Abstract
Concerns about a possible relationship between vaccination against Coronavirus Disease 2019 (COVID-19) and menstrual disorders have been raised in the media. In addition, different studies have shown that the COVID-19 vaccine may be associated with menstrual changes. This study was conducted to investigate the effects of COVID-19 vaccines on the menstrual cycle in women. This cross-sectional descriptive study was conducted between August 16 and September 17, 2021. Data were collected through a self-administered questionnaire via an online form sent to the participants through social media. Data of 586 women were included in this study. A total of 82.4% (n = 483) of the participants were aged between 31 and 50 years. The BioNTech vaccine (2 doses) was administered to 75.8% (n = 444), Sinovac (3 doses) to 9.0% (n = 53) of the participants. 53.1% (n = 311) of the women experienced changes in their menstrual cycles. The most common menstrual changes after vaccination were delayed menstruation (n = 176; 30.0%) and prolonged menstrual duration (n = 132; 22.5%). Menstrual delay, prolonged menstrual duration, heavy bleeding, and early menstruation were more common in women than prior to receiving the vaccine (P < .05). More than half of the women experienced menstrual cycle changes after receiving the COVID-19 vaccine. Women experienced significantly higher rates of menstruation delay, prolonged menstrual duration, heavy bleeding, and early bleeding compared to before vaccination.
Keywords: adverse effects, COVID-19 vaccination, menstrual cycle, menstrual regularity, menstruation disorders
1. Introduction
According to the World Health Organization, due to the Coronavirus Disease 2019 (COVID-19) outbreak that emerged in China in December 2019, approximately 770 million people worldwide were infected, and 7 million people died.[1] Vaccination is the most effective method for preventing the spread of infectious diseases and reducing morbidity and mortality rates.[2] By the end of 2022, 50 COVID-19 vaccines had been approved by at least 1 country worldwide. As of July 2023, approximately 13.5 billion doses of COVID-19 vaccine have been administered worldwide.[1,3] The COVID-19 vaccination campaign in Turkey was started on January 14, 2021, with the Sinovac vaccine (Sinovac Biotech Co. Ltd.), also known as CoronaVac, containing inactive virus particles, and then the Pfizer-BioNTech mRNA vaccine (Biontech Manufacturing GmbH) was started to be administered on April 12, 2021.[4]
In the Sinovac vaccine administration guideline, no side effects regarding menstrual changes have been reported.[5] In the Pfizer/BioNTech vaccine administration guideline, in relation to menstrual changes, it has been stated that heavy menstrual bleeding may occur, but most cases are not severe and inherently temporary, and its frequency cannot be estimated from the currently available data.[6] No information has been provided regarding other menstrual changes.
Concerns have been raised in the media that there is a possible relationship between vaccination against COVID-19 and menstrual disorders. In addition, false claims that the future fertility of young women may be affected have led to hesitation about getting vaccinated.[7] In studies conducted in different countries, the rate of menstrual changes after the COVID-19 vaccine ranged from 24.8% to 78%.[7–11] In light of these parameters, this study was conducted to investigate the effects of COVID-19 vaccines on menstrual cycles in women.
2. Material and methods
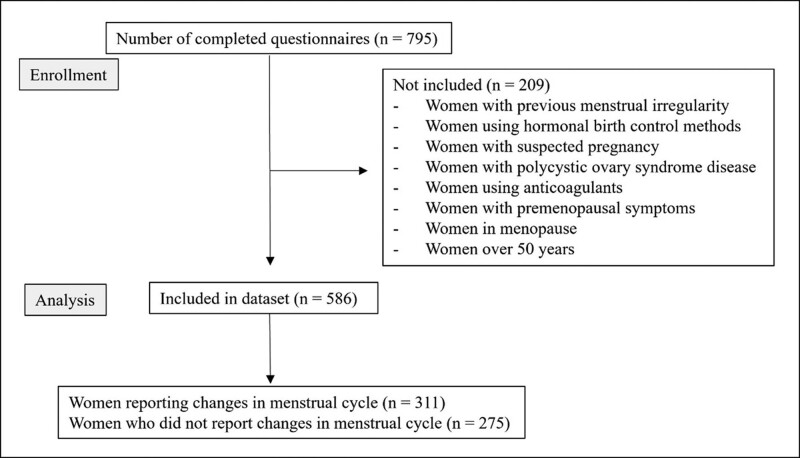
This cross-sectional descriptive study was conducted between August 16 and September 17, 2021. Approval for the study was obtained from the Health Sciences University Gazi Yaşargil Training and Research Hospital Clinical Research Ethics Committee (decision no: 2021/951). Data were collected through a self-administered questionnaire sent online to participants via the social media platform Twitter. The form contained questions about the participants’ age, presence of chronic disease, use of anti-menstrual medication, regularity of their pre-vaccination menstrual cycles, COVID-19 vaccine type received, and changes in menstruation patterns after vaccination. In order to exclude women with previous menstrual irregularities, all participants in the questionnaire were asked whether they had menstrual irregularities before vaccination. The study included women over the age of 18 years who previously had regular menstrual cycles. Women with menstrual irregularity, suspected pregnancy, polycystic ovary syndrome, anticoagulant use, premenopausal symptoms, menopause, women who are using hormonal contraception or are over 50 years of age were excluded from the study. A flowchart illustrating how the samples were obtained is shown in Figure 1.
Figure 1.
Flowchart showing how the sample was obtained.
Women who received the COVID-19 vaccine were evaluated in 3 groups:
The group that received only Sinovac vaccine (Sinovac Biotech Co. Ltd.): A total of 3 doses of the vaccine were administered, with at least 4 weeks between the first 2 doses and at least 3 months between the second and third doses.
The group that received only the Pfizer-BioNTech (Biontech Manufacturing GmbH) vaccine: A total of 2 doses of the vaccine were administered, with at least 4 weeks between the 2 doses.
The group that received both the Sinovac and the Pfizer-BioNTech vaccines (Sinovac + Biontech): After 2 doses of the Sinovac vaccine that were administered 4 weeks apart, a total of 3 doses of the vaccines were administered, with 1 dose of the Pfizer-BioNTech vaccine, which was administered at least 3 months later.
2.1. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences software (version 21). Numbers and percentages were calculated. The chi-square test was used to analyze independent variables, and the McNemar test was used to evaluate dependent variables. A P value of < .05 was considered statistically significant.
3. Results
3.1. Demographic data and types of vaccines
A total of 795 women responded to the online questionnaire. However, 209 of these women were excluded from the study because of the exclusion criteria. Therefore, the data from 586 women were included in our study. A total of 82.4% (n = 483) of the participants were aged between 31 and 50 years. Of the participants, 35% (n = 205) had chronic diseases such as hypertension and diabetes. The BioNTech vaccine (2 doses) was administered to 75.8% (n = 444) of the participants, Sinovac (3 doses) to 9.0% (n = 53), and both Sinovac and BioNTech (2 doses and 1 dose, respectively) were administered to 15.2% (n = 89) of the participants. After receiving the COVID-19 vaccine, 53.1% (n = 311) of the women experienced changes in their menstrual cycles. There were no statistically significant differences between the age of the women, the type of vaccine administered, the status of chronic diseases, and menstrual changes (Table 1).
Table 1.
Evaluation of menstrual changes according to age, vaccine type, and presence of chronic diseases in women who have been vaccinated against COVID-19.
| Number | Percentage | Menstrual changes | P | |||
|---|---|---|---|---|---|---|
| Yes n (%) |
No n (%) |
|||||
| Age | 18–30 yr | 103 | 17.6 | 58 (56.3%) | 45 (43.7%) | .46 |
| 31–50 yr | 483 | 82.4 | 253 (52.4%) | 230 (47.6%) | ||
| COVID-19 vaccine type | Sinovac (3 doses) | 53 | 9.0 | 33 (62.3%) | 20 (37.7%) | .27 |
| BioNTech (2 doses) | 444 | 75.8 | 235 (52.9%) | 209 (47.1%) | ||
| Sinovac + BioNTech (2 + 1 doses) | 89 | 15.2 | 43 (48.3%) | 46 (51.7%) | ||
| Do you have any chronic diseases? | Yes | 205 | 35.0 | 110 (53.7%) | 95 (46.3%) | .83 |
| No | 381 | 65.0 | 201 (52.8%) | 180 (47.2%) | ||
| Total | 586 | 100.0 | 311 (53.1%) | 275 (46.9%) | ||
3.2. Side effects of COVID-19 vaccines
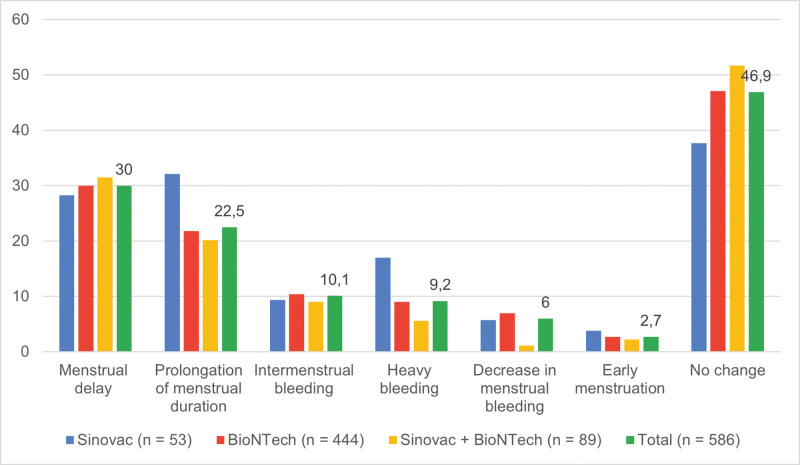
Menstrual changes occurring post-Sinovac vaccine (n = 33; 63.3%) were more common than post-BioNTech vaccine (n = 235; 52.9%). However, there was no statistically significant difference between the 2 vaccines in terms of menstrual changes (P = .27). The most common menstrual changes after vaccination were delayed menstruation (n = 176; 30.0%) and prolonged menstrual duration (n = 132; 22.5%). In contrast, 10.1% (n = 59) of the women had intermenstrual bleeding, 9.2% (n = 54) had heavy bleeding, 6.0% (n = 35) had light bleeding, and 2.7% (n = 16) had early menstruation. The other menstrual changes are shown in Figure 2 and Table 2. There was no statistically significant difference between the COVID-19 vaccines in terms of menstrual changes.
Figure 2.
Menstrual changes according to COVID-19 vaccine types. COVID-19 = Coronavirus Disease 2019.
Table 2.
Evaluation of menstrual changes according to COVID-19 vaccine type.
| Menstrual change* | COVID-19 vaccine type | Total n (%) |
P ** | ||
|---|---|---|---|---|---|
| Sinovac n (%) |
BioNTech n (%) |
Sinovac + BioNTech n (%) |
|||
| Menstrual delay | 15 (28.3%) | 133 (30.0%) | 28 (31.5%) | 176 (30.0%) | .92 |
| 1–7 d | 12 (22.6%) | 69 (15.6%) | 15 (16.9%) | 96 (16.4%) | |
| 8–14 d | 1 (1.9%) | 27 (6.1%) | 6 (6.7%) | 34 (5.8%) | |
| 15 d or more | 2 (3.8%) | 37 (8.3%) | 7 (7.9%) | 46 (7.8%) | |
| Prolongation of menstrual duration | 17 (32.1%) | 97 (21.8%) | 18 (20.2%) | 132 (22.5%) | .20 |
| 1–2 d | 6 (11.3%) | 41 (9.2%) | 7 (7.9%) | 54 (9.2%) | |
| 3 d or more | 11 (20.8%) | 56 (12.6%) | 11 (12.3%) | 78 (13.3%) | |
| Intermenstrual bleeding | 5 (9.4%) | 46 (10.4%) | 8 (9.0%) | 59 (10.1%) | .91 |
| Heavy bleeding | 9 (17.0%) | 40 (9.0%) | 5 (5.6%) | 54 (9.2%) | .06 |
| Decrease in menstrual bleeding | 3 (5.7%) | 31 (7.0%) | 1 (1.1%) | 35 (6.0%) | .11 |
| Early menstruation | 2 (3.8%) | 12 (2.7%) | 2 (2.2%) | 16 (2.7%) | .76 |
| No change | 20 (37.7%) | 209 (47.1%) | 46 (51.7%) | 275 (46.9%) | .27 |
| Total | 53 (100.0%) | 444 (100.0%) | 89 (100.0%) | 586 (100.0%) | |
Multiple responses were obtained.
Chi-square test was used for the statistical analysis.
Of the women participating in the study, 31% (n = 101) stated that they had experienced menstrual irregularities in the past, although their menses were regular at the time of the questionnaire. Menstruation delay, prolonged menstruation duration, heavy bleeding, and early menstruation were more frequent in women than before vaccination (P < .05). However, there was no significant difference in terms of intermenstrual bleeding and decrease in flow volume after vaccination in women compared to the occurrence of these symptoms prior to receiving the vaccine (P = .88) (Table 3).
Table 3.
Evaluation of similar menstrual changes before and after COVID-19 vaccination.
| Menstrual change after COVID-19 vaccination* | Have you experienced similar menstrual changes in the past? | P ** | ||
|---|---|---|---|---|
| Yes n (%) |
No n (%) |
|||
| Menstrual delay (n = 326) | Yes | 63 (34.6%) | 119 (65.4%) | <.001 |
| No | 38 (26.4%) | 106 (73.6%) | ||
| Prolongation of menstrual duration (n = 326) | Yes | 35 (26.3%) | 98 (73.7%) | .02 |
| No | 66 (34.2%) | 127 (65.8%) | ||
| Intermenstrual bleeding (n = 230) | Yes | 9 (18.4%) | 40 (81.6%) | .08 |
| No | 58 (32.0%) | 123 (68.0%) | ||
| Heavy bleeding (n = 74) | Yes | 15 (30.6%) | 34 (69.4%) | <.001 |
| No | 5 (20.0%) | 20 (80.0%) | ||
| Decrease in Menstrual Bleeding (n = 117) | Yes | 6 (20.0%) | 24 (80%) | .88 |
| No | 22 (25.3%) | 65 (74.7%) | ||
| Early menstruation (n = 196) | Yes | 1 (7.7%) | 12 (92.3%) | <.001 |
| No | 61 (33.3%) | 122 (66.7%) | ||
| Total | 101 (31.0%) | 225 (69.0%) | ||
The values in bold have a significant statistical difference.
Multiple responses were obtained.
McNemar test was used for the statistical analysis.
4. Discussion
In our study, we evaluated the post-vaccination menstrual patterns of 586 women who had regular menstrual periods before receiving the COVID-19 vaccine. In our study, 53.1% of the women experienced menstrual changes after vaccination. In studies conducted in different countries, the rate of menstrual changes after the COVID-19 vaccine ranged from 24.8% to 78%.[7–11] Our study is compatible with the literature in terms of the incidence of menstrual cycle changes after the COVID-19 vaccination.
A regular menstrual cycle is an indication that the hypothalamic-pituitary-ovarian axis is healthy. Menstrual characteristics may change from month to month throughout an individual life.[12] Many factors, such as stress, endocrine disturbances, gynecological changes, autoimmune disorders, nutrition, genetics, infection, and lifestyle changes, can affect the menstrual cycle.[13] Studies have reported that the perception of stress is higher in women experiencing menstrual irregularities after vaccination, and the menstrual periods have been found to be prolonged during the peak of the pandemic.[9,10] In addition, menstrual changes affect up to 30% of women of reproductive age, regardless of the COVID-19 vaccine.[14] This study was conducted while the third peak of the increase in deaths due to COVID-19 was occurring in Turkey.[15] In our study, it was determined that 31% of the women had experienced temporary menstrual irregularities in the past, even though their menstrual cycles were regular. The fact that menstrual changes observed after the vaccination may have been due to the stress caused by the pandemic other than the COVID-19 vaccine should be taken into consideration.
The International Federation of Gynecology and Obstetrics (FIGO) has determined normal menstrual parameters for menstrual frequency (≥24–≤ 38 days), duration (≤8 days), regularity, and flow volume in women of reproductive age.[16] Deviations from these parameters and/or intermenstrual bleeding were considered cases of abnormal uterine bleeding.[16] In a study conducted in women living in South America, in accordance with the FIGO criteria, 56.53% of women had abnormally frequent periods, 48.92% had irregular periods, 26.08% had menstruation durations of more than 9 days, and 69.02% of women had mild or heavy menstrual bleeding.[17] In a study conducted in Spain, the most common menstrual changes were heavy bleeding, excessive menstrual pain, delayed menstruation, decreased duration of bleeding, and early menstruation.[11] In a study conducted in Hungary, it was reported that the menstrual cycle was shortened by 29.9%, prolonged by 22.2%, delayed by 13.9%, and bleeding duration was prolonged in 7.8% of women.[10] In another study consisting of women over the age of 12 in Saudi Arabia, after the first dose of the COVID-19 vaccine, 27.7% of women had menstrual delays, while 16.8% had early menstruation.[18] In a meta-analysis, the prevalence of menorrhagia after the COVID-19 vaccination was 24.24%, oligomenorrhea was 22.7%, polymenorrhea was 16.2%, and abnormal menstrual cycle length was 6.6%.[19] It was found that COVID-19-related menstrual symptoms resolved within 2 months at a rate of 58% to 93.6%.[8,9] In our study, menstrual delay (30%), prolonged menstrual bleeding (22.5%), breakthrough bleeding (10.1%), and excessive bleeding (9.2%) were found to be the most common menstrual symptoms. One of the important limitations of our study was that length of the menstrual cycles and menstrual periods of the participants had not been recorded before vaccination. In our study, it was found that there was a significant increase in menstrual delay, prolonged menstrual bleeding and early menstruation compared to the routine cycles of the participants before vaccination. However, we were not able to determine whether this increase in the length of menstrual periods was within the normal range according to FIGO criteria. In studies, in which menstrual cycle lengths were measured, it was found that the menstrual cycle lengths after vaccination were not different from before vaccination.[20,21] In another prospective study conducted in women who were planning pregnancy and monitored online, it was found that the first menstrual cycle after each COVID-19 vaccine dose was an average of 1 day longer than the menstrual cycles before vaccination; however, it was reported that the cycle length returned to the pre-vaccine length with the second cycle.[22]
In the present study, according to the FIGO criteria, heavy menstrual bleeding after vaccination was more common than before vaccination, but post-vaccine intermenstrual bleeding and a decrease in menstruation flow volume were found to be similar to pre-vaccine conditions. As there are no studies in the literature comparing these post-vaccine symptoms with pre-vaccine rates, our study did not consider this aspect in any further detail.
4.1. Limitations and strengths of the research
Data were collected online via social media. While online surveys were a safe way to conduct the research during the pandemic, women prone to menstrual changes may have been more likely to participate. Therefore, the sample may not be reflective of all women in our country. Even though a short time had passed since vaccination, there may have been a recall bias.
We also excluded participants who showed menstrual cycle irregularities due to other factors that were not related to vaccine administration (e.g., women who had menstrual irregularities, used hormonal contraception, polycystic ovary syndrome). There was no difference between women with and without menstrual changes in terms of age or the presence of chronic diseases. These are the strengths of this study that reduced the potential for bias.
5. Conclusion
More than half of the women surveyed in our study experienced menstrual cycle changes after the COVID-19 vaccine. The most common symptoms were delayed menstruation, prolonged menstruation, intermenstrual bleeding, and heavy bleeding. One of the most important findings of our study was that the flow volume of menstrual bleeding after vaccination was higher than before vaccination. There was a significant increase in post-vaccine menstruation delay, prolonged menstrual duration, and early menstruation compared to the pre-vaccine routine cycles in women. However, we were not able to determine whether these increases were within the normal range according to the FIGO criteria.
Regardless of their effect on the menstrual cycle, vaccines are the most effective public health measure available for the control of infectious diseases such as COVID-19. Therefore, before vaccination, the side effects and benefits of vaccination should be well explained in order to mitigate vaccination hesitation among women. In women with bleeding diathesis, the decision to receive vaccination should be based on profit-and-loss considerations.
Acknowledgments
We express our gratitude to the chief editor and the anonymous reviewers for their valuable feedback.
Author contributions
Conceptualization: Hakan Akelma, Eşref Araç.
Formal analysis: İzzettin Toktaş.
Investigation: İzzettin Toktaş, Hakan Akelma.
Methodology: Hakan Akelma, Eşref Araç.
Resources: İzzettin Toktaş.
Supervision: Hakan Akelma.
Visualization: İzzettin Toktaş, Eşref Araç.
Writing – original draft: İzzettin Toktaş.
Writing – review & editing: Hakan Akelma, Eşref Araç.
Abbreviations:
- COVID-19
- Coronavirus Disease 2019
- FIGO
- The International Federation of Gynecology and Obstetrics
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and approved by the University of Health Sciences Gazi Yasargil Training and Research Hospital Ethics Committee (2021/951).
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Toktaş İ, Akelma H, Araç E. Examining the effect of COVID-19 vaccines on the menstrual cycle: A study from Turkey. Medicine 2023;102:50(e36638).
Contributor Information
Hakan Akelma, Email: hakanakelma@hotmail.com.
Eşref Araç, Email: esrefarac@gmail.com.
References
- [1].. WHO. Coronavirus Disease (COVID-19) Pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [access date June 20, 2023].
- [2].Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Basta NE, Moodie EMM; on behalf of the VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID-19 Vaccine Development and Approvals Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. Available at: https://covid19.trackvaccines.org/ [access date July 25, 2023].
- [4].Çelik O, Özmemiş C, Balçik B, et al. Evaluation of the Covid-19 vaccination process in family health centers: a descriptive study. J Turk Fam Phys. 2022;13:84–95. [Google Scholar]
- [5].COVID-19 Vaccine (Vero Cell), Inactivated (Brief Edition). Available at: https://www.healthbureau.gov.hk/download/our_work/health/201200/e_PI_CoronaVac_brief.pdf [access date December 2, 2023].
- [6].Package Leaflet: Information for the User. Available at: https://labeling.pfizer.com/ShowLabeling.aspx?id=15502 [access date July 25, 2023].
- [7].Alahmadi AM, Aljohani AH, Fadhloun RA, et al. The effect of the COVID-19 vaccine on the menstrual cycle among reproductive-aged females in Saudi Arabia. Cureus. 2022;14:e32473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muhaidat N, Alshrouf MA, Azzam MI, et al. Menstrual symptoms after COVID-19 vaccine: a cross-sectional investigation in the MENA region. Int J Womens Health. 2022;14:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Farland LV, Khan SM, Shilen A, et al. COVID-19 vaccination and changes in the menstrual cycle among vaccinated persons. Fertil Steril. 2023;119:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barabás K, Makkai B, Farkas N, et al. Influence of COVID-19 pandemic and vaccination on the menstrual cycle: a retrospective study in Hungary. Front Endocrinol (Lausanne). 2022;13:974788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baena-García L, Aparicio VA, Molina-López A, et al. Premenstrual and menstrual changes reported after COVID-19 vaccination: the EVA project. Womens Health (Lond). 2022;18:17455057221112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sualeh M, Uddin MR, Junaid N, et al. Impact of COVID-19 vaccination on menstrual cycle: a cross-sectional study from Karachi, Pakistan. Cureus. 2022;14:e28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taşkaldiran I, Vuraloğlu E, Bozkuş Y, et al. Menstrual changes after COVID-19 infection and COVID-19 vaccination. Int J Clin Pract. 2022;2022:3199758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Matteson KA, Abed H, Wheeler TL, 2nd, et al. A systematic review comparing hysterectomy with less-invasive treatments for abnormal uterine bleeding. J Minim Invasive Gynecol. 2012;19:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Republic of Turkey Ministry of Health. COVID-19 Information Platform. Available at: https://covid19.saglik.gov.tr/TR-66935/genel-koronavirus-tablosu.html [access date August 05, 2023].
- [16].Munro MG, Critchley HOD, Fraser IS. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynaecol Obstet. 2018;143:393–408. [DOI] [PubMed] [Google Scholar]
- [17].Rodríguez Quejada L, Toro Wills MF, Martínez-Ávila MC, et al. Menstrual cycle disturbances after COVID-19 vaccination. Womens Health (Lond). 2022;18:17455057221109375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qashqari FSI, Dahlawi M, Assaggaf HM, et al. Effect of the COVID-19 vaccine on the menstrual cycle among females in Saudi Arabia. Ethiop J Health Sci. 2022;32:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Al Kadri HM, Al Sudairy AA, Alangari AS, et al. COVID-19 vaccination and menstrual disorders among women: findings from a meta-analysis study. J Infect Public Health. 2023;16:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kajiwara S, Akiyama N, Baba H, et al. Association between COVID-19 vaccines and the menstrual cycle in young Japanese women. J Infect Chemother. 2023;29:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edelman A, Boniface ER, Benhar E, et al. Association between menstrual cycle length and coronavirus disease 2019 (COVID-19) vaccination: a US cohort. Obstet Gynecol. 2022;139:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wesselink AK, Lovett SM, Weinberg J, et al. COVID-19 vaccination and menstrual cycle characteristics: a prospective cohort study. Vaccine. 2023;41:4327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]