Abstract
Background
Cardiovascular complications after acute ischemic stroke (AIS) can be related to chronic/comorbid cardiac conditions or acute disruption of the brain–heart autonomic axis (stroke‐heart syndrome). Women are known to be more vulnerable to certain stress‐induced cardiac complications, such as Takotsubo cardiomyopathy. We investigated sex differences in cardiac troponin (cTn) elevation, cardiac events, and outcomes after AIS.
Methods and Results
We retrospectively analyzed consecutive patients with AIS from 5 stroke centers. Patients with AIS with elevated baseline cTn and at least 2 cTn measurements were included, while patients with acute comorbid conditions that could impact cTn levels were excluded. Poststroke acute myocardial injury was defined as the presence of a dynamic cTn pattern (rise/fall >20% in serial measurements) in the absence of acute atherosclerotic coronary disease (type 1 myocardial infarction) or cardiac death (type 3 myocardial infarction). From a total cohort of 3789 patients with AIS, 300 patients were included in the study: 160 were women (53%). Women were older, had a lower burden of cardiovascular risk factors, and more frequently had cardioembolic stroke and right insula involvement (P values all <0.05). In multivariate analysis, women were more likely to have a dynamic cTn pattern (adjusted odds ratio, 2.1 [95% CI, 1.2–3.6]) and develop poststroke acute myocardial injury (adjusted odds ratio, 2.1 [95% CI, 1.1–3.8]). Patients with poststroke acute myocardial injury had higher 7‐day mortality (adjusted odds ratio, 5.5 [95% CI, 1.2–24.4]).
Conclusions
In patients with AIS with elevated cTn at baseline, women are twice as likely to develop poststroke acute myocardial injury, and this is associated with higher risk of short‐term mortality. Translational studies are needed to clarify mechanisms underlying sex differences in cardiac events and mortality in AIS.
Keywords: cardiac complications, ischemic stroke, mortality, stroke‐heart syndrome, troponin
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- cTn
cardiac troponin
- PSAMI
poststroke acute myocardial injury
Clinical Perspective.
What Is New?
It is unknown whether sex affects the risk of developing poststroke acute myocardial injury.
We hypothesized that women have a higher probability of brain‐heart autonomic axis disruption (stroke‐heart syndrome) triggered by an acute ischemic stroke.
Our study reveals that women with acute ischemic stroke with elevated cardiac troponin at baseline are more susceptible to developing poststroke acute myocardial injury, and this is associated with higher risk of short‐term mortality.
What Are the Clinical Implications?
Clinicians should be aware that female sex is a risk factor for stroke‐heart syndrome.
Clinicians should consider routinely performing serial cardiac troponin measurements in patients with acute ischemic stroke to identify individuals at higher risk of cardiac complications and tailor monitoring accordingly.
Translational studies are needed to clarify sex differences in functional and structural alterations of the central autonomic network triggered by acute ischemic stroke and the possible role of hormonal factors as a cause of predisposition of women for stroke‐heart syndrome.
Cardiovascular complications in the setting of acute ischemic stroke (AIS) occur in up to 20% of patients. 1 Poststroke cardiac events are heterogeneous, including sudden cardiac death, acute coronary syndrome, heart failure, and cardiac arrhythmias, 2 and are a major cause of death in the first 4 weeks after AIS. 1 Given the strong association between AIS and cardiac events, current American Heart Association/American Stroke Association guidelines recommend measuring cardiac troponin (cTn) as a biomarker of concomitant myocardial injury in all patients with suspected stroke. 3 However, a single elevated cTn does not indicate the underlying mechanism of myocardial injury. Chronic conditions, such as congestive heart failure, impaired renal function, and coronary artery disease (CAD), as well as comorbid acute disorders (acute coronary syndrome, pulmonary embolism, sepsis, and active cancer) 4 can increase cTn levels. Patients with AIS are also susceptible to functional and structural alterations in the central autonomic network leading to dysregulation of neural cardiac control and acute cardiac damage (stroke‐heart syndrome). 5 Distinguishing stroke‐heart syndrome from other conditions causing cTn elevation is challenging, given the multiple cause–effect interactions, shared pathophysiological mechanisms, and common risk factors of AIS and cardiac events.
There is increasing recognition of sex differences in ischemic stroke epidemiology, risk factors, clinical presentation, pathophysiology, treatment, and outcome. 6 , 7 Women tend to have more severe stroke 8 and experience greater disability and worse quality of life poststroke than men. 6 , 9 , 10 , 11 , 12 Important sex differences also exist with cardiac disease. 13 Women have much greater susceptibility to developing stress‐induced cardiomyopathy 14 (90% of patients from the International Takotsubo registry study were women 15 ) and are more likely to experience myocardial infarction (MI) in the setting of nonobstructive coronary artery disease compared with men. 16 Women with AIS have a much lower burden (8 times) of atherosclerotic CAD than men. 17 Furthermore, female sex has already been reported as an independent predictor of cardiac complications after subarachnoid hemorrhage. 18 , 19 Whether there are sex differences in stroke‐heart syndrome and poststroke cardiac complications remains unexplored.
We investigated sex differences in cTn elevation and cardiac events after AIS. We hypothesized that sex impacts the risk of developing poststroke myocardial injury, with women having higher probability of brain‐heart autonomic axis disruption triggered by the AIS. We also assessed the impact of sex on in‐hospital mortality and discharge disposition in patients with evidence of acute cardiac injury as indicated by cTn elevation.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We retrospectively evaluated consecutive patients with AIS admitted to 5 stroke centers (Hospital of the University of Pennsylvania, Lancaster General Hospital, Penn Presbyterian Medical Center, Pennsylvania Hospital, and University Hospital at SUNY Downstate Health Science University) from June 2020 to July 2022. The study protocol was approved by the University of Pennsylvania and SUNY Downstate Health Science University Institutional Review Boards with waiver of informed consent. The inclusion criteria were as follows: (1) adults (>18 years of age) who had neuroimaging‐confirmed AIS, (2) hospital admission within 72 hours from symptoms onset, (3) increased cTn level (value above the assay‐specific 99th percentile upper reference limit) at admission, and (4) at least 1 additional follow‐up cTn measurement within 48 hours. Exclusion criteria were as follows: (1) in‐hospital stroke (patients who were admitted for nonneurological symptoms and later developed a stroke during the hospital course), (2) acute concomitant conditions within 2 weeks known to cause elevated cTn, defined as any of the following: sepsis, acute kidney injury or rhabdomyolysis, major cardiac surgery or MI, prior ischemic or hemorrhagic stroke, congestive heart failure exacerbation, pulmonary embolism, deep vein thrombosis, endocarditis, or illicit drug use other than marijuana and benzodiazepine use. This study complies with Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 20
Data Collection
Patients' demographics (age, sex, and race), hand dominance, presence of cardiovascular risk factors (hypertension, diabetes, dyslipidemia, smoking status, chronic kidney disease, CAD, congestive heart failure, atrial fibrillation or flutter, prior AIS or hemorrhagic stroke), information regarding stroke onset, severity, cause, and localization (radiology reports and imaging were reviewed with specific interest for stroke side and insular involvement) were collected from existing records. Sex was abstracted from medical records. Self‐identified gender was not separately available in a systematic way.
Stroke severity and functional status were assessed with the National Institutes of Health Stroke Scale and the modified Rankin Scale, respectively. Cause/subtype of AIS was classified per Trial of Org 10 172 in Acute Stroke Treatment. 21 Cardiology consultation reports, electrocardiogram, echocardiogram, advanced cardiac imaging, and coronary angiography data were reviewed. Specific cardiac events and cardiac diagnoses were based on cardiology reports. Cardiologists were involved in the evaluation of all patients with a new acute cardiac event. Coronary angiography or additional advanced cardiac imaging were performed when recommended by cardiologists, as determined by medical history, cTn pattern, cTn peak value, cardiac event clinical characteristics, and individual‐level risk–benefit assessment. Cardiac ejection fraction (EF) was classified based on 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America Guideline 22 : preserved EF (≥50%), mildly reduced EF (41%–49%), and reduced EF (≤40%). Subtype of acute myocardial injury was classified based on the 2018 Fourth Universal Definition of Myocardial Infarction. 23
Outcomes
Our primary outcomes included dynamic cTn pattern and poststroke acute myocardial injury (PSAMI). A dynamic cTn pattern was defined as a rising or falling cTn >20% in serial measurements, based on the 2018 Fourth Universal Definition of Myocardial Infarction. 23 While elevated but stable cTn levels can be seen in multiple chronic cardiac and noncardiac conditions, a dynamic cTn pattern is specific for an acute evolving myocardial injury. 24 PSAMI encompasses an acute myocardial injury secondary to a disruption of the brain‐heart autonomic axis triggered by AIS, after exclusion of Type 1 MI (caused by atherothrombotic CAD) or Type 3 MI (cardiac death before diagnostic testing). In keeping with the Fourth Universal Definition of Myocardial Infarction, PSAMI includes both type 2 MI (mismatch between oxygen supply and demand) and acute myocardial injury in the absence of ischemic signs or symptoms, specifically, Takotsubo cardiomyopathy, heart failure, and atrial fibrillation with rapid ventricular response. Patients with an isolated dynamic cTn without evidence of MI or myocardial injury were not included in PSAMI. Ischemic electrocardiogram changes included new ST‐segment depression or elevation or new bundle branch block. Ischemic echocardiogram changes included new or previously unknown regional wall motion abnormality in an ischemic cause pattern. The cause of PSAMI was based on assessment at time of AIS.
Secondary outcomes included mortality within 7 days from admission and discharge disposition. Discharge disposition was dichotomized as good if discharged to home or acute rehabilitation, or unfavorable if discharged to long‐term care facility, hospice, or death during the hospitalization.
Statistical Analysis
Continuous variables were presented as mean±SD, or median (interquartile range) based on their distribution. Categorical variables were expressed as frequency. No imputation was performed for missing data. When data were missing, we showed the denominator of the population with available data. In univariate analysis, women and men were compared using independent sample t test for continuous normally distributed variables, Wilcoxon‐Mann–Whitney U test for continuous nonnormally distributed or ordinal variables, and χ2 or Fisher exact tests for categorical variables. Unadjusted and adjusted multivariable logistic regression analyses were performed in order to calculate odds ratios (ORs) and 95% CIs for association of sex with the key outcomes. In the primary model we adjusted for age, race, medical history of CAD, chronic kidney disease, congestive heart failure, atrial fibrillation, National Institutes of Health Stroke Scale, involvement of the right insula, and stroke cause. In sensitivity analysis, we did not include stroke cause as covariate (due to possible collinearity with outcome), because the detection of a new‐onset atrial fibrillation or low EF might have been considered the cause (cardioembolic cause) rather than the consequence of the ischemic stroke. Covariates were selected a priori.
Unadjusted and adjusted logistic regression analyses were also performed for association of PSAMI with mortality within 7 days from admission and discharge disposition. Primary model and sensitivity analysis also included sex as covariate. P<0.05 was considered significant. All analyses were performed with STATA 17.0 (StataCorp, College Station, TX) for Windows.
Sample size calculation was based on several lines of evidence used to estimate the differential probability of cardiac events not ascribed to CAD in women versus men. First, there appears to be an ≈6‐fold lower prevalence of CAD (identified with computed tomography coronary angiogram) in women with AIS or transient ischemic attack compared with men, and an absolute prevalence of CAD of 4% in women compared with 24% in men. 17 The prevalence of significant cardiac complications in patients with acute ischemic stroke is estimated to be ≈10% within the first 7 days and is similar between men and women 1 ; using the assumption that these events are mostly due to CAD in men (90%), whereas they are 6 times less likely due to CAD in women compared with men (15%), this suggests a rate of cardiac events not related to CAD of ≈8.5% in women and 1% in men. 17 Using this differential effect with a power of 0.80 and a 2‐sided α=0.05, a sample size of 250 with a 1:1 ratio of men to women was needed. 25 To ensure the sample was sufficient if the event rate of the differential between men and women was lower than anticipated, we opted to use a total sample size of 300. To detect sex differences in our primary outcome (PSAMI), we aimed for a population of 300 subjects with AIS and increased cTn at baseline, assuming that women have a lower burden (8 times lower) of typical atherosclerotic CAD than men. 17 Considering that ≈10% to 30% of patients with AIS have a concomitant increased cTn, 5 , 26 , 27 we planned to screen a minimum of 2000 consecutive patients admitted for AIS. Given the possibility that not all patients with AIS in our centers would have a cTn level at admission and/or the percentage of our patients with AIS with increased cTn could be lower than reported in literature, we planned to continue screening retrospectively eligible patients until obtaining the above‐declared sample size of 300.
Results
Patient Characteristics
Of 3789 patients admitted for AIS in our 5 stroke centers, 606 patients (16%) had an elevated cTn at baseline. Of these, 104 (17%) did not have a second cTn measurement performed within 48 hours, and another 202 (33%) were excluded for presence of acute concomitant comorbidities known to cause elevated cTn, or because the hospital admission was secondary to a different medical condition than AIS (Figure 1). Our final cohort included 300 patients (mean age 74±13 years), 160 women (53%) and 140 men (47%). Women were older and more frequently right‐handed, had a lower burden of cardiovascular risk factors, and more often had cardioembolic stroke. Women were less likely to have small‐vessel occlusion or embolic stroke of undetermined source as the mechanism of their stroke. Women also had a stroke involving the right insula more frequently, while men were more frequently affected by a stroke involving the left hemisphere or bilateral hemispheres. Detailed patient demographics according to sex are described in Table 1.
Figure 1. Flowchart of patient selection.
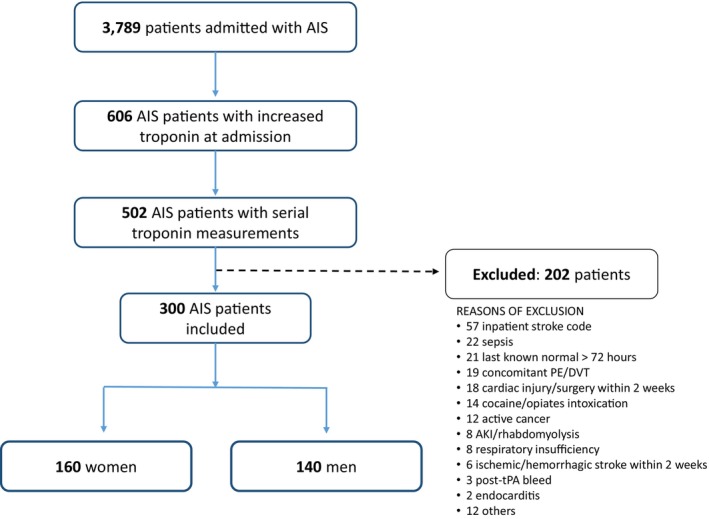
AIS indicates acute ischemic stroke; AKI, acute kidney injury; DVT, deep vein thrombosis; PE, pulmonary embolism; and tPA, tissue plasminogen activator.
Table 1.
Demographics, Comorbidities, and Stroke Causes and Localization
| Women (n=160) | Men (n=140) | P value | |
|---|---|---|---|
| Demographic | |||
| Mean age, y | 78±12 | 71±14 | <0.01 |
| Race | |||
| Asian | 1 (0.6%) | 1 (0.7%) | |
| Black | 69 (43%) | 67 (48%) | |
| White | 84 (53%) | 70 (50%) | |
| Other | 6 (4%) | 2 (1%) | 0.58 |
| BMI | 27.8±8.3 | 28±6.2 | 0.75 |
| Hand dominance (R) | 98/103 (95%) | 82/96 (82%) | 0.02 |
| Comorbidities | |||
| Hypertension | 140 (88%) | 126 (90%) | 0.50 |
| Diabetes | 59 (37%) | 67 (48%) | 0.06 |
| Dyslipidemia | 89 (56%) | 94 (67%) | 0.04 |
| Smoking (prior and current) | 59 (37%) | 77 (55%) | <0.01 |
| Chronic kidney disease | 54 (34%) | 53 (38%) | 0.46 |
| Coronary artery disease | 40 (25%) | 49 (35%) | 0.06 |
| Chronic heart failure | 35 (22%) | 40 (29%) | 0.18 |
| Atrial fibrillation/flutter | 45 (28%) | 36 (26%) | 0.64 |
| Stroke/TIA | 47 (20%) | 44 (31%) | 0.70 |
| NIHSS at admission | 6 (2–14) | 5 (3–11) | 0.21 |
| Stroke causes | |||
| LAA | 17 (11%) | 11 (8%) | |
| Cardioembolic | 65 (41%) | 44 (32%) | |
| SVO | 18 (11%) | 22 (16%) | |
| Cryptogenic | |||
| Multiple causes | 25 (15%) | 12 (8%) | |
| ESUS | 22 (14%) | 46 (33%) | |
| Incomplete work‐up | 10 (6%) | 2 (1%) | |
| Others (hypercoagulability) | 3 (2%) | 3 (2%) | <0.01 |
| Insula involvement | 51/159 (32%) | 37/140 (26%) | 0.28 |
| R Insula involvement | 32/159 (20%) | 15/140 (11%) | 0.02 |
| Side of stroke | |||
| Left | 58 (36%) | 66 (47%) | |
| Right | 82 (51%) | 49 (35%) | |
| Bilateral | 20 (12.5%) | 25 (18%) | 0.02 |
Categorical variables are presented as frequency (column percent), continuous variables are presented as mean±SD, or median and interquartile range when nonnormally distributed. BMI indicates body mass index; ESUS, embolic stroke of undetermined source; LAA, large artery atherosclerosis; NIHSS, National Institutes of Health Stroke Scale; R, right; SVO, small vessel occlusion; and TIA, transient ischemic attack.
Cardiac Events, PSAMI, and Discharge Disposition
A dynamic cTn pattern (rising or falling cTn >20% in serial measurement) was more frequent in women (53% versus 39%, P=0.02) (Table 2). Cardiac events in patients with dynamic cTn pattern are reported in in Table S1. After excluding patients with Type 1 MI (4 women and 4 men) and type 3 MI (2 women and 2 men), 74 patients with AIS met the definition of PSAMI (Figure 2). PSAMI was more common in women compared with men (31% versus 18%, P=0.01). The majority of these events were Type 2 MI (see Table 2 for details).
Table 2.
Dynamic cTn Pattern, Poststroke Myocardial Injury, and Outcomes
| Women (n=160) | Men (n=140) | P value | |
|---|---|---|---|
| Dynamic cTn pattern | 84 (53%) | 54 (39%) | 0.02 |
| PSAMI | 49 (31%) | 25 (18%) | 0.01 |
| Type 2 MI | 31 (64%) | 19 (76%) | |
| Nonischemic cardiomyopathy | 4 (8%) | 4 (16%) | |
| Takotsubo | 5 (10%) | 1 (4%) | |
| AF with RVR | 6 (12%) | 1 (4%) | |
| Heart failure | 3 (6%) | 0 | |
| Length of hospital stay | 5 (3–9) | 5 (3–10) | 0.76 |
| Mortality within 7 d* | 11 (7%) | 3 (2%) | 0.06 |
| Unfavorable discharge disposition | 70 (44%) | 36 (26%) | <0.01 |
Categorical variables are presented as frequency (column percent), and nonnormally distributed continuous variables as median and interquartile range. AF indicates atrial fibrillation; cTn, cardiac troponin; MI, myocardial infarction; PSAMI, poststroke myocardial injury; and RVR, rapid ventricular rhythm.
P values were calculated using Fisher exact test.
Figure 2. Flowchart of poststroke acute myocardial injury selection.
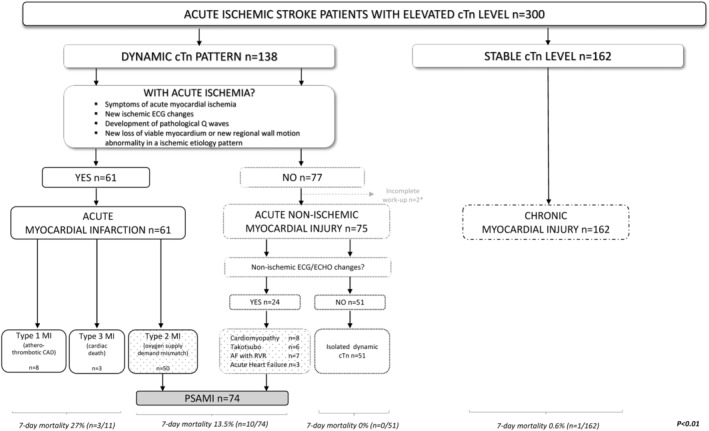
*No echocardiogram available. AF indicates atrial fibrillation; CAD, coronary artery disease; cTn, cardiac troponin; ECG, electrocardiogram; ECHO, echocardiogram; MI, myocardial infarction; PSAMI, poststroke acute myocardial injury; and RVR, rapid ventricular rhythm.
Nineteen patients (12 women and 7 men; 8% versus 5%, P=0.4) underwent advanced cardiac testing to rule out acute atherothrombotic CAD (Table S2). Two patients with a high pretest probability of Type 1 MI could not undergo coronary angiography due to severe bleeding risk (AIS with hemorrhagic conversion in 1 woman and life‐threatening retroperitoneal bleed in 1 man).
Seven‐day mortality was numerically higher in women (n=11) than men (n=3) (7% versus 2%, P=0.06) and differed based on type of myocardial injury (Figure 2). Seven patients (5 women and 2 men) died from a neurological cause; namely, malignant middle cerebral artery syndrome with severe cerebral edema (n=5) and intracranial bleeding after early anticoagulation for new atrial fibrillation (n=1). Two patients died following early withdrawal of care (2 women). Three patients (2 women and 1 man) died from cardiac complications (cardiac arrest secondary to ST‐segment–elevation myocardial infarction) and ventricular arrhythmia).
Women were more frequently discharged to a long‐term care facility or hospice or died during the admission than men (unfavorable discharge disposition) (44% versus 26%, P<0.01) (Table 2).
In univariate regression analysis, women were more likely to have a dynamic cTn pattern (OR, 1.8 [95% CI, 1.1–2.8], P=0.02) and have PSAMI (OR, 2 [1.2–3.5], P=0.01). This association was similar in primary multivariable analyses (OR, 2.1 [1.2–3.6], P<0.01 and OR 1.9 [1.1–3.7], P=0.04, respectively) and in the sensitivity analysis. Women were more likely to have an unfavorable discharge disposition outcome in univariate regression analysis (OR, 2.3 [1.4–3.7], P<0.01), but this was not upheld in multivariate analysis, with evidence of confounding by older age and higher National Institutes of Health Stroke Scale (Figure 3).
Figure 3. Multivariate analysis of association of sex and main outcome measures.
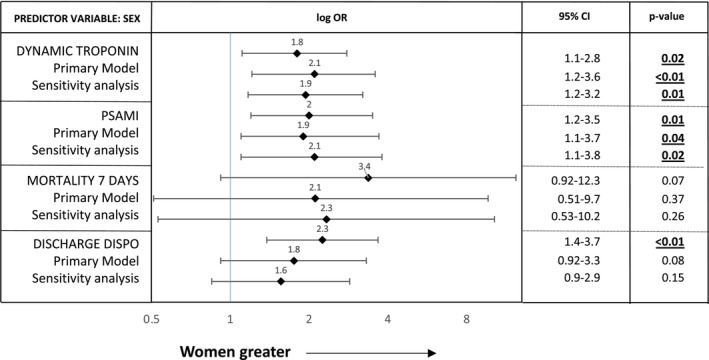
Odds ratios (ORs) for association of sex with dynamic pattern or cardiac troponin, poststroke acute myocardial injury (PSAMI), mortality within 7 days, and discharge disposition. Primary model: adjusted for age, race, medical history of chronic kidney disease, coronary artery disease, acute congestive heart failure, atrial fibrillation or flutter, National Institutes of Health Stroke Scale at admission, right insula involvement, and TOAST (Trial of Org 10 172 in Acute Stroke Treatment) classification. Sensitivity analysis: same as primary model but with removal of TOAST classification as a covariate. PSAMI indicates poststroke acute myocardial injury.
In univariate analysis, PSAMI was associated with almost an 8‐fold higher odds of death within 7 days (OR, 8.5 [2.6–28.6], P<0.01); this difference remained significant in the primary multivariable analyses (OR, 5.5 [1.2–24.4], P=0.02) and in the sensitivity analysis (OR, 4.6 [1.2–18.2], P=0.02). Patients with PSAMI had higher odds for unfavorable discharge disposition (OR, 2.3 [1.3–3.9], P<0.01), with similar magnitude but nonsignificant association in adjusted and sensitivity analysis (Table 3).
Table 3.
Unadjusted and Adjusted Logistic Regression Analysis of Association of Poststroke Acute Myocardial Injury and Main Outcomes
| Predictor variable: PSAMI | Model | OR | 95% CI | P value |
|---|---|---|---|---|
| Mortality within 7 d | Unadjusted | 8.5 | 2.6–28.6 | <0.01 |
| Primary* | 5.5 | 1.2–24.4 | 0.02 | |
| Sensitivity† | 4.6 | 1.2–18.2 | 0.02 | |
| Unfavorable discharge disposition | Unadjusted | 2.3 | 1.3–3.9 | <0.01 |
| Primary* | 1.8 | 0.9–3.7 | 0.1 | |
| Sensitivity† | 1.6 | 0.8–3.1 | 0.2 |
Odds ratio (OR) and corresponding 95% CI for association of PSAMI with mortality within 7 days and discharge disposition. PSAMI indicates poststroke acute myocardial injury.
Primary model: adjusted for sex, age, race, medical history of chronic kidney disease, coronary artery disease, acute congestive heart failure, atrial fibrillation or flutter, National Institutes of Health Stroke Scale at admission, right insula involvement, TOAST (Trial of Org 10 172 in Acute Stroke Treatment) classification.
Sensitivity analysis: same as primary model but with removal of TOAST classification as a covariate.
Discussion
Our data suggest that among patients with AIS with concomitant elevated cTN at admission, women are significantly more likely to develop a dynamic cTn pattern, and are also more likely to have PSAMI. In addition, we found that PSAMI was associated with markedly increased odds of early mortality.
The baseline differences between women and men in our cohort of patients with AIS and increased cTn at admission suggest distinct underlying pathophysiology for their cardiac injury. While women were older, they had a much lower burden of atherosclerotic risk factors and cardiac comorbidities than men. This is consistent with prior studies demonstrating that women with AIS are less affected (8 times) by CAD than men. 17 Considering that women in our cohort had a higher risk of acute myocardial injury and a lower burden of cardiovascular risk factors than men, it is possible that their cardiac injury was more acute and due to a nonatherosclerotic mechanism, such as stroke‐heart syndrome. Sex differences in the cause of cardiac injury after AIS have already been suggested by Sposato et al, 25 who investigated the sex‐specific risk of incident poststroke major adverse cardiovascular events in patients with AIS and matched individuals, without the confounding effect of pre‐existing heart disease. The risk of major cardiovascular complications was 23 to 25 times higher in patients with first‐ever AIS than in controls in the first 30 days after stroke in both sexes; this risk waned with time but remained significantly elevated, approximately doubled, even until 1 year later. Considering that men with AIS are more likely to be diagnosed with subclinical CAD than women, the authors suggested that the risk in women was driven by other factors such as selective predisposition to heart injury induced by neurogenic mechanisms. 25
An important neuroanatomic consideration in the pathogenesis of stroke‐heart syndrome is the involvement of the right insular cortex, which has a crucial role in the regulation of the central autonomic network, including autonomic control of cardiac function. 28 Specifically, lesions of the right insula might cause a downregulation of the parasympathetic activity and a consequent upregulation of sympathetic function, leading to acute myocardial injury. 29 Although we found that right insula involvement was overrepresented in women, its inclusion in multivariable models did not attenuate the independent effect of sex on PSAMI.
The pathophysiological mechanisms underlying sex differences in poststroke cardiac complications need further investigation. AIS may trigger a stronger stress‐mediated dysregulation of the central autonomic network in women, causing stroke‐heart syndrome. This could occur due to increased susceptibility to circulating catecholamine, similar to Takotsubo syndrome, 14 and/or right insula injury. 29 Estrogen is an important regulator of endothelial function 30 and catecholamine‐mediated vasoconstriction 31 : age‐related estrogen deprivation and sex differences in myocardial sensitivity to catecholamine increase postmenopausal women's susceptibility for Takotsubo syndrome during periods of acute stress. 32 , 33 Interestingly, female sex has also been recognized as an independent risk factor of cardiomyopathy after subarachnoid hemorrhage, 34 and elevated plasma norepinephrine and low estradiol levels seem to be associated with higher risk of post–subarachnoid hemorrhage cardiac injury in women. 35 The role of hormonal factors on autonomic nervous system response after AIS and on brain‐heart axis needs to be further explored.
We observed that PSAMI is independently associated with a 5 to 8 times increased risk of short‐term mortality, in line with prior reports showing that a dynamic cTn pattern is associated with an increased risk of in‐hospital death and unfavorable functional status at discharge in patients with AIS. 36 , 37 However, patients included in prior studies were affected by concomitant acute comorbidities affecting survival (nonneurological/noncardiac causes of death, such as sepsis, severe kidney failure, and end‐stage malignancy are frequently reported) and quantification of the impact of stroke‐heart syndrome on outcomes was not possible. To address this possible confounder, we specifically identified patients with PSAMI in the absence of concomitant acute conditions that could affect cTn levels as well as clinical outcomes. Nevertheless, a potential lack of validity for the adjusted association of PSAMI and short‐term mortality cannot be excluded, given the low number of patients with AIS who died within 7 days of hospitalization (n=14).
Additionally, we found a trend for higher 7‐day mortality and unfavorable discharge disposition in women. This association was not statistically significant, possibly because our study was powered specifically to analyze the effect of sex on PSAMI (our primary outcome) and not secondary outcomes. However, given that women have a higher risk of developing PSAMI, and PSAMI is associated with a severe increase of short‐term mortality (9 of the 14 patients who died within 7 days had a cardiac complication as cause of death), we can reasonably infer that women affected by AIS and elevated cTn have a higher risk of developing PSAMI and consequently dying within 7 days of admission.
Important sex differences in outcomes have already been reported in the setting of acute chest pain and acute coronary syndrome. 38 Compared with men, women presenting with acute MI are more than twice as likely to have nonobstructive coronary arteries, characterized as MI in the setting of nonobstructive coronary arteries. 16 Among the 50 patients identified as having Type 2 MI in our cohort, only 7 patients (5 women and 2 men) underwent coronary angiography with cardiac catheterization or computed tomography angiography to confirm nonobstructive CAD (Table S2). It is possible that some of these patients had MI in the setting of nonobstructive coronary arteries as a result of plaque erosion, coronary vasospasm, coronary microvascular disease, or spontaneous coronary artery dissection. However, the treating cardiologist did not identify these diagnoses as the most likely cause of acute MI.
A lower pretest probability for acute coronary syndrome among women might trigger an unconscious bias in providers, causing delays in care, lower probability to receive guideline‐directed care, or to undergo coronary angiography affecting outcomes. 38 , 39 , 40 In our cohort of patients with AIS with increased cTn levels, we did not find statistical sex differences in referral for advanced cardiac testing; however, the possibility exists of care discrepancies for women with AIS and PSAMI compared with men. The implementation of sex–specific cTn threshold might help the early identification of women at risk of future cardiac events. 41 Large, prospective studies are warranted to investigate disparities in mortality and functional outcomes between men and women with AIS and PSAMI, while thoroughly adjusting for potential confounding factors, especially age, stroke severity, and premorbid status.
While the strengths of our study include its multicenter nature and the systematic collection of all cardiac tests performed, our results should be interpreted in the context of several limitations. First, given the retrospective nature of the study, not all patients had serial testing despite an elevated initial cTn, nor was coronary angiography obtained in all with baseline elevated cTn. This may explain the low number of patients with high cTn level/dynamic pattern that ultimately underwent invasive cardiac testing. However, cardiologists were involved in the diagnostic plans of all patients with new acute cardiac event or when deemed necessary by the primary stroke team to ensure that acute CAD was ruled out. It is important to consider though that patients with AIS with increased cTn might be victim of a self‐fulfilling prophecy: cardiologists are aware that an acute brain injury disrupts the brain‐heart axis causing PSAMI; therefore a higher threshold to obtain additional and potentially invasive cardiology procedures to rule out CAD might be applied to patients with AIS. In our cohorts, when patients developed a nonlife‐threatening or self‐resolving poststroke acute myocardial injury, advance cardiac testing was frequently deferred to an outpatient setting. Moreover, some patients died before they could receive full diagnostic evaluation, and/or did not have complete diagnostic evaluations based on goals of care or increased bleeding risk. Finally, this study was underpowered to assess the direct association of sex and PSAMI with mortality and disposition outcomes in patients with AIS with increased cTn.
Conclusions
In conclusion, our study reveals that among patients with AIS with elevated cTn at baseline, women are more susceptible to developing PSAMI (2‐fold higher odds), and this is in turn associated with higher risk of short‐term mortality. Translational studies are needed to clarify sex differences in functional and structural alterations of the central autonomic network triggered by AIS and the consequent myocardial injury. Future studies should investigate the relationship between right insula injury, poststroke plasma catecholamine levels, and subsequent risk of stroke‐heart syndrome in women. Specifically, the role of hormonal factors in women with predisposition for brain‐heart autonomic axis disruption poststroke warrants investigation. Clinically, our results support the need to recognize patients with AIS at higher risk of cardiac complications and tailor monitoring accordingly, as already suggested by other groups. 21 Future clinical trials may explore therapeutic approaches to targeting the proposed autonomic imbalance in patients with AIS.
Sources of Funding
Dr Rosso was supported by the National Institutes of Health U24‐NS‐107224.
Disclosures
Dr Kasner has received grant funding from Bayer, Bristol‐Myers Squibb, Genentech, Medtronic, and Diamedica (all paid to institution); consulting fees from AstraZeneca, NovoNordisk, and Medtronic; and royalties from UpToDate. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
This manuscript was sent to Jennifer Tremmel, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.029799
See Editorial by Tweet et al.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S; VISTA Investigators . Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302. doi: 10.1161/STROKEAHA.106.471813 [DOI] [PubMed] [Google Scholar]
- 2. Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2 [DOI] [PubMed] [Google Scholar]
- 3. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 4. Scheitz JF, Endres M, Mochmann HC, Audebert HJ, Nolte CH. Frequency, determinants and outcome of elevated troponin in acute ischemic stroke patients. Int J Cardiol. 2012;157:239–242. doi: 10.1016/j.ijcard.2012.01.055 [DOI] [PubMed] [Google Scholar]
- 5. Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke‐heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 2018;17:1109–1120. doi: 10.1016/S1474-4422(18)30336-3 [DOI] [PubMed] [Google Scholar]
- 6. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, Howard VJ, Lichtman JH, Lisabeth LD, Pina IL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herson PS, Palmateer J, Hurn PD. Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res. 2013;4:413–419. doi: 10.1007/s12975-012-0238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dehlendorff C, Andersen KK, Olsen TS. Sex disparities in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4:4. doi: 10.1161/JAHA.115.001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carcel C, Wang X, Sandset EC, Delcourt C, Arima H, Lindley R, Hackett ML, Lavados P, Robinson TG, Munoz Venturelli P, et al. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology. 2019;93:e2170–e2180. doi: 10.1212/WNL.0000000000008615 [DOI] [PubMed] [Google Scholar]
- 10. Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D, et al. Sex differences in the clinical presentation, resource use, and 3‐month outcome of acute stroke in Europe: data from a multicenter multinational hospital‐based registry. Stroke. 2003;34:1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7 [DOI] [PubMed] [Google Scholar]
- 11. Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roquer J, Campello AR, Gomis M. Sex differences in first‐ever acute stroke. Stroke. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6 [DOI] [PubMed] [Google Scholar]
- 13. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 14. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046 [DOI] [PubMed] [Google Scholar]
- 15. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761 [DOI] [PubMed] [Google Scholar]
- 16. Tamis‐Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, Lerman A, Cushman M, Kumbhani DJ, Arslanian‐Engoren C, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. 2019;139:e891–e908. doi: 10.1161/CIR.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 17. Calvet D, Touze E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010;121:1623–1629. doi: 10.1161/CIRCULATIONAHA.109.906958 [DOI] [PubMed] [Google Scholar]
- 18. Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, Fink ME, Beckford A, Klebanoff LM. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–786. doi: 10.1161/01.str.30.4.780 [DOI] [PubMed] [Google Scholar]
- 19. Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–551. doi: 10.1161/01.STR.0000114874.96688.54 [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- 22. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 23. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 24. Mahajan VS, Jarolim P. How to interpret elevated cardiac troponin levels. Circulation. 2011;124:2350–2354. doi: 10.1161/CIRCULATIONAHA.111.023697 [DOI] [PubMed] [Google Scholar]
- 25. Sposato LA, Lam M, Allen B, Shariff SZ, Saposnik G, Group PS. First‐ever ischemic stroke and incident major adverse cardiovascular events in 93 627 older women and men. Stroke. 2020;51:387–394. doi: 10.1161/STROKEAHA.119.028066 [DOI] [PubMed] [Google Scholar]
- 26. Faiz KW, Thommessen B, Einvik G, Brekke PH, Omland T, Ronning OM. Determinants of high sensitivity cardiac troponin T elevation in acute ischemic stroke. BMC Neurol. 2014;14:96. doi: 10.1186/1471-2377-14-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn SH, Kim YH, Lee JS, Han JH, Kim SY, Kang DW, Kim JS, Kwon SU. Troponin I levels and long‐term outcomes in acute ischemic stroke patients. J Am Coll Cardiol. 2019;73:525–526. doi: 10.1016/j.jacc.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 28. Soros P, Hachinski V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012;11:179–188. doi: 10.1016/S1474-4422(11)70291-5 [DOI] [PubMed] [Google Scholar]
- 29. Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, Endres M, Scheitz JF, Nolte CH. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann Neurol. 2017;81:502–511. doi: 10.1002/ana.24906 [DOI] [PubMed] [Google Scholar]
- 30. Sader MA, Celermajer DS. Endothelial function, vascular reactivity and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:597–604. doi: 10.1016/s0008-6363(01)00473-4 [DOI] [PubMed] [Google Scholar]
- 31. Sung BH, Ching M, Izzo JL Jr, Dandona P, Wilson MF. Estrogen improves abnormal norepinephrine‐induced vasoconstriction in postmenopausal women. J Hypertens. 1999;17:523–528. doi: 10.1097/00004872-199917040-00010 [DOI] [PubMed] [Google Scholar]
- 32. Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of Takotsubo syndrome. Circulation. 2017;135:2426–2441. doi: 10.1161/CIRCULATIONAHA.116.027121 [DOI] [PubMed] [Google Scholar]
- 33. Wittstein IS. Why age matters in Takotsubo syndrome. J Am Coll Cardiol. 2020;75:1878–1881. doi: 10.1016/j.jacc.2020.03.030 [DOI] [PubMed] [Google Scholar]
- 34. Bybee KA, Prasad A. Stress‐related cardiomyopathy syndromes. Circulation. 2008;118:397–409. doi: 10.1161/CIRCULATIONAHA.106.677625 [DOI] [PubMed] [Google Scholar]
- 35. Sugimoto K, Inamasu J, Hirose Y, Kato Y, Ito K, Iwase M, Sugimoto K, Watanabe E, Takahashi A, Ozaki Y. The role of norepinephrine and estradiol in the pathogenesis of cardiac wall motion abnormality associated with subarachnoid hemorrhage. Stroke. 2012;43:1897–1903. doi: 10.1161/STROKEAHA.111.646893 [DOI] [PubMed] [Google Scholar]
- 36. Scheitz JF, Mochmann HC, Erdur H, Tutuncu S, Haeusler KG, Grittner U, Laufs U, Endres M, Nolte CH. Prognostic relevance of cardiac troponin T levels and their dynamic changes measured with a high‐sensitivity assay in acute ischaemic stroke: analyses from the TRELAS cohort. Int J Cardiol. 2014;177:886–893. doi: 10.1016/j.ijcard.2014.10.036 [DOI] [PubMed] [Google Scholar]
- 37. Stengl H, Ganeshan R, Hellwig S, Klammer MG, von Rennenberg R, Bohme S, Audebert HJ, Nolte CH, Endres M, Scheitz JF. Frequency, associated variables, and outcomes of acute myocardial injury according to the fourth Universal Definition of Myocardial Infarction in patients with acute ischemic stroke. Eur Stroke J. 2022;7:413–420. doi: 10.1177/23969873221120159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dawson LP, Nehme E, Nehme Z, Davis E, Bloom J, Cox S, Nelson AJ, Okyere D, Anderson D, Stephenson M, et al. Sex differences in epidemiology, care, and outcomes in patients with acute chest pain. J Am Coll Cardiol. 2023;81:933–945. doi: 10.1016/j.jacc.2022.12.025 [DOI] [PubMed] [Google Scholar]
- 39. Brush JE Jr. Sex disparities in chest pain patients: observations and opportunities. J Am Coll Cardiol. 2023;81:946–948. doi: 10.1016/j.jacc.2023.01.006 [DOI] [PubMed] [Google Scholar]
- 40. Alabas OA, Gale CP, Hall M, Rutherford MJ, Szummer K, Lawesson SS, Alfredsson J, Lindahl B, Jernberg T. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc. 2017;6:6. doi: 10.1161/JAHA.117.007123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee KK, Ferry AV, Anand A, Strachan FE, Chapman AR, Kimenai DM, Meex SJR, Berry C, Findlay I, Reid A, et al. Sex‐specific thresholds of high‐sensitivity troponin in patients with suspected acute coronary syndrome. J Am Coll Cardiol. 2019;74:2032–2043. doi: 10.1016/j.jacc.2019.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2


