Abstract
Introduction
Leucine-rich alpha-2-glycoprotein (LRG) is a potential biomarker for disease activity and reflects mucosal healing in patients with ulcerative colitis (UC). However, only a few studies have described a detailed sensitivity analysis of LRG in predicting mucosal healing in patients. This study aimed to evaluate the association between LRG and the endoscopic activity of UC and its predictability for mucosal healing and explore the utility and clinical application of LRG.
Methods
The diagnostic accuracy of biomarkers, including LRG, in predicting the endoscopic activity of UC was evaluated. All consecutive patients who underwent total colonoscopy between April 2021 and September 2022 were included. The Mayo endoscopic subscore (MES) was used for assessing endoscopic activity. Furthermore, endoscopic remission was defined as an MES of ≤1. Clinical activity was evaluated based on stool frequency and bloody stool. Receiver operating characteristic curve analysis and binary logistic regression were performed to assess the diagnostic accuracy of the biomarkers. We evaluated LRG trends and treatment response in patients with MES ≥2 who underwent induction therapy.
Results
This study comprised 214 patients. The proportions of endoscopically and clinically active patients were 33.6% and 49.1%, respectively. LRG had an area under the curve (AUC) of 0.856, with a higher diagnostic accuracy than other biomarkers, such as C-reactive protein, leukocyte, neutrophil, platelet, and albumin. The cutoff value for LRG was 15.6 μg/mL (sensitivity, 72.2%; specificity, 86.6%). Using the MES, patients with higher scores had higher LRG levels than those with lower scores. The cutoff value, AUC, sensitivity, and specificity varied with a higher AUC for left-sided colitis and pancolitis than for proctitis. Logistic regression analysis showed that LRG was an independent predictor of endoscopic remission using multivariate analysis, even with the factor of clinical activity. The change ratio of LRG pre- and post-treatment was statistically significant in the higher LRG group.
Conclusion
LRG reflected endoscopic activity independently, regardless of clinical symptoms. An LRG below the cutoff value could indicate a significantly low probability of endoscopic activity in asymptomatic patients, and follow-up endoscopy (not for cancer screening) may be unnecessary. Furthermore, a higher LRG level might be more useful as an indicator of treatment efficacy.
Keywords: Leucine-rich alpha-2-glycoprotein, Ulcerative colitis, Endoscopic remission, Biomarker
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease characterized by recurrent episodes of colonic inflammation. However, its diagnosis and management depend heavily on clinical symptoms, endoscopic findings, and histological evaluations, which are susceptible to interpatient and interobserver variability and may not accurately reflect disease activity.
Serada et al. [1] performed semiquantitative protein analysis of serum samples obtained from patients with rheumatoid arthritis pre- and post-treatment with antitumor necrosis factor-α antibodies and identified a novel acute-phase protein known as leucine-rich alpha-2-glycoprotein (LRG). LRG is a potential biomarker for disease activity and reflects mucosal healing in patients with UC. Notably, it was covered in June 2020 by the National Health Insurance System in Japan.
Several studies have evaluated the correlation between serum LRG levels and UC disease activity and demonstrated that LRG levels were significantly higher in patients with active disease than those in remission. LRG levels have also been associated with endoscopic disease activity and histological inflammation [2–4]. These findings suggest that LRG may be a useful biomarker for disease activity and monitoring the therapeutic response in patients with UC. However, only a few studies have described a detailed sensitivity analysis of LRG in predicting mucosal healing in patients with UC. Furthermore, endoscopic activity has not been compared with LRG, other biomarkers, and symptoms. Therefore, this study aimed to evaluate the association between LRG and the endoscopic activity of UC and the predictability of this marker for mucosal healing compared with other biomarkers and symptoms and explore its novel utility and clinical applicability from new perspectives.
Materials and Methods
Patients
This retrospective case series study was conducted at Kanazawa University Hospital, Ishikawa, Japan, and included all consecutive patients with UC who underwent total colonoscopy between April 2021 (since LRG could be measured at our hospital) and September 2022. UC was diagnosed based on the established diagnostic criteria [5]. All patients underwent confirmation of their symptoms and biomarkers, including LRG, C-reactive protein (CRP), leukocyte, neutrophil, platelet, and albumin, on the same day as their colonoscopy. Nanopia LRG (Sekisui Medical) was used for measuring LRG. The exclusion criteria included total colorectal resection with a stoma or ileal pouch. Furthermore, patients with other diseases that could affect the LRG and CRP levels, including extraintestinal complications, collagen disease, infectious disease, and malignancy during colonoscopy, and those who received severe acute respiratory syndrome coronavirus 2 vaccination within 1 week before blood collection were also excluded. Moreover, if multiple colonoscopies were performed during the period, only the findings of the first one were included and the others were excluded.
Assessment of the Endoscopic Activity of UC
The patients with UC were classified according to the extent of disease involvement as E1 (proctitis), E2 (left-sided colitis), or E3 (pancolitis), according to the Montreal classification [6]. Endoscopic activities were determined using the Mayo endoscopic subscore (MES) [7], and its remission (mucosal healing) was defined as an MES of ≤1.
Experienced endoscopists performed all endoscopic examinations. Two endoscopists (T.H. and K.K.), who were the principal readers, assessed MES through video recordings by blinding the results of serum markers. Consensus was reached upon discussion among the endoscopists in case of disagreement.
Assessment of the Clinical Activity of UC
In our study, the clinical activity of UC was assessed based on symptoms as follows: if daily stool frequency was greater by 3 or more stools compared to the normal frequency (Mayo subscore of ≥2) or if bloody stool was observed (Mayo subscore of ≥1). Clinical remission was defined as neither diarrhea (daily number of stools greater by 3 or more compared to the normal number) nor bloody stool. On the contrary, clinical nonremission (clinically active) was defined as daily number of stools greater by 3 or more compared to the normal number of bloody stools. The partial Mayo score, Rachmilewitz index, and Lichtiger index are commonly used for assessing the clinical activity of UC. However, these indices include the physicianʼs and investigator’s global assessments of symptomatic state or general well-being, which could significantly vary among evaluators. Therefore, we only used stool frequency and bloody stool to assess the clinical activity of UC since we aimed to assess the patient’s objective, not subjective, symptoms.
Outcome Measures
We assessed the patients’ background, including age, sex, and clinical characteristics, such as the extent of disease involvement and biomarkers. LRG was evaluated for each MES and the extent of disease involvement. Receiver operating characteristic (ROC) curve analysis was used for assessing the diagnostic accuracy of biomarkers, including LRG, for the endoscopic activity of UC. The associations between endoscopic activity and patient background, biomarkers, and symptoms were assessed to identify the parameters that could predict the endoscopic activity of UC. Therefore, to evaluate the usefulness of LRG for assessing endoscopic activity, we used multivariate analysis to examine potential confounding factors such as age, sex, biomarkers other than LRG, and symptoms (clinical activity). Endoscopic remission was the dependent variable. Explanatory variables were used in age, sex, and LRG in model 1. The body mass index (BMI) and other biomarkers were included in model 2. Additionally, clinical activity was included in model 3. LRG was analyzed with binary values (0 and 1 signify above and below the cutoff values, respectively) to validate the cutoff value. Additionally, we evaluated LRG trends and treatment response in patients with MES ≥2 who underwent induction therapy to explore the different utilities of LRG, including its potential as a prognostic marker in the longitudinal evaluation of LRG. Specifically, among the patients who subsequently underwent endoscopy to determine treatment efficacy, we evaluated the association between endoscopic remission rate and LRG trends between the high LRG (above the cutoff value) group and the low LRG (below the cutoff value) group. For LRG trends, the difference ([post-treatment] − [pre-treatment]) and change ratio {([post-treatment] − [pre-treatment])/pre-treatment} were determined. Post-treatment LRG was confirmed on the same day as the post-treatment colonoscopy. We additionally performed the same evaluation of LRG after induction therapy by clinical activity before induction therapy (between clinically active and inactive groups).
Statistical Analyses
Continuous non-parametric variables are presented as the median with range or interquartile range (IQR), and parametric variables are expressed as the mean (standard deviation [SD]). Between-group comparisons were performed using the Student’s t test or the Mann-Whitney U test. Categorical variables are expressed as percentages, and between-group comparisons were performed using Fisher’s exact tests. The association between the endoscopic activity of UC and biomarkers was determined using ROC curves and the area under the curve (AUC). The point with the smallest distance to the upper left corner was set as the cutoff value. Binomial logistic regression analysis was performed as a multivariate analysis to evaluate the usefulness of LRG for assessing endoscopic activity. Statistical significance was considered at p < 0.05. All statistical analyses were performed using the Statistical Package for the Social Sciences 22.0 (IBM Corporation, Armonk, NY, USA).
Results
Patient Characteristics
Table 1 summarizes the patients’ clinicopathological characteristics. Overall, 214 patients were included, of whom 60.7% (130/214) were male, and the patients’ median (range) age was 41 (8–71) years. Regarding the indications for colonoscopy, 5.6% (12/214 patients), 47.7% (102/214 patients), and 46.7% (100/214 patients) were diagnosed with UC, cancer surveillance, and disease activity evaluation, respectively. Based on the Montreal classification, 15.4% (33/214 patients), 35.5% (76/214 patients), and 49.1% (105/214 patients) of the patients had E1 (proctitis), E2 (left-sided colitis), and E3 (pancolitis) diseases, respectively. The proportions of endoscopically and clinically active patients were 33.6% (72/214 patients) and 49.1% (105/214 patients), respectively.
Table 1.
Baseline characteristics
| All patients (n = 214) | |
|---|---|
| Median age, years (range) | 41 (8–71) |
| Sex (female:male) n (%) | 84 (39.3):130 (60.7) |
| Indication of colonoscopy | |
| Diagnosis of UC, n (%) | 12 (5.6) |
| Cancer surveillance, n (%) | 102 (47.7) |
| Disease activity evaluation, n (%) | 100 (46.7) |
| MES (0:1:2:3), n (%) | 86 (40.2):56 (26.2):53 (24.8):19 (8.9) |
| Clinically active, n (%) | 105 (49.1) |
| ≥3 stools more than normal, n (%) | 88 (41.1) |
| Rectal bleeding, n (%) | 77 (36.0) |
| Extent of disease involvement (E1:E2:E3), n (%) | 33 (15.4):76 (35.5):105 (49.1) |
| Median LRG, μg/mL (range) | 12.6 (6.0–65.1) |
| Median CRP, mg/dL (range) | 0.05 (0.00–13.01) |
| Median leukocyte count, 103/μL (range) | 5.74 (2.58–20.60) |
| Median neutrophil count, 103/μL (range) | 3.46 (0.92–16.27) |
| Median platelet count, 104/μL (range) | 27.1 (10.4–79.6) |
| Median albumin, g/dL (range) | 4.3 (1.8–5.2) |
LRG, leucine-rich alpha-2-glycoprotein; CRP, C-reactive protein.
Association between the Endoscopic Activity of UC and Biomarkers, Including LRG
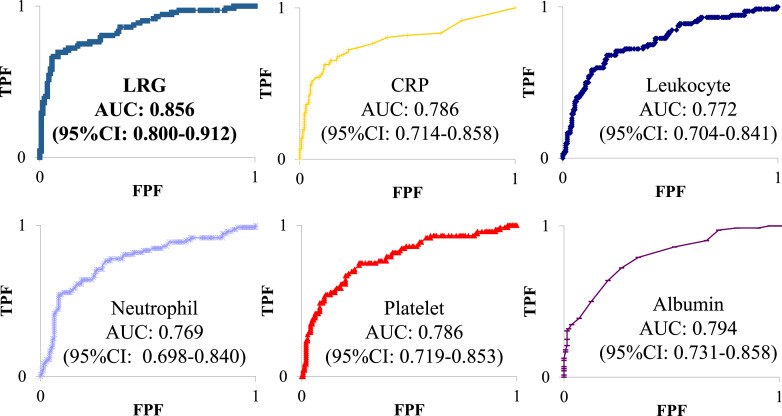
Assessing the diagnostic accuracy of biomarkers using the ROC curve analysis showed that the AUC for LRG was 0.856 (95% confidence interval [CI], 0.800–0.912), which was higher than those for CRP (0.786; 95% CI: 0.714–0.858), leukocyte (0.772; 95% CI: 0.704–0.841), neutrophil (0.769; 95% CI: 0.698–0.840), platelet (0.786; 95% CI: 0.719–0.853), and albumin (0.794; 95% CI: 0.731–0.858) (shown in Fig. 1). Furthermore, assessing the endoscopic activity of UC showed that the cutoff value for LRG in our study was 15.6 μg/mL, with a sensitivity and specificity of 72.2% and 86.6%, respectively.
Fig. 1.
Diagnostic accuracy of biomarkers based on ROC curve analysis for endoscopic activity. LRG, leucine-rich alpha-2 glycoprotein; CRP, C-reactive protein; ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
LRG Level and the Percentage of Less than the Cutoff Value for Each MES
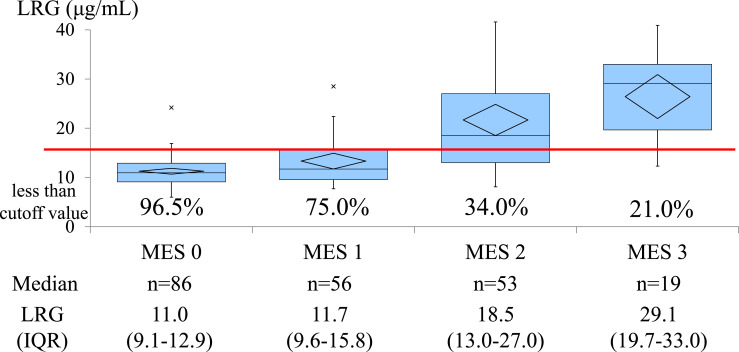
The median (IQR) serum LRG level (μg/mL) and percentage of less than the cutoff value were 12.6 (10.1–17.6) and 68.7% (147/214 patients), respectively. Based on the MES, the median serum LRG level and percentage of less than the cutoff value were 11.0 (9.1–12.9) and 96.5%, 11.7 (9.6–15.8) and 75.0%, 18.5 (13.0–27.0) and 34.0%, and 29.1 (19.7–33.0) and 21.1% for MES 0 (n = 86), MES 1 (n = 56), MES 2 (n = 53), and MES 3 (n = 19), respectively (shown in Fig. 2). Furthermore, even below the LRG cutoff value, 15.0% (22/147) of the patients were in endoscopic nonremission.
Fig. 2.
Median level of serum LRG and the percentage of less than the cutoff value based on the MES. LRG, leucine-rich alpha-2 glycoprotein; MES, Mayo endoscopic subscore; IQR, interquartile range.
Association between the Endoscopic Activity of UC and LRG Level for Each Disease Distribution
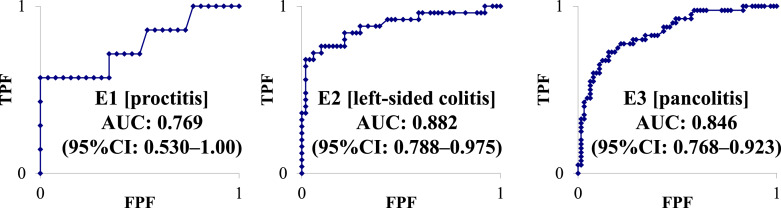
The median (IQR) levels of serum LRG (μg/mL) were as follows: E1 (proctitis) (n = 33), 11.6 (10.6–14.8); E2 (left-sided colitis) (n = 76), 12.0 (10.3–16.3); and E3 (pancolitis) (n = 105), 13.4 (9.7–20.8). Regarding E1 (proctitis), the AUC was 0.769 (95% CI: 0.530–1.00) and the cutoff value for LRG was 17.8 μg/mL, with a sensitivity and specificity of 42.9% and 100%, respectively. Regarding E2 (left-sided colitis), the AUC was 0.882 (95% CI: 0.788–0.975) and the cutoff value for LRG was 15.6 μg/mL, with a sensitivity and specificity of 76.0% and 90.2%, respectively. Considering E3 (pancolitis), the AUC was 0.846 (95% CI: 0.768–0.923) and the cutoff value for LRG was 14.7 μg/mL, with a sensitivity and specificity of 77.5% and 84.6%, respectively (shown in Fig. 3).
Fig. 3.
Diagnostic accuracy of LRG based on ROC curve analysis for endoscopic activity. ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
Independent Factors Related to the Endoscopic Activity of UC
In total, 3.7% (4/109) of the patients with clinical remission (neither daily number of stools greater by 3 or more compared to the normal number nor bloody stool) were in endoscopic nonremission. However, no cases of endoscopic nonremission were observed when the LRG was below the cutoff value, but clinical remission was observed (Table 2). Using multivariate analysis, age (p = 0.024) and LRG (p < 0.001) in model 1; LRG (p < 0.001) and albumin (p = 0.009) in model 2; and LRG (p < 0.001), albumin (p = 0.008), and clinical activity (p < 0.001) in model 3 were independent predictors of endoscopic remission (Table 3).
Table 2.
Rate of endoscopic remission
| Clinical remissiona, n (%) | Clinical nonremission, n (%) | |
|---|---|---|
| LRG < COV = 15.6 μg/mL | 96/96 (100) | 29/51 (56.9) |
| LRG ≥ COV = 15.6 μg/mL | 9/13 (69.2) | 8/54 (14.8) |
LRG, leucine-rich alpha-2-glycoprotein; COV, cutoff value.
aNeither ≥3 stools more than the normal daily number nor bloody stool.
Table 3.
Predictors of endoscopic remission − independent factors related to endoscopic activity
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p value | OR | (95% CI) | p value | OR | (95% CI) | p value | |
| Age (+1 year) | 1.03 | (1.00–1.05) | 0.024 | 1.02 | (0.99–1.04) | 0.164 | 1.03 | (1.00–1.07) | 0.070 |
| Female (vs. male) | 0.83 | (0.40–1.71) | 0.613 | 0.99 | (0.45–2.21) | 0.985 | 0.78 | (0.28–2.12) | 0.623 |
| LRG < COV = 15.6 μg/mL (vs. ≥COV) | 17.13 | (8.21-35.74) | <0.001 | 7.22 | (3.00-17.36) | <0.001 | 8.20 | (2.59-26.02) | <0.001 |
| BMI (+1 kg/m2) | 1.03 | (0.92–1.14) | 0.633 | 1.00 | (0.89–1.13) | 0.974 | |||
| CRP (−1 mg/dL) | 0.92 | (0.57–1.49) | 0.746 | 0.78 | (0.51–1.19) | 0.249 | |||
| Leukocyte (−1,000/μL) | 1.11 | (0.64–1.93) | 0.717 | 0.93 | (0.47–1.85) | 0.833 | |||
| Neutrophil (−1,000/μL) | 0.98 | (0.54–1.79) | 0.969 | 1.11 | (0.53–2.37) | 0.771 | |||
| Platelet (−10,000/μL) | 1.02 | (0.97–1.08) | 0.423 | 0.97 | (0.90–1.04) | 0.376 | |||
| Albumin (+0.1 mg/dL) | 1.19 | (1.04-1.35) | 0.009 | 1.28 | (1.07-1.53) | 0.008 | |||
| Clinical activity inactive (vs. active) | 39.17 | (10.55-145.93) | <0.001 | ||||||
OR, odds ratio; CI, confidence interval; COV, cutoff value; LRG, leucine-rich alpha-2-glycoprotein; CRP, C-reactive protein; BMI, body mass index.
Longitudinal Evaluation of LRG as a Prognostic Marker and Indicator of the Efficacy of Induction Therapy of Endoscopic Nonremission Cases
No statistical difference was found between the two groups, categorized as high and low LRG groups (above and below the cutoff value of LRG with an MES of ≥2, respectively) in age, sex, extent of disease involvement, MES before induction therapy, and medication of induction therapy. The median number of days from treatment initiation to post-treatment endoscopy was 183 in the high LRG group and 254 in the low LRG group; however, there was no significant difference between these groups. The endoscopic remission rate and MES trends did not significantly differ between these groups. However, the LRG difference and change ratio between pre- and post-treatment were statistically more prominent in the high LRG group than in the low LRG group. Even limited to endoscopic remission cases, similar trends were observed between the groups (Table 4). By the same evaluation of LRG after induction therapy by clinical activity (between the clinically active group and the inactive group) before induction therapy, no statistical difference was observed in age, sex, extent of disease involvement, MES before induction therapy, medication of induction therapy, days from treatment initiation to post-treatment endoscopy, endoscopic remission after treatment, MES differences, and LRG differences (both value and change ratio) (Table 5).
Table 4.
Longitudinal evaluation of LRG after induction therapy for endoscopic nonremission cases between the high LRG (above the cutoff value) and the low LRG (below the cutoff value) groups
| LRG ≥ COV (n = 39) | LRG < COV (n = 16) | p value | |
|---|---|---|---|
| Median age, years (range) | 40.5 (13–62) | 30 (11–73) | 0.800 |
| Sex (female:male) n (%) | 16 (41.0):23 (59.0) | 6 (37.5):10 (62.5) | 0.808 |
| Extent of disease involvement (E1:E2:E3), n (%) | 2 (5.1):14 (35.9):23 (59.0) | 2 (12.5):4 (25.0):10 (62.5) | 0.527 |
| MES before induction therapy (2:3), n (%) | 27 (69.2):12 (30.8) | 13 (81.3):3 (18.8) | 0.363 |
| Clinically activea, n (%) | 35 (89.7) | 16 (100) | 0.448 |
| Induction therapy, n (%) | 0.940 | ||
| Mesalamine | 14 (35.9) | 6 (37.5) | |
| Steroids | 9 (23.1) | 3 (18.8) | |
| Thioprines | 6 (15.4) | 2 (12.5) | |
| TNF-alpha inhibitor | 10 (25.6) | 3 (18.8) | |
| Ustekinumab | 4 (10.3) | 1 (6.3) | |
| Vedolizumab | 5 (12.8) | 1 (6.3) | |
| Janus kinase inhibitor | 2 (5.1) | 2 (12.5) | |
| Median days from treatment initiation to post-treatment endoscopy | 183 (69–682) | 254 (14–753) | 0.931 |
| Endoscopic remission after treatment, n (%) | 23 (59.0) | 9 (62.5) | 0.852 |
| MES differences (post-pre) | −1.00±0.92 | −1.00±0.97 | 1,000 |
| LRG differences (post-pre), μg/mL | −9.16±10.78 | 1.11±4.71 | <0.001 |
| LRG change ratio ([post-pre]/pre [%]) | −31.7±29.1 | 9.7±40.6 | <0.001 |
| Endoscopic remission cases | LRG ≥ COV (n = 23) | LRG < COV (n = 9) | p value |
|---|---|---|---|
| LRG differences (post-pre), μg/mL | −13.94±10.97 | −0.54±1.93 | 0.001 |
| LRG change ratio ([post-pre]/pre [%]) | −48.5±20.0 | −4.1±16.0 | <0.001 |
LRG, leucine-rich alpha-2-glycoprotein; COV, cutoff value; TNF, tumor necrosis factor; MES, Mayo endoscopic subscore.
a ≥3 stools more than the normal daily number or bloody stool.
Table 5.
Longitudinal evaluation of LRG after induction therapy for endoscopic nonremission cases by clinical activity at baseline
| Clinically activea (n = 51) | Clinically inactive (n = 4) | p value | |
|---|---|---|---|
| Median age, years (range) | 30 (11–73) | 36 (17–52) | 0.864 |
| Sex (female:male) n (%) | 20 (39.2):31 (60.8) | 2 (50.0):2 (50.0) | 0.672 |
| Extent of disease involvement (E1:E2:E3), n (%) | 3 (5.9):18 (35.3):30 (58.8) | 1 (25.0):0 (0.0):3 (75.0) | 0.179 |
| MES before induction therapy (2:3), n (%) | 36 (70.6):15 (29.4) | 4 (100):0 (0.0) | 0.203 |
| Median LRG before induction therapy, μg/mL (range) | 21.1 (8.1–65.1) | 17.4 (16.6–21.5) | 0.409 |
| Induction therapy, n (%) | 0.861 | ||
| Mesalamine | 18 (35.3) | 2 (50.0) | |
| Steroids | 10 (19.6) | 2 (50.0) | |
| Thioprines | 7 (13.7) | 1 (25.0) | |
| TNF-alpha inhibitor | 12 (23.5) | 1 (25.0) | |
| Ustekinumab | 5 (9.8) | 0 (0.0) | |
| Vedolizumab | 6 (11.8) | 0 (0.0) | |
| Janus kinase inhibitor | 4 (7.8) | 0 (0.0) | |
| Median days from treatment initiation to post-treatment endoscopy | 196 (14–753) | 312 (100–483) | 0.537 |
| Endoscopic remission after treatment, n (%) | 30 (58.8) | 2 (50.0) | 0.730 |
| MES differences (post-pre) | −1.02±0.93 | −0.75±0.96 | 0.579 |
| LRG differences (post-pre), μg/mL | −6.44±10.81 | −2.78±4.22 | 0.507 |
| LRG change ratio ([post-pre]/pre [%]) | −20.1±38.7 | −13.9±20.8 | 0.756 |
LRG, leucine-rich alpha-2-glycoprotein; COV, cutoff value; TNF, tumor necrosis factor; MES, Mayo endoscopic subscore.
a ≥3 stools more than the normal daily number or bloody stool.
Discussion
According to a meta-analysis, achieving mucosal healing could reduce the risk of recurrence and surgery and promote long-term mucosal healing [8]. Since mucosal healing has become an accepted treatment goal, there is a growing interest in determining the methods for its evaluation. Although colonoscopy is crucial for assessing mucosal healing and remains the gold standard for evaluating the disease activity in patients with UC, it is invasive and difficult to perform frequently and may worsen symptoms in them [9]. Therefore, there is a demand for reliable noninvasive surrogate markers that could substitute for colonoscopy.
In this study, we assessed the correlation between LRG level and endoscopic activity in patients with UC and also compared its efficacy as a predictor of mucosal healing with that of other biomarkers and symptoms. Blood biomarkers are convenient to measure for both patients and physicians. According to the ROC curve analysis, our study revealed that LRG was a more effective biomarker for predicting endoscopic activity than CRP, leukocyte, neutrophil, platelet, or albumin. Additionally, serum LRG levels were positively correlated with the endoscopic activity. To date, some studies have assessed the role of LRG in patients with UC. Serada et al. [2] found elevated LRG levels in patients with UC, which strongly correlated with clinical activity compared with CRP, whereas endoscopic evaluation was not included in the study. Shinzaki et al. [3] found that LRG was more strongly correlated with clinical and endoscopic activities than CRP in patients with UC (n = 129). They also demonstrated that LRG levels could be used for distinguishing between clinical remission and mucosal healing, even in patients with normal CRP levels. Yasutomi et al. [10] and Matsumoto et al. [11] found that LRG correlated with endoscopic activity and could predict mucosal healing in patients with UC (n = 166 and n = 190). Compared with the results of previous reports, our study, comprising a larger number of patients, supported previous studies’ results regarding the usefulness of LRG for predicting the endoscopic activity. Additionally, we evaluated the association between blood markers other than CRP and endoscopic activity, which has rarely been studied, and the results revealed that LRG was the most beneficial blood marker. The patients were consecutively recruited in this study, making it more representative of actual clinical settings than those arbitrarily selected with varying levels of activity in previous reports to demonstrate the performance of LRG.
Although the reference value of LRG was found to be 16.0 μg/mL [2, 3], reports on cutoff values for predicting mucosal healing in patients with UC are extremely limited. In our study, the cutoff value for LRG was 15.6 μg/mL, which was close to the reference value. Therefore, the sensitivity and specificity could be regarded as being clinically useful.
Analyzing the association between endoscopic activity and LRG levels for each disease distribution, the cutoff values for E2 (left-sided colitis) and E3 (pancolitis) were close to those previously reported, and both sensitivity and specificity were considered clinically useful. However, in E1 (proctitis), sensitivity was deemed low. Moreover, the usefulness of LRG levels as a biomarker remained unclear. Horiuchi et al. [12] reported that the LRG level was not elevated in patients with E1 (proctitis) disease and that LRG could not predict the presence of ulcerative proctitis. The degree of serum LRG level elevation may be closely related to the extent of colorectal mucosa with active inflammation, with the result that the LRG level was not elevated in our patients with E1 (proctitis) disease compared with that in participants with E2 (left-sided colitis) or E3 (pancolitis) diseases. The number of patients with E1 (proctitis) disease was insufficient even in this study. Therefore, the usefulness of LRG for E1 (proctitis) disease should be further elucidated by accumulating cases.
Our study showed that an LRG level below the cutoff value could not solely exclude endoscopic nonremission. However, all patients with LRG levels below the LRG cutoff value and without diarrhea or bloody stool were in endoscopic remission. In subsequent multivariate analysis, LRG was an independent factor of endoscopic remission. Additionally, clinical activity, such as diarrhea and bloody stool, was added to a factor of the multivariate analysis. Specifically, LRG reflected endoscopic activity independently, regardless of the symptoms. This point has not been previously reported, making it a noteworthy aspect of our study.
In models 2 and 3, higher albumin level was an independent factor in endoscopic remission. A suppressed albumin reflects a systemic inflammatory burden and is a known poor prognostic marker in multiple disease entities, including UC [13]. However, in endoscopic remission, higher age was an independent factor only in model 1, possibly because children with inactive UC tended to avoid endoscopy more than adults.
A previous study revealed that baseline LRG levels, which suggest mucosal healing, differed among patients [11]. Shinzaki et al. [4] reported that LRG levels reflected clinical and endoscopic disease activities at each predetermined time point and that they could be determined before treatment initiation for inducing remission, and subsequently measured periodically to monitor changes in disease activity. Our longitudinal evaluation revealed that the difference and change ratio of LRG pre- and post-treatment were statistically significant above the cutoff value of LRG. This suggests that a higher value of LRG could be more useful as a biomarker since the decrease in LRG has become more pronounced. Therefore, it can be inferred that the difference in LRG would be more pronounced in cases of higher LRG values. However, the change ratio of LRG pre- and post-treatment was also more pronounced in cases of higher LRG values. Although some reports have assessed the delta value of LRG [11], only a few have assessed the LRG change ratio. We expected that if this ratio of the low LRG group was prominent, as well as that of the high LRG group, we could find further usefulness of LRG as a biomarker of UC. However, the change ratio of the low LRG group was not conspicuous in our study. Thus, we could not find any usefulness of LRG in the low LRG group in the longitudinal study. Regarding clinical activity before induction therapy, no statistical difference was observed in the trends of MES and LRG. Although the number of clinically inactive cases was low in our study, this suggests that induction therapy, whether in clinically active or inactive cases, resulted in a similar decrease in LRG as endoscopic findings improved.
A question remains on how LRG should be clinically used. In asymptomatic patients, if the LRG level is below the cutoff value, the probability of endoscopic activity is considered significantly low, and endoscopy for follow-up purposes (not for cancer screening evaluation) may be omitted. However, even in asymptomatic patients, an elevated LRG level may indicate endoscopic activity and may be a criterion for the decision to perform a colonoscopy. Additionally, scoring blood biomarkers, in addition to symptoms, is crucial to predicting endoscopic activity assessment. Therefore, using a well-designed validation study protocol and collecting data directly from the participants, we hope to provide robust evidence of the potential of LRG as a diagnostic marker for UC. Particularly, we should take into account the risk of carcinogenesis and not omit endoscopy for cancer screening.
Although a recent study has reported no difference in LRG levels between healthy individuals and patients with UC [14], certain factors, such as age, sex, and BMI, could affect LRG values. A previous study demonstrated an association between CRP and fecal calprotectin levels and age, sex, and BMI [15–19]. Since we could not perform adjustments based on these variables in our analysis due to insufficient data, further data collection is necessary for a better understanding of the effects of the factors affecting LRG.
This study has some limitations. First, it was conducted at a single institution. Second, histological studies were not evaluated. Third, novel biomarkers, such as anti-integrin αvβ6, and nonblood biomarkers, such as fecal calprotectin and prostaglandin E-major urinary metabolite, were not evaluated. Finally, this retrospective study relied on existing medical records, which may contain incomplete or inaccurate information and could not be controlled for confounding variables that were not recorded in the data.
In conclusion, LRG reflected endoscopic activity independently, regardless of clinical symptoms. An LRG below the cutoff value could indicate a significantly low probability of endoscopic activity in asymptomatic patients, wherein follow-up endoscopy (not cancer screening) may be unnecessary. Furthermore, a higher LRG level might be more useful as an indicator of treatment efficacy.
Acknowledgments
The authors wish to thank Keiichiro Iki, Hideaki Shunto, Takafumi Nakagawa, Kazunori Kawaguchi, Satoko Inagaki, Takuya Seike, Yusuke Minato, and all the endoscopy medical staff at Kanazawa University Hospital and Editage (www.editage.com) for English language editing.
Statement of Ethics
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study design was observational. The study was approved by the Ethics Committee of our institution (Institutional Review Board of Kanazawa University Hospital). The IRB approval number is 2016-211. The study was performed in accordance with the ethics guidelines in Japan and was allowed implementation without obtaining individual written informed consent. This study was announced by posters in the outpatient ward or on the homepage of the participating institutions. Patients who expressed unwillingness to participate in this study were excluded.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding support to declare.
Author Contributions
Tomoyuki Hayashi was involved in designing the study, collecting and interpreting data, and writing the manuscript. Kazuya Kitamura, Hajime Takatori, Masaaki Usami, Shinya Yamada, Masaki Miyazawa, Masaki Nishitani, Akihiro Dejima, Makoto Yamamoto, Shotaro Kawase, Masaya Funaki, Noriaki Orita, Hidetoshi Nakagawa, Kouki Morita, Noriho Iida, Akihiro Seki, Kouki Nio, Hidenori Kido, Hideo Takayama, Yuta Takeuchi, Mari Shimada, Hiroto Saito, Daisuke Yamamoto, and Taro Yamashita participated in data collection and analysis. Tadashi Toyama performed statistical analysis and provided advice. All authors critically revised the report, commented on manuscript drafts, and approved the final report.
Funding Statement
The authors have no funding support to declare.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.
References
- 1. Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010 Apr;69(4):770–4. 10.1136/ard.2009.118919. [DOI] [PubMed] [Google Scholar]
- 2. Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012 Nov;18(11):2169–79. 10.1002/ibd.22936. [DOI] [PubMed] [Google Scholar]
- 3. Shinzaki S, Matsuoka K, Iijima H, Mizuno S, Serada S, Fujimoto M, et al. Leucine-rich alpha-2 glycoprotein is a serum biomarker of mucosal healing in ulcerative colitis. J Crohns Colitis. 2017 Jan;11(1):84–91. 10.1093/ecco-jcc/jjw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shinzaki S, Matsuoka K, Tanaka H, Takeshima F, Kato S, Torisu T, et al. Leucine-rich alpha-2 glycoprotein is a potential biomarker to monitor disease activity in inflammatory bowel disease receiving adalimumab: PLANET study. J Gastroenterol. 2021 Jun;56(6):560–9. 10.1007/s00535-021-01793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. 2021 Jun;56(6):489–526. 10.1007/s00535-021-01784-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006 Jun;55(6):749–53. 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987 Dec;317(26):1625–9. 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 8. Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016 Sep;14(9):1245–55.e8. 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 9. Mukewar S, Costedio M, Wu X, Bajaj N, Lopez R, Brzezinski A, et al. Severe adverse outcomes of endoscopic perforations in patients with and without IBD. Inflamm Bowel Dis. 2014 Nov;20(11):2056–66. 10.1097/MIB.0000000000000154. [DOI] [PubMed] [Google Scholar]
- 10. Yasutomi E, Inokuchi T, Hiraoka S, Takei K, Igawa S, Yamamoto S, et al. Leucine-rich alpha-2 glycoprotein as a marker of mucosal healing in inflammatory bowel disease. Sci Rep. 2021 May;11(1):11086. 10.1038/s41598-021-90441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsumoto S, Mashima H. Usefulness of the optimal cutoff value and delta value of leucine-rich alpha 2 glycoprotein in ulcerative colitis. Crohns Colitis 360. 2022 Nov;4(4):otac039. 10.1093/crocol/otac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horiuchi I, Horiuchi A, Umemura T. Serum leucine-rich α2 glycoprotein: a biomarker for predicting the presence of ulcerative colitis but not ulcerative proctitis. J Clin Med. 2022 Oct;11(21):6366. 10.3390/jcm11216366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Con D, Andrew B, Nicolaides S, van Langenberg DR, Vasudevan A. Biomarker dynamics during infliximab salvage for acute severe ulcerative colitis: C-reactive protein (CRP)-lymphocyte ratio and CRP-albumin ratio are useful in predicting colectomy. Intest Res. 2022 Jan;20(1):101–13. 10.5217/ir.2020.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kourkoulis P, Michalopoulos G, Katifelis H, Giannopoulou I, Lazaris AC, Papaconstantinou I, et al. Leucine-rich alpha-2 glycoprotein 1, high mobility group box 1, matrix metalloproteinase 3 and annexin A1 as biomarkers of ulcerative colitis endoscopic and histological activity. Eur J Gastroenterol Hepatol. 2020 Sep;32(9):1106–15. 10.1097/MEG.0000000000001783. [DOI] [PubMed] [Google Scholar]
- 15. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999 Dec;282(22):2131–5. 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 16. Chenillot O, Henny J, Steinmetz J, Herbeth B, Wagner C, Siest G. High sensitivity C-reactive protein: biological variations and reference limits. Clin Chem Lab Med. 2000 Oct;38(10):1003–11. 10.1515/CCLM.2000.149. [DOI] [PubMed] [Google Scholar]
- 17. Reece AS. High-sensitivity CRP in opiate addiction: relative and age-dependent elevations. Cardiovasc Toxicol. 2012 Jun;12(2):149–57. 10.1007/s12012-012-9154-2. [DOI] [PubMed] [Google Scholar]
- 18. Shen J, Chung SY, Azimi-Nekoo E, Jose J, Saif MW. A rare case of gemcitabine-induced pulmonary hypertension. Cancer Epidemiol. 2019 Jun;5:1–3. 10.17140/PRRMOJ-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poullis A, Foster R, Shetty A, Fagerhol MK, Mendall MA. Bowel inflammation as measured by fecal calprotectin: a link between lifestyle factors and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004 Feb;13(2):279–84. 10.1158/1055-9965.epi-03-0160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.