Abstract
The endogenous capacity of the kidney to repair is limited, and generation of new nephrons after injury for adequate function recovery remains a need. Discovery of factors that promote the endogenous regenerative capacity of the injured kidney or generation of transplantable kidney tissue represent promising therapeutic strategies. While several encouraging results are obtained after administration of stem or progenitor cells, stem cell secretome, or extracellular vesicles in experimental kidney injury models, very little data exist in the clinical setting to make conclusions about their efficacy. In this review, we provide an overview of the cutting-edge knowledge on kidney regeneration, including pre-clinical methodologies used to elucidate regenerative pathways and describe the perspectives of regenerative medicine for kidney patients.
Keywords: Pediatric nephrology, Kidney, Regenerative medicine
Introduction
Chronic kidney disease (CKD) in children poses a high burden for patients and their families and is a global health care problem. CKD is defined as abnormal kidney function that is present for more than 3 months with implications to health [1]. The childhood incidence of CKD in Europe is estimated to be around 11–12 per million of age-related population (pmarp) for stages 3–5, and its prevalence is around 55–60 pmarp [2, 3]. Both the incidence and the prevalence of CKD are higher in males due to the high frequency of congenital abnormalities of the kidney and urinary tract (CAKUT) [2]. Incomplete recovery from acute kidney injury (AKI) can also result in CKD; however, developmental defects and hereditary diseases are the main causes of CKD from birth until the age of 4. Between 5 and 14 years, hereditary diseases, nephrotic syndrome, and systemic diseases most frequently underlie permanent kidney damage. From 15 until 19 years, mainly glomerular diseases are responsible for the onset of CKD in adolescents and young adults [3]. CKD is a progressive disease that cannot be effectively treated. It is associated with numerous comorbidities, impact on child development and decreased quality of life, especially in those with kidney failure in need of kidney replacement therapy (KRT) [4]. The median incidence of KRT in children (0–19 years) is around 9 pmarp, and the prevalence is around 65 pmarp worldwide [2]. Kidney transplantation is currently the best solution for patients with kidney failure, but it is associated with a shortage of donor organs and therefore long waiting lists, risk of rejection, and limited lifetime of the donor organ. From the clinical perspective, there is a high need for novel treatments for pediatric kidney patients.
Regenerative medicine has emerged as an important research field during the past decade focusing on disease modeling and improving, renewing, or replacing tissue function. While genetic kidney diseases are a prevalent cause of CKD in children, genetically modified mouse models do not fully replicate human physiology; therefore, stem cell–derived systems emerge as a promising tool for studying human disease mechanisms and drug testing [5]. Additionally, various strategies have already been used to boost the endogenous regenerative capacity of the kidneys, or to create replacement of organs using organoids (Table 1) and 3D bioprinting. However, the kidney is an extremely challenging organ because of its anatomic and cellular complexity. Therefore, before being able to regenerate the kidney, a detailed understanding of kidney development and kidney repair mechanisms is essential as these processes seem to be interconnected.
Table 1.
Definitions widely used in regenerative medicine
| Regeneration | Generation of new nephrons to replace/renew damaged nephrons leading to effective functionality |
| Repair | Restoration of damaged cells or nephrons leading to effective functionality |
| iPSC | Induced pluripotent stem cells are somatic cells that are reprogrammed by overexpression of 4 key transcription factors (cMyc, Sox2, Klf4, Oct4) into pluripotent stem cells |
| ASC | An adult stem cell (or tissue stem cell) remains undifferentiated and can replace cells after cell division (multipotent). Their self-renewal capacity is a key characteristic |
| MSC | A mesenchymal stromal cell is an adult stem cell that can differentiate into other cell types (multipotent). Human sources of MSCs, which are of stromal origin, include bone marrow, umbilical cord tissue, adipose tissue, and amniotic fluid |
| KSPC | Kidney stem/progenitor cells represent a unique population of stem cells derived from developing kidneys. They self-renew, express markers of nephron progenitors, and can differentiate into mature epithelial cells of the kidney. They can be derived from fetal kidneys or urine of neonates born prematurely |
| Organoids | Organoids are self-organized 3D tissue cultures of stem cells and have self-renewal and differentiation capacity. They contain multiple organ-specific cell types which spatially organize and can recapitulate organ function |
| Tubuloids | Tubuloids are ASC-derived organoids consisting of polarized proximal tubule, loop of Henle, distal tubule and collecting duct cells, representing the kidney tubular epithelium |
| Secretome | The secretome is the total set of molecules secreted by cells and consists of bioactive molecules such as cytokines/chemokines and growth factors |
| EV | Extracellular vesicles are secreted by cells with a variable cargo composition. They originate from the endosome or plasma membrane and can establish cell–cell communication |
How kidney development connects to the mechanism of regeneration
Knowing the complexity of the mature kidney, one can marvel at its humble beginning. The current knowledge of kidney development is based on decades of studies in animal models such as zebrafish, frogs, mice, and rats. In this review, we will focus on human kidney development and its relation with regeneration.
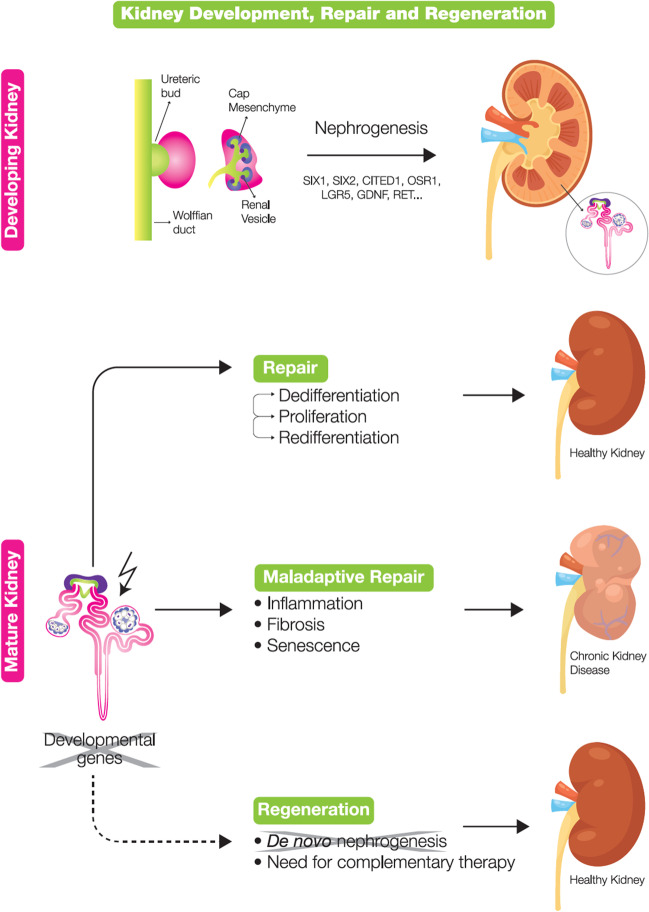
The human kidney originates from a succession of three stages: pronephros, mesonephros, and metanephros. The latter is the final kidney prototype, and it arises through cell-to-cell interactions and signaling pathways between the metanephric mesenchyme (MM) and the ureteric bud (UB), both deriving from the intermediate mesoderm [6]. The interplay of both structures is greatly influenced by the production of nephrogenic factors derived from a restricted kidney stem/progenitor cell (KSPC) population (Table 1) localized at the cap mesenchyme (CM) (Fig. 1). Expression of the transcription factor SIX2 (Sine Oculus Homeobox Homolog 2) is a determinant feature of KSPC, since SIX2 + KSPCs will give rise to all cell types of the nephron [7].
Fig. 1.
Kidney development, repair, and regeneration. Development: In kidney development, growth and branching of ureteric bud (UB) is influenced by the kidney stem/progenitor cell population localized at the cap mesenchyme (CM). With branching of UB, renal vesicles (RV) emerge by pre-tubular aggregates. Specific genes are upregulated only during nephrogenesis including SIX1, SIX2, CITED1, OSR1, LGR5, GDNF, and RET. Repair: After AKI, epithelial cells will dedifferentiate, proliferate, and redifferentiate to form new tubular cells. If the epithelial cells fail to fully re-differentiate, maladaptive repair will occur. Maladaptive repair will cause fibrosis, inflammation, and senescence which will eventually lead to CKD. Regeneration: Genes related to human development including PAX2, LHX1, and SOX9 are re-expressed in injured tubular cells during regeneration. However, specific nephrogenesis genes are not upregulated in regeneration, and therefore, complementary therapy is required
Branching of the UB tree is accompanied by the release of several factors such as Wnt9b and later Wnt4, and the concomitant downregulation of SIX2 gradually transits KSPCs into epithelial cells through a process of mesenchymal-to-epithelial transition (MET). Consequently, these epithelial cells form the pre-tubular aggregates from which the renal vesicles (RVs) emerge [6]. The RV evolves into a comma-shaped body and S-shaped body. Endothelial cells migrate into the cleft of the S-shaped body to assist in the formation of the renal corpuscle. The upper part of S-shaped bodies fuses with what has been the UB, to enable the connection to the collecting duct [8]. As a result of epithelial differentiation, the glomerular capsule, the podocytes, the descending and ascending limb, and the distal tubules are formed, all originating from the SIX2 + KSPC. Next, the kidney vasculature emerges in close contact with other structures of the nephron. When ready, the podocytes encapsulate the glomerular capillaries, and the two structures fuse to the glomerular basement membrane. The cells surrounding the nephrons (interstitial cells) originate from another pool of progenitor cells, which are FOXD1 + (Forkhead Box 1), localized at the top of the CM [9]. Stemming from the UB, larger kidney structures emerge: the collecting system and the renal pelvis. In humans, by the 36th week of gestation, the kidneys are functionally mature, and the SIX2 + KSPC population is exhausted; therefore, no new nephrons are formed [10], which limits the regenerative potential of the organ (Fig. 1). Children born premature are at increased risk of CKD, likely due to decreased nephron number and exposure to post-natal nephrotoxins. They are also at higher risk of neonatal acute kidney injury (nAKI), which may further decrease the viable nephron number and potentiate progression to CKD [11].
Kidney regeneration after birth
What do we know about the behavior of kidney cells after injury and how does this relate to potential therapeutic approaches?
Upon AKI, proximal tubular cells rapidly lose their brush border and dedifferentiate into a mesenchymal phenotype. Processes like cell migration, detachment, apoptosis, and necrosis result in denudation of the tubular basement membrane. In the glomerulus, injury can lead to podocyte loss with a complete glomerular collapse when the podocyte cell number falls below 20% of the original amount [12]. The capacity of the kidney to functionally regenerate upon AKI is a major determinant of the outcome, but no specific therapeutic approach has been shown to improve the effectiveness of regeneration so far [13]. Following AKI, a variety of intrinsic repair processes are activated rapidly, but repetitive or prolonged injury may lead to an unwanted maladaptive regenerative process. Maladaptive repair of tubular cells occurs when epithelial cells fail to fully re-differentiate or become growth arrested in G2 cell cycle phase, becoming an additional source of profibrotic factors, inflammation, and senescence leading to CKD and eventual kidney loss [14] (Fig. 1). Mechanisms of (limited) kidney regeneration have been an ongoing debate in the stem cell research field during the last decades. Whereas many studies attempted to identify cells with the ability to generate nephrons, growing evidence shows that the regeneration process arises through phenotypic and metabolic plasticity of tubular cells mediated by the microenvironment. Previously, it has been postulated that bone marrow-derived stem cells could translocate into the kidney upon damage, but that has not been substantiated, and the possibility of regeneration driven by extrarenal cells has been disregarded [15, 16].
Although it has previously been suggested that a specific population of CD133 + CD24 + , Vimentin + , and PAX2 + cells would function as stem cells in the kidney, displaying self-renewal properties and potential to differentiate into epithelial cells to repair damaged tissue [17, 18], it is now clear that after kidney injury, the repair process is accomplished by remaining reparative cells within the tubule that dedifferentiate, proliferate, and re-differentiate without any contribution from a preexisting specific progenitor cell population [19, 20]. To elucidate this process, cells from mice were genetically labeled during ischemic injury to mark individual damaged tubular cells and to follow subsequent recovery. The number of labeled cells increased significantly upon injury, indicating that tubular epithelium can arise from any surviving tubular cell and not from a fixed progenitor population [21]. The debate goes on to define the exact mechanism of repair and regeneration. Although kidney function is restored to baseline after AKI, patients frequently develop CKD, which demonstrates that re-entering the cell cycle is insufficient to fully regenerate nephrons [22]. This highlights the importance of research and development of techniques to identify target pathways and the factors that drive effective regeneration to boost this process.
Regeneration versus development
In rodent models, injured tubular cells start to re-express genes and proteins that are active during development, such as PAX2, LHX1, SOX9, followed by increased proliferation rates, release of several growth factors such as epidermal growth factor (EGF), insulin-like growth factor (IGF), and transforming growth factor-β (TGF-β) and involvement of the canonical Wnt signaling pathway [23, 24]. A recent study has demonstrated a similar mechanism in humans. Using intercellular cross-talk analysis, it has been shown that upon AKI, tubular epithelial cells activated the transcription factor SOX9, and these cells released factors such as VEGF, complement, SPP1, and CALCR, which influenced the surrounding cells to facilitate endogenous repair [25]. In the same study, the authors identified S100 calcium-binding protein 9 (S100A9) as a protein that enhances cell proliferation and might be directly related to tissue regeneration [25].
Still, upregulation of genes that are specific for nephrogenesis, such as SIX1, SIX2, CITED1, OSR1, LGR5, GDNF, and RET, has not been reported after injury or during repair (Fig. 1). Therefore, kidney regeneration does not fully recapitulate development.
Recently, it has been shown for the first time that injection of SIX2 + neonatal kidney stem/progenitor cells (nKSPC) into human deceased donor kidneys induces the de novo expression of SIX2 in proliferating proximal tubular cells. These cells were derived from the urine of neonates born prematurely; therefore, they endogenously express SIX2 [26]. nKSPCs were injected via the kidney artery into human grafts that were not used for transplantation and were perfused during 6 h in normothermic machine perfusion (NMP). Besides SIX2 expression, these kidneys showed upregulation of regenerative markers, such as SOX9 and VEGF, and had significantly lower levels of kidney injury biomarkers and reduced inflammatory cytokines [27]. The reactivation of SIX2, a nephrogenic factor, might be related to the initiation of an endogenous regenerative repair of the kidney tissue and can reflect a possibility to therapeutically re-induce nephrogenesis.
Nevertheless, the mechanisms of regeneration are not fully understood, and several technologies have been developed to study and improve the regenerative potential of the kidney tissue for therapeutic purposes.
Fundamental research pushes forward the field of regenerative medicine
Innovative technologies including genomics, transcriptomics, proteomics, metabolomics — conjunctively referred to as “omics”— have generated large data set collections and analyses to improve our understanding of basic principles and mechanisms of kidney development, repair, and regeneration. Based on knowledge acquired from omics studies, researchers were able to develop the protocols to induce pluripotent stem cells (iPSCs) to form nephrons in vitro, the so-called kidney organoids. Single cell RNA sequencing (scRNA-seq) data was used to optimize the kidney cell differentiation and to reduce the rate of non-kidney cell types [28]. Kidney organoids are nowadays one of the most important in vitro models to elucidate kidney development.
Transcriptomic characterization of repair and regeneration
Transcriptomic studies are based on RNA sequencing (RNA-seq) technology and indicate transcriptional activity of genes. RNA-seq technology has been used for analyzing kidney biopsies and supports diagnosis and prognosis in some of the kidney diseases. Bulk RNA-seq, scRNA-seq, and single-nuclei RNA-seq (snRNA-seq) are three commonly used RNA-seq techniques. Bulk RNA-seq provides an overview of the average of gene expression. ScRNA-seq and snRNA-seq can provide information about each cell and show the potential to find molecular differences which are only linked to specific cell types. ScRNA-seq can measure both cytoplasmic and nuclear transcripts whereas snRNA-seq can only measure nuclear transcripts. Not many RNA-seq studies have been performed to characterize repair and regeneration in AKI or CKD; however, some studies report molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion injury (IRI). An scRNA-seq study defined key differences in adaptive and fibrotic repair, suggesting potential druggable pathways. The authors found that specific maladaptive/profibrotic proximal tubules (PT) expressed proinflammatory, profibrotic cytokines, and myeloid cell chemotactic factors (e.g. CXCL2, IL1b, CCL3) after long IRI. Additionally, maladaptive PT cells showed a marked enrichment of ferroptosis and pyroptosis. Pharmacological targeting of pyroptosis/ferroptosis (VX-765, pyroptosis inhibitor and liproxstatin, ferroptosis inhibitor) in vivo induced cells towards adaptive repair and improvement of fibrosis [29]. This supports the potential of RNA-seq for the identification of regenerative therapeutic targets [30]. SnRNA-seq analysis of biopsies of 8 individuals with severe AKI revealed common epithelial cell response patterns including oxidative stress, hypoxia, interferon response, and epithelial-to-mesenchymal transition [31]. Similarly, scRNA-seq of urine samples demonstrated that urinary cells in adaptive states are potentially derived from the thick ascending limb and show regenerative signatures by expressing PAX2, SOX4, and SOX9, which were predominantly expressed in the presumed progenitor clusters [32]. These outcomes underline the possibility of applying RNA-seq technology in humans, but still leave a gap between research and clinical application.
Metabolomic characterization of repair and regeneration
Besides transcriptomics, metabolomic studies may reveal the molecular signatures of cells and tissues upon injury and repair. A very promising advance in metabolic pathways has been made by using isotope tracing in dynamic metabolic processes [33] based on matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI). This technology might provide a truly comprehensive understanding of the interplay between biochemical alterations and cell type-specific functions, metabolic fluxes, and dynamic interpretations of cellular states [34]. Using this technology in a mouse model of AKI, authors concluded that the maladaptive repair in tubular cells is characterized by differences in production of lactate, which could possibly be the result of higher glycolytic activity and, as injury progresses, a concomitant reduction of the tricarboxylic acid (TCA) cycle metabolite consumption [34]. Over the past decades, metabolomics has added a promising number of new biomarkers through better pathophysiology knowledge, paving the way for insightful perspectives on the management of different kidney diseases [35]. However, the metabolome greatly varies with age, diet, drug consumption, lifestyle, and, in adults, with gender, making it difficult to compare studies in adults and in children and neonates. More metabolomic studies focused on pediatric patients are required to determine the practical clinical impact of metabolomics in conditions of kidney damage and repair.
Kidney organoids and their potential for clinical implementation
In the field of regenerative medicine, organoid cultures are widely used to model disease, study physiology, and develop clinical applications, like drug screening and personalized medicine approaches, as well as their use for regenerative therapies. Organoids are self-organized 3-dimensional (3D) tissue cultures of stem cells, which have self-renewal and differentiation capacity. They contain multiple organ-specific cell types which spatially organize and can recapitulate organ function (Table 1). Here, we will describe two types of kidney organoid models which have been developed in the past 5 years: iPSC-derived kidney organoids and adult stem cell (ASC)-derived tubuloids (Table 1).
iPSC-derived organoids
iPSC-derived organoids have emerged as advanced in vitro models of kidney development, physiology, and disease. iPSC-derived organoids reflect mainly kidney developmental aspects by mimicking nephrogenesis. When iPSCs are differentiated into kidney organoids, most of the nephron segments are present: both proximal and distal tubule cells, glomerular structures, and loop of Henle as well as collecting duct cells can be found [36]. Recently, novel hybrid protocols have been designed to culture podocyte-like cells in kidney iPSC-derived organoids [37]. Patient mutations can be created by genome-editing technologies like CRISPR/Cas9 in order to model diseases, or patient-derived cells can be reprogrammed to have pluripotent features [38] (Table 2). To date, iPSC-based disease modeling has successfully been used for studying genetic kidney diseases such as cystinosis [39], nephronophthisis (NPH), and nephrotic syndrome [27, 40, 41], as well as for drug development (Table 2). Nevertheless, there are several limitations of using kidney iPSC-derived organoid models. The organoids most closely resemble human embryonic kidney tissue and not a mature organ [36]. In addition, a vascular system is missing [36]. So far, both endothelial cells (CD31 +) and interstitium could be induced in kidney organoid culture [42]; however, this still needs to be further developed into more mature and functional vascularization. Recently, iPSC-derived vascular organoids showed to be a new cell source of functional and flow-adaptive vascular cells for the creation of a perfused macrovessel model [43], which might inspire future vascularized kidney organoid culture. This new model recapitulates the bi-layer vessel architecture and allows in vitro studies of vascular disease [43]. Unfortunately, after directed differentiation protocols toward kidney organoids, still 10–20% off-target non-renal cells appear in culture, including mainly neuronal-like and muscle-like cell populations. Protocols on how to improve iPSC-derived organoid cultures are under development [44]. For example, iPSC organoid-derived tubuloid cultures showed disappearance of immature and off-target cell populations, accessibility of the apical site, and prolonged expansion capacity [45]. Notably, iPSC-derived organoids generated by knocking out a single gene of interest miss potential genetic or epigenetic modifiers that can be present in individual patients. On the other hand, this provides a model to identify potential modifiers when comparing patient-derived organoids with gene-edited iPSC-derived organoids.
Table 2.
iPSC-derived organoid or tubuloid models in pediatric genetic kidney diseases
| Disease | Gene | Approaches | Applications | Reference |
|---|---|---|---|---|
| ARPKD | PKHD1 | Patient iPSC-derived kidney organoids | Drug validation in a patient-specific manner | [78] |
| ARPKD | PKHD1 | iPSC-derived organoid-on-a-chip model subjected to flow | Therapeutic target discovery using transcriptomics | [79] |
| ARPKD | PKHD1 | iPSCs harboring loss-of-function mutations that recapitulate cystic phenotype | Disease modeling | [80] |
| NPH-RC | IFT140 | Patient-derived iPSC kidney organoids, gene corrected | Disease modeling | [81] |
| Cystinosis | CTNS | Patient urine-derived tubuloid model | Personalized drug screening | [47] |
| Cystinosis | CTNS | iPSC-derived organoids | Drug testing | [39] |
| CAKUT | WT1, HNF1β, GLI3, COL4A3 | Urine-derived patient-specific iPSCs and kidney organoids | Disease modeling | [82] |
| Nephrotic syndrome | NOS1AP | Human kidney organoids with knock-in of patient specific variant | Disease modeling | [83] |
| Nephrotic syndrome | NPHS1 | Organoid-derived glomeruli and podocytes | Disease modeling | [84] |
| Nephrotic syndrome | NPHS1 | Patient-derived iPSC organoids | Disease modeling | [40, 41] |
ARPKD autosomal recessive polycystic kidney disease, NPH-RC nephronophthisis-related ciliopathy; CAKUT congenital anomalies of the kidney and urinary tract
Tubuloids
Kidney organoids can also be generated from ASCs obtained either from kidney biopsy material or from urine, and thus carry the exact genetic and epigenetic information of the patient. These 3D structures are called tubuloids (Table 1) as they mainly represent the tubular epithelium and lack differentiation into glomerular cells. In tubuloid cultures, podocytes and stroma are absent, and like in iPSC-derived organoids, the vasculature is also absent in tubuloids. Tubuloids can be long-term expanded, without the need of genetic modification and without the risk of off-target differentiation [46]. To date, genome-editing protocols in tubuloids have not yet been published. Recently, tubuloids derived from urine of cystinosis patients were used to develop novel treatment strategies [47] (Table 2), indicating their power as a disease model for translational applications. In this study, an omics-inspired drug screen revealed a novel combination therapy, which has been tested in patient-derived tubuloids. Age- and gender-matched healthy donor-derived tubuloid cultures are used as controls. Biobanks of healthy donor and patient material-derived tubuloids will facilitate the development of personalized medicine and will create a short line from bench to bedside. Kidney organoids can be cultured non-invasively from urine from (pediatric) kidney disease patients, as being long-term expandable and genetically stable cultures. For pediatric nephrology, a urine cell-derived tubuloid biobank [48] will be of interest to study hereditary kidney disease. For children and adolescents, non-invasive ways of collecting primary patients’ cells are preferred. As an advancement of primary urine cell cultures in 2D [49], the 3D tubuloid cultures will provide an improved cell culture model for fundamental and translational research, including drug development (Fig. 2). For example, drug screening on tumor organoids derived from childhood malignancies showed successful identification of potential therapeutic agents targeting pediatric tumors [50]. Kidney organoid cells can also be cultured in flow chambers [51]. The introduction of flow in perfused cell systems of tubuloid cells on a chip reflects another way to create a more advanced in vitro kidney model for research applications [51]. ASC-derived organoids were first developed to model murine intestine [52]. To date, one key application of the intestinal organoids is the development of forskolin swelling assays allowing drug response monitoring in cystic fibrosis patients. In vitro swell responses can be monitored and correlate with the individual’s clinical response to therapy [53]. This is an important showcase stressing the value of organoids for clinical applications.
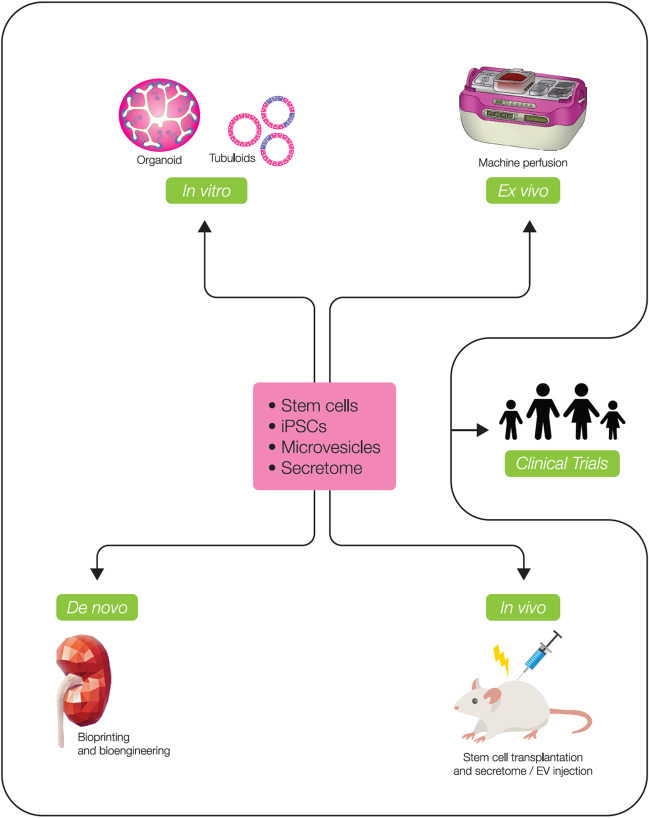
Fig. 2.
State-of-the-art tools applied in regenerative medicine approaches. In vitro: Kidney organoid cultures generated from iPSC can be used for modeling development, and together with tubuloids are valuable tools to model diseases and to test drugs. Additionally, iPSC-derived kidney organoids have potential to replace damaged kidney tissue. Ex vivo: Machine perfusion can serve as a suitable platform to locally boost the regenerative potential of donor kidneys having inferior quality prior to transplantation by means of cell therapy or drug injection. De novo: Bioprinting platforms can facilitate high throughput culture of highly consistent and reproducible organoid cultures to build kidney tissue. In vivo: Therapy including stem cell transplantations, cell secretome injection, and extracellular vesicles (EVs) injection can be used clinically to induce repair and regeneration, as already shown in pre-clinical models. All these methodologies are fundamental to elucidate the processes of kidney repair and regeneration prior to clinical trials and clinical applications
Application of organoids in future transplantable kidney tissue
Kidney organoid cultures are also further investigated in the direction of replacing damaged kidney tissue (Fig. 2). Several attempts have been made to create functional kidney structures by transplanting iPSC-derived organoids in mice. Organoid transplantation under the kidney capsule or subcutaneously resulted in functional glomerular perfusion, connection to vascular networks or vascularization, and improved morphogenesis [54, 55]. In addition, iPSC-derived nephron sheets which contain many nephrons could be transplanted in immunodeficient mice, and this scalable protocol was demonstrated to produce kidney tissue with glomerular filtration function [56]. A critical step in future experiments is to establish a robust connection between the transplanted organoids and the host’s vasculature, and to overcome graft overgrowth by stromal cells in the long term [57]. To further optimize kidney organoid engraftment, material-driven applications refine organoid culture by implementing improved hydrogel engineering [58]. Soft hydrogels demonstrate better performance of kidney organoids in comparison to stiff hydrogels [58, 59]. 3D bioprinting of kidney matrix could facilitate high throughput culture of highly consistent and reproducible organoid cultures in transplantation-compatible hydrogels in the future. Despite gaining novel fundamental knowledge, no clinical applications in pediatric nephrology of kidney organoids or tubuloids have been reported so far. Nevertheless, over the long term, kidney organoids might have the potential to advance KRT and, as an outlook, have a significant contribution to the bioengineering of kidney grafts and of bioartificial kidney tissue. Implantable bioartificial kidneys, which are attached to the systemic circulation, are not yet clinically tested, as both technical and biological challenges must be overcome. The development of a transplantable auxiliary kidney would hold great promise for people with kidney disease by (partly) implementing kidney function. In comparison to wearable and portable dialysis machines, which are cell-free, a bioartificial kidney would overcome dialysis shortcomings characterized by poor clinical outcomes and low quality of life. Functional requirements, which need to be addressed, include membrane characteristics, cell characteristics, and functional aspects like toxin excretion and nutrient and water reabsorption capacity. Finding cellular components that are capable of taking over tubular functions in a bioartificial kidney and which are biocompatible at the same time will substantially advance its development [60].
The current clinical applications of regenerative medicine: the status of stem cell transplantation and extracellular vesicle injection
In preclinical models of kidney injury, repair and regeneration have been observed upon different types of (stem) cell transplantations, cell secretome injection, and extracellular vesicle (EV) injection (Fig. 2). However, translation of these methodologies to the clinics still faces several challenges.
Cell therapy-based clinical trials
Mesenchymal stromal cells (MSC) (Table 1) have immunomodulatory properties and might play a role in tissue repair, therefore being the most widely used type of cells for cell therapy of damaged kidneys. A few clinical trials have been performed showing feasibility and safety of MSC infusion in patients [61–64]. In an 18-month follow-up study, seven patients with CKD of different etiologies such as hypertension, nephrotic syndrome, and unknown etiology were infused with 1–2 million autologous bone marrow MSC/kg for safety and tolerability evaluation. Although none of the patients had adverse events related to the therapy, kidney function (GFR and serum creatinine measurements) did not improve [62]. A similar trial of bone barrow autologous MSC transplantation in 6 ADPKD patients confirmed feasibility of the therapy, but in these patients, serum creatinine levels were significantly improved [63].
In kidney transplantation, MSC therapy shows promising results with potential to induce immunotolerance. In a phase 1 trial, four living-donor kidney transplant patients were given autologous bone marrow MSC 1 day before or 7 days after kidney transplantation while receiving induction therapy. All patients had stable graft function in 5–7-year follow-up, but the protolerogenic effect of MSC was variable. One of these patients was successfully weaned off immunosuppressive drugs and stayed free from anti-rejection therapy with optimal long-term kidney allograft function [65]. In a larger study, patients received MSC infusion 6 and 7 weeks after kidney transplantation in a randomized prospective, single-center, open-label trial. Twenty-nine patients received MSC and had early tacrolimus withdrawal, while 28 patients were in the control tacrolimus group. Early tacrolimus withdrawal with MSC therapy was feasible and safe, and there was no increased rate of rejection. Kidney function was preserved in both groups, but the MSC-treated patients were not prevented from developing progressive fibrosis [64].
These studies suggest that MSC therapy in humans is feasible and safe on a short-term basis, and that the immunomodulatory effect is promising. Nevertheless, MSCs of different tissues are devoid of differentiation capacity into mature kidney epithelial cells, and trials have not shown effective tissue repair or regeneration leading to improved kidney function. Therefore, it remains crucial to find a superior source of (stem) cells to develop effective kidney tissue regeneration, and kidney-derived cells might be the ideal candidate. Autologous selected renal cells (SRC), a pool of proximal tubule and glomerular cells and other cell subpopulations, such as interstitial cells, were used in a trial of 22 patients with advanced type 2 diabetes–related CKD (D-CKD). In this study, the cell therapy seemed to improve kidney function and possibly halted type 2 D-CKD progression [66]. In another safety study, 18 patients with CKD of unknown cause (stages 3–5) were followed for 36 months after receiving a single infusion of angiogenic/anti-fibrotic autologous adipose-derived stromal vascular fraction (SVF) cells into their kidneys bilaterally via renal artery catheterization. Both kidney structure and function were shown to be improved [67].
Ex vivo cell therapy
With the increasing need for kidney transplantation in the adult and pediatric populations, the criteria for organ recruitment have been extended as an attempt to reduce the waiting time by increasing the donor organ pool. This implies that kidneys of older donors (> or = 60 years) or donors who are aged 50 to 59 years and have two of the following three features: hypertension, serum creatinine > 1.5 mg/dl, or death from cerebrovascular accident [68] can be offered to patients. However, these kidneys are of suboptimal quality, rendering grafts more susceptible to ischemia reperfusion injury (IRI) in comparison with organs derived from living healthy donors [69]. These are the targeted organs for receiving ex vivo cell therapy in order to reduce IRI, reduce the immunogenicity of the kidney graft, and promote regeneration of already injured organs.
Ex vivo cell therapy can be performed during machine perfusion (MP), the current method of choice for preserving kidney allografts obtained from deceased donors. The first study of administration of human MSC into human kidneys during MP suggested tissue regeneration by increased cellular proliferative rates and ATP production [70]. Using preterm neonatal KSPC, we have shown that cell therapy of human deceased donor kidneys during normothermic MP (NMP) induced de novo expression of SIX2 in the donor tubular cells while it also upregulated regenerative genes such as VEGF and SOX9 [26, 27]. This suggested that kidney stem cells or developmental factors released by these cells are necessary to induce endogenous regeneration in kidneys. Still, long-term perfusions will be fundamental to prove effective regeneration of the kidney tissue leading to improved graft function.
Secretome and EVs
The described beneficial effects of cell therapy have been mainly attributed to paracrine mechanisms [5]. In preclinical studies, the secretome (Table 1) of different kinds of MSCs has shown regulatory function in cell proliferation, cell migration, cell differentiation, and modulation of the immune system. The secretome consists of bioactive molecules such as cytokines/chemokines and growth factors including granulocyte-colony stimulating factor, leukemia-inhibitory factor, macrophage-colony stimulating factor, PGE2, IL-10, TGFβ, IDO, HO-1, HGF, VEGF, FGF, and IGF-1, which can also modulate kidney regenerative responses [70, 71]. MSC-derived secretome was shown to drive kidney regeneration by inducing surviving kidney cells to dedifferentiate and replicate to restore the lost kidney cells in animal models [72, 73].
Being part of the secretome, EVs (Table 1) play an important role in inducing kidney regeneration. EVs are a type of nanoscale vesicles encapsulated by cytomembranes, which have an important function in intercellular communication, and are widely present in the body fluids, including blood, urine, and amniotic fluid. Preclinical research has proven that EVs can improve AKI by inhibiting inflammation, apoptosis, and oxidation and by regulating angiogenesis [73–75].
In human donor kidneys, MSC-EVs were infused during hypothermic oxygenated perfusion (HOPE). HOPE + EV kidneys had lower ischemic damage score and better kidney ultrastructure. They had higher HGF and VEGF levels with lower apoptosis rate than control kidneys. Moreover, HOPE + EV kidneys had lower lactate release and higher glucose levels than controls, suggesting that the gluconeogenesis system was preserved [76].
Only one clinical trial has been performed using EVs in CKD patients. In this study, cell-free cord-blood MSC-derived EVs were administered to 20 CKD patients stage III and IV (eGFR 15–60 mg/ml). The 20 patients in the treatment group exhibited improvement of eGFR, serum creatinine level, and blood urea and urinary albumin creatinine ratio compared with 20 patients in the placebo group at the end of the study period of 1 year [77]. Although the biopsies of some of the treatment group patients did not show significant histologic changes, the expression of Ki67 (a marker of proliferation) in some tubular cells confirmed the ability of MSC-EVs to activate tubular cells [77]. Longer observation and larger number of patients are required to demonstrate the efficacy and safety over the long run of EV injection as a treatment inducing kidney regeneration.
Future perspectives
Regenerative medicine is a rapidly evolving field. Here, we describe the current state of knowledge and understanding of kidney development, repair, and regeneration. Furthermore, we provide an overview of methodologies to understand and potentially enhance kidney regeneration. Unfortunately, clinical readiness of applications based on preclinical findings using omics, stem cells, or bioartificial transplants has not yet been achieved. However, the field is rapidly moving forward, and detailed knowledge about kidney development, repair, and regeneration paves the way for novel translational solutions for kidney patients (Fig. 2). Nephron-like structures are being cultured in 3D and hold promise for advanced modeling of kidney (patho)physiology, drug screening, and personalized medicine, as well as for regenerative therapies as part of a bioartificial kidney or transplantable kidney tissue. Available omics technologies are propelling our understanding of the mechanisms of kidney injury and repair, which opens opportunities for finding new druggable targets or interventional gene/cellular therapies to finally improve the outcome of kidney disease.
Currently, there are no FDA-approved stem cell-based therapies for CKD. Nevertheless, clinical trials to test efficacy and safety of stem cell-based therapies for kidney disease are being conducted. In the future, it will be important to also conduct clinical trials specifically designed for children to improve outcomes and advance knowledge. There is no doubt that clinical trials including children aiming to ameliorate CKD or find a cure for kidney failure are facing many insecurities. In addition, ethical aspects of these novel regenerative medicine therapies have to be carefully considered. Regarding the long-term goals to create donor organs and to develop personalized regenerative medicine approaches, there is a long way to go. Nevertheless, every day, we get closer to regenerative solutions for kidney patients.
Key summary points
During kidney development, functional nephrons are formed; however, this process cannot be repeated during repair and regeneration in a mature kidney.
Advanced basic science approaches and advanced organoid cell culture models enable understanding mechanisms of kidney tissue repair and regeneration.
Cell(-based) therapy is currently being tested for regenerative medicine applications. However, these cell therapies are not yet available in the clinic for kidney failure patients.
Generation of a bioartificial kidney is on the horizon; however, more knowledge about kidney development, repair, and regeneration is required for future progress.
Funding
This work was funded by the Gravitation Program “Materials Driven Regeneration,” funded by the Netherlands Organization for Scientific Research (024.003.013) (to M.C.V.). The authors acknowledge the support of the partners of “Regenerative Medicine Crossing Borders” (RegMed XB). E. L. is supported by the ERC Consolidator grant 101045467 – NEOGRAFT.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M (2013) Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158:825–830 [DOI] [PubMed]
- 2.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59:e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Greenbaum LA, Warady BA, Furth SL. Current advances in chronic kidney disease in children: growth, cardiovascular, and neurocognitive risk factors. Semin Nephrol. 2009;29:425–434. doi: 10.1016/j.semnephrol.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rota C, Morigi M, Imberti B. Stem cell therapies in kidney diseases: progress and challenges. Int J Mol Sci. 2019;20:2790. doi: 10.3390/ijms20112790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moritz KM, Wintour EM, Black MJ, Bertram JF, Caruana G. Factors influencing mammalian kidney development: implications for health in adult life. Adv Anat Embryol Cell Biol. 2008;196:1–78. doi: 10.1007/978-3-540-77768-7. [DOI] [PubMed] [Google Scholar]
- 9.Fetting JL, Guay JA, Karolak MJ, Iozzo RV, Adams DC, Maridas DE, Brown AC, Oxburgh L. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little MH, Kairath P. Does renal repair recapitulate kidney development? J Am Soc Nephrol. 2017;28:34–46. doi: 10.1681/ASN.2016070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr MC, Hingorani SR. Prematurity and future kidney health: the growing risk of chronic kidney disease. Curr Opin Pediatr. 2018;30:228–235. doi: 10.1097/MOP.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondue T, Arcolino FO, Veys KRP, Adebayo OC, Levtchenko E, van den Heuvel LP, Elmonem MA. Urine-derived epithelial cells as models for genetic kidney diseases. Cells. 2021;10:1413. doi: 10.3390/cells10061413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S. Cellular and molecular pathways of renal repair after acute kidney injury. Kidney Int. 2018;93:27–40. doi: 10.1016/j.kint.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Lin F, Moran A, Igarashi P. Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest. 2005;115:1756–1764. doi: 10.1172/JCI23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 19.Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD. Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci U S A. 2014;111:1527–1532. doi: 10.1073/pnas.1310653110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeets B, Boor P, Dijkman H, Sharma SV, Jirak P, Mooren F, Berger K, Bornemann J, Gelman IH, Floege J, van der Vlag J, Wetzels JF, Moeller MJ. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol. 2013;229:645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger K, Bangen JM, Hammerich L, Liedtke C, Floege J, Smeets B, Moeller MJ. Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci U S A. 2014;111:1533–1538. doi: 10.1073/pnas.1316177111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Chiara L, Conte C, Antonelli G, Lazzeri E. Tubular cell cycle response upon AKI: revising old and new paradigms to identify novel targets for CKD prevention. Int J Mol Sci. 2021;22:11093. doi: 10.3390/ijms222011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Liu J, Pang P, Krautzberger AM, Reginensi A, Akiyama H, Schedl A, Humphreys BD, McMahon AP. Sox9 activation highlights a cellular pathway of renal repair in the acutely injured mammalian kidney. Cell Rep. 2015;12:1325–1338. doi: 10.1016/j.celrep.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290:R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 25.Nie H, Zhao Z, Zhou D, Li D, Wang Y, Ma Y, Liu X, Zuo W (2023) Activated SOX9+ renal epithelial cells promote kidney repair through secreting factors. Cell Prolif 56(4):e13394 [DOI] [PMC free article] [PubMed]
- 26.Arcolino FO, Zia S, Held K, Papadimitriou E, Theunis K, Bussolati B, Raaijmakers A, Allegaert K, Voet T, Deprest J, Vriens J, Toelen J, van den Heuvel L, Levtchenko E. Urine of preterm neonates as a novel source of kidney progenitor cells. J Am Soc Nephrol. 2016;27:2762–2770. doi: 10.1681/ASN.2015060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arcolino FO, Hosgood S, Akalay S, Jordan N, Herman J, Elliott T, Veys K, Vermeire K, Sprangers B, Nicholson M, van den Heuvel L, Levtchenko E. De novo SIX2 activation in human kidneys treated with neonatal kidney stem/progenitor cells. Am J Transplant. 2022;22:2791–2803. doi: 10.1111/ajt.17164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman BS. Better being single? Omics improves kidney organoids. Nephron. 2019;141:128–132. doi: 10.1159/000496009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balzer MS, Doke T, Yang YW, Aldridge DL, Hu H, Mai H, Mukhi D, Ma Z, Shrestha R, Palmer MB, Hunter CA, Susztak K. Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat Commun. 2022;13:4018. doi: 10.1038/s41467-022-31772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Kumar S, Dolzhenko E, Alvarado GF, Guo J, Lu C, Chen Y, Li M, Dessing MC, Parvez RK, Cippa PE, Krautzberger AM, Saribekyan G, Smith AD, McMahon AP. Molecular characterization of the transition from acute to chronic kidney injury following ischemia/reperfusion. JCI Insight. 2017;2:e94716. doi: 10.1172/jci.insight.94716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinze C, Kocks C, Leiz J, Karaiskos N, Boltengagen A, Cao S, Skopnik CM, Klocke J, Hardenberg JH, Stockmann H, Gotthardt I, Obermayer B, Haghverdi L, Wyler E, Landthaler M, Bachmann S, Hocke AC, Corman V, Busch J, Schneider W, Himmerkus N, Bleich M, Eckardt KU, Enghard P, Rajewsky N, Schmidt-Ott KM. Single-cell transcriptomics reveals common epithelial response patterns in human acute kidney injury. Genome Med. 2022;14:103. doi: 10.1186/s13073-022-01108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klocke J, Kim SJ, Skopnik CM, Hinze C, Boltengagen A, Metzke D, Grothgar E, Prskalo L, Wagner L, Freund P, Gorlich N, Muench F, Schmidt-Ott KM, Mashreghi MF, Kocks C, Eckardt KU, Rajewsky N, Enghard P. Urinary single-cell sequencing captures kidney injury and repair processes in human acute kidney injury. Kidney Int. 2022;102:1359–1370. doi: 10.1016/j.kint.2022.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Jang C, Chen L, Rabinowitz JD. Metabolomics and isotope tracing. Cell. 2018;173:822–837. doi: 10.1016/j.cell.2018.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Heijs B, Kostidis S, Mahfouz A, Rietjens RGJ, Bijkerk R, Koudijs A, van der Pluijm LAK, van den Berg CW, Dumas SJ, Carmeliet P, Giera M, van den Berg BM, Rabelink TJ. Analyzing cell-type-specific dynamics of metabolism in kidney repair. Nat Metab. 2022;4:1109–1118. doi: 10.1038/s42255-022-00615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riccio S, Valentino MS, Passaro AP, Izzo M, Guarino S, Miraglia Del Giudice E, Marzuillo P, Di Sessa A. New insights from metabolomics in pediatric renal diseases. Children (Basel) 2022;9:118. doi: 10.3390/children9010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takasato M, Er PX, Chiu HS, Little MH. Generation of kidney organoids from human pluripotent stem cells. Nat Protoc. 2016;11:1681–1692. doi: 10.1038/nprot.2016.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jansen J, van den Berge BT, van den Broek M, Maas RJ, Daviran D, Willemsen B, Roverts R, van der Kruit M, Kuppe C, Reimer KC, Di Giovanni G, Mooren F, Nlandu Q, Mudde H, Wetzels R, den Braanker D, Parr N, Nagai JS, Drenic V, Costa IG, Steenbergen E, Nijenhuis T, Dijkman H, Endlich N, van de Kar N, Schneider RK, Wetzels JFM, Akiva A, van der Vlag J, Kramann R, Schreuder MF, Smeets B. Human pluripotent stem cell-derived kidney organoids for personalized congenital and idiopathic nephrotic syndrome modeling. Development. 2022;149:200198. doi: 10.1242/dev.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollywood JA, Przepiorski A, D'Souza RF, Sreebhavan S, Wolvetang EJ, Harrison PT, Davidson AJ, Holm TM. Use of human induced pluripotent stem cells and kidney organoids to develop a cysteamine/mTOR inhibition combination therapy for cystinosis. J Am Soc Nephrol. 2020;31:962–982. doi: 10.1681/ASN.2019070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, Era T, Nakazato H, Nakanishi K, Sakuma T, Yamamoto T, Kurihara H, Taguchi A, Nishinakamura R. Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Reports. 2018;11:727–740. doi: 10.1016/j.stemcr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohmori T, De S, Tanigawa S, Miike K, Islam M, Soga M, Era T, Shiona S, Nakanishi K, Nakazato H, Nishinakamura R. Impaired NEPHRIN localization in kidney organoids derived from nephrotic patient iPS cells. Sci Rep. 2021;11:3982. doi: 10.1038/s41598-021-83501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2016;536:238. doi: 10.1038/nature17982. [DOI] [PubMed] [Google Scholar]
- 43.Meijer EM, Koch SE, van Dijk CGM, Maas RGC, Chrifi I, Szymczyk W, Besseling PJ, Pomp L, Koomen V, Buikema JW, Bouten CVC, Verhaar MC, Smits A, Cheng C. 3D human iPSC blood vessel organoids as a source of flow-adaptive vascular cells for creating a human-relevant 3D-scaffold based macrovessel model. Adv Biol (Weinh) 2022;7:e2200137. doi: 10.1002/adbi.202200137. [DOI] [PubMed] [Google Scholar]
- 44.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomicS. Cell Stem Cell. 2018;23:869–881.e868. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousef Yengej FA, Jansen J, Ammerlaan CME, Dilmen E, Pou Casellas C, Masereeuw R, Hoenderop JG, Smeets B, Rookmaaker MB, Verhaar MC, Clevers H. Tubuloid culture enables long-term expansion of functional human kidney tubule epithelium from iPSC-derived organoids. Proc Natl Acad Sci U S A. 2023;120:e2216836120. doi: 10.1073/pnas.2216836120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, Gijzen L, Vormann M, Vonk A, Viveen M, Yengej FY, Derakhshan S, de Winter-de Groot KM, Artegiani B, van Boxtel R, Cuppen E, Hendrickx APA, van den Heuvel-Eibrink MM, Heitzer E, Lanz H, Beekman J, Murk JL, Masereeuw R, Holstege F, Drost J, Verhaar MC, Clevers H. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol. 2019;37:303–313. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 47.Jamalpoor A, van Gelder CA, Yousef Yengej FA, Zaal EA, Berlingerio SP, Veys KR, Pou Casellas C, Voskuil K, Essa K, Ammerlaan CM, Rega LR, van der Welle RE, Lilien MR, Rookmaaker MB, Clevers H, Klumperman J, Levtchenko E, Berkers CR, Verhaar MC, Altelaar M, Masereeuw R, Janssen MJ. Cysteamine-bicalutamide combination therapy corrects proximal tubule phenotype in cystinosis. EMBO Mol Med. 2021;13:e13067. doi: 10.15252/emmm.202013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schutgens F, Rookmaaker M, Verhaar M. A perspective on a urine-derived kidney tubuloid biobank from patients with hereditary tubulopathies. Tissue Eng Part C Methods. 2021;27:177–182. doi: 10.1089/ten.TEC.2020.0366. [DOI] [PubMed] [Google Scholar]
- 49.Ajzenberg H, Slaats GG, Stokman MF, Arts HH, Logister I, Kroes HY, Renkema KY, van Haelst MM, Terhal PA, van Rooij IA, Keijzer-Veen MG, Knoers NV, Lilien MR, Jewett MA, Giles RH. Non-invasive sources of cells with primary cilia from pediatric and adult patients. Cilia. 2015;4:8. doi: 10.1186/s13630-015-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calandrini C, van Hooff SR, Paassen I, Ayyildiz D, Derakhshan S, Dolman MEM, Langenberg KPS, van de Ven M, de Heus C, Liv N, Kool M, de Krijger RR, Tytgat GAM, van den Heuvel-Eibrink MM, Molenaar JJ, Drost J. Organoid-based drug screening reveals neddylation as therapeutic target for malignant rhabdoid tumors. Cell Rep. 2021;36:109568. doi: 10.1016/j.celrep.2021.109568. [DOI] [PubMed] [Google Scholar]
- 51.Gijzen L, Yousef Yengej FA, Schutgens F, Vormann MK, Ammerlaan CME, Nicolas A, Kurek D, Vulto P, Rookmaaker MB, Lanz HL, Verhaar MC, Clevers H. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat Protoc. 2021;16:2023–2050. doi: 10.1038/s41596-020-00479-w. [DOI] [PubMed] [Google Scholar]
- 52.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 53.Boj SF, Vonk AM, Statia M, Su J, Vries RR, Beekman JM, Clevers H. Forskolin-induced swelling in intestinal organoids: an in vitro assay for assessing drug response in cystic fibrosis patients. J Vis Exp. 2017;120:55159. doi: 10.3791/55159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports. 2018;10:751–765. doi: 10.1016/j.stemcr.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bantounas I, Silajdzic E, Woolf AS, Kimber SJ. Formation of mature nephrons by implantation of human pluripotent stem cell-derived progenitors into mice. Methods Mol Biol. 2020;2067:309–322. doi: 10.1007/978-1-4939-9841-8_19. [DOI] [PubMed] [Google Scholar]
- 56.Wiersma LE, Avramut MC, Lievers E, Rabelink TJ, van den Berg CW. Large-scale engineering of hiPSC-derived nephron sheets and cryopreservation of their progenitors. Stem Cell Res Ther. 2022;13:208. doi: 10.1186/s13287-022-02881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar Gupta A, Sarkar P, Wertheim JA, Pan X, Carroll TJ, Oxburgh L. Asynchronous mixing of kidney progenitor cells potentiates nephrogenesis in organoids. Commun Biol. 2020;3:231. doi: 10.1038/s42003-020-0948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruiter FAA, Morgan FLC, Roumans N, Schumacher A, Slaats GG, Moroni L, LaPointe VLS, Baker MB. Soft, dynamic hydrogel confinement improves kidney organoid lumen morphology and reduces epithelial-mesenchymal transition in culture. Adv Sci (Weinh) 2022;9:e2200543. doi: 10.1002/advs.202200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Marti E, Zalvidea D, Trepat X, Roca-Cusachs P, Gavalda-Navarro A, Cozzuto L, Campistol JM, Izpisua Belmonte JC, Hurtado Del Pozo C, Montserrat N. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater. 2019;18:397–405. doi: 10.1038/s41563-019-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Gelder MK, Mihaila SM, Jansen J, Wester M, Verhaar MC, Joles JA, Stamatialis D, Masereeuw R, Gerritsen KGF. From portable dialysis to a bioengineered kidney. Expert Rev Med Devices. 2018;15:323–336. doi: 10.1080/17434440.2018.1462697. [DOI] [PubMed] [Google Scholar]
- 61.Swaminathan M, Stafford-Smith M, Chertow GM, Warnock DG, Paragamian V, Brenner RM, Lellouche F, Fox-Robichaud A, Atta MG, Melby S, Mehta RL, Wald R, Verma S, Mazer CD (2018) ACT-AKI Investigators. Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J Am Soc Nephrol 29:260–267 [DOI] [PMC free article] [PubMed]
- 62.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: a safety study with 18 months of follow-up. Cytotherapy. 2018;20:660–669. doi: 10.1016/j.jcyt.2018.02.368. [DOI] [PubMed] [Google Scholar]
- 63.Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. doi: 10.1186/s13287-017-0557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reinders MEJ, Groeneweg KE, Hendriks SH, Bank JR, Dreyer GJ, de Vries APJ, van Pel M, Roelofs H, Huurman VAL, Meij P, Moes D, Fibbe WE, Claas FHJ, Roelen DL, van Kooten C, Kers J, Heidt S, Rabelink TJ, de Fijter JW. Autologous bone marrow-derived mesenchymal stromal cell therapy with early tacrolimus withdrawal: the randomized prospective, single-center, open-label TRITON study. Am J Transplant. 2021;21:3055–3065. doi: 10.1111/ajt.16528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casiraghi F, Perico N, Gotti E, Todeschini M, Mister M, Cortinovis M, Portalupi V, Plati AR, Gaspari F, Villa A, Introna M, Longhi E, Remuzzi G. Kidney transplant tolerance associated with remote autologous mesenchymal stromal cell administration. Stem Cells Transl Med. 2020;9:427–432. doi: 10.1002/sctm.19-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stavas J, Filler G, Jain D, Ludlow J, Basu J, Payne R, Butler E, Diaz-Gonzalez de Ferris M, Bertram T. Renal autologous cell therapy to stabilize function in diabetes-related chronic kidney disease: corroboration of mechanistic action with cell marker analysis. Kidney Int Rep. 2022;7:1619–1629. doi: 10.1016/j.ekir.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carstens MH, Garcia N, Mandayam S, Workeneh B, Pastora I, Calderon C, Bertram KA, Correa D. Safety of stromal vascular fraction cell therapy for chronic kidney disease of unknown cause (mesoamerican nephropathy) Stem Cells Transl Med. 2023;12:7–16. doi: 10.1093/stcltm/szac080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD–fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4:1827–1831. doi: 10.2215/CJN.02270409. [DOI] [PubMed] [Google Scholar]
- 69.Nagaraja P, Roberts GW, Stephens M, Horvath S, Kaposztas Z, Chavez R, Asderakis A. Impact of expanded criteria variables on outcomes of kidney transplantation from donors after cardiac death. Transplantation. 2015;99:226–231. doi: 10.1097/TP.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 70.Brasile L, Henry N, Orlando G, Stubenitsky B. Potentiating renal regeneration using mesenchymal stem cells. Transplantation. 2019;103:307–313. doi: 10.1097/TP.0000000000002455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784. doi: 10.1111/j.1523-1755.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 72.van Koppen A, Joles JA, van Balkom BW, Lim SK, de Kleijn D, Giles RH, Verhaar MC. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One. 2012;7:e38746. doi: 10.1371/journal.pone.0038746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Wang R, Jia Y, Rong R, Xu M, Zhu T. Exosomes derived from mesenchymal stem cells ameliorate renal ischemic-reperfusion injury through inhibiting inflammation and cell apoptosis. Front Med (Lausanne) 2019;6:269. doi: 10.3389/fmed.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erdbrugger U, Hoorn EJ, Le TH, Blijdorp CJ, Burger D. Extracellular vesicles in kidney diseases: moving forward. Kidney 360. 2023;4:245–257. doi: 10.34067/KID.0001892022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013;4:34. doi: 10.1186/scrt194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rampino T, Gregorini M, Germinario G, Pattonieri EF, Erasmi F, Grignano MA, Bruno S, Alomari E, Bettati S, Asti A, Ramus M, De Amici M, Testa G, Bruno S, Ceccarelli G, Serpieri N, Libetta C, Sepe V, Blasevich F, Odaldi F, Maroni L, Vasuri F, La Manna G, Ravaioli M. Extracellular vesicles derived from mesenchymal stromal cells delivered during hypothermic oxygenated machine perfusion repair ischemic/reperfusion damage of kidneys from extended criteria donors. Biology (Basel) 2022;11:350. doi: 10.3390/biology11030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nassar W, El-Ansary M, Sabry D, Mostafa MA, Fayad T, Kotb E, Temraz M, Saad AN, Essa W, Adel H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater Res. 2016;20:21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell. 2019;25:373–387.e379. doi: 10.1016/j.stem.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiratsuka K, Miyoshi T, Kroll KT, Gupta NR, Valerius MT, Ferrante T, Yamashita M, Lewis JA, Morizane R. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci Adv. 2022;8:eabq0866. doi: 10.1126/sciadv.abq0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howden SE, Wilson SB, Groenewegen E, Starks L, Forbes TA, Tan KS, Vanslambrouck JM, Holloway EM, Chen YH, Jain S, Spence JR, Little MH. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell. 2021;28:671–684.e676. doi: 10.1016/j.stem.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH. Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet. 2018;102:816–831. doi: 10.1016/j.ajhg.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulder J, Sharmin S, Chow T, Rodrigues DC, Hildebrandt MR, D'Cruz R, Rogers I, Ellis J, Rosenblum ND. Generation of infant- and pediatric-derived urinary induced pluripotent stem cells competent to form kidney organoids. Pediatr Res. 2020;87:647–655. doi: 10.1038/s41390-019-0618-y. [DOI] [PubMed] [Google Scholar]
- 83.Majmundar AJ, Buerger F, Forbes TA, Klambt V, Schneider R, Deutsch K, Kitzler TM, Howden SE, Scurr M, Tan KS, Krzeminski M, Widmeier E, Braun DA, Lai E, Ullah I, Amar A, Kolb A, Eddy K, Chen CH, Salmanullah D, Dai R, Nakayama M, Ottlewski I, Kolvenbach CM, Onuchic-Whitford AC, Mao Y, Mann N, Nabhan MM, Rosen S, Forman-Kay JD, Soliman NA, Heilos A, Kain R, Aufricht C, Mane S, Lifton RP, Shril S, Little MH, Hildebrandt F. Recessive NOS1AP variants impair actin remodeling and cause glomerulopathy in humans and mice. Sci Adv. 2021;7:eabe1386. doi: 10.1126/sciadv.abe1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, Hosawi S, Wilson S, Lawlor KT, Khan S, Oshlack A, Quinlan C, Lennon R, Little MH. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun. 2018;9:5167. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]