Abstract
Background
Hypertension and diabetes are associated with increased COVID‐19 severity. The association between level of control of these conditions and COVID‐19 severity is less well understood.
Methods and Results
This retrospective cohort study identified adults with COVID‐19, March 2020 to February 2022, in 43 US health systems in the National Patient‐Centered Clinical Research Network. Hypertension control was categorized as blood pressure (BP) <130/80, 130 to 139/80 to 89, 140 to 159/90 to 99, or ≥160/100 mm Hg, and diabetes control as glycated hemoglobin <7%, 7% to <9%, ≥9%. Adjusted, pooled logistic regression assessed associations between hypertension and diabetes control and severe COVID‐19 outcomes. Among 1 494 837 adults with COVID‐19, 43% had hypertension and 12% had diabetes. Among patients with hypertension, the highest baseline BP was associated with greater odds of hospitalization (adjusted odds ratio [aOR], 1.30 [95% CI, 1.23–1.37] for BP ≥160/100 versus BP <130/80), critical care (aOR, 1.30 [95% CI, 1.21–1.40]), and mechanical ventilation (aOR, 1.32 [95% CI, 1.17–1.50]) but not mortality (aOR, 1.08 [95% CI, 0.98–1.12]). Among patients with diabetes, the highest glycated hemoglobin was associated with greater odds of hospitalization (aOR, 1.61 [95% CI, 1.47–1.76] for glycated hemoglobin ≥9% versus <7%), critical care (aOR, 1.42 [95% CI, 1.31–1.54]), mechanical ventilation (aOR, 1.12 [95% CI, 1.02–1.23]), and mortality (aOR, 1.18 [95% CI, 1.09–1.27]). Black and Hispanic adults were more likely than White adults to experience severe COVID‐19 outcomes, independent of comorbidity score and control of hypertension or diabetes.
Conclusions
Among 1.5 million patients with COVID‐19, higher BP and glycated hemoglobin were associated with more severe COVID‐19 outcomes. Findings suggest that adults with poorest control of hypertension or diabetes might benefit from efforts to prevent and initiate early treatment of COVID‐19.
Keywords: blood pressure, COVID‐19, diabetes, glycated hemoglobin, hypertension
Subject Categories: Hypertension
Nonstandard Abbreviations and Acronyms
- PCORnet
National Patient‐Centered Clinical Research Network
Clinical Perspective.
What Is New?
In this study of 1.5 million patients with COVID‐19 in the United States, higher blood pressure and glycated hemoglobin were associated with worse COVID‐19 outcomes.
This was the largest study of its kind, and unlike prior work, assessed hypertension and diabetes control based on assessments before SARS‐CoV‐2 infection, rather than measurements taken during acute COVID‐19 illness.
What Are the Clinical Implications?
These findings highlight the importance of ongoing chronic disease management during a pandemic, including through out‐of‐office services, such as telehealth, and strategies such as disease self‐management education and support.
In addition, clinicians can encourage adults with poorly controlled hypertension or diabetes to adhere to COVID‐19 vaccination recommendations and encourage these patients to seek early treatment for COVID‐19 to prevent progression to severe disease.
Poorly managed hypertension and diabetes are known risk factors for a range of adverse health events, including cardiovascular morbidity and mortality. 1 Although these conditions can be managed through medical treatment and lifestyle modifications, such as dietary change and physical activity, control of these conditions is often suboptimal. 1 In recent national estimates, approximately one‐quarter (24.3%) of US adults with hypertension had blood pressure (BP) levels that met recommended treatment targets for control (defined as BP <130/80 mm Hg). 2 Among adults with diabetes, approximately two‐thirds (66.8%) met the standard glycated hemoglobin (HbA1c) target of <7%. 3 The COVID‐19 pandemic adversely affected chronic disease management through disruptions to health care access and daily life, leading to reduced ability for adults to obtain preventive health care or engage in behaviors known to promote control of hypertension and diabetes. 1 , 4 , 5
Hypertension and diabetes are 2 of the most common comorbidities among adults hospitalized with COVID‐19 6 and have been associated with severe disease outcomes and mortality. 7 , 8 , 9 Poor BP or glycemic control might also be associated with worse COVID‐19 outcomes, but research gaps persist regarding any independent associations between severe COVID‐19 outcomes and standard measures of chronic disease control such as BP and HbA1c. 10 , 11 , 12 A prior study described greater prevalence of severe COVID‐19 outcomes across higher strata of BP and HbA1c; however, these results did not account for differences in demographic and clinical characteristics across patient populations by BP or HbA1c level. 13 In this study, we build on these previous descriptive analyses by assessing the independent association between hypertension control and diabetes control and severe COVID‐19 outcomes among a large multisite US cohort of adults with COVID‐19 from March 2020 to February 2022. We hypothesized that patients with hypertension who had the highest preinfection BP levels and patients with diabetes who had the highest preinfection HbA1c levels would have greater odds of severe COVID‐19 outcomes relative to those with the lowest respective levels, after adjusting for demographic and clinical characteristics. These results can be used to guide clinical and public health practice by identifying populations who might be most at risk for poor COVID‐19 outcomes and who might benefit most from enhanced chronic disease management, COVID‐19 prevention strategies, and early treatment with medications known to reduce progression to severe COVID‐19. 14
Methods
Participants and Procedures
This cross‐sectional study aggregated ambulatory, emergency department, and inpatient electronic health record data in 43 sites (each representing ≥1 health systems) participating in PCORnet, the National Patient‐Centered Clinical Research Network. 15 , 16 All adults aged ≥20 years with COVID‐19 identified by positive SARS‐CoV‐2 polymerase chain reaction (98%) or antigen (3%) lab test in an ambulatory, emergency department, or inpatient care setting during March 1, 2020, to February 28, 2022 were included (N=1 494 837). (Some patients tested positive by both polymerase chain reaction and antigen tests.) If a patient had multiple positive tests for COVID‐19, we used the first positive record as the COVID‐19 index date. Patient diagnoses and characteristics were identified by querying procedure codes (ie, Current Procedure Terminology; Healthcare Common Procedure Coding Systems, 9th and 10th Revisions), diagnostic codes (ie, International Classification of Diseases, 10th Revision, Clinical Modification [ICD‐10‐CM]), laboratory codes (ie, Logical Observation Identifiers Names and Codes), or prescribing codes (ie, National Drug Code, RxNorm concept unique identifiers). This activity was deemed not to be research as defined in 45 CFR 46.102(l); informed consent and institutional review board review were not required. The data for this study were shared using existing data use agreements between sites involved. PCORnet has a standardized process called the Front Door to query and request data across participating systems. Requests can be made here: https://pcornet.org/front‐door/.
Measures
Hypertension Control
Using a 3‐year lookback period before the COVID‐19 index date, we defined patients as having hypertension based on (1) prescription for an outpatient antihypertensive medication (not including centrally acting agents, loop diuretics, or beta blockers, as these are commonly used for other conditions); (2) outpatient centrally acting agents, loop diuretics, or beta blockers and either an ICD‐10‐CM diagnostic code for hypertension (I10–I16) or outpatient BP ≥130/80 mm Hg; (3) 2 outpatient BP readings ≥130/80 on separate days; or (4) 2 ICD‐10‐CM codes for hypertension on separate days in any care setting. We identified hypertension control status by the outpatient BP measurement most recently available before the COVID‐19‐positive lab test, with values identified in the 18‐month to 2‐week period before the positive COVID‐19 lab test. Excluding the 2‐week period before infection avoided use of values during the acute illness, and including measures up to 18 months before accounted for the possibility that patients might have gone long periods without a measured BP, especially during the COVID‐19 pandemic. Patients without a BP value during this period were not classified into hypertension control categories. Hypertension control was categorized into 4 strata (BP <130/80, 130–139/80–89, 140–159/90–99, or ≥160/100 mm Hg; these cutpoints were selected a priori), using the higher systolic or diastolic value if a patient's values fell into multiple levels.
Diabetes Control
Using a 3‐year‐lookback period, we defined patients as having type 1 or type 2 diabetes based on (1) having ≥1 prescriptions for an outpatient diabetes medication, (2) HbA1c ≥6.5%, (3) 1 ICD‐10‐CM code for diabetes (E10–E11) plus metformin, or (4) 2 ICD‐10‐CM codes for diabetes in any care setting. Using an 18‐month lookback period, we categorized diabetes control into 3 strata (HbA1c <7%, 7%–<9%, ≥9%; these cutpoints were selected a priori) using the most recently measured HbA1c before the index COVID‐19 date. Adults without an HbA1c measurement during this period were not categorized into diabetes control categories.
Severe Disease Outcomes
Hospitalization (defined as receiving ≥1 diagnosis for any reason in an inpatient setting), receipt of critical care (defined by procedure codes), and receipt of mechanical ventilation (defined by procedure and diagnostic codes) were assessed from 1 day before through 16 days after the COVID‐19 index date, accounting for delayed worsening of COVID‐19 illness that can extend into the second week of illness. We also captured deaths from any cause that occurred ≤60 days from the COVID‐19 index date.
Demographic and Clinical Characteristics
Demographic variables included age in years, sex (male, female), race (White, Black, Asian, other [including American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, Multiple Race, other]), and Hispanic ethnicity (yes, no). Patients were classified as having obesity according to their body mass index (≥30 kg/m2 versus <30 kg/m2) and by smoking status by the most recent value recorded in the electronic health record (current, former, never, unknown). History of 32 comorbidities within a 3‐year lookback period was assessed individually (see Table 1; comorbidities with prevalence ≥5%) and collectively using the Combined Comorbidity Index score. 17 COVID‐19 treatment variables included use of dexamethasone, monoclonal antibodies, or remdesivir within 14 days before or after the COVID‐19 index date. COVID‐19 index dates were also classified according to date ranges corresponding to pandemic phase (March 1, 2020–April 30, 2020 early pandemic, May 1, 2020–February 28, 2021 summer and winter waves, March 1, 2021–June 30, 2021 rising vaccination rates, July 1, 2021–December 19, 2021 Delta variant predominance, and December 20, 2021–February 28, 2022 Omicron variant predominance). 18
Table 1.
Demographic and Clinical Characteristics of Patients With COVID‐19, Overall and by COVID‐19 Outcomes, PCORnet, March 2020 to February 2022
| Characteristic | All COVID‐19 | Hospitalization | Critical care | Mechanical ventilation | 60‐day mortality* | |
|---|---|---|---|---|---|---|
| Number of unique patients | 1 494 837 | 100% | 188 188 (12.6%) | 43 357 (2.9%) | 22 346 (1.5%) | 31 094 (2.1%) |
| Age group | ||||||
| 20–39 y | 607 239 | 41% | 18% | 12% | 10% | 3% |
| 40–54 y | 388 201 | 26% | 19% | 19% | 19% | 10% |
| 55–64 y | 230 500 | 15% | 20% | 22% | 24% | 17% |
| 65–74 y | 153 868 | 10% | 20% | 24% | 27% | 26% |
| 75–84 y | 81 232 | 5% | 15% | 16% | 16% | 26% |
| 85+ y | 33 797 | 2% | 8% | 7% | 5% | 19% |
| Sex† | ||||||
| Female | 835 254 | 56% | 50% | 42% | 39% | 42% |
| Male | 659 026 | 44% | 50% | 58% | 61% | 58% |
| Hispanic ethnicity† | ||||||
| Yes | 249 445 | 17% | 19% | 16% | 20% | 16% |
| No | 1 106 884 | 74% | 76% | 79% | 73% | 77% |
| Race† | ||||||
| Asian | 42 665 | 3% | 3% | 3% | 3% | 3% |
| Black | 245 015 | 16% | 23% | 23% | 23% | 21% |
| White | 942 985 | 63% | 53% | 57% | 51% | 56% |
| Pandemic phase | ||||||
| March 1, 2020–April 30, 2020 | 59 836 | 4% | 11% | 12% | 20% | 19% |
| May 1, 2020–February 28, 2021 | 660 518 | 44% | 41% | 40% | 39% | 39% |
| March 1, 2021–Jun 30, 2021 | 89 500 | 6% | 10% | 9% | 8% | 7% |
| July 1, 2021–December 19, 2021 | 270 093 | 18% | 20% | 23% | 21% | 20% |
| December 20, 2021–February 28, 2022 | 414 890 | 28% | 18% | 17% | 12% | 15% |
| Hypertension | ||||||
| No hypertension | 857 014 | 57% | 40% | 35% | 42% | 32% |
| All hypertension‡ | 637 823 | 43% | 61% | 65% | 58% | 69% |
| Diabetes | ||||||
| No diabetes | 1 311 676 | 88% | 69% | 58% | 57% | 59% |
| All diabetes‡ | 183 161 | 12% | 32% | 41% | 43% | 42% |
| Other comorbidities§ | ||||||
| Combined Comorbidity Index score (mean) | 1 494 837 | 1.1 | 3.4 | 4.7 | 4.8 | 5.2 |
| Chronic pulmonary disorders | 197 721 | 13% | 25% | 29% | 28% | 29% |
| Mental health disorders | 187 948 | 13% | 20% | 21% | 19% | 20% |
| Anemia | 141 639 | 9% | 26% | 33% | 35% | 34% |
| Arrythmia | 136 473 | 9% | 29% | 43% | 45% | 47% |
| Asthma | 119 764 | 8% | 12% | 11% | 11% | 9% |
| Severe obesity (BMI ≥40 kg/m2) | 117 400 | 8% | 16% | 20% | 21% | 15% |
| Coronary artery disease | 100 203 | 7% | 22% | 28% | 26% | 33% |
| Chronic kidney disease | 91 538 | 6% | 23% | 29% | 29% | 35% |
| Cancer | 69 582 | 5% | 11% | 12% | 10% | 17% |
| Congestive heart failure | 73 737 | 5% | 19% | 27% | 27% | 31% |
| Pregnancy | 70 442 | 5% | 6% | 1% | 1% | 0% |
| Smoking status | ||||||
| Current smoker | 72 359 | 5% | 5% | 5% | 4% | 3% |
| Former smoker | 146 896 | 10% | 13% | 16% | 13% | 16% |
| Never smoker | 506 885 | 34% | 24% | 29% | 21% | 21% |
| Unknown or missing | 768 697 | 51% | 58% | 51% | 62% | 60% |
| COVID‐19 treatments | ||||||
| Dexamethasone | 107 777 | 7% | 40% | 54% | 52% | 44% |
| Monoclonal antibodies | 34 682 | 2% | 2% | 2% | 1% | 1% |
| Remdesivir | 66 210 | 4% | 29% | 39% | 38% | 30% |
| BMI | ||||||
| <30 kg/m2 | 426 797 | 29% | 37% | 41% | 34% | 42% |
| ≥30 kg/m2 | 363 352 | 24% | 34% | 39% | 38% | 30% |
| BMI missingǁ | 704 688 | 47% | 28% | 20% | 28% | 29% |
60‐day mortality refers to in‐hospital mortality.
Missing and “Other” not shown.
All hypertension and diabetes included patients who did not have a documented blood pressure measurement or glycated hemoglobin, respectively, within 18 months before COVID‐19, so control status could not be categorized.
Comorbidities with prevalence ≥5% are displayed; other comorbidities with prevalence <5% included rheumatoid arthritis, systemic lupus erythematosus, seizures or epilepsy, alcohol use disorder, chronic obstructive pulmonary disease, cirrhosis, dementia, Down syndrome, end‐stage renal disease, HIV, hemiplegia, inflammatory bowel disorder, multiple sclerosis, peripheral vascular disorders, Parkinson disease, pulmonary circulation disorders, chronic pulmonary disorders, sickle cell disease, or weight loss.
BMI was missing if patients did not have a recorded height and weight within 18 months of the index positive test.
BMI indicates body mass index; and PCORnet, National Patient‐Centered Clinical Research Network.
Statistical Analysis
Each PCORnet site ran a statistical program that conducted descriptive and multivariable analyses using patient‐level data that remained behind each health system's firewall. They returned aggregate results to the study data coordinating center. For multivariable models, pooled logistic regression models generated the parameter estimates, SEs, covariance matrices, convergence status, and number of observations. If models did not converge at sites, typically because of limited sample size, results were discarded; this was especially true for children's hospitals that had few patients in the data with the conditions of interest (diabetes or hypertension) or with severe COVID‐19 outcomes. For example, the highest number of sites that had convergence was 38 for hypertension models and 32 for diabetes models that were unadjusted with hospitalization as the outcome; for fully adjusted models with mechanical ventilation as the outcome, convergence was achieved only for 23 sites for hypertension and 21 sites for diabetes models. Results from sites that did converge were combined using meta‐analytic techniques, with the random‐effects model based on the DerSimonian and Laird method generating pooled estimates. 19 Each COVID‐19 outcome was tested in a separate model. Models generated crude (OR) and adjusted (aOR) odds ratios and 95% CIs using a type 1 error rate of 5% to determine statistical significance. Adjusted models included the following covariates: age in years, age squared, sex, race, ethnicity, obesity, Combined Comorbidity Index score, smoking status, receipt of dexamethasone, receipt of remdesivir, receipt of monoclonal antibodies, and pandemic phase.
Results
Among 1 494 837 adults with COVID‐19, 56% were female, and the median age was 46.7 years (SD=17.5; Table 1). The largest race groups were White (63%) and Black (16%); 17% were of Hispanic ethnicity (Table 1). Overall, 637 823 (43%) patients with COVID‐19 had hypertension (among whom, n=518 293 had data available to allow classification into hypertension control categories; Table 2), and 183 161 (12%) had diabetes (among whom, n=123 557 had data available to allow classification into diabetes control categories; Table 3).
Table 2.
Demographic and Clinical Characteristics of Patients With COVID‐19, by Hypertension and Control Status, PCORnet, March 2020 to February 2022
| Hypertension | Blood pressure control category | |||||
|---|---|---|---|---|---|---|
| No | Yes* | <130/80 | 130–139/80–89 | 140–159/90–99 | ≥160/100 | |
| Number | 857 034 | 638 128 | 180 021 | 192 728 | 111 519 | 34 025 |
| Mean age, y | 41.1 | 54.1 | 52.8 | 51.1 | 55.6 | 58.5 |
| Sex | ||||||
| Female | 55% | 57% | 64% | 58% | 53% | 53% |
| Male | 45% | 43% | 36% | 42% | 47% | 47% |
| Hispanic ethnicity† | ||||||
| Yes | 19% | 13% | 13% | 13% | 12% | 12% |
| No | 67% | 83% | 85% | 85% | 85% | 86% |
| Race† | ||||||
| Asian | 3% | 2% | 2% | 2% | 2% | 2% |
| Black | 15% | 19% | 16% | 17% | 20% | 26% |
| White | 59% | 68% | 72% | 71% | 69% | 62% |
| Combined Comorbidity Index score (mean) | 0.4 | 2.0 | 2.4 | 1.6 | 2.0 | 2.5 |
| Body mass index (mean)† | 24.9 | 28.0 | 27.0 | 28.6 | 29.0 | 27.5 |
| COVID‐19 outcomes | ||||||
| Hospitalization | 9% | 18% | 15% | 11% | 16% | 22% |
| Critical care | 2% | 4% | 4% | 3% | 4% | 6% |
| Mechanical ventilation | 1% | 2% | 2% | 1% | 2% | 3% |
| 60‐d mortality‡ | 1% | 3% | 3% | 2% | 3% | 4% |
PCORnet indicates National Patient‐Centered Clinical Research Network.
Includes 119 835 who met criteria for hypertension but could not be classified by control status (ie, did not have blood pressure measurement within 18 months before COVID‐19). Blood pressure control categories shown in mm Hg.
Missing and “Other” not shown.
60‐day mortality refers to in‐hospital mortality.
Table 3.
Demographic and Clinical Characteristics of Patients With COVID‐19, by Diabetes and Control Status, PCORnet, March 2020 to February 2022
| Diabetes | HbA1c control category | ||||
|---|---|---|---|---|---|
| No | Yes* | <7% | 7%–<9% | ≥9% | |
| Number | 1 311 896 | 183 266 | 57 892 | 41 457 | 24 208 |
| Mean age, y | 44.9 | 59.5 | 60.4 | 61.5 | 55.0 |
| Sex | |||||
| Female | 56% | 52% | 54% | 50% | 49% |
| Male | 43% | 48% | 46% | 50% | 51% |
| Hispanic ethnicity† | |||||
| Yes | 16% | 18% | 15% | 18% | 26% |
| No | 73% | 78% | 82% | 79% | 71% |
| Race† | |||||
| Asian | 3% | 3% | 3% | 3% | 2% |
| Black | 15% | 24% | 24% | 21% | 29% |
| White | 64% | 58% | 60% | 61% | 49% |
| Combined Comorbidity Index score (mean) | 0.7 | 3.4 | 3.9 | 3.5 | 3.3 |
| Body mass index (mean)† | 25.8 | 30.4 | 30.6 | 30.5 | 31.5 |
| COVID‐19 outcomes | |||||
| Hospitalization | 10% | 32% | 28% | 30% | 37% |
| Critical care | 2% | 10% | 9% | 9% | 11% |
| Mechanical ventilation | 1% | 5% | 4% | 5% | 6% |
| 60‐d mortality‡ | 1% | 7% | 6% | 7% | 6% |
PCORnet indicates National Patient‐Centered Clinical Research Network.
Includes 59 709 who met criteria for diabetes but could not be classified by control status (ie, did not have glycated hemoglobin measurement within 18 months before COVID‐19).
Missing and “Other” not shown.
60‐day mortality refers to in‐hospital mortality.
Among all patients with COVID‐19, 13% required hospitalization, 3% required critical care, 2% were mechanically ventilated, and 2% died within 60 days of the COVID‐19 index date (Table 1). The most frequent COVID‐19 treatment was dexamethasone (7%), followed by remdesivir (4%) and monoclonal antibodies (2%). Older age, male sex, Black race, COVID‐19 illness early in the pandemic, and a greater number of comorbidities before COVID‐19 illness were disproportionately represented among those with severe COVID‐19 outcomes (hospitalization, critical care, mechanical ventilation, or 60‐day mortality, Table 1).
Hypertension and Severe COVID‐19 Outcomes
Among patients with COVID‐19, severe COVID‐19 outcomes were twice as frequent among those with hypertension compared with those without hypertension: 18% versus 9% for hospitalization, 4% versus 2% for critical care, 2% versus 1% for mechanical ventilation, and 3% versus 1% for 60‐day mortality (Table 2). Of 518 293 (81%) patients whose hypertension control could be classified, 35% had BP <130/80, 37% had BP 130 to 139/80 to 89, 22% had BP 140 to 159/90 to 99, and 7% had BP ≥160/100. The frequency of hospitalization, critical care, mechanical ventilation, and 60‐day mortality were lowest among patients with BP 130 to 139/80 to 89 (11% hospitalized, 3% critical care, 1% mechanically ventilated, 2% 60‐day mortality), higher among those with BP 140 to 159/90 to 99 (16% hospitalized, 4% critical care, 2% mechanically ventilated, 3% 60‐day mortality) and highest among patients with BP ≥160/100 (22% hospitalized, 6% critical care, 3% mechanically ventilated, 4% 60‐day mortality). Those with the lowest BP (<130/80) were older, had higher Combined Comorbidity Index scores, and had slightly worse COVID‐19 outcomes than those with BP 130 to 139/80 to 89.
In unadjusted analyses among patients with hypertension, the poorest BP control level was significantly associated with greater odds of hospitalization (OR, 1.48 [95% CI, 1.41–1.55] for BP ≥160/100 versus BP <130/80), critical care (OR:1.59 [95% CI, 1.48–1.71]), mechanical ventilation (OR, 1.63 [95% CI, 1.45–1.84]), and 60‐day mortality (OR, 1.34 [95% CI, 1.22–1.47]) (Table S1). In adjusted analyses, associations remained statistically significant for BP ≥160/100 versus BP <130/80 for hospitalization (aOR, 1.30 [95% CI, 1.23–1.37]), critical care (aOR, 1.30 [95% CI, 1.21–1.40]), and mechanical ventilation (aOR, 1.32 [95% CI, 1.17–1.50]), but not 60‐day mortality (aOR, 1.08 [95% CI, 0.98–1.12]) (Figure 1 and Table S2). There was a less pronounced but statistically significant association for BP 140 to 159/90 to 99 versus BP <130/80 for hospitalization (aOR, 1.05 [95% CI, 1.00–1.09]), critical care (aOR, 1.13 [95% CI, 1.10–1.20]), and mechanical ventilation (aOR, 1.17 [95% CI, 1.06–1.29]). Among patients with hypertension, Black and Hispanic adults were more likely to be hospitalized, receive mechanical ventilation, or receive critical care, compared with White adults (Table S2). Other characteristics independently associated with more severe COVID‐19 outcomes included higher comorbidity score, male sex, and COVID‐19 illness during the earliest phase of the pandemic.
Figure 1. Adjusted odds ratios for COVID‐19 outcomes by hypertension control status, PCORnet, March 2020 to February 2022.
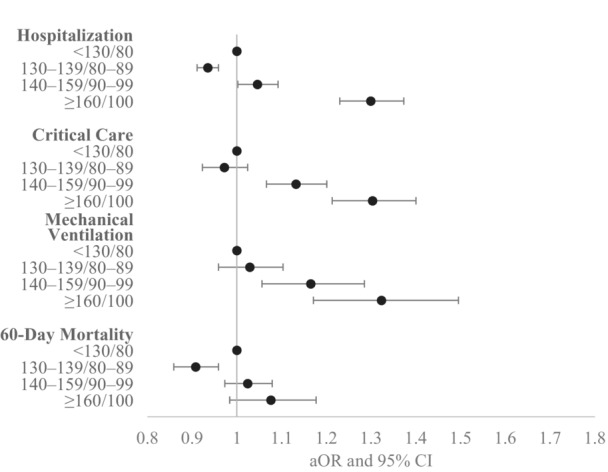
This figure shows adjusted odds ratios for inpatient hospitalization, critical care use, mechanical ventilation, and 60‐day in‐hospital mortality by strata of blood pressure control, with blood pressure <130/80 mm Hg as the referent category. Models controlled for age in years, age squared, sex, race, ethnicity, obesity, Combined Comorbidity Index score, smoking status, COVID‐19 treatments (receipt of dexamethasone, receipt of remdesivir, receipt of monoclonal antibodies), and pandemic phase. This analysis was limited to patients with hypertension whose blood pressure control status could be categorized (n=518 293 overall; model sample sizes varied depending on participating PCORnet sites). The number of sites that had model convergence was 32, 24, 23, and 29 for each of the outcomes, respectively. aOR indicates adjusted odds ratio; PCORnet, National Patient‐Centered Clinical Research Network.
Diabetes and Severe COVID‐19 Outcomes
Among patients with COVID‐19, the frequency of severe COVID‐19 outcomes was 3 to 7 times higher for those with diabetes than those without: 32% versus 10% for hospitalization, 10% versus 2% for critical care, 5% versus 1% for mechanical ventilation, and 7% versus 1% for 60‐day mortality (Table 3). Among 123 557 (67%) adults with diabetes whose control status could be classified, 47% had HbA1c <7%, 34% had HbA1c 7% to <9%, and 20% had HbA1c ≥9%. The frequency of hospitalization, critical care, and mechanical ventilation was lowest among patients with HbA1c <7% (28% hospitalized, 9% critical care, 4% mechanically ventilated) and highest among patients with HbA1c ≥9% (37% hospitalized, 11% critical care, 6% mechanically ventilated). In‐hospital 60‐day mortality was similar among patients regardless of HbA1c level.
Among patients with diabetes, there was a graded association between higher HbA1c and more severe COVID‐19 outcomes. In unadjusted analyses, higher HbA1c was significantly associated with greater odds of hospitalization (OR, 1.52 [95% CI, 1.40–1.64] for HbA1c ≥9% versus HbA1c <7%; OR, 1.10 [95% CI, 1.04–1.17] for HbA1c 7% to <9% versus <7%), critical care (OR, 1.51 [95% CI, 1.38–1.65] for HbA1c ≥9% versus HbA1c <7%, OR, 1.10 [95% CI, 1.02–1.19] for HbA1c 7%–<9% versus <7%), and mechanical ventilation (OR, 1.35 [95% CI, 1.22–1.48] HbA1c ≥9% versus HbA1c <7%; OR, 1.12 [95% CI, 1.01–1.23] for HbA1c 7% to <9% versus <7%) (Table S1). After adjustment, associations were statistically significant for hospitalization (aOR, 1.61 [95% CI, 1.47–1.76] for HbA1c ≥9% versus HbA1c <7%; OR, 1.10 [95% CI, 1.05–1.16] for HbA1c 7% to <9% versus <7%), critical care (aOR, 1.42 [95% CI, 1.31–1.54] for HbA1c ≥9% versus HbA1c <7%; OR, 1.08 [95% CI, 1.00–1.16] for HbA1c 7% to <9% versus <7%), mechanical ventilation (aOR, 1.12 [95% CI, 1.02–1.23] for HbA1c ≥9% versus HbA1c <7%; not significant for HbA1c 7% to <9% versus <7%), and 60‐day mortality (aOR, 1.18 [95% CI, 1.09–1.27] for HbA1c ≥9% versus HbA1c <7%; OR, 1.07 [95% CI, 1.01–1.15] for HbA1c 7% to <9% versus <7%) (Figure 2 and Table S3). Among adults with diabetes, Black adults were more likely to be hospitalized than White adults, and Hispanic adults were more likely to be hospitalized, receive critical care, and receive mechanical ventilation than White adults (Table S3). Higher comorbidity score, male sex, and COVID‐19 illness during the earliest phase of the pandemic were also associated with more severe COVID‐19 outcomes.
Figure 2. Adjusted odds ratios for COVID‐19 outcomes by diabetes control status, PCORnet, March 2020 to February 2022.
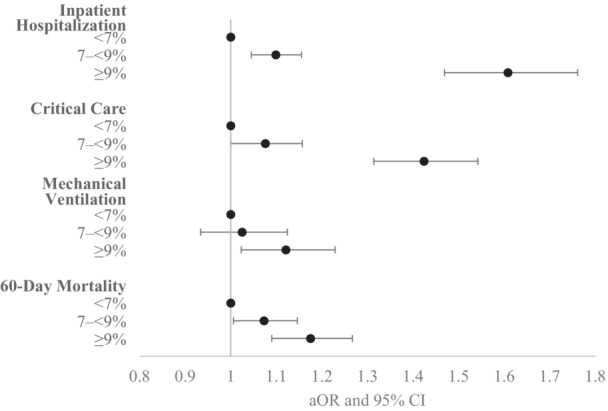
This figure shows adjusted odds ratios for inpatient hospitalization, mechanical ventilation, critical care use, and 60‐day in‐hospital mortality by strata of HbA1c control, with HbA1c <7% as the referent category. Models controlled for age in years, age squared, sex, race, ethnicity, obesity, Combined Comorbidity Index score, smoking status, COVID‐19 treatments (receipt of dexamethasone, receipt of remdesivir, receipt of monoclonal antibodies), and pandemic phase. This analysis was limited to patients with diabetes whose HbA1c control status could be categorized (n=123 557 overall; model sample sizes varied depending on participating PCORnet sites). The number of sites that had model convergence was 29, 22, 21, and 26 for each of the outcomes, respectively. aOR indicates adjusted odds ratio; HbA1c, glycated hemoglobin; PCORnet, the National Patient‐Centered Clinical Research Network.
Discussion
In a large‐scale, multisite study of nearly 1.5 million US adults, we observed that poor control of hypertension and diabetes was associated with greater odds of severe COVID‐19 outcomes. In adjusted models of adults with hypertension, those with least controlled BP levels had approximately 30% greater odds of hospitalization, 30% greater odds of critical care, and 32% greater odds of mechanical ventilation compared with those with the most controlled BP level. Among adults with diabetes, those with the least controlled HbA1c level had approximately 61% greater odds of hospitalization, 42% greater odds of critical care, 12% greater odds of mechanical ventilation, and 18% greater odds of 60‐day mortality compared with those with the most controlled HbA1c level. Overall, findings from this study address a research gap regarding the independent associations of BP and HbA1c control with COVID‐19 severity, identifying those with least control as subpopulations at greatest risk for severe COVID‐19 outcomes. Strengths of our study include the large population size across a broad geographic area, study period encompassing multiple pandemic phases, ability to assess BP and HbA1c before SARS‐CoV‐2 infection rather than upon hospital admission, and adjustment for differences in demographic and clinical characteristics among adults across BP and HbA1c control levels during a large proportion of the pandemic.
An important contribution of this study is that it clarifies the independent association between hypertension and severe COVID‐19 outcomes, which some studies have suggested might be driven by age or other comorbidities. 20 , 21 These results add to an emerging body of research that has presented conflicting findings. One study of 12 548 patients with hypertension and COVID‐19 in the Kaiser Permanente system in Southern California found that uncontrolled BP (>130/80) was not associated with hospitalization or mortality. 22 Our study included a much larger population and more refined strata of baseline BP and observed stronger associations between hypertension control and hospitalization, mechanical ventilation, and critical care in the highest stratum (BP ≥160/100). Our findings were consistent with 2 studies from Wuhan, China. One of these assessed 803 hospitalized patients with hypertension and COVID‐19 and found that higher systolic and diastolic BP were associated with adverse outcomes including intensive care unit admission and mortality. 23 The other, which consisted of 2864 hospitalized patients, also found that worse BP control was associated with worse COVID‐19 outcomes. 24 An important difference between our study and these 2 was our use of baseline, pre‐COVID‐19 measures of BP instead of in‐hospital BP assessment, during which SARS‐CoV‐2 infection itself and the hospital environment might have influenced BP levels. 25
One proposed mechanism by which hypertension could exacerbate COVID‐19 risk is through elevated immunological activity and inflammation. 26 The relationship between uncontrolled hypertension and severe COVID‐19 outcomes is especially concerning given the high prevalence of uncontrolled hypertension in the United States 2 and increases in mean systolic and diastolic BP during the COVID‐19 pandemic. 27 Such population‐level increases in BP might be related to increased alcohol consumption and stress or decreased physical activity and access to medical care. 4 , 27 , 28 To improve hypertension management, some clinical practices have made concerted efforts to increase use of telehealth services and support for self‐measured BP monitoring. 29 , 30
Counterintuitively, severe COVID‐19 outcomes were less frequent among adults with hypertension who had BP 130 to 139/80 to 89 compared with the lowest BP level of <130/80. Prior research has shown that adults with hypertension and lower BP tend to be older, with more comorbidities, and with a longer history of hypertension. 31 , 32 Adults with hypertension in our study with BP <130/80 were older than those with BP 130 to 139/80 to 89; however, worse COVID‐19 outcomes among those with BP <130/80 persisted in adjusted regression models that controlled for age and comorbidity score. Such findings suggest the possibility of residual confounding by factors we could not capture or a potential association between low BP or hypotension (which would have been grouped with BP <130/80) and frailty as well as severe COVID‐19 outcomes. 33 , 34 , 35
Severe COVID‐19 outcomes were 3 to 7 times higher for those with diabetes compared with those without, and adjusted models demonstrated a stepped association between higher HbA1c levels and more severe COVID‐19 outcomes. Our findings are consistent with prior studies that have found associations of higher HbA1c with COVID‐19 severity and mortality. 11 , 12 , 36 As with hypertension, proposed mechanisms of increased risk of severe COVID‐19 from diabetes include chronic inflammation and dysregulated immune function. 12 Also potentially related to inflammation or autoimmunity, emerging evidence suggests that COVID‐19 might increase diabetes incidence and hyperglycemia, indicating a bidirectional relationship. 37 , 38 Potentially exacerbating risk, HbA1c levels might have increased during the pandemic for some patients 5 , 39 due to less screening and monitoring and to behavior changes related to the pandemic. 40 Among young and middle‐aged adults with diabetes, a majority reported delaying medical care due to COVID‐19 concerns (63%–87%), and many reported difficulty accessing medications (26%–44%). 41 Technologies such as telehealth and home monitoring of blood glucose might help support diabetes management, especially in the context of health care disruption due to the pandemic. 39
In addition to hypertension and diabetes, other factors observed to be independently associated with more severe COVID‐19 outcomes were consistent with prior studies. Higher Combined Comorbidity Index scores, indicating more comorbidities, were associated with greater odds of severe COVID‐19 outcomes. In prior studies, multiple comorbidities have been associated with COVID‐19 risk, including obesity, chronic liver disease, chronic kidney disease, chronic neurologic disease, chronic pulmonary disease, diabetes, and heart disease. 42 , 43 We also observed greater odds of severe COVID‐19 outcomes among male adults 42 and adults whose COVID‐19 index event was in the earliest phase of the pandemic (March 1 through April 30, 2020), as other research has shown. 44 Black adults and Hispanic adults were more likely to experience severe COVID‐19 outcomes, which is consistent with prior studies demonstrating disparities in COVID‐19 risk. 42 , 45 , 46 These effects were independent of comorbidity score and control of hypertension or diabetes. It should be noted that race is not a biological construct, and observed disparities could be driven by differences in social determinants of health, 1 health care access, other societal and health system inequities, or residual confounding by other underlying health conditions. 45 , 46 , 47 At a population level, potential effects of poor hypertension and diabetes control on COVID‐19 outcomes could disproportionately affect Black and Hispanic adults, given longstanding disparities in chronic disease management 1 and potential exacerbation of these disparities during the pandemic due to factors such as reduced access to telehealth, internet access, and other technological supports for chronic disease management. 48
This study had several limitations. First, patients in this study might not be generalizable to the US population; most of the institutions included were tertiary care academic medical centers in urban settings. In addition, many patients with COVID‐19 were not captured in our data set, particularly those who tested in community settings, tested using at‐home kits, or did not test at all. This likely biases our study toward inclusion of patients at higher risk for more severe COVID‐19, which does not affect internal validity but might limit generalizability. However, if patients at higher risk for more severe COVID‐19 were also more likely to have poorly controlled hypertension or diabetes, then collider bias could have influenced our findings. Second, some patients' hypertension or diabetes control status could not be classified because they did not have a BP or HbA1c within 18 months before the COVID‐19 index date. Missing data could bias results if there was differential missingness by BP and HbA1c level. Third, patient hypertension or diabetes control status could have been misclassified if BP or HbA1c measures changed from between the date of assessment and the COVID‐19 index date or if the patient experienced transient high BP levels in health care settings (ie, the “white coat hypertension” phenomenon). Fourth, we cannot causally attribute the patient's hospitalization, critical care, mechanical ventilation, or death to their laboratory‐confirmed SARS‐CoV‐2 infection; it is possible that some patients were hospitalized, treated, or died for reasons independent of their SARS‐CoV‐2 infection. Fifth, not all sites were included in final models; if a model did not converge for a particular DataMart (typically because of small sample sizes or small number of events among patients at those sites, especially at children's hospitals that had few patients aged 20 years and older), those data were discarded rather than pooled. Sixth, we did not distinguish between type 1 and type 2 diabetes, which might have differential relationships with COVID‐19 outcomes and warrant further study. Finally, residual confounding might have occurred due to factors such as body mass index (which was missing in nearly half of patients, although we included both obesity status and an indicator for missing body mass index in our models) or unmeasured factors. We were unable to adjust for COVID‐19 vaccination status due to poor capture of COVID‐19 vaccination status in electronic health records for vaccinations that were delivered outside of health systems. Future studies could confirm our findings in different electronic health record data systems and patient populations, could explore multivariable models comparing the nonhypertension (and nondiabetes) groups against various levels of chronic disease control, and could examine robustness of results across additional stratifications, such as by age, by pre‐ and postvaccination eras, by pre‐ and post‐in‐home‐testing eras, and by additional categories of BP control (such as systolic BP 110–130).
Conclusions
In conclusion, this multisite study of nearly 1.5 million US adults demonstrated worse COVID‐19 severity among patients with poorly controlled hypertension and diabetes. These results have important implications for clinical and public health practice, especially given existing disparities in chronic disease control and the role such disparities might play in exacerbating observed disparities in COVID‐19 outcomes. Clinicians and public health professionals can encourage adults with poorly controlled hypertension or diabetes to adhere to COVID‐19 vaccination recommendations and encourage these patients to seek early treatment for COVID‐19 to prevent progression to severe disease. 14 , 49 Additionally, results underscore the importance of ongoing chronic disease management during a pandemic, including through out‐of‐office services, such as telehealth, and strategies such as disease self‐management education and support, as well as self‐measured BP monitoring and home blood glucose measurements with clinical support. 30 , 50 Ongoing efforts to adapt care delivery to the changing needs of the population during the pandemic could potentially improve population health and COVID‐19 outcomes or outcomes from other infectious diseases. Finally, improved capture of data related to COVID‐19 care, particularly vaccination status, would help to mitigate the limitations cited as well as improve the reliability of research findings.
Appendix
Collaborative Authors: PCORnet Network Partners
Faraz S. Ahmad, MD, MS, Northwestern University Feinberg School of Medicine; Saul Blecker, NYU Langone Health; H. Timothy Bunnell, PhD, Nemours Children's Health; Bernard P. Chang, MD, PhD, Columbia University; Elizabeth A. Chrischilles, PhD, The University of Iowa; Dimitri A. Christakis, MD, MPH, Seattle Children's Research Institute; Lindsay G. Cowell, MS, PhD, UT Southwestern Medical Center; Janis L. Curtis, MSPH, MA, Duke University School of Medicine; Daniel Fort, PhD, MPH, Ochsner Health; David A Hanauer, MD, MS, University of Michigan; Rachel Hess, MD, MS, Spencer Fox Eccles School of Medicine at the University of Utah; Benjamin D. Horne, PhD, MStat, MPH, Intermountain Medical Center, Stanford University; Philip Giordano, MD, FACEP, Orlando Health, Inc.; William Hogan, MD, MS, University of Florida; Wenke Hwang, PhD, Penn State University School of Medicine; Harold Lehmann, Johns Hopkins School of Medicine; Kenneth H. Mayer, MD, FACP, Fenway Health; Abu Saleh Mohammad Mosa, PhD, MS, FAMIA, University of Missouri; James C. McClay, MD, MS, University of Nebraska Medical Center; Samyuktha Nandhakumar, MS, UNC School of Medicine; Bridget Nolan, Department of Population Medicine, Harvard Pilgrim Health Care Institute; Jihad S. Obeid, MD, FAMIA, Medical University of South Carolina; Brian Ostasiewski, Wake Forest School of Medicine; Nathan M. Pajor, MD, Cincinnati Children's Hospital Medical Center, University of Cincinnati; Lav Patel, BS, MS, University of Kansas Medical Center; Suchitra Rao, MBBS, MSCS, University of Colorado School of Medicine; Patricia S. Robinson, PhD, APRN, AdventHealth; Jonathan C. Silverstein, MD, MS, University of Pittsburgh; Alexander Stoddard, MS, Medical College of Wisconsin; William E. Trick, MD, Cook County Health.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S3
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.030240
This article was sent to Ajay K. Gupta, MD, MSc, PhD, FRCP, FESC, Senior Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 11.
Contributor Information
Sandra L. Jackson, Email: sljackson@cdc.gov.
PCORnet Collaboration Authors:
Faraz S. Ahmad, Saul Blecker, H. Timothy Bunnell, Bernard P. Chang, Elizabeth A. Chrischilles, Dimitri A. Christakis, Lindsay G. Cowell, Janis L. Curtis, Daniel Fort, David A Hanauer, Rachel Hess, Benjamin D. Horne, Philip Giordano, William Hogan, Wenke Hwang, Harold Lehmann, Kenneth H. Mayer, Abu Saleh Mohammad Mosa, James C. McClay, Samyuktha Nandhakumar, Bridget Nolan, Jihad S. Obeid, Brian Ostasiewski, Nathan M. Pajor, Lav Patel, Suchitra Rao, Patricia S. Robinson, Jonathan C. Silverstein, Alexander Stoddard, and William E. Trick
References
- 1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/cir.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2. Ritchey MD, Gillespie C, Wozniak G, Shay CM, Thompson‐Paul AM, Loustalot F, Hong Y. Potential need for expanded pharmacologic treatment and lifestyle modification services under the 2017 ACC/AHA hypertension guideline. J Clin Hypertens (Greenwich). 2018;20:1377–1391. doi: 10.1111/jch.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, Feng Y‐Q, Wang H, Zhong VW. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999‐2018. JAMA. 2021;326:704–716. doi: 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Czeisler ME, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, Ali N, McMillan H, Wiley JF, Weaver MD, et al. Delay or avoidance of medical care because of COVID‐19–related concerns—United States, June 2020. Morb Mortal Wkly Rep (MMWR). 2020;69:1250–1257. doi: 10.15585/mmwr.mm6936a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Biancalana E, Parolini F, Mengozzi A, Solini A. Short‐term impact of COVID‐19 lockdown on metabolic control of patients with well‐controlled type 2 diabetes: a single‐centre observational study. Acta Diabetol. 2021;58:431–436. doi: 10.1007/s00592-020-01637-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenthal N, Cao Z, Gundrum J, Sianis J, Safo S. Risk factors associated with In‐hospital mortality in a US national sample of patients with COVID‐19. JAMA Netw Open. 2020;3:e2029058. doi: 10.1001/jamanetworkopen.2020.29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . Underlying Medical Conditions Associated with High Risk for Severe COVID‐19: Information for Healthcare Providers. Accessed August 3, 2023. https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐care/underlyingconditions.html 2023.
- 9. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merzon E, Green I, Shpigelman M, Vinker S, Raz I, Golan‐Cohen A, Eldor R. Haemoglobin A1c is a predictor of COVID‐19 severity in patients with diabetes. Diabetes Metab Res Rev. 2021;37:e3398. doi: 10.1002/dmrr.3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prattichizzo F, de Candia P, Nicolucci A, Ceriello A. Elevated HbA1c levels in pre‐Covid‐19 infection increases the risk of mortality: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2021;38:e3476. doi: 10.1002/dmrr.3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson SL, Block JP, Rolka DB, Pavkov ME, Chevinsky JR, Lekiachvili A, Carton TW, Thacker D, Denson JL, Paranjape A, et al. COVID‐19 outcomes stratified by control status of hypertension and diabetes: preliminary findings from PCORnet, U.S. AJPM Focus. 2022;1:100012. doi: 10.1016/j.focus.2022.100012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Massetti GM, Jackson BR, Brooks JT, Perrine CG, Reott E, Hall AJ, Lubar D, Williams IT, Ritchey MD, Patel P, et al. Summary of guidance for minimizing the impact of COVID‐19 on individual persons, communities, and health care systems–United States, August 2022. Morb Mortal Wkly Rep (MMWR). 2022;71:1057–1064. doi: 10.15585/mmwr.mm7133e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Califf RM. The patient‐centered outcomes research network: a national infrastructure for comparative effectiveness research. N C Med J. 2014;75:204–210. doi: 10.18043/ncm.75.3.204 [DOI] [PubMed] [Google Scholar]
- 16. Forrest CB, McTigue KM, Hernandez AF, Cohen LW, Cruz H, Haynes K, Kaushal R, Kho AN, Marsolo KA, Nair VP, et al. PCORnet® 2020: current state, accomplishments, and future directions. J Clin Epidemiol. 2021;129:60–67. doi: 10.1016/j.jclinepi.2020.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun JW, Rogers JR, Her Q, Welch EC, Panozzo CA, Toh S, Gagne JJ. Adaptation and validation of the combined comorbidity score for ICD‐10‐CM. Med Care. 2017;55:1046–1051. doi: 10.1097/mlr.0000000000000824 [DOI] [PubMed] [Google Scholar]
- 18. Lambrou AS, Shirk P, Steele MK, Paul P, Paden CR, Cadwell B, Reese HE, Aoki Y, Hassell N, Caravas J, et al. Genomic surveillance for SARS‐CoV‐2 variants: predominance of the Delta (B.1.617.2) and omicron (B.1.1.529) variants—United States, June 2021–January 2022. Morb Mortal Wkly Rep. 2022;71:206–211. doi: 10.15585/mmwr.mm7106a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DerSimonian R, Laird N. Meta‐analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McFarlane E, Linschoten M, Asselbergs FW, Lacy PS, Jedrzejewski D, Williams B. The impact of pre‐existing hypertension and its treatment on outcomes in patients admitted to hospital with COVID‐19. Hypertens Res. 2022;45:834–845. doi: 10.1038/s41440-022-00893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tadic M, Saeed S, Grassi G, Taddei S, Mancia G, Cuspidi C. Hypertension and COVID‐19: ongoing controversies. Front Cardiovasc Med. 2021;8:639222. doi: 10.3389/fcvm.2021.639222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. An J, Zhou H, Luong TQ, Wei R, Mefford MT, Harrison TN, Lee MS, Sim JJ, Brettler JW, Martin JP, et al. Risk of hospitalization and mortality associated with uncontrolled blood pressure in patients with hypertension and COVID‐19. Int J Cardiol Cardiovasc Risk Prev. 2021;11:200117. doi: 10.1016/j.ijcrp.2021.200117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ran J, Song Y, Zhuang Z, Han L, Zhao S, Cao P, Geng Y, Xu L, Qin J, He D, et al. Blood pressure control and adverse outcomes of COVID‐19 infection in patients with concomitant hypertension in Wuhan, China. Hypertens Res. 2020;43:1267–1276. doi: 10.1038/s41440-020-00541-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen R, Yang J, Gao X, Ding X, Yang Y, Shen Y, He C, Xiang H, Ke J, Yuan F, et al. Influence of blood pressure control and application of renin‐angiotensin‐aldosterone system inhibitors on the outcomes in COVID‐19 patients with hypertension. J Clin Hypertens (Greenwich). 2020;22:1974–1983. doi: 10.1111/jch.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akpek M. Does COVID‐19 cause hypertension? Angiology. 2022;73:682–687. doi: 10.1177/00033197211053903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trump S, Lukassen S, Anker MS, Chua RL, Liebig J, Thürmann L, Corman VM, Binder M, Loske J, Klasa C, et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID‐19. Nat Biotechnol. 2021;39:705–716. doi: 10.1038/s41587-020-00796-1 [DOI] [PubMed] [Google Scholar]
- 27. Laffin LJ, Kaufman HW, Chen Z, Niles JK, Arellano AR, Bare LA, Hazen SL. Rise in blood pressure observed among US adults during the COVID‐19 pandemic. Circulation. 2022;145:235–237. doi: 10.1161/CIRCULATIONAHA.121.057075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beckman AL, King J, Streat DA, Bartz N, Figueroa JF, Mostashari F. Decreasing primary care use and blood pressure monitoring during COVID‐19. Am J Manag Care. 2021;27:366–368. doi: 10.37765/ajmc.2021.88644 [DOI] [PubMed] [Google Scholar]
- 29. Robbins CL, Ford ND, Hayes DK, Ko JY, Kuklina E, Cox S, Ferre C, Loustalot F. Clinical practice changes in monitoring hypertension early in the COVID‐19 pandemic. Am J Hypertens. 2022;35:596–600. doi: 10.1093/ajh/hpac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abbas A, Hannan J, Stolp H, Coronado F, Sperling LS. Commitment to hypertension control during the COVID‐19 pandemic: million hearts initiative exemplars. Prev Chronic Dis. 2022;19:E47. doi: 10.5888/pcd19.210439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheppard JP, Nicholson BD, Lee J, McGagh D, Sherlock J, Koshiaris C, Oke J, Jones NR, Hinton W, Armitage L, et al. Association between blood pressure control and coronavirus disease 2019 outcomes in 45 418 symptomatic patients with hypertension: an observational cohort study. Hypertension. 2021;77:846–855. doi: 10.1161/hypertensionaha.120.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Somers VK, Gao X, Chen Z, Ju J, Lin Q, Mohamed EA, Karim S, Xu H, Zhang L. Evaluation of optimal diastolic blood pressure range among adults with treated systolic blood pressure less than 130 mm Hg. JAMA Netw Open. 2021;4:e2037554. doi: 10.1001/jamanetworkopen.2020.37554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hanidziar D, Bittner EA. Hypotension, systemic inflammatory response syndrome, and COVID‐19: a clinical conundrum. Anesth Analg. 2020;131:e175–e176. doi: 10.1213/ane.0000000000005062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun B, Wang H, Lv J, Pei H, Bai Z. Predictors of mortality in hospitalized COVID‐19 patients complicated with hypotension and hypoxemia: a retrospective cohort study. Front Med (Lausanne). 2021;8:753035. doi: 10.3389/fmed.2021.753035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masoli JAH, Delgado J. Blood pressure, frailty and dementia. Exp Gerontol. 2021;155:111557. doi: 10.1016/j.exger.2021.111557 [DOI] [PubMed] [Google Scholar]
- 36. Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, Barron E, Bakhai C, Khunti K, Wareham NJ, et al. Risk factors for COVID‐19‐related mortality in people with type 1 and type 2 diabetes in England: a population‐based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/s2213-8587(20)30271-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie Y, Al‐Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311–321. doi: 10.1016/s2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID‐19, hyperglycemia, and new‐onset diabetes. Diabetes Care. 2021;44:2645–2655. doi: 10.2337/dc21-1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fisher L, Polonsky W, Asuni A, Jolly Y, Hessler D. The early impact of the COVID‐19 pandemic on adults with type 1 or type 2 diabetes: a national cohort study. J Diabetes Complicat. 2020;34:107748. doi: 10.1016/j.jdiacomp.2020.107748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holland D, Heald AH, Stedman M, Hanna F, Wu P, Duff C, Green L, Robinson S, Halsall I, Gaskell N, et al. Assessment of the effect of the COVID‐19 pandemic on UK HbA1c testing: implications for diabetes management and diagnosis. J Clin Pathol. 2021;76:177–184. doi: 10.1136/jclinpath-2021-207776 [DOI] [PubMed] [Google Scholar]
- 41. Czeisler MÉ, Barrett CE, Siegel KR, Weaver MD, Czeisler CA, Rajaratnam SMW, Howard ME, Bullard KM. Health care access and use among adults with diabetes during the COVID‐19 pandemic—United States, February–March 2021. Morb Mortal Wkly Rep. 2021;70:1597–1602. doi: 10.15585/mmwr.mm7046a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carethers JM. Insights into disparities observed with COVID‐19. J Intern Med. 2021;289:463–473. doi: 10.1111/joim.13199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, Silk BJ, Gundlapalli AV, Harris AM, Boehmer TK, et al. Risk factors for severe COVID‐19 outcomes among persons aged ≥18 years who completed a primary COVID‐19 vaccination series—465 health care facilities, United States, December 2020–October 2021. Morb Mortal Wkly Rep. 2022;71:19–25. doi: 10.15585/mmwr.mm7101a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ebinger JE, Driver M, Ji H, Claggett B, Wu M, Luong E, Sun N, Botting P, Kim EH, Hoang A, et al. Temporal variations in the severity of COVID‐19 illness by race and ethnicity. BMJ Nutr Prev Health. 2021;4:166–173. doi: 10.1136/bmjnph-2021-000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dorn AV, Cooney RE, Sabin ML. COVID‐19 exacerbating inequalities in the US. Lancet. 2020;395:1243–1244. doi: 10.1016/s0140-6736(20)30893-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, Spencer H, Rusek M, Anderson J, Veazie S, et al. Racial and ethnic disparities in COVID‐19–related infections, hospitalizations, and deaths. Ann Intern Med. 2021;174:362–373. doi: 10.7326/m20-6306%m33253040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holtgrave DR, Barranco MA, Tesoriero JM, Blog DS, Rosenberg ES. Assessing racial and ethnic disparities using a COVID‐19 outcomes continuum for New York state. Ann Epidemiol. 2020;48:9–14. doi: 10.1016/j.annepidem.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bress AP, Cohen JB, Anstey DE, Conroy MB, Ferdinand KC, Fontil V, Margolis KL, Muntner P, Millar MM, Okuyemi KS, et al. Inequities in hypertension control in the United States exposed and exacerbated by COVID‐19 and the role of home blood pressure and virtual health care during and after the COVID‐19 pandemic. J Am Heart Assoc. 2021;10:e020997. doi: 10.1161/jaha.121.020997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. National Institutes of Health . Therapeutic Management of Nonhospitalized Adults with COVID‐19. 2023. Accessed August 3, 2023. https://www.covid19treatmentguidelines.nih.gov/management/clinical‐management/nonhospitalized‐adults‐‐therapeutic‐management/?utm_source=site&utm_medium=home&utm_campaign=highlights.
- 50. Chircop J, Sheffield D, Kotera Y. Systematic review of self‐monitoring of blood glucose in patients with type 2 diabetes. Nurs Res. 2021;70:487–497. doi: 10.1097/nnr.0000000000000542 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


