Abstract
Renal involvement represents the major long-term morbidity associated with IgA vasculitis (IgAV). Our aim was to evaluate clinical characteristics and long-term renal outcomes of IgAV in pediatrics and adults comparing to IgA nephropathy (IgAN). Our retrospective study included children and adults with IgAV and IgAN patients, admitted in a 13-year period (2007–2019) to rheumatology clinics and in hospital pediatric and internal medicine departments. We compared frequencies of clinical manifestations, laboratory findings, treatments, long-term outcomes at 1 year follow-up, including all-cause mortality and dialysis until the end of follow-up time. A total of 60 adult IgAV, 60 pediatric IgAV and 45 IgAN patients were evaluated. Adult IgAV patients were significantly older than IgAN patients (53.1 ± 17.4 years vs 45.1 ± 15.7 years respectively, P = .02) and had significantly higher rates of cardiovascular comorbidities. The risk and time to dialysis were similar among IgAN and adult IgAV groups. Yet, overall mortality at long term follow up was higher in IgAV adult group compared to IgAN. No dialysis or renal transplantation were reported in pediatric IgAV patients. IgAV and IgAN adult patients were comparable regarding risk of end stage renal disease. Of note, high mortality rates were observed among adult IgAV group.
Keywords: dialysis, IgA nephropathy, IgA vasculitis, kidney
1. Introduction
IgA vasculitis (IgAV), formerly known as Henoch-Schönlein purpura, is the most common form of childhood vasculitis and is often regarded as a benign disease, with a self-limited course.[1] While IgAV has been extensively studied in children, much less is known about its natural history in adults. Previous data imply significant differences between both age groups in aspects of clinical presentations and outcomes.[2] In approximately half of pediatric-onset IgAV patients, the disease onset is preceded by a bacterial infection (most frequently streptococcal pharyngitis), whereas adults-onset disease is associated with various contributing factors, such as solid tumors, medications use and comorbidities of other autoimmune diseases.[3,4] Renal involvement which is less frequently seen in pediatric patients appears at a significantly higher rate of adult patients (20–28% vs 45–85%, respectively).[5] Adults with IgAV have an increased risk of progression to chronic renal failure as compared to children (8–68% vs 2–15%).[6] Hence, adults with IgAV may benefit from an intensive treatment to prevent the progression to chronic kidney disease. A French multicenter survey study of 260 adult IgAV patients showed that corticosteroid use was found to be effective in this population, albeit its potential harms.[7]
Although the comprehensive pathogenesis of IgAV with nephritis has not been fully elucidated, it shares cardinal features with IgA nephropathy (IgAN). The onset of both IgAV and IgAN is associated with preceding episodes of upper respiratory tract infections, which suggests that mucosal antigens may play a role in their development.[8] Both IgAV with nephritis and IgAN are characterized by the hallmark finding of mesangial deposition of IgA1-containing immune complexes, which potentially trigger mesangial proliferation and glomerular damage.[9] Due to their similarities, IgAV and IgAN considered to be 2 manifestations of the same entity, however the multi-system involvement in IgAV implies that they might have different underlying origins.[9]
The aim of the current study was to investigate differences in clinical course, laboratory findings and management between adult-onset IgAV, childhood IgAV and non vasculitic IgAN. In addition, we compared long-term renal outcomes and survival between adult-onset IgAV and IgAN.
2. Methods
This collaborative retrospective study examined a total of 165 health records of patients who were admitted to the Rheumatology and Pediatric departments at Ha’Emek Medical Center and Rambam Health Care Campus, within a 13-year period (2007–2019). The population study consisted of 3 groups: 60 adults with IgAV, 60 children (age < 18 years) with IgAV and 45 adults with IgAN. Patients were included in a consecutive manner, till maximum number of 60 patients per group, according to available registries of IgAV adult patients with skin biopsy-proven disease. The minimal sample size per group was calculated based on the expected rate of the end-stage renal failure in IgAV in adults compared to children and IgAN patients to achieve an error of 0.05 and 80% power. This study was performed in accordance with the ethical standards of the Helsinki Declaration and was approved by the ethics committees of Ha’EmekMedical Center (0133-18-EMC) and Rambam Health Care Campus (0013-19-RMB).
2.1. Inclusion criteria
2.1.1. Adult IgAV.
The diagnosis of IgAV in adults met the EULAR/PRINT/PRES criteria.[10] We mandated a confirmed skin biopsy interpreted by an expert skin pathologist presenting of leukocytoclastic vasculitis under light microscopy and predominant IgA deposition by direct immunofluorescence.
2.1.2. Pediatric IgAV.
Pediatric patients also fulfilled EULAR/PRINT/PRES criteria.[10] Their diagnoses were obtained by a pediatric rheumatologist or an expert pediatrician. No skin biopsy was mandated in this group.
2.1.3. Adult IgAN.
The diagnosis of IgAN required a kidney biopsy showing mesangial IgA deposition and an expert nephrologist diagnosis.
2.1.4. Follow up.
Follow up of adult-onset IgAV patients started at the date of skin biopsy or at the time of documented rash if it occurred more than a month before biopsy. For pediatric IgAV patients, follow up started from the date of clinical diagnosis. For IgAN group, follow up started at the date of first documented major renal dysfunction or at time of retrieving the diagnostic biopsy, whichever occurred first.
2.2. Exclusion criteria
Two adult IgAV patients with biopsy-proven kidney cancer (renal cell carcinoma, transitional cell carcinoma) were excluded from our analysis. Since vasculitis can occur in the context of other autoimmune diseases, we didn’t exclude patients with concomitant rheumatic disorders. Hence, 4 subjects with the following co-morbidities were included in our study: Gout, Familial Mediterranean fever, Periodic fever, aphthous stomatitis, pharyngitis, and adenitis syndrome and Rheumatoid Arthritis.
2.3. Statistical analysis
Continuous data were described as mean and standard deviation (mean ± SD), and categorical variables as percentages. Chi-square test was used to compare categorical variables. All tests were 2-tailed, and a confidence interval of 95% (P < .05) was considered statistically significant. Subsequently, the primary endpoints, survival probability and time for dialysis, were compared between IgAN and adult IgAV groups using Kaplan–Meier curves. Categorical variables were presented as a number (percentage) and continuous variables as mean ± standard deviation (range). When existing data were based on incomplete study group, numbers of available patients for evaluation were listed near the results. Analyses were performed using SPSS Statistics 28.0.1 (IBM Inc., Chicago, IL) software.
2.4. Clinical and laboratory data
We collected demographical characteristics of patients at baseline and followed them up at 6-month intervals for 1 year. We analyzed and compared the frequencies of their clinical manifestations, laboratory data and treatments. Primary outcomes for adult-onset IgAV and IgAN groups were defined as time to dialysis and all-cause mortality, that were followed up until the end of the data collection. Hematuria and leukocyturia were defined as the presence of more than 30 red blood cells or leukocytes in urine per high power field, respectively. Dipstick proteinuria was defined as level greater than 30 mg/dL in general urine test, or “++” on dipstick urinalysis. Significant proteinuria was considered as the presence of more than 300 mg protein in 24-hours urine collection or urine protein to creatinine ratio above 300 mg/g.
3. Results
3.1. Baseline characteristics
A total of 60 adult IgAV, 60 pediatric IgAV and 45 IgAN patients were included in our study. The adult IgAV group was significantly older than the IgAN group (53.1 ± 17.4 years vs 45.1 ± 15.7 years, P = .02). The groups differed by sex distribution with male predominance in the IgAN group (35, 77.8%) as compared to the IgAV groups (25, 41.7% for adults, 33, 55% for pediatrics) (P = .001). No marked difference was observed in their ethnical origin. Table 1 describes the demographic and clinical characteristics of each study group at baseline. Adult-onset IgAV patients were associated with higher rates of preexisting comorbidities (see Table 1 for details). A history of preceding infection was more prevalent among pediatric-onset IgAV group as compared to adult IgAV and IgAN groups (44.8%, 20.0%, and 13.6% respectively, P = .001). Of note, prior antibiotic use was also more prevalent in pediatric IgAV patients but without a significant difference between groups. Further baseline characteristics are outlined in Table 1.
Table 1.
Patient demographics and clinical characteristics at presentation.
| IgAN | IgAV adults | IgAV pediatrics |
P value IgAV adults, IgAN |
P value IgAV adults, pediatrics |
Overall P value |
|
|---|---|---|---|---|---|---|
| N | 45 | 60 | 60 | |||
| Age (years) | 45.1 ± 15.7 (19.1–77.3) |
53.1 ± 17.4 (18.7–88.2) |
7.28 ± 3.5 (2.95–17.2) |
.02 | N/A | N/A |
| Gender | ||||||
| Male | 35 (77.8%) | 25 (41.7%) | 33 (55.0%) | .001 | .14 | .001 |
| Female | 10 (22.2%) | 35 (58.3%) | 27 (45.0%) | |||
| Ethnic group | ||||||
| Jewish | 12 (26.7%) | 26 (43.3%) | 21 (35.0%) | .08 | .35 | .21 |
| Arab | 33 (73.3%) | 34 (56.7%) | 39 (65.0%) | |||
| BMI (kg/m2) | 27.17 ± 4.0139 (16.9–35.7) |
33.16 ± 6.9551 (19.8–55.0) |
17.54 ± 4.2559 (13–35) |
.001 | N/A | N/A |
| Obesity (BMI > 30) | 9/39 (23.1%) | 34/51 (66.7%) | N/A | .001 | N/A | N/A |
| Smoking | 19/44 (43.2%) | 21 (35.0%) | N/A | .40 | N/A | N/A |
| Co-morbidities | ||||||
| Diabetes Mellitus type II | 11 (24.4%) | 26 (43.3%) | 0 (0.0) | .05 | N/A | N/A |
| Ischemic heart disease | 2 (4.4%) | 11 (18.3%) | N/A | .03 | N/A | N/A |
| Malignancy | 0 (0%) | 4 (6.7%) | 0 (0%) | 0.13 | N/A | N/A |
| Preceding infection | 0/44 (0%) | 4 (6.7%) | 8/58 (13.8%) | .22 | .02 | .003 |
| Positive culture Probable |
6/44 (13.6%) | 8 (13.3%) | 18/58 (31.0%) | |||
| Medications | ||||||
| Antibiotics | 4 (8.9%) | 11 (18.3%) | 15 (25.0%) | .26 | .38 | .11 |
| ACEI/ARB | 18 (40.0%) | 19 (31.7%) | N/A | .38 | N/A | N/A |
| Beta blockers | 10 (22.2%) | 16 (26.7%) | N/A | .60 | N/A | N/A |
| Antidiabetic drugs | 5 (11.1%) | 19 (31.7%) | N/A | .01 | N/A | N/A |
| Corticosteroids | 2 (4.4%) | 2 (3.3%) | N/A | >.99 | >.99 | 0.70 |
| Immunosuppressants | 1 (2.2%) | 0 (0.0%) | N/A | .43 | N/A | N/A |
| PPI or H2B | 7 (15.6%) | 22 (36.7%) | N/A | .02 | N/A | N/A |
| Diuretics | 2 (4.4%) | 19 (31.7%) | N/A | .001 | N/A | N/A |
Bold values represents significant P-values. Superscript numbers are number of patients with available information.
ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, BMI = body mass index, H2B = histamine-2 receptor blocker, IgAN = IgA nephropathy, IgAV = IgA vasculitis, N = number of patients, N/A = not available, PPI = proton-pump inhibitor.
3.2. Initial presentation
Table 2 describes the initial clinical presentation and treatments that patients received within 2 months from study entry. All IgAV patients, both children and adults, exhibited non-thrombocytopenic palpable purpura, which was a mandatory feature for their diagnosis. By contrast, no skin rash was documented among IgAN patients. A notable portion of adults and children with IgAV had joint involvement, as opposed to IgAN patients that didn’t present joint-related symptoms. Arthralgia and arthritis were most common in pediatric-onset IgAV group as compared to adult IgAV and IgAN groups (for arthritis: 68.3%, 20% and 0% respectively, P < .001). Pediatric and adult IgAV patients had also higher rates of abdominal pain, as compared to IgAN group (51.7% and 35.0% vs 11.4%, respectively, P < .01). Of note, pediatric IgAV patients were more likely to experience abdominal pain as compared to adults with IgAV (51.7% vs 35.0%, P < .06) but it did not reach a statistical significance. Regarding gastrointestinal involvement, hematochezia occurred in 5 children and 2 adults with IgAV and was not observed in IgAN patients. Two children with IgAV (3.3%) and 1 IgAN patient (2.3%) suffered from intussusception.
Table 2.
Routine laboratory and clinical findings at study entry
| IgAN | IgAV adults | IgAV pediatrics | P value IgAV adults, IgAN | P value IgAV adults, pediatrics | Overall P value | |
|---|---|---|---|---|---|---|
| N | 45 | 60 | 60 | |||
| Clinical presentation | ||||||
| Palpable purpura | 0/44 (0.0%) | 60 (100.0%) | 60 (100.0%) | N/A | N/A | N/A |
| Arthralgia | 0/44 (0.0%) | 6 (10.0%) | 27 (45.0%) | .001 | .001 | .001 |
| Arthritis | 0/44 (0.0%) | 12 (20.0%) | 41 (68.3%) | .002 | .001 | .001 |
| Abdominal pain | 4/35 (11.4%) | 21 (35.0%) | 31 (51.7%) | .01 | .06 | .001 |
| Hematochezia | 0/44 (0.0%) | 2 (3.3%) | 5 (8.3%) | .51 | .44 | .10 |
| Intussusception | 1/44 (2.3%) | 0 (0.0%) | 2 (3.3%) | .42 | .50 | .38 |
| Renal involvement | ||||||
| Serum creatinine (mg/dL) | 1.924 ± 1.717 (0.63–10.20) |
1.144 ± 1.141 (0.50–6.93) |
0.418 ± 0.13759 (0.19–0.72) |
.001 | .001 | .001 |
| Hematuria | 38/44 (86.4%) | 21/49 (42.9%) | 8 (13.6%) | .001 | .001 | .001 |
| Leukocyturia | 4/44 (9.1%) | 13/49 (26.5%) | 9/59 (15.3%) | .03 | .15 | .07 |
| Dipstick proteinuria | 40 (88.9%) | 21/49 (42.9%) | 20/59 (33.9%) | .001 | .34 | .001 |
| Proteinuria > 300 (mg/day) or Urine P/C > 300 (mg/g) | 40/42 (95.2%) | 23/38 (60.5%) | 13/31 (41.1%) | <.001 | .13 | <.001 |
| Proteinuria (mg/24h) | 2609.0 ± 1854.536 (249–7522.8) |
1303.06 ± 2486.8629 (57.2–10,648.2) |
1654.98 ± 3281.2115 (30–11,600.0) |
.001 | .25 | .001 |
| Urine P/C (mg/g) | 2224.6 ± 1661.332 (151.4–5662.3) |
1123.76 ± 1758.6229 (36–7889.6) |
2812.10 ± 8807.4519 (105.5–39,000) |
.001 | .22 | .001 |
| Laboratory studies | ||||||
| C-reactive protein (mg/dL) | 2.66 ± 4.64 24 (0.05–17.30) |
5.84 ± 6.32 57 (0.25–26.30) |
3.39 ± 2.46 43 (0.15–9.59) |
.001 | .09 | .001 |
| IgA > 300 mg/dL | (78.6%) 11/14 460.21 ± 240.16 |
19/30 (63.3%) 373.60 ± 160.54 |
2/8 (25%) 221.13 ± 91.54 |
.30 | .05 | .05 |
| IgG (mg/dL) | 988 ± 189.99 17 (669–1282) |
1273.69 ± 547.68 29 (302–2336) |
1029.71 ± 253.86 7 (607–1336) |
.02 | .26 | .08 |
| IgM (mg/dL) | 98.19 ± 48.33 16 (27–214) |
108.66 ± 83.08 28 (24–420) |
95.81 ± 41.94 7 (46–169) |
.65 | .70 | .84 |
| C3 (mg/dL) | 116.60 ± 19.84 29 (69.3–154.7) |
134.22 ± 36.05 41 (37–198.3) |
148.32 ± 24.52 16 (110–199) |
.05 | .16 | .002 |
| C4 (mg/dL) | 35.52 ± 11.36 29 (13.5–64.4) |
28.66 ± 11.84 40 (3.0–51.8) |
31.80 ± 9.14 16 (19.0–54.0) |
.04 | .35 | .10 |
| ANA Titers | ||||||
| 1:80 | 0/34 (0.0%) | 0/47 (0.0%) | 5/13 (38.5%) | .50 | .001 | .001 |
| 1:160 | 2/34 (5.9%) | 3/47 (6.3%) | 0 (0.0%) | |||
| 1:320 | 1/34 (2.9%) | 0/47 (0.0%) | 0 (0.0%) | |||
| ENA positive | 4/30 (13.3%) | 3/39 (7.7%) | 0/9 (0.0%) | .44 | >.99 | .44 |
| Initial treatment | ||||||
| NSAID | 0 (0.0%) | 6/53 (11.3%) | 35 (58.3%) | .03 | <.001 | .001 |
| Prednisone | 14 (31.1%) | 26/55 (47.3%) | 41 (68.3%) | .10 | .02 | .001 |
| IV pulse steroids | 6 (13.3%) | 7/54 (13.0%) | 13 (21.7%) | .96 | .22 | .37 |
| ACEi/ARB | 28 (62.2%) | 21/55 (38.2%) | 2 (3.3%) | .02 | <.001 | .001 |
| Cyclophosphamide | 0 (0.0%) | 1/54 (1.9%) | 0 (0.0%) | >.99 | .47 | .38 |
| Cyclosporine | 0 (0.0%) | 1/54 (1.9%) | 1 (1.7%) | >.99 | >.99 | .67 |
| Imuran | 0 (0.0%) | 1/55 (1.8%) | 0 (0.0%) | >.99 | .48 | .38 |
| Dermatologic topical drug | 0 (0.0%) | 18/55 (32.7%) | 3 (5.0%) | <.001 | <.001 | .001 |
| Colchicine | 0 (0.0%) | 7/52 (13.5%) | 0 (0.0%) | .36 | .003 | .001 |
| Dialysis | 3 (6.8%) | 7/55 (12.7%) | 0 (0.0%) | .50 | .005 | .02 |
Bold values represents significant P-values. Superscript numbers are number of patients with available information.
ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, IgAN = IgA nephropathy, IgAV = IgA vasculitis, IV = intravenous, N = number of patients, N/A = not available, NSAID = non-steroidal anti-inflammatory drug, Urine P/C = urine protein to urine creatinine ratio.
Development of renal disease was less frequently seen in childhood-onset IgAV and was more common in adults with IgAV or IgAN. At onset, IgAN versus adult and pediatric IgAV, had statistically significant different serum creatinine levels (1.92 mg/dL, 1.14 mg/dL, 0.42 mg/dL respectively, P < .001). Additional urinary abnormalities such as hematuria and proteinuria were most prominent among IgAN group (see Table 2 for details). Significant was observed in 95.2% of IgAN patients, 60.5% of adult IgAV patients and 41.1% of pediatric IgAV patients, with significant difference (P < .001) for the comparison between IgAN and adult IgAV. Measurements of significant proteinuria were not available for all patients as data was collected retrospectively. Hematuria was detected in 86.4% of IgAN patients, while presented in 42.9% of IgAV adult patients and was less common in children with IgAV (13.6%) (P < .001 for all).
To further assess disease activity, we obtained levels of inflammatory biomarkers, complement components and auto antibodies. Both IgAV groups had significantly higher levels of C-reactive protein (CRP) as compared to IgAN group (5.84 mg/dL, 3.39 mg/dL vs, 2.66 mg/dL respectively, P < .001). Increased systemic IgA levels were most prominently seen in IgAN group and in lesser extent in adult-onset IgAV patients, while it was less frequent among pediatric IgAV patients (78.6%, 63.3%, 25%, respectively, P = .05). No reduced C3 and/or C4 levels were observed, although tests were only available for approximately half of the patients.
As for initial management, different types of treatments were applied at each study group. Most of the children with IgAV were treated with nonsteroidal anti-inflammatory drugs (NSAIDs), while NSAIDs were uncommonly used in adult-onset IgAV (58.3% vs 11.3%. P < .001) and were not used in IgAN patients. Similarly, 68.3% of pediatric patients received oral prednisone during the first 2 month from presentation, compared to 47.3% of adult IgAV and 31.1% of IgAN groups (P < .01 overall, and P = .1 between adult groups). Angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) were the main initial treatment for IgAN patients and were also given to a considerable amount of IgAV adult patients, though rarely used in children (62.2%, 38.2%, 3.3% respectively, P < .001). 7 adult-onset IgAV and 3 IgAN patients and no pediatric patients commenced hemodialysis within 2 months from disease presentation.
3.3. Half year follow up
At half year follow up, data was available for 49 IgAV adult patients (81.7%), 13 IgAV pediatrics patients (21.7%) and 44 IgAN patients (97.8%). The clinical features, evaluation of renal involvement and treatments applied at 6-month follow up were described in Table 3.
Table 3.
Outcomes at half year follow-up.
| IgAN | IgAV adults | IgAV pediatrics |
P value IgAV adults, IgAN |
P value IgAV adults, pediatrics |
Overall P value |
|
|---|---|---|---|---|---|---|
| N | 44 | 49 | 13 | |||
| Clinical manifestations | ||||||
| Palpable purpura | 0/43 (0.0%) | 4/44 (9.1%) | 2/9 (22.2%) | .12 | .27 | .02 |
| Abdominal pain | 4/43 (9.3%) | 4/44 (9.1%) | 0 (0.0%) | >.99 | >.99 | .64 |
| Hematochezia | 0/43 (0.0%) | 1/44 (2.3%) | 0 (0.0%) | >.99 | >.99 | .56 |
| Arthritis/Arthralgia | 0/43 (0.0%) | 5/42 (11.9%) | 1/9 (11.1%) | .03 | .95 | .04 |
| Renal involvement | ||||||
| Serum creatinine (mg/dL) | 1.863 ± 2.439 43 (0.61–16.75) |
1.296 ± 1.442 43 (0.49–6.97) |
0.516 ± 0.137 10 (0.31–0.73) |
.001 | .001 | .001 |
| Hematuria | 23/36 (63.9%) | 13/28 (46.4%) | 6/13 (46.2%) | .16 | .99 | .30 |
| Leukocyturia | 3/38 (7.9%) | 6/27 (22.2%) | 2/12 (16.7%) | .15 | .69 | .26 |
| Dipstick proteinuria | 30/39 (76.9%) | 12/27 (44.4%) | 4/13 (30.8%) | .007 | .40 | .003 |
| Proteinuria > 300 (mg/d) or Urine P/C > 300 (mg/g) | 38/43 (88.4%) | 14/44 (31.8%) | 1/9 (11.1%) | .001 | .42 | .001 |
| Proteinuria (mg/24h) | 1703.5 ± 1625.7 36 (139.6–8463.8) |
1794.2 ± 3197.9 9 (26.0–9931.9) |
1838.9 ± 3025.9 3 (68–5332.9) |
.14 | .60 | .23 |
| Urine P/C ratio (mg/g) | 1455.6 ± 1432.6 34 (94.2–6816.17) |
495.2 ± 147.9 10 (54.2–2070.0) |
1084.3 ± 1276.8 2 (181.4–1987.1) |
.002 | .36 | .01 |
| Treatment | ||||||
| NSAID | 0/43 (0.0%) | 1/41 (2.4%) | 0/8 (0.0%) | .49 | >.99 | .53 |
| Prednisone 20 mg | 14/43 (32.6%) | 2/41 (4.9%) | 2/8 (25.0%) | .001 | .12 | .006 |
| Prednisone 10 mg | 2/43 (4.7%) | 5/41 (12.2%) | 1/8 (12.5%) | .26 | >.99 | .44 |
| Prednisone 1 mg | 1/43 (2.3%) | 3/41 (7.3%) | 1/8 (12.5%) | .35 | .52 | .39 |
| Any dose prednisone | 17/43 (39.5%) | 10/41 (24.4%) | 4/8 (50.0%) | .14 | .15 | .20 |
| Cyclophosphamide | 1/43 (2.3%) | 0/41 (0.0%) | 0/8 (0.0%) | >.99 | N/A | .56 |
| Cyclosporine | 2/43 (4.7%) | 0/41 (0.0%) | 2/8 (25.0%) | .49 | .001 | .006 |
| Imuran | 1/43 (2.3%) | 3/41 (7.3%) | 0/8 (0.0%) | .35 | >.99 | .44 |
| ACEi/ARB | 30/43 (69.8%) | 9/41 (22.5%) | 2/8 (25.0%) | .001 | >.99 | .001 |
| Colchicine | 0/43 (0.0%) | 1/40 (2.5%) | 0/8 (0.0%) | >.99 | >.99 | .77 |
| Dialysis | 1/43 (2.3%) | 4/41 (9.1%) | 0/8 (0.0%) | .36 | >.99 | .27 |
Bold values represents significant P-values. Superscript numbers are number of patients with available information.
ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, IgAN = IgA nephropathy, IgAV = IgA vasculitis, N = number of patients, N/A = not available, NSAID = non-steroidal anti-inflammatory drug, Urine P/C = urine protein to urine creatinine ratio.
Clinically, 4 adults and 2 children with IgAV had active rash after 6 months from disease presentation (P = .27). No rash was observed in IgAN group. Joint involvement was reported by 5 adults and 1 child with IgAV (P = .95) but wasn’t described in IgAN patients (P < .04).
At 6-months follow up, we found a statistically significant difference in creatinine levels between the 3 study groups. IgAN patients had the highest mean creatinine level and IgAV adult patients had higher creatinine levels as compared to IgAV pediatrics group (creatinine levels of 1.86 mg/dL vs, 1.3 and 0.51 respectively, P < .01). Notably, mean creatinine levels in the 3 groups remained similar to the values presented at initial presentation. Further findings are summarized in Table 3.
Due to their persistent renal and clinical manifestations, a subset of patients from the 3 groups still required medical treatment. IgAN patients received significantly higher rates of ACEi/ARBs (69.8% vs, 22.5% and 25% of IgAN vs adult and pediatric IgAV groups respectively, P < .01) and high dose prednisone in comparison to both IgAV groups (14, 32.6% vs 2, 4.9% and 2, 25% of IgAN vs adult and pediatric IgAV groups respectively, P < .006). Yet, prednisone, ACEi/ARBs and immunosuppressive drugs were administered to a substantial amount of IgAV adult patients (see Table 3). IgAV pediatric patients had a significantly higher rate of cyclosporine treatment as compared to adult IgAV patients (25%, 0% and 2.3% of pediatric IgAV, adult IgAV and IgAN respectively, P = .006). Finally, 4 IgAV adult patients and 1 IgAN patient underwent dialysis, still without a statistically significant difference between these groups.
3.4. One year follow up
At 1 year follow up, data was available for 45 IgAN patients and 42 IgAV adult patients, while most of the pediatric IgAV patients dropped out of medical follow up. Table 4 compares the outcomes and treatments of adults with IgAV and IgAN at 1 year follow up.
Table 4.
Outcomes and treatments of adults with IgAN and IgAV during 1 year follow up and long term mortality.
| IgAN | IgAV adults | P value | |
|---|---|---|---|
| N | 45 | 42 | |
| Clinical manifestations | |||
| Palpable purpura | 0 (0.0%) | 1/31 (3.2%) | .44 |
| Abdominal pain | 1/40 (2.5%) | 2/31 (6.5%) | .41 |
| Arthritis/Arthralgia | 1/40 (2.5%) | 0 (0.0%) | >.99 |
| Renal involvement | |||
| Serum creatinine (mg/dL) | 2.002 ± 2.275 42 (0.54–12.67) |
1.100 ± 1.313 40 (0.46–8.47) |
<.001 |
| Hematuria | 16/30 (53.3%) | 6/16 (37.5%) | .30 |
| Leukocyturia | 1/31 (3.2%) | 3/16 (18.8%) | .11 |
| Dipstick proteinuria | 23/35 (65.7%) | 6/17 (35.2%) | .02 |
| Proteinuria > 300 (mg/d) or Urine P/C > 300 (mg/g) | 31/36 (86.1%) | 6/14 (42.8%) | .002 |
| Proteinuria (mg/24h) | 1375.9 ± 2858.0 32 (113.1–5337.7) |
1478.9 ± 2446.0 7 (767.8; 36–6920) |
.30 |
| Urine P/C ratio (mg/g) | 938.0 ± 827.5 27 (44.1–3596.3) |
1196.2 ± 2858.0 9 (58.9–8781.0) |
.03 |
| Treatment | |||
| NSAID | 0 (0.0%) | 0/26 (0.0%) | N/A |
| Prednisone 20 mg | 8/40 (20.0%) | 0/26 (0.0%) | .02 |
| Prednisone 10 mg | 3/40 (7.5%) | 0/26 (0.0%) | .27 |
| Prednisone 1 mg | 3/40 (7.5%) | 1/26 (3.8%) | >.99 |
| Any dose prednisone | 14/40 (35.0%) | 1/26 (3.8%) | .003 |
| Cyclophosphamide | 0/40 (0.0%) | 0/26 (0.0%) | N/A |
| Cyclosporine | 2/40 (5.0%) | 0/26 (0.0%) | .52 |
| Imuran | 1/40 (2.5%) | 2/26 (7.7%) | .56 |
| ACEi/ARB | 31/40 (77.5%) | 4/26 (15.4%) | <.001 |
| Colchicine | 0 (0.0%) | 1/25 (4.0%) | >.99 |
| Late dialysis | 4/42 (9.5%) | 2/39 (5.1%) | .68 |
| Mortality | |||
| 1-year mortality | 0 (0%) | 7/60 (11.7%) | .02 |
| Long-term deaths | 3/45 (6.67%) | 14/60 (23.33%) | |
| COVID19 | 1 | – | |
| Sepsis | 1 | 4 | |
| Cancer | – | 2 | |
| Cardiovascular | 1 | 6 | |
| Other | – | 2 | |
Bold values represents significant P-values. Superscript numbers are number of patients with available information.
ACEi = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, IgAN = IgA nephropathy, IgAV = IgA vasculitis, N = number of patients, N/A = not available, NSAID = non-steroidal anti-inflammatory drug, Urine P/C = urine protein to urine creatinine ratio.
Of note, most of adult patients with IgAV or IgAN didn’t have documented clinical symptoms after 1 year. However, persistent renal impairment at varying degrees was observed at both groups. Comparing to previous findings at 6-months follow up, IgAN group had a slight increase in their mean creatinine level and continued to have significantly higher creatinine level as compared to IgAV adults group (2 mg/dL vs 1.1 mg/dL, respectively, P < .001). In addition, significant proteinuria was significantly more common among adults with IgAN as compared to adults with IgAV (86.1% vs 42.8%, P = .002). Correspondingly, more IgAN patients required administration of medical treatment, including prednisone and ACEi/ARBs. At 1-year follow up, 4 IgAN patients and 2 adults with IgAV underwent dialysis, but without a statistically significant difference. Finally, during this 1 year follow up, 7 adult patients with IgAV have died (4 cardiovascular, 3 sepsis), while no death was listed in the other 2 groups of our study.
3.5. Long-term dialysis
During our 1 year follow up, 6 IgAN patients and 8 adult-onset IgAV patients required dialysis. However, on long term follow up, a total of 9 adult-onset IgAV patients (15%; 6 males mean age 66.6 years) and 10 IgAN patients (22.2%; 9 males mean age 46.6 years) required dialysis. In contrast, no pediatric patient with IgAV underwent a dialysis throughout this time. Kaplan–Meier analysis revealed that there was no statistically significant difference in time to dialysis between the 2 groups (Mantel Cox χ2 = 0.69, P > .41). Mean time to dialysis of was 11.1 years for adults with IgAV (95% CI: 9.8–12.4 years) and 5.8 years for adults with IgAN (95% CI: 5.0–6.6 years). After adjusting for age and gender there was still no difference between the groups (χ2 = 0.55, P > .46). After adjusting for age, sex, hypertension, ischemic heart disease (IHD), body mass index (BMI), obesity, diabetes, dyslipidemia and chronic kidney disease (CKD), there was still no difference between groups (data not shown).
3.6. Long-term survival
Adult patients were followed for a mean period of 3.7 years (SD: 2.1 years; median 3.6 years, range 0.1 to 11.4 years). There was no statistically significant difference in follow up time between adult-onset IgAV and IgAN groups (IgAV: 3.5 ± 2.1 years vs IgAN: 4.0 ± 2.2 years, P > .23). During 1-year follow up, 7 deaths occurred in adult IgAV group, while no deaths were documented among other groups (P = .02). Over long-term follow up, a total of 14 IgAV adult patients and 3 IgAN patients have died. The leading causes of deaths among adult IgAV group were cardiovascular diseases, sepsis and cancer.
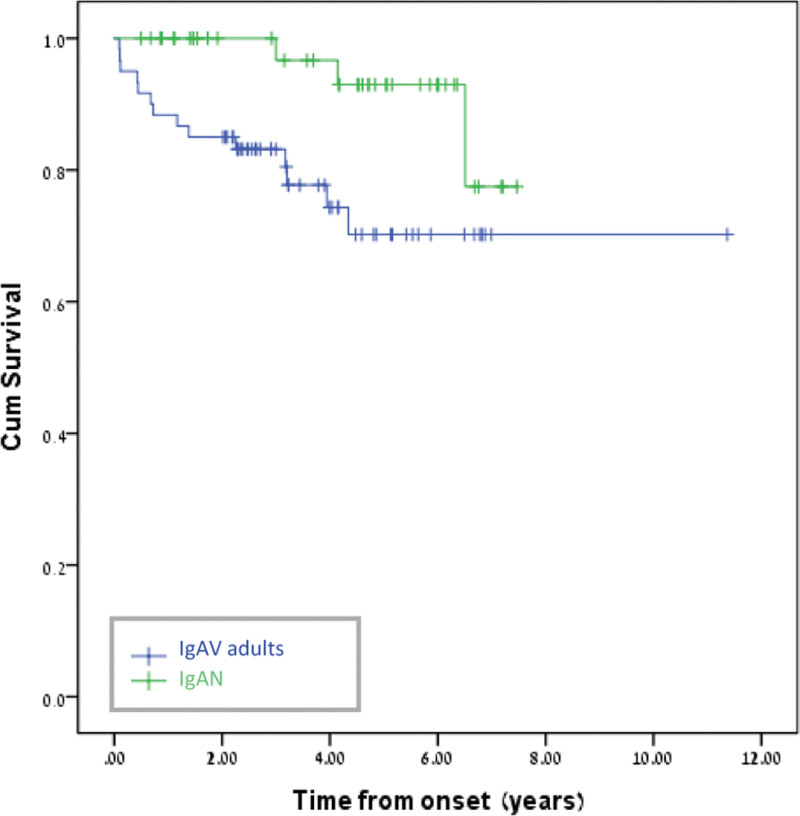
Kaplan–Meier survival analysis (Fig. 1) revealed that after removing an outlier from adult-onset IgAV group, patients from this group had significantly shorter survival time (5.5 years, 95% CI: 4.8–6.2 years) as compared to their IgAN counterparts (7.0 years, 95% CI: 6.6–7.5 years; log rank χ2 = 6.14, P < .01). The excluded outlier reflects a young IgAV adult patient that was diagnosed at 21 years old, and had the second most earlier date of disease onset, with extremely long follow up time (11 years, while the maximal follow up time for other subjects in both groups was 7.5 years). As to the IgAV adult patient with the earliest date of disease onset, he died during 5 years from disease presentation, therefore was included in our survival analysis. After adjusting for age, sex, hypertension, IHD, BMI, obesity, diabetes, dyslipidemia and CKD, there was no statistically significant difference in survival time between the 2 groups (IgAV—adults and IgAN) (χ2 = 1.72, P < .19).
Figure 1.
Kaplan–Meier survival curve for adult patients with IgAV and IgAN. IgAN = IgA nephropathy, IgAV = IgA vasculitis.
4. Discussion
This study showed that adult IgAV patients were older and suffered more from metabolic comorbidities and cardiovascular disease compared to adult IgAN group. This finding is reasonable considering the epidemiology of IgAN, which is relatively more common among children and young adults than in adults above 65 years of age.[11] Yet, our IgAN patients were all kidney biopsies proven for IgAN, which suggests a more severe disease. Also, routine treatment for IgAN does not usually include corticosteroids, hence not accelerating metabolic and vascular diseases. Of the presenting clinical picture of IgAV, it is interesting to note that only in a minority of patients from adult IgAV group, a preceding infection was proven or suspected, compared to almost half of pediatric IgAV patients. In a study by Blanco et al[12] 66% of pediatric IgAV and 33% of adult IgAV had suspected or proven prior infection, mostly upper respiratory tract infections.
4.1. Clinical presentation
Clinical presentations at disease onset are presented at Table 2. It is interesting to note that substantial portion of IgAV adult patients were included in our study due to their positive skin biopsy and suffered less from overt clinical symptoms as compared to pediatric IgAV, who had higher rates of arthritis, arthralgia and abdominal involvement. Additional Serious manifestations such as intussusception and hematochezia were observed more frequently in pediatric IgAV group. This observation was already recognized in a pivotal work assessing the differences between pediatric and adulthood IgAV.[12]
4.2. Laboratory data
Laboratory findings at presentation and follow-up data are described at Tables 2–4. Differences in creatinine levels were found between studied groups at presentation and at follow up and reflect the clinical nature of each disease. Urine dipstick at presentation was positive for hematuria in 42.9% of IgAV adults compared to 13.6% in the pediatric group (P < .01). This highlights the importance of simple urine measures during the workup of small vessel vasculitis. Significant proteinuria at presentation was observed in 95.2% of IgAN patients, 60.5% of adult IgAV patients and 41.1% of pediatric IgAV patients, with significant difference (P < .001) for the comparison between IgAN and adult IgAV These findings are hampered by the limited number of patients tested (approximately half of each study group). Of those patients who were proteinuric, pediatric IgAV patients showed a higher level of proteinuria than adult IgAV patients (UPCR, urine protein/creatinine ratio of 2812 vs 1124 mg/gram, P < .01). Calculated UPCR values of the 3 study groups correlate with previous data from the CureGN study, of which most IgAN and IgAV patients with proteinuria had UPCR of more than 1 g/gram.[13] The fact that at half year follow-up data was available for only 13 pediatric patients, and none after a year, reflects the self-limited nature of this disease. Yet, for 3 pediatric patients, levels of proteinuria were still high at 6 months follow-up (1.8 g/d). Levels of proteinuria were still higher than 1 gram per day at 6 and 12 months for adult IgAV patients who were followed up (9 pts at 6 months and 7 pts at 12 months). In IgAN group, most patients that were followed up for a year presented a persistent proteinuria (86.1%). Persistent proteinuria in IgAV adult patients that consisted more than 6 months from disease onset reflects disease chronicity and predisposition to worse outcomes as reported in a previous study.[14]
The higher inflammatory markers among both adult and pediatric IgAV groups compared to IgAN can be explained by the timing of the laboratory work, which was performed in proximity to the time of biopsy for IgAV skin vasculitis, or purpura in children. This also suggests that systemic inflammation dominates the first 8 weeks from clinical presentation. As reported in previous studies, we also found higher serum IgA levels among adults (either IgAV or IgAN) compared to pediatric IgAV. This may be a clue for the pathogenic role of IgA antibody. The pathogenic mechanism of IgA1 and its interactions with different haplotypes and immunocomplex formation play a role in kidney injury, which is the susceptible organ in adult IgAV compared to pediatrics.[15]
4.3. Treatment
We showed that 68.3% of pediatric patients received corticosteroids during the first 2 month from presentation, compared to 47.3% of adult IgAV and 31.1% of IgAN groups. Altogether, 7 adults and 13 pediatric IgAV patients, were treated with pulse corticosteroids, representing some serious clinical manifestations. In a systematic review by Weiss et al[16] pulse corticosteroid therapy was given mainly for renal and gastrointestinal indications, and in most studies reviews it succeeded in controlling inflammation and shortening disease course by a few days. A retrospective French cohort suggested that corticosteroids might be a reasonable first line therapy, with not enough information supporting the addition of cyclophosphamide.[17] Our analysis was not powered to deduce such observations. Yet, the observed rates of steroid use in our cohort represent a higher usage than expected from a self-limited disease, especially among the pediatric group. It can be explained as we collected the pediatric cases from applications to emergency room, hence presenting more serious and urgent clinical picture. Compared to previous published data, Trapani et al[18] found that only 13% of pediatric IgAV patients were treated with corticosteroids, a much lower percentage than our study. Also, NSAIDS were used in 15% of their pediatric patients, compared to 58.3% of pediatric patients in our cohort. The higher use of ACEi/ARB in the IgAN group represents the current therapy approach toward IgAN patients with proteinuria. In our cohort, about 38.2% of IgAV adult patients were treated with ACEi/ARB, while these could have been given due to worsening proteinuria, or as a treatment of hypertension or for other etiologies of proteinuria. Regarding other immunosuppressants, we found sporadic use of cyclophosphamide, cyclosporine and azathioprine especially among adult IgAV group (total of 3 patients), see Table 2. Few studies and case series reported the use of immunosuppressants and immunomodulators, yet none of them were randomized control trials and their generalizability is limited.[19–22] It is interesting to note that cyclosporine was more common approach among pediatric compared to adult IgAV patients, while cyclophosphamide and azathioprine were more common among adults.
4.4. End stage renal disease
During the first 8 weeks from presentation, 7 adult IgAV patients underwent dialysis compared to no pediatric patient and only 3 IgAN patients. At long-term follow up, we found that 22% of IgAN and 15% of adult IgAV cohorts required dialysis. Differences in time to dialysis initiation were not statistically significant, yet IgAV adult group went on dialysis sooner. Considering other differences between both groups, we cannot deduce causality between IgAV and time to dialysis. A previous report about adults with IgAV found relatively similar results, with 11% of patients reached end stage renal disease at approximately 10 years follow-up.[14]
4.5. Mortality
Our mean follow-up time was 3.7 years. As shown in Figure 1, survival was significantly shorter among IgAV adults compared to IgAN patients. Yet after controlling for age, sex, hypertension, IHD, BMI, obesity, diabetes, dyslipidemia and CKD, there was no statistically significant difference in survival time between the groups. This can be explained by the fact that these 2 groups are clinically different (as shown in Tables 1 and 2), IgAN patients were younger and had less metabolic diseases. Tracy et al[23] showed in a retrospective cohort study that IgAV adult patients were associated with excess mortality as compared to matched controls, with HR of 1.27, and presented significantly higher rates of CKD and hypertension, while IHD or VTE were not found to be related. Our cohort was older than their study population, which might account for the differences between both works, as death occurred in higher frequency in our adult IgAV group, mostly during the first follow-up year. The relatively prevalent use of immunomodulators among adult IgAV group might as well contribute to the differences in mortality between groups, yet our sample size was too small to adjust for immunomodulator medication use.
4.6. Strengths and limitations
Several limitations should be acknowledged in our study. First, this is a retrospective descriptive health record-based study. Hence, it poses the bias of non-informing or mis-informing medical records. Second, most of the pediatric patients were discharged and never followed up, so minor clinical outcomes such as urine measurements might be missed. Due to lack of data, which apparently suggests disease-free survival, we didn’t include pediatric IgAV group in our 1-year follow up. Third, we did not address the question of disease relapse and included patients only from their last manifestation, a fact that should be considered when interpreting this study. Fourth, the inclusion criteria for adults and pediatric IgAV were different, which might reflect a selection of more severe adult patients requiring a skin biopsy at presentation compared to clinically based criteria for the pediatric group. IgAN patients were included only with proven kidney biopsy, which as well might reflect a more severe form of their disease. Yet, the comparison between these 2 adult groups made sense since both were severe enough for tissue biopsy, and most had clinical and laboratory data at follow-up.
The main strength of our study was the evaluation over time of the 3 groups, which highlighted the differences between their long-term sequelae.
5. Conclusion
In our retrospective study, IgAV adult patients were sicker at presentation and had significantly shorter survival time as compared to their IgAN counterparts. Although IgAV tends to be a self-limited disease, IgAV in adults presents substantial clinical manifestations, typically high risk of progression to persistent renal impairment. Since the treatment options are not well agreed upon, we think that further studies and collaboration should be taken to guide clinicians for the best therapeutic management.
Author contributions
Data curation: Vera Gotloib, Yehudit Kraus, Irina Novofastovski, Shay Brikman, Abdallah Fawaz, Mohammad Egbaria, Yonatan Butbul Aviel, Alexandra Balbir-Gurman, Reuven Mader.
Writing – original draft: Shirel Levanon.
Writing – review & editing: Amir Bieber.
Abbreviations:
- ACEi
- angiotensin converting enzyme inhibitors
- ARBs
- angiotensin receptor blockers
- BMI
- body mass index
- IgAN
- IgA nephropathy
- IgAV
- IgA vasculitis
- IHD
- ischemic heart disease
- NSAIDs
- nonsteroidal anti-inflammatory drugs
- UPCR
- urine protein to creatinine ratio
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
How to cite this article: Levanon S, Gotloib V, Kraus Y, Novofastovski I, Brikman S, Fawaz A, Egbaria M, Butbul Aviel Y, Balbir-Gurman A, Mader R, Bieber A. IgA vasculitis in adults, pediatrics and non-vasculitic IgA nephropathy, retrospective analysis from 2 centers. Medicine 2023;102:50(e36521).
Contributor Information
Shirel Levanon, Email: shirel24@gmail.com.
Vera Gotloib, Email: vera_ko@clalit.org.il.
Yehudit Kraus, Email: krausy@clalit.org.il.
Irina Novofastovski, Email: irina_no@clalit.org.il.
Shay Brikman, Email: sbrikman@gmail.com.
Abdallah Fawaz, Email: abdallah_fa@clalit.org.il.
Mohammad Egbaria, Email: mohammad_ag@clalit.org.il.
Yonatan Butbul Aviel, Email: yonatanbutbul@gmail.com.
Alexandra Balbir-Gurman, Email: a_balbir@rambam.health.gov.il.
Reuven Mader, Email: mader@clalit.org.il.
References
- [1].Dyga K, Szczepanska M. IgA vasculitis with nephritis in children. Adv Clin Exp Med. 2020;29:513–9. [DOI] [PubMed] [Google Scholar]
- [2].Villatoro-Villar M, Crowson CS, Warrington KJ, et al. Clinical characteristics of biopsy-proven IgA vasculitis in children and adults: a retrospective cohort study. Mayo Clin Proc. 2019;94:1769–80. [DOI] [PubMed] [Google Scholar]
- [3].Nossent J, Raymond W, Isobel Keen H, et al. Morbidity and mortality in adult-onset IgA vasculitis: a long-term population-based cohort study. Rheumatology (Oxford). 2022;61:291–8. [DOI] [PubMed] [Google Scholar]
- [4].Heymann WR. The renal reality of adult Henoch-Schönlein purpura. J Am Acad Dermatol. 2020;82:1303–4. [DOI] [PubMed] [Google Scholar]
- [5].Baumrin E, Azzawi S, St John J, et al. Prognostic implications of normal or minimal urinary findings on long-term renal impairment in adults with Henoch-Schönlein purpura. J Am Acad Dermatol. 2020;82:1393–9. [DOI] [PubMed] [Google Scholar]
- [6].Kang Y, Park J, Ha YJ, et al. Differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura. J Korean Med Sci. 2014;29:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Audemard-Verger A, Terrier B, Dechartres A, et al.; French Vasculitis Study Group. Characteristics and management of IgA vasculitis (Henoch-Schönlein) in adults: data from 260 patients included in a French multicenter retrospective survey. Arthritis Rheumatol. 2017;69:1862–70. [DOI] [PubMed] [Google Scholar]
- [8].Song Y, Huang X, Yu G, et al. Pathogenesis of IgA vasculitis: an up-to-date review. Front Immunol. 2021;12:771619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heineke M, Ballering A, Jamin A, et al. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun Rev. 2017;16:1246. [DOI] [PubMed] [Google Scholar]
- [10].Ruperto N, Ozen S, Pistorio A, et al.; Paediatric Rheumatology International Trials Organisation (PRINTO). EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008 Part I: overall methodology and clinical characterisation. Ann Rheum Dis. 2010;69:790–7. [DOI] [PubMed] [Google Scholar]
- [11].Lai KN, Tang SCW, Schena FP, et al. IgA nephropathy. Nat Rev Dis Prim. 2016;2:16001. [DOI] [PubMed] [Google Scholar]
- [12].Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, et al. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997;40:859–64. [DOI] [PubMed] [Google Scholar]
- [13].Selewski DT, Ambruzs JM, Appel GB, et al.; CureGN Consortium. Clinical characteristics and treatment patterns of children and adults with IgA nephropathy or IgA vasculitis: findings from the CureGN study. Kidney Int Rep. 2018;3:1373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pillebout E, Thervet E, Hill G, et al. Henoch-Schönlein purpura in adults: outcome and prognostic factors. J Am Soc Nephrol. 2002;13:1271–8. [DOI] [PubMed] [Google Scholar]
- [15].Yang YH, Chuang YH, Wang LC, et al. The immunobiology of Henoch-Schönlein purpura. Autoimmun Rev. 2008;7:179–84. [DOI] [PubMed] [Google Scholar]
- [16].Weiss PF, Feinstein JA, Luan X, et al. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics. 2007;120:1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Audemard-Verger A, Pillebout E, Guillevin L, et al. IgA vasculitis (Henoch-Shönlein purpura) in adults: diagnostic and therapeutic aspects. Autoimmun Rev. 2015;14:579–85. [DOI] [PubMed] [Google Scholar]
- [18].Trapani S, Micheli A, Grisolia F, et al. Henoch Schonlein purpura in childhood: epidemiological and clinical analysis of 150 cases over a 5-year period and review of literature. Semin Arthritis Rheum. 2005;35:143–53. [DOI] [PubMed] [Google Scholar]
- [19].Pillebout E, Alberti C, Guillevin L, et al.; CESAR study group. Addition of cyclophosphamide to steroids provides no benefit compared with steroids alone in treating adult patients with severe Henoch Schönlein purpura. Kidney Int. 2010;78:495–502. [DOI] [PubMed] [Google Scholar]
- [20].Han F, Chen LL, Ren PP, et al. Mycophenolate mofetil plus prednisone for inducing remission of Henoch-Schönlein purpura nephritis: a retrospective study. J Zhejiang Univ Sci B. 2015;16:772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shin JI, Park JM, Shin YH, et al. Cyclosporin A therapy for severe Henoch-Schönlein nephritis with nephrotic syndrome. Pediatr Nephrol. 2005;20:1093–7. [DOI] [PubMed] [Google Scholar]
- [22].Chaudhary K, Shin JY, Saab G, et al. Successful treatment of Henoch-Schonlein purpura nephritis with plasma exchange in an adult male. NDT Plus. 2008;1:303–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tracy A, Subramanian A, Adderley NJ, et al. Cardiovascular, thromboembolic and renal outcomes in IgA vasculitis (Henoch-Schönlein purpura): a retrospective cohort study using routinely collected primary care data. Ann Rheum Dis. 2019;78:261–9. [DOI] [PubMed] [Google Scholar]