Abstract
Immunofluorescence microscopy was used to study the establishment of compartment-specific transcription during sporulation in Bacillus subtilis. Analysis of the distribution of the anti-anti-sigma factor, SpoIIAA, in a variety of mutant backgrounds supports a model in which the SpoIIE phosphatase, which activates SpoIIAA by dephosphorylation, is sequestered onto the prespore face of the asymmetric septum. Thus, prespore-specific gene expression apparently arises as a result of the compartmentalization of SpoIIE protein. The results also suggest the existence of at least two compartment-specific programs of proteolysis, one dependent on the mother cell-specific sigma factor ςE and the other dependent on the prespore-specific sigma factor ςF.
Spore formation by the gram-positive bacterium Bacillus subtilis has been extensively studied for many years as a simple example of cellular development and differentiation (reviewed in references 10 and 39). The first overt morphological change associated with sporulation is the formation of a highly asymmetric septum that produces a large mother cell and a much smaller prespore. Different, coordinated programs of gene expression are then initiated in the two compartments.
Differential gene expression is controlled by four sigma factors, two of which are active in the prespore (ςF and ςG) and two in the mother cell (ςE and ςK). These sigma factors become active sequentially and alternately in the two cells. The correct activation of ςF in the prespore is of particular importance because it is required, directly or indirectly, for the activation of all of the later sigma factors. ςF is encoded by the third gene, spoIIAC, of the spoIIA operon (15), which is strongly induced before asymmetric septation (13, 28, 42). Thus, ςF is present throughout the cell before asymmetric septation (16, 21, 27). The products of the two genes upstream of spoIIAC (spoIIAA and spoIIAB) are important regulators of ςF activity (28, 34). SpoIIAB is an anti-sigma factor which can bind to ςF, preventing it from interacting with core RNA polymerase (9, 24). SpoIIAB can also specifically phosphorylate and inactivate the SpoIIAA protein (6, 23–25). Conversely, binding of nonphosphorylated, active SpoIIAA to SpoIIAB leads to the release of ςF activity (1, 6, 8).
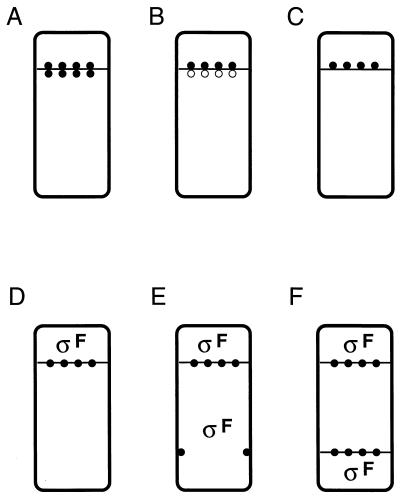
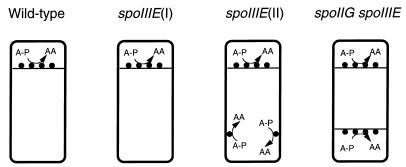
Inactive SpoIIAA-P can be restored to activity by the action of a specific phosphatase, SpoIIE (2, 7, 14). This phosphatase is a bifunctional protein that is also required for proper formation of the asymmetric septum (5, 14). Consistent with its possible role in coupling ςF activation to septation, SpoIIE protein is targeted to the potential sites of asymmetric division at both poles of the cell early in sporulation (3, 4). Later, the protein disappears, first from the prespore-distal pole of the sporangium in a ςE-dependent manner (31), then from the prespore pole itself. An important unresolved question concerns whether (and if so, how) SpoIIE phosphatase activity is regulated to bring about prespore-specific release of ςF. There are essentially three ways in which this might work (Fig. 1) (7, 14). First, SpoIIE might be active on both sides of the asymmetric septum, in which case the smaller size of the prespore compartment could lead to more active dephosphorylation of SpoIIAA-P (Fig. 1A). Second, SpoIIE activity could be distributed on both sides of the septum but regulated so as to be active only on the prespore face of the septum (Fig. 1B). Third, the protein could be exclusively localized to the prespore face of the septum (Fig. 1C). Recent results provide strong support for the third model (46).
FIG. 1.
(A to C) Schematic illustrations of the possible distribution of SpoIIE after asymmetric septation (adapted from Feucht et al. [14]). (A) SpoIIE localizes and is active on both sides of the asymmetric septum. (B) SpoIIE localizes to both sides of the asymmetric septum but is active only in the prespore. (C) SpoIIE is restricted to the prespore face of the asymmetric septum. Solid circles, active SpoIIE; open circles, inactive SpoIIE. (D to F) Cell morphology and localization of ςF activity in spoIIIE(I) (D), spoIIIE(II) (E), and spoIIG (F) mutant cells. Septa are shown as horizontal lines across the cells, and SpoIIE is shown as solid circles on the septa or at the position of the second potential septum in the spoIIIE(II) mutant.
In a previous study we used immunofluorescence microscopy to examine how ςF and its regulatory proteins, SpoIIAA (i.e., the nonphosphorylated, active form of the protein), SpoIIAA-P, and SpoIIAB, are distributed in wild-type sporulating cells (21). The main conclusion from that work was that the release of ςF activity was closely correlated with the accumulation of SpoIIAA in the prespore compartment. Here we describe the application of similar methods to various sporulation mutants, aiming to distinguish between different possible models of SpoIIE action.
spoIIIE mutants are defective in prespore chromosome segregation. In such cells, only a small segment of the chromosome (about 30%, centered near oriC) is present in the prespore (43, 44). Curiously, spoIIIE mutations can be divided into two distinct classes on the basis of secondary effects on ςF activity (43, 47). In class I mutants [e.g., spoIIIE36(I)], ςF-dependent genes that lie within the oriC region are transcribed but those that lie outside this region are not transcribed (40, 43, 44). Therefore, ςF is activated, as in wild-type cells, only in the prespore (Fig. 1D). In class II mutants [e.g., spoIIIE604(II) and spoIIIE647(II)], however, ςF activity becomes delocalized and is therefore detectable in both mother cell and prespore compartments (Fig. 1E) (43). Consequently, ςF-dependent reporter genes at any location in the chromosome can be expressed. In addition, Pogliano et al. (31) have recently analyzed the distribution of SpoIIE phosphatase in spoIIIE mutants and found that in spoIIIE(I) mutants the pattern was essentially normal, whereas in spoIIIE(II) mutants SpoIIE persists both at the asymmetric septum and at the opposite pole of the sporangium (Fig. 1D and E).
A second class of mutations we have exploited in this work lies in the spoIIG operon, encoding the precursor of ςE and its proteolytic activator, SpoIIGA (19, 38). These mutations produce a “disporic” phenotype, in which prespore-like cells are formed at both poles of the sporulating cell as a result of the failure of ςE to become active in the mother cell (18, 22, 29, 30). The distribution of SpoIIE has also been determined in these mutants as well as in spoIIG spoIIIE double mutants (Fig. 1F) (31).
Analysis of the distribution of ςF and its regulatory proteins in several mutant strains strongly supports the recent findings of Wu et al. (46) that SpoIIE phosphatase becomes sequestered to the prespore face of the asymmetric septum. Additionally, we provide further evidence for the existence of compartment-specific programs of protein degradation (21). These could play important roles in fixing the differential gene expression of the prespore and mother cell types.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used are listed in Table 1. All are isogenic with SG38. Cells were grown and resuspended to induce sporulation as described previously (26, 27, 36).
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype | Source or construction |
|---|---|---|
| B. subtilis strains | ||
| SG38 | trpC2 | 13 |
| 36.3 | trpC2 spoIIIE36(I) | 13 |
| 604.6 | trpC2 spoIIIE604(II) | 47 |
| 647 | trpC2 Ω(spoIIIE::aphA-3)647(II) | 43 |
| 713 | trpC2 Ω(spoIVA′-lacZ cat)713 | 37 |
| 902 | trpC2 spoIIIE36(I) Ω(spoIIGA::aphA-3)901 | 43 |
| 919 | trpC2 spoIIIE604(II) Ω(spoIIGA::aphA-3)901 | 44 |
| 930 | trpC2 spoIIIE36 Ω(amyE::gpr′-′lacZ cat) | 43 |
| 940 | trpC2 spoIIIE36 Ω(spoIVA′-lacZ cat)713 | 36.3 transformed with DNA from 713 |
| 941 | trpC2 (spoIIIE::aphA-3)647(II) (spoIVA′-lacZ cat)713 | 647 transformed with DNA from 713 |
| 1216 | trpC2 Ω(spoIIIE::aphA-3)647(II) chr::pPS1395 (gpr′-′lacZ cat) | 647 transformed with pPS1395 |
| 1218 | trpC2 Ω(spoIIGA::aph-A3)901 spoIIIE36(I) Ω(amyE::gpr′-′lacZ cat) | 902 transformed with pPS1326 |
| 1219 | trpC2 Ω(spoIIGA::aph-3)901 spoIIIE604(II) Ω(amyE::gpr′-′lacZ cat) | 919 transformed with pPS1326 |
| Plasmids | ||
| pPS1326 | bla cat amyE::gpr′-′lacZ | 40 |
| pPS1395 | bla cat gpr′-′lacZ | 41 |
Antibodies and immunofluorescence.
Antibodies were prepared, their specificities were verified, and they were used for cytological staining as described by Lewis et al. (21).
Epifluorescence microscopy and image analysis.
Epifluorescence microscopy was performed as described by Lewis et al. (21). Twelve-bit images were captured with a 1,200- by 800-pixel, 22-μm-pitch, cooled, charge-coupled device (spoIIIE single mutant data) or a 1,536- by 1,024-pixel, 9-μm-pitch, cooled, charge-coupled device (spoIIG spoIIIE double mutant data), both from Digital Pixel Advanced Imaging Systems, Brighton, United Kingdom. The exposure times were the same as those described by Lewis et al. (21) for single mutant data. For double mutant data, fluorescein isothiocyanate images were acquired with a 5-s exposure, and Cy3 and DAPI (4′,6-diamidino-2-phenylindole) images were acquired with a 2-s exposure. The single mutant data was acquired with Lucida version 2.x software (Kinetic Imaging). The double mutant data was acquired with IPLab Spectrum version 3.1.1 software (Signal Analytics Corporation, Vienna, Va.). All images were analyzed as described previously by Lewis et al. (21) and prepared for publication with Adobe Photoshop and PowerPoint.
Enzyme assays.
β-Galactosidase activity was measured by the method of Errington and Mandelstam (13). One unit of β-galactosidase catalyzes the production of 1 nmol of 4-methylumbelliferone per min under the standard conditions. Alkaline phosphatase (APase) activity was measured as described by Errington and Mandelstam (12). One unit of APase catalyzes the production of 1 nmol of o-nitrophenol per min under the standard reaction conditions.
RESULTS
Experimental protocol.
As described previously (21), affinity-purified antibodies from polyclonal antisera were used to determine the subcellular distributions of ςF, SpoIIAB, SpoIIAA-P, and SpoIIAA. To correlate the pattern of distribution of the protein with the developmental state of the cell, two additional cytological markers were used. First, the DNA-specific fluorescent dye DAPI was used to reveal the characteristic changes in chromosome morphology that occur during sporulation (17, 35, 47). Second, anti-β-galactosidase antibodies were used to detect the expression of a ςF-dependent gpr-lacZ fusion (present in all of the strains used), so that ςF activity could be monitored (see Materials and Methods) and as a control to check cell permeabilization in the absence of a signal for the other proteins. It should be noted that in spoIIIE mutants, ςG (which can also direct transcription of gpr) is not active (45).
Compartmentalized accumulation of nonphosphorylated SpoIIAA is coincident with ςF activity in spoIIIE mutant cells.
Since the two classes of spoIIIE mutations have very different effects on the localization of ςF activity (see the introduction), studies of the distribution of ςF and its regulatory proteins could be useful in further understanding the mechanisms involved in ςF activation. B. subtilis 930 [spoIIIE(I)] and 1216 [spoIIIE(II)] were induced to sporulate, and samples were collected at intervals for analysis by immunofluorescence microscopy. The frequency of initiation of sporulation (as judged by DNA morphology) and the proportion of cells with active ςF (i.e., having detectable β-galactosidase signals) increased with time, in accordance with our previous observation of sporulating wild-type cultures (reference 21 and data not shown). Table 2 shows the results of a detailed analysis of samples collected 3 h after the induction of sporulation (t3), when all of the different classes of cells can be readily detected (21). In the spoIIIE(I) mutant, the β-galactosidase signal was restricted to prespores (Fig. 2A), in accordance with the correct activation of ςF in the prespore (43). In addition, the behavior of the nonphosphorylated form of SpoIIAA was very similar to that of wild-type cells (21). Thus, most of the cells with active ςF showed a significantly greater SpoIIAA signal in the prespore than in the mother cell (Fig. 2A). The remaining cells had a relatively weak signal, which either covered both the prespore and mother cell compartments (column “M+P” in Table 2) or, in predivisional cells, covered the whole cell (not shown).
TABLE 2.
Distribution of ςF, SpoIIAB, SpoIIAA-P, and SpoIIAA in spoIIIE(I) and spoIIIE(II) mutants and spoIIG spoIIIE double mutantsa
| Mutation | Protein | Total no. of cells counted | % Sporulation | % β-Galactosidase | Location of antibody signalb
|
||
|---|---|---|---|---|---|---|---|
| Preseptational cells (%)c | M+P (%)c,d | Prespore (%) | |||||
| Wild typee | ςF | 874 | 79 | 63 | 12 | 53 | 12 |
| SpoIIAB | 650 | 78 | 62 | 11 | 39 | 0 | |
| SpoIIAA-P | 662 | 78 | 63 | 7 | 56 | 0 | |
| SpoIIAA | 426 | 80 | 64 | 13 | 23 | 53 | |
| spoIIIE(I) | ςF | 332 | 79 | 63 | 19 | 38 | 37 |
| SpoIIAB | 319 | 79 | 69 | 18 | 30 | 47 | |
| SpoIIAA-P | 129 | 74 | 58 | 24 | 69 | 1.6 | |
| SpoIIAA | 226 | 73 | 57 | 25 | 23 | 50 | |
| spoIIIE(II) | ςF | 237 | 75 | 57 | 20 | 69 | 0 |
| SpoIIAB | 227 | 80 | 60 | 19 | 71 | 0.9 | |
| SpoIIAA-P | 239 | 78 | 56 | 13 | 74 | 0 | |
| SpoIIAA | 297 | 74 | 58 | 20 | 71 | 1.7 | |
| spoIIG spoIIIE(I) | ςF | 563 | 75 | 55 | 25 | 68 (3) | 7 |
| SpoIIAB | 443 | 76 | 55 | 23 | 55 (16) | 21 | |
| SpoIIAA-P | 432 | 75 | 56 | 19 | 75 (16) | 0 | |
| SpoIIAA | 404 | 76 | 61 | 23 | 30 (0) | 47 | |
| spoIIG spoIIIE(II) | ςF | 444 | 75 | 55 | 22 | 63 (3) | 12 |
| SpoIIAB | 293 | 77 | 57 | 21 | 55 (8) | 22 | |
| SpoIIAA-P | 314 | 84 | 66 | 12 | 80 (24) | 0 | |
| SpoIIAA | 207 | 82 | 69 | 18 | 29 (0) | 53 | |
Cells of strains 930 [spoIIIE(I)], 1216 [spoIIIE(II)], 1218 [spoIIG spoIIIE(I)], and 1219 [spoIIG spoIIIE(II)] were induced to sporulate and were sampled 3 h after resuspension. All percentage values represent the percentage of the total number of cells counted. The sporulation frequency (cells having reached at least the stage of septation) was determined from DAPI-stained images of the nucleoids. Cells containing active ςF were scored on the basis of having a detectable fluorescent signal for β-galactosidase (from the gpr-lacZ reporter).
Preseptational cells, whole-cell fluorescence in cells with a preseptational (i.e., nonseptate) nucleoid distribution; M+P, fluorescence approximately equal over the prespore and mother cell compartments; prespore, signal significantly greater in the prespore than in the mother cell.
The signal for SpoIIAA was weak in preseptational and M+P cells, except for M+P in the spoIIIE(I) mutant (see text).
The frequency of a subset of spoIIG spoIIIE double mutant cells in which the signal in the central compartment was stronger in the prespores is shown in parentheses.
Data for the wild-type cells were taken from Lewis et al. (21).
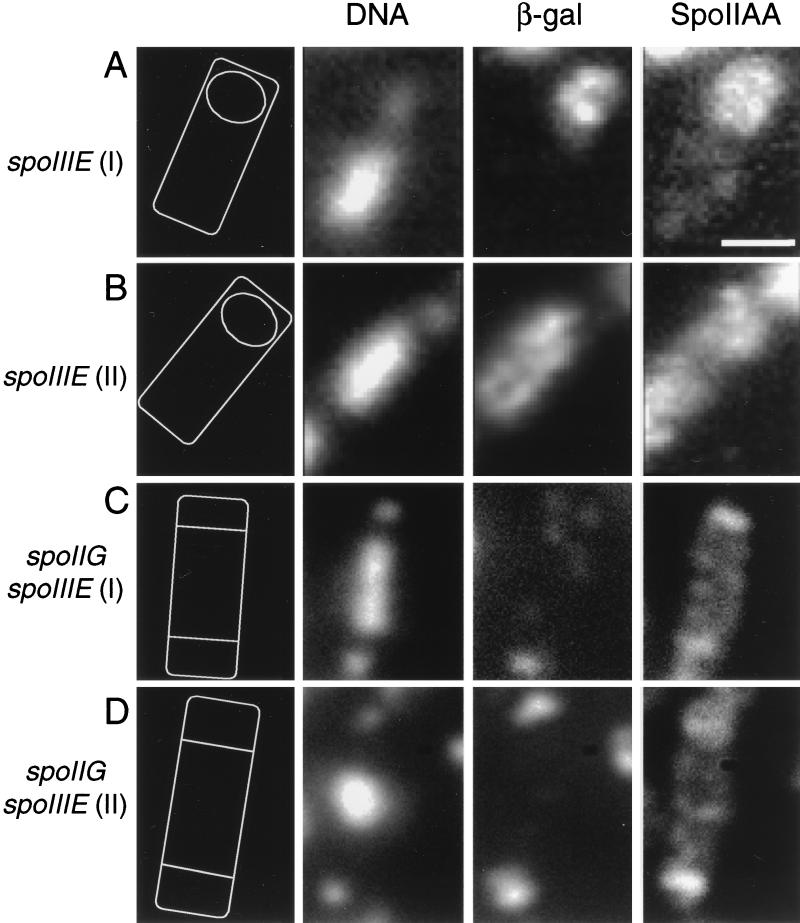
FIG. 2.
Distribution of SpoIIAA in spoIIIE and spoIIG spoIIIE mutants at t3 of sporulation. (A) Strain 930 [spoIIIE(I)]; (B) strain 1216 [spoIIIE(II)]; (C) strain 1218 [spoIIG spoIIIE(I)]; (D) strain 1219 [spoIIG spoIIIE(II)]. From left to right, the panels show a diagrammatic representation of the mother cell and prespore orientation, DNA, β-galactosidase, and SpoIIAA. Bar, 1 μm.
In the spoIIIE(II) mutant, however, almost all of the sporulating cells showed a strong SpoIIAA signal throughout the cell (Fig. 2B and Table 2) rather than restricted to the prespore. This accumulation of SpoIIAA throughout the cell in spoIIIE(II) mutants reflects the delocalized ςF activity observed in this strain and reinforces the previously observed close correlation between the accumulation of SpoIIAA and the release of ςF activity. Since Pogliano et al. (31) showed that the SpoIIE ring at the prespore-distal end of the cell persists in spoIIIE(II) mutants, rather than being degraded, the delocalization of SpoIIAA in this strain could be due to SpoIIE activity in the mother cell. (Note that the β-galactosidase signal is seen only in the mother cell because of the location of the reporter gene [43].)
Dephosphorylation of SpoIIAA is restricted to the prespore compartment in both classes of spoIIIE mutants in the presence of a spoIIG mutation.
To try to clarify why the two classes of spoIIIE mutations had different effects on SpoIIAA dephosphorylation in the mother cell, we repeated the experiments with both classes of spoIIIE mutations in a spoIIG background. This genetic background was useful for two reasons. First, the developmentally regulated degradation of the SpoIIE phosphatase that normally occurs in the mother cell is blocked, because it depends on ςE, encoded by the spoIIG operon (31). Second, the SpoIIE ring at the prespore-distal pole is “processed” by formation of a second polar septum, so that activation or sequestration of SpoIIE at that pole should occur in the same manner as at the first polar septum. Interestingly, in contrast to the disparate results obtained with the spoIIIE single mutants, the distribution patterns of SpoIIAA in the two double mutant strains were indistinguishable (compare Fig. 2A and B with C and D). SpoIIAA was enriched in the prespore compartments of the majority of the sporulating cells (Table 2 and Fig. 2C and D). Thus, it appears that in both strains, most of the phosphatase activity is now restricted to the prespores and the “aberrant” dephosphorylation of SpoIIAA that occurs in the mother cell compartment of spoIIIE(II) mutants is greatly reduced. Most likely the spoIIG mutation has this effect because it leads to formation of the second polar septum and this sequesters the SpoIIE phosphatase out the central compartment (46) (see Discussion).
As described previously for spoIIG single mutants (22), we found that ςF was activated in both of the prespores, as seen by the expression of β-galactosidase from the gpr-lacZ fusion situated at the amyE locus (Fig. 2C and D). In similar strains with the gpr-lacZ fusion at its normal chromosomal position and thus located in the central compartment (43), no ςF activity was detected (45, 46), in accordance with our finding only low levels of SpoIIAA in this compartment. We noticed that the β-galactosidase signals in these cells tended to lie closer to the cell poles than the main part of SpoIIAA signal (this is especially apparent in Fig. 2D). It seems possible that this is related to the localization of the SpoIIE phosphatase, which generates the unphosphorylated SpoIIAA, at the septum.
Changes in the patterns of protein turnover in spoIIIE mutant cells.
It was important to determine that the other proteins involved in the regulation of ςF were not responsible for the effects on ςF activity seen in the various mutant strains. The distributions of ςF, SpoIIAB, and SpoIIAA-P in cells of the spoIIIE(I) mutant are summarized in Table 2, and examples of the predominant staining patterns are shown in Fig. 3. These cells did show some differences from wild-type cells, particularly in that ςF and SpoIIAB both appeared to persist in the prespore while they disappeared from the mother cell (Fig. 3A and B and Table 2). In the wild type, these signals, particularly that of SpoIIAB, had disappeared from both compartments by t3. Therefore, it appears that there are separate programs for degrading various proteins in the mother cell and prespore and that in a spoIIIE(I) mutant the prespore-specific program fails to be activated (see Discussion).
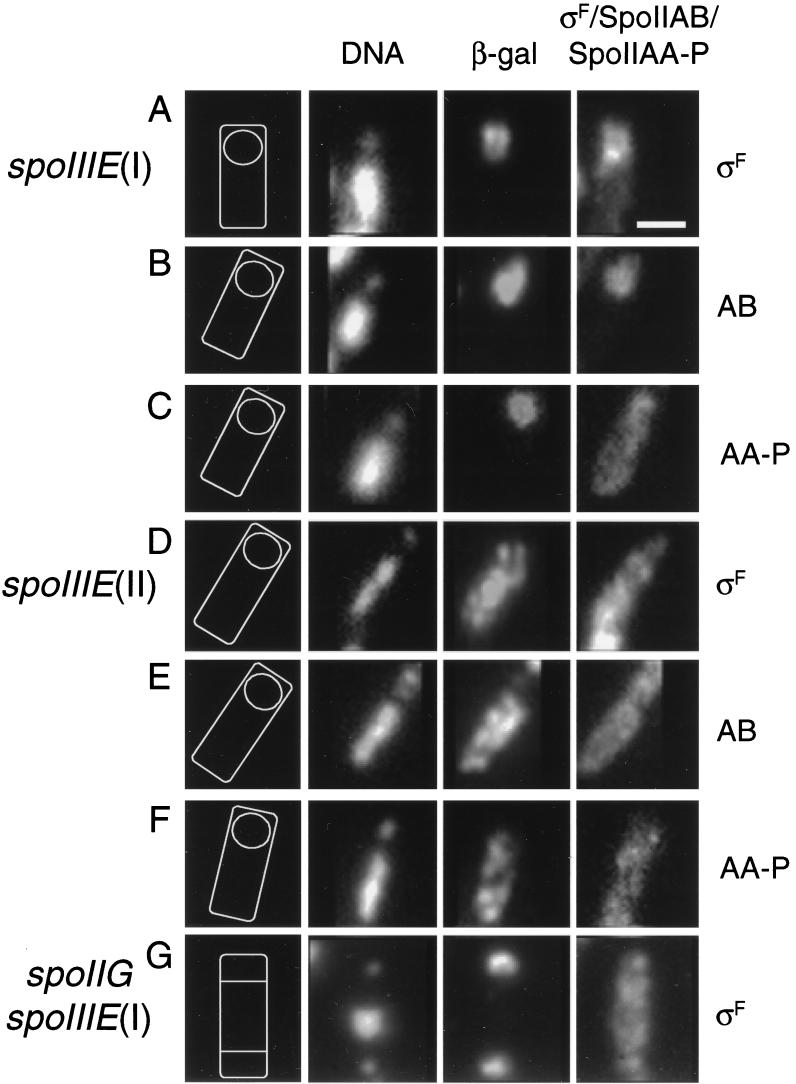
FIG. 3.
Distribution of ςF, SpoIIAB, and SpoIIAA-P in typical sporulating cells of spoIIIE single mutants and spoIIG spoIIIE double mutants (t3). (A to C) Strain 930 [spoIIIE(I)]; (D to F) strain 1216 [spoIIIE(II)]; (G) strain 1218 [spoIIG spoIIIE(I)]. (Strain 1219 gave indistinguishable results; see text.) The cells in panels A, D, and G were stained for ςF; the cells in panels B and E were stained for SpoIIAB; and the cells in panels C and F were stained for SpoIIAA-P. The diagrams and the organization of the panels are explained in the legend to Fig. 2. Bar, 1 μm.
In a spoIIIE(II) mutant the staining patterns for ςF, SpoIIAB, and SpoIIAA-P were noncompartmentalized (Fig. 3D to F and Table 2). Although this might appear to be a pattern of distribution similar to that of the wild type, unlike in the wild type, the proteins tended to persist in both compartments (compare the data in columns “M+P” and “% Sporulation” for the two strains in Table 2).
Most interestingly, in spoIIG spoIIIE double mutants, the distributions of all three proteins, ςF, SpoIIAB, and SpoIIAA-P, were the same irrespective of the type of spoIIIE mutation (Table 2). Nearly all of the cells retained a signal irrespective of the protein examined, suggesting that protein turnover is generally reduced compared with that of the wild type. The intensity of fluorescence did seem to decrease moderately from t1.5 to t3 (not shown), but nearly all of the cells still had a clearly detectable signal at t3. In general, ςF, SpoIIAB, and SpoIIAA-P were distributed equally in all compartments (Fig. 3G and Table 2), although two minor classes of compartmentalization could be detected. In the first (values in parentheses in the “M+P” column of Table 2), the signal was stronger in the central compartment than in the prespores. In the second, (column “Prespore” of Table 2), the signal was stronger in the prespores than in the central compartment. The distribution of SpoIIAA-P was either equal in all three compartments or stronger in the central compartment than in the prespores. No cells were observed with a stronger prespore signal (Table 2). The relatively high proportion of cells with a mother cell-enriched signal for this protein (Table 2) would be consistent with the phosphatase activity of SpoIIE being restricted to the prespores.
Taken together, these results suggest that these single and double mutants are deficient in the developmentally regulated degradation of several different proteins.
Reduced ςE activity in the mother cell compartment of spoIIIE(II) cells.
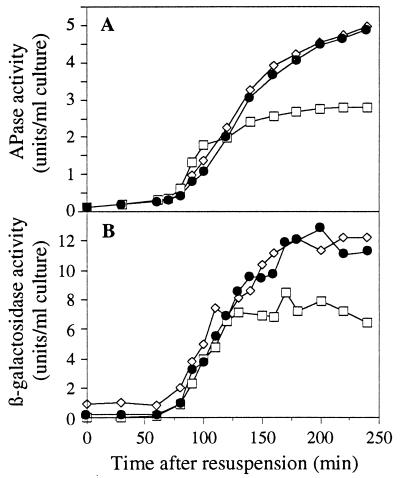
The reduction in protein turnover in the mother cell compartment of spoIIIE(II) mutants mentioned above was reminiscent of the previous report that the SpoIIE ring at the prespore-distal pole of the cell (i.e., in the mother cell) is retained in spoIIIE(II) mutants (31). The most likely explanation for a general reduction in protein turnover in the mother cell would be that it fails to synthesize or activate one or more proteases. Such events would most likely be controlled by the mother cell-specific sigma factor, ςE. To test whether spoIIIE(II) mutants were affected for ςE activity, we examined the effects of both classes of spoIIIE mutations on two mother cell-specific reporter genes. As shown in Fig. 4, spoIIIE(II) mutants showed a significant reduction in expression of both APase (a “natural” reporter enzyme [11]) and β-galactosidase from a spoIVA-lacZ fusion (33, 37), whereas spoIIIE(I) mutants were virtually indistinguishable from the wild type. It appeared that the effect was most marked in later sporulating cells than in early ones, for reasons that are not yet understood. However, it may be significant that the aberrant activation of ςF in the mother cell compartment of spoIIIE(II) mutants also seems to occur relatively late (20, 45). We conclude that spoIIIE(II) mutants are deficient in ςE activation and that ςE most likely controls the turnover of a number of proteins in the mother cell compartment.
FIG. 4.
Reduced ςE activity in a spoIIIE(II) mutant. (A) APase activity in sporulating cultures of strains SG38 (spoIIIE+) (solid circles), 36.3 [spoIIIE(I)] (open diamonds), and 647 [spoIIIE(II)] (open squares). (B) β-Galactosidase activity in sporulating cultures of strains 713 (spoIIIE+) (solid circles), 940 [spoIIIE(I)] (open diamonds), and 941 [spoIIIE(II)] (open squares).
DISCUSSION
Further correlation between the accumulation of nonphosphorylated SpoIIAA and ςF activation.
We have previously shown that in wild-type cells there is a strong correlation between the accumulation of SpoIIAA in the prespore and activation of ςF in that compartment (21). Analysis of spoIIIE(I) cells showed that SpoIIAA accumulated in the prespore, consistent with the correct compartmentalization of ςF activation in this mutant. This correlation was extended further when a spoIIIE(II) mutant was examined. In this mutant, ςF activation is delocalized, occurring in the mother cell and the prespore, and as is shown clearly in Fig. 2 and Table 2, the nonphosphorylated form of SpoIIAA was also delocalized in this strain. Surprisingly, when combined with a spoIIG mutation, resulting in a disporic phenotype, ςF activity became correctly localized to the prespore compartments, irrespective of the type of spoIIIE mutation (Fig. 2) (46). Analysis here of the accumulation of SpoIIAA showed that in both cases, it was now mainly restricted to the prespores. Thus, again, there is a close correlation between SpoIIAA accumulation and ςF activation.
The data obtained with the spoIIIE(I) mutant strain also indicate that the accumulation of SpoIIAA in the prespore cannot be due to de novo synthesis, as the structural gene for this protein, spoIIAA, lies at 208° (30), well outside the segment of the chromosome that is trapped in the prespores of spoIIIE mutants (43, 44). Thus, in the mutant, SpoIIAA must be generated by dephosphorylation, presumably by the SpoIIE phosphatase. It follows that this is also likely to be the primary origin of the nonphosphorylated SpoIIAA in the prespores of wild-type sporulating cells. How then is SpoIIE activity regulated so that SpoIIAA dephosphorylation occurs only in the prespore?
SpoIIE is restricted to the prespore face of the asymmetric septum.
The results described above, together with those of Pogliano et al. (31), support the recent results showing that SpoIIE is sequestered to the prespore face of the asymmetric septum (44). In the model shown in Fig. 5, SpoIIE is inherently active (i.e., not regulated), but on septation it is sequestered on the prespore face of the asymmetric septum. This model accounts for the distributions of SpoIIAA in all of the mutant backgrounds, as well as in the wild type. spoIIIE(I) mutants behave similarly to the wild type. In spoIIIE(II) mutants, we assume that the SpoIIE remaining at the prespore-distal pole of the cell could generate sufficient SpoIIAA to activate ςF. Indeed, the fact that ςF activation in the mother cell is slightly delayed in these mutants (20, 45) could be because it takes SpoIIE longer to generate a sufficiently large pool of SpoIIAA in the much larger mother cell. More importantly, this model explains why SpoIIAA accumulation was observed predominantly in the prespores of spoIIG spoIIIE double mutants, since there is no phosphatase in the central compartment. On the basis of these arguments, the most likely explanation for the compartmentalization of ςF activity is that it is driven by sequestration of the SpoIIE phosphatase to the prespore face of the asymmetric septum. Direct microscopic observation of protoplasts made from sporulating cells containing a SpoIIE-GFP fusion are consistent with this idea (46).
FIG. 5.
Proposed explanation for the distribution of SpoIIAA in wild-type and mutant strains. We assume that SpoIIE (solid circles) is restricted to the prespore after asymmetric septation. The curved arrow indicates the generation of SpoIIAA (AA) from SpoIIAA-P (A-P) by SpoIIE phosphatase activity.
An important issue arising from the notion that SpoIIE is regulated only by sequestration concerns its presumptive activity in the predivisional cell and at the prespore-distal pole soon after septation. We previously noted a small, but reproducibly detectable, signal for SpoIIAA in the mother cell during sporulation (Fig. 2) (21). Presumably, in wild-type cells, this small amount of SpoIIAA is not sufficient to activate ςF, and after septation, ςE-dependent degradation of SpoIIE in the mother cell helps to prevent further accumulation of SpoIIAA, and therefore ςF, from being inappropriately activated in these cells. In spoIIG spoIIIE double mutants the slightly stronger signal for SpoIIAA in the central compartment (compare Fig. 2A with C and D) is probably due to phosphatase activity in the mother cell prior to the formation of the second prespore (since the two prespores form sequentially [22]), but the level of SpoIIAA generated is presumably still less than is required for activation of ςF.
The model shown in Fig. 5 also helps to explain the loss of SpoIIE from sporulating cells of a spoIIG mutant, as observed previously by Arigoni et al. (3) and Pogliano et al. (31). If SpoIIE were distributed on both faces of the septum, the protein present in the central compartment of such cells should persist, since SpoIIE degradation in the mother cell requires ςE (31). The disappearance of SpoIIE in spoIIG mutants would be expected if the protein is all sequestered in the prespore compartments, where it could be degraded in a ςF-dependent manner. Following from this, the retention of SpoIIE in the prespores of spoIIIE mutants could be due to the fact that the putative protease-encoding gene(s) transcribed by ςF lies within the region of the chromosome that does not enter the prespore in these mutants, so the protease is not made in that compartment.
Irrespective of these detailed arguments, it is clear that restriction of SpoIIE to the prespore face of the asymmetric septum, thus abruptly concentrating it in a small compartment, could provide a strong, spatially localized signal triggering the initial release of ςF in the prespore and leading to the establishment of differential transcription in the two cells.
Compartment-specific pathways of protein degradation during sporulation.
The results discussed above, together with those described previously by Pogliano et al. (31), strongly suggest the existence of independent compartment-specific pathways of degradation of SpoIIE. Further evidence for the existence of compartment-specific degradation pathways is also apparent from the distributions of the other proteins examined in these studies and previously (21). In spoIIIE(I) mutants, ςF and SpoIIAB signals disappeared at a much greater rate from the mother cell than from the prespore, leading to an abundance of cells with stronger prespore signals. This contrasts with the results observed for wild-type cells, in which ςF and SpoIIAB disappeared at approximately equal rates from both compartments (21). In spoIIIE(I) mutants, ςE is activated normally (43), and ςF, SpoIIAB, and SpoIIE are all degraded in the mother cell. However, in these mutants the putative ςF-dependent protease gene(s) presumably does not enter the prespore compartment, so ςF and SpoIIAB persist. It should be noted that the loss of ςF from the prespores of wild-type cells coincides more or less with the appearance of the late prespore-specific sigma factor, ςG. It is possible that a ςG-dependent protease is responsible for ςF turnover in the prespore while a ςE-dependent protease turns over ςF in the mother cell. The same argument could also be applied to the turnover of SpoIIAB. However, turnover of SpoIIAB by a ςF-dependent protease could be important for the correct activation of ςG, as SpoIIAB is also thought to regulate the activity of ςG (32).
In spoIIIE(II) mutants, ςF and ςE activities are delocalized and all of the proteins examined tend to be retained in both compartments. Thus, not only is the prespore-specific degradation pathway lost, as in the spoIIIE(I) mutant, but mother cell-specific protein turnover is also reduced. The results shown in Fig. 4 provide a possible explanation by demonstrating that spoIIIE(II) mutants have reduced ςE activity, as well as aberrant ςF activity in the mother cell. It is not yet clear which of these effects is the primary one, if indeed they are interdependent.
In a spoIIG background, ςE activity is abolished, so both compartment-specific protease pathways should be abolished, irrespective of the type of spoIIIE mutation present. This was supported by the low (and approximately equal) rates of protein turnover throughout the three compartments in these cells.
Since SpoIIAA differs from all of the other proteins in its continued accumulation during development of the wild type, it is likely that specific sequence motifs identify those proteins that are to be degraded (or left intact). The availability of several mutants, differing in the extent to which these pathways are activated, should facilitate identification of the genes encoding some of the putative proteases and allow their specificities, regulation, and possible roles in the control of transcription to be elucidated.
ACKNOWLEDGMENTS
We thank Michaela Sharpe for helpful comments.
This work was funded by the Biotechnology and Biological Sciences Research Council.
REFERENCES
- 1.Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–205. doi: 10.1016/0092-8674(94)90312-3. [DOI] [PubMed] [Google Scholar]
- 2.Arigoni F, Duncan L, Alper S, Losick R, Stragier P. SpoIIE governs the phosphorylation state of a protein regulating transcription factor ςF during sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:3238–3242. doi: 10.1073/pnas.93.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. doi: 10.1126/science.270.5236.637. [DOI] [PubMed] [Google Scholar]
- 4.Barak I, Behari J, Olmedo G, Guzman P, Brown D P, Castro E, Walker D, Westpheling J, Youngman P. Structure and function of the Bacillus SpoIIE protein and its localization to sites of sporulation septum assembly. Mol Microbiol. 1996;19:1047–1060. doi: 10.1046/j.1365-2958.1996.433963.x. [DOI] [PubMed] [Google Scholar]
- 5.Barak I, Youngman P. SpoIIE mutants of Bacillus subtilis comprise two distinct phenotypic classes consistent with a dual functional role for the SpoIIE protein. J Bacteriol. 1996;178:4984–4989. doi: 10.1128/jb.178.16.4984-4989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diederich B, Wilkinson J F, Magnin T, Najafi S M A, Errington J, Yudkin M D. Role of interactions between SpoIIAA and SpoIIAB in regulating cell-specific transcription factor ςF of Bacillus subtilis. Genes Dev. 1994;8:2653–2663. doi: 10.1101/gad.8.21.2653. [DOI] [PubMed] [Google Scholar]
- 7.Duncan L, Alper S, Arigoni F, Losick R, Stragier P. Activation of cell-specific transcription by a serine phosphatase at the site of asymmetric division. Science. 1995;270:641–644. doi: 10.1126/science.270.5236.641. [DOI] [PubMed] [Google Scholar]
- 8.Duncan L, Alper S, Losick R. SpoIIAA governs the release of the cell-type specific transcription factor ςF from its anti-sigma factor SpoIIAB. J Mol Biol. 1996;260:147–164. doi: 10.1006/jmbi.1996.0389. [DOI] [PubMed] [Google Scholar]
- 9.Duncan L, Losick R. SpoIIAB is an anti-ς factor that binds to and inhibits transcription by regulatory protein ςF from Bacillus subtilis. Proc Natl Acad Sci USA. 1993;90:2325–2329. doi: 10.1073/pnas.90.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Errington J, Illing N. Establishment of cell-specific transcription during sporulation in Bacillus subtilis. Mol Microbiol. 1992;6:689–695. doi: 10.1111/j.1365-2958.1992.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 12.Errington J, Mandelstam J. Variety of sporulation phenotypes resulting from mutations in a single regulatory locus, spoIIA, in Bacillus subtilis. J Gen Microbiol. 1983;129:2091–2101. doi: 10.1099/00221287-129-7-2091. [DOI] [PubMed] [Google Scholar]
- 13.Errington J, Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986;132:2967–2976. doi: 10.1099/00221287-132-11-2967. [DOI] [PubMed] [Google Scholar]
- 14.Feucht A, Magnin T, Yudkin M D, Errington J. Bifunctional protein required for asymmetric cell division and cell-specific transcription in Bacillus subtilis. Genes Dev. 1996;10:794–803. doi: 10.1101/gad.10.7.794. [DOI] [PubMed] [Google Scholar]
- 15.Fort P, Piggot P J. Nucleotide sequence of sporulation locus spoIIA in Bacillus subtilis. J Gen Microbiol. 1984;130:2147–2153. doi: 10.1099/00221287-130-8-2147. [DOI] [PubMed] [Google Scholar]
- 16.Gholamhoseinian A, Piggot P J. Timing of spoII gene expression relative to septum formation during sporulation of Bacillus subtilis. J Bacteriol. 1989;171:5747–5749. doi: 10.1128/jb.171.10.5747-5749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harry E J, Pogliano K, Losick R. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1995;177:3386–3393. doi: 10.1128/jb.177.12.3386-3393.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing N, Errington J. Genetic regulation of morphogenesis in Bacillus subtilis: roles of ςE and ςF in prespore engulfment. J Bacteriol. 1991;173:3159–3169. doi: 10.1128/jb.173.10.3159-3169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaBell T L, Trempy J E, Haldenwang W G. Sporulation-specific ς factor ς29 of Bacillus subtilis is synthesized from a precursor protein, P31. Proc Natl Acad Sci USA. 1987;84:1784–1788. doi: 10.1073/pnas.84.7.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis, P. J., and J. Errington. Unpublished results.
- 21.Lewis P J, Magnin T, Errington J. Compartmentalized distribution of the proteins controlling the prespore-specific transcription factor ςF of Bacillus subtilis. Genes Cells. 1996;1:881–894. doi: 10.1046/j.1365-2443.1996.750275.x. [DOI] [PubMed] [Google Scholar]
- 22.Lewis P J, Partridge S R, Errington J. ς Factors, asymmetry, and the determination of cell fate in Bacillus subtilis. Proc Natl Acad Sci USA. 1994;91:3849–3853. doi: 10.1073/pnas.91.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magnin T, Lord M, Errington J, Yudkin M D. Establishing differential gene expression in sporulating Bacillus subtilis: phosphorylation of SpoIIAA (anti-anti-ςF) alters its conformation and prevents formation of a SpoIIAA/SpoIIAB/ADP complex. Mol Microbiol. 1996;19:901–907. doi: 10.1046/j.1365-2958.1996.434964.x. [DOI] [PubMed] [Google Scholar]
- 24.Min K-T, Hilditch C M, Diederich B, Errington J, Yudkin M D. ςF, the first compartment-specific transcription factor of B. subtilis, is regulated by an anti-ς factor that is also a protein kinase. Cell. 1993;74:735–742. doi: 10.1016/0092-8674(93)90520-z. [DOI] [PubMed] [Google Scholar]
- 25.Najafi S M A, Willis A C, Yudkin M D. Site of phosphorylation of SpoIIAA, the anti-anti-sigma factor for sporulation-specific ςF of Bacillus subtilis. J Bacteriol. 1995;177:2912–2913. doi: 10.1128/jb.177.10.2912-2913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: Wiley; 1990. pp. 391–450. [Google Scholar]
- 27.Partridge S R, Errington J. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol Microbiol. 1993;8:945–955. doi: 10.1111/j.1365-2958.1993.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 28.Partridge S R, Foulger D, Errington J. The role of ςF in prespore-specific transcription in Bacillus subtilis. Mol Microbiol. 1991;5:757–767. doi: 10.1111/j.1365-2958.1991.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 29.Piggot P J. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporulation operons. J Bacteriol. 1973;114:1241–1253. doi: 10.1128/jb.114.3.1241-1253.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pogliano K, Hofmeister A E, Losick R. Disappearance of the ςE transcription factor from the forespore and the SpoIIE phosphatase from the mother cell contributes to establishment of cell-specific gene expression during sporulation in Bacillus subtilis. J Bacteriol. 1997;179:3331–3341. doi: 10.1128/jb.179.10.3331-3341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rather P N, Coppolecchia R, DeGrazia H, Moran C P., Jr Negative regulator of ςG-controlled gene expression in stationary-phase Bacillus subtilis. J Bacteriol. 1990;172:709–715. doi: 10.1128/jb.172.2.709-715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roels S, Driks A, Losick R. Characterization of spoIVA, a sporulation gene involved in coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:575–585. doi: 10.1128/jb.174.2.575-585.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt R, Margolis P, Duncan L, Coppolecchia R, Moran C P, Jr, Losick R. Control of developmental transcription factor ςF by sporulation regulatory proteins SpoIIAA and SpoIIAB in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:9221–9225. doi: 10.1073/pnas.87.23.9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setlow B, Magill N, Febbroriello P, Nakhimovsky L, Koppel D E, Setlow P. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J Bacteriol. 1991;173:6270–6278. doi: 10.1128/jb.173.19.6270-6278.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterlini J M, Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to the development of actinomycin resistance. Biochem J. 1969;113:29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens C M, Daniel R, Illing N, Errington J. Characterization of a sporulation gene, spoIVA, involved in spore coat morphogenesis in Bacillus subtilis. J Bacteriol. 1992;174:586–594. doi: 10.1128/jb.174.2.586-594.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stragier P, Bonamy C, Karmazyn-Campelli C. Processing of a sporulation sigma factor in Bacillus subtilis: how morphological structure could control gene expression. Cell. 1988;52:697–704. doi: 10.1016/0092-8674(88)90407-2. [DOI] [PubMed] [Google Scholar]
- 39.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 40.Sun D, Fajardo-Cavazos P, Sussman M D, Tovar-Rojo F, Cabrera-Martinez R-M, Setlow P. Effect of chromosome location of Bacillus subtilis forespore genes on their spo gene dependence and transcription by EςF: identification of features of good EςF-dependent promoters. J Bacteriol. 1991;173:7867–7874. doi: 10.1128/jb.173.24.7867-7874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sussman M D, Setlow P. Cloning, nucleotide sequence, and regulation of the Bacillus subtilis gpr gene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991;173:291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J-J, Howard M G, Piggot P J. Regulation of transcription of the Bacillus subtilis spoIIA locus. J Bacteriol. 1989;171:692–698. doi: 10.1128/jb.171.2.692-698.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 44.Wu L J, Errington J. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol Microbiol. 1998;27:777–786. doi: 10.1046/j.1365-2958.1998.00724.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu, L. J., and J. Errington. Unpublished results.
- 46.Wu L J, Feucht A, Errington J. Prespore-specific gene expression in Bacillus subtilis is driven by sequestration of SpoIIE phosphatase to the prespore side of the asymmetric septum. Genes Dev. 1998;12:1371–1380. doi: 10.1101/gad.12.9.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]