Abstract
Objective
Proper inflammation resolution is crucial to prevent runaway inflammation during sepsis and reduce sepsis-related mortality/morbidity. Previous studies suggest that deleting TRAM, a key TLR4 signaling adaptor, can reprogram the first inflammatory responder cell-neutrophil from an inflammatory state to a resolving state. In this study, we aim to examine the therapeutic potential of TRAM-deficient neutrophils in vivo with recipient mice undergoing experimental sepsis.
Material and methods
Wild-type or Tram−/− mice were intraperitoneally injected with cecal slurry to induce either severe or mild sepsis. Phenotypic examinations of sepsis and neutrophil characteristics were examined in vivo and ex vivo. The propagations of resolution from donor neutrophils to recipient cells such as monocytes, T cells, and endothelial cells were examined through co-culture assays in vitro. The efficacies of Tram−/− neutrophils in reducing inflammation were studied by transfusing either wild-type or Tram−/− neutrophils into septic recipient mice.
Results
Tram−/− septic mice had improved survival and attenuated injuries within the lung and kidney tissues as compared to wild-type septic mice. Wild-type septic mice transfused with Tram−/− resolving neutrophils exhibited reduced multi-organ damages and improved cellular homeostasis. In vitro co-culture studies revealed that donor Tram−/− neutrophils can effectively propagate cellular homeostasis to co-cultured neighboring monocytes, neutrophils, T cells as well as endothelial cells.
Conclusions
Neutrophils with TRAM deletion render effective reprogramming into a resolving state beneficial for ameliorating experimental sepsis, with therapeutic potential in propagating cellular and tissue homeostasis as well as treating sepsis.
Keywords: Sepsis, Neutrophils, TRAM, Adoptive transfer, Therapeutics
Introduction
Sepsis is a systemic inflammatory response to infections, ultimately leading to multi-organ failure and death, with > 10 million annual mortalities worldwide [1]. Sepsis can be caused by polymicrobial infection-related immune dysregulations. With the global COVID-19 pandemic, the recent incidences of both severe and mild sepsis have been increasing [2], posing tremendous financial burdens on the healthcare system. Despite the dire medical need, there is no effective cure for sepsis. Dysregulated inflammatory responses including infiltration of monocytes and neutrophils; excessive secretion of pro-inflammatory mediators such as neutrophil elastase [3–7], likely contribute to vasculature leakages and multi-organ injuries during the course of sepsis. However, no effective approach is currently available to properly mitigate sepsis-associated inflammation to prevent multi-organ injuries and reduce long-term complications.
Neutrophils are the very first responder during the course of sepsis pathogenesis, with complex roles in promoting inflammation as well as facilitating inflammation resolution [8, 9]. However, detailed mechanisms controlling the fate of neutrophil inflammation or resolution are not well-understood. We previously reported that neutrophils can be reprogrammed into the revolving state through the pharmacological treatment with 4-PBA (a peroxisome stabilizer) or the genetic depletion of TRAM (an adaptor molecule in TRIF-mediated TLR4 downstream signaling pathway) in vitro. The reprogrammed neutrophils express elevated pro-resolving mediators (CD200R, CD86, ResolvinD1, and SerpinB1) and exhibit enhanced antimicrobial capacity [10]. Together with independent findings [11–13], we hypothesize that reprogrammed resolving neutrophils may provide therapeutic effects for treating experimental sepsis.
To test this hypothesis, we designed in vivo studies comparing wild-type and Tram−/− mice during experimental sepsis, with the well-developed cecal slurry (CS)-induced sepsis model. We further performed transfusion studies comparing the efficacies of donor wild type and Tram−/− neutrophils in treating experimental sepsis. Complementing our in vivo study design, we characterized the propagation of resolution among donor neutrophils with recipient neighboring cells including monocytes and endothelial cells through the in vitro co-culture studies. Collectively, our study demonstrated the resolution propagation mediated by Tram−/− neutrophils and the therapeutic potential of Tram−/− neutrophils in treating experimental sepsis.
Materials and methods
Mice and bacteria
Both adult male and female Tram−/− and the respective wild-type (WT) C57BL/6 as well as BALB/c mice (6–8 weeks of age) were as described [14] and housed at the animal facility of Virginia Tech. All animal protocols were approved by the Institute for Animal Care and Use Committee. Escherichia coli (# 25922) was purchased from ATCC.
Murine sepsis model
Sepsis was induced by intraperitoneal injection of cecal slurry in mice as previously described [15–17]. In brief, cecal contents were collected and weighed from the cecum of donor C57BL/6 mice, and the slurry was made by suspending fecal materials in PBS. The slurry was strained through mesh strainers (in the order of 380, 190, 100, and 70 μm) and prepared at a final concentration of 125 mg/ml of 10% glycerol in PBS. For severe sepsis, CS was injected into mice intraperitoneally with a 28-gauge needle at a dose of ~ 1 mg/g of body weight (BW). The survival was monitored for 7 days. For mild sepsis, mice were subjected to CS at a dose of ~ 0.7 mg/g of BW and observed for 14 days. On day 14, mice were injected intravenously with 5 × 106 E.coli in 200 μl PBS 30 min prior to sacrifice, and tissues were harvested for subsequent analyses.
Bone marrow-derived neutrophil and monocyte/macrophage isolation and culture
Neutrophils were purified using 62.5% Percoll gradient from WT and TRAM knock-out (KO) murine bone marrow (BM), and BM monocytes were isolated from WT mice as we described previously [10, 14, 18]. Neutrophils and monocytes were then cultured in the complete RPMI medium (containing 10% FBS, 1% L-glutamine, and 1% penicillin/streptomycin) supplemented with 100 ng/ml G-CSF and 10 ng/ml M-CSF, respectively. Monocytes were cultured for 5 days with the refreshing culture medium every 2 days as reported before [18]. For neutrophil resolving phenotype reprogramming, WT BM neutrophils were stimulated with 4-PBA (1 mM) (TOCRIS, # 2682) for 24 h or 2 h followed by a 24-h challenge with fMLP (1 μM) (Sigma, # F3506). For priming and inducing sepsis-like phenotype in vitro, WT BM neutrophils were treated with super-low-dose (100 pg/ml) and high-dose (100 ng/ml) of LPS (Sigma, # L2630), respectively, for 24 h. For the elastase inhibitory assay, WT and TRAM KO BM neutrophils were treated with 1 μM fMLP together with or without 1 μg/ml sivelestat (Selleckchem, # S8136) for 24 h. For the NAD+ assay, WT and TRAM KO BM neutrophils were stimulated with LPS (100 ng/ml) (Sigma, # L2630) for 24 h.
Adoptive neutrophil transfer
BM neutrophils from WT and TRAM KO mice were isolated by a magnetic bead-based neutrophil enrichment kit (STEMCELL, # 19762) with > 92% purity. After three time-washes with PBS, neutrophils were suspended in PBS for injection. WT mice with CS-induced mild sepsis were transfused twice with 5 × 106 WT or TRAM KO neutrophils in 200 μl of PBS twice weekly during the first week through intravenous injection during the chronic model of sepsis. On day 14, mice were sacrificed and tissues were harvested for further analyses.
Spleen-derived T cell purification
Naïve T cells were prepared using a T cell isolation kit (STEMCELL, # 19851) with > 97% purity from the spleens of BALB/c mice. The purified cells were labeled with CFSE dye (Invitrogen, # C34554) for the proliferation assay then cultured in the complete medium supplemented with HEPES, β-mercaptoethanol, and anti-CD28 antibodies (2.5 μg/ml; Bio X Cell, # BE0015-1) in an anti-CD3 antibody pre-coated plate (1 μg/ml; Bio X Cell, # BE0001-1).
Neutrophil co-culture experiments with neutrophils, macrophages, or T cells
Donor cells (WT neutrophils, TRAM KO neutrophils, or 4-PBA reprogrammed-WT neutrophils) were labeled with a red fluorescent dye (Sigma, # PKH26GL). For neutrophilneutrophil co-cultures, freshly harvested WT naïve neutrophils (recipient cells) were co-cultured with donor cells for 24 h at a ratio of 1:2 in the G-CSF supplemented complete medium. For monocyte/macrophage-neutrophil co-cultures, WT monocytes/macrophages (recipient cells) from 5 day-cultures were co-cultured with donor cells for 24 h at a ratio of 2:1 in the M-CSF supplemented complete medium. For T cell-neutrophil co-cultures, freshly harvested T cells (recipient cells) were co-cultured with donor cells for 4 days at a ratio of 2:1 in the anti-CD3/anti-CD28 antibody-supplemented conditions.
Endothelial cell culture and co-culture
bEnd.3 endothelial cells (# CRL-2299) were purchased from ATCC, and cell passage numbers from 10 to 15 were used in this study. Endothelial cells were cultured as ATCC protocol instructed in the complete DMEM medium (including 10% FBS and 1% penicillin/streptomycin). After growing to ~ 80% of confluency, endothelial cells (recipient cells) were co-cultured with donor cells (WT neutrophils, TRAM KO neutrophils, or 4-PBA reprogrammed-WT neutrophils) at a ratio of 1:10 or cultured in conditioned media from neutrophil cultures (based on the assay settings) with the additive of 0.2 μM Oleoyl-L-α-lysophosphatidic acid sodium salt (LPA; Sigma, # L7260) for 24 h.
FACS analyses
Splenocytes and lung cells in single-cell suspension (prepared as described [19]) from in vivo experiments as well as recipient cells (naïve neutrophils, monocytes/macrophages, T cells, and endothelial cells) from in co-cultures were assessed with the flow cytometry. After blocked with the Fc blocker (1:200 dilution; BD Biosciences, # 553141), cells were stained with anti-Ly6G (1:300 dilution; BioLegend, # 127606), anti-Ly6C (1:300 dilution; BioLegend, # 128018), anti-CD11b (1:200 dilution; BioLegend, # 101226), anti-CD86 (1:200 dilution; BioLegend, # 105006), anti-CD200R (1:200 dilution; BioLegend, # 123908), anti-CD24 (1:200 dilution; BioLegend, # 101813), anti-CD3 (1:200 dilution; BioLegend, # 100221), anti-CD4 (1:200 dilution; BioLegend, # 116006), anti-CD8 (1:200 dilution; BioLegend, # 100712), anti-CD31 (1:200 dilution; BioLegend, # 160204), anti-VE-Cadherin (1:200 dilution; BioLegend, # 138011), anti-ESAM (1:200 dilution; BioLegend, # 136207), or anti-JAM-1 antibodies (1:200 dilution; BioLegend, # 158503). Before samples were analyzed with a FACSCanto II (BD Biosciences), cells were resuspended in FACS buffer containing Propidium Iodide (PI; 1:400 dilution; Thermo Fisher Scientific, # P3566) for testing cell viability. In some experiments, cells were first stained with anti-CD3, anti-CD4, and anti-CD8 antibodies as well as the Live/Dead dye (Invitrogen, # L34975) then fixed and permeabilized using a transcription factor staining buffer set (Thermo Fisher, # 00-5523-00), followed by staining with anti-Foxp3 (1:200 dilution; Biolegend, # 126403) and flow cytometry. Data were analyzed with FlowJo (Ashland, OR).
Quantification of protein in bronchoalveolar lavage fluid (BALF)
BALF from WT and TRAM−/− septic mice was collected as described previously [19]. Briefly, 500 μl 0.9% NaCl with 0.1 mM EDTA was injected from the murine tracheas to the lungs using a 21-gauge plastic needle to wash lungs 3 times. The recovered BALF was then spun down to remove cell debris. The protein concentration of BALF was tested with a BCA protein assay kit (Thermo Fisher, # 23227) and then measured by a plate reader at 562 nm.
ELISA
The plasma samples from in vivo experiments were harvested and the supernatant of in vitro cell cultures as described above was collected. The levels of urea nitrogen (BUN; Thermo Fisher, # EIABUN), creatinine (Cayman, # 700460), neutrophil elastase (R&D Systems, # MELA20), sphingosine-1-phosphate receptor 3 (S1P3; NOVUS, # NBP2–82428) in samples were measured by ELISA kits.
NAD+ assay
LPS-treated WT and TRAM KO BM neutrophils were lysed and the levels of intracellular NAD+ were determined with a NAD/NADH quantification kit (Sigma, # MAK037) following manufacturer’s instructions.
Statistical analyses
GraphPad Prism 9 (La Jolla, CA) was used to perform statistical analysis. Data were representative of at least three independent experiments and were expressed as means ± SD. The statistical significance was determined by the Student’s t-test (for two groups) or one-way analysis of variance (ANOVA) (for multiple groups). P < 0.05 was considered statistically significant.
Results
Mice with TRAM deletion are more resistant to severe sepsis and less susceptible to sepsis-induced tissue injuries
We previously demonstrated that Tram−/− neutrophils constitutively exhibit pro-resolving potentials with enhanced expression of homeostatic mediators including CD200R, CD86, RvD1, and SerpinB1 [10]. CD86 and CD200R are cell surface markers for resolving neutrophils: CD86 on the myeloid cells is associated with Treg cell differentiation to maintain immune homeostasis [20], and CD200R is positively correlated with inflammation resolution [12]. In addition, Tram−/− mice are protected from DSS-induced acute colitis-related death and tissue damage [14]. Hence, we hypothesized that TRAM deficiency may similarly render immunomodulatory protection against experimental sepsis. To evaluate the role of TRAM in sepsis pathogenesis, we intraperitoneally (i.p.) injected the high dose of cecal slurry (CS; ~ 1 mg/g of BW) to both WT and Tram−/− mice to induce severe sepsis [16, 17]. We observed that consistent with our and others’ reports [14, 16], the survival of WT septic mice was ~ 30% with all the death happening within the first three days after the injection, while all Tram−/− septic mice survived throughout the 7 day observation period (Fig. 1a).
Fig. 1.
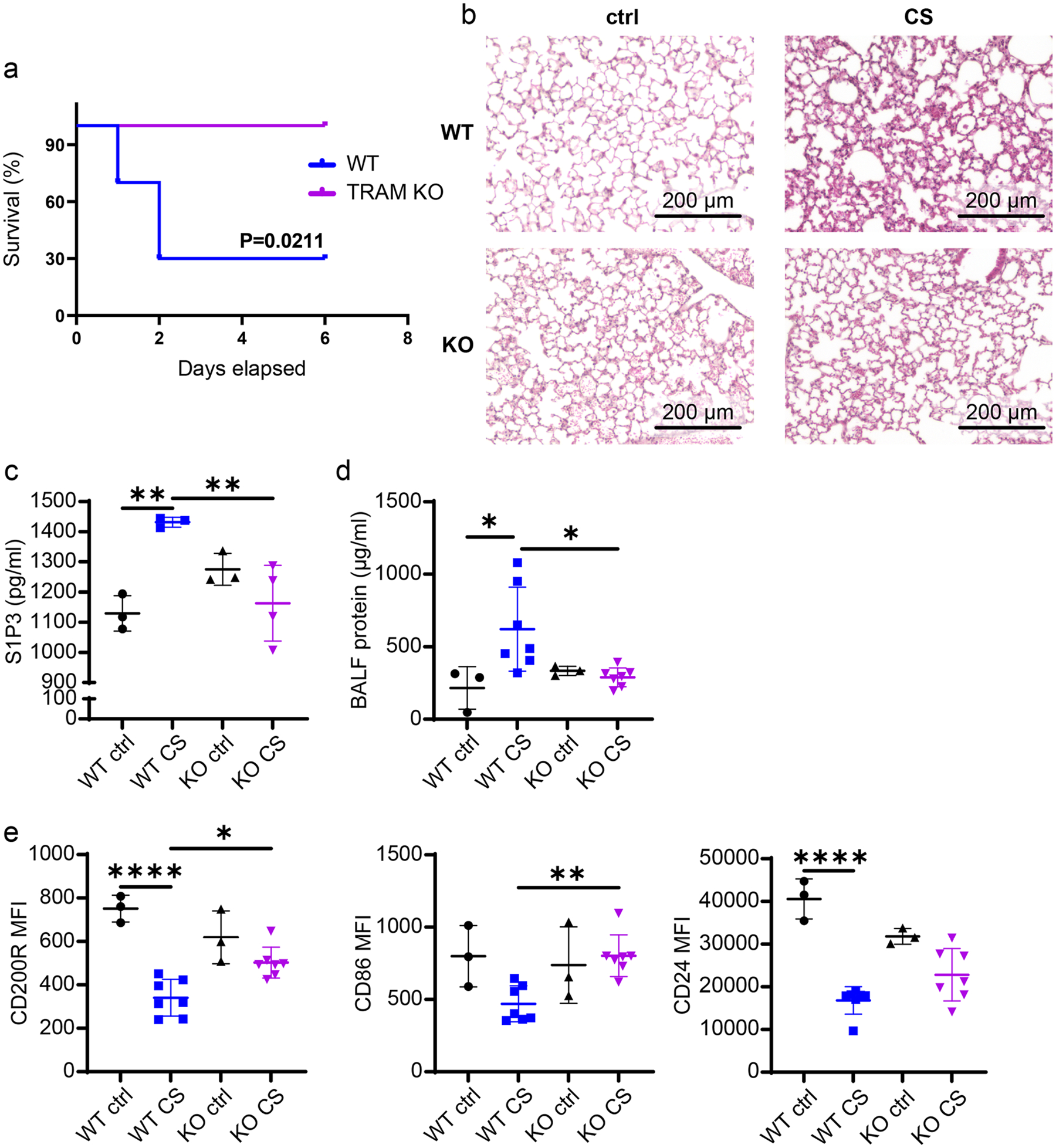
TRAM KO septic mice exhibited improved resolving signatures on neutrophils and ameliorated tissue injuries. a The Kaplan–Meier survival plot of WT and TRAM KO male mice with severe sepsis induced by the high dose CS (~ 1 mg/g of BW) i.p. injection. b To examine the long-term effects of mild sepsis, WT and TRAM KO male mice were i.p. injected with the low dose CS (~ 0.7 mg/g of BW) to induce mild sepsis. Thirty minutes prior to the time of sacrificing (day 14), all the mice (WT and TRAM KO, control and CS-induced septic mice) were subjected to i.v. injection of E. coli. H&E-stained pulmonary sections from WT control (upper left), WT septic (upper right), KO control (bottom left), and KO septic male mice (bottom right). c The plasma levels of S1P3 and d the protein concentration in BALFs from 4 groups of mice. e Flow cytometry analysis of the expression of CD200R, CD86, and CD24 on spleen-resident neutrophils harvested from 4 groups of mice. ****P < 0.0001, **P < 0.01, *P < 0.05 using Log-rank test (a) and one-way ANOVA test followed by the post-hoc Sidak multiple comparisons test (c–e). n = 3–7. Image scale bars = 200 μm. ctrl control. KO TRAM KO. CS cecal slurry i.p. injected
Next, to examine the long-term effect of sepsis, we induced mild sepsis in both strains of mice by i.p. injection with a lower dose of CS (~ 0.7 mg/g of BW) [15], followed by the intravenous (i.v.) injection of E. coli 30 min prior to the time of sacrifice. All mice survived by the termination day (Day 14). Consistent with the previous clinical observation that sepsis can cause acute lung injury (ALI) [21], we found compromised lung morphology with altered alveolar structures in WT septic mice as compared to their control counterparts (Fig. 1b). On the other hand, the disrupted alveolar integrity associated with sepsis was less apparent in Tram−/− mice (Fig. 1b). Next, we examined key plasma markers associated with tissue damages. The levels of S1P3, a biomarker for lung injury and pulmonary fibrosis [22, 23], were significantly increased in plasma from WT septic mice as compared to WT control mice regardless of gender. In contrast, Tram−/− mice were less susceptible to sepsis-mediated lung damage as assessed by a dampened induction of S1P3 (Figs. 1c and S1a). We also assessed lung vasculature leakage by measuring leaked protein levels in bronchoalveolar lavage fluid (BALF) and observed diminished levels in Tram−/− mice compared with WT mice (Fig. 1d) (though less apparent in female groups) (Fig. S1b). Taken together, our data suggest that TRAM deletion facilitates the maintenance of tissue homeostasis under septic conditions.
We then examined the cellular levels of resolving mediators from harvested neutrophils. As shown in Fig. 1e, cecalslury injection caused a twofold reduction in the levels of CD200R as well as CD24 in WT neutrophils. In contrast, there was only a negligible reduction of CD200R and CD24 in Tram−/− mice following CS injection (Fig. 1e). This phenomenon is similarly observed in female mice. As shown in Fig. S1c, CS-induced sepsis reduced the expression of CD200R, CD86, and CD24 on spleen-resident neutrophils from WT female mice. However, such an effect was attenuated in Tram−/− septic female mice (Fig. S1c). Flow cytometry further revealed significantly lower neutrophil infiltration in spleens from female Tram−/− septic mice as compared to WT septic mice (Fig. S1d). These results confirm that TRAM deletion ameliorates sepsis-related tissue damage and sustains immune homeostasis during sepsis.
Tram−/− neutrophils attenuate tissue damage and rejuvenate the immune system in WT septic mice
Our in vivo data complemented our in vitro studies revealing that high-dose LPS seen in septic conditions potently drives an “exhausted” neutrophil state with reduced resolving mediators such as Cd200r and Cd86 [10], while simultaneously expressing higher levels of Cd11b (Itgal) (Fig. S2a, b). This is in contrast with neutrophils primed with super-low-dose LPS which developed into an immune-enhanced population with reduced Cd11b (Fig. S2a, b). Re-analyses of our existing single-cell sequencing data revealed that WT neutrophils had only a minor subset of resolving neutrophils that correlates with reduced Tram expression (Fig. S2c). This is consistent with our previous report that TRAM deletion enables the expansion of resolving neutrophils [10].
We further observed that Tram−/− neutrophils are more resilient to endotoxin-induced metabolic dysregulation as reflected in sustained cellular NAD+ levels in Tram−/− neutrophils (Fig. S3). In contrast, the high-dose LPS challenge led to a drastic reduction of NAD+ levels in WT neutrophils. Enhanced resolving mediators such as CD200R, CD86, and NAD+ may collectively communicate with neighboring cells to propagate immune homeostasis [10, 24, 25]. Based on these mechanistic studies, we hypothesized that Tram−/− neutrophils may be capable of actively maintaining and propagating immune homeostasis and alleviating tissue damage. To test this, we set up the transfusion experiment as illustrated in Fig. 2a. As compared to septic mice transfused with WT neutrophils, septic mice transfused with Tram−/− neutrophils exhibited significantly reduced levels of plasma creatinine, an indicator of liver damage. Likewise, although no statistical significance, there was a ~ 20% reduction of BALF protein levels (indicator of lung damage), and a ~ 15% reduction of BUN levels (indicator of kidney damage) in septic mice transfused with Tram−/− neutrophils as compared to septic mice transfused with WT neutrophils (Fig. 2b, c). Correspondingly, we observed that septic mice transfused with Tram−/− neutrophils retained key adhesion molecule VE-Cadherin on lung endothelium as compared to septic mice transfused with WT neutrophils (Fig. S4). Furthermore, septic mice transfused with Tram−/− neutrophils exhibited reduced plasma creatinine and BUN levels (Fig. 2b, c). On the cellular level, we observed that septic recipient mice transfused with Tram−/− neutrophils sustained the expression of resolving mediators, including CD200R and CD24, on recipient neutrophils (Fig. 2d). Collectively, these data demonstrate the therapeutic potential of Tram−/− neutrophils in propagating homeostasis to recipient cells and alleviating sepsis-associated injuries.
Fig. 2.
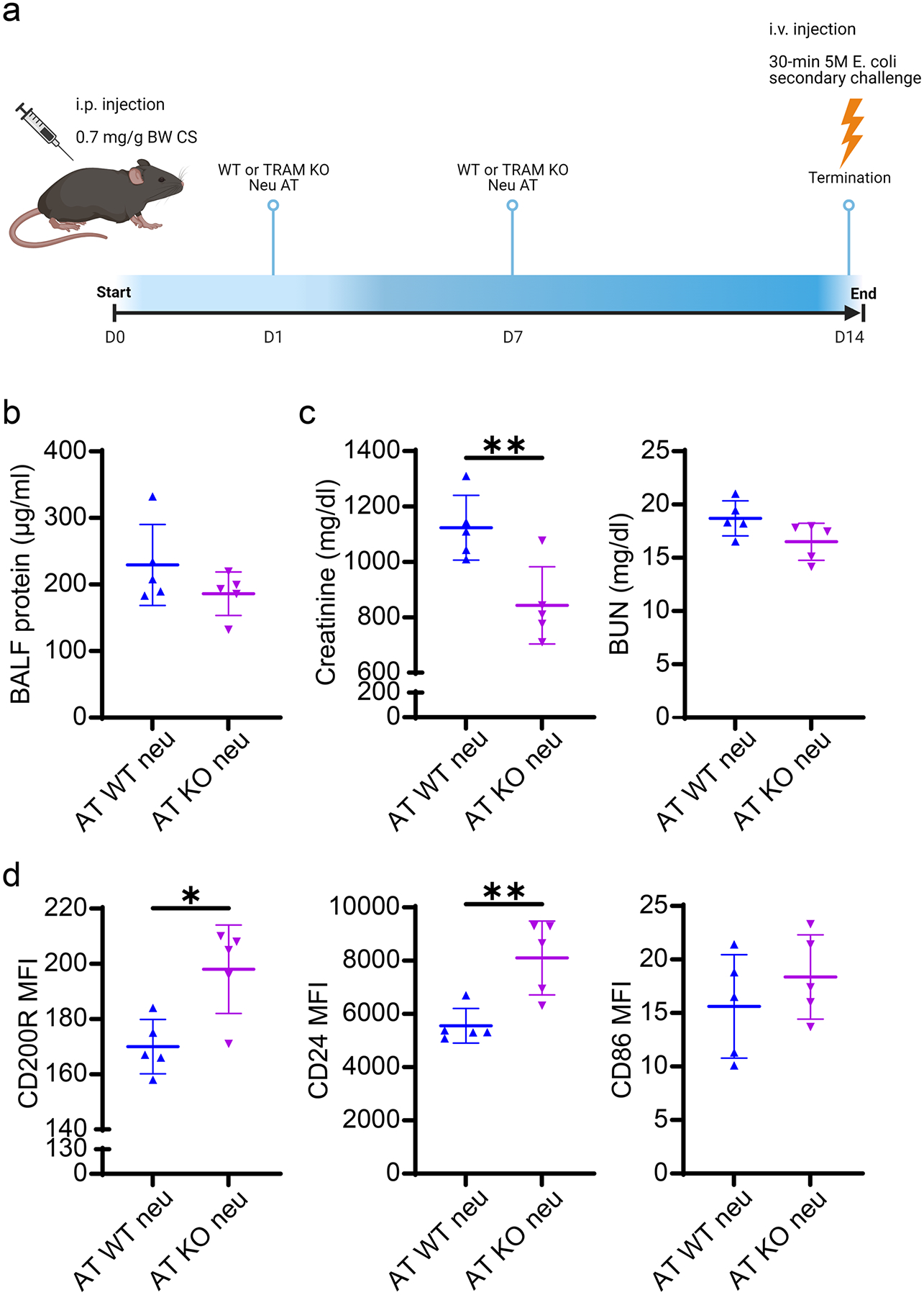
WT septic mice transfused with TRAM KO neutrophils possessed the restored resolving features on neutrophils and attenuated organ damage. a Experimental scheme of the protocol for the adoptive transfer of WT or TRAM KO neutrophils in mild septic mice. WT male mice with CS-induced mild sepsis were transfused twice with WT or TRAM KO neutrophils on day 1 and 7. Thirty minutes prior to the time of sacrificing (day 14), all the mice were subjected to i.v. injection of E. coli. b The protein concentration in BALFs and c the plasma levels of creatinine and BUN in both groups of mice. d Flow cytometry analysis of the expression of CD200R, CD24, and CD86 on spleen-resident neutrophils harvested from both groups of mice. **P < 0.01, *P < 0.05 using two-sided Student’s t-test (b–d). n = 5. KO TRAM KO. AT adoptive transfer. neu neutrophils
Innate and adaptive immune cells acquire resolving phenotypes when co-cultured with Tram−/− resolving neutrophils
Given the propagation of immune homeostasis mediated by Tram−/− neutrophils in vivo, we then further characterized the in vitro propagation of homeostasis by Tram−/− neutrophils. Hence, we investigated the phenotypic changes of naïve neutrophils, macrophages, and T cells co-cultured with TRAM KO or 4-PBA-reprogrammed neutrophils. We previously demonstrated that neutrophils treated with 4-PBA, a compound facilitating peroxisome homeostasis and inflammation resolution [26–28], exhibit a similar resolving phenotype as Tram−/− neutrophils [10]. We observed that naïve neutrophils co-cultured with 4-PBA-treated or Tram−/− neutrophils expressed ~ 10% and ~ 15% more CD86 as well as CD200R, respectively, as compared to their counterparts co-cultured with PBS control neutrophils (Fig. 3a). In addition, we observed ~ 15% increase of CD200R expression on monocytes co-cultured with resolving neutrophils (4-PBA-reprogrammed or Tram−/−) as compared to the control group (Fig. 3b).
Fig. 3.
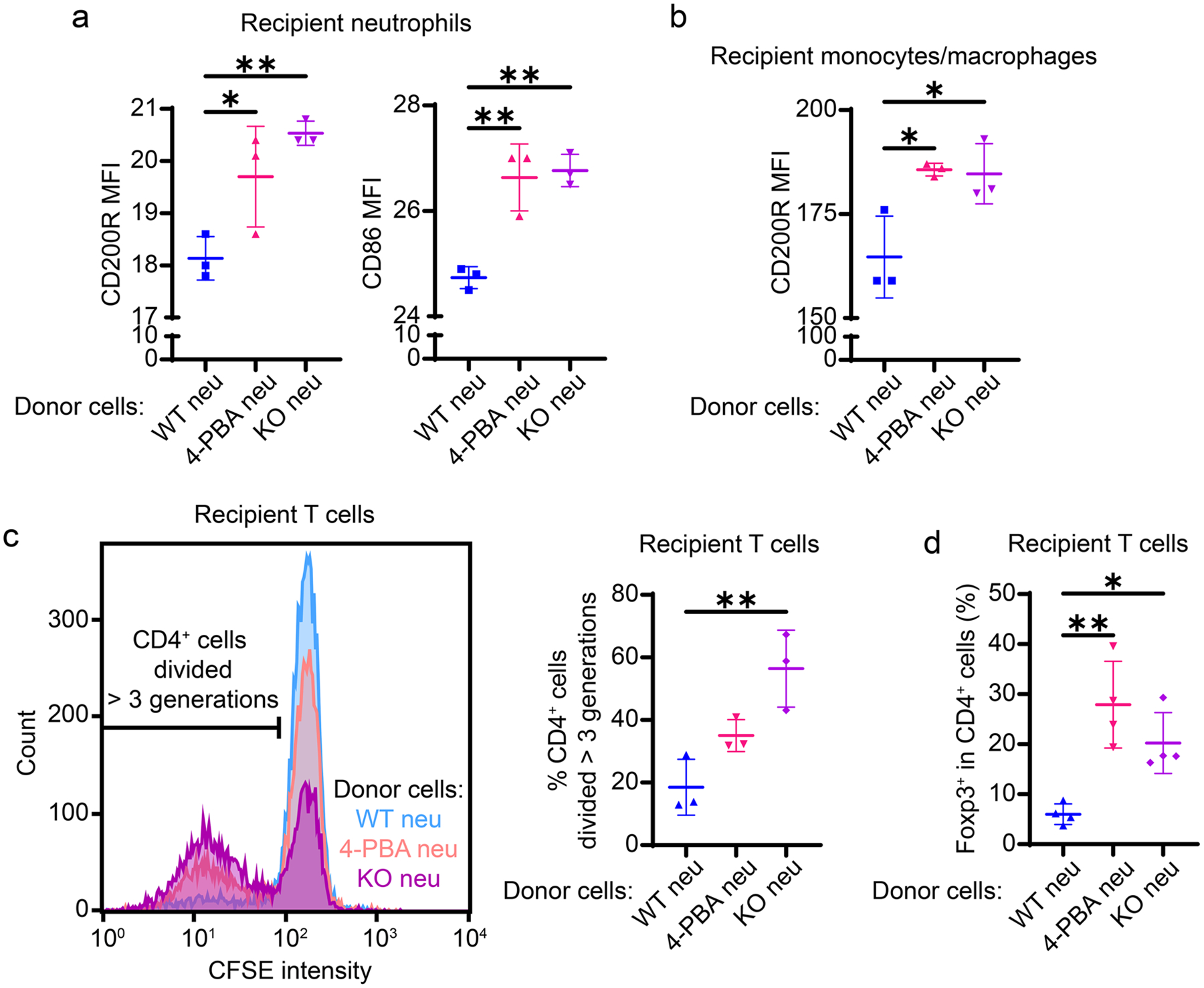
TRAM KO resolving neutrophils mediated resolving phenotypic adaptation of leukocytes in vitro. Recipient WT neutrophils, monocytes/macrophages, and T cells were cocultured with donor cells (WT PBS control neutrophils, 4-PBA-reprogrammed neutrophils, or TRAM KO neutrophils) for 1, 1, and 4 days, respectively. Flow cytometry analysis of a the expression of CD200R and CD86 on recipient neutrophils and b the expression of CD200R on recipient monocytes/macrophages. CFSE prelabeled recipient T cells were stained for CD4 and FoxP3 expression and analyzed by flow cytometry. c The representative CFSE histogram (left) and the quantification data (right) of CD4+ T cells divided more than 3 times from T cell-neutrophil cocultures. d The quantification data of FoxP3+ Treg cell proportion in CD4+ T cells from cocultures. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 using one-way ANOVA test followed by the post-hoc Sidak multiple comparisons test. n = 3–5. neu neutrophils. KO TRAM KO
We also examined neutrophil-mediated phenotypic adaption of the adaptive immune cells. Murine spleen-derived naïve T cells were co-cultured with resolving neutrophils (4-PBA-reprogrammed or Tram−/−) or PBS control neutrophils supplemented with anti-CD3 and anti-CD28 antibodies for 4 days. CD4+ T cell proliferation was elevated by ~ 1 and ~ 2 folds when co-cultured with 4-PBA-treated or Tram−/− neutrophils, respectively, as compared to the CD4+ T cell with control neutrophils (Fig. 3c). In addition, we evaluated the percentage of Treg cells among the entire CD4+ T cell population. Both 4-PBA-treated and Tram−/− resolving neutrophils increased the fractions of Treg cells in co-cultures by 2–3 folds (Fig. 3d), consistent with an independent finding about CD86-dependent Treg cell maintenance and differentiation [20]. Taken together, our data reveal that resolving neutrophils can elevate CD86 and CD200R expression on recipient neutrophils and monocytes, and facilitate homeostasis by enhancing the proliferation of CD4+ T cells as well as Treg cells.
Neutrophil-secreted soluble mediators are partially responsible for sustaining markers of endothelial integrity
Consistent with independent findings [29, 30], we observed a compromised lung endothelium integrity in WT septic mice with disrupted alveolar structure (Fig. 1b) and greater vasculature leakage in BALF (Fig. 1d). In contrast, the adoptive transfer of naïve Tram−/− neutrophils can alleviate CS-induced lung endothelial barrier dysfunction in recipient septic mice (Figs. 2b and S3). To further clarify how neutrophils influence the permeability and stability of the endothelium, we co-cultured endothelial cells with neutrophils and tested the expression of endothelial adhesion and tight junction molecules, given that endothelial integrity is regulated by junctional markers [31–34]. We observed that the expression levels of CD31, ESAM, JAM-1, and VE-Cadherin on endothelial cells co-cultured with Tram−/− neutrophils were significantly upregulated as compared to the endothelial cells co-cultured with WT neutrophils (Fig. 4a).
Fig. 4.
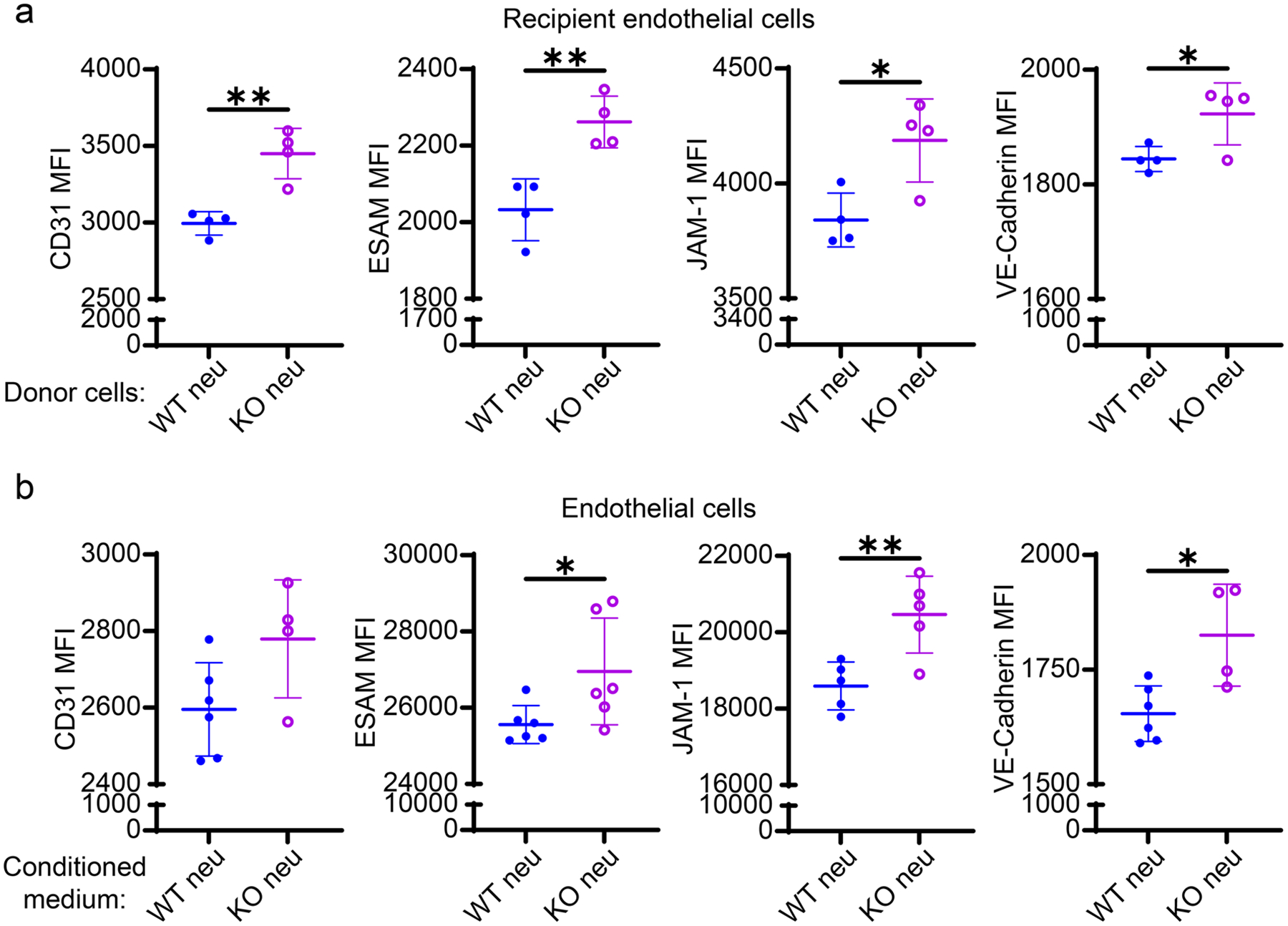
TRAM KO resolving neutrophils improved markers of endothelial integrity independent of direct intercellular interaction. Recipient endothelial cells were co-cultured with donor cells (WT or TRAM KO neutrophils) in the presence of 0.2 μM LPA for 1 day. a Flow cytometry analysis of the expression of CD31, ESAM, JAM-1, and VE-Cadherin on recipient endothelial cells from two different cocultures. In a separate experiment, endothelial cells were cultured in the neutrophil-conditioned media in the presence of LPA. The neutrophil-conditioned medium was collected from WT or TRAM KO neutrophil cultures pre-stimulated with 1 μM fMLP for 24 h. b Flow cytometry analysis of the expression of CD31, ESAM, JAM-1, and VE-Cadherin on endothelial cells cultured in two different conditioned media. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 using two-sided Student’s t-test. n = 4 or 6. neu neutrophils. KO TRAM KO
To test whether the endothelium disturbance is mediated by the soluble mediators secreted by neutrophils, we cultured endothelial cells in the conditioned medium harvested from either WT or Tram−/− neutrophil cultures stimulated with 1 μM fMLP for 24 h. We observed that endothelial cells cultured in Tram−/− neutrophil medium exhibited an enhanced expression of adhesion and tight junction molecules as compared to endothelial cells cultured in WT neutrophil medium (Fig. 4b). Together, our data suggest the involvement of neutrophil-secreted mediators in modulating endothelial integrity.
Knocking out TRAM in neutrophils decreases elastase secretion to maintain endothelial barrier markers
Elastase is a proteinase secreted by neutrophils during inflammation to limit pathogen invasion but can also cause damage to the host tissue. It has been shown that neutrophil elastase is responsible for disrupted pulmonary endothelial structures in experimental endotoxemia [35]. Neutrophil elastase was also shown to facilitate transendothelial migration and increase vascular permeability by degrading VE-Cadherin and promoting endothelium apoptosis [36, 37]. The inhibition of neutrophil elastase was shown to improve the survival of septic animals and attenuate sepsis-related lung and kidney impairment [6, 7, 38]. Hence, we hypothesized that TRAM-mediated neutrophil elastase production might be potentially involved in modulating endothelial integrity. Indeed, we found that elastase secretion by Tram−/− neutrophils upon 24-h fMLP stimulation was significantly lower than by WT neutrophils (Fig. 5a). Complementary to this finding, we also observed that 4-PBA reprogrammed resolving neutrophils secreted reduced levels of elastase as compared to their PBS control counterparts following fMLP challenge (Fig. S5). Consistent with previous studies, we found that the plasma levels of neutrophil elastase were elevated in the WT male septic mice as compared to the WT control mice (Fig. S6a). Knocking out TRAM, though not significantly, reduced the elevation of neutrophil elastase in septic male mice (Fig. S6a). Of note, in the female murine model, while CS-induced sepsis did not lead to dramatically increased neutrophil elastase in plasma from either WT and Tram−/− septic mice, the plasma level of neutrophil elastase of Tram−/− septic mice was significantly lower as compared to WT septic mice (Fig. S6b). Further, we found that the adoptive transfer of Tram−/− neutrophils potently reduced plasma levels of elastase in recipient septic mice (Fig. S6c). Taken together, these data supported our hypothesis that TRAM is required for neutrophil elastase secretion.
Fig. 5.
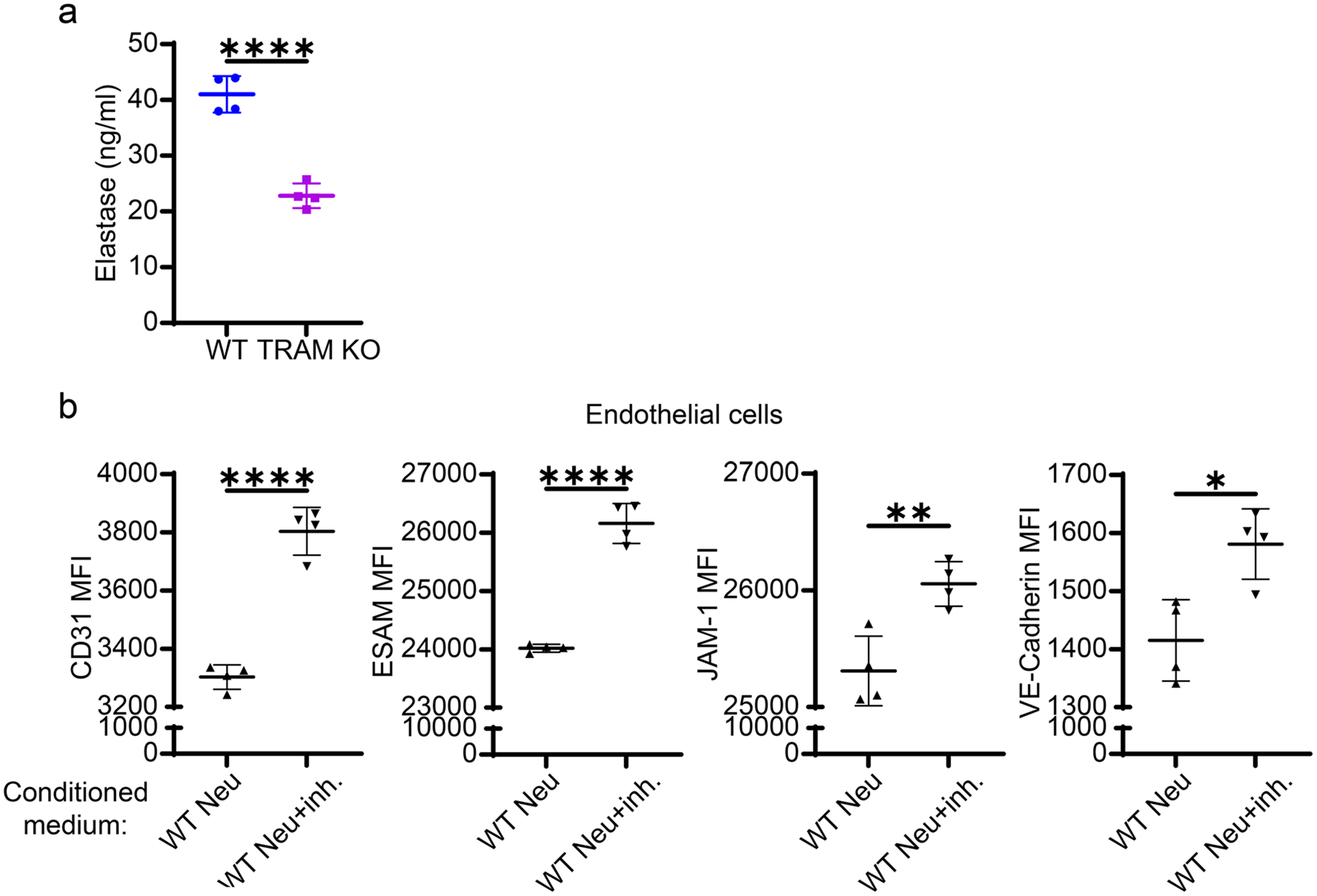
Deficiency in TRAM hindered neutrophil elastase secretion and the blockage of elastase restored markers of endothelial integrity. a The secretion of neutrophil elastase by WT and TRAM KO neutrophils upon 24-h fMLP stimulation was evaluated by ELISA. In a separate experiment, endothelial cells were cultured in the neutrophil-conditioned media in the presence of LPA. The neutrophil-conditioned medium was collected from fMLP-stimulated WT neutrophil cultures with or without 1 μg/ml neutrophil elastase inhibitor, sivelestat. b Flow cytometry analysis of the expression of CD31, ESAM, JAM-1, and VE-Cadherin on endothelial cells cultured in two different conditioned media. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 using two-sided Student’s t-test. n = 4. neu neutrophils. inh. inhibitor (sivelestat)
Next, we tested whether the blockage of neutrophil elastase could restore the integrity of endothelial cells cultured in conditioned media. WT neutrophils were simultaneously treated with fMLP and sivelestat, a widely used neutrophil elastase inhibitor, for 24 h. Indeed, we observed that endothelial cells cultured in WT neutrophil medium with the additive of sivelestat preserved higher levels of CD31, VE-Cadherin, JAM-1, and ESAM expression as compared to endothelial cells cultured in WT neutrophil medium without the inhibitor (Fig. 5b). Overall, our data suggest that elastase secreted by neutrophils may contribute to endothelial dysfunction by decreasing their expression of adhesion and tight junction molecules.
Discussion
Neutrophils are known to exhibit both inflammatory and resolving characteristics [8]. Inflammation resolution with engineered resolving neutrophils is drawing increasing interest from both the basic and clinical fields. Our group has reported that resolving neutrophils can be effectively expanded by pharmacological reprogramming with 4-PBA or by genetic deletion of TRAM [10]. TRAM is a key adaptor molecule regulating innate immunity signaling and is responsible for driving neutrophil inflammatory exhaustion and secretion of elastase which are responsible for the high mortality and morbidity of experimental sepsis. In the current study, we demonstrated that Tram−/− mice exhibiting in vivo signatures of resolving neutrophils are more resistant to CS-induced septic death and less vulnerable to sepsis-associated organ injury. Our data highlight the beneficial effects of TRAM deletion, which leads to an enhanced pro-resolving phenotype in neutrophils and provides immunomodulatory protection against severe sepsis and tissue damage.
Our data further clarify the resolving characteristics of Tram−/− neutrophils, with not only enhanced expression of CD200R and CD86, but also improved metabolic regulation, sustained NAD + levels, and reduced secretion of elastase, a proteinase involved in tissue damage. In contrast, WT neutrophils are prone to inflammatory exhaustion under septic conditions in vivo or challenges with higher doses of bacterial endotoxin lipopolysaccharide (LPS) in vitro. As we reported previously [10], naïve WT neutrophils only exhibit a small portion of resolving subsets. Re-analyses further revealed a reduced expression of TRAM in the minor subset of WT resolving neutrophils. Consistently, TRAM deletion renders the expansion of resolving neutrophils, enabling the protective effects during experimental sepsis.
While our data showed that the overall patterns of neutrophil surface markers and serological indicators upon CS challenge are consistent across male and female groups, it should be noted that neutrophil elastase production is higher from WT female mice as compared to male mice both constitutively and following CS-induced sepsis. This is consistent with previous reports showing potential sex differences among transcriptomic profiles leading to functional variations in both mice and human [39, 40]. Gupta et al. reported that neutrophils from human females exhibit a more mature/activated phenotype with elevated pro-inflammatory responses (i.e., upregulated Type I IFN signaling) [40]. In addition, female neutrophils exhibit a greater phagocytic ability as compared to male neutrophils [41], and 17β-oestradiol (E2) can promote neutrophil recruitment in human and mice to protect against influenza infection [42]. These findings may explain the stronger innate immune responses against pathogens but the predisposition of auto-immune diseases in females [43]. Our data further suggest that sex differences should be considered and further studied with regard to in vivo modulations of immune functions.
From the therapeutic perspective, our data suggest an intriguing potential of utilizing genetically engineered Tram−/− neutrophils in the treatment of experimental sepsis. We demonstrated that the adoptive transfer of Tram−/− neutrophils can confer protective effects on WT septic mice by restoring immune homeostasis and reducing tissue damage. Despite these intriguing phenotypic observations, underlying mechanisms still need extensive future studies. Our current study focused on examining the potential neutrophil-initiated inter-cellular communications with neighboring monocytes, neutrophils, T cells, and endothelial cells. We showed that the propagation of resolving characteristics to neighboring cells can also be demonstrated in vitro. Co-culturing naive neutrophils, monocytes, and T cells with Tram−/− neutrophils or 4-PBA-treated neutrophils can lead to increased expression of resolving markers, such as CD86 and CD200R, on recipient neutrophils and monocytes. In the context of T cell c-culture, donor Tram−/− neutrophils can enhance the proliferation of CD4+ T cells and regulatory T cells, which play crucial roles in maintaining immune homeostasis. Our co-culture data further demonstrate that Tram−/− donor neutrophils can effectively improve key markers correlated with endothelial integrity in vitro. These novel inter-cellular effects of neutrophils on co-cultured monocytes, neutrophils, and T cells advance our understanding of how resolving neutrophils due to TRAM deletion may potentially impact immune homeostasis. However, future studies based on our findings will be needed to examine their causal relevance to tissue damage.
It is important to note that due to the complexity of inter-cellular interactions, detailed mechanisms responsible for the propagation of either inflammatory or resolving characteristics are likely complex and remain to be further clarified through future studies. Although our effort revealed some potential mediators (e.g., soluble elastase, neutrophil membrane associated makers such as CD24), future studies are needed to systematically define relevant mediators involved in context-dependent communications among neutrophils with neighboring cells and/or tissues both in vitro and in vivo. In this regard, our scRNAseq data and analyses provided in this study will serve as an important resource for future reference. Our current data cannot exclude or differentiate the effects of direct cellular contact or paracrine effects of secreted mediators. Future live-cell imaging with three-dimensional precision will be required to resolve the complex dynamics of inter-cellular communications responsible for the inter-cellular propagations of inflammation resolution. In vivo tracking of labeled cells within the recipient hosts will also be needed for in-depth pharmacodynamics analyses of resolving neutrophils during sepsis intervention.
Together, our data provide an initial attempt of characterizing resolving neutrophils and their novel therapeutic potential in propagating resolution in vivo and in vitro, in the context of experimental innate immune therapeutics for the treatment of sepsis. Better characterization and manipulation of the pro-resolving neutrophils and their interactions with other immune cells will likely hold promise for developing future effective immune cell-based therapeutics to fill the unmet critical medical need in treating severe sepsis.
Supplementary Material
Acknowledgements
This study was supported partly by the National Institute of Health grants R01 AI172133.
Funding
National Institutes of Health, NIH AI172133.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00011-023-01779-z.
Conflict of interest There is no conflict of interest related to this publication.
Data availability
scRNAseq data were deposited at the NCBI Genebank with the accession number GSE230241. All other experimental data are presented and described in full within this manuscript.
References
- 1.Rudd KE, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–11. 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Silva Ramos FJ, de Freitas FGR, Machado FR. Sepsis in patients hospitalized with coronavirus disease 2019: how often and how severe? Curr Opin Crit Care. 2021;27(5):474–9. 10.1097/MCC.0000000000000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drosatos K, Lymperopoulos A, Kennel PJ, Pollak N, Schulze PC, Goldberg IJ. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr Heart Fail Rep. 2015;12(2):130–40. 10.1007/s11897-014-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Li S, Li S. The role of the liver in sepsis. Int Rev Immunol. 2014;33(6):498–510. 10.3109/08830185.2014.889129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schortgen F, Asfar P. Update in sepsis and acute kidney injury 2014. Am J Respir Crit Care Med. 2015;191(11):1226–31. 10.1164/rccm.201502-0307UP. [DOI] [PubMed] [Google Scholar]
- 6.Suda K, et al. Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock. 2010;33(5):526–31. 10.1097/SHK.0b013e3181cc064b. [DOI] [PubMed] [Google Scholar]
- 7.Li G, et al. The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int J Mol Med. 2016;38(3):767–75. 10.3892/ijmm.2016.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28(2):137–45. 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 9.El Kebir D, Filep JG. Role of neutrophil apoptosis in the resolution of inflammation. Sci World J. 2010;10:1731–48. 10.1100/tsw.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin R, Yi Z, Wang J, Geng S, Li L. Generation of resolving memory neutrophils through pharmacological training with 4-PBA or genetic deletion of TRAM. Cell Death Dis. 2022;13(4):345. 10.1038/s41419-022-04809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uddin M, Levy BD. Resolvins: natural agonists for resolution of pulmonary inflammation. Prog Lipid Res. 2011;50(1):75–88. 10.1016/j.plipres.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casulli J, et al. CD200R deletion promotes a neutrophil niche for Francisella tularensis and increases infectious burden and mortality. Nat Commun. 2019;10(1):2121. 10.1038/s41467-019-10156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrera BS, et al. Impact of resolvin E1 on murine neutrophil phagocytosis in type 2 diabetes. Infect Immun. 2015;83(2):792–801. 10.1128/IAI.02444-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin R, Zhang Y, Pradhan K, Li L. TICAM2-related pathway mediates neutrophil exhaustion. Sci Rep. 2020;10(1):14397. 10.1038/s41598-020-71379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starr ME, Steele AM, Saito M, Hacker BJ, Evers BM, Saito H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE. 2014;9(12): e115705. 10.1371/journal.pone.0115705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steele AM, Starr ME, Saito H. Late therapeutic intervention with antibiotics and fluid resuscitation allows for a prolonged disease course with high survival in a severe murine model of sepsis. Shock. 2017;47(6):726–34. 10.1097/SHK.0000000000000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowak JE, Harmon K, Caldwell CC, Wong HR. Prophylactic zinc supplementation reduces bacterial load and improves survival in a murine model of sepsis. Pediatr Crit Care Med. 2012;13(5):e323–9. 10.1097/PCC.0b013e31824fbd90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pradhan K, Geng S, Zhang Y, Lin RC, Li L. TRAM-related TLR4 pathway antagonized by IRAK-M mediates the expression of adhesion/coactivating molecules on low-grade inflammatory monocytes. J Immunol. 2021;206(12):2980–8. 10.4049/jimmunol.2000978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H, Ziegler SF. Bronchoalveolar lavage and lung tissue digestion. Bio Protoc. 2013. 10.21769/bioprotoc.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halliday N, et al. CD86 is a selective CD28 ligand supporting FoxP3+ regulatory T cell homeostasis in the presence of high levels of CTLA-4. Front Immunol. 2020;11: 600000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, et al. Sphingosine-1–phosphate receptor–3 is a novel biomarker in acute lung injury. Am J Respir Cell Mol Biol. 2012;47(5):628–36. 10.1165/rcmb.2012-0048oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami K, et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS ONE. 2014;9(9): e106792. 10.1371/journal.pone.0106792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Vlist M, et al. Macrophages transfer mitochondria to sensory neurons to resolve inflammatory pain. Neuron. 2022;110(4):613–6266.e9. 10.1016/j.neuron.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Pradhan K, Yi Z, Geng S, Li L. Development of exhausted memory monocytes and underlying mechanisms. Front Immunol. 2021;12: 778830. 10.3389/fimmu.2021.778830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusaczuk M, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyric Acid: simple structure-multiple effects. Curr Pharm Des. 2015;21(16):2147–66. [DOI] [PubMed] [Google Scholar]
- 27.Liu N, Qiang W, Kuang X, Thuillier P, Lynn WS, Wong PK. The peroxisome proliferator phenylbutyric acid (PBA) protects astrocytes from ts 1 MoMuLV-induced oxidative cell death. J Neurovirol. 2002;8(4):318–25. [DOI] [PubMed] [Google Scholar]
- 28.Farr RL, Lismont C, Terlecky SR, Fransen M. Peroxisome bio-genesis in mammalian cells: the impact of genes and environment. Biochimica et Biophysica Acta (BBA)-Mol Cell Res. 2016;1863(5):1049–60. [DOI] [PubMed] [Google Scholar]
- 29.Rungsung S, et al. Luteolin attenuates acute lung injury in experimental mouse model of sepsis. Cytokine. 2018;110:333–43. 10.1016/j.cyto.2018.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Kong Q, Wu X, Qiu Z, Huang Q, Xia Z, Song X. Protective effect of dexmedetomidine on acute lung injury via the upregulation of tumour necrosis factor-alpha-induced protein-8-like 2 in septic mice. Inflammation. 2020;43(3):833–46. 10.1007/s10753-019-01169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mundi S, et al. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res. 2018;114(1):35–52. 10.1093/cvr/cvx226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chistiakov DA, Orekhov AN, Bobryshev YV. Endothelial barrier and its abnormalities in cardiovascular disease. Front Physiol. 2015;6:365. 10.3389/fphys.2015.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cangara HM, et al. Role of endothelial cell-selective adhesion molecule in hematogeneous metastasis. Microvasc Res. 2010;80(1):133–41. 10.1016/j.mvr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrero E, Ferrero ME, Pardi R, Zocchi MR. The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett. 1995;374(3):323–6. 10.1016/0014-5793(95)01110-z. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, et al. Neutrophil elastase damages the pulmonary endothelial glycocalyx in lipopolysaccharide-induced experimental endotoxemia. Am J Pathol. 2019;189(8):1526–35. 10.1016/j.ajpath.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Ushakumari CJ, et al. Neutrophil elastase increases vascular permeability and leukocyte transmigration in cultured endothelial cells and obese mice. Cells. 2022. 10.3390/cells11152288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grechowa I, Horke S, Wallrath A, Vahl CF, Dorweiler B. Human neutrophil elastase induces endothelial cell apoptosis by activating the PERK-CHOP branch of the unfolded protein response. FASEB J. 2017;31(9):3868–81. 10.1096/fj.201700012R. [DOI] [PubMed] [Google Scholar]
- 38.Ishii T, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol. 2010;177(4):1665–73. 10.2353/ajpath.2010.090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu RJ, et al. Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex and age-related functional regulation. Nat Aging. 2021;1(8):715–33. 10.1038/s43587-021-00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc Natl Acad Sci U S A. 2020;117(28):16481–91. 10.1073/pnas.2003603117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spitzer J Gender differences in some host defense mechanisms. Lupus. 1999;8(5):380–3. [DOI] [PubMed] [Google Scholar]
- 42.Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta-estradiol protects females against influenza by recruiting neutrophils and increasing virus-specific CD8 T cell responses in the lungs. J Virol. 2014;88(9):4711–20. 10.1128/JVI.02081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
scRNAseq data were deposited at the NCBI Genebank with the accession number GSE230241. All other experimental data are presented and described in full within this manuscript.


