Abstract
Introduction
Advocacy for the provision of public health resources, including vaccine for the prevention of acute respiratory illnesses (ARIs) among older adults in India, needs evidence on costs and benefits. Using a cohort of community-dwelling adults aged 60 years and older in India, we estimated the cost of ARI episode and its determinants.
Methods
We enrolled 6016 participants in Ballabgarh, Chennai, Kolkata and Pune from July 2018 to March 2020. They were followed up weekly to identify ARI and classified them as acute upper respiratory illness (AURI) or pneumonia based on clinical features based on British Thoracic Society guidelines. All pneumonia and 20% of AURI cases were asked about the cost incurred on medical consultation, investigation, medications, transportation, food and lodging. The cost of services at public facilities was supplemented by WHO-Choosing Interventions that are Cost-Effective(CHOICE) estimates for 2019. Indirect costs incurred by the affected participant and their caregivers were estimated using human capital approach. We used generalised linear model with log link and gamma family to identify the average marginal effect of key determinants of the total cost of ARI.
Results
We included 2648 AURI and 1081 pneumonia episodes. Only 47% (range 36%–60%) of the participants with pneumonia sought care. The mean cost of AURI episode was US$13.9, while that of pneumonia episode was US$25.6, with indirect costs comprising three-fourths of the total. The cost was higher among older men by US$3.4 (95% CI: 1.4 to 5.3), those with comorbidities by US$4.3 (95% CI: 2.8 to 5.7) and those who sought care by US$17.2 (95% CI: 15.1 to 19.2) but not by influenza status. The mean per capita annual cost of respiratory illness was US$29.5.
Conclusion
Given the high community disease and cost burden of ARI, intensifying public health interventions to prevent and mitigate ARI among this fast-growing older adult population in India is warranted.
INTRODUCTION
Acute lower respiratory infections (ALRI) or pneumonia contributed to 4.9% of global deaths among all ages in 2015.1 Global Burden of Disease (GBD) estimates show that people aged more than 70 years had the highest incidence of lower respiratory tract infections and deaths as compared with all other age groups including children aged less than 5 years.2 GBD estimates also show that between 1990 and 2017, deaths attributed to pneumococcal pneumonia increased by 60.4%, influenza by 91.1% and respiratory syncytial virus by 100.3% among persons aged more than 70 years.3 National estimates for India, for example, suggest that pneumonia accounted for 2.9% of all deaths in persons aged more than 69 years during 2016–2018.4 These estimates will likely need revision because COVID-19 has emerged as one more cause of ALRI leading to hospitalisation and death.5
Demographic trends such as population ageing would proportionately increase the disease burden among older adults in India and worldwide. Globally between 2015 and 2050, the proportion of the world’s population aged more than 60 years will increase from 12% to 22% and the number of people aged 60 years and above will exceed that of children aged less than 5 years.6 In 2011, India had more than 100 million persons aged≥60 years (8.6% of the total population) and by the year 2050, this is projected to reach 19.5%.7
Considering the increasing burden of pneumonia among older adults, it is important to consider implementation of appropriate public health measures. Associations of geriatricians, pulmonologists and public health experts have recommended the use of vaccines (influenza and pneumococcal vaccines) for the prevention of pneumonia in older adults.8-10 The current Indian national initiative of National Programme for Health Care of Elderly, however, does not offer pneumococcal and influenza vaccines.11 Despite the benefits of vaccination, population level coverage of these vaccines in India is low (1.5% for influenza and 0.6% for pneumococcal vaccine).12
Advocacy for public health interventions that may reduce the burden of pneumonia among older adults would be strengthened by evidence that the cost is appropriate for the anticipated benefits. Unfortunately, evidence of India’s aetiology-specific pneumonia disease and economic burden is scarce. Moreover, using the current evidence coming from mainly developed countries and hospital-based studies among elderly individuals poses critical limitations and uncertainties for making robust recommendations for India’s older adults, where health utilisation can be very different from that of high-income countries13-15 The applicability and appropriateness of global cost-effective analysis for making an investment case for influenza vaccination among older adults suffers from similar limitations.16 17
As a part of the Indian Network of Population-based Surveillance Platforms for Influenza and other Respiratory viruses among the Elderly (INSPIRE) cohort, we attempted to address the need of India-specific evidence.18 In this paper, we estimate the economic burden of symptomatic respiratory illness among older adults aged≥60 years in India. Specifically, we estimated the total societal cost and cost per episode of acute respiratory illness (ARI) including pneumonia among a cohort of community-dwelling older persons at four sites in India. In addition, we identified the determinants of the cost of an episode including lab-confirmed influenza illness status.
METHODOLOGY
Study population
INSPIRE cohort had older adults aged 60 years and above from four geographically diverse sites in India: (1) northern (Ballabgarh), (2) southern (Chennai), (3) eastern (Kolkata) and (4) western (Pune). The details of INSPIRE have been published earlier.18 We initiated INSPIRE in July 2018 and continued the follow-up until March 2020, at which point we stopped it due to COVID-19 movement restrictions.
Study design
INSPIRE adopted an open-cohort design with original enrolees supplemented annually with new participants who either aged up to 60 years or immigrated to the study areas.
Sample size and sampling
A sample size of 4500 person-years per site was calculated for estimating the cumulative incidence of influenza-associated ALRI in the cohort based on annual cumulative incidence of 0.01 per person-year, an alpha of 5%, relative precision of 30% and 3-year attrition of 10%. Each participating institution chose a convenient community nearby for the study and enrolled all elderly after initial screening for eligibility and consent. All older adults aged≥60 years who had been living in the village for more than 6 months, and with reported intention of continuing to live there and who were not cognitively impaired, were eligible for inclusion.
Data collection
Information related to health insurance coverage, presence of comorbidities and household wealth was collected at the time of enrolment. Trained nurses made house visits to all participants weekly to actively identify ARI and clinically assess individuals to diagnose community-acquired pneumonia based on 2009 update of British Thoracic Society guidelines.19 ARI was defined as new onset/worsening of cough or difficulty in breathing in the last 7 days; pneumonia was defined as ARI along with dyspnoea or chest pain, a respiratory rate of ≥20 breaths/minute and either measured fever or a reported symptom complex of fever, sweating, headache and myalgia. All episodes not identified as pneumonia were classified as acute upper respiratory illness (AURI). Nasal and oropharyngeal swabs were collected from all pneumonia cases. In addition, samples were collected from a random sample of 20% of AURIs to minimise operational cost without affecting primary study objectives.
Estimation of cost
All patients who provided specimens were asked about costs they incurred for treatment of their ARI episode. A societal perspective was adopted for cost estimation. The data were collected as recommended by the WHO Manual for estimating the economic burden of seasonal influenza.20 The presence of respiratory symptoms, healthcare seeking, cost incurred on treatment and impact on their daily life were asked for each day of illness until the episode ended. Each day, persons could have taken treatment at home (self-treatment), gone to a pharmacist for over the counter (OTC) medications or visited a healthcare provider (qualified or otherwise) for consultation. Information collected for each visit included type of facility (private/public), level of care (self-medication, OTC, ambulatory, emergency and inpatient). Multiple options were allowed for a day.
For each visit, the cost of consultations, investigations and medications (direct medical) and the cost of transportation, food and lodging (direct non-medical) were collected. Information about costs borne out-of-pocket and billed costs for insured persons was collected, wherever applicable. For private facility use, the expenditure by patient was considered as a proxy for cost. For public health facilities, out-of-pocket cost was supplemented by the cost-of-service provision as estimated by updated WHO-Choosing Interventions that are Cost-Effective (CHOICE) estimates indexed to 2019 using consumer price index.21 In public facilities, the cost incurred by patients on medicines and laboratory/radiological investigations was also included.
Indirect costs incurred by the affected older adult and their caregivers were estimated using a human capital approach.22 The impact on productivity was assessed as their ability to do 0%, 1%–25%, 26%–50%, 51%–75% or 76%–100% of their routine daily work during the episode. The number of days affected multiplied by the impact on productivity was used to estimate the total days of productivity (workday) loss of the patient. The daily income of the patient (US$5.2) was based on the net national annual per capita income (US$1859) divided by 365 days.23 For sensitivity analysis, we used reported daily income among those patient who were working and median of daily income among those who were not working to calculate indirect cost incurred by affected older adults. We used caregiver-reported income to estimate the opportunity cost of caring for adults during illness. If caregiver income was unavailable, we assumed they earned net national annual per capita income. All costs were estimated in Indian rupees (INR) and converted into US$ at exchange rate for 2019 (US$1=INR 70.8).24 The cost data collected during the study period were indexed to 2019.
Statistical analysis
All cost data collection was done on handheld tablets (Android based) using the Open Data Kit platform. All data were cleaned and analysed using Stata V.14.1. The duration of an episode was calculated as the period between the date of onset and end date based on two key symptoms—cough and breathing difficulty. In those episodes for which end date was missing for either of these symptoms, the episode end date was assumed to be at the end of 7 continuous days of not seeking care or at 28 days after the date of onset, whichever was earlier. The combined cost of each episode was calculated as the sum of the direct medical, direct non-medical and indirect costs.
Costs are presented as mean with SD or median with IQR. Site-wise differences in characteristics of pneumonia and AURI episodes and their care seeking were assessed using χ2 test. For identifying key determinants of the cost of an episode, a two-part fixed-effect regression model was applied taking the total cost of pneumonia episode as the dependent variable where the first part models the episodes with zero cost using logit and the second part models the episodes with non-zero cost using generalised linear model with log link and gamma family. The equations are provided below.
Part 1: Estimating the probability of observing a positive outcome, using a logit link function.
Part 2: Estimating the conditional mean of the positive outcome given that it is positive, using a log link function.
where:
represents the probability of observing a positive outcome.
is the logit link function applied to the probability.
represents the conditional mean of the positive outcome.
is the log link function applied to the conditional mean.
are the coefficients corresponding to the predictor variables in the logit link function.
are the coefficients corresponding to the predictor variables in the log link function.
Overall, mean can be written as . We have used study site as a fixed effect.
All eligible independent variables with less than five variance inflation factor were included in the model. The mean cost of acute respiratory illness episode or the dependent variable (US$13.1) was close to the mean of the predicted value derived by the two-part model (US$13.7) indicating that the model performed well. Average marginal effect (dy/dx with dy indicating the change in cost and dx the change in the independent variable.) indicates how sensitive the change in cost of the episode of respiratory illness is to with unitary changes in a specific independent variable at the time while other variables remain unchanged at their reference value. This is presented along with its 95% CIs calculated using profile likelihood approach. The total cost of ARI in the cohort was estimated by applying cost per AURI episode to all the AURI episodes identified in the cohort (not just those enrolled for cost estimation) and adding to the total pneumonia costs. The annual cost per participant was derived by dividing this by person-years of surveillance.
Patient and public involvement
Public and/or patients were not involved in the development of the study protocol. However, the ethical committees of participating institutes had representatives from public and recommendations of the ethics committees were incorporated in the protocol. The study tools were piloted in a non-study study area among a community similar to the study area to assess the acceptability of questions asked and any sensitive questions were reframed or omitted. The study findings are planned to be communicated through public lectures and pamphlets.
RESULTS
Participant profile
We enrolled 6016 participants aged≥60 years across 4220 households who contributed to 7653 person-years of surveillance. For the cost of ARI study, we used data from 2322 participants who developed pneumonia (n=1081) or AURI (n=2648;18.6% of 14 168) episodes that were tested for influenza. Ballabgarh site enrolled more participants than other sites (37%) and male:female ratio was close to 2:3 at all sites (table 1). The study populations of all the four sites varied significantly in most characteristics except in sex distribution. Patients were relatively younger in Pune and Kolkata as compared with Ballabgarh and Chennai. Economically, 66% of Ballabgarh participants were in the upper tercile of combined wealth index distribution, whereas 83% of Kolkata participants were in the lowest tercile. Almost all participants in Chennai (95%) had comorbidities, while half of Ballabgarh’s reported comorbidities (57.5%). Health insurance coverage was low across all sites, ranging from 0.2% in Pune to 22% in Chennai. Less than one in four participants were currently employed (varying from 14.4% in Chennai to 24.8% in Pune) and their mean income ranged from US$802 to US$1043.
Table 1.
Characteristics of patients with acute respiratory illness at each participating site
| Characteristics | Subgroups | Ballabgarh | Chennai | Kolkata | Pune | P value |
|---|---|---|---|---|---|---|
| Total persons who reported an acute respiratory illness included in the study | 858 | 479 | 480 | 505 | – | |
| Characteristics (%) of patients with acute respiratory illness | ||||||
| Age in years | 60–64 | 28.1 | 38.2 | 47.7 | 52.5 | 0.001 |
| 65–69 | 33.9 | 29.8 | 31.2 | 26.1 | ||
| 70–74 | 17.0 | 18.4 | 13.3 | 13.3 | ||
| 75 and above | 21.0 | 13.6 | 7.7 | 8.1 | ||
| Sex | Female | 60.9 | 58.7 | 61.7 | 59.6 | 0.7 |
| Male | 39.1 | 41.3 | 38.3 | 40.4 | ||
| Wealth tercile* | Upper | 66.2 | 38.6 | 1.2 | 2.4 | 0.001 |
| Middle | 20.8 | 42.4 | 15.4 | 69.7 | ||
| Lower | 13.0 | 19.0 | 83.3 | 27.9 | ||
| Presence of reported comorbidity† | None | 42.5 | 4.8 | 20.2 | 37.4 | 0.001 |
| One | 33.7 | 16.1 | 32.7 | 33.7 | ||
| Multimorbidity | 23.8 | 79.1 | 47.1 | 28.9 | ||
| Coverage with health insurance | Yes | 15.4 | 22.1 | 1.2 | 0.2 | 0.001 |
| No | 84.6 | 77.8 | 98.8 | 99.8 | ||
| Per cent of patients currently employed | 17.1 | 14.4 | 20.4 | 24.8 | ||
| Mean annual income (US$) of those currently employed | 825.7 | 1042.5 | 801.6 | 1017.8 | ||
Terciles calculated based on the combined sample.
Comorbidities included were diabetes, hypertension, chronic heart, liver, kidney or respiratory disease, stroke, arthritis, malignancy, anaemia, depression, tuberculosis.
Episode characteristics
Among 2648 AURI episodes, 3.8% tested positive for influenza (varying from 2.2% in Ballabgarh to 5.3% in Pune) (table 2). Most participants (69.9%; range: 51%–79.3%) did not seek care; 23.4% (range: 14.6–45.5) sought ambulatory care in outpatient departments of health facilities. Approximately 85.5% (71.6% in Ballabgarh to 94.3% in Pune) of those who sought care went to primary care providers. Public sector care was used predominantly in Pune (67.3%) and in Kolkata (55.9%).
Table 2.
Description of episodes of acute respiratory illness and their healthcare seeking by site (n=3729)
| Characteristics | Subgroups | AURI (n=2648) |
Pneumonia (n=1081) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ballabgarh (1083) |
Chennai (386) |
Kolkata (562) |
Pune (617) |
Total (2648) |
P value | Ballabgarh (562) |
Chennai (235) |
Kolkata (139) |
Pune (145) |
Total (1081) |
P value | ||
| Virus detected (%) | Influenza | 2.2 | 4.4 | 4.6 | 5.3 | 3.8 | 0.001 | 8.5 | 9.36 | 12.2 | 8.3 | 9.2 | 0.5 |
| Type of care sought* (%) | None or self-medication | 73.1 | 79.3 | 78.1 | 50.9 | 69.9 | 0.001 | 54.1 | 63.8 | 43.9 | 39.3 | 52.9 | 0.001 |
| Pharmacy or study | 11.9 | 3.4 | 2.5 | 3.6 | 6.7 | 16.0 | 5.5 | 7.2 | 2.1 | 10.7 | |||
| Ambulatory/outpatient | 14.6 | 17.5 | 19.4 | 45.5 | 23.4 | 28.8 | 30.6 | 48.2 | 57.9 | 35.6 | |||
| Emergency/Inpatient | 0 | 0 | 0 | 0 | 0 | 1.1 | 0 | 0.7 | 0.1 | 0.7 | |||
| Level of care* (%) (n=1012) | Primary | 71.6 | 85.1 | 83.5 | 94.3 | 85.5 | 0.001 | 62.5 | 88.9 | 80.9 | 95.3 | 77.6 | 0.001 |
| Secondary | 24.1 | 5.9 | 4.6 | 4.3 | 9.7 | 32.1 | 5.6 | 4.4 | 3.5 | 16.2 | |||
| Tertiary | 4.3 | 8.9 | 11.9 | 1.4 | 4.8 | 5.4 | 5.6 | 14.7 | 1.2 | 6.1 | |||
| Sector of care sought (%) (n=1012) | Private only | 47.5 | 53.7 | 38.5 | 22.4 | 35.2 | 0.001 | 47.0 | 55.6 | 32.4 | 29.4 | 42.2 | 0.001 |
| Public only | 46.9 | 41.8 | 55.9 | 67.3 | 57.2 | 41.0 | 31.9 | 60.3 | 56.5 | 46.1 | |||
| Both | 5.6 | 4.5 | 5.5 | 10.3 | 7.6 | 11.9 | 12.5 | 7.4 | 14.1 | 11.7 | |||
Based on the highest level or type of care sought for that episode.
Among 1081 pneumonia episodes, 9.2% tested positive for influenza (8.5% in Ballabgarh to 12.2% in Kolkata). In about half (52.9%) of the episodes of pneumonia, patients (ranging from 40% in Pune to 64% in Chennai) did not seek any care. Overall, in 35.1% (28.8% in Ballabgarh to 58% in Pune) of pneumonia episodes, ambulatory care was sought with<1% being admitted as inpatients. Among those episodes where care was sought, most (77.6%) were at a primary care facility (65% at Ballabgarh to 96% in Pune). Almost 30% of episodes among Ballabgarh patients were seen at a secondary facility, whereas at other study sites it was between 3.5% and 5.6%. In Chennai, treatment was most commonly received in the private sector, whereas in Kolkata and Pune, the public sector was more common. In Delhi, both were equally used.
Cost and resource use
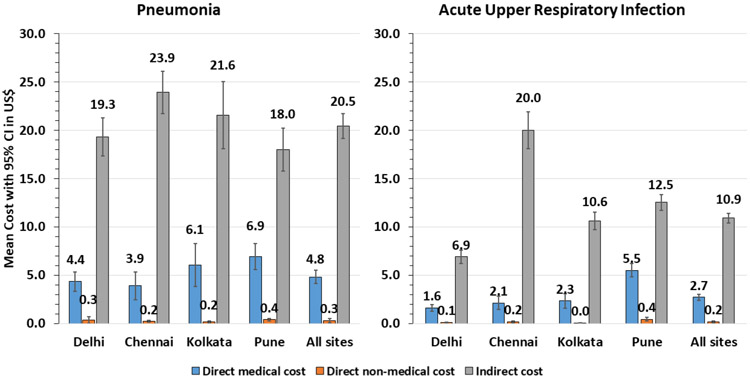
The resource use and cost of AURI are shown in table 3. The mean (SD) duration of an episode was 9.2 (7.5) days and median (IQR) was 7 days4-12. In most (73%) of the episodes, patients took some medicines, with the highest medication use in Ballabgarh (92%) and the least in Kolkata (44%). Antibiotic use was reported in 20% of episodes with the highest use being in Pune (44%). Antiviral drugs were only used in 4.6% of episodes, with the highest use being in Pune (16.4%). Investigations (laboratory or radiological) were reported by very few (1.4%) patients. On an average, participants lost 1.9 workdays and caregivers lost 0.2 workdays per AURI episode. The mean (SD) total cost of acute upper respiratory illness episode across four sites was US$13.9 (16.9) and median IQR US$9.1 (2.6–19.5). Mean cost ranged from the least costly in Ballabgarh, US$8.7 (13.8), to the costliest in Chennai, US$22.3 (22.2). Almost three-fourths of the mean cost (US$10.9 came from indirect cost of loss in wages of the patients, followed by cost of medications (US$1.5)) (figure 1)
Table 3.
Average rate of resource use and societal cost (US$) of acute upper respiratory tract illness among study participants
| Type of cost | Resource use indicators |
Ballabgarh | Chennai | Kolkata | Pune | All sites | Cost indicators | Ballabgarh (1083) |
Chennai (386) |
Kolkata (562) |
Pune (617) |
All sites |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct medical | Number of consultations, mean (SD) | 0.4 (0.7) |
0.3 (0.7) |
0.3 (0.5) |
0.7 (0.8) |
0.4 (0.7) |
Cost of consultation*, mean (SD) | 0.4 (1.2) |
0.6 (2.0) |
0.6 (1.6) |
1.8 (2.8) |
0.8 (2.0) |
| Took any medications, (%) | 92.2 | 65.5 | 44.1 | 70.0 | 72.9 | |||||||
| Took antibiotics, (%) | 11.0 | 20.5 | 12.6 | 43.9 | 20.4 | |||||||
| Took antivirals, (%) | 0.5 | 1.0 | 2.1 | 16.4 | 4.6 | Cost of medicines, mean (SD) | 0.9 (3.1) |
1.3 (4.4) |
1.0 (2.6) |
3.1 (4.7) |
1.5 (3.8) |
|
| With laboratory investigations, (%) | 0.3 | 1.0 | 0.9 | 4.1 | 1.4 | Cost of investigations, mean (SD) | 0.4 (2.6) |
0.2 (1.7) |
0.8 (7.5) |
0.5 (2.9) |
0.5 (4.1) |
|
| With radiological investigation, (%) | 3.4 | 1.0 | 1.1 | 4.7 | 2.9 | Direct medical cost, mean (SD) | 1.6 (5.6) |
2.1 (6.9) |
2.3 (9.0) |
5.5 (8.5) |
2.7 (7.5) |
|
| Direct non-medical | With travel cost, (%) | 4.2 | 6.2 | 5.3 | 23.9 | 9.3 | Direct Non-medical cost, mean (SD) | 0.1 (0.8) |
0.2 (1.1) |
0.0 (0.2) |
0.4 (2.5) |
0.2 (1.4) |
| Indirect | Duration of episode in days, mean (SD) | 11.7 (7.9) |
9.6 (8.4) |
7.4 (7.1) |
6.0 (4.0) |
9.2 (7.5) |
Cost of workdays loss of patients, mean (SD) | 6.4 (10.6) |
19.9 (19.0) |
10.6 (10.6) |
10.9 (7.5) |
10.3 (12.5) |
| Days lost by caregiver, mean (SD) | 0.1 (0.8) |
0.1 (0.4) |
0.03 (0.3) |
0.4 (1.6) |
0.2 (0.9) |
Cost of workdays loss of caregivers, mean (SD) | 0.6 (4.9) |
0.2 (1.2) |
0.1 (1.3) |
1.7 (6.5) |
0.7 (4.6) |
|
| Patient workdays lost during episode, mean (SD) | 1.2 (2.0) |
3.8 (3.6) |
2.0 (2.0) |
2.0 (1.4) |
1.9 (2.4) |
Total indirect cost, mean (SD) | 6.9 (11.6) |
20.0 (19.1) |
10.6 (10.8) |
12.5 (10.4) |
10.9 (13.3) |
|
| Total cost of acute upper respiratory tract illness, mean (SD) | 8.7 (13.8) |
22.3 (22.2) |
13.0 (14.9) |
18.5 (16.3) |
13.9 (16.9) |
|||||||
| Total cost of acute upper respiratory tract illness, median (IQR) | 3.9 (0–10.4) |
16.9 (7.7–29.5) |
7.7 (3.9–17.8) |
14.5 (7.8–24.9) |
9.1 (2.6–19.5) |
|||||||
Figure 1.
Mean direct and indirect cost per acute upper respiratory illness and pneumonia episode among older adults.
The resource use and cost accrued to participants with pneumonia are shown in table 4. Most (83.5%) took some medications, and one-third of the cases took antibiotics. Antiviral drug use in pneumonia patients was low (3.9%) with most of it coming from Pune site (20.7%). Participants had laboratory testing in 2.6% and radiological investigations in 6.6% of episodes. The mean duration of an episode of pneumonia was 10.8 days and median (IQR) was 8 days (5-14). The mean duration ranged from 7.3 days in Pune to 12.8 days in Ballabgarh . Patients with pneumonia, on an average, lost 3.7 workdays. The mean (SD) total cost of a pneumonia episode was US$25.6 (25.6) and median (IQR) cost was US$19.1 (7.7–36.3). The mean cost varied from US$24.0 in Ballabgarh to US$28.0 in Chennai. Almost four-fifths of this cost were in the form of indirect cost (US$20.5), followed by cost of medications (US$2.3). Pune consistently had higher direct medical costs and Chennai had higher indirect costs (figure 1). Costs differed by level of care; among participants who sought ambulatory care, the mean (SD) total cost was $36.5 (25.5), including $12.5 (14.5) direct medical costs, $0.5 (1.4) direct non-medical costs and $23.3 (19.2) indirect costs. All mean costs were higher among inpatient/emergency patients, with a total cost of US$68.2 (89.3) including US$20.8 (56.9) direct medical costs, US$12.1 (33.4) direct non-medical costs and US$35.3 (30.6) indirect costs (data not shown).
Table 4.
Average rate of resource use and societal cost for pneumonia among study participants
| Type of cost | Resource use indicators |
Ballabgarh | Chennai | Kolkata | Pune | All sites | Cost indicators | Ballabgarh (562) |
Chennai (235) |
Kolkata (139) |
Pune (145) |
All sites (1081) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct medical | Number of consultations, mean (SD) | 0.7 (1.0) |
0.4 (0.7) |
0.7 (0.7) |
0.9 (0.9) |
0.7 (0.9) |
Cost of consultation, mean (SD) | 0.9 (3.1) |
1.1 (2.6) |
1.3 (2.1) |
2.4 (2.8) |
1.2 (2.8) |
| Took any medications, (%) | 96.6 | 68.5 | 70.5 | 69.7 | 83.5 | |||||||
| Took antibiotics, (%) | 28.8 | 35.7 | 39.6 | 44.8 | 33.9 | |||||||
| Took antivirals, (%) | 0.2 | 0.9 | 6.5 | 20.7 | 3.9 | Cost of medicines, mean (SD) | 1.9 (4.8) |
2.1 (4.9) |
2.6 (3.7) |
3.6 (4.8) |
2.3 (4.7) |
|
| With laboratory investigations, (%) | 2.3 | 1.3 | 1.4 | 6.9 | 2.6 | Cost of investigations, mean (SD) | 1.4 (6.5) |
0.7 (5.5) |
2.1 (10.4) |
0.9 (3.3) |
1.3 (6.6) |
|
| With radiological investigation, (%) | 9.3 | 0.9 | 6.5 | 5.5 | 6.6 | Direct medical cost, mean (SD) | 4.4 (11.7) |
3.9 (11.3) |
6.1 (13.3) |
6.9 (8.3) |
4.8 (11.5) |
|
| Direct non-medical | With travel cost, (%) | 10.7 | 11.1 | 17.9 | 26.2 | 13.8 | Direct non-medical cost, mean (SD) | 0.3 (4.1) |
0.2 (0.8) |
0.2 (0.6) |
0.4 (0.8) |
0.3 (3.0) |
| indirect | Duration of episode in days, mean (SD) | 12.8 (8.3) |
9.4 (7.0) |
8.9 (7.6) |
7.3 (5.2) |
10.8 (7.8) |
Loss of wages of patients, mean (SD) | 18.6 (23.3) |
23.5 (16.7) |
20.9 (20.6) |
14.2 (9.3) |
19.5 (20.4) |
| Days of loss for caregivers, mean (SD) | 0.1 (0.8) |
0.1 (0.5) |
0.2 (0.9) |
0.9 (2.0) |
0.2 (1.1) |
Loss of wages of caregivers, mean (SD) | 0.6 (4.3) |
0.4 (2.7) |
0.6 (2.9) |
3.7 (10.9) |
0.9 (5.4) |
|
| Patient workdays lost during episode, mean (SD) | 3.6 (4.4) |
4.5 (3.2) |
4.0 (3.9) |
2.7 (1.8) |
3.7 (3.9) |
Total indirect cost, mean (SD) | 19.3 (23.8) |
23.9 (17.0) |
21.6 (20.8) |
18.0 (13.6) |
20.5 (21.0) |
|
| Total cost of pneumonia, mean (SD) | 24.0 (28.4) |
28.0 (21.7) |
27.8 (26.7) |
25.4 (17.5) |
25.6 (25.6) |
|||||||
| Total cost of pneumonia, median (IQR) | 15.1 (3.8–36.3) |
25.9 (11.6–38.9) |
20.7 (10.7–34.5) |
23.9 (14.3–34.4) |
19.1 (7.7–36.3) |
|||||||
Sensitivity analysis using actual wage loss among patient showed that the mean (SD) of total cost of AURI episode was US$7.6 (11.8) and pneumonia episode was US$13.2 (16.7).
Determinants of cost
Table 5 presents the key drivers of cost. Total cost of an episode of respiratory illness in patients aged more than 75 years was on average US$3.4 (95% CI: 1.4 to 5.3) higher when compared with patients aged 60–64 years. The cost was higher among men by US$1.3 (95% CI: 0.1 to 2.4) when compared with women. The presence of single comorbidity significantly increased the cost by US$2.0 (95% CI: 0.6 to 3.3) and by US$4.3 (95% CI: 2.8 to 5.7) if multiple comorbidities were present. Sex, wealth tercile, coverage with health insurance did not significantly alter the cost of respiratory illness episode. Receiving ambulatory care (US$17.2) or inpatient care (US$36.8) was associated with higher cost of episodes. The cost of an episode of pneumonia was US$8.1 (95% CI: 6.6 to 9.6) more than that of an AURI episode. Detection of influenza in the participants did not result in any significant change in the cost. Patients in Chennai and Pune reported a higher cost of treatment of respiratory illness than in Ballabgarh and Kolkata.
Table 5.
Average marginal effect of cost (US$) of acute respiratory illness* (n=3729)
| Covariates | Values | Cost in US$ (95% CI) |
P value |
|---|---|---|---|
| Age in years | 60–64 | Reference | – |
| 65–74 | 0.2 (−0.9 to 1.4) | 0.71 | |
| 75 and above | 3.4 (1.4 to 5.3) | <0.01 | |
| Sex | Female | Reference | – |
| Male | 1.3 (0.1 to 2.4) | <0.05 | |
| Presence of any comorbidity | None | Reference | – |
| One | 2.0 (0.6 to 3.3) | <0.01 | |
| More than one | 4.3 (2.8 to 5.7) | <0.01 | |
| Coverage with health insurance | No | Reference | – |
| Yes | 0.3 (−2.2 to 1.6) | 0.77 | |
| Wealth tercile | Lower | Reference | – |
| Middle | 0.6 (−2.0 to 0.8) | 0.41 | |
| Upper | 0.2 (−1.9 to 1.6) | 0.84 | |
| Any private sector visit during the episode | No | Reference | – |
| Yes | 0.8 (−2.2 to 0.6) | 0.28 | |
| Type of care (higher one taken if more than one) | None or pharmacy | Reference | |
| Ambulatory | 17.2 (15.1 to 19.2) | <0.01 | |
| Emergency or inpatient | 36.8 (4.9 to 68.7) | <0.05 | |
| Influenza virus detected | No | Reference | |
| Yes | 0.3 (−2.0 to 2.7) | 0.80 | |
| Type of illness | AURI | Reference | |
| Pneumonia | 8.1 (6.6 to 9.6) | <0.01 | |
| Site of study | Ballabgarh | Reference | – |
| Chennai | 7.3 (5.2 to 9.4) | <0.01 | |
| Kolkata | 0.7 (−2.5 to 1.1) | 0.45 | |
| Pune | 2.1 (0.2 to 3.9) | <0.05 |
Using generalised linear model with log link and gamma family. AURI, acute upper respiratory illness.
Overall, the cohort incurred a total cost of US$225 786 on 14168 AURI episodes and 1127 pneumonia episodes; 87% of this cost was attributable to AURI. The annual cost of acute respiratory illness per cohort participant was US$29.5.
DISCUSSION
This multisite cohort study found a substantial health and economic burden of community-acquired ARI among older adults aged ≥60 years. The mean societal cost of an episode of AURI was US$13.9 and of pneumonia was US$25.6, with three-fourths of the total cost being indirect costs. The mean per capita annual cost due to symptomatic respiratory illness was equivalent to 2% of India’s per capita income and was mainly attributed to acute upper respiratory illness. Approximately 50% of pneumonia cases did not seek healthcare and most sought ambulatory care at primary level. Non-hospitalised pneumonia episodes still incurred substantial economic costs, primarily because of lost wages for both participants and caregivers. The presence of comorbidity and care seeking was associated with higher costs. Demographics, disease burden, healthcare seeking and cost of respiratory illness varied substantially between sites.
We found that health insurance coverage was low across sites, which has also been reported in the Longitudinal Aging Study in India (LASI).25 LASI has also reported 24.9% multimorbidity and 29.3% with single comorbidity in nationally representative sample from across India, which is similar to two of our sites (Ballabgarh and Pune), whereas Chennai and Kolkata reported much higher levels of comorbidity.26 Our study reported a very low treatment seeking behaviour for respiratory illness including pneumonia. Similarly, the 75th round National Sample Survey of India, 2017–2018, reported that 33.6% of the elderly went to a public outpatient care for any illness in the last 15 days.27 While there are notably substantial differences in socioeconomic conditions, a qualitative study among older adults in Canada also reported delays in seeking professional medical care due to misperceptions about the severity of community-acquired pneumonia, trying to manage illness at home and access barriers.28
It is difficult to compare our cost estimates of pneumonia among older adults as most previous studies have used hospital-based approaches or used administrative or insurance data from developed countries. We found that almost three-fourths of the cost of respiratory illness was contributed by indirect costs in our study, so studies only focusing on treatment cost would likely underestimate the true cost. In France, the mean direct medical cost of a pneumonia episode managed ambulatorily (€118.8) in primary care had equal weight for medical time, drugs, diagnostic procedures and tests.29 It also estimated the indirect cost (€1980) per ambulatory episode contributed to more than 90% of the total cost. In Japan, the median treatment costs of pneumonia were US$346 (IQR: 195–551) per outpatient episode among those above 65 years.15 Increases in the cost of a respiratory illness in participants with comorbidities have been reported in many studies, most of which are hospital based.30 In a study by Vissink et al among Dutch elderly, being male, younger and higher socioeconomic status were associated with lower costs.31
In our study, people aged more than 75 years, men, those with comorbidities, those seeking care in outpatient or inpatient, with pneumonia had higher costs. Similar results for community-acquired pneumonia have been reported before. A higher cost among inpatients, elders and individuals with comorbidities was reported by Kosar et al.32 Most studies on cost of pneumonia are from developed countries and are hospital based or based on cost estimated through insurance pay-outs. These are not relevant to our context. Chennai and Pune reported significantly higher costs and as already seen it was driven by higher indirect costs in Chennai and higher direct costs in Pune. Interestingly, once adjusted for each other, coverage with insurance, use of private sector and being wealthier did not influence the cost.
In our study, almost 80% of the total cost of pneumonia was contributed by indirect cost and cost of medicines was the major contributor of the direct cost. In a retrospective analysis of patients at a tertiary hospital, treated between October 2015 and March 2016, the main inpatient cost driver was hospital stay (94.97%), followed by medication (3.30%) and diagnostic tests (0.87%). For outpatients, key cost drivers, in order of magnitude, were prescribed medication (38.84%), diagnostic tests (33.51%) and physician visits (17.54%).33
In this study, ARI patients with laboratory-confirmed influenza incurred similar costs to those who tested negative. Antiviral use was very low, and the use of antibiotics was not related to influenza status. Respiratory illnesses often present with similar syndromes, are seldom tested for specific pathogens at the point of care and are treated empirically in the same manner. This calls for efforts to rationalise the use of antibiotics and antivirals in these patients perhaps using standardised but context-specific test and treat algorithms as these might have implications for patient prognosis as well as antimicrobial resistance. The resource utilisation and severity of illness did not vary for influenza-positive cases as compared with others. In an analysis of a cohort of 505 adult patients hospitalised with confirmed pneumonia between 2004 and 2010 in the Netherlands, detection of Staphylococcus aureus, or Streptococcus as causative pathogen and not viral pathogens were independent cost driving factors.34 The median cost of an influenza-associated outpatient visit was US$4.80 (IQR=2.93–8.11) and an influenza-associated hospitalisation was US$82.20 (IQR=59.96–121.56) in Bangladesh.35 This ratio of ambulatory to inpatient cost of 16 was much higher than our study.
The economic burden associated with pneumonia remains substantial at >US$17 billion annually in the USA.36 Applying the cost of US$25.6 to estimate of 138 million older adults (≥60 years) in India with a pneumonia incidence of 14.72 per 100 person-years as found in the study (1127 episodes in 7653 person-years of follow-up) means that pneumonia costs for India might be approximately US$520 million annually. The total economic burden of pneumonia in adults over 50 years of age was €12.6 million (Czech Republic); €9.2 million (Slovakia); €22.4 million (Poland); and 18.3 million Hungarian forint (Hungary) per year; with hospitalisation representing over 90% of the direct costs of treatment.37 A review of literature published between 1990 and May 2010 on the clinical and economic burden of CAP among adults in Asia-Pacific concluded that pneumonia is a significant health burden with significant economic impact in this region.38 Estimates from India are much lower despite a higher population because of low healthcare seeking with low rates of hospitalisation as well as lower cost of medical and non-medical resources. Among the hospitalised, however, the admission rate to intensive care unit was not very different from other studies.39
Our study estimated the total cost of pneumonia episode was US$25.6 and the annual cost of acute respiratory illness as US$29.5. The estimated total per capita annual healthcare expenditure for Indian in 2019–2020 was US$68.7, out of which 47.1% is by out-of-pocket expenditure.40 Using data from the National Sample Survey Organization, Social Consumption in Health 2018, Sriram and Albadrani estimated mean (SD) household monthly consumption expenditure for 2018 as US$132.8.41 The total cost of pneumonia episode (US$25.6) is two times the threshold used for defining catastrophic health expenditure (10% of monthly household consumption expenditure),42 indicating the potential for impoverishment in households where the elderly develop pneumonia. We did not collect data on household level income or expenditure and therefore could not estimate the proportion of household income spent on pneumonia.
The strengths of the study include a large multisite cohort size and multiple years covered. The study was community-based and used rigorous weekly follow-up visits to capture the treatment seeking, resource use and cost data. The limitations in the study include methods adopted to estimate loss of productivity and method of its valuation using national per capita income. Given that indirect costs were the main contributors to the cost, its valuation assumes importance. While three-fourths of the participants were unemployed, researchers have questioned the notion that older adults stop being productive once they retire.40 43 The minimum daily wages of the government of India vary from US$5.2 to US$11.7, depending on skill level and area of work.44 Cost of caregivers’ productivity loss constituted very small proportion of indirect costs. Overall, 57% of the caregivers were working and their mean income was US$91 per month, much less than US$155 estimate of national per capita income used in the study. Due to the community-based nature of the study (unlike facility based), resource use might not have been well captured. The cost of public facilities could have been underestimated as cost of medicines and investigations done free of charge might not have been captured.
In conclusion, the high economic burden due to respiratory illness characterised by poor insurance coverage, poor healthcare seeking as well as low vaccination coverage demonstrated in this study sites call for contemplating public health interventions to address pneumonia in this age group. The integrated public health approach would include the use of different vaccines for prevention and a case-management approach for the management of community-acquired pneumonia among older adults delivered through a strengthened primary healthcare system.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Acute respiratory illness (ARI) is a major cause of morbidity and mortality among older adults in India.
WHAT THIS STUDY ADDS
The study provided estimates on the economic burden of ARI among older adults in India.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This study would help in providing important estimates for assessing cost-effectiveness of preventives measures such as vaccination against influenza and pneumococcal infection in older adults.
Acknowledgements
We acknowledge the support of all the investigators and coinvestigators, our research staff, study participants and other members of the community who helped us in conducting this study. Comments received from the CDC e-clearance system to improve the manuscript are acknowledged.
Funding
The study was funded by Centers for Disease Prevention and Control, Atlanta, USA under Co-operative Agreement U01IP001074.
Footnotes
Disclaimer The findings and conclusions in this publication are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics approval This study involves human participants. The study received clearance from institutional ethics committees of the All India Institute of Medical Sciences (New Delhi, India), National Institute of Cholera and Enteric Diseases, National Institute of Virology (Pune, India) and National Institute of Epidemiology (Chennai, India). (AIIMS-IEC-283/02.06.17, NIE-IHEC/2017-03, NIV-IEC/2018/D5, NICED-A-1/2017-IEC). The study was approved by the Health Ministry Screening Committee on 8 November 2017. (Reference: 2017-4262). Participants gave informed consent to participate in the study before taking part.
Data availability statement
Data are available upon reasonable request.
REFERENCES
- 1.Wang H, Naghavi M, Allen C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu HH, Vongpradith A, Sirota SB. Age–sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: results from the Global Burden of Disease Study 2019. Lancet Infect Dis 2022;22:1626–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roth GA, Abate D, Abate KH. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Office of the Registrar General and Census Commissioner of India. Cause of death statistics in India 2016-18. New Delhi, 2022. [Google Scholar]
- 5.Chan EYS, Cheng D, Martin J. Magnitude of COVID-19 deaths relative to other leading causes of death: a global analysis. BMJ Open 2022;12:e049689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Ageing and health October. 2022. Available: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- 7.United Nations Department of Economic and Social Affairs PD. World population prospects. New York: United Nations, 2019. [Google Scholar]
- 8.American Lung Association. Preventing pneumonia. n.d. Available: https://www.lung.org/lung-health-diseases/lung-disease-lookup/pneumonia/preventing-pneumonia
- 9.Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive Pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 2010;59:1102–6. [PubMed] [Google Scholar]
- 10.World Health Organization. WHO SAGE seasonal influenza vaccination recommendations during the COVID-19 pandemic. 2020. [Google Scholar]
- 11.Directorate General of Health Services, Ministry of Health & Family Welfare, Government of India. Operational guidelines. National Programme for Health Care of the Elderly (NPHCE). 2019. [Google Scholar]
- 12.Rizvi AA, Singh A. Vaccination coverage among older adults: a population-based study in India. Bull World Health Organ 2022;100:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campling J, Jones D, Chalmers J, et al. Clinical and financial burden of hospitalised community-acquired pneumonia in patients with selected underlying comorbidities in England. BMJ Open Respir Res 2020;7:e000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divino V, Schranz J, Early M, et al. The annual economic burden among patients hospitalized for community-acquired pneumonia (CAP): a retrospective US cohort study. Curr Med Res Opin 2020;36:151–60. [DOI] [PubMed] [Google Scholar]
- 15.Konomura K, Nagai H, Akazawa M. Economic burden of community-acquired pneumonia among elderly patients: a Japanese perspective. Pneumonia (Nathan) 2017;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dilokthornsakul P, Lan LM, Thakkinstian A, et al. Economic evaluation of seasonal influenza vaccination in elderly and health workers: a systematic review and meta-analysis. EClinicalMedicine 2022;47:101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loong D, Pham B, Amiri M, et al. Systematic review on the cost-effectiveness of seasonal influenza vaccines in older adults. Value Health 2022;25:1439–58. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan A, Dar L, Amarchand R, et al. Cohort profile: Indian network of population-based surveillance platforms for influenza and other respiratory viruses among the elderly (INSPIRE). BMJ Open 2021;11:e052473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009;64 Suppl 3:iii1–55. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. WHO manual for estimating the economic burden of seasonal influenza. Geneva: WHO, 2016. [Google Scholar]
- 21.Stenberg K, Lauer JA, Gkountouras G, et al. Econometric estimation of WHO-CHOICE country-specific costs for inpatient and outpatient health service delivery. Cost Eff Resour Alloc 2018;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jo C. Cost-of-illness studies: concepts, scopes, and methods. Clin Mol Hepatol 2014;20:327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Economic Affair Government of India. Economic survey 2021-2022 appendix. 2022. [Google Scholar]
- 24.Reserve Bank of India. Exchange rate of Indian Rupee vu-A-vis US dollar, puund Sterling, Euro, Japenese Yen. [Google Scholar]
- 25.Garg S, Bebarta KK, Tripathi N. Role of publicly funded health insurance in financial protection of the elderly from hospitalisation expenditure in India-findings from the longitudinal aging study. BMC Geriatr 2022;22:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauhan S, Patel R, Kumar S. Prevalence, factors and inequalities in chronic disease multimorbidity among older adults in India: analysis of cross-sectional data from the nationally representative longitudinal aging study in India (LASI). BMJ Open 2022;12:e053953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranjan A, Muraleedharan VR. Equity and elderly health in India: reflections from 75th round national sample survey, 2017-18, amidst the COVID-19 pandemic. Global Health 2020;16:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly C, Krueger P, Lohfeld L, et al. I really should've gone to the doctor": older adults and family caregivers describe their experiences with community-acquired pneumonia. BMC Fam Pract 2006;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Personne V, Chevalier J, Buffel du Vaure C, et al. CAPECO: cost evaluation of community acquired pneumonia managed in primary care. Vaccine 2016;34:2275–80. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Yoo CG, Kim HJ, et al. Disease burden of pneumonia in Korean adults aged over 50 years stratified by age and underlying diseases. Korean J Intern Med 2014;29:764–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vissink CE, Huijts SM, de Wit GA, et al. Hospitalization costs for community-acquired pneumonia in Dutch elderly: an observational study. BMC Infect Dis 2016;16:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosar F, Alici DE, Hacibedel B, et al. Burden of community-acquired pneumonia in adults over 18 Y of age. Hum Vaccin Immunother 2017;13:1673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naoum P, Athanasakis K, Kyriopoulos I, et al. Community acquired pneumonia: a cost-of-illness analysis in Greece. Rural Remote Health 2020;20:5400. [DOI] [PubMed] [Google Scholar]
- 34.Spoorenberg SMC, Bos WJW, Heijligenberg R, et al. Microbial aetiology, outcomes, and costs of hospitalisation for community-acquired pneumonia; an observational analysis. BMC Infect Dis 2014;14:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhuiyan MU, Luby SP, Alamgir NI, et al. Economic burden of influenza-associated hospitalizations and outpatient visits in Bangladesh during 2010. Influenza Other Respir Viruses 2014;8:406–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.File TM, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010;122:130–41. [DOI] [PubMed] [Google Scholar]
- 37.Tichopad A, Roberts C, Gembula I, et al. Clinical and economic burden of community-acquired pneumonia among adults in the Czech Republic, Hungary, Poland and Slovakia. PLoS One 2013;8:e71375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song J-H, Thamlikitkul V, Hsueh P-R. Clinical and economic burden of community-acquired pneumonia amongst adults in the Asia-Pacific region. Int J Antimicrob Agents 2011;38:108–17. [DOI] [PubMed] [Google Scholar]
- 39.Marrie TJ, Shariatzadeh MR. Community-acquired pneumonia requiring admission to an intensive care unit: a descriptive study. Medicine (Baltimore) 2007;86:103–11. [DOI] [PubMed] [Google Scholar]
- 40.National Health Systems Resource Centre. National health accounts estimates for India (2019-20). New Delhi, 2023. [Google Scholar]
- 41.Sriram S, Albadrani M. A study of catastrophic health expenditures in India - evidence from nationally representative survey data: 2014-2018. F1000Res 2022;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ekirapa-Kiracho E, De Broucker G, Ssebagereka A, et al. The economic burden of pneumonia in children under five in Uganda. Vaccine X 2021;8:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-Ballesteros R, Zamarrón MD, Díez-Nicolás J, et al. Productivity in old age. Res Aging 2011;33:205–26. [Google Scholar]
- 44.Ministry of Labour & Employment GoI. Rates of wages. 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.