Abstract
As the most prevalent chronic liver disease globally, NAFLD encompasses a pathological process that ranges from simple steatosis to NASH, fibrosis, cirrhosis, and HCC, closely associated with numerous extrahepatic diseases. While the initial etiology was believed to be hepatocyte injury caused by lipid toxicity from accumulated triglycerides, recent studies suggest that an imbalance of cholesterol homeostasis is of greater significance. The role of nuclear receptors in regulating liver cholesterol homeostasis has been demonstrated to be crucial. This review summarizes the roles and regulatory mechanisms of nuclear receptors in the 3 main aspects of cholesterol production, excretion, and storage in the liver, as well as their cross talk in reverse cholesterol transport. It is hoped that this review will offer new insights and theoretical foundations for the study of the pathogenesis and progression of NAFLD and provide new research directions for extrahepatic diseases associated with NAFLD.
INTRODUCTION
NAFLD is a prevalent chronic liver disease affecting approximately 2 billion people worldwide.1 The condition is defined by the presence of fatty degeneration in >5% of liver cells, often coupled with metabolic risk factors, such as obesity and type 2 diabetes, and a lack of excessive alcohol consumption (≥30 g/d for men, ≥20 g/d for women) or other chronic liver diseases.2 The disease can progress from simple hepatic steatosis to NASH, then to advanced liver fibrosis, cirrhosis, or HCC. Over the past 20 years, the burden of NAFLD in China has increased significantly; the prevalence began to increase from 23.8% in the early- to mid-2000s, accelerating in 2010, then reaching 32.9% in 2018, resulting in an average prevalence of 29.6%.3 An increase in NAFLD prevalence is associated globally with aging, obesity, and diabetes,4 whereas in China, it is associated with an increase in older and young patients exhibiting changes in diet and lifestyle. From 2010 to 2018, the incidence of NAFLD in Chinese people aged below 60 years was higher than that in those aged above 60 years.3
Patients with NAFLD commonly exhibit a series of complex metabolic dysfunctions, including insulin resistance, lipid metabolism abnormalities, gut microbiota disturbances, and abnormal uric acid metabolism. Additionally, NAFLD is positively correlated with the onset of atherosclerotic diseases, thereby increasing the risk of stroke and myocardial infarction, which can lead to systemic functional impairments or even death.5 Moreover, the increasing prevalence of NAFLD among young populations may exacerbate its burden on society. Therefore, it is imperative to further elucidate the pathogenesis of NAFLD. This review summarizes the existing literature on NAFLD pathogenesis, specifically, the roles and regulatory mechanisms of nuclear receptors (NRs) in (1) maintaining hepatic cholesterol homeostasis, (2) the development and progression of NAFLD, and (3) cross talk during reverse cholesterol transport (RCT).
NAFLD PATHOGENESIS
The primary driving factor of NAFLD is nutrient excess, which leads to the excessive accumulation of liver lipids and disrupts the balance of hepatic lipid metabolism.6 However, research increasingly suggests that triglycerides, which are lipid storage substances, serve as a buffer against lipid-induced liver damage,7,8 whereas the accumulation of free fatty acids,9 cholesterol,10 phosphatidylcholine,11 and ceramides12 plays a more critical role in the development and progression of NAFLD. Among these factors, maintaining cholesterol metabolic homeostasis is particularly crucial.10
Cholesterol homeostasis
Cholesterol is a precursor for the synthesis of bile acids, vitamins, and steroid hormones and is an essential lipid molecule in animal cells. Cholesterol is crucial not only for maintaining the barrier function and fluidity of cell membranes but also for playing an important role in intercellular signaling through the formation of lipid rafts on the cell membrane.13 Therefore, maintaining cholesterol homeostasis is essential for maintaining basic body activities.
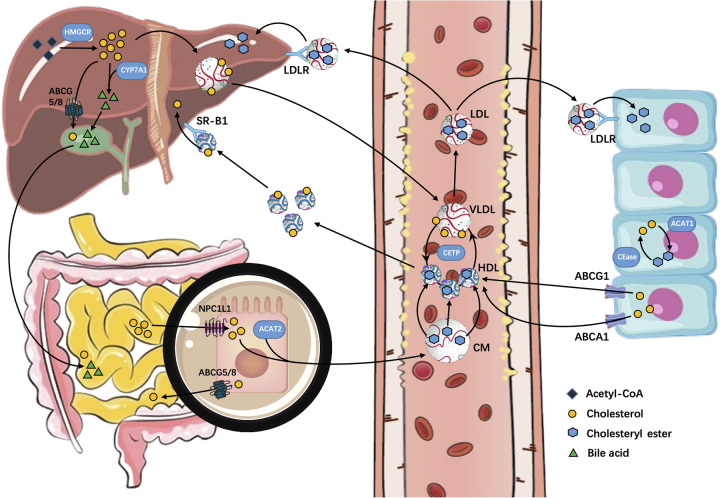
Cholesterol homeostasis refers to the dynamic balance between the production, excretion, and storage of cholesterol. Endogenous cholesterol is synthesized from acetyl-CoA through a series of >30 reactions involving over 20 enzymes. Conversely, exogenous cholesterol from food is absorbed by the intestines and transported to the liver via chylomicrons. The liver, as the central hub of cholesterol metabolism, converts both endogenous and exogenous cholesterol into VLDL and releases it into the bloodstream. VLDL is then processed into LDL, which is recognized by LDL receptors on the cell surface and internalized, allowing the cholesterol to be utilized by cells. Excess cholesterol is either esterified by acyl-CoA cholesterol acyltransferase (ACAT) and stored in lipid droplets or incorporated into plasma lipoproteins and released into the bloodstream. Cholesterol in the liver cells can also be excreted through the intestines by being converted into bile acids in the gallbladder. Excess cholesterol intake or synthesis can lead to cholesterol accumulation in the liver and bloodstream, resulting in pathological changes in organs, such as fatty liver and atherosclerosis. Furthermore, mutations in the genes regulating cholesterol homeostasis can lead to a range of inherited diseases, including Schnyder corneal dystrophy,14 Smith-Lemli-Opitz syndrome,15 familial hypercholesterolemia,16 Tangier disease,17 and sitosterolemia.18 Acquired imbalances in cholesterol homeostasis can also lead to several diseases, such as Parkinson disease,19 Alzheimer disease,20 muscular dystrophy,21 cancer,22–24 and common atherosclerosis-related conditions.25,26
As the central of cholesterol metabolism in the body, the liver plays a crucial role in cholesterol homeostasis; approximately 50% of cholesterol is produced by the liver.27 The liver also utilizes the scavenger receptor B1 (SR-B1) pathway to clear circulating cholesterol carried by HDL and maintain peripheral cholesterol homeostasis.28 Approximately 70% of retinoic acid in the human body is stored in HSCs, which play a critical role in the progression of NAFLD to liver fibrosis and cirrhosis.29 The liver is the only organ that can eliminate excess cholesterol by converting it into bile acids and excreting it with bile.30 Given the liver’s crucial role in the development, progression, and treatment prognosis of NAFLD, the regulation of cholesterol homeostasis has become a topic of interest in recent years. Studies have demonstrated the important role of NRs in regulating and stabilizing hepatic lipid metabolism31–37 (Fig. 1).
FIGURE 1.
The process of cholesterol from synthesis, absorption, utilization, and excretion. Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG5, ATP-binding cassette transporter G5; CYP7A1, cytochrome P450; LDLR, LDL receptor; SR-B1, scavenger receptor B1.
Role of cholesterol in NAFLD
The accumulation of liver cholesterol is caused by an increase in endogenous cholesterol synthesis and increased absorption of intestinal cholesterol, as well as obstructed excretion of free cholesterol in bile, conversion of bile acids, and nonbiliary transintestinal cholesterol efflux.38 In addition, animal models fed with a high-cholesterol diet exhibit NAFLD characteristics and are more likely to develop liver fibrosis and atherosclerosis manifestations than models fed with a high-fat diet alone.39 Severe liver fibrosis and cirrhosis are known risk factors for the development of liver cancer, which is the third deadliest cancer worldwide.40 Indeed, a significant proportion of liver cancer is caused by NAFLD, which is more hazardous because of the high incidence, late diagnosis, and poor prognosis of HCC induced by this type of NAFLD than HCC induced by viral hepatitis.41,42 A large body of research has shown that free cholesterol in the liver is a key pathogenic factor in promoting HCC.43–45 Overall, cholesterol plays a crucial role in the occurrence of NAFLD and the development of liver cirrhosis, HCC, and extrahepatic atherosclerotic diseases.
NUCLEAR RECEPTORS
NRs are among the most abundant transcriptional regulatory factors in metazoans and play crucial roles in metabolism, sex determination, reproductive development, and maintaining homeostasis.46–48 The human genome contains 48 NRs, in contrast to the 49 NRs found in rodents.49 NRs comprise an N-terminal regulatory region that activates transcription, DNA-binding domains containing 2 zinc finger structures, a nonconserved hinge region, a ligand-binding domain, and a variable C-terminal region.50 NRs can be divided into 7 subfamilies, ranging from NR0 to NR6.51
As the ligands for many NRs are fatty acids, steroids, and oxysterols, their role in liver lipid metabolism has received extensive attention.36,37,52–54 Table 1 lists the NRs involved in cholesterol metabolism and NAFLD. In the following sections, we summarize the roles of several NRs in maintaining hepatic cholesterol homeostasis and in the development and progression of NAFLD.
TABLE 1.
The role of nuclear receptors in hepatic cholesterol metabolism and NAFLD
| Gene name | Nuclear receptor | Ligand | Function in cholesterol metabolism and NAFLD | References |
|---|---|---|---|---|
| NR0B1 | DAX1 | Orphan | DAX-1 inhibits the transcriptional activity of LRH-1 and LXRα, and inhibits gluconeogenesis by negatively regulating HNF4A | 55–57 |
| NR0B2 | SHP | Orphan | The expression of SHP inhibits CYP7A1, and downregulation of SHP accelerates the transformation of cholesterol to bile acids by activating CPY7A1 | 58,59 |
| NR1A1 | TRα | Thyroid hormones | Downregulation of TRα alleviates diet-induced hepatic steatosis | 60,61 |
| NR1A2 | TRβ | Thyroid hormones | Loss of TRβ shows excessive lipid accumulation in both human and mouse livers | 62–64 |
| NR1B1 | RARα | Retinoic acids | Loss of RARα-induced hepatic steatosis and decreased macrophage cholesterol effection in HFD-fed mice. Upregulation of RARα could reduce hepatic lipid accumulation by decreasing CD36 expression | 65–67 |
| NR1B2 | RARβ | Retinoic acids | Upregulation of RARβ can alleviate hepatic lipid accumulation in hepatocytes | 68,69 |
| NR1B3 | RARγ | Retinoic acids | Upregulation of RARγ activates ABCA1-mediated cholesterol efflux | 70 |
| NR1C1 | PPARα | Fatty acids | Metabolomics has revealed that PPARα is an important regulator of bile acids by interacting with SREBP2, and that PPAR-α/γ agonist Saroglitazar significantly improves insulin resistance and dyslipidemia in NAFLD | 71–73 |
| NR1C2 | PPARβ | Fatty acids | PPARβ/δ inhibits CYP7a1 expression by upregulating FGF21 | 74 |
| NR1C3 | PPARγ | Fatty acids | PARγ inhibitor GW9662 can reduce the development of NAFLD by inhibiting TLR4, but PPARγ can promote cholesterol effluence by inducing the expression of ABCA1 in gallbladder epithelium | 75,76 |
| NR1D1 | REV-ERBα | Heme | REV-ERB coordinates the regulation of most genes encoding important enzymes in the cholesterol biosynthesis pathway, and downregulation of Rev-ERbα promotes bile acid metabolism through upregulation of CYP7A1 | 77–79 |
| NR1D2 | REV-ERBβ | Heme | Activation of REV-ERBα/β reduces hepatic triglyceride storage and inhibits cholesterol synthesis, and can increase promoter activity of CYP7A1 | 80,81 |
| NR1F1 | RORα | Sterols | Overexpression of RORα reduces diet-induced hepatic lipid accumulation, and its inverse agonist SR1001 regulates intestinal excretion of cholesterol by upregulating ABCG5/G8 | 82,83 |
| NR1F3 | RORγ | Sterols | Knockout of RORγ may reduce bile acid synthesis by decreasing levels of Cyp8b1, Cyp7b1, and Cyp27a1 | 84 |
| NR1H4 | FXRα | Bile acids | FXRα can promote CYP7A1 expression through FGF19 and SHP/LRH-1 pathway, competitively inhibit LXRα to promote CETP transcription and reduce liver cholesterol uptake and accumulation | 85–87 |
| NR1H3 | LXRα | Oxysterols | LXRα activates ABCG5/8 to promote the excretion of cholesterol in bile. LXRα can promote cholesterol effection in gallbladder epithelium by inducing the expression of ABCA1. LXRα deficiency leads to downregulation of CYP7A1 level, leading to liver cholesterol accumulation | 76,88,89 |
| NR1H2 | LXRβ | Oxysterols | LXRβ agonists induce the expression of cholesterol pump ABCA1 in bile duct cells and promote the output of cholesterol from the basolateral membrane of bile duct cells | 90 |
| NR1I1 | VDR | 1α,25‐dihydroxyvitamin D3 | VDR is significantly highly expressed in NAFLD, and its deficiency leads to reduced fat accumulation when aging and adult mice are fed a high-fat diet | 91,92 |
| NR1I2 | PXR | Endobiotics and xenobiotics | PXR aggravated hepatic steatosis caused by a high-fat diet, and PXR KO mice had significantly reduced levels of liver triglycerides, hepatic steatosis, serum total bile acids, and liver gene expression of enzymes involved in the bile acid synthesis pathway | 93,94 |
| NR1I3 | CAR | Xenobiotics | CAR is able to confer hepatoprotection from bile acids by increasing their sulfation and excretion | 95,96 |
| NR2A1 | HNF4α | Fatty acids | Loss of HNF4α enhances hepatic cholesterol accumulation by inhibiting CYP7A1/CYP8B1, FXR, and ABCAS1, and loss of HNF4α in mice shows hepatic steatosis and reduced plasma cholesterol levels | 97–99 |
| NR2B1 | RXRα | 9‐Cis retinoic acid | RXRα serves as a heterodimerization partner for PPARα, PXR, LXR, and FXR to participate in the regulation of cholesterol metabolism | 100–104 |
| NR2C2 | TR4 | Orphan | TR4 knockout reduces liver lipid accumulation and can reduce apoE levels | 105,106 |
| NR2E1 | TLX | Orphan | Mice with NR2E1 knocked out display a significant hepatic steatosis phenotype | 107 |
| NR2F1 | COUP-TFα | Orphan | COUP-TFα exerts a transcriptional repression effect by binding to the promoter region of apoCIII | 108 |
| NR2F2 | COUP-TFβ | Orphan | HNF4 and coup-TF-β synergistically activate the transcription of the CYP7A1 promoter | 109,110 |
| NR2F6 | EAR2 | Orphan | EAR2 inhibits the transcription of the genes encoding apoB, apoCIII, and apoAII | 111 |
| NR3A1 | ERα | Estrogens | ERα upregulates the expression of intestinal Npc1l1, Abcg5 and Abcg8, inhibits the transcriptional activity of LXRα in liver, and interacts with FXR in an estradiol-dependent manner to inhibit its function in vitro | 112–114 |
| NR3A2 | ERβ | Estrogens | Absence of Erβ alleviates the disruption of bile acid and cholesterol metabolism induced by perfluorooctane sulfonate | 115 |
| NR3B1 | ERRα | Orphan | VLDL-TG secretion is reduced in ERRα KO mice, leading to hepatic steatosis | 116 |
| NR3B3 | ERRγ | Orphan | Overexpression of ERRγ upregulates the expression of CYP7A1 both in vitro and in vivo | 117 |
| NR3C1 | GR | Glucocorticoids | GR interacts with FXR to reduce FXR transcriptional activity and promote hepatic cholestasis in mice by recruiting CtBP coblocking complex, and GR regulator CORT118335 can reverse hepatic cholesterol accumulation | 118,119 |
| NR3C2 | MR | Mineralocorticoids and glucocorticoids | Specific blockade of MR exhibits hepatic antisteatotic effects | 120 |
| NR3C4 | AR | Androgens | AR reduces cholesterol synthesis by mediating phosphorylation of HMGCR and promotes cholesterol synthesis and accumulation by activating sterol-regulatory element-binding protein isoform 2 | 121,122 |
| NR4A1 | Nur77 | Orphan | Nur77 regulates liver lipid metabolism by inhibiting SREBP1c activity, and the levels of TCHO, LDLR, HMGCR, and Nur77 in HepG2 cells are negatively correlated | 123,124 |
| NR5A2 | LRH‐1 | Phospholipids | LRH-1 regulates hepatic cholesterol excretion through CYP7A1 and CYP8B1 | 125 |
Abbreviations: ABCA1, ATP-binding cassette transporter A1; CYP7A1, cytochrome P450; FXR, farnesoid X receptor; HNF4α, hepatocyte nuclear factor 4 alpha; KO, knockout; LDLR, LDL receptor; LXR, liver X receptor; PPAR, peroxisome proliferator-activated receptor; SHP, small heterodimer partner.
Liver X receptor
As a cholesterol sensor, liver X receptor (LXR) activates the expression of a series of genes related to cholesterol absorption, efflux, transport, and excretion under increased cellular cholesterol levels.126 LXR has 2 homologous subtypes, LXRα and LXRβ, which have different tissue distributions. LXRα is highly expressed in metabolically active tissues and cell types, including liver, intestine, adipose tissue, and macrophages, whereas LXRβ is more widely expressed. Its physiological ligands include oxysterols, including 24(S), 25-epoxycholesterol, 25-hydroxycholesterol, and 22(R)-hydroxycholesterol, which are metabolites of cholesterol.127 Previous studies have shown that high-cholesterol diets result in significantly more cholesterol accumulation in the livers of LXRα-knockout mice than in those of wild-type mice.128
NPC1L1 is expressed in the brush border membrane of intestinal cells and is essential for intestinal cholesterol absorption.129 Treatment of Caco-2 cells with the LXR agonists T0901317 and GW3965 results in a significant decrease in hNPC1L1 mRNA levels,130 indicating that LXR activation can inhibit cholesterol absorption via dietary intake. SREBP2 regulates cellular cholesterol levels at the transcriptional level131; in the liver of LXR (−/−) mice fed a low-cholesterol diet, the mRNA levels of cholesterol synthesis-related genes comprising SREBP-2 were significantly higher than those in wild-type mice,128 further demonstrating the crucial role of LXR in maintaining cholesterol uptake and synthesis.
Cytochrome P450 (CYP7A1) is the rate-limiting enzyme in bile acid synthesis. Binding of LXRα to the promoter region of CYP7A1 in rodents induces the conversion of excess cholesterol to bile acids,127 whereas LXRα deficiency in mice leads to cholesterol accumulation in the liver.128 However, the same phenomenon is not observed because the binding site of LXRα is missing in the human CYP7a1 promoter region.132,133 ATP-binding cassette transporter A1 (ABCA1) is a crucial mediator for cholesterol efflux from cells to apolipoprotein AI (apoA-I) and the generation of HDL, which transfers peripheral cholesterol to the liver for metabolism to prevent atherosclerotic diseases.134 The transfection of LXRα and retinoid X receptor (RXR) can transactivate the transcription of ABCA1 in reverse in 293 cells.135 Moreover, activating the AMPK pathway can upregulate the mRNA and protein levels of LXRα and ABCA1. Additionally, knocking out LXRα can eliminate the upregulation effect of AMPK on ABCA1.136 Apart from ABCA1, ABCG1 also mediates cholesterol efflux to HDL.137 Multiple LXR and RXR heterodimer response elements have also been found in the ABCG1 gene.138 However, the mechanism by which cholesterol efflux is mediated by ABCG1, which is regulated by LXR remains unclear. ATP-binding cassette transporter G5 (ABCG5)/8 is expressed as a heterodimer on the surface of hepatocytes and intestinal cells and mediates cholesterol excretion into bile and the intestines.139 ABCG5/8 levels are also regulated by LXR.140 Thus, downregulation of LXR inhibits cholesterol efflux from macrophages, whereas upregulation of LXR increases the levels of ABCG5/8 in the liver and small intestine, promoting cholesterol reverse transport.141
The expression of LXR is correlated with the severity of NAFLD,142–144 with significant cholesterol accumulation observed in LXR-knockout mice fed a high-cholesterol diet.145 Although some LXR agonists, such as GW6340, 22(R)-hydroxycholesterol, and LXR-623, exhibit well tolerated,146,147 LXR activation can promote hepatic lipid synthesis and inhibit VLDL degradation148,149; thus, it is rarely used for the clinical treatment of NAFLD. Nevertheless, its potential as a drug target is gradually being recognized.
Hepatocyte nuclear factor 4 alpha
Hepatocyte nuclear factor 4 alpha (HNF4α) is a nuclear transcription factor expressed in the liver, kidney, intestine, and pancreas150; it binds to DNA as a homodimer and is the main regulatory factor for the expression of bile acid, lipid, glucose, and drug metabolism genes.36,151 Mutations in the HNF4α gene can cause maturity-onset diabetes of the young in adolescents.152 HNF4α is critical for pancreatic islet β-cell proliferation, as mice with HNF4α knockout in their β cells cannot respond to insulin resistance-induced proliferation.153 Mutations in HNF4α are also associated with changes in HDL cholesterol,154,155 which has prompted suggestions that HNF-4α is a central regulator of glucose and lipid metabolism.156 Previous animal studies have shown that HNF4α-knockout mice exhibit significant accumulation of liver cholesterol, as well as significant decreases in total cholesterol, HDL cholesterol, and triglyceride levels in serum relative to the control serum, with a significant increase in serum bile acid concentration.157 These changes in serum lipid profiles may be attributed to liver dysfunction or defects in lipid transport and metabolism.
SR-B1 mediates cholesterol uptake in the liver, and HNF4α can enhance the transcription of SR-B1 mediated by another NR, peroxisome proliferator-activated receptor (PPAR)γ.158 A decrease in HNF4α levels can inhibit the expression of NPC1L1 in Caco-2 cells and inhibit the uptake of cholesterol by cells.159 ApoA-1 is the main carrier protein of HDL and exerts atherosclerosis protective properties by participating in the ABCA1 and ABCG1 pathways involved in RCT.160,161 ApoA-1-defective mice do not form normal HDL particles and cannot effectively transport cholesterol to liver tissue, leading to cardiovascular diseases such as atherosclerosis. HNF4α, PPARα, and LXR participate in the downregulation of human ApoA-I gene expression and ApoA-I protein secretion mediated by TNFα in HepG2 cells.162 Moreover, HNF4α is an important transcription factor that binds to the CYP7A1 promoter region. Unlike LXR, HNF4α regulation of CYP7A1 levels appears to be bidirectional, with previous results showing that HNF4α inhibits CPY7A1 transcription levels.163 In another study, bile acid-induced dissociation of the HNF-4α co-activator complex HNF-4/PGC-1α/cAMP response element binding protein also inhibited CYP7A1 transcription.164 However, in mice with liver-specific knockout/overexpression of HNF4α, reduced de novo synthesis of fat and cholesterol was detected in the knockout mice, whereas overexpression of Hnf4α significantly induced the expression of genes related to cholesterol absorption, storage, and excretion, such as Mtp, Apob, Cyp7a1, Cyp8b1, Lrp, Ldlr, SR-B1, Acat2, Lcat, Abca1, Abcg5, Abcg8, Apoa1, Apoa2, and Apoc2. In addition, overexpression of Hnf4α showed no significant effect on Srebp-2, Hmgcr, Srebp-1c, or Fas,97 indicating that HNF4α plays a critical role in cholesterol metabolism but may not affect lipid synthesis, suggesting the potential role of HNF4α as a cholesterol sensor.
The pathogenesis of NAFLD is related to the abnormal concentration, structure, and function of HDL.165 In a study on HNF4α-knockout mice, the livers were enlarged and showed obvious lipid changes.166 Furthermore, research indicates that HNF4α is required for the preventive effect of liver cell-activating transcription factor 3 on the formation of NASH.167 Additionally, HNF4α can prevent the progression of NAFLD to NASH by regulating P53 and bile acid signaling pathways.98 These findings indicate that HNF4α not only participates in the pathogenesis of NAFLD but also plays an important role in disease progression. Recent research has shown that small molecule-activated RNA can activate HNF4α and significantly lower blood cholesterol and glucose levels.151 Furthermore, HNF4α mRNA therapy has been shown to restore the metabolic activity of liver cells in mice with liver fibrosis and in vitro human liver cells.168
Peroxisome proliferator-activated receptor (PPAR)
The PPAR family members are vertebrate-specific nutrient-sensing NRs. Three members have been identified in this family: PPARα, β/δ, and γ. The expression and function of the 3 subtypes of PPAR are unique, as is their tissue distribution. PPARαPPARα is highly expressed in the liver, skeletal muscle, and brown adipose tissue, whereas PPARγ is mainly expressed in white adipose tissue, and PPARβ/δ is expressed ubiquitously. The function of PPAR is closely related to energy homeostasis and nutrient sensing. The α and β/δ subtypes are involved in energy utilization, whereas γ contributes to energy storage in fat.32 PPAR acts as a transcription factor in the form of homologous or heterodimer binding to a cis-acting element called a peroxisome proliferator response element (PPRE) of the target gene.169
PPARα plays a crucial role in regulating lipid homeostasis by promoting fatty acid oxidation.170 Recent research on the NZO mouse model, which simulates human metabolic syndrome, has revealed significant changes in the pathways and targets involved in fatty acid metabolism mediated by PPARα, as well as notable changes in cholesterol-related targets.171 Interestingly, mice with liver-specific inactivation of fatty acid synthase exhibited lower serum and liver cholesterol levels, decreased SREBP-2, increased HMG-CoA, and reduced cholesterol biosynthesis. However, these phenotypes were corrected after the application of PPARα agonists.172 Bile acids, formed from cholesterol in the liver, represent an important pathway for eliminating cholesterol from the body. Sterol 12α-hydroxylase is a branching enzyme in the bile acid biosynthesis pathway that determines the ratio of cholic acid to chenodeoxycholic acid. Administration of the PPARα agonist WY-14,643 to mice results in a several-fold increase in sterol 12α-hydroxylase mRNA.173 Several studies have suggested that alterations in small, dense LDL may increase the risk of NAFLD-associated atherosclerosis and cardiovascular disease.174,175 Pparα agonists fibrate reduce small dense LDL particles and TG to regulate dyslipidemia in atherosclerotic disease.176 Additionally, PPARα synergizes with LXR to promote cholesterol excretion, with the co-application of PPARα and LXR agonists increasing fecal cholesterol excretion in mice by more than 12 times that observed with a single agonist.177 Although the primary biological function of PPARα is related to fatty acid metabolism, researchers are increasingly investigating its role in cholesterol homeostasis and have shown that fenofibrate, a PPARα agonist widely used to treat hyperlipidemia, can also be used to treat NAFLD.178
Nur77
The gene induced by nerve growth factor B, also known as Nur77, belongs to the NR subfamily 4A and is encoded by the NR4A1 gene. Nur77 is a type of orphan receptor that can function independent of a ligand despite not having a clear endogenous ligand.179 Structural studies of the ligand-binding domains of all 3 NR4A members have shown that these receptors lack a conventional-sized ligand-binding pocket because of the presence of large hydrophobic amino acid residue side chains.180–183 Members of the NR4A subfamily bind to DNA as monomers, homodimers, and heterodimers. As monomers of the NR4A subfamily, they bind to NGFI-B response elements; however, as homodimers and heterodimers with other NR4A members, all 3 members of the NR4A subfamily bind to Nur response elements. Nur77 and Nurr1 can also heterodimerize with the retinoid X receptor.184–188 In terms of function, the NR4A family is closely related to metabolic diseases.189–191
Overexpression of Nur77 in mouse livers can reduce the hepatic triglyceride content and lower the expression of Srebp1c, an important regulator of cholesterol metabolism.123 The levels of genes involved in hepatic cholesterol metabolism, such as LDLR and HMGCoA reductase, increase as Nur77 expression is downregulated and decrease as Nur77 expression is upregulated.124 Treatment with Csn-b, which is a Nur77 transcriptional activator, gradually reduces the gene levels of LDLR, ABCG5, SREBP1c, and SREBP2 in mouse livers, while increasing the gene levels of SR-B1 and hepatic lipase. When Nur77 was knocked out, the expression of these genes was downregulated, and the lipid content in mouse liver was reduced by 39.9% after Csn-b treatment.192 Another study showed that Nur77 reduces the expression of Abcg5 and Abcg8, which are 2 LXR target genes in mice.123
Although the overexpression of Nur77 can help the liver to excrete cholesterol and reduce the hepatotoxicity caused by cholesterol, Nur77 is highly expressed in both HCC and cancerous cirrhosis,193 which suggests its potential role in the progression of NAFLD to HCC.
Farnesoid X receptor
Farnesoid X receptor (FXR) is a bile acid-activated receptor that is mainly expressed in the liver and intestinal tissues and has 2 members in mammals: FXRα and FXRβ.194 Existing studies have shown that FXR regulates the metabolism of bile acids, carbohydrates, and lipids.195,196 After activation, FXR and RXR form a heterodimer and induce expression of the small heterodimer partner (SHP) gene, leading to the transcriptional inhibition of the rate-limiting enzyme in bile acid synthesis, 7α-hydroxylase (CYP7A1).197 FXR also stimulates the synthesis of FGF-19, which inhibits the expression of CYP7A1 and sterol 12α-hydroxylase (CYP8B1) through the fibroblast growth factor receptor 4 (FGFR4) pathway in hepatocytes.198–200 The FXR/SHP and FXR/FGF19/FGFR4 pathways constitute the main negative regulators of bile acid synthesis. FXR inhibits the uptake of bile acids in the liver by suppressing the expression of sodium taurocholate cotransporting polypeptide via an SHP-dependent mechanism.201 FXR also upregulates the expression of genes encoding bile salt export pump and multidrug resistance protein 3 and increases the efflux of bile acids from hepatocytes into the canaliculus.202,203 Moreover, FXR enhances the expression of organic solute transporter α/β, thereby increasing the efflux of bile acids from hepatocytes into the portal vein.204 In addition, FXR regulates key enzymes involved in bile acid conjugation and detoxification.202 Overall, FXR is closely related to the entire metabolic process of bile acid synthesis, transport, and reabsorption.205,206 In both in vivo and in vitro models of cholestasis in the liver, FXR activation can improve bile stasis, thereby protecting the liver from the high cytotoxicity of bile acids.207 Furthermore, FXR induces the synthesis of FGF15/19 and upregulates FGF15/19-FGFR4 signaling, which may increase the risk of HCC.208 According to previous research, FXR activation exhibits potential antitumor activity in colorectal cancer,209 HCC,210 and cholangiocarcinoma.211
In a multicentre study, FXR activation inhibited cholesterol uptake and conversion to bile acids but also affected cholesterol synthesis and excretion. Furthermore, FXR activation promoted the expression of liver scavenger receptors, thereby enhancing RCT. Moreover, obeticholic acid not only increased LDL but also decreased HDL,212 which was further supported by the low HDL levels in animals treated with GW4064 and XL335.213,214 In contrast, FXR antagonists are more beneficial for hypercholesterolemia.215–217 Thus, more data are required to better understand the potential of selective FXR agonists for modulating the cholesterol levels of humans, as well as the potential of statins for mitigating the associated adverse effects.
RXRα
RXR consists of 3 subtypes: α, β, and γ. The ligand of RXR is 9-cis RA; however, high concentrations of all-trans RA can also activate RXR by conversion to 9-cis RA.218 Conventional research on NRs has been limited to identifying ligands and determining their biological functions. However, the discovery of RXR and its ability to serve as a heterodimerization partner for other NRs has ushered in a new era of NR research. To date, studies have revealed that RXR can form heterodimers with other NRs219–221 and also form homodimers.222 NRs bind to a DNA sequence called hormone response elements, which contain at least 6 core nucleotides—AGGTCA—and can be constructed into various structured motifs.223 RXR and its dimerization partners recognize hormone response elements on DNA that are spaced 1 to 5 nucleotides apart. The complexity resulting from these various combinations can be partially explained by the tissue-specific expression of NRs.
The 3 subtypes of RXR are widely expressed in vivo, with RXRα being the most abundant subtype expressed in the liver.220 High doses of all-trans RA have been used to treat acne and reportedly cause hyperlipidemia and hepatotoxicity224; the mechanism behind this effect may be that all-trans RA inhibits the transcriptional activation of CYP7A1 by the FXR/RXR dimer.100 The expression of ABCG1 in the liver is closely related to cholesterol efflux to HDL, and as a heterodimerization partner, RXR participates in almost the entire process of cholesterol efflux through ABCG1. Overexpression of LXR/RXR can activate the transcription of ABCG1 in HepG2 cells,225 and activation of the RXR/RAR dimer can upregulate the expression of the main component of HDL in the liver (apoA-I).226 Additionally, the RXR agonist LGD1069 can activate the RXR/PPAR pathway to increase HDL cholesterol levels without changing apoA-I levels in the liver.227 CYP3A4 catalyzes the 25-hydroxylation of cholesterol, and 25-hydroxycholesterol is metabolized more quickly by CYP7A1 and CYP8B1 than 4β-hydroxycholesterol.228 Furthermore, CAR/RXR enhances the transcription of CYP3A4 in the form of heterodimers, and interestingly, LXR can inhibit the transcription of PXR-dependent CYP3A4.229
The ligand of RXR is a metabolite of vitamin A, and absorption of vitamin A in the human body requires the assistance of intestinal bile acids. Additionally, as RXR can serve as a dimerization partner for several important NRs in bile acid metabolism, RXR links vitamin A and bile acid metabolism.230 Both in vivo and in vitro experiments have demonstrated that vitamin A metabolites can directly regulate the expression of bile acid homeostasis-related genes through RXR or RAR and can also regulate gene transcriptional activity through FXR/RXRα. Furthermore, SHP and FGF19/15, 2 pathways that inhibit bile acid synthesis, are also mediated by vitamin A.100,231,232 Currently, obstacles to the application of vitamin A for NAFLD treatment include uncertainty regarding vitamin A status in the liver and the cell toxicity caused by high vitamin A concentrations. (Fig. 2).
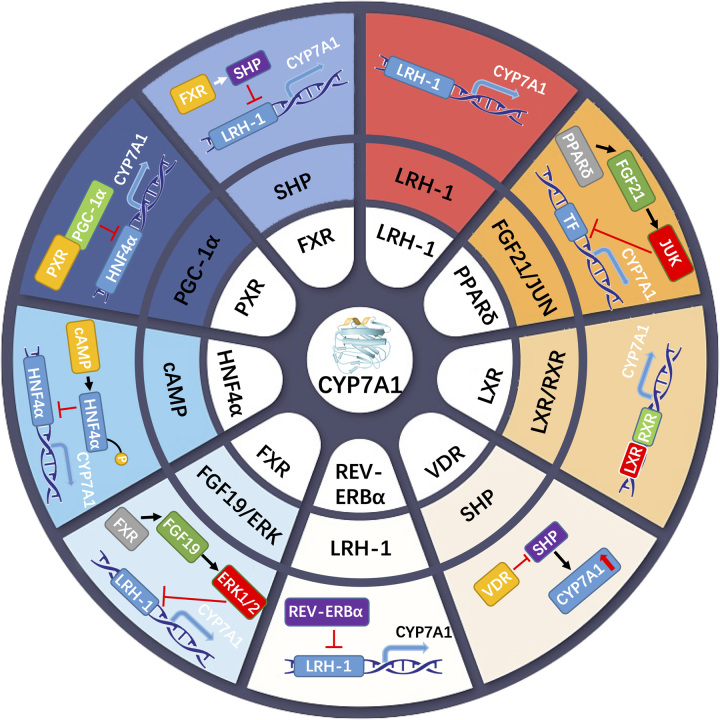
FIGURE 2.
The molecular mechanism of nuclear receptor regulation on the rate-limiting enzyme CYP7a1 in bile acid synthesis. The inner ring represents the key nuclear receptor. The intermediate ring represents the factor or pathway that the nuclear receptor acts on. The outer ring is a model graph. Abbreviations: CYP7A1, cytochrome P450; FXR, farnesoid X receptor; HNF4α, hepatocyte nuclear factor 4 alpha; LXR, liver X receptor; PPAR, peroxisome proliferator-activated receptor; retinoid X receptor; SHP, small heterodimer partner.
Reverse cholesterol transport
Although the process from NAFLD to HCC and eventual mortality may persist for decades, it is the accompanying atherosclerotic disease of NAFLD that serves as the primary cause of death.233 The prevailing view is that the hallmarks of atherosclerosis are uncontrolled uptake of oxidized LDL by macrophages, impaired cholesterol efflux, and accumulation of cholesterol esters as cytoplasmic lipid droplets, leading to foam cell formation in the arterial intima. RCT refers to the process by which excess cholesterol is transported from peripheral tissue cells into circulation, metabolized in the liver, and ultimately excreted in feces, which is the main pathway for the liver to clear excess cholesterol in circulation.
Cholesterol efflux from cells to HDL marks the onset of RCT, in which NRs play a crucial role. Macrophages absorb cholesterol from oxidized LDL through CD36 and scavenger receptor A. Internalized oxidized LDL provides PPARγ activation ligands and fatty acids, which induce the expression of CD36.234 The loss of NCOR1, an NR corepressor, relieves the inhibition of PPARγ on CD36 transcriptional activity, leading to increased foam cell formation.235 Tamoxifen can relieve PPARγ activation of CD36 transcription.236 In a previous study, overexpression of Nur77 reduced CD36 and scavenger receptor A levels, inhibiting the process of macrophage differentiation into foam cells.237 After macrophages take up cholesterol, ACAT1 catalyzes the formation of cholesterol esters to maintain a balance with free cholesterol. The excessive accumulation of cholesterol esters promotes foam cell formation. PPARα is the target of fibrate drugs, whose activation reduces the cholesterol ester content in macrophages but does not downregulate ACAT1 gene expression. Instead, PPARα promotes the entry of ACAT1 substrates, which are long-chain fatty acids, into mitochondria for β-oxidation, reducing the efficiency of cholesterol esterification and thus reducing the ratio of cholesterol esters to free cholesterol.238 Excess cholesterol in cells is transported out to mature HDL via SR-B1 and ATP-binding cassette protein G1 (ABCG1) and A1 (ABCA1). Over 70% of cholesterol efflux in macrophages is mediated by ABCA1 and ABCG1.239 As mentioned previously, the transcription of ABCA1 and ABCG1 is regulated by LXR/RXR heterodimers. Subsequent studies have clarified that the ligand activation of PPARγ amplifies the cholesterol clearance mediated by LXR-ABCA1.240 An investigation of 131 patients with coronary artery disease also revealed that the use of the PPARγ inhibitor GW9662 led to a decrease in LXR-α and ABCA1 levels in the group, accompanied by impaired cholesterol efflux capacity.241 Cholesterol carried by HDL is eventually taken up by hepatic SR-B1 receptors after circulation and metabolized in the liver, which brings us back to the liver function discussed at the beginning of this review.
PROGRESS IN RELATED DRUG RESEARCH
Cholesterol originates from endogenous synthesis or diet and is eliminated primarily through biliary secretion. A significant increase in cholesterol synthesis was found in patients with NAFLD and was positively correlated with liver fat content,242 while increased cholesterol intake led to more severe steatosis.243 Although statins have been shown to be effective in reducing the risk of NAFLD and have cardiovascular protective abilities,244,245 and treating mice with atorvastatin mitigated liver and blood vessel damage caused by HFD while increasing bile acid synthesis and excretion.246 Moreover, in view of its potential liver damage and the exact effects of cholesterol metabolic homeostasis on atherosclerotic disease, drug research targeting NR therapy for NAFLD is still particularly important. GFT505, a dual PPAR-α/δ agonist, is a promising novel agent for the treatment of NAFLD and has been shown to improve insulin sensitivity, lipids, and liver enzymes in patients with MetS or prediabetic abdominal obesity in phase II clinical trials.247,248 Low doses of thyroid hormone effectively and safely reduced hepatic fat content in men with type 2 diabetes mellitus and NAFLD,249 and oral selective THR-β agonist MGL-3196 significantly reduced hepatic fat accumulation in a multicenter study.250 LXR, a key NR regulating cholesterol effection and transport, and its reverse stimulant SR9238 inhibited liver steatosis induced by high-fat diet in mice251 and alleviated inflammation and fibrosis in mouse models of NASH.252 Obeticholic acid (OCA), also known as INT747, is a selective FXR agonist that has been entered into clinical studies. OCA can significantly improve insulin sensitivity and reduce markers of liver inflammation and fibrosis, thereby improving NAFLD progression.253 OCA reduced blood cholesterol levels in a Western diet and STZ-induced insulin deficiency and hyperglycemia mouse model.254 In addition, in a multicenter study, OCA therapy improved NAFLD-induced steatosis and hepatocellular ballooning and was well tolerated, with pruritus being the most common adverse event.212
CONCLUSIONS
The dysregulation of NRs disrupts the comprehensive control of energy metabolism via the gut-liver-adipose axis, leading to the onset of NAFLD, with the imbalance of cholesterol homeostasis playing a crucial role. The effects of NRs on cholesterol metabolism are complex, as they are linked to both fatty acid and glucose metabolism and may appear simultaneously. Although the activation of LXR and PXR increases steatosis, PPAR and FXR reduce steatosis; interestingly, hepatic inflammation is downregulated by the activation of these receptors. This may represent a protective mechanism as it simulates the weakened activation of TLR4 by lipopolysaccharides, making the liver highly resistant to lipotoxicity. During the progression of NAFLD, FXR appears to have an antifibrotic effect; however, its role in human NASH requires further investigation. Nur77 is highly expressed in HCC and cirrhosis; however, this does not negate its protective role in the initial stages of NAFLD. Novel treatment strategies are required that provide the beneficial effects of NR activation while minimizing adverse metabolic effects. Such strategies, which are currently under development, include dual receptor agonists, tissue-specific agonists/antagonists, and the use of FGF21. Chinese herbal medicine has also shown substantial therapeutic potential in targeting some NR targets in RCT.255,256 Furthermore, the impact of NR cross talk in NAFLD may be profound; for example, the synergistic or regulatory effects of HNF4α, LXR, and Nur77 on the regulation of cholesterol efflux and the cascade effect of PPARγ and LXR in RCT regulation. However, more research is warranted to explore the mechanisms of cross talk between NRs.
FUNDING INFORMATION
This study was supported by the Major Research Plan of the National Natural Science Foundation of China (Grant No.92249305),“Announce the List and Take Charge” Major Scientific and Technological Projects of Liaoning Province (Grant No.2022JH1/10400001), and the Joint Programme on Science and Technology for the People’s Livelihood of Liaoning Province(Grant No.2021JH2/10300090).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG5, ATP-binding cassette transporter G5; ACAT, acyl-CoA cholesterol acyltransferase; AMPK, AMP-activated protein kinase; apoA-I, apolipoprotein AI; CETP, cholesteryl ester transfer protein; CtBP, C-terminal binding protein; CYP7A1, cytochrome P450; FGFR4, fibroblast growth factor receptor 4; FXR, farnesoid X receptor; GR, Glucocorticoid receptor; HFD, high-fat diet; HNF4α, hepatocyte nuclear factor 4 alpha; LXR, liver X receptor; NRs, nuclear receptors; NZO, New Zealand Obese mice; PPAR, peroxisome proliferator-activated receptor; PPRE, peroxisome proliferator response element; RA, Retinoic acid; RCT, reverse cholesterol transport; RXR, retinoid X receptor; SHP, small heterodimer partner; SR-B1, scavenger receptor B1; TCHO, total cholesterol; TG, Triglyceride.
Contributor Information
Zhichi Li, Email: lizhichi_cmu@126.com.
Dantong Zheng, Email: zdt_cmu@163.com.
Tiantian Zhang, Email: zhangtiantian0502@163.com.
Shan Ruan, Email: ruanshan88@163.com.
Na Li, Email: nli@cmu.edu.cn.
Yang Yu, Email: yyu90@cmu.edu.cn.
Yang Peng, Email: pengy@sj-hospital.org.
Difei Wang, Email: dfwang@cmu.edu.cn.
REFERENCES
- 1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2. European Association for the Study of the, L., D. European Association for the Study of, and O . European Association for the Study of, EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia. 2016;59:1121–40. [DOI] [PubMed] [Google Scholar]
- 3. Zhou J, Zhou F, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology. 2020;71:1851–64. [DOI] [PubMed] [Google Scholar]
- 4. Papatheodoridi AM, Chrysavgis L, Koutsilieris M, Chatzigeorgiou A. The role of senescence in the development of nonalcoholic fatty liver disease and progression to nonalcoholic steatohepatitis. Hepatology. 2020;71:363–74. [DOI] [PubMed] [Google Scholar]
- 5. Simon TG, Roelstraete B, Hagström H, Sundström J, Ludvigsson JF. Non-alcoholic fatty liver disease and incident major adverse cardiovascular events: Results from a nationwide histology cohort. Gut. 2022;71:1867–75. [DOI] [PubMed] [Google Scholar]
- 6. Powell EE, Wong VWS, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24. [DOI] [PubMed] [Google Scholar]
- 7. Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774–88. [DOI] [PubMed] [Google Scholar]
- 8. Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–74. [DOI] [PubMed] [Google Scholar]
- 9. Hirsova P, Ibrabim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Yu XH, Ou X, Ouyang XP, Tang CK. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog Lipid Res. 2021;83:101109. [DOI] [PubMed] [Google Scholar]
- 11. Mota M, Banini BA, Cazanave SC, Sanyal AJ. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism. 2016;65:1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lunghi G, Fazzari M, Ciampa MG, Mauri L, Di Biase E, Chiricozzi E, et al. Regulation of signal transduction by gangliosides in lipid rafts: Focus on GM3-IR and GM1-TrkA interactions. FEBS Lett. 2022;596:3124–32. [DOI] [PubMed] [Google Scholar]
- 14. Weiss JS. More on Schnyder corneal dystrophy. Ophthalmology. 2009;116:2260; author reply 2260. [DOI] [PubMed] [Google Scholar]
- 15. Nowaczyk MJM, Irons MB. Smith-Lemli-Opitz syndrome: phenotype, natural history, and epidemiology. Am J Med Genet C Semin Med Genet. 2012;160C:250–62. [DOI] [PubMed] [Google Scholar]
- 16. Henderson R, O’Kane M, McGilligan V, Watterson S. The genetics and screening of familial hypercholesterolaemia. J Biomed Sci. 2016;23:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolovou G, Mikhailidis D, Anagnostopoulou K, Daskalopoulou S, Cokkinos D. Tangier disease four decades of research: A reflection of the importance of HDL. Curr Med Chem. 2006;13:771–82. [DOI] [PubMed] [Google Scholar]
- 18. Escolà-Gil JC, Quesada H, Julve J, Martín-Campos JM, Cedó L, Blanco-Vaca F. Sitosterolemia: Diagnosis, investigation, and management. Curr Atheroscler Rep. 2014;16:424. [DOI] [PubMed] [Google Scholar]
- 19. Huang X, Sterling NW, Du G, Sun D, Stetter C, Kong L, et al. Brain cholesterol metabolism and Parkinson’s disease. Mov Disord. 2019;34:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li D, Zhang J, Liu Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022;45:401–14. [DOI] [PubMed] [Google Scholar]
- 21. White Z, Theret M, Milad N, Tung LW, Chen WWH, Sirois MG, et al. Cholesterol absorption blocker ezetimibe prevents muscle wasting in severe dysferlin-deficient and mdx mice. J Cachexia Sarcopenia Muscle. 2022;13:544–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874:188394. [DOI] [PubMed] [Google Scholar]
- 23. Liu W, Chakraborty B, Safi R, Kazmin D, Chang C, McDonnell DP. Dysregulated cholesterol homeostasis results in resistance to ferroptosis increasing tumorigenicity and metastasis in cancer. Nat Commun. 2021;12:5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. King RJ, Singh PK, Mehla K. The cholesterol pathway: Impact on immunity and cancer. Trends Immunol. 2022;43:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: Triglycerides, remnant cholesterol, and atherosclerotic cardiovascular disease risk. Endocr Rev. 2019;40:537–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poznyak AV, Kashirskikh DA, Sukhorukov VN, Kalmykov V, Omelchenko AV, Orekhov AN. Cholesterol transport dysfunction and its involvement in atherogenesis. Int J Mol Sci. 2022;23:1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–81. [DOI] [PubMed] [Google Scholar]
- 28. Shen WJ, Asthana S, Kraemer FB, Azhar S. Scavenger receptor B type 1: Expression, molecular regulation, and cholesterol transport function. J Lipid Res. 2018;59:1114–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blaner WS, O'Byrne SM, Wongsiriroj N, Kluwe J, D'Ambrosio DM, Jiang H, et al. Hepatic stellate cell lipid droplets: A specialized lipid droplet for retinoid storage. Biochim Biophys Acta. 2009;1791:467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vourakis M, Mayer G, Rousseau G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int J Mol Sci. 2021;22:8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Z, Roth K, Agarwal M, Liu W, Petriello MC. The transcription factors CREBH, PPARa, and FOXO1 as critical hepatic mediators of diet-induced metabolic dysregulation. J Nutr Biochem. 2021;95:108633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. He J, Gao J, Xu M, Ren S, Stefanovic-Racic M, O'Doherty RM, et al. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes. 2013;62:1876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uppal H, Toma D, Saini SPS, Ren S, Jones TJ, Xie W. Combined loss of orphan receptors PXR and CAR heightens sensitivity to toxic bile acids in mice. Hepatology. 2005;41:168–76. [DOI] [PubMed] [Google Scholar]
- 35. Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, et al. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ren H, Hu F, Wang D, Kang X, Feng X, Zhang L, et al. Sirtuin 2 prevents liver steatosis and metabolic disorders by deacetylation of hepatocyte nuclear factor 4alpha. Hepatology. 2021;74:723–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liang H, Xie X, Song X, Huang M, Su T, Chang X, et al. Orphan nuclear receptor NR4A1 suppresses hyperhomocysteinemia-induced hepatic steatosis in vitro and in vivo. FEBS Lett. 2019;593:1061–71. [DOI] [PubMed] [Google Scholar]
- 38. Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–45. [DOI] [PubMed] [Google Scholar]
- 39. Kim EJ, Kim B, Seo HS, Lee YJ, Kim HH, Son HH, et al. Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. 2014;9:e97841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 41. Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGlynn KA, Petrick JL, El‐Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021;70:761–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liang JQ, Teoh N, Xu L, Pok S, Li X, Chu ESH, et al. Dietary cholesterol promotes steatohepatitis related hepatocellular carcinoma through dysregulated metabolism and calcium signaling. Nat Commun. 2018;9:4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Che L, Chi W, Qiao Y, Zhang J, Song X, Liu Y, et al. Cholesterol biosynthesis supports the growth of hepatocarcinoma lesions depleted of fatty acid synthase in mice and humans. Gut. 2020;69:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giguere V. Orphan nuclear receptors: From gene to function. Endocr Rev. 1999;20:689–725. [DOI] [PubMed] [Google Scholar]
- 47. Sonoda J, Pei L, Evans RM. Nuclear receptors: Decoding metabolic disease. FEBS Lett. 2008;582:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–10. [DOI] [PubMed] [Google Scholar]
- 49. Xie CQ, Jeong Y, Fu M, Bookout AL, Garcia-Barrio MT, Sun T, et al. Expression profiling of nuclear receptors in human and mouse embryonic stem cells. Mol Endocrinol. 2009;23:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Owen GI, Zelent A. Origins and evolutionary diversification of the nuclear receptor superfamily. Cell Mol Life Sci. 2000;57:809–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, et al. Molecular actions of PPARalpha in lipid metabolism and inflammation. Endocr Rev. 2018;39:760–802. [DOI] [PubMed] [Google Scholar]
- 53. Liang N, Jakobsson T, Fan R, Treuter E. The nuclear receptor-co-repressor complex in control of liver metabolism and disease. Front Endocrinol (Lausanne). 2019;10:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335–47. [DOI] [PubMed] [Google Scholar]
- 55. Nedumaran B, Hong S, Xie YB, Kim YH, Seo WY, Lee MW, et al. DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4alpha and negatively regulates gluconeogenic enzyme gene expression. J Biol Chem. 2009;284:27511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nedumaran B, Kim GS, Hong S, Yoon YS, Kim YH, Lee CH, et al. Orphan nuclear receptor DAX-1 acts as a novel corepressor of liver X receptor alpha and inhibits hepatic lipogenesis. J Biol Chem. 2010;285:9221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sablin EP, Woods A, Krylova IN, Hwang P, Ingraham HA, Fletterick RJ. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. Proc Natl Acad Sci U S A. 2008;105:18390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Y, Tian Y, Cai W, Wang Q, Chang Y, Sun Y, et al. Novel iota-carrageenan tetrasaccharide alleviates liver lipid accumulation via the bile acid-FXR-SHP/PXR pathway to regulate cholesterol conversion and fatty acid metabolism in insulin-resistant mice. J Agric Food Chem. 2021;69:9813–21. [DOI] [PubMed] [Google Scholar]
- 59. Xiao Y, Wang Y, Liu Y, Wang W, Tian X, Chen S, et al. A nonbile acid farnesoid X receptor agonist tropifexor potently inhibits cholestatic liver injury and fibrosis by modulating the gut-liver axis. Liver Int. 2021;41:2117–31. [DOI] [PubMed] [Google Scholar]
- 60. Jornayvaz FR, Lee HY, Jurczak MJ, Alves TC, Guebre-Egziabher F, Guigni BA, et al. Thyroid hormone receptor-alpha gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 2012;153:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pelletier P, Gauthier K, Sideleva O, Samarut J, Silva JE. Mice lacking the thyroid hormone receptor-alpha gene spend more energy in thermogenesis, burn more fat, and are less sensitive to high-fat diet-induced obesity. Endocrinology. 2008;149:6471–86. [DOI] [PubMed] [Google Scholar]
- 62. Krause C, Grohs M, El Gammal AT, Wolter S, Lehnert H, Mann O, et al. Reduced expression of thyroid hormone receptor beta in human nonalcoholic steatohepatitis. Endocr Connect. 2018;7:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Araki O, Ying H, Zhu XG, Willingham MC, Cheng SY. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol Endocrinol. 2009;23:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chaves C, Bruinstroop E, Refetoff S, Yen PM, Anselmo J. Correction to: Increased hepatic fat content in patients with resistance to thyroid hormone beta, by Chaves et al. Thyroid 2021;31(7):1127-1134. doi: 10.1089/thy.2020.0651. Thyroid. 2021;31:1447–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao Z, Deng ZT, Huang S, Ning M, Feng Y, Shen Y, et al. Alisol B alleviates hepatocyte lipid accumulation and lipotoxicity via regulating RARalpha-PPARgamma-CD36 cascade and attenuates non-alcoholic steatohepatitis in mice. Nutrients. 2022;14:2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cassim Bawa FN, Xu Y, Gopoju R, Plonski NM, Shiyab A, Hu S, et al. Hepatic retinoic acid receptor alpha mediates all-trans retinoic acid’s effect on diet-induced hepatosteatosis. Hepatol Commun. 2022;6:2665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cassim Bawa FN, Gopoju R, Xu Y, Hu S, Zhu Y, Chen S, et al. Retinoic acid receptor alpha (RARalpha) in macrophages protects from diet-induced atherosclerosis in mice. Cells. 2022;11:3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tang XH, Melis M, Lu C, Rappa A, Zhang T, Jessurun J, et al. A retinoic acid receptor beta2 agonist attenuates transcriptome and metabolome changes underlying nonalcohol-associated fatty liver disease. J Biol Chem. 2021;297:101331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li Y, Wong K, Walsh K, Gao B, Zang M. Retinoic acid receptor beta stimulates hepatic induction of fibroblast growth factor 21 to promote fatty acid oxidation and control whole-body energy homeostasis in mice. J Biol Chem. 2013;288:10490–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Costet P, Lalanne F, Gerbod-Giannone MC, Molina JR, Fu X, Lund EG, et al. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol Cell Biol. 2003;23:7756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu HY, Hu P, Li Y, Sun MA, Qu H, Zong Q, et al. Targeted inhibition of PPARalpha ameliorates CLA-induced hypercholesterolemia via hepatic cholesterol biosynthesis reprogramming. Liver Int. 2022;42:1449–66. [DOI] [PubMed] [Google Scholar]
- 72. Li F, Patterson AD, Krausz KW, Tanaka N, Gonzalez FJ. Metabolomics reveals an essential role for peroxisome proliferator-activated receptor alpha in bile acid homeostasis. J Lipid Res. 2012;53:1625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K, et al. Saroglitazar, a PPAR-alpha/gamma Agonist, for Treatment of NAFLD: A randomized controlled double-blind phase 2 trial. Hepatology. 2021;74:1809–24. [DOI] [PubMed] [Google Scholar]
- 74. Kouno T, Liu X, Zhao H, Kisseleva T, Cable EE, Schnabl B. Selective PPARdelta agonist seladelpar suppresses bile acid synthesis by reducing hepatocyte CYP7A1 via the fibroblast growth factor 21 signaling pathway. J Biol Chem. 2022;298:102056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baumann A, Burger K, Brandt A, Staltner R, Jung F, Rajcic D, et al. GW9662, a peroxisome proliferator-activated receptor gamma antagonist, attenuates the development of non-alcoholic fatty liver disease. Metabolism. 2022;133:155233. [DOI] [PubMed] [Google Scholar]
- 76. Wang JM, Wang D, Tan YY, Zhao G, Ji ZL. 22(R)-hydroxycholesterol and pioglitazone synergistically decrease cholesterol ester via the PPARgamma-LXRalpha-ABCA1 pathway in cholesterosis of the gallbladder. Biochem Biophys Res Commun. 2014;447:152–7. [DOI] [PubMed] [Google Scholar]
- 77. Zhang T, Zhao M, Lu D, Wang S, Yu F, Guo L, et al. REV-ERBalpha regulates CYP7A1 through repression of liver receptor homolog-1. Drug Metab Dispos. 2018;46:248–58. [DOI] [PubMed] [Google Scholar]
- 78. Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, Sasso GL, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sitaula S, Zhang J, Ruiz F, Burris TP. Rev-erb regulation of cholesterologenesis. Biochem Pharmacol. 2017;131:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Noshiro M, Usui E, Kawamoto T, Kubo H, Fujimoto K, Furukawa M, et al. Multiple mechanisms regulate circadian expression of the gene for cholesterol 7alpha-hydroxylase (Cyp7a), a key enzyme in hepatic bile acid biosynthesis. J Biol Rhythms. 2007;22:299–311. [DOI] [PubMed] [Google Scholar]
- 82. Kim EJ, Yoon YS, Hong S, Son HY, Na TY, Lee MH, et al. Retinoic acid receptor-related orphan receptor alpha-induced activation of adenosine monophosphate-activated protein kinase results in attenuation of hepatic steatosis. Hepatology. 2012;55:1379–88. [DOI] [PubMed] [Google Scholar]
- 83. Billon C, Sitaula S, Burris TP. Inhibition of RORalpha/gamma suppresses atherosclerosis via inhibition of both cholesterol absorption and inflammation. Mol Metab. 2016;5:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Takeda Y, Kang HS, Lih FB, Jiang H, Blaner WS, Jetten AM. Retinoid acid-related orphan receptor gamma, RORgamma, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res. 2014;42:10448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. [DOI] [PubMed] [Google Scholar]
- 87. Park SS, Choi H, Kim SJ, Kim OJ, Chae KS, Kim E. FXRalpha down-regulates LXRalpha signaling at the CETP promoter via a common element. Mol Cells. 2008;26:409–14. [PubMed] [Google Scholar]
- 88. Wang B, Tontonoz P. Liver X receptors in lipid signalling and membrane homeostasis. Nat Rev Endocrinol. 2018;14:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–70. [DOI] [PubMed] [Google Scholar]
- 90. Xia X, Jung D, Webb P, Zhang A, Zhang B, Li L, et al. Liver X receptor beta and peroxisome proliferator-activated receptor delta regulate cholesterol transport in murine cholangiocytes. Hepatology. 2012;56:2288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Barchetta I, Cimini FA, Chiappetta C, Bertoccini L, Ceccarelli V, Capoccia D, et al. Relationship between hepatic and systemic angiopoietin-like 3, hepatic Vitamin D receptor expression and NAFLD in obesity. Liver Int. 2020;40:2139–47. [DOI] [PubMed] [Google Scholar]
- 92. Weber K, Erben RG. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J Anim Physiol Anim Nutr (Berl). 2013;97:675–83. [DOI] [PubMed] [Google Scholar]
- 93. Kim S, Choi S, Dutta M, Asubonteng JO, Polunas M, Goedken M, et al. Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity- and inflammation-prone gut microbiome signature. Biochem Pharmacol. 2021;193:114698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhao LY, Xu JY, Shi Z, Englert NA, Zhang SY. Pregnane X receptor (PXR) deficiency improves high fat diet-induced obesity via induction of fibroblast growth factor 15 (FGF15) expression. Biochem Pharmacol. 2017;142:194–203. [DOI] [PubMed] [Google Scholar]
- 95. Guo GL, Lambert G, Negishi M, Ward JM, Brewer HB, Kliewer SA, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71. [DOI] [PubMed] [Google Scholar]
- 96. Assem M, Schuetz EG, Leggas M, Sun D, Yasuda K, Reid G, et al. Interactions between hepatic Mrp4 and Sult2a as revealed by the constitutive androstane receptor and Mrp4 knockout mice. J Biol Chem. 2004;279:22250–7. [DOI] [PubMed] [Google Scholar]
- 97. Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol. 2011;31:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Xu Y, Zhu Y, Hu S, Xu Y, Stroup D, Pan X, et al. Hepatocyte nuclear factor 4alpha prevents the steatosis-to-NASH progression by regulating p53 and bile acid signaling (in mice). Hepatology. 2021;73:2251–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ohoka N, Okuhira K, Cui H, Wu W, Sato R, Naito M, et al. HNF4alpha increases liver-specific human ATP-binding cassette transporter A1 expression and cholesterol efflux to apolipoprotein A-I in response to cholesterol depletion. Arterioscler Thromb Vasc Biol. 2012;32:1005–14. [DOI] [PubMed] [Google Scholar]
- 100. Cai SY, He H, Nguyen T, Mennone A, Boyer JL. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res. 2010;51:2265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Marrapodi M, Chiang JYL. Peroxisome proliferator-activated receptor alpha (PPARalpha) and agonist inhibit cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Lipid Res. 2000;41:514–20. [PubMed] [Google Scholar]
- 102. He Y, Gong L, Fang Y, Zhan Q, Liu HX, Lu Y, et al. The role of retinoic acid in hepatic lipid homeostasis defined by genomic binding and transcriptome profiling. BMC Genomics. 2013;14:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yoshinari K, Ohno H, Benoki S, Yamazoe Y. Constitutive androstane receptor transactivates the hepatic expression of mouse Dhcr24 and human DHCR24 encoding a cholesterogenic enzyme 24-dehydrocholesterol reductase. Toxicol Lett. 2012;208:185–91. [DOI] [PubMed] [Google Scholar]
- 104. Luo Y, Tall AR. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest. 2000;105:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kang HS, Okamoto K, Kim YS, Takeda Y, Bortner CD, Dang H, et al. Nuclear orphan receptor TAK1/TR4-deficient mice are protected against obesity-linked inflammation, hepatic steatosis, and insulin resistance. Diabetes. 2011;60:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kim E, Xie S, Yeh SD, Lee YF, Collins LL, Hu YC, et al. Disruption of TR4 orphan nuclear receptor reduces the expression of liver apolipoprotein E/C-I/C-II gene cluster. J Biol Chem. 2003;278:46919–26. [DOI] [PubMed] [Google Scholar]
- 107. Xiong Q, Wu Y, Yang M, Wu G, Wang Y, Wang H, et al. Nr2e1 ablation impairs liver glucolipid metabolism and induces inflammation, high-fat diets amplify the damage. Biomed Pharmacother. 2019;120:109503. [DOI] [PubMed] [Google Scholar]
- 108. Mietus-Snyder M, Sladek F, Ginsburg GS, Frank Kuo C, Ladias JAA, Darnell JE, et al. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992;12:1708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stroup D, Crestani M, Chiang JYL. Orphan receptors chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and retinoid X receptor (RXR) activate and bind the rat cholesterol 7alpha-hydroxylase gene (CYP7A). J Biol Chem. 1997;272:9833–99. [DOI] [PubMed] [Google Scholar]
- 110. Stroup D, Chiang JYL. HNF4 and COUP-TFII interact to modulate transcription of the cholesterol 7alpha-hydroxylase gene (CYP7A1). J Lipid Res. 2000;41:1–11. [PubMed] [Google Scholar]
- 111. Ladias JA, Hadzopoulou-Cladaras M, Kardassis D, Cardot P, Cheng J, Zannis V, et al. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J Biol Chem. 1992;267:15849–60. [PubMed] [Google Scholar]
- 112. Kondo K, Harada N, Masuda H, Sugo N, Terazono S, Okonogi S, et al. A neurosurgical simulation of skull base tumors using a 3D printed rapid prototyping model containing mesh structures. Acta Neurochir (Wien). 2016;158:1213–9. [DOI] [PubMed] [Google Scholar]
- 113. Duan LP, Wang HH, Ohashi A, Wang DQH. Role of intestinal sterol transporters Abcg5, Abcg8, and Npc1l1 in cholesterol absorption in mice: gender and age effects. Am J Physiol Gastrointest Liver Physiol. 2006;290:G269–76. [DOI] [PubMed] [Google Scholar]
- 114. Milona A, Owen BM, Cobbold JFL, Willemsen ECL, Cox IJ, Boudjelal M, et al. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology. 2010;52:1341–9. [DOI] [PubMed] [Google Scholar]
- 115. Xu C, Jiang ZY, Liu Q, Liu H, Gu A. Estrogen receptor beta mediates hepatotoxicity induced by perfluorooctane sulfonate in mouse. Environ Sci Pollut Res Int. 2017;24:13414–23. [DOI] [PubMed] [Google Scholar]
- 116. Yang M, Liu Q, Huang T, Tan W, Qu L, Chen T, et al. Dysfunction of estrogen-related receptor alpha-dependent hepatic VLDL secretion contributes to sex disparity in NAFLD/NASH development. Theranostics. 2020;10:10874–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhang Y, Kim DK, Lee JM, Park SB, Jeong WI, Kim SH, et al. Orphan nuclear receptor oestrogen-related receptor gamma (ERRgamma) plays a key role in hepatic cannabinoid receptor type 1-mediated induction of CYP7A1 gene expression. Biochem J. 2015;470:181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Koorneef LL, van den Heuvel JK, Kroon J, Boon MR, 't Hoen P, Hettne KM, et al. Selective glucocorticoid receptor modulation prevents and reverses nonalcoholic fatty liver disease in male mice. Endocrinology. 2018;159:3925–36. [DOI] [PubMed] [Google Scholar]
- 119. Lu Y, Zhang Z, Xiong X, Wang X, Li J, Shi G, et al. Glucocorticoids promote hepatic cholestasis in mice by inhibiting the transcriptional activity of the farnesoid X receptor. Gastroenterology. 2012;143:1630–40. e8. [DOI] [PubMed] [Google Scholar]
- 120. Pizarro M, Solís N, Quintero P, Barrera F, Cabrera D, Rojas‐de Santiago P, et al. Beneficial effects of mineralocorticoid receptor blockade in experimental non-alcoholic steatohepatitis. Liver Int. 2015;35:2129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res. 2013;54:345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Li TP, Sun SW, Xiong GZ, Qiu F, Yang DM, Sun SY, et al. Direct interaction of Daxx and androgen receptor is required for their regulatory activity in cholesterol biosynthesis. Pharmacology. 2021;106:29–36. [DOI] [PubMed] [Google Scholar]
- 123. Pols TWH, Ottenhoff R, Vos M, Levels JHM, Quax PHA, Meijers JCM, et al. Nur77 modulates hepatic lipid metabolism through suppression of SREBP1c activity. Biochem Biophys Res Commun. 2008;366:910–6. [DOI] [PubMed] [Google Scholar]
- 124. Zhang P, Hu Y, Yang J, Zheng L, Wang Q. The orphan nuclear receptor Nur77 regulates hepatic cholesterol metabolism through the suppression of LDLR and HMGCR expression. Mol Med Rep. 2012;5:1541–7. [DOI] [PubMed] [Google Scholar]
- 125. Sun Y, Demagny H, Schoonjans K. Emerging functions of the nuclear receptor LRH-1 in liver physiology and pathology. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166145. [DOI] [PubMed] [Google Scholar]
- 126. Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40. [DOI] [PubMed] [Google Scholar]
- 128. Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JMA, Hammer RE, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. [DOI] [PubMed] [Google Scholar]
- 129. Altmann SW, Davis HR, Zhu L, Yao X, Hoos LM, Tetzloff G, et al. Niemann-pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–4. [DOI] [PubMed] [Google Scholar]
- 130. Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, et al. Niemann-pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun. 2006;340:1259–63. [DOI] [PubMed] [Google Scholar]
- 131. Bindesbøll C, Aas A, Ogmundsdottir MH, Pankiv S, Reine T, Zoncu R, et al. NBEAL1 controls SREBP2 processing and cholesterol metabolism and is a susceptibility locus for coronary artery disease. Sci Rep. 2020;10:4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chiang JYL, Kimmel R, Stroup D. Regulation of cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription by the liver orphan receptor (LXRalpha). Gene. 2001;262:257–65. [DOI] [PubMed] [Google Scholar]
- 133. Agellon LB, Drover VAB, Cheema SK, Gbaguidi GF, Walsh A. Dietary cholesterol fails to stimulate the human cholesterol 7alpha-hydroxylase gene (CYP7A1) in transgenic mice. J Biol Chem. 2002;277:20131–4. [DOI] [PubMed] [Google Scholar]
- 134. Chen W, Li L, Wang J, Zhang R, Zhang T, Wu Y, et al. The ABCA1-efferocytosis axis: a new strategy to protect against atherosclerosis. Clin Chim Acta. 2021;518:1–8. [DOI] [PubMed] [Google Scholar]
- 135. Costet P, Luo Y, Wang N, Tall AR. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem. 2000;275:28240–5. [DOI] [PubMed] [Google Scholar]
- 136. Kemmerer M, Wittig I, Richter F, Brüne B, Namgaladze D. AMPK activates LXRalpha and ABCA1 expression in human macrophages. Int J Biochem Cell Biol. 2016;78:1–9. [DOI] [PubMed] [Google Scholar]
- 137. Kennedy MA, Barrera GC, Nakamura K, Baldán Á, Tarr P, Fishbein MC, et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–31. [DOI] [PubMed] [Google Scholar]
- 138. Kennedy MA, Venkateswaran A, Tarr PT, Xenarios I, Kudoh J, Shimizu N, et al. Characterization of the human ABCG1 gene: Liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J Biol Chem. 2001;276:39438–47. [DOI] [PubMed] [Google Scholar]
- 139. Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, et al. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–82. [DOI] [PubMed] [Google Scholar]
- 140. Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–800. [DOI] [PubMed] [Google Scholar]
- 141. Errico TL, Méndez-Lara KA, Santos D, Cabrerizo N, Baila-Rueda L, Metso J, et al. LXR-dependent regulation of macrophage-specific reverse cholesterol transport is impaired in a model of genetic diabesity. Transl Res. 2017;186:19–35. e5. [DOI] [PubMed] [Google Scholar]
- 142. Ni M, Zhang B, Zhao J, Feng Q, Peng J, Hu Y, et al. Biological mechanisms and related natural modulators of liver X receptor in nonalcoholic fatty liver disease. Biomed Pharmacother. 2019;113:108778. [DOI] [PubMed] [Google Scholar]
- 143. Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ahn SB, Jang K, Jun DW, Lee BH, Shin KJ. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2014;59:2975–82. [DOI] [PubMed] [Google Scholar]
- 145. Endo-Umeda K, Nakashima H, Umeda N, Seki S, Makishima M. Dysregulation of Kupffer cells/macrophages and natural killer T cells in steatohepatitis in LXRalpha knockout male mice. Endocrinology. 2018;159:1419–32. [DOI] [PubMed] [Google Scholar]
- 146. Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–44. [DOI] [PubMed] [Google Scholar]
- 147. Muse ED, Yu S, Edillor CR, Tao J, Spann NJ, Troutman TD, et al. Cell-specific discrimination of desmosterol and desmosterol mimetics confers selective regulation of LXR and SREBP in macrophages. Proc Natl Acad Sci U S A. 2018;115:E4680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lima-Cabello E, García-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillón J, Lozano-Rodríguez T, Fernández-Bermejo M, et al. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond). 2011;120:239–50. [DOI] [PubMed] [Google Scholar]
- 149. Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–9. [DOI] [PubMed] [Google Scholar]
- 150. Ihara A, Yamagata K, Nammo T, Miura A, Yuan M, Tanaka T, et al. Functional characterization of the HNF4alpha isoform (HNF4alpha8) expressed in pancreatic beta-cells. Biochem Biophys Res Commun. 2005;329:984–90. [DOI] [PubMed] [Google Scholar]
- 151. Huang KW, Reebye V, Czysz K, Ciriello S, Dorman S, Reccia I, et al. Liver activation of hepatocellular nuclear factor-4alpha by small activating RNA rescues dyslipidemia and improves metabolic profile. Mol Ther Nucleic Acids. 2020;19:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Broome DT, Pantalone KM, Kashyap SR, Philipson LH. Approach to the patient with MODY-monogenic diabetes. J Clin Endocrinol Metab. 2021;106:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Barth R, Ruoso C, Ferreira SM, de Ramos FC, Lima FB, Boschero AC, et al. Hepatocyte Nuclear Factor 4-alpha (HNF4alpha) controls the insulin resistance-induced pancreatic beta-cell mass expansion. Life Sci. 2022;289:120213. [DOI] [PubMed] [Google Scholar]
- 154. Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HAJ, Lumb PJ, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48:878–85. [DOI] [PubMed] [Google Scholar]
- 155. Zhu Q, Yamagata K, Miura A, Shihara N, Horikawa Y, Takeda J, et al. T130I mutation in HNF-4alpha gene is a loss-of-function mutation in hepatocytes and is associated with late-onset type 2 diabetes mellitus in Japanese subjects. Diabetologia. 2003;46:567–73. [DOI] [PubMed] [Google Scholar]
- 156. Weissglas-Volkov D, Huertas-Vazquez A, Suviolahti E, Lee J, Plaisier C, Canizales-Quinteros S, et al. Common hepatic nuclear factor-4alpha variants are associated with high serum lipid levels and the metabolic syndrome. Diabetes. 2006;55:1970–7. [DOI] [PubMed] [Google Scholar]
- 157. Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Malerod L, Sporstøl M, Juvet LK, Mousavi A, Gjøen T, Berg T, et al. Hepatic scavenger receptor class B, type I is stimulated by peroxisome proliferator-activated receptor gamma and hepatocyte nuclear factor 4alpha. Biochem Biophys Res Commun. 2003;305:557–65. [DOI] [PubMed] [Google Scholar]
- 159. Thang SK, Chen PY, Gao WY, Wu MJ, Pan MH, Yen JH. Xanthohumol suppresses NPC1L1 gene expression through downregulation of HNF-4alpha and inhibits cholesterol uptake in Caco-2 cells. J Agric Food Chem. 2019;67:11119–28. [DOI] [PubMed] [Google Scholar]
- 160. Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4:193–205. [DOI] [PubMed] [Google Scholar]
- 161. Rosenson RS, Brewer HB, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2016;13:48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Mogilenko DA, Dizhe EB, Shavva VS, Lapikov IA, Orlov SV, Perevozchikov AP. Role of the nuclear receptors HNF4 alpha, PPAR alpha, and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry. 2009;48:11950–60. [DOI] [PubMed] [Google Scholar]
- 163. De Fabiani E, Mitro N, Anzulovich AC, Pinelli A, Galli G, Crestani M. The negative effects of bile acids and tumor necrosis factor-alpha on the transcription of cholesterol 7alpha-hydroxylase gene (CYP7A1) converge to hepatic nuclear factor-4: A novel mechanism of feedback regulation of bile acid synthesis mediated by nuclear receptors. J Biol Chem. 2001;276:30708–16. [DOI] [PubMed] [Google Scholar]
- 164. De Fabiani E, Mitro N, Gilardi F, Caruso D, Galli G, Crestani M. Coordinated control of cholesterol catabolism to bile acids and of gluconeogenesis via a novel mechanism of transcription regulation linked to the fasted-to-fed cycle. J Biol Chem. 2003;278:39124–32. [DOI] [PubMed] [Google Scholar]
- 165. McCullough A, Previs SF, Dasarathy J, Lee K, Osme A, Kim C, et al. HDL flux is higher in patients with nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2019;317:E852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Thymiakou E, Othman A, Hornemann T, Kardassis D. Defects in high density lipoprotein metabolism and hepatic steatosis in mice with liver-specific ablation of hepatocyte nuclear factor 4A. Metabolism. 2020;110:154307. [DOI] [PubMed] [Google Scholar]
- 167. Xu Y, Hu S, Jadhav K, Zhu Y, Pan X, Bawa FC, et al. Hepatocytic activating transcription factor 3 protects against steatohepatitis via hepatocyte nuclear factor 4alpha. Diabetes. 2021;70:2506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Yang T, Poenisch M, Khanal R, Hu Q, Dai Z, Li R, et al. Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J Hepatol. 2021;75:1420–33. [DOI] [PubMed] [Google Scholar]
- 169. Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications.. Eur J Med Chem. 2019;166:502–13. [DOI] [PubMed] [Google Scholar]
- 170. Gu Y, Duan S, Ding M, Zheng Q, Fan G, Li X, et al. Saikosaponin D attenuates metabolic associated fatty liver disease by coordinately tuning PPARalpha and INSIG/SREBP1c pathway. Phytomedicine. 2022;103:154219. [DOI] [PubMed] [Google Scholar]
- 171. Knebel B, Göddeke S, Hartwig S, Hörbelt T, Fahlbusch P, Al‐Hasani H, et al. Alteration of liver peroxisomal and mitochondrial functionality in the NZO mouse model of metabolic syndrome. Proteomics Clin Appl. 2018;12. doi: 10.1002/prca.201700028 [DOI] [PubMed] [Google Scholar]
- 172. Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1:309–22. [DOI] [PubMed] [Google Scholar]
- 173. Hunt MC, Yang YZ, Eggertsen G, Carneheim CM, Gåfvels M, Einarsson C, et al. The peroxisome proliferator-activated receptor alpha (PPARalpha) regulates bile acid biosynthesis. J Biol Chem. 2000;275:28947–53. [DOI] [PubMed] [Google Scholar]
- 174. Sonmez A, Nikolic D, Dogru T, Ercin CN, Genc H, Cesur M, et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol. 2015;9:576–82. [DOI] [PubMed] [Google Scholar]
- 175. Sugino I, Kuboki K, Matsumoto T, Murakami E, Nishimura C, Yoshino G. Influence of fatty liver on plasma small, dense LDL-cholesterol in subjects with and without metabolic syndrome. J Atheroscler Thromb. 2011;18:1–7. [DOI] [PubMed] [Google Scholar]
- 176. Katsiki N, Nikolic D, Montalto G, Banach M, Mikhailidis DP, Rizzo M. The role of fibrate treatment in dyslipidemia: An overview. Curr Pharm Des. 2013;19:3124–3131. [DOI] [PubMed] [Google Scholar]
- 177. Srivastava RAK, Cefalu AB, Srivastava NS, Averna M. NPC1L1 and ABCG5/8 induction explain synergistic fecal cholesterol excretion in ob/ob mice co-treated with PPAR-alpha and LXR agonists. Mol Cell Biochem. 2020;473:247–62. [DOI] [PubMed] [Google Scholar]
- 178. Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA, et al. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J Clin Lipidol. 2018;12:1390–403. e4. [DOI] [PubMed] [Google Scholar]
- 179. Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–300. [DOI] [PubMed] [Google Scholar]
- 180. Wansa KDSA, Harris JM, Muscat GEO. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277:33001–11. [DOI] [PubMed] [Google Scholar]
- 181. Wansa KDSA, Harris JM, Yan G, Ordentlich P, Muscat GEO. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278:24776–90. [DOI] [PubMed] [Google Scholar]
- 182. Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–60. [DOI] [PubMed] [Google Scholar]
- 183. Flaig R, Greschik H, Peluso-Iltis C, Moras D. Structural basis for the cell-specific activities of the NGFI-B and the Nurr1 ligand-binding domain. J Biol Chem. 2005;280:19250–8. [DOI] [PubMed] [Google Scholar]
- 184. Wilson TE, Fahrner TJ, Milbrandt J, Wilson TE, Fahrner TJ, Milbrandt J. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol. 1993;13:5794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, et al. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19:7549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–82. [DOI] [PubMed] [Google Scholar]
- 188. Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T, et al. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10:1656–66. [DOI] [PubMed] [Google Scholar]
- 189. Chao LC, Wroblewski K, Zhang Z, Pei L, Vergnes L, Ilkayeva OR, et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58:2788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Fu Y, Luo L, Luo N, Zhu X, Garvey WT. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: Potential role in insulin resistance. J Biol Chem. 2007;282:31525–33. [DOI] [PubMed] [Google Scholar]
- 191. Weyrich P, Staiger H, Stančáková A, Schäfer SA, Kirchhoff K, Ullrich S, et al. Common polymorphisms within the NR4A3 locus, encoding the orphan nuclear receptor Nor-1, are associated with enhanced beta-cell function in non-diabetic subjects. BMC Med Genet. 2009;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Hu YW, Zhang P, Yang JY, Huang JL, Ma X, Li SF, et al. Nur77 decreases atherosclerosis progression in apoE(-/-) mice fed a high-fat/high-cholesterol diet. PLoS One. 2014;9:e87313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Zeng Y, Ye X, Liao D, Huang S, Mao H, Zhao D, et al. Expressions of orphan nuclear receptor TR3/Nur77 in chronic hepatopathy and its clinical significance. J Clin Exp Oncol. 2017;6:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572–80. [DOI] [PubMed] [Google Scholar]
- 195. Kemper JK. Regulation of FXR transcriptional activity in health and disease: Emerging roles of FXR cofactors and post-translational modifications. Biochim Biophys Acta. 2011;1812:842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Fu T, Coulter S, Yoshihara E, Oh TG, Fang S, Cayabyab F, et al. FXR regulates intestinal cancer stem cell proliferation. Cell. 2019;176:1098–112. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chiang JYL, Kimmel R, Weinberger C, Stroup D. Farnesoid X receptor responds to bile acids and represses cholesterol 7alpha-hydroxylase gene (CYP7A1) transcription. J Biol Chem. 2000;275:10918–24. [DOI] [PubMed] [Google Scholar]
- 198. Al-Aqil FA, Monte MJ, Peleteiro-Vigil A, Briz O, Rosales R, González R, et al. Interaction of glucocorticoids with FXR/FGF19/FGF21-mediated ileum-liver crosstalk. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):2927–37. [DOI] [PubMed] [Google Scholar]
- 199. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25. [DOI] [PubMed] [Google Scholar]
- 200. Shapiro H, Kolodziejczyk AA, Halstuch D, Elinav E. Bile acids in glucose metabolism in health and disease. J Exp Med. 2018;215:383–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Hoeke MO, Plass JRM, Heegsma J, Geuken M, van Rijsbergen D, Baller JFW, et al. Low retinol levels differentially modulate bile salt-induced expression of human and mouse hepatic bile salt transporters. Hepatology. 2009;49:151–9. [DOI] [PubMed] [Google Scholar]
- 202. Ijssennagger N, Janssen AWF, Milona A, Ramos Pittol JM, Hollman DAA, Mokry M, et al. Gene expression profiling in human precision cut liver slices in response to the FXR agonist obeticholic acid. J Hepatol. 2016;64:1158–66. [DOI] [PubMed] [Google Scholar]
- 203. Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276:28857–65. [DOI] [PubMed] [Google Scholar]
- 204. Dash A, Figler RA, Blackman BR, Marukian S, Collado MS, Lawson MJ, et al. Pharmacotoxicology of clinically-relevant concentrations of obeticholic acid in an organotypic human hepatocyte system. Toxicol In Vitro. 2017;39:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ovadia C, Perdones‐Montero A, Spagou K, Smith A, Sarafian MH, Gomez‐Romero M, et al. Enhanced microbial bile acid deconjugation and impaired ileal uptake in pregnancy repress intestinal regulation of bile acid synthesis. Hepatology. 2019;70:276–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Cao Y, Xiao Y, Zhou K, Yan J, Wang P, Yan W, et al. FXR agonist GW4064 improves liver and intestinal pathology and alters bile acid metabolism in rats undergoing small intestinal resection. Am J Physiol Gastrointest Liver Physiol. 2019;317:G108–15. [DOI] [PubMed] [Google Scholar]
- 207. Chen HL, Wu SH, Hsu SH, Liou BY, Chen HL, Chang MH. Jaundice revisited: recent advances in the diagnosis and treatment of inherited cholestatic liver diseases. J Biomed Sci. 2018;25:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Cui G, Martin RC, Jin H, Liu X, Pandit H, Zhao H, et al. Up-regulation of FGF15/19 signaling promotes hepatocellular carcinoma in the background of fatty liver. J Exp Clin Cancer Res. 2018;37:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Li S, Xu Z, Guo J, Zheng J, Sun X, Yu J. Farnesoid X receptor activation induces antitumour activity in colorectal cancer by suppressing JAK2/STAT3 signalling via transactivation of SOCS3 gene. J Cell Mol Med. 2020;24:14549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Attia YM, Tawfiq RA, Ali AA, Elmazar MM. The FXR agonist, obeticholic acid, suppresses HCC proliferation & metastasis: Role of IL-6/STAT3 signalling pathway. Sci Rep. 2017;7:12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Erice O, Labiano I, Arbelaiz A, Santos-Laso A, Munoz-Garrido P, Jimenez-Agüero R, et al. Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1335–44. [DOI] [PubMed] [Google Scholar]
- 212. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Evans MJ, Mahaney PE, Borges-Marcucci L, Lai K, Wang S, Krueger JA, et al. A synthetic farnesoid X receptor (FXR) agonist promotes cholesterol lowering in models of dyslipidemia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G543–52. [DOI] [PubMed] [Google Scholar]
- 214. Claudel T, Sturm E, Duez H, Torra IP, Sirvent A, Kosykh V, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via a negative FXR response element. J Clin Invest. 2002;109:961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–6. [DOI] [PubMed] [Google Scholar]
- 216. Huang H, Yu Y, Gao Z, Zhang Y, Li C, Xu X, et al. Discovery and optimization of 1,3,4-trisubstituted-pyrazolone derivatives as novel, potent, and nonsteroidal farnesoid X receptor (FXR) selective antagonists. J Med Chem. 2012;55:7037–53. [DOI] [PubMed] [Google Scholar]
- 217. Xu X, Xu X, Liu P, Zhu Z, Chen J, Fu H, et al. Structural basis for small molecule NDB (N-Benzyl-N-(3-(tert-butyl)-4-hydroxyphenyl)-2,6-dichloro-4-(dimethylamino) benzamide) as a selective antagonist of farnesoid X receptor alpha (FXRalpha) in stabilizing the homodimerization of the receptor. J Biol Chem. 2015;290:19888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Heyman RA, Mangelsdorf DJ, Dyck JA, Stein RB, Eichele G, Evans RM, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. [DOI] [PubMed] [Google Scholar]
- 219. Hiebl V, Ladurner A, Latkolik S, Dirsch VM. Natural products as modulators of the nuclear receptors and metabolic sensors LXR, FXR and RXR. Biotechnol Adv. 2018;36:1657–98. [DOI] [PubMed] [Google Scholar]
- 220. Li B, Cai SY, Boyer JL. The role of the retinoid receptor, RAR/RXR heterodimer, in liver physiology. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Li X, Bai R, Bai Y, Shi X, Yang Y, Xu S. ROS-mediated PPAR/RXR inhibition contributes to acetochlor-induced apoptosis and autophagy in Ctenopharyngodon idella hepatic cells. Fish Shellfish Immunol. 2022;128:684–694. [DOI] [PubMed] [Google Scholar]
- 222. Zhang X, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, et al. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992;358:587–91. [DOI] [PubMed] [Google Scholar]
- 223. Glass CK. Differential recognition of target genes by nuclear receptor monomers, dimers, and heterodimers. Endocr Rev. 1994;15:391–407. [DOI] [PubMed] [Google Scholar]
- 224. Bershad S, Rubinstein A, Paterniti JR, Le NA, Poliak SC, Heller B, et al. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313:981–5. [DOI] [PubMed] [Google Scholar]
- 225. Sabol SL, Brewer HB, Jr, Santamarina-Fojo S. The human ABCG1 gene: Identification of LXR response elements that modulate expression in macrophages and liver. J Lipid Res. 2005;46:2151–67. [DOI] [PubMed] [Google Scholar]
- 226. Rottman JN, Widom RL, Nadal-Ginard B, Mahdavi V, Karathanasis SK, Rottman JN, et al. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991;11:3814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Mukherjee R, Strasser J, Jow L, Hoener P, Paterniti JR, Heyman RA. RXR agonists activate PPARalpha-inducible genes, lower triglycerides, and raise HDL levels in vivo. Arterioscler Thromb Vasc Biol. 1998;18:272–6. [DOI] [PubMed] [Google Scholar]
- 228. Norlin M, Andersson U, Björkhem I, Wikvall K. Oxysterol 7 alpha-hydroxylase activity by cholesterol 7 alpha-hydroxylase (CYP7A). J Biol Chem. 2000;275:34046–53. [DOI] [PubMed] [Google Scholar]
- 229. Watanabe K, Sakurai K, Tsuchiya Y, Yamazoe Y, Yoshinari K. Dual roles of nuclear receptor liver X receptor alpha (LXRalpha) in the CYP3A4 expression in human hepatocytes as a positive and negative regulator. Biochem Pharmacol. 2013;86:428–36. [DOI] [PubMed] [Google Scholar]
- 230. Saeed A, Hoekstra M, Hoeke MO, Heegsma J, Faber KN. The interrelationship between bile acid and vitamin A homeostasis. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:496–512. [DOI] [PubMed] [Google Scholar]
- 231. Schmidt DR, Holmstrom SR, Fon Tacer K, Bookout AL, Kliewer SA, Mangelsdorf DJ. Regulation of bile acid synthesis by fat-soluble vitamins A and D. J Biol Chem. 2010;285:14486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hoeke MO, Heegsma J, Hoekstra M, Moshage H, Faber KN. Human FXR regulates SHP expression through direct binding to an LRH-1 binding site, independent of an IR-1 and LRH-1. PLoS One. 2014;9:e88011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Duell PB, Welty FK, Miller M, Chait A, Hammond G, Ahmad Z, et al. Nonalcoholic fatty liver disease and cardiovascular risk: A scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2022;42:e168–85. [DOI] [PubMed] [Google Scholar]
- 234. Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–40. [DOI] [PubMed] [Google Scholar]
- 235. Oppi S, Nusser-Stein S, Blyszczuk P, Wang X, Jomard A, Marzolla V, et al. Macrophage NCOR1 protects from atherosclerosis by repressing a pro-atherogenic PPARgamma signature. Eur Heart J. 2020;41:995–1005. [DOI] [PubMed] [Google Scholar]
- 236. Yu M, Jiang M, Chen Y, Zhang S, Zhang W, Yang X, et al. Inhibition of macrophage CD36 expression and cellular oxidized low density lipoprotein (oxLDL) accumulation by tamoxifen: A peroxisome proliferator-activated receptor (PPAR)gamma-dependent mechanism. J Biol Chem. 2016;291:16977–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bonta PI, van Tiel CM, Vos M, Pols TWH, van Thienen JV, Ferreira V, et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–94. [DOI] [PubMed] [Google Scholar]
- 238. Chinetti G, Lestavel S, Fruchart JC, Clavey V, Staels B. Peroxisome proliferator-activated receptor alpha reduces cholesterol esterification in macrophages. Circ Res. 2003;92:212–7. [DOI] [PubMed] [Google Scholar]
- 239. Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–71. [DOI] [PubMed] [Google Scholar]
- 241. Liang L, Xie Q, Sun C, Wu Y, Zhang W, Li W. Phospholipase A2 group IIA correlates with circulating high-density lipoprotein cholesterol and modulates cholesterol efflux possibly through regulation of PPAR-gamma/LXR-alpha/ABCA1 in macrophages. J Transl Med. 2021;19:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Simonen P, Kotronen A, Hallikainen M, Sevastianova K, Makkonen J, Hakkarainen A, et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J Hepatol. 2011;54:153–9. [DOI] [PubMed] [Google Scholar]
- 243. Enjoji M, Yasutake K, Kohjima M, Nakamuta M. Nutrition and nonalcoholic Fatty liver disease: The significance of cholesterol. Int J Hepatol. 2012;2012:925807–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ekstedt M, Franzén LE, Mathiesen UL, Holmqvist M, Bodemar G, Kechagias S. Statins in non-alcoholic fatty liver disease and chronically elevated liver enzymes: A histopathological follow-up study. J Hepatol. 2007;47:135–41. [DOI] [PubMed] [Google Scholar]
- 245. Pignatelli P, Carnevale R, Pastori D, Cangemi R, Napoleone L, Bartimoccia S, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation. 2012;126:92–103. [DOI] [PubMed] [Google Scholar]
- 246. Marchiano S, Biagioli M, Roselli R, Zampella A, Di Giorgio C, Bordoni M, et al. Atorvastatin protects against liver and vascular damage in a model of diet induced steatohepatitis by resetting FXR and GPBAR1 signaling. FASEB J. 2022;36:e22060. [DOI] [PubMed] [Google Scholar]
- 247. Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–52. [DOI] [PubMed] [Google Scholar]
- 248. Cariou B, Hanf R, Lambert-Porcheron S, Zaïr Y, Sauvinet V, Noël B, et al. Dual peroxisome proliferator-activated receptor alpha/delta agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Bruinstroop E, Dalan R, Cao Y, Bee YM, Chandran K, Cho LW, et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J Clin Endocrinol Metab. 2018;103:2698–706. [DOI] [PubMed] [Google Scholar]
- 250. Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–24. [DOI] [PubMed] [Google Scholar]
- 251. Griffett K, Solt LA, El-Gendy BEDM, Kamenecka TM, Burris TP. A liver-selective LXR inverse agonist that suppresses hepatic steatosis. ACS Chem Biol. 2013;8:559–67. [DOI] [PubMed] [Google Scholar]
- 252. Griffett K, Welch RD, Flaveny CA, Kolar GR, Neuschwander-Tetri BA, Burris TP. The LXR inverse agonist SR9238 suppresses fibrosis in a model of non-alcoholic steatohepatitis. Mol Metab. 2015;4:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82. e1. [DOI] [PubMed] [Google Scholar]
- 254. Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Li C, Gong D, Chen L, Zhang M, Xia X, Cheng H, et al. Puerarin promotes ABCA1-mediated cholesterol efflux and decreases cellular lipid accumulation in THP-1 macrophages. Eur J Pharmacol. 2017;811:74–86. [DOI] [PubMed] [Google Scholar]
- 256. Zheng S, Huang H, Li Y, Wang Y, Zheng Y, Liang J, et al. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARgamma-LXRalpha-ABCA1/ABCG1 pathway. Pharmacol Res. 2021;169:105639. [DOI] [PubMed] [Google Scholar]