Abstract
Individuals diagnosed with cancer often turn to the use of herbal remedies with the intention of treating and ameliorating the condition, impeding the progression of metastasis, enhancing immune function, mitigating stress, and inducing relaxation. Recently, medicinal plants were combined with conventional chemotherapy to decrease the side effects and increase the effectiveness of chemotherapy. This study showed the effectiveness of gemcitabine (Gem) was significantly increased after being used together with ethyl acetate extract obtained from Vernonia amygdalina (Eav) leaves. The combination doses of Eav and Gem were determined based on cytotoxic activity using the MTT assay method. The anticancer effect of this combination was identified by several parameters including the apoptosis effect, anti-migration, and anti-invasion activities of PANC-1 cells. Furthermore, this effect was explained via protein expression evaluation using immunohistochemical and flow cytometry. The Eav has a better Inhibitory Concentration 50 (IC50) than Gem of 21.19 ± 0.64 µg/mL and 164.78 ± 1.40 µg/mL. The combination of Eav and Gem at IC50 (1:1) has the strongest activity than Eav and Gem alone at 500.00 µg/mL. The anti-cancer effect of this combination showed significantly increased levels of apoptosis, particularly in the early phase of 17.46 ± 0.35 % (p < 0.0001) than Eav and Gem alone of 7.76 ± 0.25 % and 7.06 ± 0.20 %. A similar impact was evaluated in the migration and invasion of PANC-1 cells after the combination treatment. The % relative migration and cell invasion were significantly decreased compared to the control group and Eav or Gem alone by 21.49 ± 0.96 % and 125.25 ± 5.25 cells, respectively (p < 0.0001). This study found that signature molecules of VEGF, COX2, RAS, and MEK were down-regulated after treatment. Our study suggested that the Eav ameliorates the Gem effect against PANC-1 cells through apoptosis, migration, and invasion influence via RAS/MEK pathways.
Keywords: Vernonia amygdalina, Gemcitabine, Anticancer, PANC-1 cells, RAS/MEK pathways
1. Introduction
Pancreatic cancer is a significant unresolved healthcare issue worldwide, with the highest mortality rate among all primary cancers. In the United States alone, it is projected to cause the deaths of over 49,830 individuals in 2022 (Park et al., 2021). Pancreatic cancer is a challenging malignancy to identify. Upon diagnosis, over 50 % of pancreatic cancer cases have already metastasized, resulting in a more 3 % five-year survival rate for affected individuals. The imprecise administration of chemotherapy contributes to the challenging nature of treating pancreatic cancer in this phase (McGuigan et al., 2018, Hu et al., 2021).
The present chemotherapy regimen, predominantly employing gemcitabine as the benchmark (with or without nab-paclitaxel or cisplatin), has exhibited marginal enhancements. However, they are considerably inadequate in attaining satisfactory outcomes (Saung and Zheng, 2017, Springfeld et al., 2019). The FOLFIRINOX regimen, consisting of folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin, not only has demonstrated increased efficacy but also has a concomitant increase in toxicity (Suker et al., 2016, Conroy et al., 2018). Tyrosine-kinase inhibitors, exemplified by erlotinib, have demonstrated efficacy in preclinical studies, but have not yielded similar outcomes in the clinical context (Lakkakula et al., 2019). Clinical testing has demonstrated the ineffectiveness of antiangiogenic treatments (Tamburrino et al., 2013). The aforementioned reasoning is based on the fact that antiangiogenic therapy exacerbates hypoxia and reduces vascularization in pancreatic cancer, thereby limiting the efficacy of additional chemotherapeutic agents in accessing the tumor (Annese et al., 2019). An urgent requirement exists for a novel combination chemotherapy regimen to address the treatment of pancreatic cancer (Miller et al., 2020).
The utilization of natural products as a source of bioactive anticancer agents has been extensively demonstrated. Natural products possess the advantage of exhibiting lower toxicity in comparison to conventional chemotherapy drugs (Kashyap et al., 2021, Huang et al., 2021, Lubis et al., 2022). The co-administration of natural compounds and conventional chemotherapeutic agents has the potential to produce additive or synergistic effects in the eradication of cancerous cells. This approach may facilitate the use of reduced and less hazardous dosages (Tan and Norhaizan, 2019, Ashrafizadeh et al., 2020). A study reported a synergistic effect in inhibiting pancreatic cancer cell lines when gemcitabine was combined with extracts of Clinacanthus nutans (Hii et al., 2019). Another study demonstrated the similar impact of the combination of gemcitabine and Pao pereira extract on PANC-1 cells (Yu et al., 2013). Furthermore, the concomitant administration of a Chinese herbal blend and gemcitabine exhibited a synergistic impact on PANC-1 cells (Pak et al., 2021). Further investigation is required to examine the potential impact of incorporating Vernonia amygdalina leaf extract in conjunction with gemcitabine, as an additional outcome of utilizing natural products.
The leaf of Vernonia amygdalina has been documented as a co-chemotherapeutic agent (Alara et al., 2017). According to various studies, the extract derived from Vernonia amygdalina leaf has demonstrated the ability to inhibit the growth of cancer cell lines such as breast cancer cells (Yedjou et al., 2008, Oyugi et al., 2009, Wong et al., 2013, Yedjou et al., 2013), colon cancer cells (Bestari et al., 2017, Burhan et al., 2022), prostate cancer cells (Johnson et al., 2017), liver cancer cells (Thongnest et al., 2019), and pancreatic cancer cells (Lubis et al., 2022). According to the report, the Vernonia amygdalina leaf's ethyl acetate extract was found to induce cell death in 4 T1 cells through the activation of the phosphoinositide 3-kinase (PI3K) pathway (Hasibuan et al., 2020). The observation of the extract activity has the potential to induce cell death in T47D cells through the activation of cell cycle arrest and apoptotic pathways (Lifiani et al., 2018). The ethanolic extract of Vernonia amygdalina leaf has been observed to induce apoptosis in MDA-MB-231 and MCF-7 cancer cell lines through the mediation of caspase and p53. This effect has been noted in other cancer cell lines as well (Wong et al., 2013). The n-hexane extract of Vernonia amygdalina leaf was utilized to induce cell cycle arrest and apoptosis in PANC-1 cells (Fauzan et al., 2019). The leaves of Vernonia amygdalina have demonstrated anticancer properties. Nevertheless, there is currently no report on the combined effect of Vernonia amygdalina and chemotherapy.
The present study aims to investigate the synergistic impact of Vernonia amygdalina leaf in combination with gemcitabine on PANC-1 cells. The leaves of Vernonia amygdalina contain phytochemical compounds such as polyphenols, flavonoids, glycosides, vernoniosides, vernodaline, and vernomygdine. These compounds have been found to exhibit anticancer activity against cancer cell lines. Additionally, Vernonia amygdalina leaf has been shown to enhance the effectiveness of gemcitabine through various mechanisms (Evbuomwan et al., 2018, Yusoff et al., 2020, Oladele et al., 2021). The identification of the anticancer mechanism can be accomplished through the observation of cancer cell migration and invasion (Wang et al., 2018). The RAS/MEK pathways exhibit a noteworthy association with the migratory and invasive behavior of PANC-1 cells at the molecular level (Buscail et al., 2020). The study reports that the combination of ginsenoside and cisplatin effectively inhibited cell migration in HeLa cells by suppressing the RAS/MEK/ERK pathways (Li et al., 2023). Anthocyanins derived from black rice were found to inhibit cell migration and invasion by suppressing MEK in various other cell lines (Chen et al., 2015). Therefore, in order to address the treatment challenges associated with pancreatic cancer, it is imperative to evaluate the efficacy of gemcitabine and Vernonia amygdalina combination therapy against PANC-1 cells, with a focus on the proteins that regulate their migration and invasion.
2. Materials and methods
2.1. Cell lines and reagents
The PANC-1 human pancreatic cancer cell lines were procured from the culture collection of the parasitology laboratory at Universitas Gadjah Mada, Indonesia. The PANC-1 cell lines was maintained in vitro at a temperature of 37 °C under a gaseous atmosphere consisting of 5 %, CO2, and 95 % air. The recommended culture medium supplemented with 10 % fetal bovine serum was utilized to support cell growth. The leaf of Vernonia amygdalina was collected from the botanical garden located at the Faculty of Pharmacy, Universitas Sumatera Utara. The chemical reagents utilized in this investigation possess a certification from Sigma Aldrich. The study employed a detection kit with DAB, FITC goat anti-rabbit IgG, annexin V, and propodium iodide (PI) that was procured from Abclonal for the primary and secondary antibodies in the immunohistochemical analysis.
2.2. Extract preapration
The maceration method was employed to prepare the ethyl acetate extract of Vernonia amygdalina leaf, denoted as Eav. In summary, a quantity of 500 g of dry Vernonia amygdalina leaf powder was subjected to a soaking process with 5 L of ethyl acetate for a duration of 3 cycles of 24 h each, while being stored in a light-free environment. The specimen was obtained from a macerated sample after the evaporation of ethyl acetate via employment of a rotary evaporator (Type RE100-S, Dlab). The specimen is stored at a temperature of 2 °C until it is utilized for the examination (Rosidah et al., 2021, Lubis et al., 2022).
2.3. Phytochemical analysis
The present study involved the examination of phytochemicals obtained from the ethyl acetate extract of Vernonia amygdalina. The analysis was conducted using TSQ Exactive (Thermo) (LSIH, Brawijaya University) and involved the use of mobile phase A (0.1 % formic acid in water) and phase B (0.1 % formic acid in acetonitrile) in accordance with the gradient method. The Hypersil GOLD aQ column, measuring 50 × 1 mm × 1.9 μm, was subjected to a flow rate of 40 μL/min during the analysis, which lasted for a duration of 70 min. The outcomes were scrutinized through the utilization of Compound Discoverer software, employing mzCloud (Hasibuan et al., 2020).
2.4. Viability test
The viability of cells was evaluated through the utilization of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay after 24 h of treatment. The study involved subjecting cells in the exponential growth phase to serial dilutions of Eav, Gemcitabine (Gem), or a combination of the two for a period of 24 h. The cells did not attain a state of plateau phase within the designated period of incubation. The concentration of a drug that resulted in a 50 % inhibition of cell growth relative to the untreated control was determined as the Fifty Percent Inhibitory Concentration (IC50) (Hasibuan and Ilyas, 2013, Lubis et al., 2022).
2.5. Apoptosis detection by flow cytometry
The cells were subjected to a 48-hour exposure to IC50 concentrations of Eav, Gem, or a combination of both. The cells underwent a washing process utilizing PBS, followed by resuspension in binding buffer. Subsequently, they were subjected to staining with FITC conjugated annexin V and propidium iodide (PI) in accordance with the manufacturer's protocol (BD Biosciences, San Jose, CA, USA). Flow cytometry was employed to analyze the cells. The present study identified Annexin V positive cells as early-stage apoptotic cells, while Annexin V-PI double positive cells were identified as late-stage apoptotic cells. Necrotic cells were identified as PI single-positive cells (Hasibuan and Sumaiyah, 2019, Lubis et al., 2023).
2.6. Cell migration assay
The PANC-1 cells were cultured in 35 mm dishes until they reached full confluence, at which point a wound was created by scratching the surface with a sterile pipette tip. Subsequently, the cells were subjected to treatment with IC50 Eav, either alone or in combination with Gem at IC50, for a duration of 24 h. The photomicroscope was utilized to capture images of cells for the purpose of evaluating cell migration. Statistical analysis was conducted based on a minimum of three biological replicates (Harahap et al., 2018).
2.7. Cell invasion assay
The experiment involved conducting a cell invasion assay through the utilization of a polyethylene terephthalate membrane (with a pore size of 8 µm) in conjunction with Millicell hanging cell culture inserts and BD Matrigel (Millipore). The PANC-1 cells that were introduced into the upper chamber underwent treatment with IC50 Eav, either alone or in combination with Gem of IC50. In this experiment, PANC-1 cells were subjected to invasion assay by incubating them with a conditioned medium containing 10 % (v/v) FBS in each well. The cells were allowed to penetrate through the membrane during a 24-hour incubation period at 37 °C. The cells that traversed the filter pores were immobilized with methanol, and subjected to crystal violet staining, and the invasiveness of PANC-1 cells was assessed through microscopic observation by quantifying the count of cells that had invaded. Cell invasion evaluation was conducted using three biological replicates (Luo et al., 2020).
2.8. Immunohistochemical analysis
The PANC-1 cells were seeded onto a 24-well plate, with a coverslip included in each well. The experiment involved subjecting the cells to IC50 Eav treatment, both in the presence and absence of combined treatment with Gem of IC50, for a duration of 24 h. In addition, the cells were rinsed with phosphate-buffered saline (PBS) and transferred to a glass receptacle where they were subjected to incubation at ambient temperature for a duration of 10–15 min. Following incubation, the cells were subjected to two washes with phosphate-buffered saline (PBS) and subsequently exposed to monoclonal antibodies targeting vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX2). The cells were then incubated for a duration of 1 h at ambient temperature. The cells underwent a PBS rinse and were subsequently treated with a secondary antibody, followed by incubation at ambient temperature for a duration of 10 min. The expression of proteins was observed and documented using a light microscope. The data was quantified as a proportion of cells that exhibited protein expression in 10 distinct fields of view for every group (Sari et al., 2018).
2.9. RAS and MEK expression analysis
A total of 5 × 105 PANC cells were seeded per well in a six-well plate and incubated for 24 h. Subsequently, the cells were subjected to IC50 Eav treatment, either alone or in conjunction with Gem at IC50, for a duration of 24 h. Both floating and adherent cells were harvested into a conical tube utilizing 0.025 % trypsin. The cellular samples underwent three rounds of washing with cold phosphate-buffered saline (PBS) and were subsequently subjected to centrifugation at a rate of 2500 rpm for a duration of 5 min. The liquid portion was isolated, while the solid residue was gathered. The sediment cells were treated with 70 % ethanol and left to incubate for a duration of 2 h at a temperature of −20 °C. Following this, RAS FITC and MEK PE antibodies were introduced and left to incubate at a temperature of 37 °C for a duration of 10 min. The specimens underwent analysis through employment of the FACScan flow cytometer (Hasibuan et al., 2020).
2.10. Statistical analysis
The statistical analysis for this study was conducted utilizing the GraphPad Prism software. The data were presented as the mean value plus or minus the standard deviation and were analyzed using the student’s t-test to determine the statistical significance of the differences observed between the two groups. Each assay was replicated biologically a minimum of three times. Statistical significance was determined based on the P-values of < 0.05, <0.001, or < 0.0001.
3. Results
3.1. Phytochemical compounds of extract
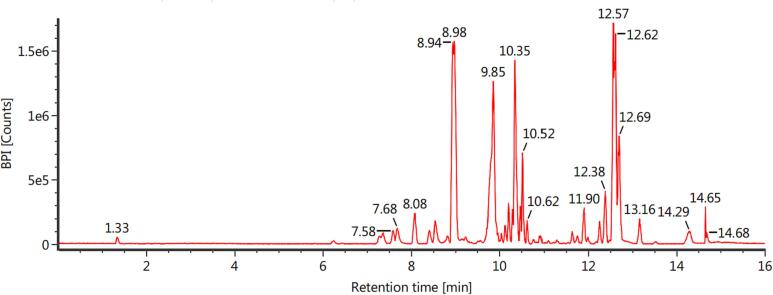
The 49.50 g Eav was obtained after the maceration process using ethyl acetate for 3 × 24 h. It means this extraction was given % a yield extract of 9.90 %. Phytochemical constituent analysis of EAF was carried out with LC-MS/MS to obtain information about its compounds. The results are given in Fig. 1.
Fig. 1.
LC-MS/MS analysis of Eav compounds identification. The analysis was carried out using 0.1 % formic acid in water (Solvent A), and 0.1 % formic acid in acetonitrile (Solvent B) in accordance with the gradient method. Meanwhile, the Hypersil GOLD aQ column (50 × 1 mm × 1.9 μm) as a coloumn system.
The results showed that Eav contains various phytochemicals. Five compounds were successfully identified based on the structure fragmentation of compounds. That is C19H20O7 candidate absence, C29H40O5 candidate, C34H46O9 candidate, C25H46O14 candidate, and C54H78O9 candidate with retention time sequentially 8.96 min, 9.85 min, 10.52 min, 10.36 min, and 12.59 min. Other tests need to be carried out to determine these compounds.
3.2. Percentage viability of PANC-1 cells
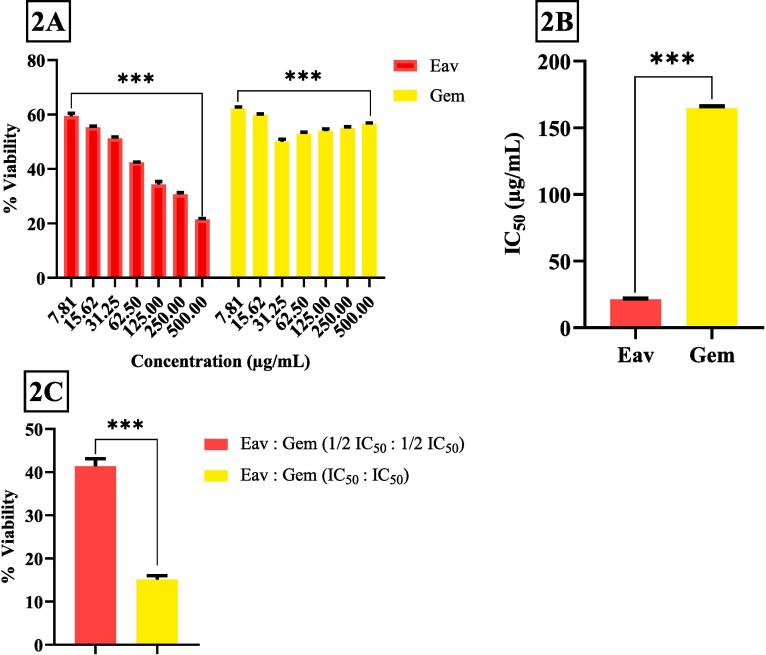
The viability of PANC 1 cells was evaluated using the MTT assay 24 h after Eav and Gem treatment. The results revealed that Eav and Gem treatment inhibited cell viability in a dose-dependent manner (Fig. 2A). The PANC-1 cells were treated using various concentrations of Eav and Gem of 7.81 µg/mL, 15.62 µg/mL, 31.25 µg/mL, 62.50 µg/mL, 125.00 µg/mL, 250.00 µg/mL, and 500.00 µg/mL, respectively. The Eav concentrations led to significant differences in % cell viability (p < 0.0001). The 500.00 µg/mL Eav was the strongest concentration causing PANC-1 cell death with a % viability of 21.43 ± 0.40 %. Meanwhile, 500.00 µg/mL Gem treatment was identified lead PANC-1 cell death but the % viability (56.70 ± 0.19 %) is significantly different compared to the Eav group (p < 0.0001), indicating the cytotoxic activity of Gem was resistant although the concentrations have increasing.
Fig. 2.
The percentage cell viability of PANC-1 cells after being treated with various concentrations of Eav, Gem, and/or co-treatment of Eav-Gem. *** The results showed a significant difference between group tests with p < 0.0001. (2A) % viability of PANC-1 cells, (2B) Inhibitory concentration 50 (IC50) of Eav and Gem, (2C) % viability of PANC-1 cells after treatment with combination of Eav and Gem.
The IC50 was utilized to characterize the cytotoxicity of the samples. This concentration elucidates that the potency of the samples results in a 50 % fatality rate of cells. A lower IC50 value in samples would indicate a higher potential as an anticancer agent (Paudel et al., 2019, Hasibuan et al., 2020). In this study, the IC50 of Eav and Gem can be shown in Fig. 2B. The cytotoxic activity of Eav is better than Gem described from IC50 of 21.19 ± 0.64 µg/mL and 164.78 ± 1.40 µg/mL. This study determined the % viability of PANC-1 cells after treatment with the combination of Eav and Gem of IC50 and ½ IC50, respectively (Fig. 2C). % Viability of these combinations shows slightly down compared with Gem of 500.00 µg/mL. Furthermore, the combination of Eav and Gem at IC50 (1:1) has activity strongest than Eav alone at 500.00 µg/mL. This result indicated potential anti-pancreatic activity after Eav combined with Gem at IC50 (1:1).
3.3. Analysis of apoptotic cell death
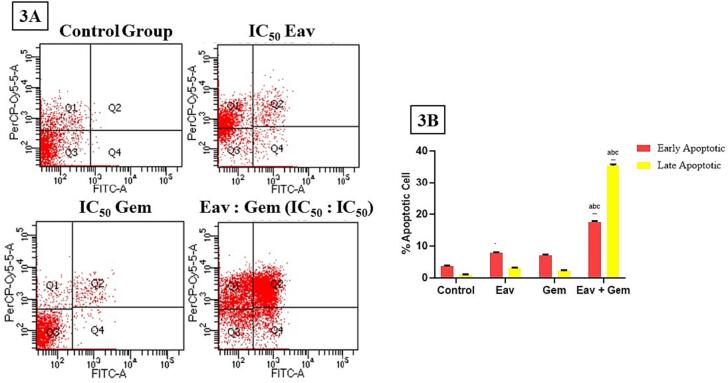
In order to examine the impact of Eav on apoptotic processes, PANC-1 cells were subjected to a 72-hour treatment with Eav and/or Gem. The resulting number of apoptotic cells was subsequently analyzed through Annexin V/PI double staining (Fig. 3A). The present study observed a significant increase (p < 0.0001) in the levels of early apoptotic in PANC-1 cells after Eav treatment (7.76 ± 0.25 %) in comparison to the control group (3.70 ± 0.17 %) (Fig. 3B). In addition, the levels of early apoptotic following Eav treatment (7.76 ± 0.25 %) are significantly different (p < 0.05) than the gemcitabine-induced levels of early apoptotic (7.06 ± 0.20 %). Moreover, the levels of early apoptotic following the co-treatment were significantly different increased (17.46 ± 0.35 %) than Eav or Gem alone (p < 0.0001). The evaluation of late apoptotic events was conducted after the administration of Eav and/or Gem (Fig. 3A). The co-treatment resulted in a significant increase (p < 0.0001) in the levels of late apoptotic, which were measured at 35.46 ± 0.45 %. In comparison, the levels of late apoptotic following Eav and Gem treatment were 3.11 ± 0.10 % and 2.26 ± 0.15 %, respectively (Fig. 3B). The general pattern exhibited a strong resemblance to that which was noted in the context of initial apoptosis. The findings indicate that the combined treatment may exhibit a synergistic impact on apoptotic and cytotoxic functions.
Fig. 3.
The percentage of apoptotic cells after treatment with Eav and Gem in combination or alone is significantly different (p < 0.0001). (3A) The distribution of PANC-1 cells using Annexin V/PI double staining, (3B) % apoptotic cell, a the result is significantly different with the control group (p < 0.0001), b the result is significantly different with Eav group (p < 0.0001), c the result is significantly different with Gem group (p < 0.0001).
3.4. Co-treatment inhibited PANC-1 cell migration and invasion
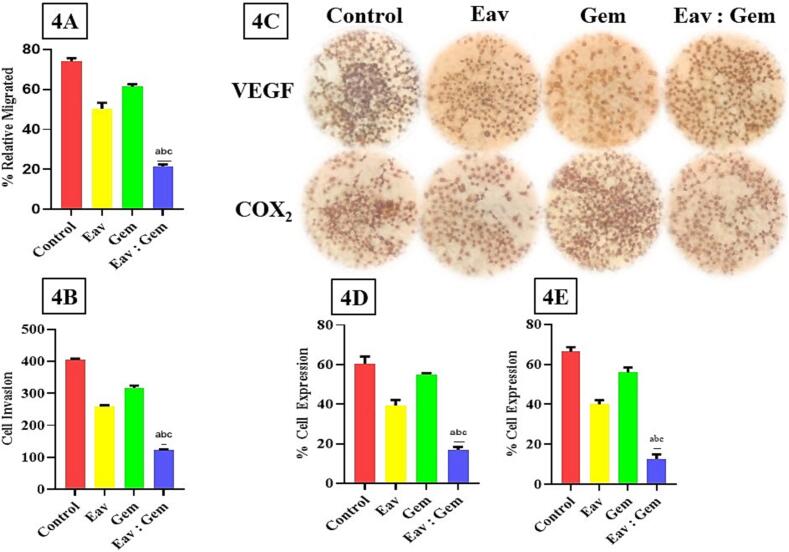
To evaluate the potential antimetastatic effect of Eav and/or Gem, we analyzed its ability to inhibit the migration and invasion of PANC-1 cells. It was demonstrated that co-treatment inhibits migration of PANC-1 cells significantly different (p < 0.0001) with the control group, Eav, and Gem with % relative migrated index subsequently 21.49 ± 0.96 %, 74.00 ± 1.70 %, 50.32 ± 2.99 %, and 61.35 ± 1.30 % (Fig. 4A). The following observation was to determine the co-treatment effect of PANC-1 cell invasion. These results are not different from migration results, that co-treatment has strongest activity inhibited PANC-1 cells invasion (p < 0.0001) than the control group, Eav, and Gem with invaded cells result of 125.25 ± 5.25 cells, 405.73 ± 5.15 cells, 260.15 ± 5.70 cells, and 315.35 ± 5.40 cells (Fig. 4B). Hence, these results suggested that the co-treatment is stronger than Eav and Gem alone in inhibiting migration and invasion of PANC-1 cells.
Fig. 4.
The effect of Eav and Gem Combination Against migration and invasion of PANC-1 cells. The results showed significantly different (p < 0.0001) with each group treatment. (4A) %relative migrated of PANC-1 cells, (4B) Cell invasion value after treatment with each group, (4C) Immunohistochemical analysis of VEGF and COX2 proteins in PANC-1 cells, (4D) % expression of VEGF in PANC-1 cells, (4E) % expression of COX2 in PANC-1 cells.
3.5. Effect of co-treatment on the VEGF, COX2, RAS, and MEK expressions
Migration and invasion of PANC-1 cells are dependent on protein expressions like VEGF and COX2 (Shi et al., 2015). In this study, we investigated VEGF and COX2 levels on PANC-1 cells after treatment with Eav and/or Gem. The expression of proteins was carried out using immunohistochemical analysis (Fig. 4C). The expressions of VEGF were significantly decreased with co-treatment compared to the control group of 16.88 ± 1.45 % and 60.51 ± 3.87 %, respectively. The co-treatment (16.88 ± 1.45 %) of Eav with Gem is superior to each treatment of Eav and Gem. The percentage expression showed significant differences compared with single treatments of 39.14 ± 1.91 % and 55.70 ± 0.63 % (Fig. 4D). The following test described the effect of co-treatment on COX2 expression (Fig. 4C). The percentage of COX2 expression measured with the co-treatment significantly decreased COX2 expression compared with the control group, Eav, and Gem of 10.70 ± 1.51 %, 68.70 ± 2.31 %, 39.70 ± 1.81 %, and 56.70 ± 1.96 % were obtained (Fig. 4E). These results showed possible mechanisms of co-treatment of Eav and Gem against inhibiting migration and invasion of PANC-1 cells.
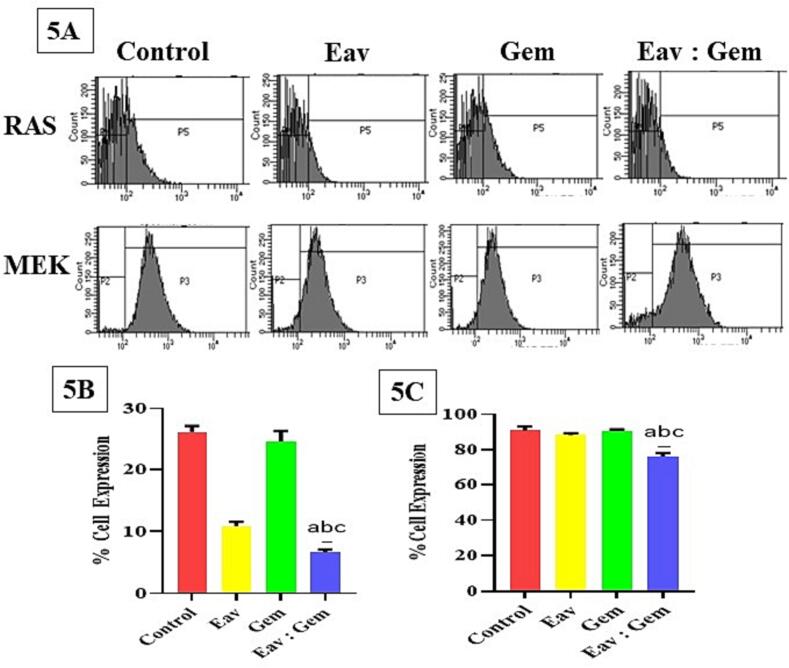
Furthermore, the flow cytometer was utilized to determine the expressions of RAS and MEK. The combined treatment of Eav and Gem resulted in a significant reduction of RAS expression in PANC-1 cells, as compared to both the control group and individual treatments of Eav and Gem (Fig. 5A). In contrast, the co-treatment of Eav and Gem caused cell accumulation in the P5 area (6.66 ± 0.49 %). While the control group and each treatment with Eav/Gem have a cell accumulation in the P5 region of 26.23 ± 0.96 %, 10.88 ± 0.69 %, and 24.69 ± 1.65 % were obtained (Fig. 5B). On the other hand, MEK expression was obtained after PANC-1 cells were treated using the combination of Eav and Gem. The PANC-1 cells have accumulated in the P3 region of 76.06 ± 2.34 %. This value is significantly decreased if compared with the control group and each treatment of Eav and Gem of 90.95 ± 2.18 %, 88.28 ± 1.18 %, and 90.90 ± 0.57 % (Fig. 5C), respectively. Furthermore, the PANC-1 cells have described overexpression of RAS and MEK, and the combination of Eav and Gem significantly reduced RAS and MEK expression compared to other groups.
Fig. 5.
The expression of RAS and MEK proteins using a flow cytometer. (5A) Distribution of cells is significantly different after the treatment of samples with n = 3 ± SD. The P5 and P3 area was described as cell distributions after treatment with each sample. The increasing cell accumulation in P5 and P3 illustrated the expression of each protein after being treated with each sample, (5B) % expression of RAS in PANC-1 cells is significantly different with p < 0.001, (5C) % expression of MEK in PANC-1 cells is significantly different with p < 0.001.
4. Discussion
The limited availability of efficacious chemotherapeutic agents has been a significant challenge in the management of pancreatic cancer. Currently, Gem is utilized as the primary treatment for pancreatic cancer. However, it yields minimal effects on the median survival rate of individuals diagnosed with locally advanced or metastatic pancreatic cancer (Schultheis et al., 2017). According to another study, the tumor inhibitory effects of Gem were observed only during the initial stages of the treatment regimen when the tumors were small. The drug was found to be ineffective in inhibiting tumor metastasis (Jamshed et al., 2020). This study reports a reduction in Gem activity as observed in the migration and invasion assays conducted on PANC-1 cells. The investigation of combination chemotherapeutic strategies was undertaken to aid in the advancement of a more efficacious treatment for pancreatic cancer. Several studies have indicated that natural products may inhibit the growth of pancreatic cancer by triggering apoptosis, suppressing migration and invasion, and blocking critical cellular signaling pathways (Wang et al., 2013). Nevertheless, the studies were limited to the utilization of monotherapy exclusively in cancer cell lines, and there was a dearth of information regarding the impact of combining Eav with conventional chemotherapeutic agents. The current investigation aimed to assess the potential of Eav alone or in conjunction with Gem to elicit enhanced anticancer outcomes.
The study at hand has documented that Eav, a form of traditional medicine, could potentially serve as a valuable adjunct to chemotherapeutic agents in enhancing the effectiveness of chemotherapy for pancreatic cancer cells. This is achieved through a reduction in the required dosage of Gem, thereby minimizing the treatment volume. The study conducted by Hasibuan et al. (2020) provided evidence that the expression of PI3K/Akt/mTOR pathways mediated the eradication of breast cancer cells by Eav. These pathways have been linked to the migration and invasion of cancer cells (Xiao et al., 2021). In the present study, secondary metabolites of Eav were identified using LC-MS/MS (Fig. 1). Five compounds success in determining, which C19H20O7 candidate, C29H40O5 candidate, C34H46O9 candidate, C25H46O14 candidate, and C54H78O9 candidate with retention time sequentially 8.96 min, 9.85 min, 10.52 min, 10.36 min, and 12.59 min. In a previous study, LC-MS/MS analysis of Eav identified that some of components, including diosmetin, 4-methyllumbelliferyl glucuronide, chlorogenic acid, 4-methylumbelliferone, scutellarin, rhoifolin, 7-hydroxycoumarine, apigetrin, apigenin, baicalin, luteolin, ingenol-3-angelate, and 4-methoxycinnamic acid (Hasibuan et al., 2020). Meanwhile, our study reported Eav contains vernonioside D and vernonioside E and these compounds were shown activity to bond with epidermal growth factor and mammalian target of rapamycin proteins (Hasibuan et al., 2021). Another study reported apigenin affects vascular epidermal growth factor (VEGF), inhibiting migration and invasion of pancreatic cancer cells (Ashrafizadeh et al., 2020). King et al. (2012) reported that apigenin-induced apoptosis in human pancreatic cells through p53 modulation. Similarly, luteolin was found to induce apoptosis through BCL-2-mediated pathways (Li et al., 2018).
Initially, the optimization of the treatment conditions for PANC-1 cells with Eav was conducted in the presence or absence of Gem. The viability of PANC-1 cells was found to be inhibited by Eav treatment via the induction of apoptosis. Subsequently, it was found that Eav triggers cellular apoptosis, a process that differs significantly from the early apoptotic effects of Gem. The preceding research demonstrated the apoptotic-inducing efficacy of Eav against HTB-182 cells (Hasibuan et al., 2020). The findings indicate that the combined administration of Eav and Gem exhibited a significantly higher efficacy in triggering early apoptotic events, as opposed to the singular administration of either treatment. This study has demonstrated the potential efficacy of combining Eav and Gem therapies in inhibiting the migration and invasion of PANC-1 cells. The efficacy of this combination in reducing the percentage of relative migration and inhibiting cell invasion is superior to that of individual treatments with Eav and Gem. The combined treatment exhibited increased inhibitory activity on proteins associated with migration and invasion, such as VEGF, COX2, RAS, and MEK, compared to the individual treatments of Eav or Gem.
The RAS/MEK pathway is a crucial factor in the metastasis of pancreatic cancer (Bylsma et al., 2020). According to the report, a mutation in over 90 % of the RAS gene was observed in pancreatic cancer, leading to the excessive activation of the downstream signaling pathway (Bannoura et al., 2021). Several genetic studies have demonstrated that the activation and mutations of RAS are essential for the initiation of pancreatic cancer (Di Magliano and Logsdon., 2013). Previously, it has been reported that the Vernonia amygdalina leaf compounds (Vernonioside D and Vernonioside E) inhibit EGFR and mTOR (Hasibuan et al., 2021). EGFR is essential for RAS oncogene-driven pancreatic cancer (Shankar et al., 2020). The EGFR protein is a member of the erbB family of tyrosine kinase receptors and plays a crucial role in the processes of tumor growth, metastasis, and disease recurrence (Zakaria et al., 2019). The research findings suggest that Eav has the potential to hinder migration and invasion through the inhibition of the RAS/MEK signaling pathway. The concurrent administration of Gem and Eav demonstrates a notable decrease in the expressions of RAS and MEK, in comparison to the individual treatments. The results of this study suggest that Eav has potential as a monotherapy or adjunctive therapy in combination with the standard chemotherapy drug Gem for managing pancreatic cancer. A forthcoming investigation will verify the safety and effectiveness of combined therapy in vivo, with the aim of establishing its theoretical foundation for clinical implementation. Furthermore, the potential impact of Eav in conjunction with other chemotherapeutic agents will be investigated.
5. Conclusion
The present investigation has furnished initial proof that Eav has the capability to trigger apoptosis and hinder the migration and invasion of PANC-1 cells in an in vitro setting. The results were obtained after the combination of Eav and Gem in each IC50 (1:1). This study succeeded in providing a significantly different effect of the combination effect than the control group and Eav or Gem alone in entire parameters. The potential of Eav to enhance Gem-induced cytotoxicity against PANC-1 cells warrants further investigation to elucidate the underlying synergistic mechanisms. The potential toxic and anticancer properties of this combination require in vivo testing as a preliminary step in the development of clinical trials.
CRediT authorship contribution statement
Poppy Anjelisa Zaitun Hasibuan: Conceptualization, Supervision, Methodology, Data curation, Validation, Formal analysis, Investigation, Funding acquisition, Writing – original draft. Jane Melita Keliat: Data curation, Validation, Formal analysis. Muhammad Fauzan Lubis: Resources, Data curation, Conceptualization, Methodology, Formal analysis. Annisa Nasution: Project administration, Software, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by funds from Ministry of Education, Culture, Research, and Technology (Indonesia) through “Hibah Penelitian Dasar Unggulan Perguruan Tinggi” research grant 2021-2023 (Contract No: 32/UN5.2.3.1/PPM/KP-DRTPM/L/2022).
Footnotes
Peer review under responsibility of King Saud University.
References
- Alara O.R., Abdurahman N.H., Mudalip S.K.A., Olalere O.A. Phytochemical and pharmacological properties of Vernonia amygdalina: a review. J. Chem. Eng. Indus. Biotechnol. 2017;2(1):80–96. [Google Scholar]
- Annese T., Tamma R., Ruggieri S., Ribatti D. Angiogenesis in pancreatic cancer: pre-clinical and clinical studies. Cancers. 2019;11(3):381. doi: 10.3390/cancers11030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M., Bakhoda M.R., Bahmanpour Z., Ilkhani K., Zarrabi A., Makvandi P., Mirzaei H. Apigenin as tumor suppressor in cancers: Biotherapeutic activity, nanodelivery, and mechanisms with emphasis on pancreatic cancer. Front. Chem. 2020;8:829. doi: 10.3389/fchem.2020.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafizadeh M., Zarrabi A., Hashemi F., Moghadam E.R., Hashemi F., Entezari M., Najafi M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020;256 doi: 10.1016/j.lfs.2020.117984. [DOI] [PubMed] [Google Scholar]
- Bannoura S.F., Uddin M.H., Nagasaka M., Fazili F., Al-Hallak M.N., Philip P.A., Azmi A.S. Targeting KRAS in pancreatic cancer: new drugs on the horizon. Cancer Metastasis Rev. 2021:1–17. doi: 10.1007/s10555-021-09990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestari, R., Ichwan, M. F., Mustofa, M., & Satria, D. (2017, December). Anticancer activity of Vernonia amygdalina del. extract on WiDr colon cancer cell line. In 2nd Public Health International Conference (PHICo 2017) (pp. 1-5). Atlantis Press.
- Burhan A., Kamaruddin M., Ahmad R., Marzuki I. Anticancer and cytotoxic potentials of Vernonia amygdalina Delile on WiDr cell lines. Phytopharmacol. Res. J. 2022;1(1):1–7. [Google Scholar]
- Buscail L., Bournet B., Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020;17(3):153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- Bylsma L.C., Gillezeau C., Garawin T.A., Kelsh M.A., Fryzek J.P., Sangaré L., Lowe K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020;9(3):1044–1057. doi: 10.1002/cam4.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. Y., Zhou, J., Luo, L. P., Han, B., Li, F., Chen, J. Y., ... & Yu, X. P. (2015). Black rice anthocyanins suppress metastasis of breast cancer cells by targeting RAS/RAF/MAPK pathway. BioMed research international, 2015. [DOI] [PMC free article] [PubMed]
- Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Bachet J.B. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl. J. Med. 2018;379(25):2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- Di Magliano M.P., Logsdon C.D. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144(6):1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evbuomwan L., Chukwuka E.P., Obazenu E.I., Ilevbare L. Antibacterial activity of Vernonia amygdalina leaf extracts against multidrug resistant bacterial isolates. J. Appl. Sci. Environ. Manag. 2018;22(1):17–21. [Google Scholar]
- Fauzan M.L., Hasibuan P.A.Z., Harahap U. Phytocemicals screening and cell cycle arrest activity of n-hexane extract of Vernonia amygdalina Delile leaves against pancreatic cancer cell line. Asian J. Pharma. Res. Develop. 2019;7(4):12–16. [Google Scholar]
- Harahap U., Hasibuan P.A.Z., Sitorus P., Arfian N., Satria D. Antimigration activity of an ethylacetate fraction of Zanthoxylum acanthopodium DC. fruits in 4T1 breast cancer cells. Asian Pac. J. Cancer Prev. 2018;19(2):565. doi: 10.22034/APJCP.2018.19.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasibuan P.A.Z., Ilyas S. Antioxidant and cytotoxic activities of Plectranthus amboinicus (Lour.) Spreng. extracts. Int. J. Pharm. Teach. Pract. 2013;4(3):1–3. [Google Scholar]
- Hasibuan P.A.Z., Munir D., Pertiwi D., Satria D., Lubis M.F. Flavonoids constituent analysis and cell cycle inhibition activity of ethylacetate extract of vernonia amygdalina delile. Leaves on lung cancer cell line. Leaves on lung cancer cell line. Rasayan J. Chem. 2020;13(4):2577–2581. [Google Scholar]
- Hasibuan P.A.Z., Harahap U., Sitorus P., Lubis M.F., Satria D. In-silico analysis of Vernonioside D and Vernonioside E from Vernonia Amygdalina Delile. leaves as inhibitor of Epidermal Growth Factor Receptor (EGFR) and Mammalian Target of Rapamycin (mTOR) Rasayan J. Chem. 2021;14(3):1539–1543. [Google Scholar]
- Hasibuan P.A.Z., Sumaiyah S. The anti-proliferative and pro-apoptotic properties of ethanol Plectranthus amboinicus (Lour.) Spreng. leaves ethanolic extract nanoparticles on T47D cell lines. Asian Pacific journal of cancer prevention. APJCP. 2019;20(3):897. doi: 10.31557/APJCP.2019.20.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasibuan P.A.Z., Harahap U., Sitorus P., Satria D. The anticancer activities of Vernonia amygdalina Delile. Leaves on 4T1 breast cancer cells through phosphoinositide 3-kinase (PI3K) pathway. Heliyon. 2020;6(7):e04449. doi: 10.1016/j.heliyon.2020.e04449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hii L.W., Lim S.H.E., Leong C.O., Chin S.Y., Tan N.P., Lai K.S., Mai C.W. The synergism of Clinacanthus nutans Lindau extracts with gemcitabine: Downregulation of anti-apoptotic markers in squamous pancreatic ductal adenocarcinoma. BMC Complement. Altern. Med. 2019;19(1):1–13. doi: 10.1186/s12906-019-2663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J.X., Zhao C.F., Chen W.B., Liu Q.C., Li Q.W., Lin Y.Y., Gao F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021;27(27):4298. doi: 10.3748/wjg.v27.i27.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Lu J.J., Ding J. Natural products in cancer therapy: Past, present and future. Nat. Prod. Bioprospect. 2021;11:5–13. doi: 10.1007/s13659-020-00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshed M.B., Munir F., Shahid N., Sadiq U., Muhammad S.A., Ghanem N.B., Zhang Q. Antitumor activity and combined inhibitory effect of ceritinib with gemcitabine in pancreatic cancer. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020;318(1):G109–G119. doi: 10.1152/ajpgi.00130.2019. [DOI] [PubMed] [Google Scholar]
- Johnson W., Tchounwou P.B., Yedjou C.G. Therapeutic mechanisms of Vernonia amygdalina delile in the treatment of prostate cancer. Molecules. 2017;22(10):1594. doi: 10.3390/molecules22101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap, D., Tuli, H. S., Yerer, M. B., Sharma, A., Sak, K., Srivastava, S., ... & Bishayee, A. (2021, February). Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. In Seminars in cancer biology (Vol. 69, pp. 5-23). Academic Press. [DOI] [PubMed]
- King J.C., Lu Q.Y., Li G., Moro A., Takahashi H., Chen M., Hines O.J. Evidence for activation of mutated p53 by apigenin in human pancreatic cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012;1823(2):593–604. doi: 10.1016/j.bbamcr.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakula, B. V. K. S., Farran, B., Lakkakula, S., Peela, S., Yarla, N. S., Bramhachari, P. V., ... & Nagaraju, G. P. (2019, June). Small molecule tyrosine kinase inhibitors and pancreatic cancer—Trials and troubles. In Seminars in cancer biology (Vol. 56, pp. 149-167). Academic Press. [DOI] [PubMed]
- Li X.L., Yin Q., Wang W., Ma R.H., Ni Z.J., Thakur K., Wei Z.J. Effect of ginsenoside CK combined with cisplatin on the proliferation and migration of human cervical cancer HeLa cells via Ras/ERK/MAPK pathway. J. Funct. Foods. 2023;102 [Google Scholar]
- Li Z., Zhang Y., Chen L., Li H. The dietary compound luteolin inhibits pancreatic cancer growth by targeting BCL-2. Food Funct. 2018;9(5):3018–3027. doi: 10.1039/c8fo00033f. [DOI] [PubMed] [Google Scholar]
- Lifiani R., Harahap U., Hasibuan P.A.Z., Satria D. Anticancer effect of african leaves (vernonia amygdalina del). to T47D cell resistant. Asian J. Pharma. Clin. Res. 2018;11(1):2–5. [Google Scholar]
- Lubis M.F., Syahputra H., Illian D.N., Kaban V.E. Antioxidant activity and nephroprotective effect of Lansium parasiticum leaves in doxorubicin-induced rats. J. Res. Pharm. (Online) 2022;26(3):565–573. [Google Scholar]
- Lubis M.F., Hasibuan P.A.Z., Syahputra H., Surbakti C., Astyka R. Saurauia vulcani (Korth.) as herbal medicine potential from North Sumatera, Indonesia: A literature review. Heliyon. 2022:e09249. doi: 10.1016/j.heliyon.2022.e09249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubis M.F., Hasibuan P.A.Z., Harahap U., Satria D., Syahputra H., Muhammad M., Astyka R. The molecular approach of natural products as pancreatic cancer treatment: A review. Rasayan J. Chem. 2022;15(2):1362–1377. [Google Scholar]
- Lubis M.F., Hasibuan P.A.Z., Syahputra H., Astyka R., Baruna I. Phytochemical profile and pharmacological activity of Vernonia amygdalina Delile stem bark extracts using different solvent extraction. Open Access Macedonian J. Med. Sci. 2022;10(A):860–866. [Google Scholar]
- Lubis M.F., Hasibuan P.A.Z., Syahputra H., Keliat J.M., Kaban V.E., Astyka R. Duku (Lansium domesticum) leaves extract induces cell cycle arrest and apoptosis of HepG2 cells via PI3K/Akt pathways. Trends Sci. 2023;20(2):6437. [Google Scholar]
- Luo C., Wang Y., Wei C., Chen Y., Ji Z. The anti migration and anti invasion effects of Bruceine D in human triple negative breast cancer MDA MB 231 cells. Exp. Ther. Med. 2020;19(1):273–279. doi: 10.3892/etm.2019.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuigan A., Kelly P., Turkington R.C., Jones C., Coleman H.G., McCain R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018;24(43):4846. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.L., Garcia P.L., Yoon K.J. Developing effective combination therapy for pancreatic cancer: An overview. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladele J.O., Oyeleke O.M., Oladele O.T., Oladiji A.T. Covid-19 treatment: Investigation on the phytochemical constituents of Vernonia amygdalina as potential Coronavirus-2 inhibitors. Comput. Toxicol. 2021;18 doi: 10.1016/j.comtox.2021.100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyugi D.A., Luo X., Lee K.S., Hill B., Izevbigie E.B. Activity markers of the anti-breast carcinoma cell growth fractions of Vernonia amygdalina extracts. Exp. Biol. Med. 2009;234(4):410–417. doi: 10.3181/0811-RM-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak P.J., Lee D.G., Sung J.H., Jung S.H., Han T.Y., Park S.H., Chung N. Synergistic effect of the herbal mixture C5E on gemcitabine treatment in PANC 1 cells. Mol. Med. Rep. 2021;23(5):1–9. doi: 10.3892/mmr.2021.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W., Chawla A., O’Reilly E.M. Pancreatic cancer: A review. Jama. 2021;326(9):851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel M.R., Chand M.B., Pant B., Pant B. Assessment of antioxidant and cytotoxic activities of extracts of Dendrobium crepidatum. Biomolecules. 2019;9(9):478. doi: 10.3390/biom9090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosidah, R., Yuandani, Y., Widjaja, S. S., Auliafendri, N., Lubis, M. F., Muhammad, M., & Satria, D. (2021). Phytochemicals Analysis and Immunomodulatory Activity of Saurauia vulcani Korth. Leaves Extracts Towards Raw 264.7 Cell.
- Sari D.P., Basyuni M., Hasibuan P.A.Z., Wati R. The inhibition of polyisoprenoids from Nypa fruticans leaves on cyclooxygenase 2 expression of widr colon cancer cells. Asian J. Pharm. Clin. Res. 2018;11(8):156. [Google Scholar]
- Saung M.T., Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin. Ther. 2017;39(11):2125–2134. doi: 10.1016/j.clinthera.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheis B., Reuter D., Ebert M.P., Siveke J., Kerkhoff A., Berdel W.E., Strumberg D. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen inKRAS wildtype patients with locally advanced or metastatic pancreatic cancer: A multicenter, randomized phase IIb study. Ann. Oncol. 2017;28(10):2429–2435. doi: 10.1093/annonc/mdx343. [DOI] [PubMed] [Google Scholar]
- Shankar S., Tien J.C.Y., Siebenaler R.F., Chugh S., Dommeti V.L., Zelenka-Wang S., Chinnaiyan A.M. An essential role for Argonaute 2 in EGFR-KRAS signaling in pancreatic cancer development. Nat. Commun. 2020;11(1):2817. doi: 10.1038/s41467-020-16309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Liu F., Zhang W., Liu X., Lin B., Tang X. Epigallocatechin-3-gallate inhibits nicotine induced migration and invasion by the suppression of angiogenesis and epithelial mesenchymal transition in non-small cell lung cancer cells. Oncol. Rep. 2015;33(6):2972–2980. doi: 10.3892/or.2015.3889. [DOI] [PubMed] [Google Scholar]
- Springfeld C., Jäger D., Büchler M.W., Strobel O., Hackert T., Palmer D.H., Neoptolemos J.P. Chemotherapy for pancreatic cancer. La Presse Medicale. 2019;48(3):e159–e174. doi: 10.1016/j.lpm.2019.02.025. [DOI] [PubMed] [Google Scholar]
- Suker M., Beumer B.R., Sadot E., Marthey L., Faris J.E., Mellon E.A., Koerkamp B.G. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol. 2016;17(6):801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburrino A., Piro G., Carbone C., Tortora G., Melisi D. Mechanisms of resistance to chemotherapeutic and anti-angiogenic drugs as novel targets for pancreatic cancer therapy. Front. Pharmacol. 2013;4:56. doi: 10.3389/fphar.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.L., Norhaizan M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules. 2019;24(14):2527. doi: 10.3390/molecules24142527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongnest S., Chawengrum P., Keeratichamroen S., Lirdprapamongkol K., Eurtivong C., Boonsombat J., Ruchirawat S. Vernodalidimer L, a sesquiterpene lactone dimer from Vernonia extensa and anti-tumor effects of vernodalin, vernolepin, and vernolide on HepG2 liver cancer cells. Bioorg. Chem. 2019;92 doi: 10.1016/j.bioorg.2019.103197. [DOI] [PubMed] [Google Scholar]
- Wang J., Liu Y., Zhao J., Zhang W., Pang X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013;93(6):1492–1498. doi: 10.1002/jsfa.5922. [DOI] [PubMed] [Google Scholar]
- Wang H., Luo Y., Qiao T., Wu Z., Huang Z. Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. J. Ovarian Res. 2018;11:1–12. doi: 10.1186/s13048-018-0468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F.C., Woo C.C., Hsu A., Tan B.K.H. The anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One. 2013;8(10):e78021. doi: 10.1371/journal.pone.0078021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Liu Y., Gao Z., Li X., Weng M., Shi C., Sun L. Fisetin inhibits the proliferation, migration and invasion of pancreatic cancer by targeting PI3K/AKT/mTOR signaling. Aging (Albany NY) 2021;13(22):24753. doi: 10.18632/aging.203713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedjou C., Izevbigie E., Tchounwou P. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int. J. Environ. Res. Public Health. 2008;5(5):337–341. doi: 10.3390/ijerph5050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedjou C.G., Izevbigie E.B., Tchounwou P.B. Vernonia amygdalina—induced growth arrest and apoptosis of breast cancer (MCF-7) cells. Pharmacol. Pharm. 2013;4(1) doi: 10.4236/pp.2013.41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J., DRISkO, J. E. A. N. N. E., & Chen, Q. (2013). Inhibition of pancreatic cancer and potentiation of gemcitabine effects by the extract of Pao Pereira. Oncology reports, 30(1), 149-156. [DOI] [PubMed]
- Yusoff, S. F., Haron, F. F., Tengku Muda Mohamed, M., Asib, N., Sakimin, S. Z., Abu Kassim, F., & Ismail, S. I. (2020). Antifungal activity and phytochemical screening of Vernonia amygdalina extract against Botrytis cinerea causing gray mold disease on tomato fruits. Biology, 9(9), 286. [DOI] [PMC free article] [PubMed]
- Zakaria Z., Zulkifle M.F., Hasan W.A.N.W., Azhari A.K., Raub S.H.A., Eswaran J., Husain S.N.A.S. Epidermal growth factor receptor (EGFR) gene alteration and protein overexpression in Malaysian triple-negative breast cancer (TNBC) cohort. OncoTargets Ther. 2019;12:7749. doi: 10.2147/OTT.S214611. [DOI] [PMC free article] [PubMed] [Google Scholar]