Abstract
Diagnostic limitations challenge management of clinically indistinguishable acute infectious illness globally. Gene expression classification models show great promise distinguishing causes of fever. We generated transcriptional data for a 294-participant (USA, Sri Lanka) discovery cohort with adjudicated viral or bacterial infections of diverse etiology or non-infectious disease mimics. We then derived and cross-validated gene expression classifiers including: 1) a single model to distinguish bacterial vs. viral (Global Fever-Bacterial/Viral [GF-B/V]) and 2) a two-model system to discriminate bacterial and viral in the context of noninfection (Global Fever-Bacterial/Viral/Non-infectious [GF-B/V/N]). We then translated to a multiplex RT-PCR assay and independent validation involved 101 participants (USA, Sri Lanka, Australia, Cambodia, Tanzania). The GF-B/V model discriminated bacterial from viral infection in the discovery cohort an area under the receiver operator curve (AUROC) of 0.93. Validation in an independent cohort demonstrated the GF-B/V model had an AUROC of 0.84 (95% CI 0.76–0.90) with overall accuracy of 81.6% (95% CI 72.7–88.5). Performance did not vary with age, demographics, or site. Host transcriptional response diagnostics distinguish bacterial and viral illness across global sites with diverse endemic pathogens.
Subject terms: Computational biology and bioinformatics, Microbiology, Biomarkers
Introduction
Infectious diseases are leading causes of morbidity and mortality worldwide1–3. The toll is greatest in low- and middle-income countries (LMIC), where infections are frequently caused by pathogens that cannot be identified when patients present with fever and resources for testing and treatment are limited. High rates of malnutrition and HIV exacerbate the problem by contributing to increased susceptibility to infection and diversity of pathogens4–8. Without sensitive and specific point-of-care diagnostics to rapidly confirm or refute multiple etiologies of fever, bacterial infections remain untreated and viral infections are treated with antibiotics unnecessarily. The result has been unprecedented inappropriate antibiotic use and associated increasing antimicrobial resistance9–17. The World Health Organization estimates that by 2050 antimicrobial resistance will lead to 10 million lives lost and cost 100 trillion USD per year, leading to an urgent called for new diagnostic assays and approaches to combat the problem18.
Host-response transcription patterns could fill this diagnostic gap by distinguishing between bacterial and viral etiologies early19–27, including before symptoms, to limit spread and guide resource allocation28–30. Gene expression classification models have shown great promise for the classification of causes of fever in high-income countries (HIC)31,32 with progress extending to atypical pathogens present in LMIC20,26,33–35. These multi-analyte gene expression models can be translated to rapid diagnotic platforms that inform clinical care32–34,36. In this study, we generated host response biomarkers for the varied etiologies of suspected infection important worldwide, translated them to a quantitative RT-PCR multiplex platform, and validated them in a globally diverse independent cohort.
Methods
Global fever discovery and validation cohorts
Participants were prospectively enrolled within 48 h of presentation to academic hospitals in the USA25,37–39, Sri Lanka40–43, Tanzania44,45, Cambodia46–48, and Australia (Supplemental Table 1). Samples from participants were stored in a Duke University international biorepository and selected for analysis if they met inclusion critieria for suspected infection defined as: 1) a qualifying vital sign or lab abnormalities (fever ≥ 38.0 °C or ≤ 36 °C, heart rate ≥ 90, respiratory rate ≥ 20, and/or white blood cell count ≥ 12 (cells × 109L), 2) clinical symptoms consistent with acute infection, and 3) adjudicated as meeting bacterial, viral, or noninfectious case definitions (Supplemental Table 2). A committee inclusive of clinical and statistical teams made final cohort selections, ensuring adequate balance among demographic and infectious phenotypes. The discovery cohort included 294 participants presenting to academic hospitals in the USA (n = 152) or Sri Lanka (n = 142). The validation cohort included 101 participants enrolled in the USA (n = 19), Sri Lanka (n = 53), Tanzania (n = 15), Cambodia (n = 10), and Australia (n = 4).
Samples and etiologic testing
Blood was collected at enrollment in PAXgene RNA tubes (QIAGEN) at all sites. Sera were collected at both enrollment (acute phase) and 2–6 week follow-up (convalescent phase) in Sri Lanka and Tanzania. Naso-pharyngeal swabs were collected at enrollment in the USA, Sri Lanka, and Australia. All samples were processed according by standardized protocols, stored at − 70 °C, and shipped on dry ice.
Etiologic testing was performed using reference standard methods to confirm or refute possible bacterial and viral causes of suspected infection endemic to the region. Blood culture and/or urine antigen tests performed as part of clinical care confirmed bacteremia for USA subjects. Bacterial isolates and urine collected in Cambodia confirmed Burkholderia pseudomallei by blood culture, sputum culture, and/or urine antigen testing47,49. For participants enrolled in Sri Lanka and Tanzania, bacterial zoonoses were confirmed by a ≥ fourfold rise in titer of microscopic agglutination testing for Leptospira spp. and Brucella spp.44,45, or indirect immunofluorescence assay for Rickettsia spp. (Spotted Fever Group, Typhus Group, and Orientia tsutsugamushi) and Coxiella burnetii, and/or by polymerase chain reaction (PCR) in a USA reference laboratory. For participants enrolled in the USA and Sri Lanka, respiratory viral infections were confirmed by PCR on nasopharyngeal samples (Luminex Integrated System NxTAG Respiratory Pathogen Panel; Luminex Corporation; Austin, TX)50. For those enrolled in Sri Lanka, acute dengue was confirmed by fourfold rise in antibody titer, viral isolation, and/or PCR at a reference laboratory41,51. The Tanzania study performed blood culture and/or blood smears for malarial pathogens.
Reference standard adjudication of etiology
Phenotypic adjudication of bacterial, viral, or noninfectious etiology independent of cohort selection (described above) was performed by a panel of ≥ 2 physicians who reviewed all available microbiologic data, de-identified clinical data extracted from case report forms (international), or the full medical records (USA) (Supplemental Table 2). Participants known to have malaria by blood smear were excluded due to insufficient frequency required to generate a parasitic classifier. Non-infectious cases had supportive clinical and radiographic data along with negative testing for infectious etiologies. Infectious cases were defined by positive etiology testing and supportive clinical data. Participants included from Tanzania had confirmed bacterial etiologic testing, but did not undergo testing for viral co-infection because dengue testing and respiratory viral swab were not available as part of this study (Supplemental Table 1).
Generation and normalization of transcriptomic data
Total RNA was extracted from whole blood collected and stored at − 70 °C in PAXgene Blood RNA tubes using the PAXgene miRNA Extraction Kit (QIAGEN) according to manufacturer’s instructions. RNA yield and integrity were assessed using NanoDrop ND-2000 spectrophotometer (ThermoFisher Scientific, Wilmington, DE) and 2100 Bioanalyzer with RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA), respectively. All RNA was purified under BSL3 conditions by approved protocols at Duke Regional Biocontainment Laboratory, except B. pseudomallei mRNA isolated under BSL4 conditions by standard procedures at the Navel Medical Research Laboratory.
RNA sequencing was performed at EAGenomics/Q2 Labs (Durham, NC) for 183 samples and the Duke Sequencing and Genomic Techologies Facility for 111 samples. Library preparation resulted in selected poly-A mRNA for sequencing using GlobinClear RNA Reduction (Invitrogen) and TruSeq Stranded mRNA Library Kit (Illumina) for the EA Genomics/Q2 Labs batch, and NuGEN Universal Plus mRNA-Seq Library Prep Kit with AnyDeplete Globin depletion (NuGEN/Tecan) for the Duke Sequencing Facility batch. Sequencing libraries were sequenced on Illumina HiSeq 2500 instrument (EA Genomics/Q2 Labs) or NovaSeq 6000 instrument (Duke Sequencing Facility) with 50 bp paired-end reads and target of > 40 million reads per sample, including crossover of 24 samples between the two batches to allow for quality control and batch corrections.
Nanostring multiplex transcript detection platform
Quantitative RT-PCR assays for genes in both the Global Fever Bacterial/Viral (GF-B/V) and Global Fever-Bacterial/Viral/Noninfectious (GF-B/V/N) models were developed using the NanoString platform. Total RNA (100 ng) from each participants was analyzed using a NanoString nCounter XT custom transcriptional response probe panel (NanoString Technologies, Seattle, WA). Nanostring assay processing was performed by the Duke Microbiome Core Facility according to manufacturer instructions.
Statistical analysis
We used Limma-voom modeling to obtain differential expression of transcripts ≥ tenfold in bacteria versus virus infected participants with an adjusted p-value < 0.01 in the discovery cohort. A cutoff of ≥ tenfold was used to identify the most highly differentially expressed genes. A significance threshold of 5% false discovery rate (FDR) was used. Pathway analysis used Database for Annotation, Visualization and Integrated Discovery (DAVID) and ENRICHr programs to create broad functional groups. Transcripts that did not fit into well-defined ontologic clusters were categorized by literature review.
To develop predictive models, the discovery cohort included Duke and Sri Lanka participants because these sites had similar extensive phenotypic analysis for both bacterial and viral pathogens and adequate populations of at least two of the phenotypes classes. We developed a simple binary GF-B/V model including only participants with bacterial or viral infection (Fig. 1A). Since fever or suspected infections may be neither bacterial nor viral, we incorporated participants with non-infectious illness as a control group in a second modeling approach (GF-B/V/N). The GF-B/V/N model used two binary predictive classifiers for discrimination: bacterial vs. non-bacterial (viral or non-infectious), and viral vs. non-viral (bacterial or non-infectious). The categorization of bacterial or viral illness by the GF-B/V/N test is made for each participant by comparing the probabilities of each binary classifier (Supplemental Fig. 1A). High-confidence noninfectious samples were only available from the USA, but there were no significant difference in expression of control house-keeping genes that would suggest a site specific or confounding affect.
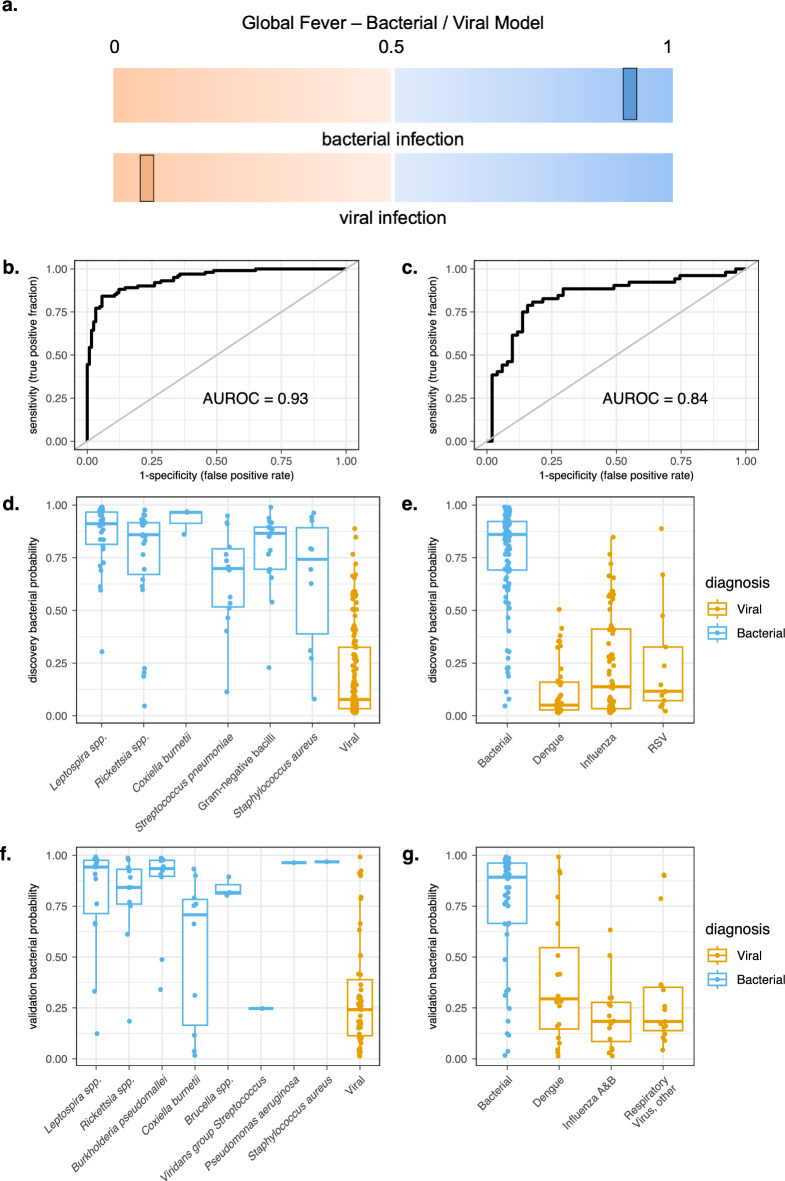
Figure 1.
Performance of GF-B/V model to classify bacterial and viral disease in a global cohort. (A) A binary model (GF-B/V) provides a single score that discriminates bacterial from viral infection. High probabilities closer to 1 are associated with bacterial infection and low probabilities closer to 0 indicate viral infection. (B) AUROC curve of the discovery cohort (RNA sequencing) for GF-B/V model. (C) AUROC curve of the validation cohort (NanoString platform) for GF-B/V model. (D) Predicted probabilities for the GF-B/V model in the discovery cohort for bacterial pathogens (blue) compared to viral pathogens (orange) using RNA sequencing. (E) Predicted probabilities for the GF-B/V model in the discovery cohort for bacterial pathogens (blue) compared to viral pathogens (orange) using NanoString assay. Bacterial abbreviations: Gram negative bacilli = Escherichia coli, Klebsiella pneumoniae, Rickettsia spp. = Spotted fever group, Typhus group, Orientia tsutsugamushi. Viral abbreviations: Other Resp. Virus = human Rhinovirus, Parainfluenza, human Metapneumovirus, Respiratory Syncytial Virus.
Standard quality control and principal component analysis was performed and ensured there were no site dependent effects or inappropriate clustering of the data. We then conducted supervised regularized regression (Least Absolute Shrinkage and Selection Operator [LASSO]) analysis of the entire transcriptome. Nested, repeated (500 repeats) fivefold cross-validation was performed to estimate predicted probabilities. All model-building steps were performed on training data only to maintain unbiased estimates generated on the test fold. Predicted probabilities were utilized to estimate area under the receiver operating curve (AUROC) and ROC01 method was used to select a cutoff to estimate accuracy and characterize performance. Use of 500 sets of predictions for the discovery cohort limited calculation of predicted confidence intervals by the standard approach52, but was more representative of model development.
The validation cohort was designed to represent a more typical global population; thus, sites representative of a single class or with less extensive phenotyping were included. To generate NanoString nCounter assays, we expanded feature prediction to include correlated transcripts that can substitute for one another with respect to class prediction (bacterial, viral, or noninfectious). Feature selection was performed using elastic net regression and the selection frequency across resampling iterations measured variable importance. Characterizing performance in a targeted validation study required selecting 263 transcripts (Supplementary Table 3). Endogenous control transcripts (TRAP1, DECR1, TBP, and PPIB) were incorporated to normalize for differences in sample input and correct for technical variability. A model was trained on the NanoString data using 91 participants from the discovery cohort (47 bacterial, 34 viral, 10 noninfectious), accommodating known positive control normalization to reduce technical variability and allow background subtraction using negative controls. Discovery cohort participants selected for model training on NanoString prioritized three goals in the following order: 1) balance of infectious etiologies and phenotypes, 2) robust performance in the discovery models, and 3) representation from diverse geographic regions and pathogens. Noninfectious samples were not incorporated into the validation cohort due to availability of unique specimens and a desire to incorporate increased infectious etiologies. The NanoString GF-B/V and GF-B/V/N models were then fixed and applied to the independent validation cohort.
Confidence intervals were calculated using the epiR package in R. exact binomial for the sensitivity, specificity, and model accuracy53. The approach of Simel et al. was used to calculate confidence intervals for the positive and negative likelihood ratios54. Confidence intervals for the validation AUROCs were calculated using the method of DeLong52. A confidence interval for the overall accuracy of the GF-B/V/N model for the discovery cohort was estimated by taking 10,000 bootstrapped samples. We used the nonparametric Mood’s median test to calculate the p-value estimating the differences in median ages and to evaluate whether the proportion of women in bacterial samples was different than non-bacterial samples.
Ethical approval
Prospective collection of specimens and data after written informed consent by subjects or their legally authorized representatives, and assent was obtained for minors less than 18 years old. Studies were approved by Institutional Review Boards of Duke University Health System, Faculty of Medicine, University of Ruhuna, Johns Hopkins University, Naval Medical Research Center, Kilimanjaro Christian Medical Center Research Ethics Committee, Tanzania National Institute for Medical Research National Research Ethics Coordinating Committee, University of Otago Human Ethics Committee (Health), and the USA CDC. This study used deidentified specimens and clinical data, and was approved by Duke University Health System (Durham, NC) Institutional Revew Board (Duke IRB Pro00072857). All research was conducted in accordance with the Declaration of Helsinki.
Results
Participants and pathogens
The discovery cohort consisted of participants from the USA and Sri Lanka with median age 48 years (IQR 31–61; range 10–86 years), 48% female, 1.4% Hispanic, 28.5% White, 19% Black/African American, 49.5% Asian/South Asian (Table 1). The median age of the USA cohort was higher than the Sri Lankan cohort (54 years [(IQR 42–66] vs. 37 years [IQR 26–51], p = 0.51), although this was not statistically significant. Those with bacterial infections were more likely to be male (p = 0.001), but this was not site or pathogen specific. USA participants had more severe illness (intensive care, 16.4% [n = 25/152], mechanical ventilation, 8.5% [n = 13/152], and mortality 7.2% [n = 11/152]) than those in Sri Lanka (intensive care, 0.7% [n = 1/142], mechanical ventilation, 0.7% [n = 1/142], and mortality, 0.7% [n = 1/142]). However, determining severity of illness between internationally diverse clinical settings, types of infection, and standards of care may be misleading. Chronic HIV was low across the total cohort (3 USA in discovery cohort, 3 Tanzania in validation cohort), and although HIV status was not collected for Sri Lanka the incidence in the country is < 0.01%.
Table 1.
Demographics and participant characteristics of discovery and validation cohort.
| Discovery cohort | USA | Sri Lanka | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 294) | Bacterial (n = 42) | Viral (n = 43) | Noninfectious (n = 67) | Bacterial (n = 60) | Viral (n = 82) | |||
| Age, median (IQR) | 48 (31, 61) | 58 (49, 66) | 50 (28, 66) | 54 (42, 66) | 41 (31, 50) | 34 (23, 51) | ||
| Sex, n (%) | ||||||||
| Male | 155 (52.7) | 28 (66.7) | 14 (32.6) | 30 (44.7) | 39 (66.7) | 38 (51.2) | ||
| Female | 139 (47.3) | 14 (33.3) | 29 (67.4) | 37 (55.3) | 21 (33.3) | 44 (48.8) | ||
| Race, n (%) | ||||||||
| Hispanic | 142 (48.3) | 38 (90.5) | 39 (93.0) | 65 (97.0) | – | – | ||
| Non-Hispanic | 4 (1.4) | 2 (4.8) | 1 (2.3) | 1 (1.5) | – | – | ||
| 5 (1.7) | 2 (4.7) | 2 (4.6) | 1 (1.5) | – | – | |||
| Ethnicity, n (%) | ||||||||
| White | 87 (29.5) | 27 (64.3) | 25 (58.1) | 35 (52.2) | – | – | ||
| Black | 56 (19.0) | 13 (31.0) | 14 (32.6) | 29 (43.3) | – | – | ||
| Asian/S. Asian | 144 (49.0) | 0 (0.0) | 0 (0.0) | 2 (4.7) | 60 (100) | 82 (100) | ||
| American In/Alaska Na | 5 (1.7) | 2 (4.8) | 1 (2.3) | 2 (3.0) | – | – | ||
| Hawaiian/Pacific Is | 1 (0.3) | 0 (0.0) | 1 (2.3) | 0 (0.0) | – | – | ||
| Other/unknown | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (1.5) | – | – | ||
| Duration ill, median days (IQR) | 4 (3, 6) | 3 (2, 5.5) | 4 (2. 5, 5.5) | 3 (2, 11) | 5 (3, 8) | 4 (3, 5) | ||
| Hospital length of stay, median days (IQR) | 4 (2, 6) | 6 (3, 9) | 0 (0, 15) | 2 (1, 6) | 5 (4, 6) | 4 (3, 6) | ||
| Intensive care, n (%) | 26 (8.8) | 11 (26.2) | 3 (7.0) | 11 (16.4) | 1 (1.7) | 0 (0.0) | ||
| Mech. ventilation, n (%) | 14 (4.8) | 6 (14.3) | 1 (2.3) | 6 (9.0) | 1 (1.7) | 0 (0.0) | ||
| Mortality, n (%) | 12 (4.0) | 5 (11.9) | 0 (0.0) | 6 (9.0) | 0 (0.0) | 1 (1.2) | ||
| Pathogens | – | 11 S. aureus | 17 FluA | NA | 30 Leptospira spp. | 43 Dengue | ||
| 14 S. pneumo | 12 FluB | 27 Rickettsia spp. | 23 FluA | |||||
| 9 E. coli | 14 RSV | 3 C. burnetii | 16 FluB | |||||
| 9 K. pneumo | ||||||||
| Validation cohort | USA | Sri Lanka | Cambodia | Tanzania | Australia | |||
|---|---|---|---|---|---|---|---|---|
| Total (n = 101) | Bacterial (n = 3) | Viral (n = 16) | Bacterial n = 24) | Viral (n = 29) | Bacterial (n = 10) | Bacterial (n = 15) | Viral (n = 4) | |
| Age, median (IQR) | 36.5 (27.8, 54.3) | 61 (60.5, 73.5) | 49 (29, 60) | 37 (29.5, 58) | 30 (25, 47) | 51 (36.3, 58.8) | 34 (30.5, 41.5) | 27 (27, 35.5) |
| Sex, n (%) | ||||||||
| Male | 55 (54.5) | 3 (100) | 5 (31.3) | 14 (58.3) | 21 (72.4) | 5 (50.0) | 6 (40.0) | 1 (25.0) |
| Female | 46 (45.5) | 0 (0.0) | 11 (68.8) | 10 (41.7) | 8 (27.6) | 5 (50.0) | 9 (60.0) | 3 (75.0) |
| Race, n (%) | ||||||||
| Hispanic | – | 3 (100) | 16 (100) | – | – | – | – | 4 (100) |
| Non-Hispanic | – | 0 (0.0) | 0 (0.0) | – | – | – | – | 0 (0.0) |
| Unknown | – | 0 (0.0) | 0 (0.0) | – | – | – | – | 0 (0.0) |
| Ethnicity, n (%) | ||||||||
| White | 12 (11.9) | 2 (66.7) | 6 (37.5) | – | – | – | – | 4 (100) |
| Black | 26 (25.7) | 1 (33.3) | 10 (62.5) | – | – | – | 15 (100) | 0 (0.0) |
| Asian/S. Asian | 63 (62.4) | 0 (0.0) | 0 (0.0) | 24 (100) | 29 (100) | 10 (100) | – | 0 (0.0) |
| Pathogens | 1 S. aureus | 2 Dengue | 15 Leptospira spp. | 18 Dengue | 10 B. pseudomallei | 3 Brucella spp. | 4 FluA | |
| 1 VGS | 4 FluA | 9 Rickettsia spp. | 7 FluA | 2 Rickettsia spp. | ||||
| 1 P. aerug | 2 RSV | 4 HRV | 10 C. burnetii | |||||
| 2 HRV | ||||||||
| 3 Paraflu | ||||||||
| 3 hMPV | ||||||||
Days ill number of days ill prior to presentation, ICU Intensive care unit, Mech. Ventilation Invasive Mechanical Ventilation, NA not applicable, S. aureus Staphylococus aurus, S. pneumo Steptococcus pneumoniae, E. coli Escherichia coli, K. pneumo Klebsiella pneumoniae, FluA Influenza A, FluB Influenza B, RSV Respiratory Syncitial Virus, C. burnetii Coxiella burnetii, VGS Viridians group Streptococcus, P. aerug Pseudomonas aeruginosa, HRV Human Rhinovirus, Paraflu ParaInfluenza, hMPV. human Metapneumovirus, B. pseudomallei Burkholderia pseudomallei.
The discovery cohort included 102 participants with bacterial (42 with bloodstream infections and 60 bacterial zoonoses), 125 with viral (82 respiratory, 43 dengue), and 67 with non-infectious illness (e.g., pulmonary embolus, congestive heart failure, COPD/Asthma, cancer, autoimmune disorders). The validation cohort had 101 participants (52 bacterial, 49 viral) and represented a wider range of demographics, geographic locations (USA, Sri Lanka, Tanzania, Cambodia, and Australia), and pathogens (Table 1). Patients with non-infectious illness were not analyzed in the validation cohort.
Differential gene expression of global pathogens
To identify differentially expressioned genes, we employed a conservative approach, using a 5% FDR and a ≥ tenfold change in expression. We identified 38 unique genes increased at least tenfold in participants with bacterial illness, and these were divided into 18 primary clusters (Table 2). Transcripts corresponded to known pathways for acute phase reactants, antimicrobial killing, innate immunity, and immune response. Similarly, we identified 65 unique genes associated with increased expression by tenfold or greater in viral infection, and these were divided into 17 primary clusters (Table 2) primarily corresponding to interferon response and chemokine/cytokine pathways.
Table 2.
Differential expression of genes upregulated at least tenfold in bacterial versus viral illness.
| Functional category | Genes |
|---|---|
| Upregulated in bacterial infection | |
| Acid/base equilibrium | CA4 |
| Acute phase reactants | ALPL, C4BPA, HP, HPR, ORM1, ORM2 |
| Antimicrobial killing | ARG1, PGLYRP1, PI3, S100A12, SLPI |
| Apoptosis/development | KREMEN1 |
| Cell division | SPATC1 |
| Cell migration | ITGA7 |
| Cell motility | CFAP126 |
| Epigenetics | KDM5D |
| Extracellular matrix integrity | ADAMTS2, PCOLCE2 |
| Heavy metal binding | MT1H |
| Immune regulation (cell surface receptors) | CD177, CD300LD, VSIG4 |
| Innate immune response | IL1R2 |
| Metabolism | OLAH, SLC51A, VNN1 |
| Protein degradation | MMP8, PGA4 |
| Protein processing/sorting | AP3B2, FAM20A, GALNT14, ZDHHC19 |
| Signal transduction | BMX, NECAB1, RCVRN |
| Transcription | KLF14 |
| Translation | EIF1AY |
| Upregulated in viral infection | |
| Amino acid metabolism | IL4I1, SDS |
| Antimicrobial killing | DEFB1 |
| Apoptosis | BCL2L14 |
| Autophagy | RUFY4 |
| Cell activation receptors | LY6E |
| Cell–cell interactions | AGRN, DSP, SIGLEC1 |
| Cell differentiation/growth | AXL, EPHB2 |
| Cell motility | DZIP1L, TTC21A |
| Cell structure/junctional | JUP, KRT5, NEXN, OTOF, SAMD4A |
| Electrochemical gradiant | NKAIN1 |
| Exocytosis | EXOC3L1 |
| Interferon response/chemokines/cytokines | DDX60, HERC5, HERC6, IFI27, IFI44, IFIT1, IFIT2, IFIT3, ISG15, LAMP3, MX1, NRIR, OAS1, OAS2, OAS3, OASL, RSAD2, USP18, CCL2, CCL8, CXCL10, CXCL11, FPR3, LIP |
| Intracellular trafficking | FBX039, RABGAP1L, RIN2 |
| Mitochondrial DNA synthesis | CMPK2 |
| Pattern recognition receptors | CLEC4F, TLR3 |
| Transcription | HES4, HESX1, ZNF684 |
| Non-coding or poorly characterized/unknown | ALMS1P1, ERICH3, HSPB9, KCTD14, LINC00487, LOC100133669, LOC101927027, LOC105369192, SPATS2L, TMEM252, TMEM255A, XIST |
Genes upregulated in bacterial disease are conversely downregulated in viral disease and vice versa.
Bacterial versus viral classification: a simple binary model
We conducted predictive analysis to develop a binary model (Fig. 1A) using the entire transcriptome from the discovery cohort. The Global Fever-Bacterial/Viral (GF-B/V) model classified bacterial from viral disease with high accuracy when internally validated using fivefold cross-validation: AUROC of 0.93 (Fig. 1B), sensitivity of 84.2% (95% CI 75.6–90.7), specificity of 94.7% (95% CI 88.6–97.7), and overall accuracy of 89.7% (95% CI 85.0–93.4). Additional performance characteristics are shown in Table 3. The model demonstrated similar performance after stratifying for specific pathogen (Fig. 1D), site, age, sex, or race (Supplemental Fig. 2).
Table 3.
Performance characteristics for Global Fever classifier models for acute bacterial and viral infection.
| Cohort | Comparison | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Model accuracy, % (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) |
|---|---|---|---|---|---|---|
| Global fever bacterial/viral model (GF-B/V) | ||||||
| Discovery | Bacterial versus viral | 84.2 (75.6–90.7) | 94.7 (88.6–97.7) | 89.7 (85.0–93.4) | 14.7 (7.2–30.5) | 0.2 (0.1–0.3) |
| Validation | Bacterial versus viral | 78.8 (65.3–88.9) | 84.3 (71.4–93.0) | 81.6 (72.7–88.5) | 5.0 (2.6–9.6) | 0.3 (0.1–0.4) |
| Global fever bacterial/viral/noninfectious model (GF-B/V/N) | ||||||
| Discovery | Bacterial versus nonbacterial | 87.7 (79.0–89.8) | 84.2 (78.2–89.1) | 85.2 (80.6–89.1) | 5.5 (3.9–7.7) | 0.2 (0.1–0.3) |
| Viral versus nonviral | 83.7 (76.0–89.8) | 81.5 (74.8–87.1) | 82.5 (77.6–86.7) | 4.5 (3.3–6.3) | 0.2 (0.1–0.3) | |
| Validation | Bacterial versus nonbacterial | 82.7 (69.7–91.8) | 80.4 (66.9–90.2) | 81.6 (72.7–88.5) | 4.2 (2.4–7.5) | 0.2 (0.1–0.4) |
| Viral versus nonviral | 76.5 (62.5–87.2) | 80.8 (67.5–90.4) | 78.6 (69.5–86.1) | 4.0 (2.237.1) | 0.3 (0.2–0.5) | |
The top of the table provides performace characteristics for the GF-B/V model and the bottom of the table shows performance of the GF-B/V/N model. In the discovery cohort, performance characteristics are calculated using nested cross validation on the original RNA sequencing data. In the validation cohort, the model is fixed and applied to NanoString data of an independent bacterial and viral cohort. Positive and negative predictive value requires knowledge of prevalence in the community which is not known for global infections. Thus, these could not be calculated.
To independently validate this model using a quantitative RT-PCR system that more closely approximates a clinical assay, we used the NanoString system to measure expression levels of 27 highly predictive genes (Supplemental Table 4A). After training a classification model on subjects from the discovery cohort, the model and its parameters were fixed and applied to the validation cohort. We incorporated both pathogen and geographic diverisity (Table 1). For the discrimination of bacterial and viral infection, the GF-B/V model an AUROC of 0.84 (95% CI 0.76–0.9) (Fig. 1C), sensitivity of 78.8% (95% CI 65.3–88.9), specificity of 84.3% (95% CI 71.4–93.0), and overall accuracy of 81.6% (95% CI 72.7–88.5) with additional performance characteristics reported (Table 3). Additionally, GF-B/V discriminated difficult-to-diagnose bacterial zoonotic pathogens not included in the discovery cohort, such as spotted fever group rickettsiae, B. pseudomallei, and Brucella spp. (Fig. 1E).
Classification of bacterial and viral infections in the setting of other illness: a complex model
The Global Fever-Bacterial/Viral/Noninfectious (GF-B/V/N) classifier provides two probabilities, a measure of bacterial infection or viral infection in the context of nonbacterial/nonviral illness as a control (Supplemental Fig. 1A). Theoretically, this model has the potential for identifying a co-infection if both the probability of bacterial and viral infection were high (Supplemental Fig. 1A). For classification of bacterial infection (bacterial vs. nonbacterial model) the AUROC was 0.92 (Supplemental Fig. 1B), with sensitivity 87.7% (95% CI 79.0–89.8), specificity 84.2% (95% CI 78.2–89.1), and accuracy 85.2% (95% CI 80.6–89.1) (Table 3). For the classification of viral infection (viral vs. nonviral model), AUROC was 0.91 (Supplemental Fig. 1C), with sensitivity 83.7% (95% CI 76.0–89.8), specificity 81.5% (95% CI 74.8–87.1), and accuracy 82.5% (95% CI 77.6–86.7) (Table 3). Similar to the binary model, the GF-B/V/N test demonstrated good performance for a broad range of bacterial and viral pathogens (Supplemental Fig. 1D,E).
Translation of the 2-model GF-B/V/N system to NanoString was exploratory in nature because it only validated the GF-B/V/N test for bacterial and viral illness, evaluating how often bacterial or viral disease was misclassified in the context of nonbacterial/nonviral illness. We measured expression of 33 genes for the bacterial model and 19 for the viral model (Supplemental Table 4B,C). In the validation cohort, the bacterial model had an AUROC of 0.84 (95% CI 0.76–0.93) (Supplemental Fig. 1F), sensitivity of 82.7% (95% CI 69.7–91.8), specificity of 80.4% (95% CI 66.9–90.2), and accuracy of 81.6% (95% CI 72.7–88.5) (Table 3). The viral model had an AUROC of 0.85 (95% CI 0.77–0.93) (Supplemental Fig. 1G), sensitivity of 76.5% (95% CI 62.5–87.2), specificity of 80.8% (95% CI 67.5–90.4), and accuracy of 78.6% (95% CI 69.5–86.1) for viral infection (Table 3). Performance was similar across pathogens (Supplemental Fig. 1H,I), except for a single Viridans group streptococcus case.
Discordant classifications
Discordant cases in the validation cohort were similar between the two classifiers (19 GF-B/V, 19 GF-B/V/N; with overlap of 15 for both models) (Supplemental Table 5). A review of these discordant cases did not identify any pattern with respect to site or pathogen. The relative increased number of Sri Lanka patients was nearly proportional to the total number in the whole cohort. Interestingly, when predictive genes were fixed and the model weights were allowed to vary among the validation cohort, performance improved.
Discussion
We utilized a 294-participant multinational prospectively enrolled cohort to develop a bacterial versus viral host-response classifier that incorporates LMIC with representation of zoonotic bacteria and arboviruses. While others have utilized publically available data to apply host-response transcriptional classifiers to atypical global infections33, this cohort is the largest prospectively enrolled with robust clinical, phenotypic, and adjudication data. Translation of the GF-B/V test to a multiplex gene expression detection platform demonstrated good performance (overall accuracy of 81.6% [95% CI 72.7–88.5]) in independent validation despite different genetic backgrounds, geographies (five countries), and pathogens. For example, a person with a positive GF-B/V NanoString test in the validation cohort was 5-times more likely to have a bacterial infection and 3-times less likely with a negative test. Such a test could provide timely diagnostic reassurance to inform antibiotic use and guide clinical care.
Decreasing morbidity, mortality, and misuse of antimicrobials from infections requires improved diagnosis at the time a patient presents to care. LMIC have decreased laboratory infrastructure, so performing multiple pathogen-based tests is unrealistic. Accurate acute-phase pathogen-based diagnostics do not exist for many bacterial zoonotic infections, such as ricktettsial infections, that require different treatment from antibiotics empirically used for routinely cultivatable organisms. Point-of-care biomarkers commonly utilized in high-resource settings, like C-reactive protein and procalcitonin, have yielded mixed performance in LMIC (e.g., low specificity, poorer performance for bacterial zoonotic pathogens)27,50,55–57, and are potentially affected by higher rates of malnutrition, parasitic disease, HIV, and co-infection58. Host-response gene expression assays are poised to fill this void25–27,31–33,59,60.
Tremendous progress has been made developing host-response diagnostics in HIC in multiple disciplines, including infectious diseases31,59–61. Recently, an algorithmic approach utilizing publically available data extended this method to intracellular and atypical pathogens prevalent globally33. Rao et al., utilize a co-normalization technique to diminish study variability and batch effects. While the signal for the bacterial versus viral classifier was preserved, the co-normalization technique could potentially reduce biological variability and artificially improve overall performance in a population with increased variability of pathogens and genetic ancestry. Additionally, use of publically available data does not align enrollment criteria or apply an even reference standard. Prospective validation of this promising work will be critical to determine performance in a real world population of global infections.
Taking a different approach, our study utilized existing biorepository specimens of prospectively enrolled patients that meet reliable eligibility criteria and apply a consistent diagnostic reference. This approach preserves biological variability while avoiding potential bias and confounding. Access to participant-level clinical, biologic, and etiologic data allows refinement of the cohort not possible for publically available data. Additionally, the GF-B/V incorporates a significant number of zoonotic bacterial pathogens that are both extracellular (e.g. Leptospirosis spp.) and intracellular (e.g. Ricketsial spp.) at the model development and validation phase, while other studies have a low percentage of Leptospirosis or other extracellular pathogens representated in LMIC settings33.
A binary bacterial versus viral classifier provides a simple approach to identifying bacterial infections, but does not account for other treatable etiologies of suspected infection. Layered diagnostic tests using multiple binary classifiers, like GF-B/V/N, are more generalizable for a global population, and are attractive given the breadth of pathogen diversity and febrile illness globally. Precedent exists for layered transcriptional expression classifiers that incorporate other classes of illness25,32. We demonstrate a more complex model can discriminate bacterial from viral infection in an independent validation cohort, but the absence of noninfectious samples in the validation cohort limits full evaluation in a real world population. Thus, we cannot comment on noninfectious illness, but simply on nonbacterial or nonviral disease. However, we demonstrate that misclassification by GF-B/V or GF-B/V/N is largely overlapping, reassuringly demonstrating that incorporating more complexity does not reduce performance in a limited population of bacterial and viral illness. Incorporating multiple models for this and other work has previously been shown and will need to be addressed going forward62. While exploratory, a model with this complexity is not available in other published work on global pathogens, such as leptospirosis or rickettsial infection31,33,63,64. The composite model could provide a path forward in the complex milleu of global illness.
Host response biomarkers could change clinical practice, but expansion of these diagnostics to LMIC must be inexpensive, easy to operate, and clinically interpretable. Host gene expression diagnostics for non-infectious applications are considered high complexity tests, often run in referral laboratories. However, technical advances have enabled highly multiplexed quantitative, real-time PCR systems that operate in a sample-in, answer-out format with results available in < 1-h32,36,60. As simpler host gene expression tests continue to be developed, cost-of-goods and simplicity will be key parameters for their implementation in LMIC settings65. Host response-based biomarker panels have also extended to proteomics and metabolomics64,66,67, which may be less expensive and amenable to field deployable diagnostics. Progress refining host-response biomarkers in international cohorts must occur alongside technological advances in platform development to allow more rapid translation to LMIC. The results presented here suggest easy translatability of this approach to LMIC.
GF-B/V and GF-B/V/N multi-analyte biomarkers have attractive features, but there are limitations to this study. Translation to a PCR-based detection system revealed lower accuracy in the validation cohort compared to the RNA-seq based classification in the discovery cohort. This could be due to technical differences (e.g., going from RNAseq to NanoString) but is also an expected difference between discovery and validation, the latter of which includes a wider array of infections and variability of illness. Analysis of discordant classifications suggests that genes used in the models have strong predictive power, but that individuals have variability in the amount, or weight, each gene contributes to the model. Consistent with this is the observation that both classifiers had a reduction of performance on pathogens not hightly represented in the discovery cohort (Viridians group Streptococcus, non-influenza respiratory viruses, Coxiella burnetii). The GF-B/V/N model is constrained by reliance on non-infectious illness as a control rather than being representative of febrile illness globally. Additional limited availability of high confidence noninfectious samples prevented incorporation into the validation cohort, prohibiting validation of the performance of the GF-B/V/N test for nonbacterial/nonviral illness or co-infection. It will be critical for future studies to perform iterations and optimization on expanded cohorts with increased pathogen (e.g. atypical viruses, tuberculosis, malaria, cryptococcus) and host diversity (e.g., a larger cohort of children and immunocompromised hosts) that would be expected to improve model weights, overall performance, and be more representative of febrile illnesses62.
We found that novel host transcriptional biomarkers could accurately discriminate diverse bacterial and viral infections, including those endemic in not only high-income temperate regions but also LMIC in the tropics. Translation of these tests to a custom multiplex gene expression platform, such as the NanoString, shows promise for identification of infections in increasingly diverse populations with the future possibility of point-of-care application. Host-response biomarkers to distinguish bacterial from viral infection could improve clinical care and antibiotic stewardship across the globe.
Supplementary Information
Acknowledgements
We thank the 395 participants worldwide for the provision of samples and clinical data and the study teams that recruited them.
Disclaimer
The opinions expressed herein are those of the author(s) and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD); or, the United States Army, Navy, or Air Force. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation. In the conduct of research where humans are the subjects, the investigator(s) adhered to the policies regarding the protection of human subjects as prescribed by Code of Federal Regulations (CFR) Title 45, Volume 1, Part 46; Title 32, Chapter 1, Part 219; and Title 21, Chapter 1, Part 50 (Protection of Human Subjects).
Author contributions
E.R.K. and M.E.R. wrote the manuscript. Project conception, design, planning and data interpretation were carried out by E.R.K., M.E.R., L.G.T., T.W.B., R.H., M.T.M., S.S., B.N., E.P., E.L.T., G.S.G., T.D.M., and C.W.W. Analysis and figures prep were performed by E.R.K., C.M., A.B., R.H., and S.S. Sample acquisition at global sites conducted by M.E.R., L.G.T., C.K.B., A.N., V.D., M.P.R., V.P.M., B.F.L., D.M., W.K.-A., R.K., A.D.D., D.V.C., K.L.S., and J.A.C. Laboratory work and etiology testing performed by T.W.B., C.K., R.D., J.S.D. and B.N.
Funding
This work is supported by the US Army Medical Research and Materiel Command under Contract No. W81XWH-16-C-0147. Tanzania studies were supported by US NIH NIAID R01TW009237.
Data availability
All data in this article were generated as part of this work. All RNA sequencing data has been submitted to GEO under accession number GSE211567. NanoString transcripts are included in supplemental information. Token to access GSE211567: obqzkkoarjwpfct.
Competing interests
CWW is a consultant for Biomeme, Arena Pharmaceuticals, Biofire, FHI Clinical and sits on the advisory board for Biomeme, FHI Clinical, and Regeneron. CWW is also the acting Chief Medical Officer for Biomeme. CWW is a member of the board of directors for Global Health Innovation Alliance Accelerator. TWB hold equity in and is consultant for Biomeme. ELT consults for Biomeme and is employed by Danaher Diagnostics. CWW, ELT, MTM, TWB, and RH hold patents on genomic methods to diagnose and treat acute infections. All other authors reported no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Emily R. Ko and Megan E. Reller.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-49734-6.
References
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrar, J. J. J. T., Kang, G., Lalloo, D., & White, N. J. Manson's Tropical Diseases (Saunders, 2009).
- 3.Naghavi M, Murray CJL, Ikuta KS, Mestrovic T, Swetschinski L, Sartorius B. Global burden of antimicrobial resistance: Essential pieces of a global puzzle—Authors' reply. Lancet. 2022;399:2349–2350. doi: 10.1016/S0140-6736(22)00947-3. [DOI] [PubMed] [Google Scholar]
- 4.Barr DA, Lewis JM, Feasey N, Schutz C, Kerkhoff AD, Jacob ST, et al. Mycobacterium tuberculosis bloodstream infection prevalence, diagnosis, and mortality risk in seriously ill adults with HIV: A systematic review and meta-analysis of individual patient data. Lancet Infect. Dis. 2020;20:742–752. doi: 10.1016/S1473-3099(19)30695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crump JA, Morrissey AB, Ramadhani HO, Njau BN, Maro VP, Reller LB. Controlled comparison of BacT/Alert MB system, manual Myco/F lytic procedure, and isolator 10 system for diagnosis of Mycobacterium tuberculosis Bacteremia. J. Clin. Microbiol. 2011;49:3054–3057. doi: 10.1128/JCM.01035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang LY, Chow SC, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected children and infants in northern Tanzania. Trop. Med. Int. Health. 2011;16:830–837. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin. Infect. Dis. 2011;52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray KD, Cunningham CK, Clifton DC, Afwamba IA, Mushi GS, Msuya LJ, et al. Prevalence of mycobacteremia among HIV-infected infants and children in northern Tanzania. Pediatr. Infect. Dis. J. 2013;32:754–756. doi: 10.1097/INF.0b013e318286957f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havers FP, Hicks LA, Chung JR, Gaglani M, Murthy K, Zimmerman RK, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Netw. Open. 2018;1:e180243. doi: 10.1001/jamanetworkopen.2018.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee GC, Reveles KR, Attridge RT, Lawson KA, Mansi IA, Lewis JS, 2nd, et al. Outpatient antibiotic prescribing in the United States: 2000 to 2010. BMC Med. 2014;12:96. doi: 10.1186/1741-7015-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J. Antimicrob. Chemother. 2014;69:234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 12.Tillekeratne LG, Bodinayake CK, Dabrera T, Nagahawatte A, Arachchi WK, Sooriyaarachchi A, et al. Antibiotic overuse for acute respiratory tract infections in Sri Lanka: A qualitative study of outpatients and their physicians. BMC Fam. Pract. 2017;18:37. doi: 10.1186/s12875-017-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Wang P, Wang X, Zheng Y, Xiao Y. Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 2014;174:1914–1920. doi: 10.1001/jamainternmed.2014.5214. [DOI] [PubMed] [Google Scholar]
- 14.Al-Hadidi SH, Alhussain H, Abdel Hadi H, Johar A, Yassine HM, Al Thani AA, et al. The spectrum of antibiotic prescribing during COVID-19 pandemic: A systematic literature review. Microb. Drug Resist. 2021;27:1705–1725. doi: 10.1089/mdr.2020.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhimal M, Dumre SP, Sharma GN, Khanal P, Ranabhat K, Shah LP, et al. An outbreak investigation of scrub typhus in Nepal: Confirmation of local transmission. BMC Infect. Dis. 2021;21:193. doi: 10.1186/s12879-021-05866-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dittrich S, Rattanavong S, Lee SJ, Panyanivong P, Craig SB, Tulsiani SM, et al. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: A prospective study. Lancet Glob. Health. 2015;3:e104–112. doi: 10.1016/S2214-109X(14)70289-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinbrink JM, Myers RA, Hua K, Johnson MD, Seidelman JL, Tsalik EL, et al. The host transcriptional response to Candidemia is dominated by neutrophil activation and heme biosynthesis and supports novel diagnostic approaches. Genome Med. 2021;13:108. doi: 10.1186/s13073-021-00924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Antimicrobial Resistance—Global Report on Surveillance (World Health Organization, 2014).
- 19.Zaas AK, Chen M, Varkey J, Veldman T, Hero AO, 3rd, Lucas J, et al. Gene expression signatures diagnose influenza and other symptomatic respiratory viral infections in humans. Cell Host. Microbe. 2009;6:207–217. doi: 10.1016/j.chom.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloom CI, Graham CM, Berry MP, Rozakeas F, Redford PS, Wang Y, et al. Transcriptional blood signatures distinguish pulmonary tuberculosis, pulmonary sarcoidosis, pneumonias and lung cancers. PLoS ONE. 2013;8:e70630. doi: 10.1371/journal.pone.0070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihret A, Loxton AG, Bekele Y, Kaufmann SH, Kidd M, Haks MC, et al. Combination of gene expression patterns in whole blood discriminate between tuberculosis infection states. BMC Infect. Dis. 2014;14:257. doi: 10.1186/1471-2334-14-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhania A, Verma R, Graham CM, Lee J, Tran T, Richardson M, et al. A modular transcriptional signature identifies phenotypic heterogeneity of human tuberculosis infection. Nat. Commun. 2018;9:2308. doi: 10.1038/s41467-018-04579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valim C, Ahmad R, Lanaspa M, Tan Y, Acacio S, Gillette MA, et al. Responses to bacteria, virus, and malaria distinguish the etiology of pediatric clinical pneumonia. Am. J. Respir. Crit. Care Med. 2016;193:448–459. doi: 10.1164/rccm.201506-1100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolayeva I, Bost P, Casademont I, Duong V, Koeth F, Prot M, et al. A blood RNA signature detecting severe disease in young dengue patients at hospital arrival. J. Infect. Dis. 2018;217:1690–1698. doi: 10.1093/infdis/jiy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsalik EL, Henao R, Nichols M, Burke T, Ko ER, McClain MT, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci. Transl. Med. 2016;8:322ra311. doi: 10.1126/scitranslmed.aad6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson M, Sweeney TE, Barouch-Bentov R, Sahoo MK, Kalesinskas L, Vallania F, et al. A 20-gene set predictive of progression to severe dengue. Cell Rep. 2019;26:1104–1111e1104. doi: 10.1016/j.celrep.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tillekeratne LG, Suchindran S, Ko ER, Petzold EA, Bodinayake CK, Nagahawatte A, et al. Previously derived host gene expression classifiers identify bacterial and viral etiologies of acute febrile respiratory illness in a south asian population. Open Forum Infect. Dis. 2020;7:ofaa194. doi: 10.1093/ofid/ofaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ockenhouse CF, Hu WC, Kester KE, Cummings JF, Stewart A, Heppner DG, et al. Common and divergent immune response signaling pathways discovered in peripheral blood mononuclear cell gene expression patterns in presymptomatic and clinically apparent malaria. Infect. Immun. 2006;74:5561–5573. doi: 10.1128/IAI.00408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods CW, McClain MT, Chen M, Zaas AK, Nicholson BP, Varkey J, et al. A host transcriptional signature for presymptomatic detection of infection in humans exposed to influenza H1N1 or H3N2. PLoS ONE. 2013;8:e52198. doi: 10.1371/journal.pone.0052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClain MT, Constantine FJ, Nicholson BP, Nichols M, Burke TW, Henao R, et al. A blood-based host gene expression assay for early detection of respiratory viral infection: An index-cluster prospective cohort study. Lancet Infect. Dis. 2021;21:396–404. doi: 10.1016/S1473-3099(20)30486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RR, 3rd, Lopansri BK, Burke JP, Levy M, Opal S, Rothman RE, et al. Validation of a host response assay, SeptiCyte LAB, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am. J. Respir. Crit. Care Med. 2018;198:903–913. doi: 10.1164/rccm.201712-2472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsalik EL, Henao R, Montgomery JL, Nawrocki JW, Aydin M, Lydon EC, et al. Discriminating bacterial and viral infection using a rapid host gene expression test. Crit. Care Med. 2021;49:1651–1663. doi: 10.1097/CCM.0000000000005085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao AM, Popper SJ, Gupta S, Davong V, Vaidya K, Chanthongthip A, et al. A robust host-response-based signature distinguishes bacterial and viral infections across diverse global populations. Cell Rep. Med. 2022;3:100842. doi: 10.1016/j.xcrm.2022.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland JS, van der Spuy G, Gindeh A, Thuong NT, Namuganga AR, Owolabi O, et al. Diagnostic accuracy of the Cepheid 3-gene host response fingerstick blood test in a prospective, multi-site study: interim results. Clin. Infect. Dis. 2021;6:66. doi: 10.1093/cid/ciab839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warsinske HC, Rao AM, Moreira FMF, Santos PCP, Liu AB, Scott M, et al. Assessment of validity of a blood-based 3-gene signature score for progression and diagnosis of tuberculosis, disease severity, and treatment response. JAMA Netw. Open. 2018;1:e183779. doi: 10.1001/jamanetworkopen.2018.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsalik EL, Khine A, Talebpour A, Samiei A, Parmar V, Burke TW, et al. Rapid, sample-to-answer host gene expression test to diagnose viral infection. Open Forum Infect. Dis. 2019;6:ofz466. doi: 10.1093/ofid/ofz466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsalik EL, Langley RJ, Dinwiddie DL, Miller NA, Yoo B, van Velkinburgh JC, et al. An integrated transcriptome and expressed variant analysis of sepsis survival and death. Genome Med. 2014;6:111. doi: 10.1186/s13073-014-0111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langley RJ, Tsalik EL, van Velkinburgh JC, Glickman SW, Rice BJ, Wang C, et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013;5:195ra195. doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glickman SW, Cairns CB, Otero RM, Woods CW, Tsalik EL, Langley RJ, et al. Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad. Emerg. Med. 2010;17:383–390. doi: 10.1111/j.1553-2712.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tillekeratne LG, Bodinayake CK, Nagahawatte A, Vidanagama D, Devasiri V, Arachchi WK, et al. An under-recognized influenza epidemic identified by rapid influenza testing, southern Sri Lanka, 2013. Am. J. Trop. Med. Hyg. 2015;92:1023–1029. doi: 10.4269/ajtmh.14-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodinayake CK, Tillekeratne LG, Nagahawatte A, Devasiri V, Kodikara Arachichi W, Strouse JJ, et al. Emergence of epidemic dengue-1 virus in the Southern Province of Sri Lanka. PLoS Negl. Trop. Dis. 2016;10:e0004995. doi: 10.1371/journal.pntd.0004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uehara A, Tissera HA, Bodinayake CK, Amarasinghe A, Nagahawatte A, Tillekeratne LG, et al. Analysis of dengue Serotype 4 in Sri Lanka during the 2012–2013 Dengue Epidemic. Am. J. Trop. Med. Hyg. 2017;97:130–136. doi: 10.4269/ajtmh.16-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bodinayake CK, Tillekeratne LG, Nagahawatte A, Devasiri V, Kodikara Arachchi W, Strouse JJ, et al. Evaluation of the WHO 2009 classification for diagnosis of acute dengue in a large cohort of adults and children in Sri Lanka during a dengue-1 epidemic. PLoS Negl. Trop. Dis. 2018;12:e0006258. doi: 10.1371/journal.pntd.0006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maze MJ, Cash-Goldwasser S, Rubach MP, Biggs HM, Galloway RL, Sharples KJ, et al. Risk factors for human acute leptospirosis in northern Tanzania. PLoS Negl. Trop. Dis. 2018;12:e0006372. doi: 10.1371/journal.pntd.0006372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pisharody S, Rubach MP, Carugati M, Nicholson WL, Perniciaro JL, Biggs HM, et al. Incidence estimates of Acute Q fever and spotted fever group rickettsioses, Kilimanjaro, Tanzania, from 2007 to 2008 and from 2012 to 2014. Am. J. Trop. Med. Hyg. 2021;106:494–503. doi: 10.4269/ajtmh.20-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schully, K. L., & Clark, D. V. Aspiring to Precision Medicine for Infectious Diseases in Resource Limited Settings 105–115 (Elsevier, 2019).
- 47.Schully KL, Berjohn CM, Prouty AM, Fitkariwala A, Som T, Sieng D, et al. Melioidosis in lower provincial Cambodia: A case series from a prospective study of sepsis in Takeo Province. PLoS Negl. Trop. Dis. 2017;11:e0005923. doi: 10.1371/journal.pntd.0005923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rozo M, Schully KL, Philipson C, Fitkariwala A, Nhim D, Som T, et al. An observational study of sepsis in Takeo Province Cambodia: An in-depth examination of pathogens causing severe infections. PLoS Negl. Trop. Dis. 2020;14:e0008381. doi: 10.1371/journal.pntd.0008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schully KL, Young CC, Mayo M, Connolly AL, Rigas V, Spall A, et al. Next-generation diagnostics for melioidosis: Evaluation of a Prototype i-STAT Cartridge to detect Burkholderia pseudomallei biomarkers. Clin. Infect. Dis. 2019;69:421–427. doi: 10.1093/cid/ciy929. [DOI] [PubMed] [Google Scholar]
- 50.Do NT, Ta NT, Tran NT, Than HM, Vu BT, Hoang LB, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: A randomised controlled trial. Lancet Glob. Health. 2016;4:e633–641. doi: 10.1016/S2214-109X(16)30142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheng T, Wijayaratne GB, Dabrera TM, Drew RJ, Nagahawatte A, Bodinayake CK, et al. Point-prevalence study of antimicrobial use in public hospitals in southern Sri Lanka identifies opportunities for improving prescribing practices. Infect. Control Hosp. Epidemiol. 2019;40:224–227. doi: 10.1017/ice.2018.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 53.Stevenson, M., E.S.w.c.f.T.N., Heuer, C., Marshall, J., Sanchez, J., Thornton, R., Reiczigel, J., Robison-Cox, J., Sebastiani, P., Solymos, P., Yoshida, K., Jones, G., Pirikahu, S., Firestone, S., Kyle, R., Popp, J., Jay, M., Reynard, C., Cheung, A., Singanallur, N., Szabo, A. & Rabiee, A. epiR: Tools for the Analysis of Epidemiological Data. R package version 2.0.50. https://CRAN.R-project.org/package=epiR (2022).
- 54.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: Sample size estimation for diagnostic test studies. J. Clin. Epidemiol. 1991;44:763–770. doi: 10.1016/0895-4356(91)90128-V. [DOI] [PubMed] [Google Scholar]
- 55.Tan TL, Kang CW, Ooi KS, Tan ST, Ahmad NS, Nasuruddin DN, et al. Comparison of sPLA2IIA performance with high-sensitive CRP neutrophil percentage PCT and lactate to identify bacterial infection. Sci. Rep. 2021;11:11369. doi: 10.1038/s41598-021-90894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Althaus T, Greer RC, Swe MMM, Cohen J, Tun NN, Heaton J, et al. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: An open-label, randomised, controlled trial. Lancet Glob. Health. 2019;7:e119–e131. doi: 10.1016/S2214-109X(18)30444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lubell Y, Blacksell SD, Dunachie S, Tanganuchitcharnchai A, Althaus T, Watthanaworawit W, et al. Performance of C-reactive protein and procalcitonin to distinguish viral from bacterial and malarial causes of fever in Southeast Asia. BMC Infect. Dis. 2015;15:511. doi: 10.1186/s12879-015-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Hecke O, Raymond M, Lee JJ, Turner P, Goyder CR, Verbakel JY, et al. In-vitro diagnostic point-of-care tests in paediatric ambulatory care: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0235605. doi: 10.1371/journal.pone.0235605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sweeney TE, Wynn JL, Cernada M, Serna E, Wong HR, Baker HV, et al. Validation of the sepsis metascore for diagnosis of neonatal sepsis. J. Pediatr. Infect. Dis. Soc. 2018;7:129–135. doi: 10.1093/jpids/pix021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko ER, Henao R, Frankey K, Petzold EA, Isner PD, Jaehne AK, et al. Prospective validation of a rapid host gene expression test to discriminate bacterial from viral respiratory infection. JAMA Netw. Open. 2022;5:e227299. doi: 10.1001/jamanetworkopen.2022.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martinez-Ledesma E, Verhaak RG, Trevino V. Identification of a multi-cancer gene expression biomarker for cancer clinical outcomes using a network-based algorithm. Sci. Rep. 2015;5:11966. doi: 10.1038/srep11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodkin N, Ross M, McClain MT, Ko ER, Woods CW, Ginsburg GS, et al. Systematic comparison of published host gene expression signatures for bacterial/viral discrimination. Genome Med. 2022;14:18. doi: 10.1186/s13073-022-01025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mor M, Paz M, Amir L, Levy I, Scheuerman O, Livni G, et al. Bacterial vs viral etiology of fever: A prospective study of a host score for supporting etiologic accuracy of emergency department physicians. PLoS ONE. 2023;18:e0281018. doi: 10.1371/journal.pone.0281018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papan C, Argentiero A, Porwoll M, Hakim U, Farinelli E, Testa I, et al. A host signature based on TRAIL, IP-10, and CRP for reducing antibiotic overuse in children by differentiating bacterial from viral infections: a prospective, multicentre cohort study. Clin. Microbiol. Infect. 2021;6:66. doi: 10.1016/j.cmi.2021.10.019. [DOI] [PubMed] [Google Scholar]
- 65.Manabe YC, Betz J, Jackson O, Asoala V, Bazan I, Blair PW, et al. Clinical evaluation of the BioFire Global Fever Panel for the identification of malaria, leptospirosis, chikungunya, and dengue from whole blood: A prospective, multicentre, cross-sectional diagnostic accuracy study. Lancet Infect. Dis. 2022;22:1356–1364. doi: 10.1016/S1473-3099(22)00290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eden E, Srugo I, Gottlieb T, Navon R, Boico O, Cohen A, et al. Diagnostic accuracy of a TRAIL, IP-10 and CRP combination for discriminating bacterial and viral etiologies at the Emergency Department. J. Infect. 2016;73:177–180. doi: 10.1016/j.jinf.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Langley RJ, Tipper JL, Bruse S, Baron RM, Tsalik EL, Huntley J, et al. Integrative "omic" analysis of experimental bacteremia identifies a metabolic signature that distinguishes human sepsis from systemic inflammatory response syndromes. Am. J. Respir. Crit. Care Med. 2014;190:445–455. doi: 10.1164/rccm.201404-0624OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in this article were generated as part of this work. All RNA sequencing data has been submitted to GEO under accession number GSE211567. NanoString transcripts are included in supplemental information. Token to access GSE211567: obqzkkoarjwpfct.