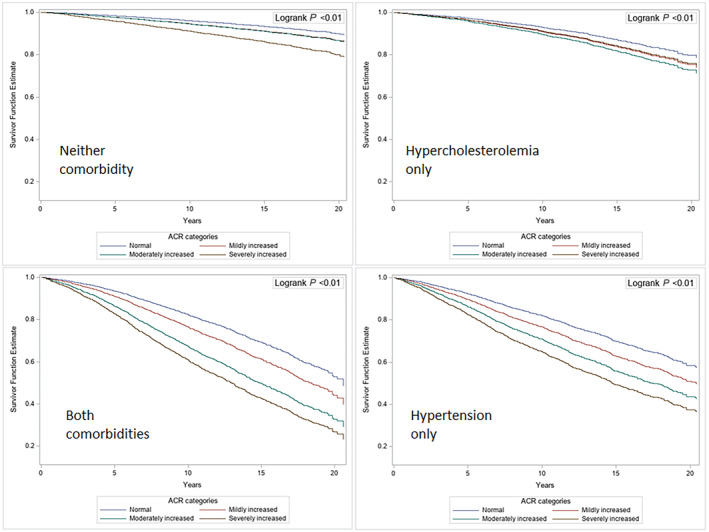
*Log‐rank test for differences between groups was significant (P<0.01). ACR_4cat indicates albumin‐to‐creatinine ratio in 4 levels according to the Kidney Disease: Improving Global Outcomes guideline.
Sensitivity Analyses
Results from our sensitivity analysis using the 2017 American College of Cardiology/American Heart Association hypertension definition are consistent with our main findings (Table S1). In Table S2, we show that higher ACR is associated with greater age‐adjusted cause‐specific mortality rates for the following causes of death: disease of the heart, cerebrovascular disease, diabetes, and all other causes. In Table S3, we show the association between ACR category and all‐cause mortality stratified by other comorbidities (diabetes, obesity, CVD) and eGFR categories. In Table S4, we show that compared with men with ACR <10 mg/g, risk of all‐cause mortality was lower among women with normal (<10 mg/g) or mildly increased (10 to <30 mg/g) ACR, but higher among women with moderately or severely increased ACR and among all other men. Lastly, in Table S5, we show that mortality risk was lowest among individuals without hypertension or hypercholesterolemia and with ACR <10 mg/g compared with nearly all other groups.
Discussion
In a nationally representative study, we found strong graded associations between albuminuria and risk of all‐cause mortality across all strata of sex, race or ethnicity, and comorbid conditions. We identified subtle distinctions across sex and comorbidity categories. For example, women were less likely to have normal albuminuria (ACR <10 mg/g) than men and the association between albuminuria and mortality was more pronounced among women compared with men at severely increased levels of albuminuria. Conversely, although Black and Hispanic individuals were more likely to have increased albuminuria compared with non‐Hispanic White individuals, we found similar associations between albuminuria and mortality risk across race or ethnicity groups. Finally, albuminuria was associated with increased mortality risk across all comorbidity categories, even at mildly increased levels of albuminuria, with the strongest association between albuminuria and mortality among individuals with ACR >300 mg/g and both hypertension and hypercholesterolemia.
Although ACR ≥30 mg/g has been established as a marker of kidney disease and risk of progression to end‐stage kidney disease, 26 , 27 , 28 ACR across the entire spectrum is increasingly being studied as an independent predictor of all‐cause and CVD mortality. The association between albuminuria and mortality has been demonstrated in general population cohorts with and without diabetes, in diabetic patients without cardiovascular disease, and in other high‐risk groups. 2 , 5 , 27 , 29 , 30 , 31 , 32 , 33 , 34 , 35 For example, a large meta‐analysis that included data from NHANES III (1988–1994) demonstrated that an ACR as low as 10 mg/g is associated with an increased risk of mortality, independent of eGFR and conventional risk factors. 1 Our study extends these older findings to the US population using recent data and confirms a strong independent association between elevated ACR and mortality. An ACR as low as 10 to 30 mg/g is associated with a significantly increased mortality risk compared with ACR <10 mg/g across all categories of sex, race or ethnicity, presence or absence of comorbidities (hypertension, hypercholesterolemia, diabetes, obesity, CVD), and eGFR strata.
We found a higher prevalence of increased albuminuria among women than men, consistent with prior studies. It has been proposed that sex‐specific cutoffs be used to define increased albuminuria, with higher ACR thresholds in women due to sex differences in urine creatinine excretion. 36 , 37 Findings from the Chronic Kidney Disease Prognosis Consortium demonstrated sex differences in the association between albuminuria and all‐cause mortality, such that the slope of the risk relationship for all‐cause mortality was steeper in women than in men. 10 It has been postulated that albuminuria may reflect more advanced microvascular disease among women and may contribute to early mortality after CVD events and other sex‐related disparities in CVD outcomes. 10 , 38 In our study, though we found more pronounced associations between albuminuria and mortality among women compared with men at higher levels of ACR (ie, severely increased albuminuria), the overall associations were similar and were independent of eGFR and typical risk factors.
Corroborated by other studies, 39 , 40 Black adults had higher rates of albuminuria compared with non‐Hispanic White adults. Findings from the Reasons for Geographic and Racial Differences in Stroke study showed stronger associations between ACR with CVD among Black compared with White individuals. 40 , 41 In contrast, a meta‐analysis 1 and an analysis of the US community‐based ARIC (Atherosclerosis Risk in Communities) Study 11 found that the association between albuminuria and cardiovascular and all‐cause mortality risk did not differ between Black versus White individuals. Our study, consistent with ARIC, extends these findings to a diverse, nationally representative sample of the general US population and indicates that the association between increasing levels of albuminuria and increased mortality risk is equally strong across race and ethnicity groups.
The current findings showing that the association between albuminuria and mortality is more pronounced in individuals with both hypertension and hypercholesterolemia support albuminuria as a marker of systemic vascular dysfunction. 1 , 2 , 3 , 4 , 5 , 29 The leading theory describing this interaction highlights the glomeruli as a window to the state of the vasculature, where albuminuria is accompanied by diffuse endothelial dysfunction and altered hemostasis, fibrinolysis, leukocyte adhesion, and nitric oxide synthesis, all key components in the initiation and progression of atherosclerosis and cardiovascular events. 42 , 43 , 44 , 45 , 46 , 47 Though a prior meta‐analysis found a stronger association between albuminuria and mortality among individuals without hypertension than in those with hypertension, 12 our current findings are biologically plausible and highlight the importance of albuminuria as a marker of risk across subgroups with and without relevant comorbidities. Importantly, we found that albuminuria was associated with increased risk of all‐cause mortality independent of diabetes status, obesity, or history of CVD and across all eGFR categories. These results further highlight the importance of albuminuria as a marker of risk in both low‐ and high‐risk populations.
In conjunction with a prior meta‐analysis, 48 our results support the use of ACR as a powerful marker of future risk in the general population and among those with comorbidities, particularly those individuals with both hypertension and hypercholesterolemia. Current US guidelines recommend ACR monitoring in patients with diabetes in the management of diabetic kidney disease, 13 , 16 suggesting optional testing among those with hypertension. 24 However, the 2018 European Society of Cardiology and European Society of Hypertension Guidelines for the management of arterial hypertension 15 and the 2021 European Society of Cardiology Guidelines on CVD prevention 14 recommend measurement of ACR in all patients with hypertension. The present findings showing an increased risk of mortality across all levels of albuminuria and all categories of sex, race or ethnicity, comorbidities (hypertension, hypercholesterolemia, diabetes, obesity, CVD), and eGFR strata suggest a benefit for ACR monitoring among a broad subset of the general population, regardless of comorbidity status. The optimal approach to identifying individuals at risk for increased albuminuria, and the benefit of clinical interventions among those with even mildly increased albuminuria, warrants further study. In current practice, this is especially relevant due to the emergence of cost‐effective therapies such as sodium‐glucose cotransporter‐2 inhibitors that reduce albuminuria and the risk of adverse kidney and cardiovascular outcomes among patients at increased risk, including those with history of CVD, heart failure, or chronic kidney disease, with or without diabetes. 49 , 50 In fact, a recent study has shown that screening for albuminuria in the general US population, followed by treatment if indicated, is cost effective. 51
In addition, our findings highlight the remarkable increase in absolute mortality risk among individuals with hypertension alone or with both hypertension and hypercholesterolemia across all categories of albuminuria, compared with individuals with neither comorbidity or with hypercholesterolemia alone. Surprisingly, we did not find a strong association between albuminuria and mortality among individuals with high cholesterol alone. Though the lack of significant findings could be attributed to a low sample size, absolute mortality rates among individuals with high cholesterol alone were similar to rates observed among individuals with neither hypertension nor high cholesterol (as shown in Figure 2). In fact, prior research has noted a J‐ or U‐shaped association between total cholesterol and mortality among patients with ischemic stroke, acute coronary syndrome, chronic kidney disease, and general population cohorts. 52 , 53 , 54 , 55 Such a “cholesterol paradox” may be explained by factors such as systemic inflammation and malnutrition that are strongly associated with higher mortality and lower cholesterol levels. For example, among patients with CKD, the cytokine response to chronic inflammation, mediated by tumor necrosis factor‐α and interleukin‐6, leads to increased catabolism, hypocholesterolemia, and progressive atherosclerosis, resulting in attenuated or even inverse associations between cholesterol and mortality. 55 , 56 In contrast, hypertension, whether alone or in the presence of hypercholesterolemia, is associated with markedly increased mortality risk. Overall, this has important public health implications for the large proportion of US adults with hypertension (>100 million, or nearly half of US adults), 57 who are at an increased risk of experiencing adverse cardiovascular outcomes but are not universally included in US guideline recommendations or protocols for ACR monitoring. In particular, the identification of increased albuminuria may affect the decision to initiate antihypertensive therapy in an individual with stage 1 hypertension not otherwise meeting criteria for pharmacologic therapy according to current hypertension treatment guidelines. 24
The current study is not without limitations. First, the presence of albuminuria was denoted by a random single spot measurement rather than a 24‐hour urine collection or multiple spot samples over time. ACR measured on random urine samples may slightly overestimate the prevalence of albuminuria. 58 However, comparisons between early morning void ACR and 24‐hour urinary albumin excretion have demonstrated that despite occasional under‐ or overestimation of albuminuria, early morning void ACR correlates with mortality risk, 59 suggesting that the same may be true for random single spot measurements. Further, the initial assessment of albuminuria using a random urine sample rather than an early morning void ACR reflects current clinical practice. In addition, because of the cross‐sectional design of NHANES, we were unable to account for changing levels of albumin excretion over time. Finally, although the NDI is accurate in ascertaining vital status, 60 it is less accurate in identifying cause of death. However, our primary outcome of interest was all‐cause mortality, and NDI‐derived versus expert‐adjudicated overall mortality rates appear to be highly consistent. 60 Despite these limitations, the present study has many strengths. Most notably, we aggregated 20 years' worth of data resulting in a large sample representative of the US population with linkage to death information. With complete information on serum and urine creatinine, BP, and fasting cholesterol, we were able to examine the impact of even mildly increased albuminuria and stratify by important demographic and clinical factors. Further, we were able to complete various sensitivity analyses, including an analysis incorporating the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline hypertension definition. Finally, extensive longitudinal data from the NDI allowed us to assess cause‐specific mortality data, opening doors for future studies of associations between albuminuria and mortality in specific subgroups according to vascular‐related cause of death.
Conclusions
In summary, in a nationally representative study, we found strong graded associations between albuminuria and all‐cause mortality, even at mildly increased levels of albuminuria, across categories of sex, race or ethnicity, and CVD‐related comorbidities. Associations were more pronounced among women in comparison with men and among those with hypertension and hypercholesterolemia. Thus, the presence of albuminuria identifies individuals across a broad range of subgroups by sex, race or ethnicity, and comorbidities who are at increased risk of all‐cause mortality and may benefit from more aggressive risk factor modification to help reduce long‐term adverse health outcomes and mortality risk. However, the optimal approach to clinical interventions, particularly among those with mildly increased albuminuria, remains to be elucidated. These findings have potentially important implications for risk assessment in clinical practice and for albuminuria monitoring guidelines in the United States. Whether the implementation of broader albuminuria screening will translate into improved survival, and the cost‐effectiveness of such an approach, are important areas for further study.
Sources of Funding
Dr Drexler is supported by grant number UL1TR002736, Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Dr Elfassy is supported by the National Institute on Minority Health and Health Disparities (K01MD014158).
Disclosures
None.
Supporting information
Tables S1–S5
Figures S1–S2
This article was sent to Tazeen H. Jafar, MD MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030773
For Sources of Funding and Disclosures, see page 11.
References
- 1. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality. A collaborative meta‐analysis of high‐risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536 [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end‐stage renal disease. A collaborative meta‐analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421 [DOI] [PubMed] [Google Scholar]
- 6. Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang M, Kwon S, Lee J, Shin JI, Kim YC, Park JY, Bae E, Kim EY, Kim DK, Lim CS, et al. Albuminuria within the normal range can predict all‐cause mortality and cardiovascular mortality. Kidney360. 2022;3:74–82. doi: 10.34067/KID.0003912021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoue K, Streja E, Tsujimoto T, Kobayashi H. Urinary albumin‐to‐creatinine ratio within normal range and all‐cause or cardiovascular mortality among U.S. adults enrolled in the NHANES during 1999‐2015. Ann Epidemiol. 2021;55:15–23. doi: 10.1016/j.annepidem.2020.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483 [DOI] [PubMed] [Google Scholar]
- 10. Nitsch D, Grams M, Sang Y, Black C, Cirillo M, Djurdjev O, Iseki K, Jassal SK, Kimm H, Kronenberg F, et al. Associations of estimated glomerular filtration rate and albuminuria with mortality and renal failure by sex: a meta‐analysis. BMJ. 2013;346:f324. doi: 10.1136/bmj.f324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hui X, Matsushita K, Sang Y, Ballew SH, Fülöp T, Coresh J. CKD and cardiovascular disease in the Atherosclerosis Risk in Communities (ARIC) study: interactions with age, sex, and race. Am J Kidney Dis. 2013;62:691–702. doi: 10.1053/j.ajkd.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmoodi BK, Matsushita K, Woodward M, Blankestijn PJ, Cirillo M, Ohkubo T, Rossing P, Sarnak MJ, Stengel B, Yamagishi K, et al. Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without hypertension: a meta‐analysis. Lancet. 2012;380:1649–1661. doi: 10.1016/S0140-6736(12)61272-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–S83. doi: 10.2337/diacare.27.2007.S79 [DOI] [PubMed] [Google Scholar]
- 14. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 15. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 16. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102:S1–s127. doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2022. Accessed October 9, 2023. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 18.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey. NCHS Ethics Review Board (ERB) Approval. 2022. Accessed October 9, 2023. https://www.cdc.gov/nchs/nhanes/irba98.htm
- 19. Chavers BM, Simonson J, Michael AF. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int. 1984;25:576–578. doi: 10.1038/ki.1984.57 [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). NHANES 2007‐2008 Laboratory Methods. Published September 2009. Accessed April 10, 2021. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?Cycle=2007‐2008
- 21. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1–s115. doi: 10.1016/j.kint.2020.06.019 [DOI] [PubMed] [Google Scholar]
- 22. Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol. 2009;170:515–518. doi: 10.1093/aje/kwp155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 25. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New creatinine‐ and cystatin c–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–1077. doi: 10.1681/ASN.2008070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin‐to‐creatinine ratio and association with progression to end‐stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta‐analysis of general and high‐risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nichols GA, Déruaz‐Luyet A, Brodovicz KG, Kimes TM, Rosales AG, Hauske SJ. Kidney disease progression and all‐cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020;21:167. doi: 10.1186/s12882-020-01792-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, Gans RO, Janssen WM, Grobbee DE, de Jong PE. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106:1777–1782. doi: 10.1161/01.CIR.0000031732.78052.81 [DOI] [PubMed] [Google Scholar]
- 31. Seo MH, Lee J‐Y, Ryu S, Won YS, Sung KC. The effects of urinary albumin and hypertension on all‐cause and cardiovascular disease mortality in Korea. Am J Hypertens. 2017;30:799–807. doi: 10.1093/ajh/hpx051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Solbu MD, Kronborg J, Jenssen TG, Njølstad I, Løchen M‐L, Mathiesen EB, Wilsgaard T, Eriksen BO, Toft I. Albuminuria, metabolic syndrome and the risk of mortality and cardiovascular events. Atherosclerosis. 2009;204:503–508. doi: 10.1016/j.atherosclerosis.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 33. Fangel MV, Nielsen PB, Kristensen JK, Larsen TB, Overvad TF, Lip GY, Jensen MB. Albuminuria and risk of cardiovascular events and mortality in a general population of patients with type 2 diabetes without cardiovascular disease: a Danish cohort study. Am J Med. 2020;133:e269–e279. doi: 10.1016/j.amjmed.2019.10.042 [DOI] [PubMed] [Google Scholar]
- 34. Nichols GA, Déruaz‐Luyet A, Hauske SJ, Brodovicz KG. The association between estimated glomerular filtration rate, albuminuria, and risk of cardiovascular hospitalizations and all‐cause mortality among patients with type 2 diabetes. J Diabetes Complicat. 2018;32:291–297. doi: 10.1016/j.jdiacomp.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 35. Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS. Low‐grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132 [DOI] [PubMed] [Google Scholar]
- 36. Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. doi: 10.1681/ASN.V1341034 [DOI] [PubMed] [Google Scholar]
- 37. de Jong PE, Curhan GC. Screening, monitoring, and treatment of albuminuria: public health perspectives. J Am Soc Nephrol. 2006;17:2120–2126. doi: 10.1681/ASN.2006010097 [DOI] [PubMed] [Google Scholar]
- 38. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401 [DOI] [PubMed] [Google Scholar]
- 39. Jolly SE, Burrows NR, Chen S‐C, Li S, Jurkovitz CT, Narva AS, Norris KC, Shlipak MG. Racial and ethnic differences in albuminuria in individuals with estimated GFR greater than 60 mL/min/1.73 m(2): results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55:S15–S22. doi: 10.1053/j.ajkd.2009.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gutiérrez OM, Khodneva YA, Muntner P, Rizk DV, McClellan WM, Cushman M, Warnock DG, Safford MM. Association between urinary albumin excretion and coronary heart disease in black vs white adults. JAMA. 2013;310:706–714. doi: 10.1001/jama.2013.8777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gutiérrez OM, Judd SE, Muntner P, Rizk DV, McClellan WM, Safford MM, Cushman M, Kissela BM, Howard VJ, Warnock DG. Racial differences in albuminuria, kidney function, and risk of stroke. Neurology. 2012;79:1686–1692. doi: 10.1212/WNL.0b013e31826e9af8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stehouwer CD, Smulders YM. Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. J Am Soc Nephrol. 2006;17:2106–2111. doi: 10.1681/ASN.2005121288 [DOI] [PubMed] [Google Scholar]
- 43. Deckert T, Feldt‐Rasmussen B, Borch‐Johnsen K, Jensen T, Kofoed‐Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno Hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287 [DOI] [PubMed] [Google Scholar]
- 44. Ritz E. Albuminuria and vascular damage–the vicious twins. N Engl J Med. 2003;348:2349–2352. doi: 10.1056/NEJMe030066 [DOI] [PubMed] [Google Scholar]
- 45. Danziger J. Importance of low‐grade albuminuria. Mayo Clin Proc. 2008;83:806–812. doi: 10.4065/83.7.806 [DOI] [PubMed] [Google Scholar]
- 46. Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol. 2007;2:581–590. doi: 10.2215/CJN.03190906 [DOI] [PubMed] [Google Scholar]
- 47. Kramer H, Jacobs DR, Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18 [DOI] [PubMed] [Google Scholar]
- 48. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta‐analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 50. Heerspink HJL, Stefánsson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou F‐F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 51. Cusick MM, Tisdale RL, Chertow GM, Owens DK, Goldhaber‐Fiebert JD. Population‐wide screening for chronic kidney disease. Ann Intern Med. 2023;176:788–797. doi: 10.7326/M22-3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291:451–459. doi: 10.1001/jama.291.4.451 [DOI] [PubMed] [Google Scholar]
- 53. Yeramaneni S, Kleindorfer DO, Sucharew H, Alwell K, Moomaw CJ, Flaherty ML, Woo D, Adeoye O, Ferioli S, de Los Rios La Rosa F, et al. Hyperlipidemia is associated with lower risk of poststroke mortality independent of statin use: a population‐based study. Int J Stroke. 2016;12:152–160. doi: 10.1177/1747493016670175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang TY, Newby LK, Chen AY, Mulgund J, Roe MT, Sonel AF, Bhatt DL, DeLong ER, Ohman EM, Gibler WB, et al. Hypercholesterolemia paradox in relation to mortality in acute coronary syndrome. Clin Cardiol. 2009;32:E22–E28. doi: 10.1002/clc.20518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kalantar‐Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3:493–506. doi: 10.1038/ncpneph0570 [DOI] [PubMed] [Google Scholar]
- 56. Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL. Interleukin‐6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. doi: 10.1053/ajkd.1998.v32.pm9669431 [DOI] [PubMed] [Google Scholar]
- 57. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential us population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. doi: 10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, Narva AS, Eggers PW, Williams DE. Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009‐2010. Clin Chem. 2013;59:675–683. doi: 10.1373/clinchem.2012.195644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vart P, Scheven L, Lambers Heerspink HJ, de Jong PE, de Zeeuw D, Gansevoort RT. Urine albumin‐creatinine ratio versus albumin excretion for albuminuria staging: a prospective longitudinal cohort study. Am J Kidney Dis. 2016;67:70–78. doi: 10.1053/j.ajkd.2015.05.025 [DOI] [PubMed] [Google Scholar]
- 60. Olubowale OT, Safford MM, Brown TM, Durant RW, Howard VJ, Gamboa C, Glasser SP, Rhodes JD, Levitan EB. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2017;6:e004966. doi: 10.1161/JAHA.116.004966 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S2


