Abstract
Background
Completion of tuberculosis (TB) treatment presents several challenges to patients, including long treatment duration, medication adverse-effects and heavy pill burden. WHO emphasize the need for patient-centered TB care, but such approaches require understanding of patient experiences and perceptions.
Methods
In 2020, we nested a qualitative study within a clinical trial that recruited 128 HIV-TB co-infected adults in Kampala receiving rifampicin-based TB treatment, alongside anti-retroviral therapy. A purposively selected sub-sample of 46 trial participants contributed to nine gender segregated focus group discussions. Of these, 12 also participated in in-depth interviews. Sessions were recorded, transcribed verbatim and translated from local languages into English. Thematic analysis focused on drug adverse-effects, use of self-prescribed medications and barriers to treatment adherence.
Results
Patients seemed more concerned about adverse effects that clinicians sometimes overlook such as change in urine color. Those who remembered pre-treatment counselling advice were disinclined to manage adverse-effects by self-prescription. Difficulty in accessing a medical practitioner was reported as a reason for self-medication. Obstacles to adherence included stigma (especially from visible adverse-effects like “red urine”), difficulties with pill size and number, discomfort with formulation and medication adverse effects.
Conclusion
Tailored pre-treatment counselling, improved access to clinical services, and simpler drug administration will deliver more patient-centered care.
Keywords: Tuberculosis treatment, Adherence, Experiences, Side effects
1. Introduction
Approximately 10 million people acquired Tuberculosis (TB) in 2019 with Africa accounting for up to 25% [1]. Uganda had an incidence of 201 cases per 100,000 in 2017 with a mortality rate of 26 per 100,000 cases [2].
TB treatment has had a great impact in averting TB deaths and reducing the disease incidence [1]. However, in Uganda, current programmatic therapy for drug sensitive TB takes six months and the pill burden is often cited as an adherence challenge [3]. Adverse-effects are common and are often managed with additional medication, further increasing the complexity of therapy, especially for patients with other comorbidities such as HIV [4]. Qualitative studies have described that barriers to TB treatment adherence include pill burden, adverse effects, stigma, financial constraints, forgetfulness and health system-related factors like provider attitudes [4], [5], [6], [7]. These barriers influence patient perspectives and shape their experience of treatment programs [8].
The World Health Organization has recommended patient-centered care and research and innovation among the crucial pillars to improving TB care and achieving the ‘End TB Strategy’ targets [9]. Research must include holistic exploration of patients’ experiences to design appropriate patient-centered treatment-support interventions [10]. While the biological symptoms of adverse effects may be relatively similar across populations, their interpretation by patients is likely to vary culturally/geographically. Therefore, qualitative methods are essential to investigate patients’ experiences and perceptions of treatment and their effects on compliance in particular settings.
This study sought to provide insights into the experiences and behaviours, including use of concurrent medications and treatment adherence, of participants receiving TB therapy in an out-patient clinic during a randomized clinical trial in Uganda. We aimed to improve our understanding of the challenges faced by patients to help inform interventions which address their needs and concerns.
2. Methodology
This was a qualitative study nested within a clinical trial conducted to study the safety, efficacy and effect of higher dose rifampicin on pharmacokinetics of dolutegravir and efavirenz amongst TB-HIV outpatients in Uganda. The trial protocol [11] and the results [12] have been reported elsewhere.
All participants were ≥ 18 years, newly diagnosed with TB, and taking or imminently due to commence anti-retroviral therapy (ART).
2.1. Study setting
Data were collected from SAEFRIF trial participants attending the Integrated HIV/TB outpatient clinic at the Infectious Diseases Institute Mulago in Kampala, which treats an average of 150 TB cases annually.
2.2. Sampling approach
Our sample strategy was purposive. We aimed to include males and females from both the standard and intervention arms of the trial as well as participants who had reported toxicities and those who had not during their participation in the clinical trial. Recruitment was on the basis of convenience; the first participants to be reached by phone or come into the clinic for appointments were included in the study. This notwithstanding, we were able to recruit both male and female participants.
2.3. Data collection methods
Data were collected first using in-depth interviews (IDIs) to elicit participant experiences of adherence to TB medication, and then focus group discussions (FGDs), to provide both normative views on TB medication adherence and to take advantage of the benefits of FGDs where patients discuss among themselves. The IDIs and FGDs were audio-recorded and were conducted in a secluded area outside the clinic for privacy.
2.3.1. In depth interviews
Using a semi-structured interview guide, IDIs were conducted for 20–30 min, with 12 primary respondents (6 males and 6 females) from the main trial.
2.3.2. Focus group discussions
Using a FGD guide, we elicited information from nine groups, comprising four female and five male groups, with five persons on average per group. Twenty-six males and twenty females participated in 45–60-minute FDGs. Male and female FGDs were run separately to mitigate discomfort regarding sensitive information.
2.4. Data analysis
The audio data were transcribed and then coded using NVivo 12 [13], to segment sentences and/or paragraphs into categories as informed by prior literature on TB medication adverse effects, patterns within the transcript, and/or intuitive interpretations. We developed a codebook comprising a list of codes, their definitions, labels, and code use. This was used to code the data, generating themes, which appear as major findings in the study. Coding was done by a separate individual from the team that collected and transcribed data. Participants were assigned gender defining names (pseudonyms) while reporting the results.
2.5. Ethical considerations
Ethical permission was obtained from the Joint Clinical Research Centre Research Ethics Committee, the Uganda National Council of Science and Technology, and the University Teaching and Research Ethics Committee at the School of Medicine in the University of St Andrews. Written informed consent was obtained from all participants and risks of confidentiality addressed through use of pseudonyms.
3. Findings of the study
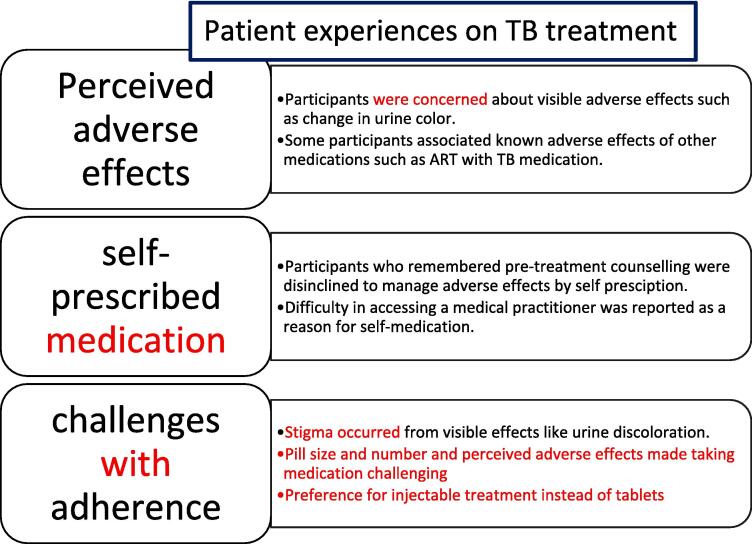
The study provides insights into the perceived adverse-effects of TB treatment among TB-HIV co-infected Ugandan adults; concurrent use of other medications as well as, challenges related to adherence to TB treatment. Fig. 1 summarizes the findings from the study. Table 1 shows the baseline demographic and clinical characteristics of participants. All participants received 6 months of TB treatment.
Fig. 1.
Showing summary of major findings from the study.
Table 1.
Baseline demographic and clinical characteristics of participants.
| Characteristic | Number (%) N = 46 | |
|---|---|---|
| Age in years | Median (IQR) | 37 (30 – 43) |
| Age group | 20–29 | 10 (21.7) |
| 30–39 | 18 (39.1) | |
| 40–49 | 14 (30.4) | |
| 50+ | 4 (8.7) | |
| Sex | Male | 27 (58.7) |
| Female | 19 (41.3) | |
| Marital status | Single | 5 (10.9) |
| Married/cohabiting | 19 (41.3) | |
| Separated | 19 (41.3) | |
| Widowed | 3 (6.5) | |
| TB treatment course | New TB | 46 (100%) |
| TB Retreatment | 0 | |
| ART regimen | DTG-based | 27 (58.7) |
| EFV-based | 19 (41.3) | |
| Adherence | Reported at least one missed dose | 6 (13.0) |
| No reported missed doses | 40 (87.0) | |
| Herbal Use | Reported herbal use | 16 (34.8) |
| Reported no herbal use | 30 (65.2) | |
3.1. Perceived Adverse-Effects of TB treatment
The most frequently reported adverse effect was change in urine color to red or orange. Many participants understood this as a known adverse effect of the drug as reported by Mary, 51 years. However, this did not prevent some patients expressing this as unpleasant or linking it to potentially more serious causes; as was posed by Fred, 43 years old. Other patients like Kenneth, 29-years, found the change in urine color embarrassing. Table 2 shows participants' quotes.
Table 2.
Showing participants’ quotes.
| Factors | Representative quotes | Participant (age) | Proposed strategies to address | |
|---|---|---|---|---|
| Perceived adverse effects | “I was very worried that maybe my bladder’s heart is bleeding.” | Fred (43) |
|
|
| “that colour irritates me so much. I have to go in hiding when I am urinating.” | Kenneth (29) | |||
| I would hold it like this and vomit….if I swallow it at once, however much I try to force it in, it comes back.” | Jane (45) | |||
| “I become dizzy when I take before meals.” | Fred (43) | |||
| “Loss of stamina started after I begun TB treatment…joints shivering and no energy to stand.” | Moses (37) | |||
| “I would feel like putting my feet in water after swallowing medication.” | Peter (45) | |||
| “I got joint pains immediately that when I went to the bathroom to take a shower, I could not squat. I could only bend…” | Hillary (54) | |||
| They reddened, pained and my vision was unclear | Leonard (25) | |||
| “I urinate here and there and not aim like other men.” | John (45) | |||
| “With TB drugs, I had nightmares, dreaming about beasts.” | Diana (32) | |||
| “the challenge is that we are advised to take drugs before eating… Perhaps if there is any way for changing this…It would be better.” | John (45) | |||
|
“It started at week 5…with the legs and I thought it was witchcraft but because am a born-again Christian… I started praying and fasting.” |
Ross (33) | |||
| Concurrent use of other medication | Reasons for | “So at last I ruled myself that I will never go to the clinic to buy it because it was giving me another problem.” | Sarah (30) |
|
| “The body itch refused to stop but because of the tablets I buy from the pharmacy……. If you do not resume swallowing it again on the following day, the itching starts again and worsens.” | Michael (44) | |||
| “If she does not chew them, the medicine would come back (vomit it out).” | Sarah (30) | |||
| “The lemon…can increase immunity…” | Ahmed (35) | |||
| “Because there is a time you can get some attack and you cannot access any medical personnel at that time.” | Fred (43) | |||
| “I took drugs to prevent the increasing vomiting.” | Sarah (38) | |||
| “They make it from palm fruit seeds…it detoxifies the body and you get better…it made me energetic.” | John (45) | |||
| “the herbal medicine has benefits but it also has its limitations just like western medicine with successes and failures.” | Caleb (56) | |||
| “if medical doctors work with herbalists, they could get better results.” | Caleb (56) | |||
| Reasons against | “I cannot take it because my doctor told me not to take herbal drugs.” | Joseph (28) | ||
| “The doctors had told me that I would get side effects…I did not panic.” | Jacob (36) | |||
| “after 6 months, I became weak…thin…coughing…I developed TB…Herbal can kill you.” | Moses (37) | |||
| “the sellers…never tell you the duration,dose,diagnosis.” | Ritah (45) | |||
| “the herbals are not well checked like the western medicines.” | Vicent (37) | |||
| Challenges with adherence | “they should reduce the “obukambwe” [strength] of the medicine…because of its effects on us.” | Shamim (25) | ||
| “If it finds you weak, it makes you weaker.” | Ritah (45) | |||
| “We should be injected…sometimes, depending on what you are doing you forget…Why don’t they have any alternative?” | Fred (43) | Patients’ preferences should be considered when designing drug formulations | ||
| “The drugs are too many and too strong.” | Jane (45) | |||
| “could they change the flavour…smell?” | Fred (43) | |||
| “people say ‘eehh! How do you swallow that big tablet?” | Silas (43) | |||
Further, concerns were raised about constipation, flatulence, nausea, diarrhoea, heartburn, changes in appetite and. abdominal pain. Jane (45 years) complained of nausea which sometimes resulted in vomiting of the medicine..Some participants attributed their abdominal swelling to taking medication on an empty stomach and this was affirmed by. Fred (43 years) who also attributed his dizziness to taking drugs on an empty stomach.
Further still, joint pains, difficulty in walking, heat in the feet, and fatigue were reported and sometimes embodied as painful and unbearable. Moses (37 years) experienced feeling weak while for Peter, (45 years), it was unbearable heat in the feet whereas Hillary (54 years) reported joint pains that interrupted his daily activities.: Some participants attributed the adverse effects to other causes like Ross (33 years) who had initially attributed the pain in his legs to witchcraft.
Other known side effects reported included visual and skin related adverse effects such as itching and skin rash.
Finally, some respondents raised complaints that are not previously documented to be known side effects of TB medication while others are known to be side effects of other drugs such as anti-retroviral therapy (ART). These included effects such as nightmares, loss of sleep, headaches and weak urinary stream. (refer to table 2 for specific quotes).
3.2. Concurrent use of other medications
To mitigate the adverse effects of TB medication, some participants took self-prescribed medicines alongside those prescribed by clinicians. Leah (47 years) used diclofenac for the joint pains and magnesium for the heartburn. Sarah (30 years) used magnesium just before swallowing the TB drugs to alleviate the nausea. However, it caused fever and sweating which led her to resorting to not using self-prescribed mediation again.
Some participants reported that they had previously received prescriptions for medications that had brought them relief and upon persistence of their symptoms, they bought the previously prescribed over-the-counter medications without further medical consultation.
Other participants reported use of products whose mechanism of action is not scientifically known. Products included cod liver oil, lemon, ginger, and coffee beans among others. Further, Sarah reported that her friend chewed raw coffee beans to prevent nausea whereasWinnie chewed clay soil to avert nausea, a habit she learnt from her friend.
Twenty of the 46 participants interviewed however, did not report taking any self-prescribed or herbal medication, explaining that the doctors had discouraged them. Others like Jacob (36 years) demonstrated that pre-treatment counselling eased his fears even when side effects started manifesting. Moses narrated his experience with herbal medication, which he had previously used to replace antiretroviral therapy and how from that experience, he developed TB. The quality of these herbs was also questioned by a number of participants including Ritah (45 years) who reported that the herbal sellers do not tell you your diagnosis nor the dose or duration of the herbal medication.Vincent (37 years) agreed with her reporting that herbal medication does not go through the same quality checks as does western medicines.
However, some participants explained the reasons for going against the doctor’s directive regarding self-prescribed medications; Fred (43 years) explained that the lack of access to doctors at certain times promoted the self-prescription. Similarly, Sarah (38 years) attributed her uptake of self-prescribed magnesium tablets to the unbearable vomiting. John (45 years) who had suffered reduced urinary stream resorted to taking herbal medication which he put in his tea.
In addition, participants such as Caleb (56 years) decried the generalization regarding herbal medicine emphasizing that it does have some benefits as well as limitations just like western medicine. He argued that it is through joint efforts with western medicine that the benefits of herbal medication can be leveraged.
3.3. Challenges of adherence to TB treatment
When asked about factors that affected adherence to medication, participants did not directly admit to not taking their medication however, they raised the following as possible hindrances to adherence.
Some of the previously described side effects of TB medication were raised. Embarrassment due to the change in urine color was echoed. Shamim (25 years) complained about the strength of the medication, which was emphasized by Ritah, a 45 year old.
The administration of the drugs orally was also questioned, suggesting that the injectable option would provide more guarantees of adherence, unlike the oral method, which requires them to remember to swallow the tablets.
The pill burden, size, color and smell were also problematized as was explained by Jane (45 years). In addition, Fred (43 years) questioned why they were given too many strong drugs and required to take them on an empty stomach. He further questioned if the smell of the medication could be modified. Moreover Silas (43 years) explained the stigma of big drugs which tend to attract attention if swallowed in public.
Other participants mentioned ways of improving adherence to the medication such as close monitoring on treatment and shortened duration of treatment.
Ritah (45 years) complimented the TB clinic’s requirement for patients to return every two weeks. Caleb (56 years) who had previously been treated for TB with the longer course of 8 months also commended the reduction in treatment duration from 8 to 6 months appealing for more research towards further reducing this duration.
4. Discussion
Experiences of TB treatment are reflected through patients’ understandings of adverse effects related to TB drugs. While most adverse-effects reported have been well documented [5], [8], [14], this study revealed that some effects usually considered clinically minor by clinicians, may affect patients’ adherence to medication. The change in the urine color for example, usually overlooked by health practitioners, might have affected their urination practices due to embarrassment. However, we are not certain if this then affected adherence. In relating the change in urine color to a bleeding bladder, a participant in this study echoed a similar narrative in a study which assessed factors affecting adherence among Southern Ethiopian patients [6]. This highlights the differences in the clinician and patient perceptions of severity of adverse effects. This calls for critical self-reflection on the part of medical practitioners in re-thinking patient care practices in ways that actively listen and make proactive changes in order to achieve patient centered care. We also noted that participants raised some adverse effects that are not known documented adverse effects of TB medication while others linked some known adverse effects to ART to their taking of TB medication. This calls for health workers to always distinguish between the adverse effects of various drugs for patients taking more than one drug concurrently.
The concurrent use of other medication while on TB treatment also illuminated that adequate counseling prior to initiation of treatment improves coping with adverse effects, equipping patients to refrain from use of un-prescribed products. We also noted that patients who experienced severe effects or those that involved pain, were more likely to end up self-medicating given the pervasiveness of daytime outpatient clinics like ours, which run on weekdays, leaving limited options for patient care outside clinic hours. The concept of adequate counseling corroborates findings from a study conducted in South Africa, where participants confirmed that receiving adequate TB messaging had a positive impact on adherence [15]. The authors of that study further point out that communication is a two-way process and, that reception of information by patients may not always transform into the required action due to reasons such as adverse effects, stigma, pill burden, access and loss of income—some of which have been reported in our study. Also noteworthy was how some participants reported experiences of their peers other than their own, when asked about the use of herbal medication. This suggests that the use of third parties may serve to distance them from a practice likely to rouse disapproval from health practitioners. This provides insights into the possible magnitude in under-reporting the use of herbs, which has implications for drug-drug interactions, subsequent increase in side effects as well as adherence to TB medication [6]. It is also important to note that some participants reported adverse effects as a result of self-prescription which highlights the importance of patient education to enlighten them about the risks associated with self-medication.
Finally, the challenges in adherence reported in this study were quite diverse. While forgetfulness, adverse effects and pill burden were similar to what has been reported elsewhere [5], [6], [7], perceived stigma as a result of adverse effects as well as the nature of administration of TB medicines have rarely been reported previously. The study revealed that some participants prefer the use of an intravenous route of administration instead of the current oral route for treating drug sensitive TB, suggesting that injectables would mitigate challenges of forgetfulness and the large number of big tablets. This was surprising considering the recent move to an all oral regimen for drug resistant TB following WHO recommendation since it resulted in better treatment outcomes and completion rates [16]. This calls for more research among patients with drug sensitive TB to explore their opinions on the preferred route of drug administration. Although stigma has previously been reported in TB patients ([5], in our study, participants expressed perceived stigma resulting from the adverse effects experienced while taking TB medication. This suggests that the stigma caused by adverse effects can affect adherence however, no respondent admitted to missing medication due to the adverse effects. This has implications for health practitioners in preparing patients during counselling, for example, in regard to what to expect as well as how to cope with the adverse effects. Further, swallowing TB medication on an empty stomach, a recommended practice for TB treatment [17], [18], seemed to worsen the adverse effects, according to some participants, hampering adherence. This supports similar findings reported in a study from Southern Ethiopia where participants reported aggravated adverse effects as a result of taking TB medication on an empty stomach [6].
Our study had some limitations such as, the purposive type of sampling used could present a selection bias and may not be a true representative of the population. We also acknowledge that this study did not fully assess whether the reported adverse events actually led to poor adherence and neither did we assess the severity and duration of the reported side effects which would have provided a better understanding of the impact of TB treatment. In addition, the adverse effects were by self-report and there was no objective measures done in this study to assess these. Additionally, this study was conducted among participants attending a clinical trial which is usually a more controlled context that may not be generalizable to a program setting. Patients’ experiences and perceptions regarding treatment many vary across different populations for example among people living without HIV and those from other cultures and geographic settings.
5. Conclusion
Seeking to understand patient experiences while on TB treatment may contribute towards patient centered care. This qualitative study nested within a clinical trial provided insights into behavioral factors including concurrent medication use; patient-reported adherence to prescribed anti-HIV and TB drugs as well as, challenges related to adherence to TB treatment. Tailored pre-treatment patient education, peer-support, and simpler drug administration may deliver more patient-centered care.
Ethical statement
Ethical permission was obtained from the Joint Clinical Research Centre Research Ethics Committee, the Uganda National Council of Science and Technology, and the University Teaching and Research Ethics Committee at the School of Medicine in the University of St Andrews. Written informed consent was obtained from all participants and risks of confidentiality addressed through use of pseudonyms.
Author contribution: This study was conceptualized by CSW, DJS and MK. . CSW and DJK were in charge of project administration. RK provided insight in data curation and methodology. CSW and RNA were in charge of supervision. RNA, JN, FA, AL, JB and BO conducted investigation and data curation. JB, JN and AL transcribed the data while LNS conducted formal analysis and validation of the data. RN, LNS, CSW and DS wrote the manuscript. All authors reviewed and contributed to the manuscript.
Funding
This study was funded by the Global Challenges Research Fund through the Scottish Funding Council administered via the University of St Andrews reference SFC/AN/18/2020, and the EDCTP2 programme supported by the European Union (grant number TMA2016CDF-1580). Additional research support was provided by the Fogarty Interntional Centre, National Institutes of Health (grant # 2D43TW009771-06 "HIV and co-infections in Uganda." The views and opinions of authors expressed herein do not necessarily state or reflect those of the funders.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the participants for their time and participation in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2023.100385.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization (WHO). Global Tuberculosis Report. Geneva; 2020.
- 2.Centres for Disease Control and Prevention C. CDC Division of Global HIV and TB Uganda. Atlanta, TB DoGHa; 2019 July 2019.
- 3.World Health Organization (WHO). Adherence to Long-term Therapies: Evidence for action. Geneva; 2003.
- 4.Gebremariam M.K., Bjune G.A., Frich J.C. Barriers and facilitators of adherence to TB treatment in patients on concomitant TB and HIV treatment: a qualitative study. BMC public health [Internet] 2010;10(651) doi: 10.1186/1471-2458-10-651. https://bmcpublichealth.biomedcentral.com/track/pdf/10.1186/1471-2458-10-651.pdf Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aibana O., Dauria E., Kiriazova T., Makarenko O., Bachmaha M., Rybak N., et al. Patients’ perspectives of tuberculosis treatment challenges and barriers to treatment adherence in Ukraine: a qualitative study. BMJ Open [Internet] 2020;10(1):e032027. doi: 10.1136/bmjopen-2019-032027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gugssa Boru C., Shimels T., Bilal A.I. Factors contributing to non-adherence with treatment among TB patients in Sodo Woreda, Gurage Zone, Southern Ethiopia: A qualitative study. J Infect Public Health. 2017;10(5):527–533. doi: 10.1016/j.jiph.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Sahile Z., Yared A., Kaba M. Patients' experiences and perceptions on associates of TB treatment adherence: a qualitative study on DOTS service in public health centers in Addis Ababa, Ethiopia. BMC Public Health. 2018;18(1):462. doi: 10.1186/s12889-018-5404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gateri AM. I lost close friends when they realized I had TB” – The lived experiences of respondents with TB in Nairobi City County, Kenya. Tuberculosis 2020, 2nd World Congress on Advancements in Tuberculosis and Lung Diseases Webinar; July 02-03, 2020; Amsterdam, Netherlands: Journal of Clinical Respiratory: Open Access; 2020.
- 9.World Health Organization (WHO). The End TB Strategy. 2015.
- 10.World Health Organization (WHO) WHO; care: 2018. A patient centered approach to TB. [Google Scholar]
- 11.Nabisere R., Musaazi J., Denti P., Aber F., Lamorde M., Dooley K.E., et al. Pharmacokinetics, SAfety/tolerability, and EFficacy of high-dose RIFampicin in tuberculosis-HIV co-infected patients on efavirenz- or dolutegravir-based antiretroviral therapy: study protocol for an open-label, phase II clinical trial (SAEFRIF) Trials. 2020;21(1) doi: 10.1186/s13063-020-4132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekaggya-Wiltshire C, Nabisere R, Musaazi J, Otaalo B, Aber F, Alinaitwe L, et al. Decreased Dolutegravir and Efavirenz Concentrations With Preserved Virological Suppression in Patients With Tuberculosis and Human Immunodeficiency Virus Receiving High-Dose Rifampicin. Clin Infect Dis. 2023;76(3):e910-e9. [DOI] [PMC free article] [PubMed]
- 13.Ltd. QIP. NVivo. 12 ed2020.
- 14.Ting NCH, El-Turk N, Chou MSH, Dobler CC. Patient-perceived treatment burden of tuberculosis treatment. PLoS One. 2020;15(10):e0241124. [DOI] [PMC free article] [PubMed]
- 15.Moodley N., Saimen A., Zakhura N., Motau D., Setswe G., Charalambous S., et al. 'They are inconveniencing us' - exploring how gaps in patient education and patient centred approaches interfere with TB treatment adherence: perspectives from patients and clinicians in the Free State Province, South Africa. BMC Public Health. 2020;20(1):454. doi: 10.1186/s12889-020-08562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization W. WHO Consolidated guidelines on Tuberculosis: Module 4: treatment-Drug-Resistant tuberculosis treatment. Geneva; 2020 2020. [PubMed]
- 17.Kumar AKH, Chandrasekaran V, Kumar AK, Kawaskar M, Lavanya J, Swaminathan S, et al. Food significantly reduces plasma concentrations of first-line anti-tuberculosis drugs. Indian J Med Res [Internet]. 2017 03 Aug 2021; 145(4):[530-5 pp.]. Available from: https://pubmed.ncbi.nlm.nih.gov/28862186 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5663168/. [DOI] [PMC free article] [PubMed]
- 18.Lin MY, Lin SJ, Chan LC, Lu YC. Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: systematic review and meta-analysis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease [Internet]. 2010 03 Aug 2021; 14(7):[806-18 pp.]. Available from: https://pubmed.ncbi.nlm.nih.gov/20550762/. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.