Abstract
Alzheimer’s disease (AD) is a disabling neurodegenerative disease. The prognosis is poor, and currently there are no proven effective therapies. Most likely, the etiology is related to cerebral inflammatory processes that cause neuronal damage, resulting in dysfunction and apoptosis of nerve cells. Pathogens that evoke a neuroinflammatory response, collectively activate astrocytes and microglia, which contributes to the secretion of pro-inflammatory cytokines. This leads to the deposit of clustered fragments of beta-amyloid and misfolded tau proteins which do not elicit an adequate immune reaction. Apart from the function of astrocytes and microglia, molecular entities such as TREM2, SYK, C22, and C33 play a role in the physiopathology of AD. Furthermore, bacteria and viruses may trigger an overactive inflammatory response in the brain. Pathogens like Helicobacter pylori, Chlamydia pneumonia, and Porphyromonas gingivalis (known for low-grade infection in the oral cavity) can release gingipains, which are enzymes that can damage and destroy neurons. Chronic infection with Borrelia burgdorferi (the causative agent of Lyme disease) can co-localize with tau tangles and amyloid deposits. As for viral infections, herpes simplex virus 1, cytomegalovirus, and Epstein-Barr virus can play a role in the pathogenesis of AD. Present investigations have resulted in the development of antibodies that can clear the brain of beta-amyloid plaques. Trials with humanized aducanumab, lecanemab, and donanemab revealed limited success in AD patients. However, AD should be considered as a continuum in which the initial preclinical phase may take 10 or even 20 years. It is generally thought that this phase offers a window for efficacious treatment. Therefore, research is also focused on the identification of biomarkers for early AD detection. In this respect, the plasma measurement of neurofilament light chain in patients treated with hydromethylthionine mesylate may well open a new way to prevent the formation of tau tangles and represents the first treatment for AD at its roots.
Keywords: Alzheimer’s disease, Neuroinflammation, Continuum, Therapy
Highlights of the Study
-
•
Pathogens that cause neuroinflammation may cause Alzheimer’s disease.
-
•
Alzheimer’s disease is presently considered a continuum.
-
•
Early, preclinical Alzheimer’s disease could be a window for Alzheimer’s disease treatment.
-
•
Biomarkers for early detection of Alzheimer’s disease need to be included in neurological diagnostic work-up.
Introduction
Alzheimer’s disease (AD) is the most common cause of senile dementia in developed countries. The UK-based National Health Service (NHS) estimates that the prevalence of AD will double every 20 years and will affect one in six individuals over the age of 80 (accessed on May 1, 2022, available online at www.nhs.uk/conditions/alzheimers-disease). This illustrates that aging within a population is a prominent risk factor and represents a major public burden. Currently, there is no cure for this disorder, which compromises the quality of life of patients, caregivers, and loved ones [1].
Neurodegeneration is one of the irreversible human pathologies that is only partly explained. Experimental and postmortem human studies have provided ample evidence that inflammation of the brain is the main feature of AD. Neurons contain large amounts of unsaturated fats, which makes them vulnerable to inflammatory processes like the ones invoked by reactive oxygen species (ROS) and reactive nitrogen species, collectively called oxidative stress [2]. This inflammatory stress invokes neuronal damage, which in turn gives rise to the release of inflammatory cytokines, resulting in neuronal dysfunction and apoptosis, thus creating a vicious circle. Such dysfunctional neurons and their fragments are largely found in the hippocampus, amygdala, and cortex, and explain the structural losses in the pathognomonic CT images of the patient’s brain atrophy. The associated clinical signs of AD are characterized by cognitive and behavioral dysfunction as well as speech difficulties, memory impairment, and motor impairment.
Research on the etiology of AD is largely related to the accumulation of beta-amyloid (βA) and neurofibrillary (aberrantly phosphorylated) tau tangles, usually called P-tau or p-tau, in the neuronal area leading to an inflammatory response by microglia and astrocytes to remove these corpora aliena. While neuroscience has revealed the basic characteristics of microglia and astrocytes, the fundamental biological mechanisms of AD are still poorly understood and remain to be elucidated [3, 4]. Nevertheless, the amyloid plaque and tau hypothesis is still the most accepted model of inherited AD. Plaques represent large clumps of interneuronal amyloid protein aggregates, while neurofibrillary tangles are intraneuronal aggregates of hyperphosphorylated tau protein. The molecule apolipoprotein e4 (APOEe4) secreted by astrocytes and microglia plays a considerable role as it is associated with an earlier age of AD onset and its presence in individuals doubles or triples the risk of AD [5]. Fresh experimental findings on the function of APOE and a developed mathematical model suggest that oxidative stress in the skin fibroblasts of AD patients favors the accumulation of ataxia telangiectasia-mutated (ATM)-pAPOE, a crown-like perinuclear complex. This prevents or delays the ATM shuttling from the cytoplasm to the nucleus and blocks the repair of damaged DNA. This knowledge shows, perhaps for the first time, that ATM-kinase is involved in the pathophysiology of AD and that the presence of this crown may support early diagnosis and treatment monitoring in AD [6].
At present, a plethora of scientists are investigating specific roles of microglia and astrocyte nerve cells in AD. Herein, we provide a narrative synopsis of recent knowledge on the role of these cells and the various biochemical pathways in the pathogenesis of AD that may open avenues to relevant therapeutic options.
Astrocytes and βA Formation
Astrocytes originate from radial glial cells. During astrogenesis, these cells turn into neural stem cells, which create astrocyte precursor cells migrating to a final destination in the CNS. There, they mature and acquire the typical complex stellate morphology. Through the input of nearby neurons, they obtain a characteristic spongiform shape with myriads of small protrusions. These mature astrocytes influence synapse function by clearing and recycling inactive derivatives of neurotransmitters back to the presynaptic terminal [7–9]. In addition, mature astrocytes control the homeostasis of the CNS and are involved in maintaining the blood-brain barrier (BBB). The BBB regulates local blood flow, the concentration of oxygen and water, and ion homeostasis according to physiological needs. It prevents the entrance of toxic molecules and blood cells into the brain [5, 10]. The integrity of the BBB can be disturbed by pathogens, eventually resulting in neuroinflammation induced by the cerebral production of ROS and oxidative stress. For instance, experiments with humanized APOEe4 have shown that this entity can initiate a leaky BBB and that removal of this molecule results in amelioration. This suggests that the detrimental effects of astrocyte production of APOEe4 are a key threat to the integrity of the BBB. Astrogliosis is a cerebral defense reaction in which astrocytes are transformed into reactive cells, featuring elongated nuclei and distinctive palisading (a process called “shapeshifting”) [11]. During this process, their number and size increases as a response to a stressful environment and disturbed homeostasis [12]. Reactive astrocytes contribute to upregulation of endothelial receptor advanced glycation end products, mediating secretion of pro-inflammatory cytokines and disruption of the BBB, allowing toxic elements to enter the brain [13]. This causes the increased accumulation of active astrocytes, collectively increasing a vicious circle of cerebral βA deposits.
βA originates from native amyloid precursor protein (APP), a 770 amino acid glycoprotein present in cell membranes [14]. APP is cleaved by β-secretase followed by gamma-secretase, resulting in the formation of βA40 and βA42. These cleavage fragments promote the molecular clustering of toxic ROS resulting in plaques of βA in the neuronal environment, disrupting their cellular function, a generally accepted cause of AD. Indeed, preclinical investigations have demonstrated that activated astrocytes can identify amyloid plaques [15, 16]. Moreover, postmortem studies have shown their presence in cerebral amyloid in AD patients [17]. On an interesting note, recent experimental and human studies have shown that medin, a most common human amyloid, interacts with vascular βA and promotes the aggregation of βA in AD, where it leads to vascular stiffening in the human brain arterioles and cognitive decline. It has been suggested that medin could be a therapeutic target to prevent its co-aggregation with vascular βA [18].
Astroglia and Scar Formation
After brain-related diseases (including injuries and neurodegeneration), the severed neurons organize a regenerative response. However, in contrast to injured Schwann cells in the peripheral nervous system, the CNS does not show tissue regeneration and typically depends on persisting scar-forming cells such as endothelial cells, inflammatory immune cells, stromal fibroblasts, and astrocytes to create fibrotic layers. This protective glial scar is impenetrable for upregulated pro-regenerative genes and transcriptional mechanisms, which results in further inflammation and degeneration. D’Ambrosi and Apolloni [19] have described the function of this glial scar in neurodegenerative disease as AD; the scar has apparently contrasting roles of promoting tissue protection and inhibiting repair. Indeed, scar-forming astrocytes are regarded as inhibitors of axon regeneration. As reactive astrocytes, they may act as pro-inflammatory entities turning the scar into a source of neuroinflammation and leading to a vicious circle in which degeneration continues. Conversely, astrocytes at the border of the scar can elongate their processes and form so-called glial bridges along which axons may regrow when appropriate growth factors are present through intrinsic neuronal pathways [20]. Nevertheless, Verkhratsky et al. [20] aver that “the role of astrocytes in the pathogenesis and progression of AD remains to be fully characterized, primarily because of the lack of longitudinal studies assessing the status of astroglia at different stages of the disease.”
p-TAU
Research on the role of tau in AD has stayed behind in favor of investigations on the role of βA, whereas its presence in the brain is considered an important hallmark of disease [21]. Indeed, hyperphosphorylated aggregates of the microtubule-associated tau protein belong to the main pathological components that are aberrantly accumulated in AD brains. The family of tau consists of six isoforms, whereas only 3-R and 4-R tau, occurring at an equal ratio are present in the human brain. The presence of AD may change this ratio in favor of the increased presence of 4-R. The AD brain tau is known to be three- to four-fold more hyperphosphorylated than in the normal human brain. This p-tau molecule can disrupt microtubules and in its oligomeric state it is self-assembled. This pathological tau is seen as intraneuronal neurofibrillary tangles. Aggregates of this malformed protein are present around the core of amyloid plaques and are considered a prerequisite for the clinical expression of AD [22].
Microglia
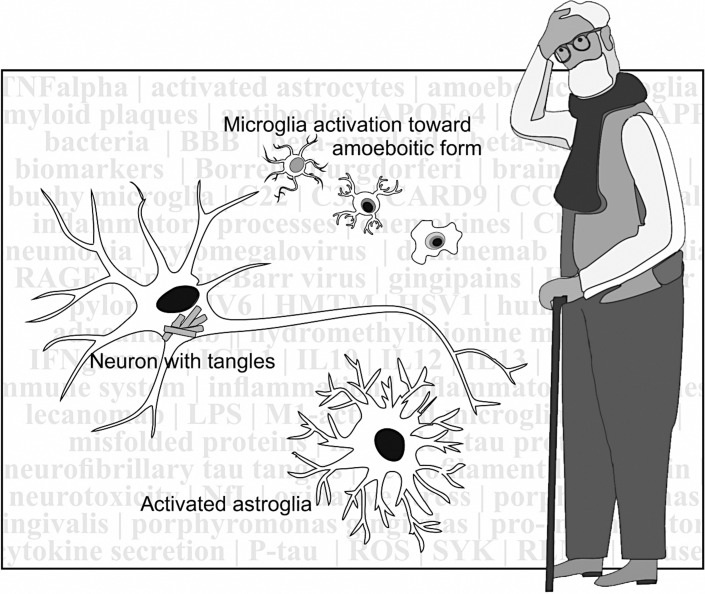
Microglia are part of the immune system and are present in the brain. Their main function is the surveillance of changes that could jeopardize cerebral homeostasis. As such, they have a ramified appearance with processes being expended or retracted. As a response to harmful events their processes can extend and become bushy, while finally the cells get their typical amoeboid phagocytic appearance (see Fig. 1), in which they can engulf apoptotic neurons [23]. In this context, experiments by Diaz-Aparicio et al. [24] suggest that microglia act as a sensor of local cell death and that the secretome of phagocytic microglia produces a homeostatic response that balances the proliferation and survival of neurons on one side and the cell death at the other side. This also prevents a deviant inflammatory reaction, which can occur when microglia enter an activated state following the detection of neural damage. They play a pivotal role in the pathogenesis of AD by changing their phenotypes, usually classified as M1 and M2, although a continuum exists of different phenotypes [25]. Activated M1 microglia are induced by interferon γ (IFNγ) and lipopolysaccharide, and produce inflammatory cytokines and chemokines such as tumor necrosis factor α (TNFα), interleukin (IL)-6, and IL-12. Activated M2 microglia are induced by anti-inflammatory cytokines such as IL-4 and IL-13. The M2 type produces the anti-inflammatory cytokines as IL-10, insulin-like growth factor-1 and promotes the phagocytosis of cell debris and misfolded proteins and supports neuronal survival through neurotrophic factors. Interesting reviews on this subject have been published by Li et al. [26], Colonna and Butovsky [27], and Guo et al. [25], from which the abovementioned data are taken. To summarize, M1 microglia induce inflammation and neurotoxicity, while M2 microglia induce anti-inflammatory and healing processes to prepare for the containment and removal of neurotoxic debris [27]. Further studies have substantiated that activated microglia might play a protective role in early AD, while in late chronic AD they may secrete several cytokines and ROS, causing excessive neural damage, which will accelerate the disease and overrule the healing effect of other (M2 type) microglia [26].
Fig. 1.
Activated astrocyte and amoeboid microglia ready to approach a damaged neuron.
Other Players in the Field
Triggering receptor expressed in myeloid cells 2 (TREM2) is a receptor that recognizes the accumulation of βA and its neuronal toxicity [28]. It is upregulated on microglia that surround amyloid plaques in AD. Its soluble ectodomain sTREM2 binds to βA and reverses oligomerization and fibrillation and is consequently an important opponent of AD. Inhibition of TREM2 and its ectodomain attenuates the microglia response to βA deposition, thus turning them into an activated toxic state, which makes it conceivable that TREM2 protects from AD [29]. La Rosa et al. [30] confirmed this hypothesis in 15 AD patients and 12 age-matched healthy controls; these researchers found that plasma levels of sTREM2 and TREM2 mRNA were significantly upregulated in the plasma of patients with AD and βA phagocytosis was diminished.
A recent publication by Ennerfelt et al. [31] elaborates on the innate immune signaling molecule caspase recruitment domain-containing protein 9 (CARD9). The authors demonstrated that deletion of this molecule in an experimental model worsens cognitive decline and aberrant microglial activation. Conversely, pharmacological activation of CARD9 increases the clearance of βA, suggesting that this signaling molecule mediates βA deposits and microglia responses. The same research group has elucidated the crucial role of spleen tyrosine kinase (SYK) in the coordination of microglial activation and phagocytosis. This intracellular regulator is deployed to limit βA pathology. Essentially, disruption of microglial SYK signaling in an experimental mouse AD model exacerbates disease [32]. Furthermore, further research has identified increased (lysophosphatidic acid) C22 expression on microglia, as a negative regulator of microglial phagocytosis in aged (20–24-month-old) mice. The long-term inhibition of C22 promotes clearance of βA in vivo and reprograms microglia toward the M2 state and improves the cognitive function of these mice [33]. Likewise, it has been shown that inhibition of cluster of differentiation 33 (CD33), an immunomodulating receptor expressed by microglia and genetically linked to AD susceptibility, attenuates βA pathology and improves cognitive capabilities in knockout mice. Interestingly, cancellation of TREM2 in these CD33 knockout mice exacerbated βA pathology [34]. While research on the abovementioned molecular entities clearly illustrates that they are important players at the roots of AD and are interesting targets for future treatment, it is clear that further studies need to clarify which signaling processes form the cornerstone of their working mechanisms before they can be tested in humans.
The Infection Hypothesis
Neuronal cell damage due to infection may induce an acute inflammatory response in various organs, including the brain. The infection hypothesis as a cause for AD starts from the assumption that microorganisms like bacteria and viruses may trigger an overactive inflammatory response; this may lead to chronic inflammation in a subset of patients.
Bacterial Infections
One of the first articles mentioning the presence of bacteria in the brain of AD patients was published by MacDonald and Miranda in 1987 [35]. In this context, Koehler et al. described the importance of the bidirectional gut-brain interaction. The enteric nervous system is often referred to as the “second brain,” as it produces neurotransmitters and signaling molecules. Alterations in the composition of the gut microbiome, e.g., through dietary changes or ingestion of pathogenic bacteria, may result in the breakdown of the gut barrier, resulting in the presence of bacteria and pro-inflammatory cytokines and other toxic molecules in the circulation. This, in turn, may lead to impairment of the aforementioned BBB and neuroinflammation and subsequent neuronal loss, which reveals itself in neurological disorders such as Parkinson’s disease and AD [36].
Various studies have demonstrated an association between AD and infectious pathogens like Helicobacter pylori [37], Chlamydia pneumonia [38], and Porphyromonas gingivalis [39]. Once in the brain, bacteria release gingipains, enzymes that can damage and destroy neurons. Senejani et al. [40] have reported that chronic infection by Borrelia burgdorferi, known for causing Lyme disease, creates biofilms in patients with autopsy-confirmed AD neuropathology. These authors showed that the B. burgdorferi biofilm co-localizes with tau protein and amyloid deposits which suggests a strong relationship with AD. Recently, these findings suggesting a strong causal relationship have been confirmed by Herrera et al. [41]. Indeed, the invasion of this bacterium stimulates the release of pro-inflammatory signaling molecules such as IL-6, IL-8, IL-10, IL-12, and TNFα as demonstrated in autopsy tissue and in vitro experiments [42–44]. Therefore, it is plausible that bacterial infection with B. burgdorferi triggers an inflammatory response involving astrocytes, which may lead to chronic inflammation and neuronal apoptosis as in AD.
Also on an interesting note, infection by the Porphyromonas gingivalis, can cause a low-grade infection in the oral cavity. Its prevalence is around 20–50% of the global population [45]. This bacterium is considered a key pathogen for neuropathogenesis; chronic infection may contribute to AD when systemic spread takes place and reaches the CNS. Recent studies on the link between periodontitis caused by Porphyromonas gingivalis and AD have revealed that toxic proteases from this bacterium are present in the brain of AD patients [46]. This toxic action requires survival in the periodontal pocket in order to overcome the antimicrobial environment. Ximinies et al. [47] have demonstrated that its gene 0686 and related proteins may offer defense against oxidative stress through a signaling system that can coordinate the necessary pathways for protection. As far as bacterial infection is considered, Siddiqui et al. [48] consider Porphyromonas gingivalis as a most important pathogen for periodontal disease and a significant cause for AD.
Viral Infections
Commonly, viruses invade the body before they arrive in the CNS. It is postulated that the virus particles are present in a latent manner in the ganglia of the trigeminal nerve. Presumably the virus is reactivated when aging has its weakening effect on the immune system that would otherwise keep the infection under control. Sait et al. [49] have provided evidence that the herpes simplex virus 1 (HSV1) is a key pathogen contributing to AD, although also other viruses including herpes virus type 6 (HVV6) [50], cytomegalovirus [51], and Epstein-Barr virus [52] may play a role. Further evidence for pathogenic HSV1, compiled in 2018 [53] and in 2021 [54] by Itzhaki’s group, has supported the compelling link between the role of the virus HSV1 in the pathogenesis of AD. HSV1 infection in a 3-dimensional bioengineered human brain model caused changes similar to those found in the brain of AD patients [55]. Laboratory-grown brain cells infected with varicella zoster virus were reported to upregulate pro-inflammatory cytokines which can reactivate latent HSV1 [56]. Induced human neural stem cell cultures, infected with HSV1 and/or varicella zoster virus, showed reactivation of HSV1 and consequent AD-like changes, including the accumulation of βA and P-tau.
Vaccine studies showed a reduced frequency of reactivation of HSV1 in the brain [57], while epidemiological data on the use of anti-HSV serum antibodies showed a link between the diminishing rate of cognitive decline in which HSV1 is implicated [58]. Zhao et al. [59] demonstrated an association of APOEe4 carriers with HSV1 infection in terms of cognitive capabilities [59]. This observation suggests that HSV1 infection plays a role in the development of AD among individuals with the APOEe4 allele as a genetic susceptibility factor [60]. On the other hand, in non-APOEe4 carriers, no association exists between HSV1 and AD [57].
Recent Insights into the Various Stages of AD
Late onset of AD or sporadic AD (i.e., with no genetic link) is the most common form, affecting over 90% of sufferers. Changes in mood, disturbed sleep patterns, and memory loss are among the earliest symptoms of AD. Although AD primarily affects the brain, during the course of the disease, extracranial body systems can be affected, including the respiratory system [61], digestive system [62], and urinary system [63] as well as changes in the epidermal layer of the skin [64]. Considering these and other organ-related symptoms, AD is increasingly being recognized as a multi-organ disorder [65].
At later stages, when features such as depression, anxiety, disorientation, agitation, and serious memory impairment are manifest, pharmacotherapy and dedicated multidisciplinary care are usually applied. Indeed, modest but relevant gains have been secured with FDA-approved anti-AD pharmacotherapy relying on inhibitors of acetylcholinesterase and the activation of synaptic N-methyl-d-aspartase receptors, both critical for neuron survival. However, although this type of pharmacotherapy can modify cognitive and/or behavioral symptoms of AD, it does not even allude to the pathophysiological root of the disease, which explains the recent setbacks in clinical trials. This has made researchers change the target and focus on immunotherapy, an often successful way of treating various malignant diseases. In the present decade, dedicated experimental and clinical research has been intensified on a possible strategy for AD treatment with monoclonal antibodies [66, 67]. Indeed, over the past few years, a number of monoclonal antibodies have appeared to be successful in clearing the brain from βA plaques, considered the culprit of AD. These recently developed antibodies include aducanumab [68], lecanemab [69, 70], both FDA-approved. A third antibody, donanemab [71] has not yet obtained this status. The US-based Alzheimer’s Society wrote on their website that “three promising drugs for treating Alzheimer’s disease bring fresh hope.” However, the publication on the clinical results was received with reservations by the European EMA which declined the request for approval. As for donanemab, side effects may include cerebral edema, headaches, confusion, changes in mental state, vomiting, nausea, tremor, and gait disturbances. In the Trailblazer-alz number 2 and number 3 clinical trials, 27.5% of the donanemab patients experienced one or more of these side effects, and 0.8% in the placebo group. Furthermore, the outcome of clinical results in 1,700 patients after 18 months of use of lecanemab versus placebo is poor; the score of a dementia test was 1.21 versus 1.66 points. This non-compelling difference of 0.45 points will not mean a difference in daily life for which a minimum clinically important difference of about 3.5 points is necessary [72].
AD as a Continuum
Presently, AD is considered a continuum that begins in a latent, preclinical phase of many years. This represents the onset of the disease spectrum ranging from an asymptomatic phase to severe and multifaceted impairment [73]. This preclinical stage of AD has become a major focus of research, as there is consensus that early diagnosis may offer the best chance of multidisciplinary therapy for slowing the progression of disease [74, 75]. During this early period, deposition of βA plaques takes place, followed by (hyper) phosphorylated tau formation and neuronal damage may become evident. Therefore, in the preclinical phase of AD dementia, when βA fibrils and soluble P-tau levels have plateaued, cognitive decline is related to the rate of accumulation of insoluble aggregates of tau [76].
Looking at a particular aspect of early detection of AD, it is worthwhile to consider brain imaging as an important subject. Structural MRI has been a first effort to visualize early AD. Various studies [77] have shown loss of hippocampus volume. Reduction of the cortical thickness, in accordance with postmortem abnormalities, has been identified as another marker for AD by Lech et al. [78], who confirmed a reduced cortical thickness in 19 AD patients and 17 control cases using this imaging technique. Functional MRI has been used to explore blood flow and volume changes in AD. A study on 35 AD patients and 27 subjects with mild cognitive impairment (MCI) showed reduced neuronal connectivity in various areas of the thalamocortical network in AD cases and more moderate disturbances in MCI cases, which has significance for pathophysiological investigations [79].
Positron emission tomography (PET) offers the possibility to study brain metabolism with fluorodeoxyglucose, radiolabeled with F-18. Brain imaging with this glucose analog allows the study of regional brain glucose consumption and the locally corresponding integrated neuronal activity [80]. As abnormal neuronal activity is associated with neurodegeneration, fluorodeoxyglucose is not suited for early, preclinical detection of AD and more specific radiotracers have been developed to resolve this problem. Amyloid deposits, considered the earliest pathological hallmark of AD. The so-called Pittsburgh-B agent, radiolabeled with C-11 (PiB) is an FDA-approved agent that has been used in clinical practice as an amyloid-seeking radiotracer [81]. PET studies by Shen et al. [82] suggest that PiB tests at 30 min after application detect “mild” AD, but its accumulation pattern in the white matter of aging individuals may interfere with appropriate interpretation in healthy individuals is poorly understood [83]. On the other hand, PiB localization has been identified before clinical evidence is present, but this appeared to be a nonspecific phenomenon for AD [84]. To the best of our knowledge, no large-scale multicenter studies have been performed on PiB, probably due to the short half-life of the C-11 radionuclide, requiring specialized production equipment and operators. This is different for tracers labeled with F-18 (half-life of around 110 min), namely, florbetapir and flutemetamol which can be transported to imaging centers about 2 h away from the manufacturing site. Both F-18-labeled tracers provide results that have similar diagnostic potential as PiB [85].
As far as p-tau PET imaging is concerned, most radiolabeled molecules tend to concentrate in βA, whereas only the C-11-labeled tracer, under the code name PBB3 has been offered a more favorable concentration for p-tau than for βA [86]. Considering that tau pathology is more specifically associated with AD than with βA, it would be logical to further develop tau tracers for longitudinal studies in patient care and (experimental) treatment monitoring [87]. Undeniably, PET studies have contributed much to further understanding the pathophysiology of AD. Nevertheless, availability of tracers, the preparation costs, the imaging equipment, and their quality control hamper a widespread use of PET studies. Therefore, the development of tests that can be applied easily to AD patients in all clinical categories is ongoing. Against this background, recent developments have been reported by Mattsson-Carlsson et al. [88] and Horie et al. [89, 90]. Both groups have focused their research on the tracking of longitudinal cognition decline in the preclinical phase, which is symptomatic for early AD. The former group of researchers reported on the plasma biomarker P-Tau 217. This appeared to be the best biomarker to predict longitudinal cognitive decline assessed by the Mini-Mental State Examination (MMSE) and the modified Preclinical Alzheimer Cognitive Composite (mPACC) over a median of 6 years (range 2–10 years). The latter group demonstrated that the microubule binding region of tau that contains the residue 243 (MTBR-tau243), is a cerebrospinal fluid (CSF) biomarker specific for insoluble tau aggregates. This marker longitudinally increased with aggregates of insoluble tau and was associated with PET imaging results and cognitive function testing. They conclude that the CSF MTBR-tau243 is a specific biomarker of tau aggregate pathology, but it should be mentioned that a (repetitive) lumbar puncture has been linked to adverse effects, though especially in younger female patients [91], and that plasma progression tracking may be more suitable for AD patients.
Conclusions
Most relevant, present developments concentrate on the discovery and the evaluation of clinical utility of biomarkers in blood plasma during the continuum of AD. Such markers will provide the framework for patient staging and further advances in the field of pharmacotherapy [92, 93]. In this respect, captivating findings were recently published by Altomare et al. [94], who confirmed the excellent correlation, up to p < 0.001, between plasma markers and traditional scintigraphy and CSF markers. Their results showed that “overall, plasma p-tau (both alone and in combination with Aβ42) showed the best performance both in terms of correlation with traditional biomarkers and of diagnostic accuracy over traditional biomarkers.” Along this line, interesting recent progress has been made by Giacomucci et al. [95] on the quantitative measurement of the blood concentration of neurofilament light chain (NfL), a well-studied biomarker for AD, as an effect of treatment with hydromethylthionine mesylate (HMTM), a molecule which prevents the aggregation of tau that forms toxic fibrils causing damage to neurons. Their investigation showed a correlation with AD disease severity and cognitive decline, which is the basis of the LUCIDITY trial (no trial number). Herein, tau pathology is targeted with HMTM. Subsequently, a phase 3 randomized, double-blind study, including a pseudo-placebo, in 105 AD participants with MCI demonstrated a statistically significant improvement. In addition, in 147 participants with mild to moderate impairment, the stabilization of cognitive decline has been reported in the first 9 months of treatment, whereas no further decline was observed in the following 9 months. Interestingly and to the best of our knowledge, this HMTM approach is the first study that prevents the formation of tangles, rather than treating symptoms of AD. In this context, Bateman et al. [96] studied a group of 128 patients with autosomal dominant AD with a predictable onset of symptoms; they observed clinical, cognitive, structural, metabolic, and biochemical changes at various years before the expected symptom onset. Their groundbreaking results, using fMRI, PET, and clinical dementia rating, made clear that CSF deposition of βA fibrils is followed by increased Tau, which is followed by volume diminishment of the hippocampus and hypometabolic areas of glucose consumption. This sequential pathophysiological development showed that βA deposition occurred 20 years earlier, whereas Tau was detected 15 years earlier. The hippocampus volume change was also detectible around the latter period, whereas mild dementia occurred 3.3 years before expected symptom onset. These pertinent observations represent a biomedical invitation to study whether noninvasive blood biomarkers as mentioned above, would keep up with the progression in autosomal dominant disease.
Acknowledgments
The authors would like to thank Mr Richard B. Wolf and Ms Saskia L. La Grouw for providing excellent editorial assistance.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was not supported by any sponsor or funder.
Author Contributions
Ernest K.J. Pauwels conceived the first and subsequent drafts. Gerard J. Boer provided written input on scientific content of drafts and designed Figure 1. Both authors approved the final draft for submission.
Funding Statement
This study was not supported by any sponsor or funder.
References
- 1. Barbe C, Jolly D, Morrone I, Wolak-Thierry A, Dramé M, Novella JL, et al. Factors associated with quality of life in patients with Alzheimer’s disease. BMC Geriatr. 2018;18(1):159. 10.1186/s12877-018-0855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falomir-Lockhart LJ, Cavazzutti GF, Giménez E, Toscani AM. Fatty acid signaling mechanisms in neural cells: fatty acid receptors. Front Cell Neurosci. 2019;13:162. 10.3389/fncel.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson RJ, Meltzer JC, Nguyen H, Commins C, Bennett RE, Hudry E, et al. APOE4 derived from astrocytes leads to blood-brain barrier impairment. Brain. 2022;145(10):3582–93. 10.1093/brain/awab478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valles SL, Singh SK, Campos-Campos J, Colmena C, Campo-Palacio I, Alvarez-Gamez K, et al. Functions of astrocytes under normal conditions and after a brain disease. Int J Mol Sci. 2023;24(9):8434. 10.3390/ijms24098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu CC, Zhao J, Fu Y, Inoue Y, Ren Y, Chen Y, et al. Peripheral apoE4 enhances Alzheimer’s pathology and impairs cognition by compromising cerebrovascular function. Nat Neurosci. 2022;25(8):1020–33. 10.1038/s41593-022-01127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berthel E, Pujo-Menjouet L, Le Reun E, Sonzogni L, Al-Choboq J, Chekroun A, et al. Toward an early diagnosis for Alzheimer’s disease based on the perinuclear localization of the ATM protein. Cells. 2023;12(13):1747. 10.3390/cells12131747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Victor MB, Leary N, Luna X, Meharena HS, Scannail AN, Bozzelli PL, et al. Lipid accumulation induced by APOE4 impairs microglial surveillance of neuronal-network activity. Cell Stem Cell. 2022;29(8):1197–212.e8. 10.1016/j.stem.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol. 2013;4:102. 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuellar-Santoyo AO, Ruiz-Rodríguez VM, Mares-Barbosa TB, Patrón-Soberano A, Howe AG, Portales-Pérez DP, et al. Revealing the contribution of astrocytes to glutamatergic neuronal transmission. Front Cell Neurosci. 2022;16:1037641. 10.3389/fncel.2022.1037641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HG, Wheeler MA, Quintana FJ. Function and therapeutic value of astrocytes in neurological diseases. Nat Rev Drug Discov. 2022;21(5):339–58. 10.1038/s41573-022-00390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schiweck J, Eickholt BJ, Murk K. Important shapeshifter: mechanisms allowing astrocytes to respond to the changing nervous system during development, injury and disease. Front Cell Neurosci. 2018 Aug 21;12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matusova Z, Hol EM, Pekny M, Kubista M, Valihrach L. Reactive astrogliosis in the era of single-cell transcriptomics. Front Cell Neurosci. 2023;17:1173200. 10.3389/fncel.2023.1173200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim H, Leng K, Park J, Sorets AG, Kim S, Shostak A, et al. Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat Commun. 2022;13(1):6581. 10.1038/s41467-022-34412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Twarowski B, Herbet M. Inflammatory processes in Alzheimer’s disease: pathomechanism, diagnosis and treatment: a review. Int J Mol Sci. 2023;24(7):6518. 10.3390/ijms24076518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verkhratsky A, Zorec R, Parpura V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017;27(5):629–44. 10.1111/bpa.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frost GR, Li YM. The role of astrocytes in amyloid production and Alzheimer's disease. Open Biol. 2017 Dec;7(12):170228. 10.1098/rsob.170228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beach TG, McGeer EG. Lamina-specific Arrangement of astrocytic gliosis and senile plaques in Alzheimer’s disease visual cortex. Brain Res. 1988;463(2):357–61. 10.1016/0006-8993(88)90410-6. [DOI] [PubMed] [Google Scholar]
- 18. Wagner J, Degenhardt K, Veit M, Louros N, Konstantoulea K, Skodras A, et al. Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease. Nature. 2022;612(7938):123–31. 10.1038/s41586-022-05440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. D’Ambrosi N, Apolloni S. Fibrotic scar in neurodegenerative diseases. Front Immunol. 2020 Aug 14;11:1394. 10.3389/fimmu.2020.01394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verkhratsky A, Parpura V, Rodriguez-Arellano JJ, Zorec R. Astroglia in Alzheimer’s disease. Adv Exp Med Biol. 2019;1175:273–324. 10.1007/978-981-13-9913-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci U S A. 1994;91(12):5562–6. 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Samudra N, Lane-Donovan C, VandeVrede L, Boxer AL. Tau pathology in neurodegenerative disease: disease mechanisms and therapeutic avenues. J Clin Invest. 2023;133(12):e168553. 10.1172/JCI168553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanRyzin JW. Phagocytic microglia in development: are they what they eat? Brain Behav Immun Health. 2021;18:100373. 10.1016/j.bbih.2021.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodríguez-Iglesias N, Márquez-Ropero M, et al. Microglia actively remodel adult hippocampal neurogenesis through the phagocytosis secretome. J Neurosci. 2020;40(7):1453–82. 10.1523/JNEUROSCI.0993-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo S, Wang H, Yin Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front Aging Neurosci. 2022;14:815347. 10.3389/fnagi.2022.815347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Tan MS, Jiang T, Tan L. Microglia in Alzheimer’s disease. BioMed Res Int. 2014;2014:437483. 10.1155/2014/437483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–68. 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Ulland TK, Ulrich JD, Song W, Tzaferis JA, Hole JT, et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016;213(5):667–75. 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ulland TK, Colonna M. TREM2: a key player in microglial biology and Alzheimer disease. Nat Rev Neurol. 2018;14(11):667–75. 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 30. La Rosa F, Agostini S, Piancone F, Marventano I, Hernis A, Fenoglio C, et al. TREM2 expression and amyloid-beta phagocytosis in Alzheimer’s disease. Int J Mol Sci. 2023;24(10):8626. 10.3390/ijms24108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ennerfelt H, Holliday C, Shapiro DA, Zengeler KE, Bolte AC, Ulland TKet al. CARD9 attenuates Aβ pathology and modifies microglial responses in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2023;120(24):e2303760120. 10.1073/pnas.2303760120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ennerfelt H, Frost EL, Shapiro DA, Holliday C, Zengeler KE, Voithofer G, et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell. 2022;185(22):4135–52.e22. 10.1016/j.cell.2022.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pluvinage JV, Haney MS, Smith BAH, Sun J, Iram T, Bonanno L, et al. CD22 blockade restores homeostatic microglial phagocytosis in ageing brains. Nature. 2019;568(7751):187–92. 10.1038/s41586-019-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Griciuc A, Federico AN, Natasan J, Forte AM, McGinty D, Nguyen H, et al. Gene therapy for Alzheimer’s disease targeting CD33 reduces amyloid beta accumulation and neuroinflammation. Hum Mol Genet. 2020;29(17):2920–35. 10.1093/hmg/ddaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. MacDonald AB, Miranda JM. Concurrent neocortical borreliosis and Alzheimer’s disease. Hum Pathol. 1987;18(7):759–61. 10.1016/s0046-8177(87)80252-6. [DOI] [PubMed] [Google Scholar]
- 36. Kaur H, Singh Y, Singh S, Singh RB. Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome. 2021;64(4):355–71. 10.1139/gen-2020-0136. [DOI] [PubMed] [Google Scholar]
- 37. Kountouras J, Boziki M, Polyzos SA, Katsinelos P, Gavalas E, Zeglinas C, et al. Impact of reactive oxygen species generation on Helicobacter pylori-related extragastric diseases: a hypothesis. Free Radic Res. 2017;51(1):73–9. 10.1080/10715762.2016.1271122. [DOI] [PubMed] [Google Scholar]
- 38. Balin BJ, Hammond CJ, Little CS, Hingley ST, Al-Atrache Z, Appelt DM, et al. Chlamydia pneumoniae: an etiologic agent for late-onset dementia. Front Aging Neurosci. 2018;10:302. 10.3389/fnagi.2018.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol. 2019;11(1):1563405. 10.1080/20002297.2018.1563405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Senejani AG, Maghsoudlou J, El-Zohiry D, Gaur G, Wawrzeniak K, Caravaglia C, et al. Borrelia burgdorferi co-localizing with amyloid markers in Alzheimer’s disease brain tissues. J Alzheimers Dis. 2022;85(2):889–903. 10.3233/JAD-215398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Herrera-Landero A, Amaya-Sánchez LE, D Hyver de Las-Deses C, Solórzano-Santos F, Gordillo-Pérez MG. Borrelia burgdorferi as a risk factor for Alzheimer’s dementia and mild cognitive impairment. Eur Geriatr Med. 2019;10(3):493–500. 10.1007/s41999-018-0153-0. [DOI] [PubMed] [Google Scholar]
- 42. Cerar T, Ogrinc K, Lotric-Furlan S, Kobal J, Levicnik-Stezinar S, Strle F, et al. Diagnostic value of cytokines and chemokines in lyme neuroborreliosis. Clin Vaccin Immunol. 2013;20(10):1578–84. 10.1128/CVI.00353-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van de Schoor FR, Vrijmoeth HD, Brouwer MAE, Ter Hofstede HJM, Lemmers HLM, Dijkstra H, et al. Borrelia burgdorferi is a poor inducer of gamma interferon: amplification induced by interleukin-12. Infect Immun. 2022;90(3):e0055821. 10.1128/iai.00558-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hurtenbach U, Museteanu C, Gasser J, Schaible UE, Simon MM. Studies on early events of Borrelia burgdorferi-induced cytokine production in immunodeficient SCID mice by using a tissue chamber model for acute inflammation. Int J Exp Pathol. 1995;76(2):111–23. [PMC free article] [PubMed] [Google Scholar]
- 45. Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci. 2017;11(2):72–80. [PMC free article] [PubMed] [Google Scholar]
- 46. Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, et al. Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5(1):eaau1173333. 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ximinies AD, Dou Y, Mishra A, Zhang K, Deivanayagam C, Wang C, et al. The oxidative stress-induced hypothetical protein PG_0686 in Porphyromonas gingivalis W83 is a novel diguanylate cyclase. Microbiol Spectr. 2023;11(2):e0441122. 10.1128/spectrum.04411-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siddiqui H, Yoder-Himes DR, Mizgalska D, Nguyen KA, Potempa J, Olsen I. Genome sequence of Porphyromonas gingivalis strain HG66 (DSM 28984). Genome Announc. 2014;2(5):e00947–14. 10.1128/genomeA.00947-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sait A, Angeli C, Doig AJ, Day PJR. Viral involvement in Alzheimer’s disease. ACS Chem Neurosci. 2021;12(7):1049–60. 10.1021/acschemneuro.0c00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Allnutt MA, Johnson K, Bennett DA, Connor SM, Troncoso JC, Pletnikova O, et al. Human Herpesvirus 6 detection in Alzheimer’s disease cases and controls across multiple cohorts. Neuron. 2020;105(6):1027–35.e2. 10.1016/j.neuron.2019.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barnes LL, Capuano AW, Aiello AE, Turner AD, Yolken RH, Torrey EF, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–7. 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang SY, Yang YX, Kuo K, Li HQ, Shen XN, Chen SD, et al. Herpesvirus infections and Alzheimer’s disease: a Mendelian randomization study. Alzheimers Res Ther. 2021;13(1):158. 10.1186/s13195-021-00905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Itzhaki RF. Corroboration of a major role for herpes simplex virus Type 1 in Alzheimer’s disease. Front Aging Neurosci. 2018;10:324. 10.3389/fnagi.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Itzhaki RF. Overwhelming evidence for a major role for herpes simplex virus type 1 (HSV1) in alzheimer’s disease (AD); underwhelming evidence against. Vaccines. 2021;9(6):679. 10.3390/vaccines9060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. A 3D human brain-like tissue model of herpes-induced Alzheimer’s disease. Sci Adv. 2020;6(19):eaay8828. 10.1126/sciadv.aay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cairns DM, Itzhaki RF, Kaplan DL. Potential involvement of varicella zoster virus in Alzheimer’s disease via reactivation of quiescent herpes simplex virus type 1. J Alzheimers Dis. 2022;88(3):1189–200. 10.3233/JAD-220287. [DOI] [PubMed] [Google Scholar]
- 57. Lehrer S, Rheinstein PH. Vaccination reduces risk of Alzheimer’s disease, Parkinson’s disease and other neurodegenerative disorders. Discov Med. 2022;34(172):97–101. [PMC free article] [PubMed] [Google Scholar]
- 58. Itzhaki RF. Herpes simplex virus type 1 and Alzheimer’s disease: increasing evidence for a major role of the virus. Front Aging Neurosci. 2014;6:202. 10.3389/fnagi.2014.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao C, Strobino K, Moon YP, Cheung YK, Sacco RL, Stern Y, et al. APOE ϵ4 modifies the relationship between infectious burden and poor cognition. Neurol Genet. 2020;6(4):e462. 10.1212/NXG.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lopatko Lindman K, Weidung B, Olsson J, Josefsson M, Kok E, Johansson A, et al. A genetic signature including apolipoprotein Eε4 potentiates the risk of herpes simplex-associated Alzheimer’s disease. Alzheimers Dement. 2019;5:697–704. 10.1016/j.trci.2019.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Khosroazad S, Gilbert CF, Aronis JB, Daigle KM, Esfahani M, Almaghasilah A, et al. Sleep movements and respiratory coupling as a biobehavioral metric for early Alzheimer’s disease in independently dwelling adults. BMC Geriatr. 2023;23(1):252. 10.1186/s12877-023-03983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Aggarwal M, Alkhayyat M, Abou Saleh M, Sarmini MT, Singh A, Garg R, et al. Alzheimer disease occurs more frequently in patients with inflammatory bowel disease: insight from a nationwide study. J Clin Gastroenterol. 2023;57(5):501–7. 10.1097/MCG.0000000000001714. [DOI] [PubMed] [Google Scholar]
- 63. Chang CW, Juan YS, Yang YH, Lee HY. The relationship between lower urinary tract symptoms and severity of Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2021;36:1533317521992657–162. 10.1177/1533317521992657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu CY, Ho CY, Yang YH. Developing biomarkers for the skin: biomarkers for the diagnosis and prediction of treatment outcomes of Alzheimer’s disease. Int J Mol Sci. 2023;24(10):8478. 10.3390/ijms24108478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eiser AR, Fulop T. Alzheimer’s disease is a multi-organ disorder: it may already be preventable. J Alzheimers Dis. 2023;91(4):1277–81. 10.3233/JAD-221078. [DOI] [PubMed] [Google Scholar]
- 66. Ruthirakuhan M, Herrmann N, Suridjan I, Abraham EH, Farber I, Lanctôt KL. Beyond immunotherapy: new approaches for disease modifying treatments for early Alzheimer’s disease. Expert Opin Pharmacother. 2016;17(18):2417–29. 10.1080/14656566.2016.1258060. [DOI] [PubMed] [Google Scholar]
- 67. Plotkin SS, Cashman NR. Passive immunotherapies targeting Aβ and tau in Alzheimer’s disease. Neurobiol Dis. 2020;144:105010. 10.1016/j.nbd.2020.105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alzheimers Dis. 2022;9(2):197–210. 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 69. McDade E, Cummings JL, Dhadda S, Swanson CJ, Reyderman L, Kanekiyo M, et al. Lecanemab in patients with early Alzheimer's disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study. Alzheimers Res Ther. 2022;14(1):191. 10.1186/s13195-022-01124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. Erratum in: Alzheimers Res Ther. 2022;14(1): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384(18):1691–704. 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 72. Liu KY, Schneider LS, Howard R. The need to show minimum clinically important differences in Alzheimer’s disease trials. Lancet Psychiatr. 2021 Nov;8(11):1013–6 Epub 2021 Jun 1. 10.1016/S2215-0366(21)00197-8. [DOI] [PubMed] [Google Scholar]
- 73. Aisen PS, Cummings J, Jack CR Jr, Morris JC, Sperling R, Frölich L, et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res Ther. 2017;9(1):60. 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rafii MS, Aisen PS. Detection and treatment of Alzheimer’s disease in its preclinical stage. Nat Aging. 2023;3(5):520–31. 10.1038/s43587-023-00410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pichet Binette A, Franzmeier N, Spotorno N, Ewers M, Brendel M, Biel D, et al. Amyloid-associated increases in soluble tau relate to tau aggregation rates and cognitive decline in early Alzheimer’s disease. Nat Commun. 2022;13(1):6635. 10.1038/s41467-022-34129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mak E, Zhang L, Tan CH, Reilhac A, Shim HY, Wen MOQ, et al. Longitudinal associations between β-amyloid and cortical thickness in mild cognitive impairment. Brain Commun. 2023;5(4):fcad192. 10.1093/braincomms/fcad192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex. 2005;15(7):995–1001. 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- 79. Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P, et al. Impaired functional connectivity of the thalamus in Alzheimer’s disease and mild cognitive impairment: a resting-state fMRI study. Curr Alzheimer Res. 2013;10 (7):754–66. 10.2174/15672050113109990146. [DOI] [PubMed] [Google Scholar]
- 80. Xu K, Niu N, Li X, Chen Y, Wang D, Zhang J, et al. The characteristics of glucose metabolism and functional connectivity in posterior default network during nondemented aging: relationship with executive function performance. Cereb Cortex. 2023;33(6):2901–11. 10.1093/cercor/bhac248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ikonomovic MD, Buckley CJ, Abrahamson EE, Kofler JK, Mathis CA, Klunk WE, et al. Post-mortem analyses of PiB and flutemetamol in diffuse and cored amyloid-β plaques in Alzheimer’s disease. Acta Neuropathol. 2020;140(4):463–76. 10.1007/s00401-020-02175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shen C, Wang Z, Chen H, Bai Y, Li X, Liang D, et al. Identifying Mild Alzheimer’s disease with first 30-min 11C-PiB PET scan. Front Aging Neurosci. 2022;14:785495. 10.3389/fnagi.2022.785495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lowe VJ, Lundt ES, Senjem ML, Schwarz CG, Min HK, Przybelski SA, et al. White matter reference region in PET studies of 11C-Pittsburgh compound B uptake: effects of age and amyloid-β deposition. J Nucl Med. 2018 Oct;59(10):1583–9. 10.2967/jnumed.117.204271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang S, Smailagic N, Hyde C, Noel-Storr AH, Takwoingi Y, McShane Ret al. (11)C-PIB-PET for the early diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2014 Jul 23;2014(7):CD010386. 10.1002/14651858.CD010386.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lesman-Segev OH, La Joie R, Iaccarino L, Lobach I, Rosen HJ, Seo SW, et al. Diagnostic accuracy of amyloid versus 18 F-fluorodeoxyglucose positron emission tomography in autopsy-confirmed dementia. Ann Neurol. 2021;89(2):389–401. 10.1002/ana.25968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yousefzadeh-Nowshahr E, Winter G, Bohn P, Kneer K, von Arnim CAF, Otto M, et al. Quantitative analysis of regional distribution of tau pathology with 11C-PBB3-PET in a clinical setting. PLoS One. 2022;17(4):e0266906. 10.1371/journal.pone.0266906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gogola A, Minhas DS, Villemagne VL, Cohen AD, Mountz JM, Pascoal TA, et al. Direct comparison of the Tau PET tracers 18F-flortaucipir and 18F-MK-6240 in human subjects. J Nucl Med. 2022;63(1):108–16. 10.2967/jnumed.120.254961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mattsson-Carlgren N, Salvadó G, Ashton NJ, Tideman P, Stomrud E, Zetterberg H, et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 2023;80(4):360–9. 10.1001/jamaneurol.2022.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Horie K, Barthélemy NR, Sato C, Bateman RJ. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer’s disease. Brain. 2021;144(2):515–27. 10.1093/brain/awaa373 Erratum in: Brain. 2021; 144(9):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Horie K, Salvadó G, Barthélemy NR, Janelidze S, Li Y, He Y, et al. CSF MTBR-tau243 is a specific biomarker of tau tangle pathology in Alzheimer’s disease. Nat Med. 2023;29(8):1954–63 Epub ahead of print. 10.1038/s41591-023-02443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Baldaranov D, Garcia V, Miller G, Donohue MC, Shaw LM, Weiner M, et al. Safety and tolerability of lumbar puncture for the evaluation of Alzheimer’s disease. Alzheimers Dement. 2023;15(2):e12431–35643092. 10.1002/dad2.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022;21(8):726–34. 10.1016/S1474-4422(22)00168-5. [DOI] [PubMed] [Google Scholar]
- 93. Altomare D, Stampacchia S, Ribaldi F, Tomczyk S, Chevalier C, Poulain G, et al. Plasma biomarkers for Alzheimer’s disease: a field-test in a memory clinic. J Neurol Neurosurg Psychiatry. 2023;94(6):420–7. 10.1136/jnnp-2022-330619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Coulthard E, Hosseini AA. Blood biomarkers: ready for clinical practice? J Neurol Neurosurg Psychiatry. 2023;94(6):409–10. 10.1136/jnnp-2022-330958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Giacomucci G, Mazzeo S, Bagnoli S, Ingannato A, Leccese D, Berti V, et al. Plasma neurofilament light chain as a biomarker of Alzheimers disease in subjective cognitive decline and mild cognitive impairment. J Neurol. 2022;269(8):4270–80. 10.1007/s00415-022-11055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]