Abstract
Background
Proximal radial artery (pRA) access for cardiac catheterization is safe but can jeopardize subsequent use of the artery because of occlusion. Distal radial artery (dRA) access in the anatomical snuffbox preserves the radial artery, but safety and potential detrimental effects on hand function are unknown.
Methods and Results
In the DIPRA (Distal Versus Proximal Radial Artery Access for Cardiac Catheterization and Intervention) study, a single‐center trial, 300 patients were randomized 1:1 to cardiac catheterization through dRA or pRA. The primary end point of change in hand function from baseline to 30 days was a composite of the QuickDASH (Quick Disabilities of the Arm, Shoulder and Hand) questionnaire, hand‐grip test, and thumb forefinger pinch test. Secondary end points included access feasibility and complications; 254 of 300 patients completed follow‐up at 30 days; of these, 128 were randomized to dRA and 126 to pRA with balanced demographic and procedural characteristics. Both groups had similar rates of access site bleeding (dRA 0% versus pRA 1.4%; P=0.25). Six patients with dRA failed access compared with 2 patients with pRA. Radial artery occlusion occurred in 2 pRA versus none in dRA. There were no significant differences in change in hand function, median hand‐grip (dRA 0 [−3.2, 3.3] versus pRA 0.7 [−2.3, 3.3] kg; P=0.21), pinch‐grip (dRA −0.3 [−1.2, 0.5] versus pRA 0 [−0.9, 0.9] kg; P=0.09), and QuickDASH (dRA 0 [−4.6, 2.3] versus pRA 0 [−4.6, 2.3] points, P=0.96). There was no significant difference in the composite of hand function between pRA and dRA.
Conclusions
dRA is a safe strategy for cardiac catheterization with a low complication rate. Compared with pRA, there is no increased risk of hand dysfunction at 30 days.
Registration
URL: https://www.ClinicalTrials.gov. Unique identifier: NCT04318990.
Keywords: closure, complications, coronary and vascular access, management
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Revascularization, Stent, Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- DASH
Disabilities of the Arm, Shoulder and Hand Questionnaire
- dRA
distal radial artery
- pRA
proximal radial artery
- RA
radial artery
Clinical Perspective.
What Is New?
Change in hand function at 30 days was assessed between the distal and proximal radial access for cardiac catheterization using the QuickDASH (Quick Disabilities of the Arm, Shoulder and Hand) questionnaire, hand grip test, and thumb forefinger pinch test.
Compared with proximal radial artery, there is no increased risk of hand dysfunction or radial artery occlusion at 30 days.
What Are the Clinical Implications?
Distal radial access is a safe access strategy for cardiac catheterization with low complication rates.
Access methods for cardiac catheterization are controversial aspects that have changed several times over the years. Femoral artery access has traditionally been the most commonly used method. However, radial artery (RA) catheterization has become the preferred approach for percutaneous coronary interventions. Indeed, RA access has been associated with lower bleeding and vascular complications, leading to better patient outcomes compared with femoral artery access. 1 , 2 , 3 , 4 , 5 The American College of Cardiology/American Heart Association/Society of Cardiovascular Intervention and the European Society of Cardiology/European Association for Cardio‐Thoracic Surgery now support RA catheterization as the default vascular approach for percutaneous coronary interventions. 6 , 7
Recently, distal radial artery (dRA) access in the anatomic snuffbox has emerged as a promising alternative to conventional proximal radial artery (pRA) access, with several small studies reporting the safety and feasibility of this approach. 8 , 9 , 10 , 11 , 12 In addition to improved procedure ergonomics, particularly with the left dRA access, 13 the dRA access has been a promising approach to further reduce the rates of RA occlusion and access site hematoma compared with pRA access, but the dRA access has been associated with higher access site crossover. 14 This is particularly important, especially in patients with end‐stage renal disease or coronary artery disease, who may require a patent RA for arteriovenous fistula for hemodialysis or as a conduit for coronary artery bypass surgery. However, because the dRA is a smaller artery and passes close to the radial nerve, there are concerns that the dRA may affect hand function. To date, despite positive accumulating data on the feasibility and safety of the dRA access, 15 , 16 there have been no randomized control trials addressing concerns about hand function and other potential complications, especially when compared with the gold standard and widely accepted pRA. Herein, we report the 30‐day outcomes of the DIPRA (Distal Versus Proximal Radial Artery Access for Cardiac Catheterization and Intervention) study to evaluate the safety and effectiveness of dRA compared with pRA in patients undergoing cardiac catheterization.
DIPRA Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Objectives
The DIPRA study is a prospective, randomized, parallel controlled, open‐label, single center trial evaluating hand function, feasibility, and safety of dRA access compared with pRA access in patients undergoing cardiac catheterization. The trial design and study protocol has been described in detail previously. 17 Patients were enrolled and randomized in a 1:1 fashion from March 2020 to December 2021. The DIPRA study is registered at ClinicalTrials.gov, identifier NCT04318990, and funded by a philanthropic gift from Satish and Yasmin Gupta to Baylor Scott & White The Heart Hospital, Plano, Texas. The study, conducted in accordance with Good Clinical Practice from the International Council for Harmonization and the Declaration of Helsinki, was approved by the ethics committee of the Baylor Scott & White Research Institute Institutional Review Board (identifier number: 019‐504). Participation in the study was voluntary and required written informed consent before any procedure could be performed.
Primary and Secondary End Points
The primary end point is the change from baseline to 1 month of hand function in patients undergoing cardiac catheterization using dRA versus pRA. Several independent primary end points were included to assess hand function, including the QuickDASH questionnaire, the hand grip test, and the thumb‐forefinger pinch test. Secondary end points included success rate of arterial access and safety outcomes, such as access site bleeding defined by the EASY (Early Discharge After Transradial Stenting of Coronary Arteries Study) criteria, access site hematoma defined using an easy‐to‐use hematoma scale, other vascular access site complications, and rates of RA occlusion assessed by 1‐month Doppler ultrasound.
Procedure Description
Patients with a clinical indication for percutaneous coronary procedure and patent dRA and pRA on initial screening were considered for enrollment. The patency and accessibility of dRA and pRA were assessed at bedside by palpation and by ultrasonography of the dRA and pRA access sites before the procedure. Once the remaining inclusion criteria were met, participants were randomized using closed envelopes. Patients randomized to pRA underwent coronary procedure using the standard of care RA access. Participants randomized to dRA underwent the procedure as previously described in the published literature. 8 , 18 The right or left hand is placed on the ipsilateral side of the patient. In conventional transradial access, the patient's arm was placed in an extended position with the palm supinated. For dRA, the patient's hand was placed in a mid‐supination position and placed on a comfortable arm support, bringing the wrist in passive ulnar flexion. The patient is asked to grasp his thumb toward the palm. This brings the RA to the surface, allowing easier puncture. A brachial drape is used to expose the anatomical snuffbox, dRA, and pRA site of the hand. After a subcutaneous anesthetic injection of lidocaine in the anatomical snuffbox above the artery, and under ultrasound guidance, the artery was punctured at the site of the greatest pulse using a cannulated 21 gauge needle (Terumo) with the modified Seldinger technique. After a successful anterior wall puncture, a Nitrol sheath wire is carefully introduced while retaining the patient's wrist in the same position. Proper position is confirmed by fluoroscopy to ensure the wire has not crossed the palmar arch. Following this, a hydrophilic radial sheath (5 or 6 French slender radial sheaths) is advanced through the micropuncture needle into the radial artery. Arterial dilation is achieved by administering 200 to 400 mg of nitroglycerin and 2.5 to 5 mg of verapamil through the radial sheath, blood pressure permitting. At this point, successful arterial access has been achieved. Maneuvering the catheter and wire through the forearm could result in potential vascular injury and perforation, so wire insertion can be done under fluoroscopy and with a roadmap if necessary. Periprocedural anticoagulation was administrated appropriately for all patients based on the standard hospital protocol of 60 units/kg up to 5000 international units or heparin intravenously or weight‐based according to operator preference.
Hemostasis was achieved according to local practice, using a standard distal radial hemostatic band (Merit Medical Preclude SYNC DISTAL radial compression device), with the lowest possible pressure and the shortest possible time. As for proximal radial access, patent hemostasis technique is used. After the procedure, the patient can use both arms without restrictions and without the need for special support measures. All relevant procedural data were recorded.
Assessment of Hand Function and Strength QuickDASH Questionnaire
The QuickDASH questionnaire is a widely used tool for measuring self‐reported outcomes related to musculoskeletal disorders of the upper limbs. It stands for Disabilities of the Arm, Shoulder, and Hand. The QuickDASH questionnaire is a shorter version of the original DASH questionnaire, making it easier to administer and less burdensome for patients, while maintaining a high correlation with the original DASH questionnaire. 19 The QuickDASH questionnaire consists of 11 questions to assess the functional status and progress of patients with upper limb injuries or diseases. 19 , 20 Each question in the QuickDASH is scored on a 5‐point Likert scale, ranging from 1 to 5. A score of 1 indicates no difficulty, whereas a score of 5 indicates extreme difficulty or inability to perform the activity. The questionnaire was completed at baseline and after 1 month.
Pinch Grip Test by Use of a Pinch Grip Dynamometer and Hand Grip Test by Use of Jamar Hand Hydraulic Dynamometer
Both the Pinch Grip Test and the Hand Grip Test are objective and valuable tools for assessing hand strength, monitoring progress during rehabilitation and evaluating the impact of hand‐related conditions on hand function. The Pinch Grip Test evaluates the strength and coordination of the thumb and fingers during a pinch grip motion. It typically involves 3 variations: Tip pinch, key pinch, and palmar pinch. Baseline® Mechanical Pinch Gauge (Baseline Medical, Quakertown, PA) measures the force exerted during each type of pinch. The Hand Grip Test assesses overall hand strength by measuring the maximal isometric force generated during a gripping motion. The Jamar® Hand Hydraulic dynamometer, 200‐pound capacity, (Fabrication Enterprises, White Plains, NY) was used for this test. The values of both tests were expressed in kilograms (kg). These tests were performed bilaterally, regardless of side of access, before the procedure and at 1 month.
Patient Population
The study population includes both men and women aged >18 years who underwent cardiac catheterization at Baylor Scott and White – The Heart Hospital Plano. The DIPRA trial's criteria for participant inclusion and exclusion have been previously published. 17
Subject Screening, Enrollment, Randomization, and Follow‐Up
Eligible patients who met the screening inclusion criteria and have not met any of the trial exclusion criteria were randomly assigned, in a 1:1 ratio, to undergo coronary catheterization by either dRA or pRA access. Screening assessments were conducted by reviewing medical records and conducting interviews after obtaining informed consent. In addition, for research purposes, a mandatory screening examination was performed to assess the patency of the pRA and dRA. This assessment was initially performed by palpation, and if both arteries were palpable, Doppler ultrasound was performed.
Participants randomized to pRA received conventional access catheterization according to the standard of care. Required follow‐up visit assessment was performed at 1 month (±10 days). Assessments included: hand function assessment using the QuickDASH questionnaire, hand grip assessment using the hand dynamometer, pinch grip assessment using a pinch grip dynamometer, any clinical change from baseline, reinterventions using the RA between the index procedure and follow‐up, and measurement of the pRA and dRA patency with Doppler ultrasound. Deviations from the follow‐up window are summarized in Table S1.
Statistical Analysis
The statistical analysis plan was published before patient enrollment 17 and is available with the protocol. The analysis for the primary and secondary end points was performed as modified intention‐to‐treat including all subjects who completed follow‐up at 30 days regardless of the access method. Patient demographics and characteristics are analyzed and compared between the dRA and pRA groups. Continuous variables were expressed as means with corresponding SDs or as medians with quartile ranges [quartile 1, quartile 3], if skewed. Categorical variables were presented as frequencies and percentages. Statistical analysis to identify differences in demographic and clinical variables, as well as safety and compliance, involved appropriate tests such as 2‐sample t‐tests and chi‐square tests (or Wilcoxon rank‐sum test and Fisher exact test).
Sample Size and Power
A Monte Carlo simulation study was used to calculate the minimum sample size required to achieve at least 80% power to detect a small clinical difference between dRA and pRA in the primary composite outcome of hand function. A small clinical effect was defined as a 10%±10% and 0%±10% reduction in 1 of the 3 pre‐ to post‐operation test scores for pRA and dRA, respectively. Assumptions used to simulate pre‐operation scores and means±SDs for the simulated difference in test scores are reported in Table S2. The resulting mean±SD of the composite average z‐scores from 1000 Monte Carlo simulations were 0.14±0.69 versus −0.14±0.69 for a 10% reduction in hand grip strength, 0.14±0.69 versus −0.14±0.69 for a 10% reduction in pinch grip strength, and 0.13±0.68 versus −0.13±0.70 for a 10% reduction in QuickDASH score comparing dRA versus pRA, respectively. A sample size of n=125 per group is sufficient to achieve at least 80% power under a correlation of 0.3 between the 3 tests. Allowing for a loss‐to‐follow‐up rate of 15%, 300 patients (150 per arm) were enrolled in the study. Subjects lost to follow‐up were excluded from the primary analyses, and demographic and baseline characteristics were compared between included and lost‐to‐follow‐up subjects.
DIPRA Primary End Point Analysis
The primary composite outcome of hand function was measured using the average Z‐score method. 21 , 22 The average z‐score for each subject was defined as the average of the z‐scores of the differences between the measurements at baseline and 1‐month post‐operation for 3 individual tests. The 3 tests that measure a subject's daily functionality and hand strength and are included in the composite outcome are: (a) the QuickDASH questionnaire score (0–100) calculated as [(sum of n responses/n)−1] * 25, (b) thumb and forefinger pinch strength test (in kilograms), and (c) hand grip strength test (in kilograms). The average of these 3 z‐scores of the difference between pre‐ and 1‐month post‐operation was compared between dRA and pRA using the Wilcoxon rank‐sum test.
DIPRA Secondary End Points Analysis
A chi‐square test (or Fisher exact test) was used to evaluate the arterial access success rate, access site bleeding, access site hematoma, and RA occlusion in both dRA and pRA cohorts at 1 month. Associations with RA occlusion and access site bleeding were not performed because of the small number of events (<5).
All P values were reported as 2‐sided tests, and P<0.05 was considered statistically significant. R version 4.1.1 (R Foundation for Statistical Computing) was used to perform the statistical analysis.
Results
Trial Participants and Baseline Characteristics
A total of 300 patients were randomly assigned to distal radial access (150 patients) or proximal radial access (150 patients) (Figure 1). The primary modified intention‐to‐treat analysis at 30 days included 254 patients (128 dRA and 126 pRA), with the remaining 46 patients excluded who did not have 30‐day assessments completed. Patient demographic characteristics were similar between the randomized groups, with a mean age of 66.6±9.6 years and 75.3% being male (Table 1). Comparing baseline patient characteristics and hand function at screening between those included and excluded, no significant differences were noted, with the exception of those excluded being 3.7 years younger on average (P=0.03) (Table S3).
Figure 1. Patient enrollment flow diagram.
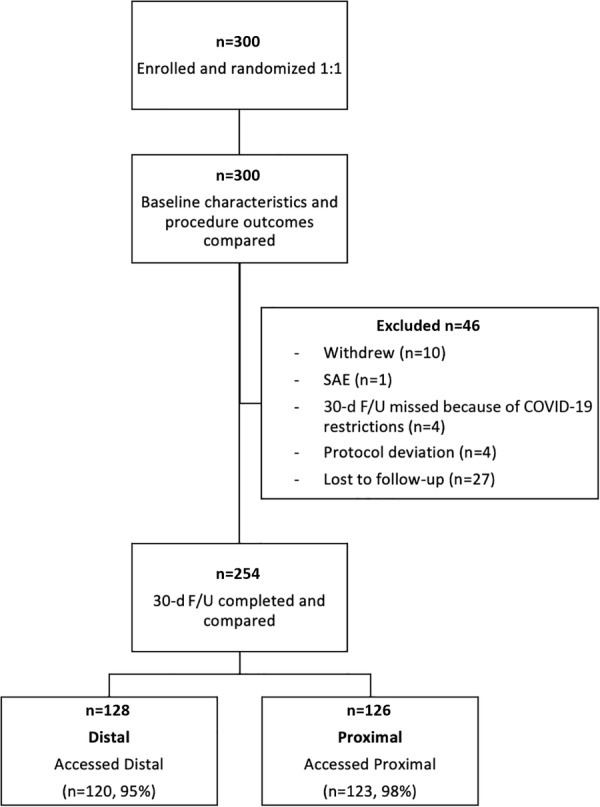
Among 300 patients enrolled and randomized 1:1 to distal and proximal radial access, 46 patients did not complete follow‐up assessments at 30 days and were excluded. F/U indicates follow‐up; and SAE, severe adverse event.
Table 1.
Baseline Patient Characteristics at Enrollment
| Variable | Overall (n=300) | Distal (n=150) | Proximal (n=150) | P |
|---|---|---|---|---|
| Age, y | 66.6±9.6 | 65.9±8.7 | 67.3±10.5 | 0.23 |
| BMI, kg/m2 | 30.0 [26.8, 34.4] | 29.9 [26.8, 34.1] | 30.1 [26.9, 35.0] | 0.98 |
| Ethnicity | ||||
| Hispanic or Latino | 7 (2.3%) | 2 (1.3%) | 5 (3.3%) | 0.46 |
| Not Hispanic or Latino | 290 (96.7%) | 147 (98%) | 143 (95.3%) | |
| Unknown/Not reported | 3 (1%) | 1 (0.7%) | 2 (1.3%) | |
| Sex | ||||
| Women | 74 (24.7%) | 31 (20.7%) | 43 (28.7%) | 0.14 |
| Men | 226 (75.3%) | 119 (79.3%) | 107 (71.3%) | |
| Race | ||||
| American Indian/Alaska Native | 1 (0.3%) | 0 (0%) | 1 (0.7%) | 0.72 |
| Asian | 13 (4.3%) | 8 (5.3%) | 5 (3.3%) | |
| Black or African American | 17 (5.7%) | 8 (5.3%) | 9 (6%) | |
| More than 1 race | 1 (0.3%) | 1 (0.7%) | 0 (0%) | |
| Unknown/Not reported | 8 (2.7%) | 5 (3.3%) | 3 (2%) | |
| White | 260 (86.7%) | 128 (85.3%) | 132 (88%) | |
| Diabetes | 96 (32%) | 51 (34%) | 45 (30%) | 0.54 |
| Hypercholesteremia | 56 (18.7%) | 27 (18%) | 29 (19.3%) | 0.88 |
| Hypertension | 232 (77.3%) | 112 (74.7%) | 120 (80%) | 0.33 |
| Prior myocardial infarction | 39 (13%) | 23 (15.3%) | 16 (10.7%) | 0.3 |
| Prior CABG | 6 (2%) | 3 (2%) | 3 (2%) | 1 |
| Prior PCI | 56 (18.7%) | 25 (16.7%) | 31 (20.7%) | 0.46 |
Values are frequency (%), mean ± SD, or median [quartiles].
BMI indicates body mass index; CABG, coronary artery bypass graft; and PCI, percutaneous coronary intervention.
Procedure Characteristics
There was no statistical difference in procedure characteristics between treatment groups, including the sheath size used (6 French 99.3% in dRA versus 99.3% in pRA) and type of procedure completed (percutaneous coronary intervention 35.9% in dRA versus 32.9% in pRA) (Table 2). Six patients randomized to dRA failed successful access and converted to pRA while 2 pRA patients failed successful access. Catheterization was performed on the right hand in 97.7% of patients. Baseline hand grip measure and QuickDASH score were not statistically different at screening before the procedure, with a median hand grip strength of 26 kg (interquartile range [IQR, 18.7–32.8] in the right hand and median QuickDASH score of 4.6 [IQR, 0–15.9]). Median pinch grip strength was 7.4 kg [IQR, 5.4–8.9] in the right hand, with higher values among dRA patients compared with pRA (dRA 7.9 [6.0, 9.1] versus pRA 7.3 [5.3, 8.5] kg; P=0.02) (Table 3).
Table 2.
Catheterization Procedure Characteristics
| Variable | Overall (n=300) | Distal (n=150) | Proximal (n=150) | P |
|---|---|---|---|---|
| Dominant hand | ||||
| Both | 1 (0.3%) | 0 (0%) | 1 (0.7%) | 0.58 |
| Left | 31 (10.3%) | 14 (9.3%) | 17 (11.3%) | |
| Right | 268 (89.3%) | 136 (90.7%) | 132 (88%) | |
| Artery size, mm | ||||
| Proximal | 2.7 [1.9, 3.6] | 2.7 [2.0, 3.5] | 2.5 [1.8, 3.5] | 0.09 |
| Distal | 2.3 [1.8, 3.2] | 2.4 [1.9, 3.3] | 2.2 [1.7, 3.1] | 0.08 |
| Sheath size | ||||
| 5 French | 2 (0.7%) | 1 (0.7%) | 1 (0.7%) | 1 |
| 6 French | 296 (99.3%) | 149 (99.3%) | 147 (99.3%) | |
| Type of procedure | 0.68 | |||
| Diagnostic | 185 (65.6%) | 91 (64.1%) | 94 (67.1%) | |
| Interventional/PCI | 97 (34.4%) | 51 (35.9%) | 46 (32.9%) | |
| Catheterization hand | 0.45 | |||
| Left | 7 (2.3%) | 2 (1.3%) | 5 (3.3%) | |
| Right | 293 (97.7%) | 148 (98.7%) | 145 (96.7%) | |
| Catheterization access | ‐ | |||
| Distal | 144 (48%) | 144 (96%) | 0 (0%) | |
| Femoral | 2 (0.7%) | 0 (0%) | 2 (1.3%) | |
| Proximal | 154 (51.5%) | 6 (4%) | 148 (98.7%) | |
| Hemostasis time (s)* | 160 [135, 195] | 166 [135, 201] | 160 [138, 192] | 0.57 |
| Hemostasis technique | <0.001 | |||
| Preclude SYNC BAND | 125 (45%) | 125 (88.7%) | 0 (0%) | |
| TR BAND | 153 (55%) | 16 (11.3%) | 137 (100%) | |
Values are frequency (%) or median [quartiles].
PCI indicates percutaneous coronary intervention; and TR, tricuspid regurgitation.
Hemostasis time was obtained by chart in 68% of patients.
Table 3.
QuickDASH, Hand Grip, and Pinch Grip at Baseline Screening
| Variable | Overall (n=300) | Distal (n=150) | Proximal (n=150) | P |
|---|---|---|---|---|
| Hand grip, kg | ||||
| Left hand | 21.2 [15.3, 28.7] | 21.5 [15.5, 29.5] | 20.3 [15.3, 28] | 0.23 |
| Right hand | 26 [18.7, 32.8] | 26 [19.1, 36] | 24.3 [18.2, 31.2] | 0.07 |
| Pinch grip, kg | ||||
| Left hand | 6.8 [5.1, 8.5] | 7.3 [5.1, 8.5] | 6.5 [5.1, 8.2] | 0.20 |
| Right hand | 7.4 [5.4, 8.9] | 7.9 [6.0, 9.1] | 7.3 [5.3, 8.5] | 0.02 |
| QuickDASH | ||||
| Score | 4.6 [0, 15.9] | 5.7 [0, 13.6] | 4.6 [0, 15.9] | 0.84 |
Values are median [quartiles].
DASH indicates Disabilities of the Arm, Shoulder, and Hand Questionnaire.
Primary Outcome
The primary composite outcome of hand function was measured in the catheterization hand as the average of the 3 z‐scores for change at 30 days from baseline in the QuickDASH, hand grip strength, and pinch grip strength. The average z‐scores were not statistically different between dRA (median −0.01, IQR: −0.32 to 0.24) and pRA (median 0.11, IQR: −0.30 to 0.51) (P=0.09) (Table 4; Figure 2).
Table 4.
Average Z‐Score Test for the Composite Primary Outcome at 30 Days
| Variable | Overall (n=254) | Distal (n=128) | Proximal (n=126) | P |
|---|---|---|---|---|
| Average Z‐score | 0.04 [−0.32, 0.35] | −0.01 [−0.32, 0.24] | 0.11 [−0.30, 0.51] | 0.09 |
| Change in QuickDASH Z‐score | 0.01 [−0.19, 0.40] | 0.01 [−0.19, 0.40] | 0.01 [−0.19, 0.40] | 0.96 |
| Change in hand grip Z‐score | −0.04 [−0.58, 0.50] | −0.1 [−0.67, 0.50] | 0.02 [−0.51, 0.51] | 0.21 |
| Change in pinch grip Z‐score | 0.06 [−0.55, 0.62] | 0 [−0.61, 0.52] | 0.21 [−0.41, 0.82] | 0.09 |
The primary composite outcome is defined as the average of the z‐scores for change from baseline hand grip strength, pinch grip strength, and QuickDASH score in the catheterization hand. Values are median [quartiles].
DASH indicates Disabilities of the Arm, Shoulder, and Hand Questionnaire.
Figure 2. Average Z‐score test for the composite primary outcome at 30 days.
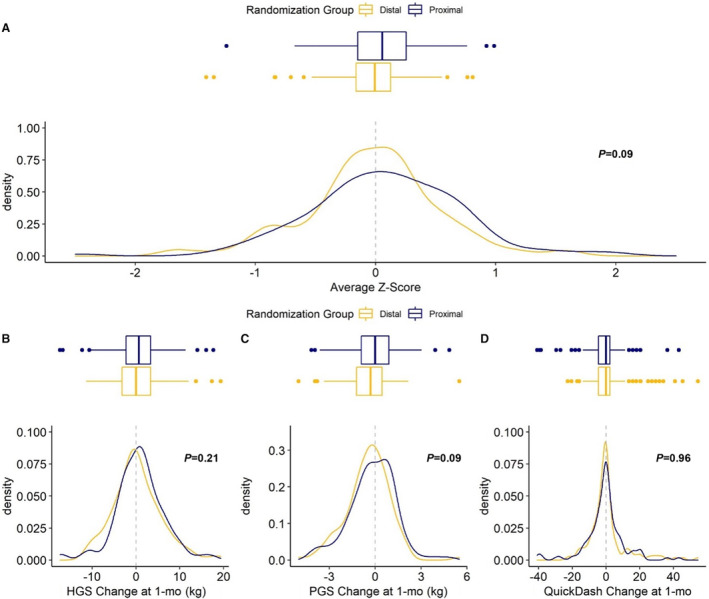
The primary composite outcome is defined as the average of the z‐scores for change from baseline hand grip strength, pinch grip strength, and QuickDash score in the catheterization hand. A, Average of the z‐score for change from baseline hand grip at 1 month (B). Average of the z‐score for change from baseline pinch grip at 1 month (C). Average of the z‐score for change from baseline DASH score at 1 month (D). DASH indicates Disabilities of the Arm, Shoulder, and Hand Questionnaire; HGS, hand grip strength; PCI, percutaneous coronary intervention; and PGS, pinch grip strength.
Secondary Outcomes
Secondary outcomes at catheterization and 30‐day follow‐up are reported in Table 5. Both groups had similar rates of access site bleeding (dRA 0% versus pRA 1.4%; P=0.25) and hematoma (dRA 0% versus pRA 0.7%; P=0.50). Successful access to the radial artery was not statistically different between groups (dRA 96.7% versus pRA 98%; P=0.72). There were no significant differences in the change of hand function in the catheterization hand between groups measured as hand grip strength (dRA 0 [IQR, −3.2 to 3.3] versus pRA 0.7 [IQR, −2.3 to 3.3] kg; P=0.21), pinch grip strength (dRA −0.3 [IQR, −1.2 to 0.5] versus pRA 0 [IQR, −0.9 to 0.9] kg; P=0.09), and QuickDASH (dRA 0 [IQR, −4.6 to 2.3] versus pRA 0 [IQR, −4.6 to 2.3] points; P=0.96), with the median change being less than 5% of the baseline level for each measure and group. Similarly, changes in the function of the non‐catheterization hand were not statistically different between dRA and pRA groups (Table S4). Radial artery occlusion occurred in 2 pRA patients versus none in dRA. The rate of reinterventions using the RA was not statistically different (dRA 3.2% versus pRA 6.4%, P=0.38) between groups.
Table 5.
Comparison of Secondary Outcomes During the Catheterization Procedure and at 30 Days
| Variable | Overall | Distal | Proximal | P |
|---|---|---|---|---|
| Catheterization procedure | n=300 | n=150 | n=150 | |
| Bleeding | 2 (0.7%) | 0 (0%) | 2 (1.4%) | 0.25 |
| Hematoma | 1 (0.4%) | 0 (0%) | 1 (0.7%) | 0.50 |
| Radial artery accessed | 289 (97.3%) | 145 (96.7%) | 144 (98%) | 0.72 |
| 30‐day outcomes | n=254 | n=128 | n=126 | |
|---|---|---|---|---|
| Hand grip strength, kg | ||||
| Baseline | 25.8 [18.7, 32.7] | 26 [19.2, 34.8] | 24.3 [18.7, 31.1] | 0.20 |
| 30 days | 26.3 [19.2, 32.7] | 27.3 [19, 33] | 25.3 [19.3, 31.3] | 0.28 |
| Change from baseline* | 0.3 [−2.7, 3.3] | 0 [−3.2, 3.3] | 0.7 [−2.3, 3.3] | 0.21 |
| Pinch grip strength, kg | ||||
| Baseline | 7.4 [5.4, 8.8] | 7.9 [5.9, 9.1] | 7.3 [5.4, 8.4] | 0.06 |
| 30 days | 6.9 [5.1, 8.7] | 7.3 [5.3, 8.7] | 6.4 [5, 8.3] | 0.43 |
| Change from baseline* | −0.2 [−1.1, 0.6] | −0.3 [−1.2, 0.5] | 0 [−0.9, 0.9] | 0.09 |
| QuickDASH | ||||
| Baseline | 4.6 [0, 13.6] | 4.6 [0, 13.6] | 4.6 [0, 15.9] | 0.87 |
| At 30 days | 4.6 [0, 15.9] | 4.6 [0, 15.9] | 4.6 [0, 15.3] | 0.75 |
| Change from baseline† | 0 [−4.6, 2.3] | 0 [−4.6, 2.3] | 0 [−4.6, 2.3] | 0.96 |
| Radial artery occlusion | ||||
| Distal | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Proximal | 2 (0.8%) | 0 (0%) | 2 (1.6%) | 0.24 |
| Reinterventions using the radial artery | 12 (4.8%) | 4 (3.2%) | 8 (6.4%) | 0.38 |
Values are frequency (%) or median [quartiles].
DASH indicates Disabilities of the Arm, Shoulder, and Hand questionnaire.
Hand grip and pinch grip change were calculated as 30 days – baseline such that negative values indicate worsening.
QuickDASH change was calculated as baseline – 30 days such that negative values indicate worsening.
Discussion
Randomized controlled trials comparing the effects of dRA and pRA accesses on neurological functions are essential to better gauge safety. The aim of the DIPRA trial was to compare hand function and access site safety of dRA versus pRA access. To our knowledge, this was the first randomized controlled trial to assess hand function following dRA access for cardiac catheterization. The main finding of the DIPRA trial was that dRA access is safe and did not impair hand function based on a systematic multidimensional evaluation compared with the gold standard pRA (Figure 3).
Figure 3. Thirty‐day results of the DIPRA study — a randomized controlled trial.
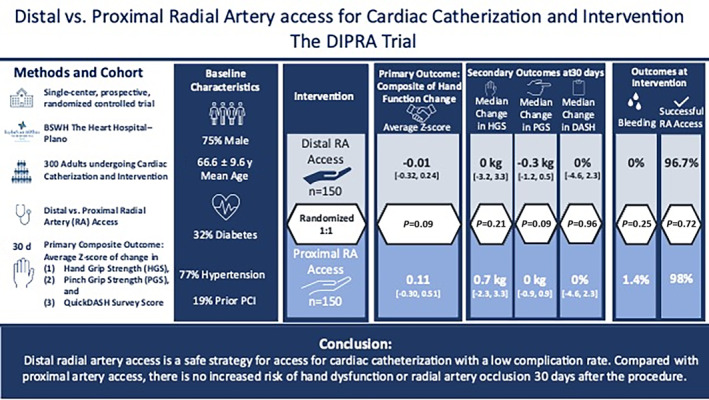
Safety of cardiac catheterization comparing distal versus radial access was assessed for feasibility and complications, including hand function as a composite of hand grip strength, pinch grip strength, and QuickDASH questionnaire score post‐radial access. DASH indicates Disabilities of the Arm, Shoulder, and Hand Questionnaire; BSWH, Baylor scott and white health; DIPRA, Distal Versus Proximal Radial Artery Access for Cardiac Catheterization and Intervention; HGS, hand grip strength; PCI, percutaneous coronary intervention; PGS, pinch grip strength; and RA, radial artery.
The distal segment of the RA is characterized by an unpredictable course at the level of the hand as it winds and twists in the anatomic snuffbox. 23 The dRA courses more superficial to the skin than the pRA, which can sometimes cause the dRA to slide during needle insertion, requiring redirection. 23 In addition, the dRA is superficially crossed by the commencement of the cephalic vein, which can sometimes result in intravenous cannulation instead of intra‐arterial. 24 These features may entail multiple puncture attempts, the potential consequences of which have not yet been investigated. The superficial branches of the radial nerve run in close proximity to the dRA within the anatomic snuffbox. 24 Iatrogenic radial nerve injury or compression following dRA access for cardiac catheterization can potentially cause neurological impairment, including hand dysfunction. To our knowledge, the “RATATOUILLE” study by Sgueglia et al was the only study that assessed hand function after dRA access and demonstrated the safety of dRA on hand function; however, the study lacked a randomized control group. 16
The DIPRA trial implemented a comprehensive set of multidomain tests, to capture the possible multifactorial nature of hand impairment. Careful selection of reliable and validated tests is crucial to determine general hand function and evaluate the consequences of a novel invasive procedure. The QuickDASH questionnaire, previously validated for monitoring upper limb musculoskeletal function, has been demonstrated to be a reliable tool even among patients with variable upper extremity conditions. 19 , 20 The QuickDASH score was used in our study to measure self‐reported physical symptoms and functional status. Although the “RATATOUILLE” study assessed hand sensory function using the monofilament test, 16 we believe that the QuickDASH score provides a more general sensory assessment of the hand as it covers different aspects of sensory function, is not subject to test variability, and is self‐reported. At 30‐day follow‐up, there was no significant change in QuickDASH score in either the pRA or dRA groups, reflecting good sensorineural response and preservation of hand function for daily household activities.
Hand grip and pinch grip strength measurements were performed by an independent specialist and provided a more objective assessment of the upper extremity function. Changes from baseline in these tests were not statistically different between pRA and dRA groups at 30‐day follow‐up for both hands. In some patients, hand grip strength improved slightly after dRA and pRA, which can be explained by the different clinical conditions of the patients at baseline. It is even possible that underlying cardiovascular disease, such as acute myocardial infarction, affects baseline handgrip strength, which could explain the progressive improvement in handgrip strength over time. 25 Deterioration in pinch grip strength of the catheterization hand was reported by some patients in both the dRA and pRA groups. However, the difference between both groups in the change in overall pinch grip strength at 30 days was not statistically significant. The reason for this deterioration in pinch grip strength for some patients is probably because of access‐related factors, such as local hematoma and edema. In our study, the patients with dRA experiencing a worsening in their pinch grip strength did not report significant changes in test results between their catheterization hand and the contralateral one, thus indicating that dRA access does not affect hand function more than other nonvascular access circumstances.
In the current study, dRA and pRA groups had similar rates of access site bleeding and hematoma. Given the subcutaneous course of the distal branches of the radial artery, access site hematoma may be more visible after dRA access than pRA access. However, because of the small subcutaneous space to contain bleeding, dRA‐related hematoma makes compartment syndrome unlikely. Recent studies have shown dRA access to be a safer alternative to pRA access in terms of conventional complications such as bleeding and hematoma formation. 14
In the current study, RA occlusion occurred in 2 patients with pRA versus none in dRA. dRA may preserve the RA for future procedures/access with lower RA occlusion rates compared with pRA, as evidenced by recent studies. 14 , 15 Indeed, the RA makes multiple anastomotic connections before its entry in the anatomic snuffbox, providing collateral circulation to the hand and wrist in case of vessel occlusion at the puncture site. 23 Our study is further evidence of the much lower incidence of RA occlusion after dRA. Overall, the extremely low complication rate demonstrates safety as a particular advantage of dRA.
Limitations
Evaluation of hand function is complex, and the timing of assessment may influence study outcomes. For instance, in the 30 days after cardiac catheterization, access site hematoma or edema can cause functional hand impairment, impacting study outcomes. Long‐term follow‐up is needed to demonstrate a more accurate causality‐effect relationship. Another limitation is that data were pooled from a single health care center; therefore, generalizability to a larger percutaneous coronary intervention population with varying operator experience and beyond our geographical region is uncertain.
Conclusions
In this single‐center randomized controlled trial, dRA was not associated with an increased risk of hand dysfunction or RA occlusion at 30 days compared with pRA. This is the first randomized controlled trial to highlight the safety and noninferiority of dRA compared with pRA.
Sources of Funding
Data acquisition efforts of this study were funded by a philanthropic gift from Satish and Yasmin Gupta to Baylor Scott & White The Heart Hospital, Plano, TX.
Disclosures
Dr Al‐Azizi serves as a consultant for Edwards LifeSciences, a consultant and advisory board member for Medtronic, a consultant for Boston Scientific, and member of the speakers bureau of Philips. Dr Szerlip is a proctor, speaker, and consultant of Edwards LifeSciences, a consultant, proctor, and member of the advisory board of Abbott Vascular, a member of the Medtronic steering committee, and a speaker and consultant for Boston Scientific. Dr Mack is a trial co‐PI for Abbott, a trial co‐PI for Edwards Lifesciences, and a trial study chair for Medtronic – all uncompensated. Dr Potluri is an advisory board member, proctor, and speaker for Medtronic, Boston Scientific, Abbott, and Cordis, and a proctor and speaker for Edwards, Terumo, and AstraZeneca. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
This work was presented in part at the SCAI Scientific Abstracts sessions, May 19 – 22, 2022, and published in abstract form Journal of the Society for Cardiovascular Angiography & Interventions. 2022;1:100320. doi:10.1016/j.jscai.2022.100320.
This manuscript was sent to Jennifer Tremmel, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030774
For Sources of Funding and Disclosures, see page 10.
References
- 1. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. doi: 10.1016/S0140-6736(11)60404-2 [DOI] [PubMed] [Google Scholar]
- 2. Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007‐2012). Circulation. 2013;127:2295–2306. doi: 10.1161/CIRCULATIONAHA.112.000536 [DOI] [PubMed] [Google Scholar]
- 3. Mehta SR, Jolly SS, Cairns J, Niemela K, Rao SV, Cheema AN, Steg PG, Cantor WJ, Džavík V, Budaj A, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST‐segment elevation. J Am Coll Cardiol. 2012;60:2490–2499. doi: 10.1016/j.jacc.2012.07.050 [DOI] [PubMed] [Google Scholar]
- 4. Cooper CJ, El‐Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138:430–436. doi: 10.1016/S0002-8703(99)70143-2 [DOI] [PubMed] [Google Scholar]
- 5. Mitchell MD, Hong JA, Lee BY, Umscheid CA, Bartsch SM, Don CW. Systematic review and cost‐benefit analysis of radial artery access for coronary angiography and intervention. Circ Cardiovasc Qual Outcomes. 2012;5:454–462. doi: 10.1161/CIRCOUTCOMES.112.965269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, et al. ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823a5596 [DOI] [PubMed] [Google Scholar]
- 7. Sousa‐Uva M, Neumann FJ, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. ESC/EACTS guidelines on myocardial revascularization. Eur J Cardiothorac Surg. 2018;2019(55):4–90. doi: 10.15829/1560-4071-2019-8-151-226 [DOI] [PubMed] [Google Scholar]
- 8. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. 2017;13:851–857. doi: 10.4244/EIJ-D-17-00079 [DOI] [PubMed] [Google Scholar]
- 9. Aoi S, Htun WW, Freeo S, Lee S, Kyaw H, Alfaro V, Coppola J, Pancholy S, Kwan T. Distal transradial artery access in the anatomical snuffbox for coronary angiography as an alternative access site for faster hemostasis. Catheter Cardiovasc Interv. 2019;94:651–657. doi: 10.1002/ccd.28155 [DOI] [PubMed] [Google Scholar]
- 10. Koutouzis M, Kontopodis E, Tassopoulos A, Tsiafoutis I, Katsanou K, Rigatou A, Didagelos M, Andreou K, Lazaris E, Oikonomidis N, et al. Distal versus traditional radial approach for coronary angiography. Cardiovasc Revasc Med. 2019;20:678–680. doi: 10.1016/j.carrev.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 11. Gajurel RM, Sahi R, Shrestha H, Thapa S, Khanal R. Initial experience on anatomical snuff box approach for coronary angiogram & percutaneous coronary intervention in a tertiary care center Nepal. World J Cardiovasc Dis. 2018;8:578–587. doi: 10.4236/wjcd.2018.812057 [DOI] [Google Scholar]
- 12. Amin MR, Singha C, Banerjee S, Hoque H, Mahabub SEE, Hoque M, Biswas E. Comparison of distal transradial in the anatomical snuffbox versus conventional transradial access for coronary angiography and intervention‐an experience in 100 cases. Univ Heart J. 2017;13:40–45. doi: 10.3329/uhj.v13i2.37657 [DOI] [Google Scholar]
- 13. Al‐Azizi KM, Grewal V, Gobeil K, Maqsood K, Haider A, Mohani A, Giugliano G, Lotfi AS. The left distal transradial artery access for coronary angiography and intervention: a US experience. Cardiovasc Revasc Med. 2019;20:786–7899. doi: 10.1016/j.carrev.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 14. Ferrante G, Condello F, Rao SV, Maurina M, Jolly S, Stefanini GG, Reimers B, Condorelli G, Lefèvre T, Pancholy SB, et al. Distal vs conventional radial access for coronary angiography and/or intervention: a meta‐analysis of randomized trials. JACC Cardiovasc Interv. 2022;15:2297–2311. doi: 10.1016/j.jcin.2022.09.006 [DOI] [PubMed] [Google Scholar]
- 15. Aminian A, Sgueglia GA, Wiemer M, Kefer J, Gasparini GL, Ruzsa Z, van Leeuwen MAH, Ungureanu C, Leibundgut G, Vandeloo B, et al. Distal versus conventional radial access for coronary angiography and intervention: the DISCO RADIAL trial. JACC Cardiovasc Interv. 2022;15:1191–1201. doi: 10.1016/j.jcin.2022.04.032 [DOI] [PubMed] [Google Scholar]
- 16. Sgueglia GA, Hassan A, Harb S, Ford TJ, Koliastasis L, Milkas A, Zappi DM, Navarro Lecaro A, Ionescu E, Rankin S, et al. International hand function study following distal radial access: the RATATOUILLE study. JACC Cardiovasc Interv. 2022;15:1205–1215. doi: 10.1016/j.jcin.2022.04.023 [DOI] [PubMed] [Google Scholar]
- 17. Al‐Azizi KM, Idris A, Christensen J, Hamandi M, Hale S, Martits‐Chalangari K, Van Zyl JS, Ravindranathan P, Banwait JK, Mcckracken J, et al. Distal versus proximal radial artery access for cardiac catheterization and intervention: design and rationale of the DIPRA trial. Cardiovasc Revasc Med. 2022;35:104–109. doi: 10.1016/j.carrev.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 18. Al‐Azizi KM, Lotfi AS. The distal left radial artery access for coronary angiography and intervention: a new era. Cardiovasc Revasc Med. 2018;19:35–40. doi: 10.1016/j.carrev.2018.03.020 [DOI] [PubMed] [Google Scholar]
- 19. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med 1996;30:372]. Am J Ind Med. 1996;29:602–608. doi: [DOI] [PubMed] [Google Scholar]
- 20. Beaton DE, Wright JG, Katz JN; Upper Extremity Collaborative Group . Development of the QuickDASH: comparison of three item‐reduction approaches. J Bone Joint Surg Am. 2005;87:1038–1046. [DOI] [PubMed] [Google Scholar]
- 21. O'Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079–1087. doi: 10.2307/2531158 [DOI] [PubMed] [Google Scholar]
- 22. Sun H, Davison BA, Cotter G, Pencina MJ, Koch GG. Evaluating treatment efficacy by multiple end points in phase II acute heart failure clinical trials: analyzing data using a global method. Circ Heart Fail. 2012;5:742–749. doi: 10.1161/CIRCHEARTFAILURE.112.969154 [DOI] [PubMed] [Google Scholar]
- 23. Sgueglia GA, Di Giorgio A, Gaspardone A, Babunashvili A. Anatomic basis and physiological rationale of distal radial artery access for percutaneous coronary and endovascular procedures. JACC Cardiovasc Interv. 2018;11:2113–2119. doi: 10.1016/j.jcin.2018.04.045 [DOI] [PubMed] [Google Scholar]
- 24. Narsinh KH, Mirza MH, Duvvuri M, Caton MT Jr, Baker A, Winkler EA, Higashida RT, Halbach VV, Amans MR, Cooke DL, et al. Radial artery access anatomy: considerations for neuroendovascular procedures. Neurointerv Surg. 2021;13:1139–1144. doi: 10.1136/neurintsurg-2021-017871 [DOI] [PubMed] [Google Scholar]
- 25. Leong DP, Teo KK, Rangarajan S, Lopez‐Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, et al. Prognostic value of grip strength: findings from the prospective urban rural epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


