Abstract
Background:
Opioid use disorder is a leading cause of death through the year postpartum.
Objectives:
To identify the association of neighborhood-level social determinants of health and prenatal opioid use disorder treatment receipt with medication for opioid use disorder outcomes through the year postpartum among a cohort of birthing people.
Design:
Population-based retrospective cohort study that utilized state Medicaid claims and enrollment data for the 1,690 individuals who delivered a live infant between July 1, 2016 to December 31, 2020 receiving medication for opioid use disorder at delivery. The primary exposure was the state Health Opportunity Index, a composite measure of social determinants of health linked at the census-tract level. Secondary exposures included comprehensiveness of opioid use disorder treatment and medication for opioid use disorder duration received prenatally. Outcomes included postpartum medication for opioid use disorder duration and continuity, operationalized as time from delivery to medication for opioid use disorder discontinuation and percentage of days covered by medication for opioid use disorder within the 12 months after delivery, respectively.
Findings:
Within the study sample, 711 deliveries were to birthing people living in the lowest state Health Opportunity Index tercile (indicating high burden of negative social determinants of health), 647 in the middle state Health Opportunity Index tercile, and 332 in the highest state Health Opportunity Index tercile. Using stepwise multivariable regression (Cox proportional hazards and negative binomial models) guided by a socioecological framework, prenatal receipt of more comprehensive opioid use disorder treatment and/or longer prenatal medication for opioid use disorder duration was associated with improved 1-year postpartum opioid use disorder treatment outcomes (medication for opioid use disorder duration and continuity). When the state Health Opportunity Index was added to the models, these significant associations remained stable, with the state Health Opportunity Index not demonstrating an association with the outcomes (Duration HR 1.39, 95% CI 0.551, 3.512; Continuity RR 1.024, 95% CI 0.323, 3.247).
Conclusions:
Targeted efforts at expanding access to and quality of evidence-based opioid use disorder treatments for reproductive-age people across the lifecourse should be prioritized within the spectrum of work aimed at eradicating disparities in pregnancy-related mortality.
Introduction
Substance use related deaths, namely opioid overdose, are the leading cause of pregnancy-related mortality in the United States, with most deaths occurring in the year after delivery.1–5 In addition to maternal morbidity and mortality, untreated opioid use disorder (OUD) carries additional risks for the dyad and increased healthcare costs.6
Expanding access and utilization of medication treatments for OUD (MOUD) is the most effective path forward to combat the overdose crisis.7 However, stark disparities in MOUD outcomes exist,8–10 including among birthing people.11–13 These disparities are hypothesized to be reflections of a culmination of higher order factors (e.g., structural and systemic racism, inequities in healthcare access) rather than solely contributions by individual-level factors demarcating the social group burdening the disparity (e.g., race, ethnicity).9,14 The use of a socioecological framework to guide investigations of OUD treatment outcomes is an innovative avenue to achieve a more comprehensive understanding of the underlying contributors to inequities,15,16 especially social determinants of health (SDoH),17,18 to ultimately inform intervention development.19 However, investigations like these have largely focused on individual-level SDoH, rather than SDoH at the neighborhood level, despite other areas of medicine indicating their important contributions to health disparities.20–22
The objective of this study was to identify the association of neighborhood-level SDoH with MOUD outcomes (duration, continuity of receipt) through the 1-year postpartum period among a cohort of birthing people in a Medicaid-expanded state, using Medicaid claims data (2016–2020). Secondarily we assess associations with measures of prenatal OUD treatment receipt (comprehensiveness and MOUD duration). Within an adapted socioecological model (Figure 1), we stepwise evaluated the contribution of four categories of factors: individual-level demographics, parent-infant dyad and health related variables, aspects of prenatal OUD treatment delivery, and neighborhood-level SDoH. SDoH of the birthing person’s community was comprehensively captured using the state Department of Health’s Health Opportunity Index (HOI). We hypothesized that the neighborhood-level SDoH for the birthing person, as measured by the HOI, along with the degree of prenatal MOUD treatment, would be associated with improved postpartum MOUD outcomes.
Figure 1:
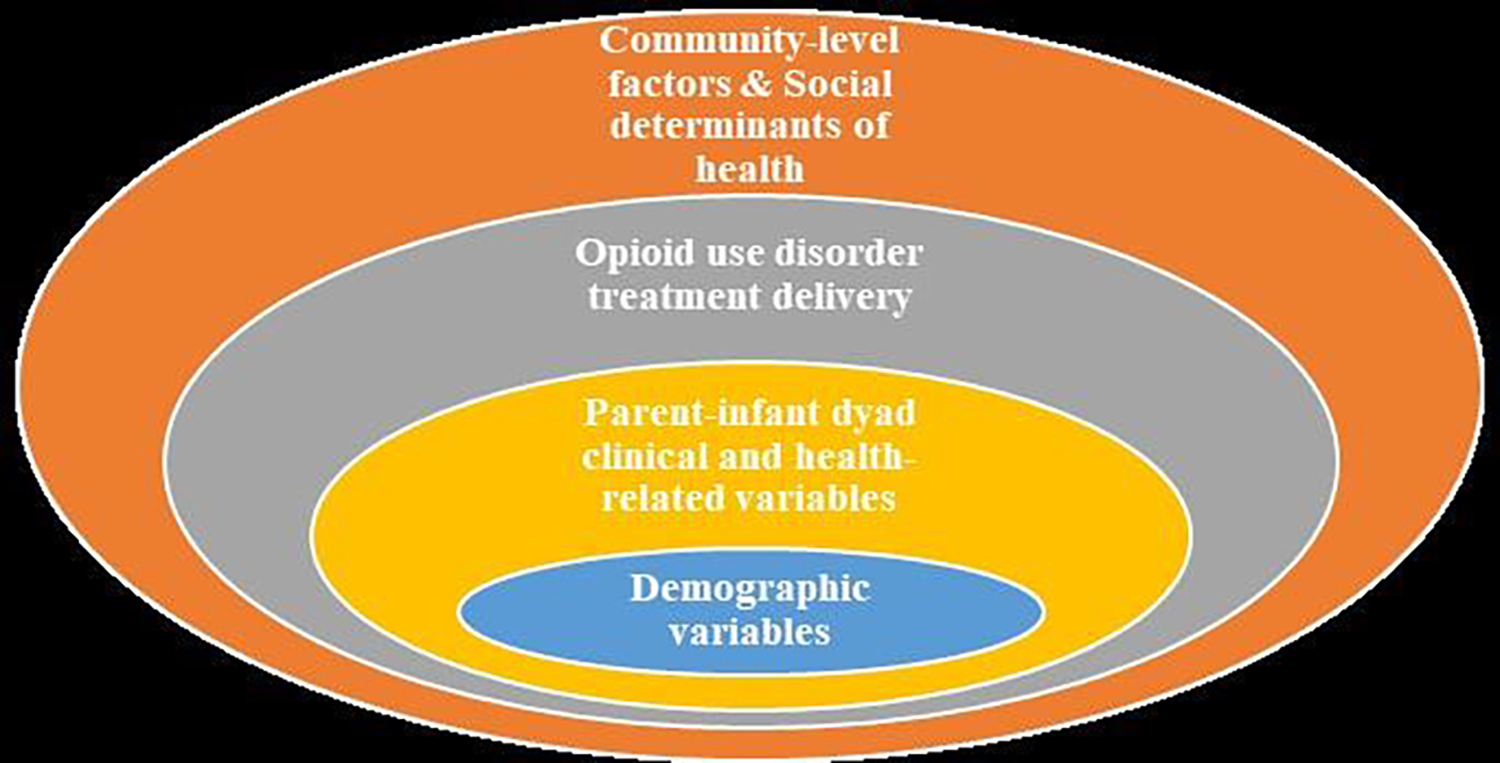
Socioecological framework that guided study design to investigate factors contributing to opioid use disorder medication treatment outcomes through the 1-year postpartum period among a cohort of Medicaid members, July 2016 to December 2020
Materials and Methods:
Data source and study sample
This population-level retrospective cohort study used state Medicaid claims and enrollment data from the Department of Medical Services for individuals who delivered a live infant between July 1, 2016 to December 31, 2020. This timeframe was selected as it was the most recent data available from state Medicaid claims. For all deliveries covered by full Medicaid benefits, member IDs for the birthing individual and infants were linked (stillbirths not included in linkage by the Department of Medical Services) and then made available to the study team. We constructed measures of diagnosed conditions, treatment, and service utilization. The unit of analysis for this study is the delivery. Singleton and multiple gestation births are treated as a single delivery, and multiple deliveries from a single birthing individual during the study period are treated as independent observations with adjustments to standard errors clustered at the patient level. We restricted our sample to birthing individuals receiving MOUD at the time of delivery; to account for mistiming of claims to actual medication provision, we included MOUD claims within 14 days after delivery. After excluding deliveries without valid census tract information to facilitate HOI linkage, the final sample included 1,690 deliveries (See eFigure 1). A University Institutional Review Board approved this study.
Outcome measures
The primary outcomes were postpartum MOUD duration and continuity of MOUD receipt. MOUD was captured by pharmacy claims (i.e., buprenorphine) and procedure codes (i.e., methadone). MOUD duration was operationalized as the number of days to first MOUD discontinuity after delivery, allowing for up to a 30-day gap between MOUD claim dates. An ‘allowable’ 30-day gap was intentionally selected based on clinical experience and to account for mistiming of claims to medication provision. If individuals switched between MOUD type, this was not considered a gap nor discontinuation. Also, overlapping pharmacy claims’ dates were considered to allow for surplus days’ supplies to accumulate (i.e., shifting refill date forward for early refills). MOUD continuity was operationalized as the cumulative number of days covered by MOUD divided by the cumulative number of days enrolled in full benefit Medicaid within the 12 months after delivery.
Main exposure of interest: neighborhood-level SDoH
Neighborhood-level SDoH was measured by the state Department of Health’s Health Opportunity Index (HOI).23 The HOI is derived from dozens of social, economic, educational, demographic, and environmental community variables.24 These variables are sequentially aggregated into a single composite HOI (Supplemental Table 1), similar to other established neighborhood-level SDoH measures.25 Each delivery’s HOI was identified at the census-tract level using the birthing individual’s address at the time of delivery (or if not available, the address corresponding to the closest timeframe to delivery). We describe the variability in outcomes of our cohort by composite HOI terciles. We used the composite HOI as a continuous variable in our multivariable models.
Secondary exposures of interest: Prenatal OUD treatment receipt
Prenatal OUD treatment receipt was captured in detail using two measures: duration of MOUD before delivery and prenatal utilization of comprehensive OUD treatment services. Prenatal MOUD duration was captured by pharmacy and procedure claims in the 12 months preceding delivery (to encompass both individuals who initiated MOUD during pregnancy and individuals who became pregnant already on MOUD). To capture OUD treatment services in addition to MOUD, we used the OUD Treatment Comprehensiveness Index, a measure previously devised by our group 13 based on the work of Edwards et al.26 Nine service categories were included in the index: peer support, case management, provider assessment, behavioral health, medication management, drug monitoring, health screening, family therapy and psychiatric evaluation. If a member had a claim for at least one service within its category during the 12 months prior to birth, that was counted as having received that service category. For each delivery, the OUD Treatment Comprehensiveness Index was calculated as the total number of service categories with at least one received service (range 0 to 9).
Other predictors of postpartum MOUD outcomes
Guided by our study framework (Figure 1), we evaluated in a stepwise manner the contributions of (a) individual-level demographics, (b) parent-infant dyad and health related variables, (c) prenatal OUD treatment (secondary exposure variables) and (d) the HOI (main exposure of interest) on postpartum MOUD outcomes. These variables were selected based on existing literature indicating their associations with OUD treatment outcomes.27,28
Individual-level variables included: age (years), race/ethnicity, and urban/rural location. Race and ethnicity were defined by member self-report on intake survey at the time of Medicaid enrollment (with no missing race/ethnicity values) and categorized as: Black, non-Hispanic, White, non-Hispanic, and other race/ethnicity. Individuals included in the ‘Other’ race/ethnicity category included: individuals self-identifying as Hispanic ethnicity (with any stated race due to small numbers) and any other categories except for Black or White, non-Hispanic.
Parent-infant dyad and health variables included: prenatal maternal diagnosis of comorbid mental health condition or substance use disorder, three or more emergency department visits or overdose event in 12 months preceding delivery, delivery type, preterm delivery, and neonatal abstinence syndrome diagnosis. Comorbid diagnoses and overdose events were captured by ICD-10 codes. Notably, overdose events not involving medical care with a Medicaid claim were not included in our dataset. Delivery type was categorized as vaginal, cesarean or undetermined. Preterm birth was defined as delivery before 37 weeks gestation. Neonatal Abstinence Syndrome (NAS) diagnosis was captured by Medicaid claims for the infant.
Analysis
First, descriptive analyses, including for unadjusted MOUD outcomes, report on the total sample as well as by terciles of the composite HOI. Next, multivariable regression identified factors associated with our MOUD outcomes. Specifically, a Cox Proportional Hazards model was used for the outcome of MOUD duration, and a negative binomial regression model was used for the outcome of MOUD continuity. For MOUD duration, we allowed for delays (up to 14 days) in their MOUD claim dates, and censoring occurred at either death or disenrollment from Medicaid. Following our study framework, we stepwise included our four categories of predictor variables into the models, with the HOI being the last variable added. Hazard ratios and 95% confidence intervals are reported for Cox Proportional Hazards models; rate ratios and 95% confidence intervals were reported for negative binomial regression models. All models controlled for secular changes over time (e.g., COVID-19 pandemic) by including the calendar quarter in which the member delivered. Analyses were conducted in SAS and STATA 17.29,30
Results:
Among the sample of 1,690 Medicaid-covered deliveries occurring between July 1, 2016 to December 31, 2020 who were receiving MOUD, 711 (42%) were to birthing people living in the lowest HOI tercile (indicating high burden of negative SDoH), 647 (38%) in the middle HOI tercile, and 332 (20%) in the highest HOI tercile (Table 1). The majority of deliveries were to White, Non-Hispanic birthing people (89.0%) with less than a tenth to Black, Non-Hispanic birthing people (8.7%) and people with other race/ethnicity. However, these proportions differed across HOI terciles, with 14.4% of deliveries in the lowest tercile being to Black, Non-Hispanic birthing people compared to only 3.9% in the highest tercile.
Table 1.
Demographic, parent-infant dyad health and OUD treatment related variables for study sample of postpartum Medicaid members, by HOI tercile group representing neighborhood-level SDoH.*
| Full sample | Lowest HOI tercile | Middle HOI tercile | Highest HOI tercile | Chi-square test | |
|---|---|---|---|---|---|
|
| |||||
| n=1690 | n=711 | n=647 | n=332 | ||
| Individual-level demographic variables | |||||
| Age | |||||
| 24 years or less | 14.3 | 14.8 | 13.8 | 14.2 | 5.7 |
| 25–34 years | 72.4 | 71.2 | 75.1 | 69.9 | p=0.22 |
| 35 years or more | 13.3 | 14.1 | 11.1 | 16.0 | |
| Race/ethnicity | |||||
| Non-Hispanic White | 89.0 | 84.3 | 92.8 | 53.0 | |
| Non-Hispanic Black | 8.7 | 14.4 | 5.0 | 3.9 | P<0.001 |
| Other | 2.3 | 1.4 | 2.8 | 3.3 | |
| Residence type | |||||
| Urban | 701 | 60.5 | 70.9 | 89.2 | 89.2 |
| Rural | 299 | 39.5 | 29.1 | 10.8 | P<0.001 |
| Parent-infant dyad and health-related variables | |||||
| Comorbid mental health | |||||
| disorder | |||||
| Yes | 62.9 | 63.6 | 60.6 | 66.0 | 3.0 |
| No | 37.1 | 36.4 | 39.4 | 34.0 | p=0.23 |
| Comorbid substance use | |||||
| disorder | |||||
| Yes | 81.7 | 82.7 | 80.2 | 82.2 | 1.5 |
| No | 18.3 | 17.3 | 19.8 | 17.8 | p=0.48 |
| Three or more emergency | |||||
| department visits (prior year) | |||||
| Yes | 29.9 | 30.0 | 29.7 | 30.4 | 0.1 |
| No | 70.1 | 70.0 | 70.3 | 69.6 | p=0.97 |
| Overdose event (prior year) | |||||
| Yes | 1.5 | 1.3 | 1.6 | 1.8 | 0.5 |
| No | 98.5 | 98.7 | 98.5 | 98.2 | p=0.78 |
| Delivery type | |||||
| Cesarean | 38.1 | 37.0 | 38.9 | 1.0 | |
| Vaginal | 56.0 | 56.7 | 55.6 | 55.4 | p=0.91 |
| Undetermined | 5.9 | 6.3 | 5.4 | 5.7 | |
| Preterm delivery | |||||
| Yes | 17.8 | 19.3 | 17.5 | 15.1 | 2.8 |
| No | 82.3 | 80.7 | 82.5 | 84.9 | |
| Infant diagnosis of neonatal | p=0.25 | ||||
| abstinence syndrome | |||||
| Yes | 68.9 | 68.5 | 68.2 | 71.1 | 1.0 |
| No | 31.1 | 31.5 | 31.8 | 28.9 | p=0.62 |
| OUD treatment delivery variables | |||||
| Prenatal MOUD duration | |||||
| None | 2.9 | 2.5 | 3.3 | 3.0 | 4.9 |
| 1 month or less | 9.8 | 9.7 | 11.0 | 7.8 | p=0.77 |
| 1–4 months | 26.7 | 27.7 | 25.0 | 27.7 | |
| 5–8 months | 32.0 | 32.8 | 31.4 | 31.6 | |
| 9–12 months | 28.6 | 27.3 | 29.4 | 29.8 | |
| OUD treatment | |||||
| comprehensiveness index | |||||
| 0 | 13.6 | 11.8 | 15.5 | 13.6 | 9.1 |
| 1 | 9.0 | 8.6 | 10.4 | 7.2 | p=0.33 |
| 2 | 13.8 | 13.2 | 14.4 | 13.9 | |
| 3 | 17.5 | 18.0 | 16.1 | 19.0 | |
| 4+ | 46.2 | 48.4 | 43.7 | 46.4 | |
p values < 0.05 denote significance
At the time of delivery, birthing people had been receiving MOUD for different durations that did not vary significantly across HOI terciles: 9.8% for less than 1 month, 26.7% for 1–4 months, 32.0% for 5–8 months, and 28.6 for at least 9 months. Similarly, almost half of the sample received at least four OUD treatment components, in addition to MOUD, during pregnancy (46.2%), with 13.6% receiving no additional OUD treatment services, 9.0% receiving one service, 13.8% two services, and 17.5% three services (Table 1).
Unadjusted study outcomes did not vary across HOI terciles. For postpartum MOUD duration, 61.5% of deliveries continued MOUD for at least 9 of 12 months after delivery (60.2% in the lowest tercile, 61.7% in the middle tercile, 64.2% in the highest tercile; eFigure 2A). Similarly, for postpartum MOUD continuity, 75% of days in the year after delivery were covered by MOUD receipt for all deliveries (75% days in the lowest tercile, 74% days in the middle tercile, 77% days in the highest tercile; eFigure 2B).
In the stepwise Cox Proportional Hazard models for the outcome of postpartum MOUD duration (Table 2), models 1 (individual-level) and 2 (parent-infant dyad) demonstrated a positive association between young age and MOUD discontinuation (HR 1.262, 95% CI 1.1039, 1.533). However, these associations no longer demonstrated significance in models 3 (OUD treatment) and 4 (neighborhood-level SDoH). In model 3, both duration of prenatal MOUD receipt and the OUD treatment comprehensiveness index were negatively associated with postpartum MOUD discontinuation with a demonstrated dose-response relationship for MOUD duration; in other words, incremental increases in the duration of MOUD received prenatally were associated with longer MOUD durations in the year postpartum. Notably, these significant associations remained stable in model 4, with the HOI not demonstrating an association with the outcome of postpartum MOUD duration (HR 1.39, 95% CI 0.551, 3.512).
Table 2.
Cox proportional hazard ratios of the time-to-initial postpartum MOUD discontinuation among Medicaid members who received MOUD at the time of delivery (n=1,690).*
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
|
| ||||||||
| Individual-level demographic variables | ||||||||
| Age | ||||||||
| 24 years or less | 1.272 | (1.048, 1.544) | 1.262 | (1.039, 1.533) | 1.116 | (0.954, 1.412) | 1.161 | (0.955, 1.413) |
| 25–34 years | -- | -- | -- | -- | -- | -- | -- | -- |
| 35 years or more | 0.843 | (0.681, 1.043) | 0.846 | (0.683, 1.047) | 0.913 | (0.735, 1.133) | 0.911 | (0.734, 1.132) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Hispanic Black | 1.131 | (0.886, 1.443) | 1.096 | 0.856, 1.402) | 1.014 | (0.788, 1.304) | 1.033 | (0.798, 1.338) |
| Other | 1.214 | (0.774, 1.901) | 1.231 | (0.782, 1.939) | 1.032 | (0.637, 1.671) | 1.024 | (0.632, 1.659) |
| Residence type | ||||||||
| Urban | -- | -- | -- | -- | -- | -- | -- | |
| Rural | 1.065 | (0.915, 1.238) | 1.058 | (0.908, 1.233) | 1.094 | (0.939, 1.274) | 1.106 | (0.946, 1.295) |
| Parent-infant dyad and health related variables | ||||||||
| Comorbid mental health | ||||||||
| disorder | ||||||||
| Yes | 0.893 | (0.771, 1.035) | 0.977 | (0.84, 1.136) | 0.976 | (0.84, 1.135) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Comorbid substance use | ||||||||
| disorder | ||||||||
| Yes | 0.863 | (0.721, 1.034) | 0.907 | (0.754, 1.871) | 0.908 | (0.755, 1.092) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Three or more emergency | ||||||||
| department visits (prior year) | ||||||||
| Yes | 1.105 | (0.948, 1.288) | 1.134 | (0.972, 1.323) | 1.132 | (0.971, 1.321) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Overdose event (prior year) | ||||||||
| Yes | 1.314 | (0.827, 2.088) | 1.147 | (0.703, 1.871) | 1.142 | (0.699, 1.864) | ||
| No | -- | -- | -- | -- | ||||
| Delivery type | ||||||||
| Cesarean | 1.045 | (0.904, 1.208) | 1.063 | (0.919, 1.228) | 1.062 | (0.918, 1.228) | ||
| Vaginal | -- | -- | -- | -- | -- | -- | ||
| Undetermined | 1.233 | (0.9.7, 1.41) | 1.121 | (0.84, 1.496) | 1.116 | (0.836, 1.49) | ||
| Preterm delivery | ||||||||
| Yes | (0.889, 1.273) | 1.03 | (0.856, 1.238) | 1.033 | (0.859, 1.244) | |||
| No | -- | -- | -- | -- | -- | -- | ||
| Infant diagnosis of neonata | ||||||||
| abstinence syndrome | ||||||||
| Yes | 0.931 | (0.805, 1.076) | 0.894 | (0.771, 1.037) | 0.892 | (0.769, 1.034) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| OUD treatment delivery | ||||||||
| OUD treatment | ||||||||
| comprehensiveness index | ||||||||
| 0 | -- | -- | -- | -- | ||||
| 1 | 0.857 | (0.653, 1.124) | 0.861 | (0.656, 1.31) | ||||
| 2 | 0.701 | (0.541, 0.907) | 0.702 | (0.543, 0.909) | ||||
| 3 | 0.607 | (0.472, 0.782) | 0.61 | (0.473, 0.786) | ||||
| 4+ | 0.632 | (0.496, 0.805) | 0.635 | (0.498, 0.809) | ||||
| Prenatal MOUD duration | ||||||||
| None | 1.117 | (0.818, 1.526) | 1.113 | (0.815, 1.52) | ||||
| 1 month or less | -- | -- | -- | -- | ||||
| 1–4 months | 0.654 | (0.52, 0.822) | 0.653 | (0.52, 0.821) | ||||
| 5–8 months | 0.501 | (0.396, 0.633) | 0.498 | (0.294, 0.629) | ||||
| 9–12 months | 0.’85 | (0.303, 0.49) | 0.383 | (0.301, 0.488) | ||||
| Neighborhood-level SDoH | ||||||||
| HOI Composite | 1.39 | (0.551, 3.512) | ||||||
Findings are adjusted for quarter of enrollment, and * p values < 0.05 denote significance
In the negative binomial regression models for the outcome of postpartum MOUD continuity (Table 3), in addition to age, race/ethnicity also demonstrated an association with MOUD continuity in models 1 (individual-level) and 2 (parent-infant dyad), with deliveries by Black, Non-Hispanic people having less days covered by MOUD in the year postpartum (RR 0.704, 95% CI 0.517, 0.949). However, this association was no longer significant in models 3 (OUD treatment) and 4 (neighborhood-level SDoH). Similar to postpartum MOUD duration models, prenatal OUD treatment variables (MOUD duration and level of additional OUD treatment services received prenatally) remained significantly associated with the proportion of postpartum days covered by MOUD in both models 3 and 4. However, a dose-response relationship with prenatal MOUD duration was not as clearly demonstrated for this outcome. For example, only MOUD receipt of at least 9 months before delivery was positively associated with postpartum MOUD continuity, whereas prenatal MOUD durations of 8 or less months did not demonstrate a significant association.
Table 3.
Negative binomial regression models of the proportion of postpartum days covered among Medicaid members who received MOUD at the time of delivery (n=1,690).*
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | RR | 95% CI | RR | 95% CI | RR | 95% CI | |
|
| ||||||||
| Individual-level demographic variables | ||||||||
| Age | ||||||||
| 24 years or less | 0.738 | (0.85, 0.931) | 0.741 | (0.586, 0.936) | 0.819 | (0.646, 1.039) | 0.819 | (0.646, 1.039) |
| 25–34 years | -- | -- | -- | -- | -- | -- | ||
| 35 years or more | 1.302 | (0.988, 1.717) | 1.306 | (0.99, 1.721) | 1.223 | (0.928, 1.611) | 1.223 | (0.928, 1.607) |
| Race/ethnicity | ||||||||
| Non-Hispanic White | -- | -- | -- | -- | -- | -- | -- | -- |
| Non-Hispanic Black | 0.676 | (0.497, 0.921) | 0.704 | (0.517, 0.959) | 0.755 | (0.557, 1.022) | 0.756 | (0.554, 1.047) |
| Other | 0.801 | (0.471, 1.364) | 0.788 | (0.461, 1.346) | 0.943 | (0.534, 1.665) | 0.943 | (0.534, 1.632) |
| Residence type | ||||||||
| Urban | -- | -- | -- | -- | -- | -- | -- | |
| Rural | 0.872 | (0.726, 1.048) | 0.872 | (0.725, 1.05) | 0.846 | (0.702, 1.019) | 0.846 | (0.698, 1.003) |
| Parent-infant dyad and health related variables | ||||||||
| Comorbid mental health | ||||||||
| disorder | ||||||||
| Yes | 1.073 | (0.891, 1.292) | 0.96 | (0.794, 1.16) | 0.96 | (0.794, 1.116) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Delivery type | ||||||||
| Cesarean | 0.892 | (0.747, 1.064) | 0.888 | (0.744, 1.059) | 0.888 | (0.744, 1.059) | ||
| Vaginal | -- | -- | -- | -- | -- | -- | ||
| Undetermined | 0.791 | (0.549, 1.141) | 0.881 | (0.61, 1.274) | 0.881 | (0.61, 1.273) | ||
| Three or more emergency | ||||||||
| department visits (prior year) | ||||||||
| Yes | 0.833 | (0.689, 1.008) | 0.833 | (0.689, 1.007) | 0.833 | (0.689, 1.006) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Preterm delivery | ||||||||
| Yes | 0.965 | (0.77, 1.209) | 0.973 | (0.776, 1.222) | 0.974 | (0.775, 1.222) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Infant diagnosis of neonatal | ||||||||
| abstinence syndrome | ||||||||
| Yes | 1.08 | (0.903, 1.292) | 1.121 | (0.935, 1.344) | 1.121 | (0.935, 1.344) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| Comorbid substance use | ||||||||
| disorder | ||||||||
| Yes | 1.225 | (0.978, 1.534) | 1.25 | (0.89, 1.421) | 1.125 | (0.89, 1.421) | ||
| No | - | -- | -- | -- | -- | -- | ||
| Overdose event (prior year) | ||||||||
| Yes | 0.797 | (0.471, 1.347) | 0.884 | (0.501, 1.557) | 0.883 | (0.501, 1.557) | ||
| No | -- | -- | -- | -- | -- | -- | ||
| OUD treatment delivery variables | ||||||||
| OUD treatment | ||||||||
| comprehensiveness index | ||||||||
| 0 | -- | -- | -- | -- | ||||
| 1 | 1.304 | (0.924, 1.842) | 1.305 | (0.924, 1.843) | ||||
| 2 | 1.853 | (1.337, 2.568) | 1.854 | (1.337, 2.57) | ||||
| 3 | 1.849 | (1.33, 2.57) | 1.85 | (1.329, 2.574) | ||||
| 4+ | 1.769 | (1.294, 2.42) | 1.77 | (1.293, 2.423) | ||||
| Prenatal MOUD duration | ||||||||
| None | -- | -- | -- | -- | ||||
| LTE 1 month | 0.757 | (0.439,1.306) | 0.757 | (0.439, 1.305) | ||||
| 1–4 months | 1.053 | (0.627,1.767) | 1.053 | (0.628, 1.766) | ||||
| 5–8 months | 1.519 | (0.9, 2.563) | 1.519 | (0.90, 2.564) | ||||
| 9–12 months | 2.385 | (1.41, 4.034) | 2.385 | (1.409, 4.035) | ||||
| Neighborhood-level SDoH | ||||||||
| Composite HOI | 1.024 | (0.323, 3.247) | ||||||
Findings are adjusted for quarter of enrollment, and * p values < 0.05 denote significance
Comment:
Principal Findings
Study findings highlight the important role that expanding OUD treatment utilization in the prenatal period can play in reducing morbidity and mortality through the year postpartum. Specifically, Medicaid covered birthing people with longer durations of MOUD and receipt of more OUD treatment services before delivery received MOUD for longer durations with fewer gaps in MOUD receipt through the year after delivery. Notably, after accounting for individual-level variables and neighborhood-level SDoH, these associations between receipt of prenatal OUD treatment and postpartum MOUD outcomes remained significant.
Results in the Context of What is Known
OUD is a leading contributor to morbidity and mortality in the postpartum period. MOUD are life-saving, and expanding MOUD utilization is the strongest path forward to combat postpartum morbidity and mortality in the United States.31 As efforts progress to combat the overdose and maternal mortality crises, new, innovative approaches are needed to increase postpartum MOUD access, uptake, and continuity.19 Our study stresses this point, despite finding our measure of neighborhood-level SDoH to not be associated with study outcomes, after accounting for deliveries’ health and OUD treatment related variables. Neighborhood-level HOI measures have demonstrated associations with multiple outcomes in medicine,25 including OUD,32 with neighborhood-level SDoH demonstrating associations with overdose rates 20 and inequities in MOUD access.33 Among birthing people, Krans et al. reported on disparities in MOUD uptake during pregnancy by regions among a Pennsylvania Medicaid cohort, hypothesizing these findings were related to access differences along urban/rural lines.34 Gao et al. expanded this investigation, finding higher county-level percentage of individuals living in poverty to be associated with MOUD receipt, yet other county-level factors did not demonstrate consistent associations with outcomes.15 Our findings extend this existing literature in its investigation of how neighborhood-level SDoH are related to MOUD duration and continuity through the year postpartum. Similar to Gao et al, our global measure of neighborhood-level SDoH was not associated with our outcomes, whereas the magnitude of prenatal OUD treatment received was strongly associated with postpartum MOUD duration and continuity. Nonetheless, the postpartum period is a highly vulnerable time for the parent-infant dyad. Further investigations of how neighborhood-level social factors contribute to postpartum OUD outcomes are warranted in other samples, such as within historically marginalized groups like rural and racial/ethnic minoritized people.
Clinical Implications
The degree of OUD treatment received prenatally by the birthing person emerged as the strongest predictor of both MOUD duration and continuity through the year postpartum. For example, the longer a birthing person had been receiving MOUD before delivery and the higher number of OUD treatment services (e.g., case management, psychiatric care, behavioral health therapy, etc.) received prenatally were incrementally associated with remaining on MOUD longer through the year postpartum. Longer MOUD durations are associated with greater benefits, including reduced overdose risk 35 and improved parent-infant dyad health outcomes.36 Thus, our findings emphasize the urgent need to not only expand MOUD access but also improve OUD treatment quality across the lifecourse, such as from preconception through postpartum for reproductive-age people. Importantly, even though the degree of more comprehensive OUD treatment receipt was associated with improved outcomes in our study, this should not be interpreted as a recommendation for a ‘kitchen sink’ approach to the provision of OUD treatment. Person-centered, harm-reductionist approaches should be prioritized, where patients are offered MOUD, other pharmacologic and non-pharmacologic therapies, as well as recovery-oriented wrap-around services, yet the combination of services provided is tailored to individuals’ needs.37 In line for the push for personalized addiction medicine,38 our findings overall underscore the urgent need for more evidence to provide a data-driven avenue to individualize OUD treatments.
Research Implications
Deliveries by Black, Non-Hispanic birthing people had fewer days covered by MOUD through the year postpartum compared to their White counterparts, when accounting for individual-level demographic and parent-infant dyad health variables. However, after prenatal OUD treatment variables were added into the model, this race association was no longer significant. We hypothesize that this finding is due to our prenatal OUD treatment variables confounding the relationship between race and postpartum MOUD continuity. In other words, the racial disparity in postpartum MOUD continuity appears to be driven by underlying differences in receipt of comprehensive OUD treatment between Black and White non-Hispanic individuals. This finding juxtaposes evidence highlighting the persistence of racial disparities in postpartum OUD outcomes, even after accounting for prenatal MOUD receipt,13,27 reflecting disparities seen nationally among pregnant and non-pregnant OUD cohorts.39 Racial and ethnic disparities are pervasive throughout the OUD treatment cascade, from diagnosis through treatment and recovery.9 The opioid epidemic, which once disproportionately impacted White populations has shifted, now with opioid-related deaths among Black individuals superseding those among White individuals.40 More work is needed to elucidate evidence-based, bias-free, person-centered approaches to increase OUD treatment utilization beyond the pregnancy window, to encompass the entire preconception and postpartum periods, that simultaneously target proximal consequences of structural and systemic racism.
Strengths and Limitations
While we utilized data, which are the most extensive and detailed patient-level data available for our state, our study findings should be interpreted within the inherent limitations of data from billing databases not originally developed for research. Some recovery services are not paid for by Medicaid (e.g., self-help groups) and some people with OUD do not seek medical care, and if they do access treatment, they may seek care at programs that do not allow Medicaid coverage. Additionally, our sample identified predominantly as White, non-Hispanic. Together, these factors limit generalizability of our findings. Yet Medicaid claims encompass the most complete information on substance use disorder treatment outcomes that are typically unavailable in other sources, including those that do account for the lack of a clinician diagnosis, such as the National Survey on Drug Use and Health.41 Additionally, many Medicaid members disenroll or have lapses in coverage due to loss of eligibility, relocations outside of the state, or other reasons. Although postpartum Medicaid enrollment was included as a covariate in all models, care utilization that occurs during these lapses of coverage is not observed. Additionally, we assumed that active prescription fills or dispensing of MOUD were equivalent to medication consumption; MOUD adherence is not captured by claims data. Lastly, both state Medicaid expansion and the onset of the COVID-19 pandemic occurred during our study period. We addressed these notable events in our models by adjusting for calendar quarter, yet it is possible that residual confounding may have introduced bias.
Conclusions
Untreated OUD is a leading cause of pregnancy-related mortality, especially in the highly vulnerable 1-year postpartum period. Our results underscore how supporting communities, health systems, and families in expanding equitable access to MOUD and high-quality, evidence-based OUD treatments across the lifecourse should be highly prioritized by policymakers and public health endeavors. In the ongoing overdose crisis, bold and decisive action is urgently needed to eradicate barriers to long-term MOUD receipt for all, such as addiction stigma,42 systemic and structural racism.16
Supplementary Material
AJOG at a Glance:
Why was this study conducted? To identify the association of neighborhood-level social determinants of health and prenatal opioid use disorder treatment receipt with medication for opioid use disorder outcomes through the year postpartum among a cohort of birthing people.
What are the key findings? This retrospective cohort study linked Medicaid claims (2016–2020) with a census tract-level measure of 32 social determinants for members receiving medication for opioid use disorder at delivery (N=1,690). Prenatal receipt of more comprehensive opioid use disorder treatment and/or longer prenatal medication for opioid use disorder duration was associated with improved 1-year postpartum opioid use disorder medication treatment outcomes across neighborhood levels of social determinants.
What does this study add to what is already known? To improve outcomes of postpartum people with opioid use disorder, interventions should prioritize the expansion of medication treatments and targeting of neighborhood-level social determinants.
Acknowledgements
The authors would like to acknowledge Chethan Bachireddy, Ashley Harrell and Neil McCray with the Virginia Department of Medical Assistance Services, and Xue Zhao with Virginia Commonwealth University, Richmond, VA, for their assistance with data interpretation and manuscript preparation.
Primary Funding
This study was supported by the Thomas F. and Kate Miller Jeffress Memorial Trust. NIDA award No. K23 DA053507 from the National Institute on Drug Abuse supports Dr. Caitlin E. Martin. The Substance Use Disorder Prevention that Promotes Opioid Use Recovery and Treatment Act from the Virginia Department of Medical Assistance Service supported Dr. Peter Cunningham. The Addiction and Recovery Treatment Services Program and Substance Use Disorder Prevention that Promotes Opioid Use Recovery and Treatment Act from the Virginia Department of Medical Assistance Services supported Erin Britton and Dr. Peter Cunningham.
Footnotes
Disclosure of potential conflicts of interest
All the authors declare no potential financial or non-financial conflicts of interest.
Tweetable statement: To improve outcomes of postpartum people with opioid use disorder, interventions should prioritize the expansion of medication treatments and targeting of neighborhood-level social determinants.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Trost SL, Beauregard J, Njie F. Pregnancy-Related Deaths: Data from Maternal Mortality Review Committees in 36 US States, 2017–2019. 2022. [Google Scholar]
- 2.Gemmill A, Kiang MV, Alexander MJ. Trends in pregnancy-associated mortality involving opioids in the United States, 2007–2016. American journal of obstetrics and gynecology. Jan 2019;220(1):115–116. doi: 10.1016/j.ajog.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 3.Goldman-Mellor S, Margerison CE. Maternal drug-related death and suicide are leading causes of postpartum death in California. American journal of obstetrics and gynecology. Nov 2019;221(5):489.e1–489.e9. doi: 10.1016/j.ajog.2019.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smid MC, Stone NM, Baksh L, et al. Pregnancy-Associated Death in Utah: Contribution of Drug-Induced Deaths. Obstetrics and gynecology. Jun 2019;133(6):1131–1140. doi: 10.1097/aog.0000000000003279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margerison CE, Roberts MH, Gemmill A, Goldman-Mellor S. Pregnancy-Associated Deaths Due to Drugs, Suicide, and Homicide in the United States, 2010–2019. Obstetrics & Gynecology. 2022;139(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Admon LK, Dalton VK, Kolenic GE, et al. Comparison of Delivery-Related, Early and Late Postpartum Severe Maternal Morbidity Among Individuals With Commercial Insurance in the US, 2016 to 2017. JAMA network open. 2021;4(12):e2137716–e2137716. doi: 10.1001/jamanetworkopen.2021.37716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies--tackling the opioid-overdose epidemic. The New England journal of medicine. May 29 2014;370(22):2063–6. doi: 10.1056/NEJMp1402780 [DOI] [PubMed] [Google Scholar]
- 8.Tan SB, deSouza P, Raifman M. Structural Racism and COVID-19 in the USA: a County-Level Empirical Analysis. Journal of racial and ethnic health disparities. Jan 19 2021:1–11. doi: 10.1007/s40615-020-00948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entress RM. The intersection of race and opioid use disorder treatment: A quantitative analysis. Journal of substance abuse treatment. Aug 19 2021:108589. doi: 10.1016/j.jsat.2021.108589 [DOI] [PubMed] [Google Scholar]
- 10.Schuler MS, Dick AW, Stein BD. Growing racial/ethnic disparities in buprenorphine distribution in the United States, 2007–2017. Drug and alcohol dependence. 2021/06/01/ 2021;223:108710. doi: 10.1016/j.drugalcdep.2021.108710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peeler M, Gupta M, Melvin P, et al. Racial and Ethnic Disparities in Maternal and Infant Outcomes Among Opioid-Exposed Mother-Infant Dyads in Massachusetts (2017–2019). American journal of public health. Dec 2020;110(12):1828–1836. doi: 10.2105/ajph.2020.305888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiff DM, Nielsen T, Hoeppner BB, et al. Assessment of Racial and Ethnic Disparities in the Use of Medication to Treat Opioid Use Disorder Among Pregnant Women in Massachusetts. JAMA network open. May 1 2020;3(5):e205734. doi: 10.1001/jamanetworkopen.2020.5734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin CE, Britton E, Shadowen H, et al. Disparities in opioid use disorder-related hospital use among postpartum Virginia Medicaid members. J Subst Use Addict Treat. Feb 2023;145:208935. doi: 10.1016/j.josat.2022.208935 [DOI] [PubMed] [Google Scholar]
- 14.Shim RS. Dismantling Structural Racism in Psychiatry: A Path to Mental Health Equity. The American journal of psychiatry. Jul 2021;178(7):592–598. doi: 10.1176/appi.ajp.2021.21060558 [DOI] [PubMed] [Google Scholar]
- 15.Gao YA, Drake C, Krans EE, Chen Q, Jarlenski MP. Explaining Racial-ethnic Disparities in the Receipt of Medication for Opioid Use Disorder During Pregnancy. Journal of addiction medicine. Mar 3 2022;doi: 10.1097/adm.0000000000000979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiff DM, Work EC, Foley B, et al. Perinatal Opioid Use Disorder Research, Race, and Racism: A Scoping Review. Pediatrics. Mar 1 2022;149(3)doi: 10.1542/peds.2021-052368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giuse NBMD Koonce TYMPH, Kusnoor SVP, et al. Institute of Medicine Measures of Social and Behavioral Determinants of Health: A Feasibility Study. American journal of preventive medicine. 2016;52(2):199–206. doi: 10.1016/j.amepre.2016.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolf SH, Johnson RE, Phillips RL, Philipsen M. Giving Everyone the Health of the Educated: An Examination of Whether Social Change Would Save More Lives Than Medical Advances. American journal of public health. 2007/04/01 2007;97(4):679–683. doi: 10.2105/AJPH.2005.084848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin CE, Parlier-Ahmad AB. Addiction treatment in the postpartum period: an opportunity for evidence-based personalized medicine. International review of psychiatry (Abingdon, England). 2021:1–12. doi: 10.1080/09540261.2021.1898349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casillas SM, Pickens CM, Stokes EK, Walters J, Vivolo-Kantor A. Patient-Level and County-Level Trends in Nonfatal Opioid-Involved Overdose Emergency Medical Services Encounters - 491 Counties, United States, January 2018-March 2022. MMWR Morbidity and mortality weekly report. Aug 26 2022;71(34):1073–1080. doi: 10.15585/mmwr.mm7134a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogojiaku CN, Allen JC, Anson-Dwamena R, et al. The Health Opportunity Index: Understanding the Input to Disparate Health Outcomes in Vulnerable and High-Risk Census Tracts. International journal of environmental research and public health. Aug 10 2020;17(16)doi: 10.3390/ijerph17165767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler DC, Boyle J, Barsell DJ, et al. Neighborhood Deprivation is Associated with Increased Risk of Prenatal Smoke Exposure. Prevention science : the official journal of the Society for Prevention Research. Feb 18 2022;doi: 10.1007/s11121-022-01355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VDH. Virginia Health Opportunity Index. Virginia Department of Health Office of Health Equity. Accessed 7 February, 2021. https://apps.vdh.virginia.gov/omhhe/hoi/ [Google Scholar]
- 24.Winter JD, Kerns JW, Winter KM, Richards A, Sabo RT. Community, Social, and Facility Factors and Long-stay Antipsychotic Use. Clinical gerontologist. Apr 21 2022:1–9. doi: 10.1080/07317115.2022.2063777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Acevedo-Garcia D, Noelke C, McArdle N, et al. Racial And Ethnic Inequities In Children’s Neighborhoods: Evidence From The New Child Opportunity Index 2.0. Health affairs (Project Hope). Oct 2020;39(10):1693–1701. doi: 10.1377/hlthaff.2020.00735 [DOI] [PubMed] [Google Scholar]
- 26.Edwards JR, Knight DK, Flynn PM. Organizational correlates of service availability in outpatient substance abuse treatment programs. The journal of behavioral health services & research. Oct 2011;38(4):432–43. doi: 10.1007/s11414-010-9231-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiff DM, Nielsen TC, Hoeppner BB, et al. Methadone and Buprenorphine Discontinuation among Postpartum Women with Opioid Use Disorder. American journal of obstetrics and gynecology. Apr 9 2021;doi: 10.1016/j.ajog.2021.04.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parlier-Ahmad AB, Radic M, Svikis DS, Martin CE. Short communication: Relationship between social determinants and opioid use disorder treatment outcomes by gender. Drug and alcohol dependence. Jan 28 2022;232:109337. doi: 10.1016/j.drugalcdep.2022.109337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institution Inc. NC: SAS Institute Inc, ed. SAS® 9.4 Guide to Software Updates and Product Changes. 2013. [Google Scholar]
- 30.StataCorp. Stata: Release 17. StataCorp LLC; 2021. [Google Scholar]
- 31.Martin CE, Scialli A, Terplan M. Unmet substance use disorder treatment need among reproductive age women. Drug and alcohol dependence. Oct 28 2019:107679. doi: 10.1016/j.drugalcdep.2019.107679 [DOI] [PubMed] [Google Scholar]
- 32.Joudrey PJ, Kolak M, Lin Q, Paykin S, Anguiano V, Jr., Wang EA. Assessment of Community-Level Vulnerability and Access to Medications for Opioid Use Disorder. JAMA network open. Apr 1 2022;5(4):e227028. doi: 10.1001/jamanetworkopen.2022.7028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goedel WC, Shapiro A, Cerdá M, Tsai JW, Hadland SE, Marshall BDL. Association of Racial/Ethnic Segregation With Treatment Capacity for Opioid Use Disorder in Counties in the United States. JAMA network open. Apr 1 2020;3(4):e203711. doi: 10.1001/jamanetworkopen.2020.3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krans EE, Kim JY, James AE 3rd, Kelley D, Jarlenski MP. Medication-Assisted Treatment Use Among Pregnant Women With Opioid Use Disorder. Obstetrics and gynecology. May 2019;133(5):943–951. doi: 10.1097/aog.0000000000003231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA network open. 2020;3(2):e1920622–e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krans EE, Kim JY, Chen Q, et al. Outcomes associated with the use of medications for opioid use disorder during pregnancy. Addiction (Abingdon, England). Dec 2021;116(12):3504–3514. doi: 10.1111/add.15582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis LP, Parlier-Ahmad AB, Scheikl M, Martin CE. An Integrated Care Model for Pregnant and Postpartum Individuals Receiving Medication for Opioid Use Disorder. Journal of addiction medicine. Aug 16 2022;doi: 10.1097/adm.0000000000001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkow ND. Personalizing the Treatment of Substance Use Disorders. The American journal of psychiatry. Feb 1 2020;177(2):113–116. doi: 10.1176/appi.ajp.2019.19121284 [DOI] [PubMed] [Google Scholar]
- 39.Lagisetty PA, Ross R, Bohnert A, Clay M, Maust DT. Buprenorphine Treatment Divide by Race/Ethnicity and Payment. JAMA psychiatry. 2019;76(9):979–981. doi: 10.1001/jamapsychiatry.2019.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furr-Holden D, Milam AJ, Wang L, Sadler R. African Americans now outpace whites in opioid-involved overdose deaths: a comparison of temporal trends from 1999 to 2018. Addiction (Abingdon, England). Mar 2021;116(3):677–683. doi: 10.1111/add.15233 [DOI] [PubMed] [Google Scholar]
- 41.SAMHSA. Data from: National Survey of Drug Use and Health. 2019. Rockville, MD. [Google Scholar]
- 42.Schmidt R, Wolfson L., Stinson J., Poole N., Greaves L. Mothering and Opioids: Addressing Stigma and Acting Collaboratively. 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


