Abstract
Krebs cycle enzyme activity in Bacillus subtilis was examined under aerobic and anaerobic conditions. Citrate synthase and aconitase activities in cells grown anaerobically in the presence of nitrate were reduced by as much as 10- and 30-fold, respectively, from levels observed under aerobic culture conditions. The maximum level of isocitrate dehydrogenase activity during anaerobic growth was only twofold lower than that in aerobic cultures. These reductions in activity under conditions of anaerobiosis were found to be primarily the result of reduced Krebs cycle gene transcription. This repression was not dependent on either the fnr or resDE gene products, which have been shown to regulate expression of other B. subtilis genes in response to anaerobic conditions. Additionally, catabolite control proteins CcpA and CcpB were not responsible for the repression. A dyad symmetry element located between positions −73 and −59 relative to the transcription start site of the aconitase gene (citB) promoter was previously shown to be a target of catabolite repression and the binding site for a putative negative regulator during aerobic growth. The deletion of the upstream arm of the dyad symmetry region abolished the citB repression observed during anaerobic growth. Furthermore, neither citZ or citB was repressed in an anaerobically grown citB mutant, an effect that was very likely the result of citrate accumulation. These results suggest that catabolite repression and anaerobic repression of citZ and citB are regulated by a common mechanism that does not involve CcpA, CcpB, Fnr, or ResDE.
The genes encoding the enzymes of the tricarboxylic acid (TCA) branch of the Krebs cycle in Bacillus subtilis, citrate synthase (CS), aconitase (ACN), and isocitrate dehydrogenase (ICDH), have been isolated, and some aspects of their regulation have been described (15, 16, 28). Genes for three CS isozymes have been found. The citA and citZ genes encode minor and major contributors, respectively, to total CS enzyme activity during the late exponential growth phase in nutrient broth (DS) medium (27) or during growth in a defined medium containing a poor carbon source. The third CS is encoded by mmgD, a gene expressed during sporulation but for which no function during development has been demonstrated (1). ACN is encoded by citB, a gene that is not linked to either citA or citZ (10, 38). The third TCA branch enzyme, ICDH, is the product of citC, the second gene of the citZCH operon (25, 27). (The citH gene encodes malate dehydrogenase, the enzyme that forms oxaloacetate, which is the substrate for CS [25].) The citC gene is transcribed both from the citZ operon promoter and from its own promoter located within the C-terminal coding sequence of citZ (28).
The Krebs cycle has three major metabolic functions: supplying biosynthetic precursors (α-ketoglutarate, succinyl coenzyme A [CoA], and oxaloacetate), generating energy, and creating reducing power when glycolysis is unable to fulfill the cell’s needs. In accordance with these physiological roles, the specific activities of CS and ACN in B. subtilis are reduced when glucose is available and are more severely depressed when glucose and a source of α-ketoglutarate (such as glutamate or glutamine) are both supplied (5, 12, 21, 22, 36). This regulation is exerted at the transcriptional level (28, 39), and regulatory sites for catabolite (glucose) repression have been identified in the citB promoter region (15, 16). Mutations that relieve catabolite repression of citB expression do not significantly affect temporal regulation of citB in DS (sporulation) medium, suggesting that catabolite repression and temporal regulation are mediated by different mechanisms (15).
In Escherichia coli, Krebs cycle enzyme activities are affected both by the medium composition and by the level of oxygen in the medium. During aerobic growth of E. coli in minimal medium with acetate as the sole carbon source, Krebs cycle activity is at its highest and abundant energy is generated by the oxidation of reduced coenzymes through the aerobic respiratory chain. Under anaerobic conditions, especially during fermentative growth, during which NADH cannot be reoxidized by the respiratory chain, Krebs cycle activity is reduced to the minimum level needed to supply biosynthetic pathways and is transformed from a cyclic pathway to two oppositely oriented half-cycles. Synthesis of α-ketoglutarate dehydrogenase is repressed, and succinate dehydrogenase is replaced by fumarate reductase (20, 41). Other Krebs cycle enzyme activities, including the TCA branch enzymes ACN and CS, are also reduced (17–19, 37, 43, 44). Expression of E. coli ACN genes acnA (19) and acnB (18) was shown to be subject to catabolite and anaerobic repression. Furthermore, it was shown that catabolite repression of acnA and acnB is mediated by cyclic AMP receptor protein (CRP) and that ArcA (24) functions as an anaerobic repressor of acnA transcription (8, 19). ArcA also functions as a repressor of the citrate synthase gene, gltA, of E. coli under anaerobic as well as aerobic conditions (37). Unlike the case for acn, a crp mutation does not significantly affect gltA expression under almost any set of conditions tested (37). The level of expression of E. coli icd, which encodes ICDH, was also shown to be fivefold lower under anaerobic cell growth conditions than under aerobic conditions. This negative control is mediated by the arcA and the fnr gene products (3).
Analogous studies on the regulation of Krebs cycle enzymes in B. subtilis have not been reported, primarily because B. subtilis has long been regarded as a strict aerobe and is unable to grow with acetate as the sole carbon source (23). The recent discovery that B. subtilis grows anaerobically (reviewed in reference 33) offers an opportunity to examine how B. subtilis Krebs cycle enzymes are affected by oxygen availability and what role, if any, they play in anaerobic growth. In this paper, we show that the activities of Krebs cycle enzymes of B. subtilis, particularly ACN, are depressed during anaerobic growth and that the dyad symmetry sequence located in the citB promoter region, which was previously identified as the target for carbon catabolite repression (15, 16), is needed for anaerobic repression of citB. The anaerobic repression of citB and citZ was relieved in a citB mutant, presumably due to accumulation of citrate, which likely inactivates the repressor of citB.
MATERIALS AND METHODS
Bacterial strains.
All B. subtilis strains used in this study (Table 1) are derivatives of JH642. All lacZ fusions described here are transcriptional fusions. lacZ fusions transcribed from promoters of citB (ACN gene), citZ (CS gene), and citC (ICDH gene) were constructed as follows. MAB100 and MAB101 were constructed by transformation of JH642 with chromosomal DNA from derivatives of strain SMY in which plasmid pAF23 or pAF24, respectively, is integrated at the amyE locus. The plasmids pAF23 and pAF24 contain a promoterless E. coli lacZ gene fused to citB promoter sequences from positions −84 to +36 and −67 to +36, respectively, relative to the transcription start site (16). LAB2163, carrying a citZ-lacZ fusion, was also constructed by transformation with SJB49 chromosomal DNA. SJB49 was previously constructed by integration of pCS46, a citZ-lacZ fusion plasmid, at the citZ locus (28). Two citZ-lacZ-carrying strains, LAB2164 and LAB2851, with resDE and fnr mutations, respectively, were constructed by transforming strain LAB2163 with chromosomal DNA isolated from strain LAB2135 (for resDE) or LAB2136 (for fnr). Strains MAB109 (citB-lacZ resDE) and LAB2852 (citB-lacZ fnr) were constructed in the same way, using MAB100 cells as the recipient. citB mutants carrying citZ-lacZ (LAB2907) or citB-lacZ (LAB2905) fusions were generated by transforming MAB160 (citB::spc) (6) with chromosomal DNA from LAB2163 or MAB100, respectively. Two citZAB mutants carrying citZ-lacZ (LAB2935) or citB-lacZ (LAB2936) fusions were constructed by transforming JCB61 (6) with pCS46 or chromosomal DNA from MAB100, respectively. A citC-lacZ transcriptional fusion was constructed in two steps. First, the 0.9-kb PstI-SspI fragment from pCS34 (28), which contains the citC promoter, was inserted into PstI- and SmaI-cleaved pBluescript II SK(−) (Stratagene), creating plasmid pMMN325. Plasmid pMMN332 was constructed by inserting the EcoRI-BamHI fragment of pMMN325 into EcoRI- and BamHI-cleaved pTKlac (30), a transcriptional fusion vector. pMMN332 was used to transform ZB307A by homologous recombination at the SPβ prophage locus (46), and the phage lysate prepared from the transformants was used to transduce ZB278. Strain MAB173, carrying citC-lacZ, was constructed by transducing JH642 with the lysate prepared from the ZB278 transductant. Strain LAB2962, carrying the ccpA and ccpB mutations, was constructed by transforming JH642 with chromosomal DNA obtained from strain ST125, with selection for phleomycin resistance carried by Tn917 and spectinomycin resistance conferred by Tn10 (4). LAB2962 was transformed with MAB100 DNA or LAB2163 DNA to generate ccpA ccpB double mutants carrying citB-lacZ (LAB2970) DNA or citZ-lacZ (LAB2971) fusions, respectively. Two lacZ fusions containing catabolite-responsive elements (cre), HUT924 and BGL2, were obtained from L. Wray and S. Fisher (45). The HUT924 lacZ fusion contains the cre site of the histidine utilization (hut) operon downstream of the tms promoter (32). The BGL2 lacZ fusion contains the promoter and the cre site of the bglPH operon that encodes two enzymes involved in β-glucoside assimilation. Strains LAB2960 and LAB2961 were JH642 cells carrying the HUT924 and BGL2 fusions, respectively.
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| JCB61 | pheA1 citA::neo citZ471 citB::spc | 6 |
| JH642 | trpC2 pheA1 | J. A. Hoch |
| SJB49 | trpC2 pheA1 citZ-lacZ cat | 28 |
| SMY::pAF23 | trpC2 pheA1 amyE::pAF23 (citB-lacZ) cat | 16 |
| SMY::pAF24 | trpC2 pheA1 amyE::pAF24 (citB-lacZ) cat | 16 |
| ST125 | trpC2 pheA1 ccpA::Tn917 ccpB::Tn10 | 4 |
| ZB278 | trpC2 SPβc | 46 |
| ZB307A | SPβc2del2::Tn917::pSK10Δ6 | 46 |
| LAB2135 | trpC2 pheA1 resDE::tet | 35 |
| LAB2136 | trpC2 pheA1 fnr::spc | 35 |
| LAB2163 | trpC2 pheA1 citZ-lacZ cat | JH642 × SJB49 DNA |
| LAB2164 | trpC2 pheA1 citZ-lacZ cat resDE::tet | LAB2163 × LAB2135 |
| LAB2851 | trpC2 pheA1 citZ-lacZ cat fnr::spc | LAB2163 × LAB2136 |
| LAB2852 | trpC2 pheA1 amyE::pAF23 (citB-lacZ) fnr::spc | MAB100 × LAB2136 |
| LAB2905 | trpC2 pheA1 amyE::pAF23 (citB-lacZ) citB::spc | MAB160 × MAB100 |
| LAB2907 | trpC2 pheA1 citZ-lacZ cat citB::spc | MAB160 × LAB2163 |
| LAB2935 | pheA1 citA::neo citZ471 citB::spc citZ-lacZ cat | JCB61 × pCS46 |
| LAB2936 | pheA1 citA::neo citZ471 citB::spc amyE::pAF23 (citB-lacZ) | JCB61 × MAB100 |
| LAB2960 | trpC2 pheA1 amyE::pHUT924 (tms-hut cre-lacZ) | 45 |
| LAB2961 | trpC2 pheA1 amyE::pBGL2 (bglPH-lacZ) | 45 |
| LAB2962 | trpC2 pheA1 ccpA::Tn917 ccpB::Tn10 | ST125 × JH642 |
| LAB2970 | trpC2 pheA1 ccpA::Tn917 ccpB::Tn10 amyE::pAF23 (citB-lacZ) cat | LAB2962 × MAB100 |
| LAB2971 | trpC2 pheA1 ccpA::Tn917 ccpB::Tn10 citZ-lacZ cat | LAB2962 × LAB2163 |
| MAB100 | trpC2 pheA1 amyE::pAF23 (citB-lacZ) | JH642 × SMY::pAF23 |
| MAB101 | trpC2 pheA1 amyE::pAF24 (citB-lacZ) | JH642 × SMY::pAF24 |
| MAB109 | trpC2 pheA1 amyE::pAF23 (citB-lacZ) resDE::tet | MAB100 × LAB2135 |
| MAB160 | trpC2 pheA1 citB::spc | 6 |
| MAB173 | trpC2 pheA1 SPβc2del2::Tn917::pMMN332 (citC-lacZ) cat | SPβ::citC-lacZ to JH642 |
Selection for antibiotic resistance was performed on DS medium (34) containing chloramphenicol (5 μg/ml), spectinomycin (75 μg/ml), tetracycline (12.5 μg/ml), phleomycin (5 μg/ml), or neomycin (5 μg/ml).
Measurement of β-galactosidase activity.
For both aerobic and anaerobic cultures, cells from fresh plates were used to inoculate liquid medium to an optical density at 600 nm of 0.02. To compare aerobic and anaerobic expression of various lacZ fusions in the same medium, KNO3 was added to all cultures. Cells were grown in DS medium (34) supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM potassium phosphate buffer (pH 7.0). For aerobic growth, the culture medium occupied about 10% of the volume of the flask and was aerated by vigorous agitation. Anaerobic conditions were achieved by filling tubes with cell suspensions, flushing with N2 gas for 1 min, capping the tubes, and incubating without shaking. After removal of each sample, N2 gas was used to flush O2 from the culture to maintain anaerobic conditions. Assays of β-galactosidase activity were performed as described elsewhere (31).
Assays for CS, ACN, and ICDH activities.
B. subtilis cells were grown in 100 ml (aerobic) or 500 ml (anaerobic) of DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM potassium phosphate buffer (pH 7.0). Anaerobic cultures were grown in bottles as described above. Cells were harvested by centrifugation (12,000 × g for 5 min) about 1 h after the end of the exponential growth phase, washed with buffer, and frozen on dry ice. The buffer used to wash cells prior to the measurement of CS activity contained 20 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 20% glycerol; that used for washing cells before measuring ACN activity consisted of 20 mM Tris–20 mM citric acid (pH 7.35), 150 mM KCl, and 0.5 mM phenylmethylsulfonyl fluoride; and the buffer utilized prior to measurement of ICDH activity contained 20 mM Tris-HCl (pH 7.5), 1 mM trisodium citrate, 5 mM MnCl2, 5 mM β-mercaptoethanol, 10% glycerol, and 0.5 mM phenylmethylsulfonyl fluoride. Cell pellets were resuspended in the corresponding buffer prior to sonication (30-s pulse, 3 to 5 min). Cell debris was removed by centrifugation (8 min at 10,000 × g and 4°C), and supernatants (crude extracts) were used for measurement of enzyme activity. The activities of CS and ICDH were assayed according to published procedures (14, 22, 27), and ACN activity was measured by a previously described method (6, 10). Units of CS, ACN, and ICDH were expressed as micromoles of CoA produced per minute per milligram of protein, nanomoles of cis-aconitate produced per minute per milligram of protein, and nanomoles of NADPH produced per minute per milligram of protein, respectively. Protein concentrations were determined by the Bio-Rad protein assay.
Immunoblotting analysis of CS, ACN, and ICDH.
Crude extracts (50 μg for ACN and 10 μg for CS and ICDH) prepared as described above were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide (8% for ACN and 12% for CS and ICDH) gel electrophoresis. Proteins were transferred to an Immobilon P membrane and exposed either to rabbit antibodies against CS or ICDH or to mouse antibodies against ACN. Antigen-antibody reactions were detected by binding of the appropriate anti-rabbit or anti-mouse immunoglobulin G coupled to alkaline phosphatase and incubating with 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium. The antibody to CS was raised against the product of the citZ gene but shows some cross-reactivity with the product of the citA gene (26).
Measurement of intracellular citrate concentration.
Wild-type and mutant cells were cultured anaerobically in DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM potassium phosphate buffer (pH 7.0). Cells from 25-ml cultures were collected by centrifugation (10,000 × g for 5 min) and washed with 0.1 M Tris-HCl (pH 8.0). The cell pellet was resuspended in 0.5 ml of 1 M perchloric acid and kept in an ice bath for 30 min. The supernatant fluid was recovered by centrifugation and neutralized with 0.25 ml of a 0.75 M potassium carbonate solution. The mixture was placed on ice for 15 min and centrifuged. The supernatant fluid was used for measurement of citrate with a kit obtained from Boehringer Mannheim. The principle of the assay is as follows: citrate is converted to oxaloacetate and acetate by citrate lyase, coupled to reduction of oxaloacetate to malate by malate dehydrogenase and concomitant oxidation of NADH. The oxidation of NADH is measured as the reduction in absorbance at 340 nm. The protein concentration in the extract was determined by the Bio-Rad protein assay.
RESULTS
The levels of expression of Krebs cycle enzymes were lower under anaerobic conditions than under aerobic conditions.
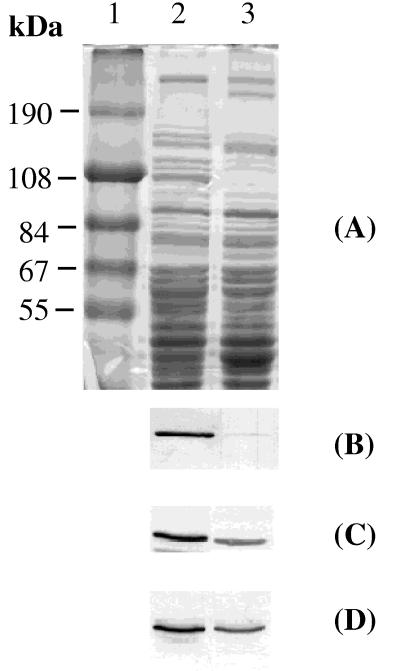
Since carbon and electron flow as well as energy generation are altered by a shift between aerobiosis and anaerobiosis, expression of the Krebs cycle enzyme genes could be regulated in response to oxygen availability. The recent finding that B. subtilis grows anaerobically prompted us to examine how expression of genes specifying Krebs cycle enzymes in B. subtilis is regulated in response to an anaerobic environment. The activities of CS, ACN, and ICDH in cells grown anaerobically were 10, 3, and 54%, respectively, of those measured in cells of aerobic cultures (Table 2). To determine if the depressed activity of each of these Krebs cycle enzymes was caused by enzyme inhibition or a reduced level of enzyme protein, equal amounts of crude protein extracts of aerobic and anaerobic cultures were applied to SDS-polyacrylamide gels and the Krebs cycle proteins were detected by immunoblotting (Fig. 1). Coomassie blue staining of the gel showed that some cellular proteins are predominantly or exclusively detected in either the anaerobic or aerobic cultures (Fig. 1A). When anti-ACN antibody was used, the crude extract from the aerobic cultures gave a strong band at the expected position corresponding to ACN, but the band was barely detectable in the extract prepared from the anaerobic cultures (Fig. 1B). Densitometric scanning showed that the intensity of the ACN band in the anaerobic culture was 3% of that of the aerobic one. CS and ICDH proteins were detectable in both the aerobic and anaerobic cultures, but the intensity of the band of CS from the anaerobic cultures was 45% of that of the aerobic cultures (Fig. 1C), and the intensity of ICDH from the anaerobically grown cells was 53% of that of the aerobic ones (Fig. 1D). The levels of ACN and ICDH proteins corresponded well to the enzyme activity determinations (Table 2), indicating that the low specific activities detected in anaerobic cultures were mainly due to reduced amounts of the protein products. CS behaved differently; the enzyme activity under anaerobic conditions was 10% of that observed in the aerobic cultures, but the amount of protein was reduced only twofold by anaerobiosis, suggesting that CS activity may also be controlled by the oxygen level.
TABLE 2.
Enzyme activities under aerobic and anaerobic conditions
| Enzyme | Activity under conditions that werea:
|
|
|---|---|---|
| Aerobic | Anaerobic | |
| CS | 11.4 | 1.1 |
| ACN | 182 | 5.4 |
| ICDH | 480 | 259 |
Enzyme activities in extracts of cells grown aerobically or anaerobically in DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM potassium phosphate buffer (pH 7.0) and harvested 1 h after the end of exponential growth were determined (see Materials and Methods). Specific activities of CS, ACN, and ICDH were calculated as micromoles of CoA produced per minute per milligram of protein, nanomoles of cis-aconitate produced per minute per milligram of protein, and nanomoles of NADPH produced per minute per milligram of protein, respectively. The data are averages of values obtained in two experiments.
FIG. 1.
(A) SDS-polyacrylamide gel electrophoresis analysis of proteins from B. subtilis. Cell lysates were prepared as described in Materials and Methods from cells grown until T1 in DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM phosphate buffer (pH 7.0) under aerobic (lane 2) and anaerobic (lane 3) conditions. Lane 1, molecular size markers. (B to D) Immunoblot analysis of the cell lysates, using anti-ACN (B), anti-CS (C), or anti-ICDH (D) immunoglobulin G as the primary antibody as described in Materials and Methods.
Anaerobic reduction of the Krebs cycle enzyme level is exerted at the transcriptional level.
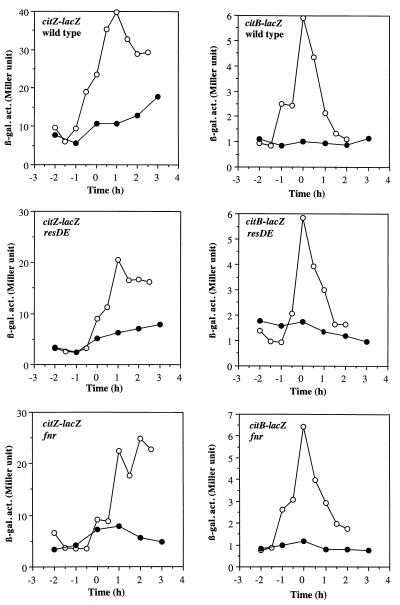
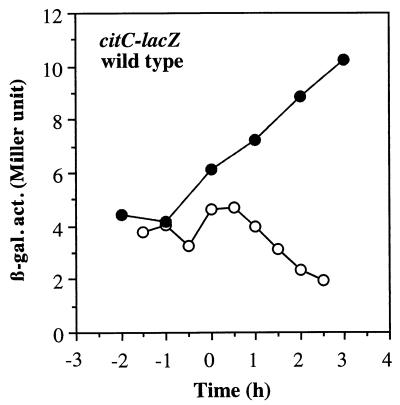
To determine if the reduction in Krebs cycle enzyme levels observed in the experiment described above was the result of transcriptional control, expression of lacZ fusions transcribed from the citZ, citB, or citC promoter was examined. Previous studies showed that citZ-lacZ (28) and citB-lacZ (9) fusions were repressed during early exponential growth in DS medium and reached a peak of expression when cultures enter the end of exponential phase. In this study, B. subtilis cells were grown in DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM phosphate buffer (pH 7.0) to support anaerobic growth. A low concentration of glucose was chosen for supplementation of the medium so that anaerobic growth would be enhanced but catabolite repression of Krebs cycle gene transcription would be minimized. In this medium, the aerobically grown cells had patterns and levels of citZ- and citB-lacZ expression similar to those of cells grown in unsupplemented DS medium, as previously shown (Fig. 2). This confirmed that 0.1% glucose does not cause catabolite repression of the Krebs cycle enzymes. The maximum level of anaerobic citZ expression was threefold lower than that observed in an aerobic culture, while maximum anaerobic expression of citB-lacZ reached only 10% of the maximum aerobic expression. In contrast, the level of citC-lacZ expression was higher during anaerobic growth than during aerobic growth (Fig. 3). The citC gene is known to be transcribed from the upstream citZ promoter as well as from its own promoter (28). Therefore, threefold repression of the citZ promoter and twofold induction of transcription from the citC promoter under anaerobic conditions could explain the overall 50% reduction of ICDH activity during anaerobic growth shown in Table 2. Among the three enzymes, synthesis of ACN was the most severely repressed, as judged by enzyme activity, immunoblot analysis, and lacZ fusion expression.
FIG. 2.
Expression of citZ-lacZ and citB-lacZ fusions in wild-type, resDE, and fnr strains. Cells of wild-type (LAB2163), resDE (LAB2164), and fnr (LAB2851) strains carrying citZ-lacZ fusions and cells of wild-type (MAB100), resDE (MAB109), and fnr (LAB2852) strains carrying citB-lacZ fusions were grown in DS medium supplemented with 0.1% glucose, 0.2% KNO3, and 20 mM phosphate buffer (pH 7.0) under aerobic (open circles) and anaerobic (closed circles) conditions. Time zero is the end of exponential growth. β-gal. act., β-galactosidase activity (in Miller units).
FIG. 3.
Expression of a citC-lacZ fusion in a wild-type strain. Cells of strain MAB173 were grown as described in the legend to Fig. 2 under aerobic (open circles) and anaerobic (closed circles) conditions. β-gal. act., β-galactosidase activity (in Miller units).
Anaerobic repression of Krebs cycle enzymes is not mediated by either CcpA or CcpB.
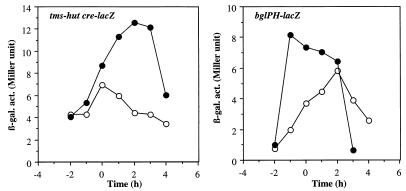
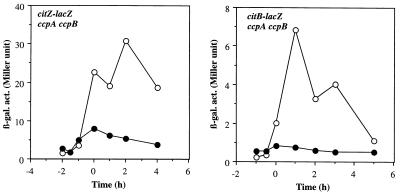
Although the concentration of glucose used for the cultures described above does not cause catabolite repression of Krebs cycle enzymes during aerobiosis, it is possible that this amount of glucose is sufficient to result in catabolite repression under anaerobic conditions. To examine this possibility, we tested whether other carbon catabolite-controlled genes are repressed during anaerobic growth under the culture conditions used for the expression of Krebs cycle enzymes. Two lacZ fusions used for this purpose are under the control of carbon catabolite repression mediated by a trans-acting factor, catabolite control protein A (CcpA), and cis-acting cre sequences. The HUT924 lacZ fusion contains the tms promoter and the cre site of the hut operon. The BGL2 lacZ fusion contains both the promoter and the cre site of the bglPH operon (45). Figure 4 shows that the expression of these lacZ fusions was not repressed (but instead increased) during anaerobic growth compared to aerobic growth. This result indicates that the repression of citZ and citB under anaerobic conditions is not caused by glucose but rather by oxygen limitation. It also shows that CcpA is not responsible for the anaerobic repression of Krebs cycle enzymes. A recent study identified a novel transcription factor, CcpB, which is involved in catabolite repression in B. subtilis (4). It was demonstrated that CcpA alone functioned in catabolite repression during growth in liquid medium with high agitation but that both CcpA and CcpB functioned when cells were grown on solid medium or in liquid medium with little agitation, conditions under which the oxygen level could be reduced. A possible role for CcpB (and CcpA) in the anaerobic repression of the citZ and citB genes was examined. As shown in Fig. 5, citZ and citB were still anaerobically repressed in the absence of CcpA and CcpB, indicating that CcpA and CcpB do not have any significant role in the anaerobic repression of citZ and citB.
FIG. 4.
Expression of tms-hut cre-lacZ and bglPH-lacZ fusions. Cells of LAB2960 (tms-hut cre-lacZ) and LAB2961 (bglPH-lacZ) were grown as described in the legend to Fig. 2 under aerobic (open circles) and anaerobic (closed circles) conditions. β-gal. act., β-galactosidase activity (in Miller units).
FIG. 5.
Effect of ccpA and ccpB mutations on expression of citZ-lacZ and citB-lacZ fusions. Cells of strains LAB2971 (ccpA ccpB citZ-lacZ) and LAB2970 (ccpA ccpB citB-lacZ) were grown as described in the legend to Fig. 2 under aerobic (open circles) and anaerobic (closed circles) conditions. β-gal. act., β-galactosidase activity (in Miller units).
ResDE and FNR are not involved in anaerobic repression of Krebs cycle enzymes.
Since the ResDE two-component regulatory signal transduction pathway (35, 42) and Fnr (7) are known to regulate gene expression in B. subtilis in response to oxygen limitation, we examined the effect of resDE and fnr mutations on citZ and citB expression. In either the resDE or fnr mutant, expression of citZ-lacZ was reduced by 40 to 50% during both aerobic and anaerobic growth (Fig. 2). However, the anaerobic repression ratio of citZ expression in the mutant strains was almost identical to that in the wild-type strain. The expression of citB-lacZ under aerobic and anaerobic conditions was not affected by either the resDE or fnr mutation, although the resDE mutation resulted in a slight but reproducible increase in citB expression during exponential growth (Fig. 2). These results indicate that the known global anaerobic regulators in B. subtilis, ResDE and Fnr, are not responsible for anaerobic repression of citB and citZ.
A regulatory site in the citB promoter involved in carbon catabolite repression is also important for anaerobic repression.
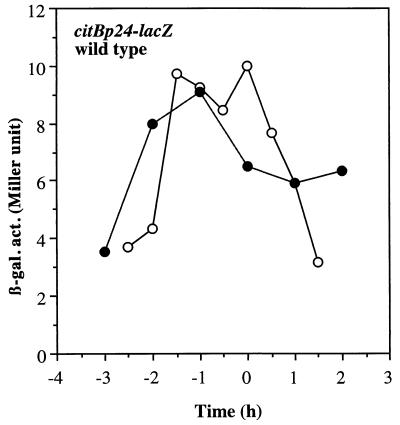
Among the genes for the Krebs cycle enzymes, only the promoter region of the citB gene has been analyzed in detail. The dyad symmetry sequence centered at position −66 relative to the transcription start site has been shown to be required for catabolite repression (15, 16). To determine if the dyad symmetry sequence mutation that relieves catabolite repression (15, 16) also affects citB expression under anaerobic conditions, we measured β-galactosidase activity driven from a mutant promoter (citBp24), which lacks the left arm of the dyad symmetry element (16). Figure 6 shows that MAB101 cells carrying the citBp24 promoter exhibited a level of lacZ expression under anaerobic conditions that was equal to that observed under aerobic conditions, indicating that the dyad symmetry element involved in catabolite repression is also critical for anaerobic repression of citB.
FIG. 6.
Expression of lacZ from a mutant citB promoter. Cells of wild-type strain MAB101 were grown as described in the legend to Fig. 2 under aerobic (open circles) and anaerobic (closed circles) conditions. β-gal. act., β-galactosidase activity (in Miller units).
Accumulation of citrate relieves anaerobic repression of citZ and citB.
How are the citZ and citB genes coordinately regulated by the oxygen level? One possibility is that a common repressor is responsible for the regulation of both genes. The other possibility is that the primary effect of anaerobiosis is to reduce CS activity, resulting in a decrease in the intracellular citrate concentration, leading to low-level citB expression. (Catabolite repression of the citB gene is known to be alleviated by accumulation of citrate [6, 15, 36]). The two possibilities are not mutually exclusive. In attempts to determine if citrate deficiency caused the anaerobic repression, expression of citB in strain MAB100 which was grown in the presence of added citrate (5 and 10 mM) was measured. If a low citrate level in the anaerobically grown cells was the cause of the reduced citB expression, addition of citrate to the culture medium might lead to a higher level of citB expression. The results showed that exogenous citrate had no significant effect on either aerobic or anaerobic citB expression (data not shown), indicating either that anaerobic repression of the citB gene is not caused by a low intracellular citrate concentration or that citrate transport is repressed under anaerobic conditions.
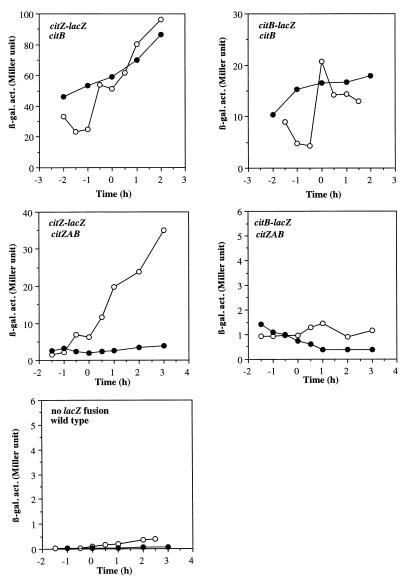
We then took an alternative approach to examine the effect of citrate on anaerobic expression of citZ and citB. It was shown that aerobic cultures of a citB null mutant accumulated a high level of citrate in the medium and also contained a twofold-higher intracellular concentration of citrate than do wild-type cells (6). Extracts from anaerobic cultures of MAB160 cells also contained a higher citrate concentration than did wild-type extracts (Table 3), and an almost 10-fold-higher citrate accumulation was observed in the citB mutant at 2 h after the end of exponential growth compared to the wild type at the same growth stage. To examine further the effect of citrate on anaerobic citZ and citB expression, we measured citZ- and citB-lacZ activities in the citB mutant. As seen in Fig. 7, aerobic citZ and citB expression was threefold higher in the citB mutant than in the wild-type strain, and anaerobic repression of citZ and citB was lost in the mutant. This result strongly suggests that accumulation of citrate can overcome the anaerobic repression of citZ and citB but does not eliminate the possibility that ACN itself is involved in the regulation.
TABLE 3.
Intracellular citrate levels in wild-type and mutant cells
| Strain (relevant genotype) | Citric acid concn (μg/mg of protein)a
|
|
|---|---|---|
| T0 | T2 | |
| JH642 (wild type) | 0.45; 0.12 | 0.34; 0.17 |
| MAB160 (citB) | 1.06; 0.83 | 2.14; 2.26 |
| JCB61 (citZAB) | 0.46; 0.23 | 0.15; 0.13 |
The concentration of citric acid (calculated as the anhydrous acid) in cells harvested at the end of (T0) and 2 h after (T2) exponential growth was measured as described in Materials and Methods. Data from two independent assays are shown.
FIG. 7.
Expression of citZ-lacZ and citB-lacZ fusions in citB and citZAB mutants. citB strains carrying citZ-lacZ (LAB2907) and citB-lacZ (LAB2905) fusions and citZAB strains carrying citZ-lacZ (LAB2935) and citB-lacZ (LAB2936) fusions were grown as described in the legend to Fig. 2 under aerobic (open circles) and anaerobic (closed circles) conditions. Also shown are endogenous β-galactosidase activities (β-gal. act.) of JH642, which carries no lacZ fusion, during aerobic (open circles) and anaerobic (closed circles) growth.
To determine which possibility is more probable, citZ- and citB-lacZ fusions were introduced into a triple mutant (JCB61) lacking ACN and both CS isozymes, encoded by citZ and citA. Mutations in citZ and citA abolished the citrate accumulation observed in the citB mutant, as shown in Table 3. If derepression of citB and citZ by the citB mutation were caused by accumulation of citrate, the level of expression of these genes in the triple mutant would be decreased. Figure 7 shows that the level of aerobic citZ expression in the triple mutant was two- to threefold lower than that in the citB single mutant but was comparable to the level in the wild type. Anaerobic citZ expression was more severely repressed in the triple mutant than in the wild-type strain, indicating that citrate deficiency causes anaerobic citZ repression. The effect of the citZAB mutations on anaerobic citB repression was difficult to assess since the level of citB expression in the triple mutant was highly reduced (although it was slightly higher than the endogenous β-galactosidase activity, as shown in Fig. 7) even under aerobic conditions, which is in agreement with the previous observation that citrate is a prerequisite for citB expression (9, 28). This result demonstrates that hyperaccumulation of citrate in the citB mutant is responsible for derepression of citZ and citB under anaerobic conditions and hence suggests that the anaerobic repression of the genes is mainly due to a citrate deficiency in anaerobically grown cells.
DISCUSSION
Expression of genes encoding enzymes of the TCA cycle branch of the Krebs cycle enzymes was previously shown to be subject to at least two different modes of transcriptional regulation, catabolite repression and temporal regulation in DS medium. Although the three Krebs cycle enzymes studied here are necessary to generate α-ketoglutarate, the regulation of the first two enzymes (CS and ACN) is somewhat different from that of ICDH. Both CS and ACN are partially repressed in cells grown in a glucose minimal medium, and the addition of glutamate to this medium leads to a severe reduction of the levels of these enzymes (12, 21, 22), whereas the synthesis of ICDH is not significantly altered in the presence of glucose and glutamate (22). These results suggest that CS and ACN, but not ICDH, are coordinately regulated, which may be due in part to the fact that citrate is an inducer of ACN (36) and that citB expression is severely repressed in a strain that lacks CS activity (9, 28). Citrate synthesis is not a prerequisite for citB expression driven from a catabolite repression-insensitive promoter (15), supporting the earlier hypothesis that a repressor for citB is activated by α-ketoglutarate and antagonized by citrate (36). In fact, there is an inverse correlation between ACN activity and the intracellular concentration of α-ketoglutarate (11). In α-ketoglutarate dehydrogenase (citK) mutants, in which the α-ketoglutarate concentration is high, the level of ACN is low (2, 13, 40) and citB expression (9) is repressed. Furthermore, both ACN and CS activities were shown to be derepressed in a strain carrying multiple copies of a DNA fragment with a dyad symmetry sequence in the citB regulatory region, the target sequence for catabolite repression, probably due to titration of a negative regulator(s) (16). This indicates the presence of a regulatory loop in which CS activity is required for inactivation of the repressor that functions in catabolite repression of both citZ and citB. A gel mobility shift assay showed that a protein(s) binds to the dyad symmetry sequence in the citB promoter region (15). Since citZ does not appear to have citB-like operator sites in its regulatory region (28), it is not known how citZ is regulated by the repressor.
As reported herein, the B. subtilis Krebs cycle enzyme genes are also controlled in response to anaerobic growth conditions. Again, citZ and citB are more susceptible to anaerobic repression than is citC. The anaerobic regulation of the citB and citZ genes was shown to be exerted independently of ResDE and Fnr, two control systems known to function in anaerobic gene regulation in B. subtilis. Anaerobic repression of citB can be overcome in two ways: by deletion of the cis-acting sequence in the citB promoter, the target for the catabolite repression, and by mutation of the citB gene, which results in derepression of citZ as well. The effect of the citB mutation on anaerobic citZ and citB expression was shown to be caused by accumulation of citrate, which also partially overcomes catabolite repression. Taken together, these results suggest that anaerobic regulation and catabolite repression of citB and citZ might be controlled by the same mechanism. It is not likely that the catabolite repression is mediated by catabolite control proteins CcpA and CcpB, because these regulators have no effect on the anaerobic repression of citZ and citB. A signal(s) generated by anaerobiosis or by the presence of excess glucose and glutamate probably leads to reduced citZ expression (and/or decreased CS activity) by a mechanism which remains to be elucidated. Reduced levels of citrate due to low CS activity further repress citZ as well as citB, the latter being the target of the repressor which binds to the dyad symmetry sequence of the citB promoter region. Citrate may interact directly with the putative repressor or may inactivate the repressor by chelating divalent cations essential for repressor activity. Chelation of divalent cations by accumulated citrate was also suggested to cause the early block of sporulation in the citB mutant, possibly because divalent cations are necessary for the activity of the Spo0A phosphorelay (6). Whether the repressor also controls citZ expression by interacting with the citZ regulatory region is unknown at present. A study involving the isolation of the gene encoding the repressor (29) is in progress, and the identification of this gene will likely shed light on the mechanism of the catabolite and anaerobic regulation of citB and citZ.
ACKNOWLEDGMENTS
We thank S. Jin, D. Blaydon, D. Acheson, and K. Matsuno for antibodies to Krebs cycle enzymes. We also thank B. Belitsky, J. Craig, and K. Matsuno for helpful discussions and advice. We are grateful to S. Chauvaux and M. H. Saier for a ccpA ccpB mutant and to S. H. Fisher and L. V. Wray for tms-hut cre-lacZ and bglPH-lacZ fusions. M.M.N. thanks the individuals in the Department of Molecular Biology and Microbiology at Tufts University School of Medicine for their warm hospitality and help during her sabbatical stay.
This work was supported by research grants from the National Science Foundation to M.M.N. (MCB-9722885) and from the National Institutes of Health (U.S. Public Health Service) to P.Z. (GM45898) and to A.L.S. (GM36718).
REFERENCES
- 1.Bryan E M, Beall B W, Moran C P., Jr A ςE-dependent operon subject to catabolite repression during sporulation in Bacillus subtilis. J Bacteriol. 1996;178:4778–4786. doi: 10.1128/jb.178.16.4778-4786.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carls R A, Hanson R S. Isolation and characterization of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1971;106:848–855. doi: 10.1128/jb.106.3.848-855.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chao G, Shen J, Tseng C P, Park S-J, Gunsalus R P. Aerobic regulation of isocitrate dehydrogenase gene (icd) expression in Escherichia coli by the arcA and fnr gene products. J Bacteriol. 1997;179:4299–4304. doi: 10.1128/jb.179.13.4299-4304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauvaux S, Paulsen I T, Saier M H., Jr CcpB, a novel transcription factor implicated in catabolite repression in Bacillus subtilis. J Bacteriol. 1998;180:491–497. doi: 10.1128/jb.180.3.491-497.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox D P, Hanson R S. Catabolite repression of aconitate hydratase in Bacillus subtilis. Biochim Biophys Acta. 1968;158:36–44. doi: 10.1016/0304-4165(68)90069-x. [DOI] [PubMed] [Google Scholar]
- 6.Craig J E, Ford M J, Blaydon D C, Sonenshein A L. A null mutation in the Bacillus subtilis aconitase gene causes a block in Spo0A-phosphate-dependent gene expression. J Bacteriol. 1997;179:7351–7359. doi: 10.1128/jb.179.23.7351-7359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz Ramos H, Boursier L, Moszer I, Kunst F, Danchin A, Glaser P. Anaerobic transcription activation in Bacillus subtilis: identification of distinct FNR-dependent and -independent regulatory mechanisms. EMBO J. 1995;14:5984–5994. doi: 10.1002/j.1460-2075.1995.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham L, Gruer M J, Guest J R. Transcriptional regulation of the aconitase genes (acnA and acnB) of Escherichia coli. Microbiology. 1997;143:3795–3805. doi: 10.1099/00221287-143-12-3795. [DOI] [PubMed] [Google Scholar]
- 9.Dingman D W, Rosenkrantz M S, Sonenshein A L. Relationship between aconitase gene expression and sporulation in Bacillus subtilis. J Bacteriol. 1987;169:3068–3075. doi: 10.1128/jb.169.7.3068-3075.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingman D W, Sonenshein A L. Purification of aconitase from Bacillus subtilis and correlation of its N-terminal amino acid sequence with the sequence of the citB gene. J Bacteriol. 1987;169:3062–3067. doi: 10.1128/jb.169.7.3062-3067.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher S H, Magasanik B. 2-Ketoglutarate and the regulation of aconitase and histidase formation in Bacillus subtilis. J Bacteriol. 1984;158:379–382. doi: 10.1128/jb.158.1.379-382.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flechtner V R, Hanson R S. Coarse and fine control of citrate synthase from Bacillus subtilis. Biochim Biophys Acta. 1969;184:252–262. doi: 10.1016/0304-4165(69)90027-0. [DOI] [PubMed] [Google Scholar]
- 13.Fortnagel P. The regulation of aconitase and isocitrate dehydrogenase in sporulation mutants of Bacillus subtilis. Biochim Biophys Acta. 1970;222:290–298. doi: 10.1016/0304-4165(70)90116-9. [DOI] [PubMed] [Google Scholar]
- 14.Fortnagel P, Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968;95:1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fouet A, Jin S, Raffel G, Sonenshein A L. Multiple regulatory sites in the Bacillus subtilis citB promoter region. J Bacteriol. 1990;172:5408–5415. doi: 10.1128/jb.172.9.5408-5415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouet A, Sonenshein A L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990;172:835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray C T, Wimpenny J W T, Mossman M R. Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta. 1966;117:33–41. doi: 10.1016/0304-4165(66)90149-8. [DOI] [PubMed] [Google Scholar]
- 18.Gruer M J, Bradbury A J, Guest J R. Construction and properties of aconitase mutants of Escherichia coli. Microbiology. 1997;143:1837–1846. doi: 10.1099/00221287-143-6-1837. [DOI] [PubMed] [Google Scholar]
- 19.Gruer M J, Guest J R. Two genetically-distinct and differentially-regulated aconitases (AcnA and AcnB) in Escherichia coli. Microbiology. 1994;140:2531–2541. doi: 10.1099/00221287-140-10-2531. [DOI] [PubMed] [Google Scholar]
- 20.Gunsalus R P, Park S-J. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res Microbiol. 1994;145:437–450. doi: 10.1016/0923-2508(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 21.Hanson R S, Blicharska J, Arnaud M, Szulmajster J. Observations on the regulation of the synthesis of the tricarboxylic acid cycle enzymes in Bacillus subtilis, Marburg. Biochem Biophys Res Commun. 1964;17:690–695. [Google Scholar]
- 22.Hanson R S, Cox D P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967;93:1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hederstedt L. The Krebs citric acid cycle. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C: American Society for Microbiology; 1993. pp. 181–197. [Google Scholar]
- 24.Iuchi S, Lin E C C. ArcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA. 1988;85:1888–1892. doi: 10.1073/pnas.85.6.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin S, De Jesús-Berríos M, Sonenshein A L. A Bacillus subtilis malate dehydrogenase gene. J Bacteriol. 1996;178:560–563. doi: 10.1128/jb.178.2.560-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin S, Sonenshein A L. Characterization of the major citrate synthase of Bacillus subtilis. J Bacteriol. 1996;178:3658–3660. doi: 10.1128/jb.178.12.3658-3660.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin S, Sonenshein A L. Identification of two distinct Bacillus subtilis citrate synthase genes. J Bacteriol. 1994;176:4669–4679. doi: 10.1128/jb.176.15.4669-4679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin S, Sonenshein A L. Transcriptional regulation of Bacillus subtilis citrate synthase genes. J Bacteriol. 1994;176:4680–4690. doi: 10.1128/jb.176.15.4680-4690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jourlin, C., and A. L. Sonenshein. Personal communication.
- 30.Kenney T J, Moran C P., Jr Genetic evidence for interaction of ςA with two promoters in Bacillus subtilis. J Bacteriol. 1991;173:3282–3290. doi: 10.1128/jb.173.11.3282-3290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Moran C, Jr, Lang N, LeGrice S, Lee G, Stephens M, Sonenshein A, Pero J, Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186:339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- 33.Nakano M M, Hulett F M. Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol Lett. 1997;157:1–7. doi: 10.1111/j.1574-6968.1997.tb12744.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakano M M, Marahiel M A, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano M M, Zuber P, Glaser P, Danchin A, Hulett F M. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178:3796–3802. doi: 10.1128/jb.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohné M. Regulation of aconitase synthesis in Bacillus subtilis: induction, feedback repression, and catabolite repression. J Bacteriol. 1974;117:1295–1305. doi: 10.1128/jb.117.3.1295-1305.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park S-J, McCabe J, Turna J, Gunsalus R P. Regulation of the citrate synthase (gltA) gene of Escherichia coli in response to anaerobiosis and carbon supply: role of the arcA gene product. J Bacteriol. 1994;176:5086–5092. doi: 10.1128/jb.176.16.5086-5092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose M, Entian K-G. New genes in the 170° region of the Bacillus subtilis genome encode DNA gyrase subunits, a thioredoxin, a xylanase and an amino acid transporter. Microbiology. 1996;142:3097–3101. doi: 10.1099/13500872-142-11-3097. [DOI] [PubMed] [Google Scholar]
- 39.Rosenkrantz M S, Dingman D W, Sonenshein A L. Bacillus subtilis citB gene is regulated synergistically by glucose and glutamine. J Bacteriol. 1985;164:155–164. doi: 10.1128/jb.164.1.155-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutberg B, Hoch J A. Citric acid cycle: gene-enzyme relationship in Bacillus subtilis. J Bacteriol. 1970;104:826–833. doi: 10.1128/jb.104.2.826-833.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiro S, Guest J R. Adaptive responses to oxygen limitation in Escherichia coli. Trends Biochem. 1991;16:310–314. doi: 10.1016/0968-0004(91)90125-f. [DOI] [PubMed] [Google Scholar]
- 42.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan M F, Sorokin A, Pujic P, Ehrlich S D, Hulett F M. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J Bacteriol. 1996;178:1374–1385. doi: 10.1128/jb.178.5.1374-1385.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wimpenny J W T, Cole J A. The regulation of metabolism in facultative bacteria. III. The effect of nitrate. Biochim Biophys Acta. 1967;148:233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto I, Ishimoto M. Effect of nitrate reduction on the enzyme levels in carbon metabolism in Escherichia coli. J Biochem. 1975;78:307–315. doi: 10.1093/oxfordjournals.jbchem.a130909. [DOI] [PubMed] [Google Scholar]
- 45.Zalieckas J M, Wray L V, Ferson A E, Fisher S H. Transcription-repair coupling factor is involved in carbon catabolite repression of the Bacillus subtilis hut and gnt operons. Mol Microbiol. 1998;27:1031–1038. doi: 10.1046/j.1365-2958.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- 46.Zuber P, Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987;169:2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]