Abstract
Background
Since laparoscopic anatomical resection (LAR) for tumors, especially located in the posterosuperior (PS) segments of the liver remains difficult, laparoscopic non-anatomical resection (LNAR) are generally preferred. To compare the clinical outcomes between LAR and LNAR for hepatocellular carcinoma (HCC) located in the PS segments.
Methods
We retrospectively reviewed the data for 1,029 patients who underwent hepatectomy for HCC between 2004 and 2019. Of 167 patients who underwent laparoscopic hepatectomy for HCC in PS segments, 64 underwent LNAR and 103 underwent LAR. Patients were matched one-to-one using propensity score matching (46:46).
Results
LNAR was associated with significantly shorter operation time (P=0.001), lower estimated blood loss (P=0.001), lower transfusion rate (P=0.006) and shorter hospital stay (P=0.012) than LAR. The respective 1- ,3-, and 5-year overall survival rates (LAR: 95.3%, 87.1%, and 77.8%; LNAR: 96.7%, 91.6%, and 85.0%; P=0.262) and recurrence-free survival rates (LAR: 75.7%, 70.3%, and 68.9%; LNAR: 81.8%, 58.3%, and 55.3%; P=0.879) were similar. The intrahepatic recurrence rate was significantly higher in LNAR group than in LAR group (78.6% vs. 57.1%, P=0.023), but the post-recurrence treatments differed significantly between the two groups (P=0.016); the re-resection rate was much greater in the LNAR group (45.0% vs. 0%) group. The respective 1-, 3-, and 5-year post-recurrence survival rates were similar in the LAR and LNAR groups (P=0.212). After recurrence, survival in re-resection group was significantly greater than not (P=0.026).
Conclusions
LNAR is safe and feasible for HCC located in PS segments, and provided acceptable oncologic outcomes that are comparable to those of LAR. LNAR can be considered for patient with tumor located in PS segment when LAR is not feasible.
Keywords: Laparoscopy; hepatectomy; carcinoma, hepatocellular; recurrence
Introduction
Liver resection is a potentially curative treatment for hepatocellular carcinoma (HCC). However, recurrence after surgery remains a major challenge in the management of HCC (1,2). Post-surgical recurrence is thought to be due to tumor invasion of portal venous branches that allows the tumor cells to be disseminate to other regions of the liver via the portal venous flow (3). Thus, it is believed that anatomical resection (AR), which removes the tumor-bearing portal tributaries supplied by a major branch of the portal vein and hepatic artery, confers a survival benefit (4). Although several studies have demonstrated the oncological benefits of AR over non-anatomical resection (NAR) for HCC, the optimum liver resection technique remains controversial (5-7).
Recent advances in minimally invasive surgery have gradually accelerated the adoption of laparoscopic liver resection (LLR), and its role in liver surgery is becoming increasingly important. Several studies have demonstrated that LLR could provide favorable short- and long-term outcomes compared with open liver resection (OLR) (8-10). However, these earlier studies included a limited number of patients who underwent laparoscopic anatomical resection (LAR), especially in the posterosuperior (PS) segments of the liver (segments 1, 7, 8, and the superior part of segment 4). Although minor LLR is accepted as a curative treatment for HCC, LAR is not widely performed (11). LAR is a challenging procedure that should be performed by surgeons with experience of performing LLR. Although several studies have demonstrated feasible outcomes of LAR, the oncological benefits of LAR for HCC remain unclear.
LLR of PS segments is considered technically challenging because these anatomical locations are difficult to access laparoscopically (12,13). Because LLR is rarely performed for tumors located in the PS segments, to our knowledge, there have been no studies comparing LAR and laparoscopic non-anatomical resection (LNAR) for tumors in PS segments. Therefore, in this study, we compared the clinical outcomes between LAR and LNAR for HCC located in the PS segments. Patients in both groups were matched using propensity score matching (PSM). We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-578/rc).
Methods
Patients and data collection
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In accordance with guidelines for human subjects’ research, this retrospective study was approved by the institutional review board of Seoul National University Bundang Hospital, Seongnam, Korea, an academic hospital affiliated to Seoul National University, College of Medicine (Approval No. B-2021-633).
Figure 1 shows the patient flowchart. A total of 1,029 consecutive patients with HCC underwent hepatectomy between January 2004 and December 2019. Patients included in this study were identified as follows. First, we extracted the records for 268 patients who underwent LLR for a tumor located in the PS segments by the same surgical team. We subsequently excluded cases who required open conversion [N=29, 18 patients in LAR (14.9%) vs. 11 patients in LNAR (14.6%), P=0.891], patients with combined HCC-cholangiocarcinoma (N=9), patients with other malignancies (N=27), and patients with incomplete data (N=36). A total of 167 patients, including 103 who underwent LAR and 64 who underwent LNAR, were finally included in this study.
Figure 1.
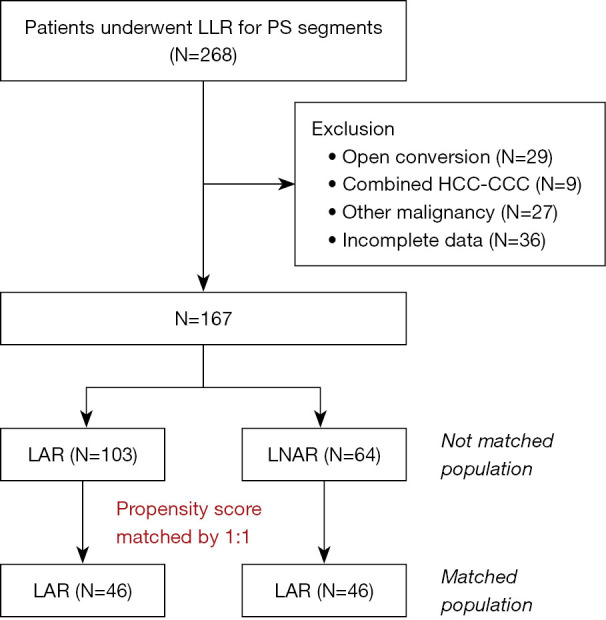
Patient flow chart. LLR, laparoscopic liver resection; PS, posterosuperior; HCC-CCC, hepatocellular carcinoma-cholangiocarcinoma; LAR, laparoscopic anatomical resection; LNAR, laparoscopic non-anatomical resection.
The decision to perform LAR or LNAR was made based on the tumor size, location, and hepatic function. LAR was preferred, if feasible, for patients with a centrally located tumor and normal hepatic function. Right hemihepatectomy is preferred when the tumor is located close to large vessel such as right hepatic vein and right posterior Glissonean pedicle with sufficient remnant liver volume and normal liver function. LNAR was performed for patients with peripherally located tumor and/or impaired liver function.
LAR and LNAR procedures
The LLR procedure for tumors located in the PS segments has been reported (14-17). The patients were placed in the lithotomy position and were tilted to a 30° reverse Trendelenburg position with right-side-up adjustment. The surgeon was positioned between the patient’s legs or the patient’s right side. After establishing pneumoperitoneum through a sub-umbilical 11-mm camera port, four additional ports were created. In some patients, additional intercostal ports were placed at the seventh and ninth intercostal spaces (18). A flexible laparoscope was used.
When performing LAR for tumors located in PS segments, we generally used the Glissonean approach. First, after performing cholecystectomy with usual methods, the right liver was mobilized from the inferior vena cava and the diaphragm. After dissecting the major Glissonean pedicle of the right posterior section, further hepatic parenchymal dissection was performed from the peripheral side until we reached the branches of the Glissonean pedicles of the PS segment. After isolating and ligating the Glissonean pedicle, the transection plane was determined based on the ischemic line. This ischemic line and laparoscopic intraoperative ultrasonography (IOUS) help guide the plane of parenchymal dissection.
The surgical procedures for LNAR differed according to the tumor locations. First, laparoscopic IOUS was performed to detect the tumor and its relationship with the nearby vasculature. Moreover, we frequently used the IOUS to obtain a sufficient surgical margin. Then, parenchymal dissection was performed using a harmonic scalpel or cavitron ultrasonic surgical aspirator to obtain an adequate resection margin (Figure 1) .
Variables
The demographic and preoperative data (etiology, liver function, tumor factors) were compared between patients who underwent LAR and LNAR. We used 1:1 PSM to compensate for selection bias and to match patients based on preoperative clinical factors. A total of 92 patients were selected, with 46 patients per each group. After adjusting for these factors, the short- and long-term operative outcomes were compared between the two matched groups. Postoperative complications that occurred within 30 days after surgery were graded using the Clavien-Dindo classification (19).
Recurrence
Patients were followed by abdominal computed tomography and blood tests, including measurement of tumor markers every 3 months for the first 2 years after primary surgery, and then every 3–6 months thereafter. Intrahepatic recurrence was divided into right or left lobe. Early recurrence was defined as disease relapse within 2 years after hepatectomy.
Patients with postsurgical recurrence were managed by locoregional treatment (LT) or palliative treatment (PT). LT comprised re-resection or radiofrequency ablation (RFA) if the recurrence was localized. LT in extrahepatic recurrence is performed when the patient showed the oligometastatic disease, absent/controlled intrahepatic hepatocellular carcinoma and satisfactory liver function and performed status were deemed for eligible for extrahepatic metasectomy. PT comprised received transcatheter arterial chemoembolization (TACE) or conservative management with sorafenib if the tumor was not amenable to locoregional treatment.
Survival
Overall survival (OS) was defined as the time from primary surgery to the date of death regardless of cause. Recurrence-free survival (RFS) was defined as the time from primary surgery to the first documented detection of recurrence during regular follow-up. Survival after recurrence (SAR) was the time from the first day of identified recurrence to the date of death or the date of last follow-up. A subgroup analysis was performed to compare the SAR according to the treatment method after recurrence (i.e., LT vs. PT).
Statistical analysis
All statistical analyses were performed using SPSS software for Windows, version 25 (IBM Corporation, Armonk, NY, USA). Clinical data were summarized using descriptive analyses, and all continuous values are presented as the mean and standard deviation. Student’s t-test or the Mann-Whitney U test were used to compare continuous variables, and Pearson’s χ2 test was used to compare categorical variables. All P values of <0.05 were considered statistically significant. Survival was estimated using the Kaplan-Meier method, and compared using the log-rank test.
Results
Patient demographics
Table 1 shows the patient characteristics of all patients, as well as both groups matched by PSM. Before PSM (LAR vs. LNAR), preoperative alanine aminotransferase (50.9±65.8 vs. 36.1±27.2 IU/L, P=0.028), α-fetoprotein (836.0±6,953.3 vs. 217.8±1,113.7 ng/mL, P=0.046), and tumor size (4.0±2.3 vs. 2.0±0.9 cm, P=0.001) were significantly difference between groups. After PSM, the patient characteristics were well balanced in both groups, with no significant differences in demographic variables (age, sex, body mass index, hypertension, diabetes mellitus, previous abdominal surgery), liver-related variables (virology, Model for End-stage Liver Disease score, Child-Pugh score) and tumor-related factors (previous TACE, RFA, and tumor size).
Table 1. Patient characteristics.
| Variables | Unmatched | After applying PSM | |||||
|---|---|---|---|---|---|---|---|
| LAR (N=103) | LNAR (N=64) | P value | LAR (N=46) | LNAR (N=46) | P value | ||
| Demographic data | |||||||
| Age (years) | 59.4±11.4 | 60.2±9.9 | 0.342 | 59.8±10.9 | 61.6±8.2 | 0.252 | |
| Male | 77 (80.8) | 54 (84.4) | 0.177 | 29 (63.0) | 38 (82.6) | 0.060 | |
| BMI (kg/m2) | 24.7±3.6 | 25.1±2.9 | 0.491 | 24.8±3.6 | 25.1±2.9 | 0.444 | |
| HTN | 42 (40.8) | 28 (43.8) | 0.748 | 12 (26.1) | 16 (34.7) | 0.831 | |
| Diabetes | 23 (22.3) | 14 (21.9) | 1.000 | 6 (13.0) | 10 (21.7) | 0.410 | |
| Prior abdominal surgery | 22 (21.4) | 19 (29.7) | 0.268 | 10 (21.7) | 14 (30.4) | 0.336 | |
| Preoperative data | |||||||
| Etiology | 0.965 | 0.470 | |||||
| Hepatitis B | 66 (64.1) | 41 (64.1) | 32 (69.6) | 27 (58.7) | |||
| Hepatitis C | 7 (6.8) | 5 (7.8) | 5 (10.9) | 5 (10.9) | |||
| MELD score | 7.6±1.6 | 7.7±1.2 | 0.220 | 7.7±1.3 | 7.8±1.4 | 0.277 | |
| Child-Pugh score | 0.372 | 1.000 | |||||
| A | 101 (98.1) | 61 (95.3) | 45 (97.8) | 45 (97.8) | |||
| B | 2 (1.9) | 3 (4.7) | 1 (2.2) | 1 (2.2) | |||
| Bilirubin (mg/dL) | 0.8±0.4 | 0.8±0.3 | 0.364 | 0.8±0.4 | 0.8±0.4 | 0.481 | |
| ALT (IU/L) | 50.9±65.8 | 36.1±27.2 | 0.028 | 42.5±61.3 | 39.9±32.7 | 0.515 | |
| AST (IU/L) | 51.1±80.0 | 41.7±33.3 | 0.307 | 49.1±109.5 | 47.3±42.5 | 0.336 | |
| Albumin (g/dL) | 4.3±0.4 | 4.2±0.4 | 0.724 | 4.2±0.4 | 4.3±0.4 | 0.185 | |
| INR | 1.1±0.1 | 1.1±0.1 | 0.639 | 1.1±0.1 | 1.1±0.1 | 0.232 | |
| Platelet count (×103/μL) | 193.4±66.9 | 163.6±65.0 | 0.276 | 183.9±78.7 | 170.4±62.6 | 0.396 | |
| Prior TACE | 20 (19.4) | 16 (25.0) | 0.441 | 12 (26.1) | 9 (19.6) | 0.620 | |
| Prior RFA | 9 (8.7) | 3 (4.7) | 0.375 | 5 (10.8) | 2 (4.3) | 0.158 | |
| AFP (ng/mL) | 836.0±6,953.3 | 217.8±1,113.7 | 0.046 | 351.9±826.3 | 332.6±658.6 | 0.874 | |
| Tumor size (cm) | 4.0±2.3 | 2.0±0.9 | 0.001 | 2.6±1.0 | 2.3±1.0 | 0.741 | |
All variables are presented as the mean ± standard deviation or n (%) of patients. PSM, propensity score matching; LAR, laparoscopic anatomical resection; LNAR, laparoscopic non-anatomical resection; BMI, body mass index; HTN, hypertension; MELD, Model for End-stage Liver Disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; AFP, α-fetoprotein.
Surgical outcomes
Table 2 shows the surgical outcomes in the PSM groups. The mean operation time (LAR vs. LNAR; 408.9±142.9 vs. 143.1±50.8 min, P=0.001), estimated blood loss (EBL; 917.5±152.9 vs. 333.8±259.3 mL, P=0.001), and intra-operative transfusion rate (30.4% vs. 6.5%, P=0.006) were significantly greater in the LAR group than in the LNAR group. The mean length of hospitalization after surgery was significantly longer in the LAR group than in the LNAR group (7.8±3.3 vs. 5.7±2.7 days, P=0.012). The frequency of major postoperative complications (Clavien-Dindo grade ≥ IIIa) was not significantly different between the two groups (P=0.134). There were no in-hospital deaths.
Table 2. Outcomes of surgery in the propensity-matched groups.
| Variables | LAR (N=46) | LNAR (N=46) | P value |
|---|---|---|---|
| Operative type | 0.001 | ||
| Right hemihepatectomy | 12 (26.1) | – | |
| Right anterior sectionectomy | 4 (8.7) | – | |
| Right posterior sectionectomy | 14 (30.4) | – | |
| Segmentectomy | 16 (34.8) | – | |
| Tumorectomy | – | 46 (100.0) | |
| Operative data | |||
| Operation time (min) | 408.9±142.9 | 143.1±50.8 | 0.001 |
| Estimated blood loss (mL) | 917.5±152.9 | 333.8±259.3 | 0.001 |
| Transfusion | 14 (30.4) | 3 (6.5) | 0.006 |
| Pringle maneuver | 19 (41.3) | 21 (45.7) | 0.834 |
| Postoperative data | |||
| Hospital stay (days) | 7.8±3.3 | 5.7±2.7 | 0.012 |
| C-D complications | 0.134 | ||
| IIIa | 3 (6.5) | 4 (8.7) | |
| IIIb | 1 (2.2) | 0 | |
| IV | 0 | 0 | |
| V | 0 | 0 | |
| Pathologic data | |||
| Tumor size (cm) | 2.7±1.0 | 2.3±0.7 | 0.088 |
| Surgical margin (cm) | 1.1±1.3 | 1.0±0.5 | 0.162 |
| Microvascular invasion | 14 (40.0) | 12 (34.3) | 0.805 |
| Serosal invasion | 10 (21.7) | 10 (21.7) | 1.000 |
| Tumor stage | 0.268 | ||
| I | 24 (52.2) | 27 (58.7) | |
| II | 18 (39.1) | 17 (37.0) | |
| III | 2 (4.3) | 0 | |
| IV | 0 | 0 | |
| Total necrosis | 2 (4.3) | 2 (4.3) | |
| Cirrhosis | 19 (41.3) | 28 (60.9) | 0.095 |
All variables are presented as the mean ± standard deviation or n (%) of patients. LAR, laparoscopic anatomical resection; LNAR, laparoscopic non-anatomical resection; C-D, Clavien-Dindo.
Regarding pathological data (LAR vs. LNAR), tumor size (2.7±1.0 vs. 2.3±0.7 cm, P=0.088) and surgical margin (1.1±1.3 vs. 1.0±0.5 cm, P=0.162) were similar in both groups. The frequencies of microvascular invasion (40.0% vs. 34.3%, P=0.805) and serosal invasion (21.7% vs. 21.7%, P=1.000), and tumor stage (P=0.268) were similar in both groups. The frequency of patients with pathologically confirmed liver cirrhosis was also similar in the LAR and LNAR groups (41.3% vs. 60.9%, P=0.095).
Clinical characteristics of patients with recurrent tumors
Table 3 compares the recurrence patterns and secondary treatment for two groups. After a median follow-up of 50 months (range, 7–175 months), 14 patients (30.4%) in the LAR group and 14 patients (30.4%) in the LNAR group experienced recurrence (P=1.000). The intrahepatic recurrence rate was significantly higher in LNAR group than in LAR group (78.6% vs. 57.1%, P=0.023). Among the patients who experienced intrahepatic recurrence, patients with LNAR more frequently experienced recurrence in the right lobe. The incidence of early recurrence was similar in the LAR and LNAR groups (42.9% vs. 35.7%, P=0.695).
Table 3. Clinical characteristics of recurrent HCC in the propensity score-matched groups.
| Variables | LAR (N=46) | LNAR (N=46) | P value |
|---|---|---|---|
| Recurrence, n (%) | 14 (30.4) | 14 (30.4) | 1.000 |
| Recurrence site, n (%) | 0.023 | ||
| Intrahepatic | 8 (57.1) | 11 (78.6) | |
| Right lobe | 2 | 11 | |
| Left lobe | 6 | 0 | |
| Extrahepatic | 5 (35.7) | 1 (7.1) | |
| Both | 1 (7.1) | 2 (14.3) | |
| Time to recurrence, n (%) | 0.695 | ||
| Early recurrence (≤24 months) | 6 (42.9) | 5 (35.7) | |
| Late recurrence (>24 months) | 8 (57.1) | 9 (64.3) | |
| Treatment after recurrence, n (%) | 0.016 | ||
| Curative intent treatment | |||
| Re-resection | 0 | 5 (35.7) | |
| RFA | 1 (7.1) | 3 (21.4) | |
| RFA + TACE | 3 (21.4) | 0 | |
| Palliative treatment | |||
| TACE | 2 (14.3) | 3 (21.4) | |
| Chemotherapy or supportive therapy | 8 (57.1) | 3 (21.4) |
HCC, hepatocellular carcinoma; LAR, laparoscopic anatomical resection; LNAR, laparoscopic non-anatomical resection; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Following recurrence, the treatment options were categorized as LT or PT. The secondary treatment differed significantly between the two groups (P=0.016). In particular, the re-resection rate was significantly greater in the LNAR group (45.0% vs. 0%). The resection type among patients who underwent re-resection was tumorectomy.
Survival
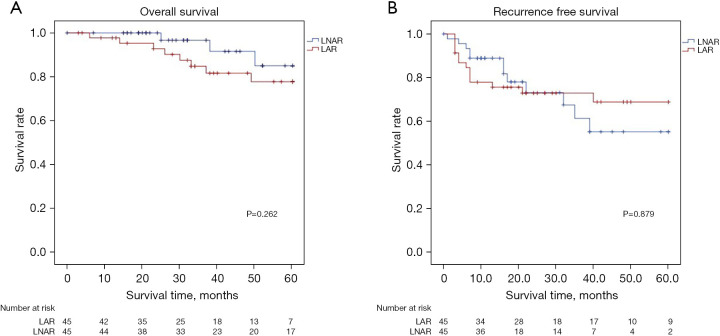
Figure 2 shows the cumulative survival curve for the LAR and LNAR groups (with PSM). The respective 1-, 3-, and 5-year OS (LAR: 95.3%, 87.1%, and 77.8%; LNAR: 96.7%, 91.6%, and 85.0%; P=0.262) and the 1-, 3-, and 5-year RFS (LAR: 75.7%, 70.3%, and 68.9%; LNAR: 81.8%, 58.3%, and 55.3%; P=0.879) were not significantly different between the two groups.
Figure 2.
Survival curve of patients undergoing LAR and LNAR for PS lesions in the propensity-matched group. (A) Overall survival; (B) recurrence free survival. LNAR, laparoscopic non-anatomical resection; LAR, laparoscopic anatomical resection; PS, posterosuperior.
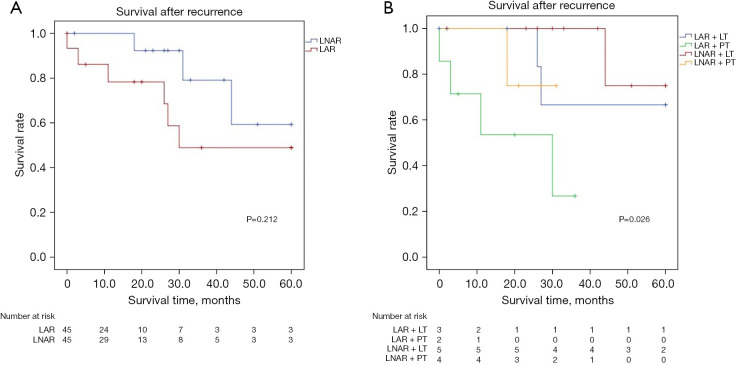
Figure 3A shows the overall SAR curves. The 1-, 3-, and 5-year SAR rates were 78.3%, 49.0%, and 49.0% in the LAR group vs. 92.3%, 79.1%, and 59.3% in the LNAR group, respectively (P=0.212). Figure 3B shows the SAR curve based on the treatment performed after recurrence. The SAR was significantly greater in the LT group than in the PT group (P=0.026).
Figure 3.
SAR of patients in the propensity-matched group. (A) Overall SAR curves; (B) SAR curve based on the treatment type after recurrence. LNAR, laparoscopic non-anatomical resection; LAR, laparoscopic anatomical resection; LT, locoregional treatment; PT, palliative treatment; SAR, survival after recurrence.
Discussion
LLR for a tumor located in PS segments is considered particularly challenging because of its location in the deepest region of the abdominal cavity, and its relationship to the hepatic veins and Glissonean pedicles (20-22). LAR procedures for PS segments, including laparoscopic posterior sectionectomy and laparoscopic S6 or S7 segmentectomy, are often considered major hepatectomy, despite not satisfying the International Hepato-Pancreato-Biliary Association (IHPBA) Brisbane definition of major hepatectomy (23). LNAR of small tumors located in the PS segments in patients with normal hepatic function is also considered technically challenging because the transection planes can be curved or angled (24). For these reasons, it is controversial whether LAR or LNAR is the optimal approach for a tumor located in the PS segments. Although, several studies have demonstrated the feasibility of LAR (8), there are no studies comparing the clinical outcomes between LAR and LNAR for tumors located in PS segments. General, normal liver function allows either LAR or LNAR to be performed safely. However, in patients with liver cirrhosis, LAR sometimes leads to post-hepatectomy liver failure (25). Thus, preoperative liver function and tumor characteristics are important confounding factors that should be considered when comparing LAR and LNAR, and they may impact on the clinical outcomes (26). Therefore, we used PSM to minimize potential confounding effects in this study. Thereby, we compared the short- and long-term outcomes between LAR and LNAR for tumors located in PS segments by applying PSM.
This study revealed better short-term outcomes of LNAR vs. LAR in terms of operation time, EBL, and hospital study. Troisi et al. reported that resection involving PS segments was an independent risk factor for open conversion during LLR, and bleeding was the main reason for conversion (21). When performing LAR in our institution, we generally adopt the Glissonean approach. LAR using the Glissonean approach is a challenging procedure associated with increased risk of bleeding. Besides, the LAR should exposure the main trunk of the hepatic vein, which can also result in excessive blood loss. This may explain the greater blood loss and longer operation time in the LAR group. Several studies have demonstrated that increased intraoperative blood loss is significantly correlated with poor prognosis following hepatectomy for HCC (27-29). In this context, reducing intraoperative blood loss should be considered in order to improve the oncological outcomes.
In terms of long-term outcomes, the OS and RFS were not significantly different between the LAR and LNAR groups, but some differences in the recurrence pattern and SAR were detected. Early recurrence (≤2 years postoperatively) is associated with aggressive tumor biology (29). In this study, both groups showed an even distribution in the time to recurrence. Thus, we consider that the tumor biology was similar in both groups. However, there were differences in the pattern of recurrence and the type of post-recurrence treatment between the two groups. Intrahepatic recurrence, especially in the right lobe, was more prevalent in the LNAR group. One possible explanation is that remnant ischemia in the LNAR group may influence the intrahepatic recurrence after surgery (30). By comparison, recurrence in the left lobe and extrahepatic metastasis were more common in the LAR group. A recent meta-analysis found no significant difference in the type of recurrence between open AR and NAR (31). However, in our study, despite the similar tumor biology in both groups, the reason for the higher incidence of extrahepatic recurrence in the LAR group is unclear. It may be suggested that excessive blood loss may promote tumor spillage and hematogenous spread during the operation, which could accelerate recurrence (28,32,33). Alternatively, high volumes of blood loss may be associated with systemic hypotension and impaired organ delivery to vital organs. This is turn may promote systemic inflammation and a cytokine milieu that may impede antitumor immunity (28,34). Due to the different patterns of recurrence, the types of post-recurrence treatment differed between the two groups. The prognosis of recurrent HCC after surgery is strongly related to whether the patient is a candidate for local treatment of the lesion (35-37). According to a study by Shimada et al., the survival rate was higher in patients with recurrent HCC who underwent locoregional treatment (e.g., hepatectomy, ablation, and percutaneous ethanol injection) than in patients with systemic recurrence who were ineligible for local treatment (38). In this study, many patients in the LNAR group underwent locoregional treatment after recurrence. For this reason, although the 3-, and 5-year RFS rate of the LNAR group is relatively lower than in LAR group, the OS of the two groups was similar. The reason for this trend is thought to be the difference in treatment option after recurrence. The main benefit of LNAR is that the chance of performing multimodal treatment is higher on the tumor recurrence, and repeat resection can be performed.
There are several limitations in this study. First, this study includes its retrospective nature and that data were obtained from a single center. Although both groups were well balanced by applying PSM, the small sample size is a major limitation. A multicenter, prospective study is necessary to help reach a firm conclusion. Second, in most patients who underwent LNAR, the tumor size was less than 3 cm. Therefore, we performed subgroup analysis based on tumor size (Table S1). According to results, although the short-term outcomes, such as operation time, estimated blood loss and hospital stays were significantly higher in LNAR group, the surgical margin was significantly lower in LNAR group. Therefore, the results of this study appear to be applicable to relatively small-sized tumors.
Conclusions
In conclusion, our study has demonstrated that LNAR offers better short-term outcomes than LAR in terms of operation time, EBL, and length of hospital stay. Although, there were no significant differences in OS and RFS between LAR and LNAR, many patients in the LNAR group underwent treatment with a curative intent following recurrence. The present results suggest that LNAR can provide acceptable oncological outcome that are comparable to those of LAR for patients with HCC located in PS segments of the liver. LNAR can be considered for patients with relatively small-sized tumor located in PS segment when LAR is not feasible.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all the patients prior to treatment, and for their data to be used for research purposes. In accordance with guidelines for human subjects’ research, this retrospective study was approved by the institutional review board of Seoul National University Bundang Hospital, Seongnam, Korea, an academic hospital affiliated to Seoul National University, College of Medicine (Approval No. B-2021-633).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-578/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-578/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-578/coif). H.S.H. serves as an unpaid editorial board member of Hepatobiliary Surgery and Nutrition. J.Y.C. receives support (Grant No. 02-2021-046) from Seoul National University Bundang Hospital Research Fund. The other authors have no conflicts of interest to declare.
References
- 1.Kim JM, Kwon CH, Joh JW, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol 2014;21:458-65. 10.1245/s10434-013-3302-1 [DOI] [PubMed] [Google Scholar]
- 2.Ishii M, Mizuguchi T, Kawamoto M, et al. Propensity score analysis demonstrated the prognostic advantage of anatomical liver resection in hepatocellular carcinoma. World J Gastroenterol 2014;20:3335-42. 10.3748/wjg.v20.i12.3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang KJ, Ahn KS. Anatomical resection of hepatocellular carcinoma: A critical review of the procedure and its benefits on survival. World J Gastroenterol 2017;23:1139-46. 10.3748/wjg.v23.i7.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-50. [PubMed] [Google Scholar]
- 5.Torimura T, Iwamoto H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin Mol Hepatol 2021;27:236-45. 10.3350/cmh.2020.0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamura H, Matsuyama Y, Miyagawa Y, et al. Prognostic significance of anatomical resection and des-gamma-carboxy prothrombin in patients with hepatocellular carcinoma. Br J Surg 1999;86:1032-8. 10.1046/j.1365-2168.1999.01185.x [DOI] [PubMed] [Google Scholar]
- 7.Kaibori M, Kon M, Kitawaki T, et al. Comparison of anatomic and non-anatomic hepatic resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2017;24:616-26. 10.1002/jhbp.502 [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. 10.1097/SLA.0000000000001184 [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Jin YJ, Shin SK, et al. Surgery versus radiofrequency ablation in patients with Child- Pugh class-A/single small (≤3 cm) hepatocellular carcinoma. Clin Mol Hepatol 2022;28:207-18. 10.3350/cmh.2021.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korean Association for the Study of the Liver (KASL) . KASL clinical practice guidelines for liver cirrhosis: Varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol 2020;26:83-127. 10.3350/cmh.2019.0010n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu T, Honda G, Kurata M, et al. Perioperative and oncological outcomes of laparoscopic anatomical hepatectomy for hepatocellular carcinoma introduced gradually in a single center. Surg Endosc 2018;32:790-8. 10.1007/s00464-017-5745-0 [DOI] [PubMed] [Google Scholar]
- 12.Lee KF, Wong J, Cheung YS, et al. Resection margin in laparoscopic hepatectomy: a comparative study between wedge resection and anatomic left lateral sectionectomy. HPB (Oxford) 2010;12:649-53. 10.1111/j.1477-2574.2010.00221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon Y, Cho JY, Han HS, et al. Improved Outcomes of Laparoscopic Liver Resection for Hepatocellular Carcinoma Located in Posterosuperior Segments of the Liver. World J Surg 2021;45:1178-85. 10.1007/s00268-020-05912-5 [DOI] [PubMed] [Google Scholar]
- 14.Choi H, Han HS, Yoon YS, et al. Laparoscopic Anatomic Segment 6 Liver Resection Using the Glissonian Approach. Surg Laparosc Endosc Percutan Tech 2017;27:e22-5. 10.1097/SLE.0000000000000391 [DOI] [PubMed] [Google Scholar]
- 15.Yoon YS, Han HS, Cho JY, et al. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg Endosc 2010;24:1630-7. 10.1007/s00464-009-0823-6 [DOI] [PubMed] [Google Scholar]
- 16.Jang JY, Han HS, Yoon YS, et al. Three-Dimensional Laparoscopic Anatomical Segment 8 Liver Resection with Glissonian Approach. Ann Surg Oncol 2017;24:1606-9. 10.1245/s10434-017-5778-6 [DOI] [PubMed] [Google Scholar]
- 17.Lee B, Cho JY, Choi Y, et al. Laparoscopic liver resection in segment 7: Hepatic vein first approach with special reference to sufficient resection margin. Surg Oncol 2019;30:87-9. 10.1016/j.suronc.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 18.Lee W, Han HS, Yoon YS, et al. Role of intercostal trocars on laparoscopic liver resection for tumors in segments 7 and 8. J Hepatobiliary Pancreat Sci 2014;21:E65-8. 10.1002/jhbp.123 [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 20.Laurent A, Cherqui D, Lesurtel M, et al. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg 2003;138:763-9; discussion 769. 10.1001/archsurg.138.7.763 [DOI] [PubMed] [Google Scholar]
- 21.Troisi RI, Montalti R, Van Limmen JG, et al. Risk factors and management of conversions to an open approach in laparoscopic liver resection: analysis of 265 consecutive cases. HPB (Oxford) 2014;16:75-82. 10.1111/hpb.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Lopez V, Ome Y, Kawamoto Y, et al. Laparoscopic Liver Resection of Segments 7 and 8: from the Initial Restrictions to the Current Indications. J Minim Invasive Surg 2020;23:5-16. 10.7602/jmis.2020.23.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang L, Xiao L, Li J, et al. Safety and feasibility of laparoscopic hepatectomy for hepatocellular carcinoma in the posterosuperior liver segments. World J Surg 2015;39:1202-9. 10.1007/s00268-015-2946-3 [DOI] [PubMed] [Google Scholar]
- 24.Teo JY, Kam JH, Chan CY, et al. Laparoscopic liver resection for posterosuperior and anterolateral lesions-a comparison experience in an Asian centre. Hepatobiliary Surg Nutr 2015;4:379-90. 10.3978/j.issn.2304-3881.2015.06.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Liu F, Hao X, et al. Laparoscopically anatomical versus non-anatomical liver resection for large hepatocellular carcinoma. HPB (Oxford) 2020;22:136-43. 10.1016/j.hpb.2019.06.008 [DOI] [PubMed] [Google Scholar]
- 26.Sasaki K, Shindoh J, Margonis GA, et al. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg 2017;152:e165059. 10.1001/jamasurg.2016.5059 [DOI] [PubMed] [Google Scholar]
- 27.Yang T, Zhang J, Lu JH, et al. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg 2011;35:2073-82. 10.1007/s00268-011-1161-0 [DOI] [PubMed] [Google Scholar]
- 28.Katz SC, Shia J, Liau KH, et al. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg 2009;249:617-23. 10.1097/SLA.0b013e31819ed22f [DOI] [PubMed] [Google Scholar]
- 29.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-7. 10.1016/s0168-8278(02)00360-4 [DOI] [PubMed] [Google Scholar]
- 30.Cho JY, Han HS, Choi Y, et al. Association of Remnant Liver Ischemia With Early Recurrence and Poor Survival After Liver Resection in Patients With Hepatocellular Carcinoma. JAMA Surg 2017;152:386-92. 10.1001/jamasurg.2016.5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan Y, Zhang W, Jiang L, et al. Efficacy and safety of anatomic resection versus nonanatomic resection in patients with hepatocellular carcinoma: A systemic review and meta-analysis. PLoS One 2017;12:e0186930. 10.1371/journal.pone.0186930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FISHER ER , TURNBULL RB Jr. The cytologic demonstration and significance of tumor cells in the mesenteric venous blood in patients with colorectal carcinoma. Surg Gynecol Obstet 1955;100:102-8. [PubMed] [Google Scholar]
- 33.Rajendran L, Ivanics T, Claasen MP, et al. The management of post-transplantation recurrence of hepatocellular carcinoma. Clin Mol Hepatol 2022;28:1-16. 10.3350/cmh.2021.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jubert AV, Lee ET, Hersh EM, et al. Effects of surgery, anesthesia and intraoperative blood loss on immunocompetence. J Surg Res 1973;15:399-403. 10.1016/0022-4804(73)90110-8 [DOI] [PubMed] [Google Scholar]
- 35.Byeon J, Cho EH, Kim SB, et al. Extrahepatic recurrence of hepatocellular carcinoma after curative hepatic resection. Korean J Hepatobiliary Pancreat Surg 2012;16:93-7. 10.14701/kjhbps.2012.16.3.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahn KS, Kang KJ. Appropriate treatment modality for solitary small hepatocellular carcinoma: Radiofrequency ablation vs. resection vs. transplantation? Clin Mol Hepatol 2019;25:354-9. 10.3350/cmh.2018.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyayama S. Ultraselective conventional transarterial chemoembolization: When and how? Clin Mol Hepatol 2019;25:344-53. 10.3350/cmh.2019.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimada M, Takenaka K, Gion T, et al. Prognosis of recurrent hepatocellular carcinoma: a 10-year surgical experience in Japan. Gastroenterology 1996;111:720-6. 10.1053/gast.1996.v111.pm8780578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as