Abstract
Developing meat analogues of superior amino acid (AA) profiles in the food industry is a challenge as plant proteins contain less of some essential AA than animal proteins. Mathematical optimisation models such as linear/non-linear programming models were used to overcome this challenge and create high-moisture meat analogues (HMMA) with AA profiles as close as possible to chicken breast meat. The effect on the physiochemical properties and specific mechanical energy (SME) of the HMMA was investigated. The AA content of HMMA was generally lower than chicken. Strong intermolecular bonds present in the globulin fraction could hinder protein acid hydrolysis of HMMA. Plant proteins also affect the HMMA colour as certain AA forms Maillard reaction products with higher browning intensity. Lastly, different characteristics of plant proteins resulted in different SME values under the same extrusion conditions. While mathematical programming can optimise plant protein combinations, fortification is required to match the AA profile of HMMA to an animal source.
Keywords: Mathematical optimisation, Plant protein, Meat analogues, Amino acid, Extrusion
Graphical abstract
Highlights
-
•
Amino acid content of meat analogues was still lower than the target animal source.
-
•
Plant proteins high in alanine and asparagine resulted in darker meat analogue.
-
•
Mechanical energy of meat analogues differed between plant protein combinations.
-
•
Fortification is still needed to improve amino acid profile of meat analogue.
Abbreviations
- AA
amino acid
- SPC
soy protein concentrate
- WG
wheat gluten
- CPI
chickpea protein isolate
- PPI
pea protein isolate
- MBPI
mung bean protein isolate
- RPI
rice protein isolate
- HMMA
high-moisture meat analogues
1. Introduction
The consumption of plant-based foods has stagnated since the year 2018 due to the lack of technological innovation that could lower costs and environmental impact while improving consumers' satisfaction and well-being (Bedoya et al., 2022). New food products are often developed via “trial and error", which is costly and time-consuming (Sheibani et al., 2018). As such, computational modelling and optimisation can be used to accelerate the development of new food products. Mathematical models have been used in food research for several purposes, such as classifying foods, determining bioactive compounds, and optimising product formulation for better nutrition and consumer acceptability (Bedoya et al., 2022). Linear programming has been used to solve diet problems, which aims to find a diet meeting nutritional needs while minimising economic and environmental costs (van Dooren, 2018), whereas non-linear and mixed integer programming have been applied to the design of vegetarian diets (Kesse-Guyot et al., 2022) and sustainable diet (García-Leal et al., 2023). Such technologies could minimise food waste, improve the food production system's efficiency, fulfil nutritional requirements, and reduce environmental impact (Hassoun et al., 2022).
While the market for meat substitutes has experienced significant growth in recent years, it is still a fraction of traditional meat (e.g., poultries, red meats, and seafood). There is definite potential for further growth, especially in countries like China and India (Szenderák et al., 2022). In the food industry, extrusion is a well-developed process involving mixing, shearing, and cooking raw materials before the mixture is forced through a die under high pressure. As the mixture leaves the opening, material expansion occurs due to the sudden drop in pressure and water is converted into steam (Leonard et al., 2020). The extrusion of protein powders with twin-screw extruders is often used for the production of high-moisture meat analogue (HMMA) with well-defined fibre structures (Choton et al., 2020; He et al., 2020). HMMA comprises plant proteins from common plant materials such as legumes and cereals (Kumar, 2016). Although the growth and harvesting of plants are significantly more energy efficient than meat production, the nutritional aspect of HMMA remains a challenge to the food industry (Szenderák et al., 2022). This is because animal proteins are complete proteins, whereas plant proteins typically lack at least one essential amino acid (AA). As all AAs are needed for protein synthesis, deficiency in one or more AAs could affect the postprandial muscle protein synthetic response (Gorissen et al., 2018). De Marchi, Costa, Pozza, Goi, and Manuelian (2021) reported that alanine, glycine and methionine contents were lower in plant-based burgers than in meat-based burgers. The authors explained that as plant-based burgers were mainly made of soy and pea protein, using a blend of plant proteins would help lower the difference. This presents an opportunity for using linear/non-linear programming to optimise and improve the nutritional profile of HMMA.
This study used the AA composition of various meat samples (e.g., chicken, pork, beef, and salmon) and plant protein powders as data. These meat samples were selected as they are widely consumed animal proteins in the world (Govoni et al., 2021; Torrissen et al., 2011). Mathematical optimisation models were then used to generate combinations of plant protein powders at different compositions that best matched the AA profile of a target meat source. Mathematical optimisation techniques have been applied to plant-based meat design in many aspects. For instance, the response surface method (RMS) has been applied to optimise the proportions of hydrocolloid, water, and encapsulated anthocyanins to obtain meat analogues with high sensory acceptability and physicochemical parameters similar to traditional meat products (Szpicer et al., 2022). The combinations were then shortlisted for extrusion to produce HMMA. The effect of HMMA with different protein combinations on their physicochemical properties and the specific mechanical energy (SME) was then investigated.
2. Materials and methods
2.1. Materials
Chicken breast, minced pork, and minced beef were purchased from a local supermarket (NTUC FairPrice Co-operative Ltd, Singapore). Salmon fillet was bought from Oriental Food & Services Pte Ltd, Singapore. Plant proteins were obtained from various commercial sources, including Wilmar International Ltd, Connell Caldic Malaysia Sdn Bhd, Roquette Singapore, Growthwell Foods Singapore, Nutraonly Nutrition Inc China, ETprotein China, Beneo Asia-Pacific Pte Ltd, and Ingredion Singapore.
Each meat sample was packed into a polyamide/polyethene pouch and vacuum sealed (C200, Multivac, Germany). The meat samples were then placed into a water bath (SD28H170-A12P, PolyScience, Singapore) at 90 °C for 20 min for cooking. The samples were cooled and drained before further analysis.
2.2. Protein content analysis
The meat samples were ground before protein content and total AA composition analysis. Protein content was determined using Dumas combustion, which was conducted using an automated protein analyser (Dumatherm® N Pro, Gerhardt GmbH, Germany). Protein content was calculated with a nitrogen conversion factor of 6.25 for animal and plant proteins, and 5.7 for wheat gluten (WG).
2.3. Total amino acids composition analysis
The total AA composition for all samples was carried out as described by Schuster (1988), Barkholt and Jensen (1989), Henderson et al. (2000), and Henderson and Brooks (2010) with modifications. Samples were hydrolysed with 6M HCl containing 0.1% w/v phenol at 110 °C for 24 h. The amino acid residues were converted into cysteic acid and methionine sulphone by performic acid before acid hydrolysis. The analysis was determined by injecting 0.5 μl of each sample into high-performance liquid chromatography (HPLC) (Agilent 1200SL, Agilent Technologies, USA) after pre-column derivatisation with flourenylmethoxycarbonyl (FMOC)-chloride for primary amino acids and ortho-phthaldialdehyde (OPA) for proline. A C18 stationary phase (150 mm × 2.1 mm id, 3.5 μm particle size) column (ZORBAX Eclipse Plus, Chrom Tech, USA) was used for separation at a flow rate of 0.42 ml/min at 40 °C. Two mobile phases were used: Mobile A comprised of 0.01M disodium phosphate, 0.01M sodium tetraborate decahydrate and 0.005M sodium azide at pH8.2; Mobile B composed of acetonitrile: methanol: ultrapure water (45:45:10, v: v: v). A fluorescence detector operated at 230 nm (excitation) and 450 nm (emission) was used to estimate AA concentrations in the sample. AA standards (Sigma-Aldrich, Singapore) were used to identify compounds based on retention time. Quantification was determined using external calibration curves.
2.4. Optimisation-based replicate formula design
In the food industry, existing studies that apply optimisation techniques mainly focus on linear programming approaches. On the other hand, the replication problem in this study was cast into a more general non-linear programming framework to allow more flexibility on optimisation criteria. The general form of a non-linear programming model was as follows:
subject to:
This meant that to find satisfying constraints, , and while is minimised.
Contrary to linear programming, non-linear programming allows objective function () and constraints ( and ) to be non-linear. In special cases, it can also include linear programming. Non-linear programming has been widely used in many other areas, such as path planning, petroleum production, statistical inference, and many others. Although non-linear programming for complex problems consisting of big data, such as training large neural network models (e.g., millions to billions of variables) remains challenging, small- and medium-sized problems (e.g., less than a few thousand variables) can be solved relatively efficiently. The optimisation problems formulated in this study shall be considered as small-sized ones.
The replicate formula design was described as determining the ratio of different plant protein powders such that the AA profile of the mixed proteins was similar to a given animal source. This objective could be cast into linear/non-linear mathematical programming models given as follows:
It was assumed to have AA profiles of plant protein sources, and they were denoted as a column vector , where is the total number of amino acids, is the number of candidate plant protein sources, and represents dimensional Euclidean space. The AA profile of the target animal source is denoted as . It was further assumed that the sum of the components of each equals that of , which means that each animal and plant protein source sample has the same weight. For each plant protein source , its ratio is denoted as and thus, the AA profile of the mixed proteins can be expressed as . Further, , , and were introduced. With the above notations, finding the optimal ratio vector is equivalent to sol1ving the following mathematical optimisation problem:
subject to:
where function measures the difference between two vectors and .
There were multiple options for the function , which have been used for our formula design as follows:
-
1)
Cosine similarity: the cosine similarity between and is given by , where denotes the transpose of and denotes the 2-norm operator. This function essentially measures the angle between and and the negative sign is for minimization.
-
2)
-norm (Manhattan distance): the Manhattan distance between and is given by , where and denotes the -th component of and .
-
3)
-norm (Euclidean distance): the Euclidean distance between and is given by .
-
4)
-norm (Chebyshev distance): the Chebyshev distance between and is given by .
The above functions are symmetric with respect to their arguments. However, in our problem setup, is the AA profile of the replicate, and is that of the target animal protein source. They are naturally asymmetric, i.e., they have different roles in this replica process. To capture such property, the following functions are considered:
-
5)
Sum of deficiencies: the sum of deficiencies from to is given by . In other words, this function measures the sum of deficiencies of each amino acid of compared with .
-
6)
Greatest deficiency: the greatest deficiency from to is given by . In other words, this function measures the greatest deficiencies of each amino acid of compared with .
Nevertheless, applying the above models to the convex combination of the plant protein powders and the target animal sources led to some trivial solutions using a single plant protein to replicate the target animal source. For instance, under the cosine similarity criterion, the optimal formula for replicating chicken breast meat was to mix 10% WG (the minimal amount as the base) and 90% pea protein isolate (PPI). Mathematically, it was correct, but the lack of variety made such a formula undesirable. The fundamental mathematical reason was that the convex cone spanned by vectors representing plant AA profiles was not large enough to contain the vector representing the target animal source. From a nutrition perspective, the variety of plant protein sources was insufficient. Thus, one can only replicate the projection of the target vector onto the convex cone. Unfortunately, when the projection happened to be one of the edges of the cone, the resulting formula became a trivial solution by picking a single plant protein.
One possible remedy was to add more plant protein powders as candidates such that the size of the convex cone can be enlarged, suggesting the variety of protein powders not from a nutrition perspective but from a mathematical perspective. Ten plant protein powders were introduced in the experimental design to reduce the possibility of trivial solutions. Another approach was introducing more criteria to measure the difference between two AA profiles to get more replicate formulas and pick those non-trivial ones from them. In what follows, two non-linear transformations that were applied to the AA profiles were described, resulting in 12 more candidate formulas for each target animal source. It was noted that the above criteria only concerned the absolute difference between the AA profile of and . Table 1 shows an example that motivated another formulation concerning the relative ratio of components in and .
Table 1.
Components comparison of chicken breast meat and optimal mixed proteins replicate under -norm criterion.
| AA Component | Threonine | Valine | Isoleucine | Leucine | Phenylalanine | Lysine | Histidine | Methionine | Non-essential AA |
|---|---|---|---|---|---|---|---|---|---|
| Chicken | 4.62 | 5.23 | 5.00 | 8.21 | 4.20 | 10.26 | 3.32 | 2.97 | 56.18 |
| Replicate | 3.56 | 5.27 | 4.88 | 8.55 | 6.00 | 6.48 | 2.58 | 1.45 | 56.18 |
Table 1 shows that the amount of methionine in the replicate was 1.52 less than that of chicken breast meat. This absolute difference was less than that of lysine, which was 3.78. However, considering the relative ratio, lysine was 63.16% of chicken breast meat in the replicate, while methionine was less than 50%. Although the absolute difference in methionine was lesser, its difference in the ratio was more. In this case, the absolute difference between AA profiles might not comprehensively quantify their similarity.
One of the extra optimisation criteria was derived from a function which computed the relative ratio of each component in and . Intuitively, a relative ratio vector between the replicate and the target animal source should be close to the all-one vector as much as possible. Consequently, the linear/non-linear programming formulation was considered with the objective function , where is defined in (1) to (6). The optimisation model under this category was named with the prefix ‘relative’. Similarly, one can define a slightly more complex transformation . This gives the relative ratio of each amino acid of the given AA profile. The objective functions was then considered, where is defined in (1) to (6). The optimisation model under this category was named with the prefix ‘ratio’. In summary, six different objective functions were adopted to quantify the difference between two vectors and , each of which had been further applied to three vector-couples: ; ; and .
As a result of optimisation algorithms and computational device development, each of the above models can be solved in less than 1 s on an ordinary personal computer, with multiple choices of open-source optimizers such as IPOPT (Waechter et al., 2006), ADMB (Fournier et al., 2012), and others. The numerical tools chosen were CasADi (Andersson et al., 2019), which is an automatic differentiation tool for computing gradients and Hessian matrices, and IPOPT, which accepts gradients and Hessian matrices computed by CasADi to accelerate computation. Therefore, one can conveniently derive formulas for different plant protein powders and animal sources. Another advantage of mathematical optimisation was its flexibility in handling arbitrary quantitative requirements. For example, in numerical examples, a constraint that required the minimum WG to be 10% would be included. Similarly, it was easy to include constraints requiring minimal and/or maximal amounts of other plant protein powders. The objective function could also be revised to include more criteria where one wants to optimise. For instance, different plant powders had different price points, so one may want to seek a balance between the total price and the similarity of the AA profile. This could be achieved by coding an extra term into the objective function as follows: Suppose that the price of each plant is per unit weight. Then, the cost of raw material for producing a unit weight of mixed plant-based protein is without considering loss during the extrusion process. This can thus minimise to seek a balance between the similarity of AA profiles represented by , and the total price represented by .
On the other hand, it could also be seen that under this mathematical optimisation framework, every feature must be quantified, and the non-quantifiable properties of plant protein powders and animal sources cannot be optimised directly. Consequently, this study focused mainly on AA profile optimisation without considering the textural properties of HMMA, which were somewhat difficult to quantify.
2.5. High-moisture extrusion
Extrusion experiments were performed with a laboratory, co-rotating and intermeshing twin screw extruder (Process 16 Hygienic, Thermo Fisher Scientific, Karlsruhe, Germany) with a screw diameter of 16 mm, a smooth barrel, and a length-diameter ratio of 40:1. The extrusion formulations of HMMA were based on Table 2. Formulations consisting of different plant protein combinations were derived from the mathematical optimisation process discussed in the previous section. The extrusion screw profile comprised of (from feed to exit): fifteen 16 mm feed screws (240 mm); twelve 4 mm mixing elements (48 mm); eight 16 mm feed screws (128 mm); ten 4 mm mixing elements (40 mm); ten 16 mm forward screws (160 mm); and one 24 mm discharge element (24 mm). The barrel was segmented into the feeding zone, and seven temperature-controlled zones heated by an electric cartridge system and cooled with water. A long die with dimensions of 83 × 49 × 188 mm (W × H × L) was attached at the end of the extruder, with water at 80 °C as a cooling medium. A gravimetric feeder (Brabender Technology GmbH, Duisburg, Germany) was used to feed the dry materials into the extruder at a feed rate of 1.8 kg/h. At the same time, salt solution at 1% w/w was injected into the extruder via a peristaltic pump (Masterflex L/S, Vernon Hills, IL, USA) into the second barrel through an inlet port at different flow rates for different formulations to obtain a moisture content of approximately 55% w/w (wet basis) in the HMMA. The screw speed was kept at 400 rpm, and the barrel had a temperature profile of 40, 60, 80, 100, 120, 120, 140 and 140 °C in the eight zones from feed to die.
Table 2.
Portion of plant protein powders for mixed protein matrices’ combinations selected for extrusion.
| Optimisation criterion (Formulation) | Plant protein (%) a |
||||||
|---|---|---|---|---|---|---|---|
| SPC-1 | SPC-2 | WG | PPI | MBPI | CPI | RPI | |
| ratio_cos_sim (F1) | – | 92.11 | 7.89 | – | – | – | – |
| relative_chebyshev_dist (F2) | – | 44.92 | 7.32 | – | – | 47.76 | – |
| relative_manhattan_dist (F3) | 62.05 | – | – | 37.95 | – | – | – |
| ratio_euclidean (F4) | 34.70 | – | – | – | 13.58 | – | 51.72 |
| relative_manhattan_dist (F5) | – | 39.89 | 10.00 | 50.11 | – | – | – |
| euclidean_dist (F6) | – | – | 10.00 | 66.81 | 23.19 | – | – |
Abbreviation: SPC-1, soy protein concentration (brand 1); SPC-2, soy protein concentration (brand 2); WG, wheat gluten; PPI, pea protein isolate; MBPI, mung bean protein isolate; CPC, chickpea protein isolate; RPI, rice protein isolate.
Extrusion parameters such as product pressure, temperature and torque were monitored. Following Osen (2017), the specific mechanical energy (SME) was then calculated using the equation below:
where N is the rotational screw speed (min−1), T is the motor torque (kJ), and ṁ is the mass flow rate (kg/min).
The HMMA produced were then subjected to pH, moisture, and colour analyses. The pH of the HMMA was measured after blending the samples at 20% w/w concentration in ultrapure water at 12,000 rpm with a high-shear mixer (T25 digital Ultra Turrax®, IKA, Germany). The moisture content of the HMMA was determined using the air-oven method. Five grams of samples were first weighed on separate pans and dried at 105 °C for 24 h. The dried samples' final weight was recorded after cooling down in a desiccator for 2 h. The colour of the HMMA was measured using a benchtop spectrophotometer (CM-5, Konica Minolta, Japan). A black calibration cup was used to calibrate the instrument while the measured colours were expressed in the Hunter-Lab parameters as L*, a*, and b* values. The Petri dish measurement mode with a 30 mm measurement area was selected, and three measurements were recorded using random surface locations of the extrudates. L* represents the lightness (0 = black, 100 = white), -a/+a represents greenness or redness, and –b/+b represents blueness or yellowness.
2.6. Statistical analyses
All treatments were conducted in triplicates, and the results were reported as the mean value ± standard deviation (SD). The data were analysed using a one-way analysis of variance (ANOVA) with Tukey pairwise comparison of means (p ≤ 0.05) using Minitab 17 statistical software (Minitab Inc., USA).
3. Results and discussion
3.1. Animal and plant proteins
3.1.1. Protein content
The protein content and total AA profiles in the meat samples and plant proteins are shown in Table 3, Table 4, respectively. Comparing the animal sources, cooked chicken breast meat had the highest protein content, while cooked salmon had the lowest. From Table 3, the protein content of cooked chicken breast, pork, and beef in this study was slightly higher than those reported in the literature (Hong et al., 2015; Villalobos-Delgado et al., 2020; Witte et al., 2022). There were multiple reasons for these discrepancies. Tougan et al. (2013) reported that extrinsic and intrinsic factors such as feed, age and gender played a role in the protein content of chickens. Daszkiewicz et al. (2005) mentioned that fat content was inversely correlated with crude protein content in pork. On the other hand, the protein content found in salmon (23.33% w/w) was similar to the protein range of salmon fillets (18.8%–24.2% w/w) reported by Sprague et al. (2020). Salmon had a lower protein content than other meat types, possibly because it had a higher fat content of 11.0%–17.0% w/w (Sprague et al., 2020).
Table 3.
Total amino acid composition of proteins from different animal sources.
| Samples | Total amino acids (mg/100 mg protein)a |
|||
|---|---|---|---|---|
| Cooked chicken breast | Cooked minced pork | Cooked minced beef | Cooked salmon | |
| Protein (g/100g) | 30.74 ± 0.81 | 25.62 ± 1.65 | 27.43 ± 0.17 | 23.33 ± 1.45 |
| Essential amino acids | ||||
| Histidine | 2.77 ± 0.14 | 3.32 ± 0.10 | 3.01 ± 0.04 | 2.61 ± 0.01 |
| Isoleucine | 4.16 ± 0.13 | 4.49 ± 0.20 | 4.57 ± 0.15 | 4.33 ± 0.12 |
| Leucine | 6.84 ± 0.23 | 7.78 ± 0.33 | 8.15 ± 0.08 | 7.11 ± 0.11 |
| Lysine | 8.54 ± 0.44 | 8.98 ± 0.12 | 8.77 ± 0.83 | 8.58 ± 0.53 |
| Methionine | 2.47 ± 0.09 | 2.78 ± 0.13 | 2.76 ± 0.09 | 2.92 ± 0.04 |
| Phenylalanine | 3.50 ± 0.14 | 4.09 ± 0.14 | 4.18 ± 0.04 | 3.85 ± 0.06 |
| Threonine | 3.85 ± 0.18 | 4.36 ± 0.12 | 4.48 ± 0.07 | 4.44 ± 0.04 |
| Valine | 4.36 ± 0.14 | 4.91 ± 0.23 | 4.92 ± 0.17 | 4.95 ± 0.04 |
| Total EAA | 36.51 ± 1.49 | 40.69 ± 1.37 | 40.85 ± 1.44 | 38.79 ± 0.95 |
| Non-essential amino acids | ||||
| Alanine | 4.99 ± 0.17 | 5.78 ± 0.22 | 6.11 ± 0.17 | 5.81 ± 0.16 |
| Arginine | 6.61 ± 0.24 | 6.56 ± 0.26 | 6.85 ± 0.10 | 7.11 ± 0.04 |
| Aspartic acid | 8.41 ± 0.28 | 9.46 ± 0.30 | 9.60 ± 0.23 | 9.58 ± 0.16 |
| Cystine | 1.03 ± 0.06 | 1.16 ± 0.08 | 1.30 ± 0.13 | 1.09 ± 0.04 |
| Glutamic acid | 12.35 ± 0.44 | 14.29 ± 0.49 | 15.32 ± 0.18 | 12.50 ± 0.27 |
| Glycine | 3.72 ± 0.18 | 5.07 ± 0.18 | 5.52 ± 0.81 | 5.24 ± 0.72 |
| Proline | 3.14 ± 0.10 | 4.09 ± 0.26 | 4.56 ± 0.15 | 3.56 ± 0.22 |
| Serine | 3.45 ± 0.12 | 3.98 ± 0.12 | 4.07 ± 0.12 | 3.84 ± 0.11 |
| Tyrosine | 3.10 ± 0.12 | 3.54 ± 0.11 | 3.60 ± 0.04 | 3.43 ± 0.05 |
Data are presented as the mean and standard deviation of three replicates.
Table 4.
Total amino acid composition of various plant proteins from legume and cereal origins.
| Samples | Total amino acids (mg/100 mg protein)a,b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPC-1 | SPC-2 | PPI | CPI | CPC | MBPI | FBP | LP | RPI | WG | |
| Protein (g/100g) | 71.53 ± 0.07 | 62.56 ± 0.13 | 79.01 ± 0.01 | 81.98 ± 0.18 | 69.68 ± 0.01 | 81.73 ± 0.17 | 53.25 ± 0.07 | 52.76 ± 0.03 | 83.88 ± 0.18 | 74.20 ± 0.06 |
| Essential amino acids | ||||||||||
| Histidine | 2.60 ± 0.10 | 2.68 ± 0.07 | 2.66 ± 0.01 | 2.94 ± 0.11 | 2.78 ± 0.12 | 3.08 ± 0.01 | 2.73 ± 0.02 | 2.28 ± 0.02 | 2.55 ± 0.04 | 2.21 ± 0.09 |
| Isoleucine | 4.63 ± 0.24 | 4.69 ± 0.11 | 5.31 ± 0.15 | 5.34 ± 0.16 | 5.25 ± 0.18 | 5.12 ± 0.06 | 4.29 ± 0.03 | 4.14 ± 0.03 | 4.51 ± 0.05 | 3.98 ± 0.13 |
| Leucine | 7.59 ± 0.38 | 7.75 ± 0.27 | 9.09 ± 0.18 | 9.44 ± 0.34 | 9.01 ± 0.31 | 9.40 ± 0.08 | 7.77 ± 0.01 | 7.22 ± 0.01 | 8.85 ± 0.06 | 7.38 ± 0.23 |
| Lysine | 6.29 ± 0.19 | 5.81 ± 0.30 | 7.56 ± 0.77 | 6.96 ± 0.36 | 7.17 ± 1.37 | 7.00 ± 0.52 | 6.79 ± 0.10 | 6.79 ± 0.26 | 3.37 ± 0.23 | 1.35 ± 0.36 |
| Methionine | 1.59 ± 0.11 | 1.67 ± 0.08 | 1.43 ± 0.12 | 1.87 ± 0.05 | 1.86 ± 0.10 | 1.63 ± 0.07 | 0.81 ± 0.01 | 0.75 ± 0.01 | 3.00 ± 0.11 | 1.83 ± 0.04 |
| Phenylalanine | 5.09 ± 0.23 | 5.12 ± 0.26 | 6.01 ± 0.06 | 7.42 ± 0.27 | 7.20 ± 0.29 | 7.45 ± 0.08 | 4.57 ± 0.01 | 4.92 ± 0.01 | 5.86 ± 0.07 | 5.61 ± 0.14 |
| Threonine | 3.84 ± 0.18 | 4.06 ± 0.12 | 4.09 ± 0.04 | 3.97 ± 0.17 | 3.83 ± 0.13 | 3.15 ± 0.02 | 3.72 ± 0.02 | 3.79 ± 0.01 | 3.93 ± 0.06 | 2.75 ± 0.06 |
| Valine | 4.52 ± 0.25 | 4.66 ± 0.17 | 5.65 ± 0.15 | 5.29 ± 0.15 | 5.28 ± 0.15 | 5.86 ± 0.08 | 4.58 ± 0.02 | 4.41 ± 0.04 | 6.14 ± 0.06 | 4.02 ± 0.11 |
| Total EAA | 36.15 ± 1.68 | 36.44 ± 1.38 | 41.81 ± 1.47 | 43.23 ± 1.61 | 42.38 ± 2.65 | 42.69 ± 0.91 | 35.25 ± 0.21 | 34.29 ± 0.40 | 38.22 ± 0.67 | 29.11 ± 1.16 |
| Non-essential amino acids | ||||||||||
| Alanine | 4.24 ± 0.16 | 4.39 ± 0.15 | 4.66 ± 0.05 | 4.98 ± 0.20 | 4.78 ± 0.24 | 4.40 ± 0.05 | 4.25 ± 0.02 | 3.94 ± 0.02 | 6.21 ± 0.11 | 2.69 ± 0.13 |
| Arginine | 7.13 ± 0.34 | 7.33 ± 0.23 | 8.94 ± 0.08 | 10.49 ± 0.40 | 9.77 ± 0.36 | 7.89 ± 0.03 | 9.83 ± 0.03 | 8.81 ± 0.26 | 9.14 ± 0.11 | 3.70 ± 0.12 |
| Aspartic acid | 11.34 ± 0.43 | 11.62 ± 0.31 | 12.32 ± 0.32 | 14.19 ± 0.69 | 13.15 ± 0.84 | 13.01 ± 0.19 | 11.40 ± 0.07 | 11.14 ± 0.08 | 9.81 ± 0.32 | 3.36 ± 0.17 |
| Cystine | 1.34 ± 0.14 | 1.52 ± 0.07 | 1.26 ± 0.10 | 1.34 ± 0.04 | 1.20 ± 0.07 | 0.52 ± 0.11 | 1.15 ± 0.01 | 0.79 ± 0.03 | 2.23 ± 0.17 | 2.49 ± 0.36 |
| Glutamic acid | 17.52 ± 0.81 | 17.96 ± 0.56 | 17.68 ± 0.29 | 19.44 ± 0.86 | 17.80 ± 0.80 | 19.09 ± 0.24 | 16.99 ± 0.06 | 15.52 ± 0.08 | 19.15 ± 0.38 | 38.86 ± 1.63 |
| Glycine | 4.36 ± 0.17 | 4.06 ± 0.12 | 4.10 ± 0.30 | 4.11 ± 0.25 | 4.11 ± 0.60 | 3.43 ± 0.22 | 4.14 ± 0.07 | 3.76 ± 0.12 | 4.66 ± 0.37 | 3.22 ± 0.79 |
| Proline | 4.87 ± 0.26 | 4.98 ± 0.16 | 4.64 ± 0.07 | 5.59 ± 0.23 | 4.74 ± 0.15 | 4.60 ± 0.07 | 5.01 ± 0.02 | 4.43 ± 0.03 | 5.29 ± 0.01 | 13.18 ± 0.38 |
| Serine | 4.89 ± 0.17 | 5.06 ± 0.19 | 5.50 ± 0.13 | 6.38 ± 0.30 | 5.88 ± 0.32 | 5.71 ± 0.10 | 5.17 ± 0.02 | 5.03 ± 0.05 | 5.53 ± 0.18 | 5.04 ± 0.30 |
| Tyrosine | 3.62 ± 0.15 | 3.79 ± 0.13 | 4.28 ± 0.05 | 3.61 ± 0.18 | 3.52 ± 0.15 | 3.49 ± 0.05 | 3.73 ± 0.02 | 3.27 ± 0.01 | 5.77 ± 0.09 | 3.67 ± 0.14 |
Data are presented as the mean and standard deviation of three replicates.
Abbreviation: SPC-1, soy protein concentration (brand 1); SPC-2, soy protein concentration (brand 2); PPI, pea protein isolate; CPI, chickpea protein isolate; CPC, chickpea protein concentrate; MBPI, mung bean protein isolate; FBP, faba bean protein; LP, lentil protein; RPI, rice protein isolate; WG, wheat gluten.
As shown in Table 4, plant protein powders were typically classified as a concentrate or isolate, with isolates having a protein content of at least 90% dry weight basis (Ma et al., 2022). The difference in the protein content was mainly due to the type of extraction method utilised, with the additional defatting and filtration steps creating highly purified protein isolates.
3.1.2. Amino acid compositions
In Table 3, the meat samples contained all essential AA (tryptophan not analysed), making them a good source of complete protein, with cooked minced beef having the highest leucine content. Cooked minced beef and pork had the highest essential AA among the four samples. The AA profiles of these meat samples slightly differed from past literature (Colombo and Mazal, 2020; Jensen et al., 2020; Purchas et al., 2014; Tan et al., 2012), which could have been due to the different agricultural practices in various geographical locations. These conditions affected how the animals were reared, which could have affected the AA profiles of the meat.
Among the ten plant protein powders, rice protein isolate (RPI) and WG belong to the cereal family, while the other proteins were sourced from legumes. RPI and WG contained a good amount of sulphur-containing AA (i.e., cysteine and methionine). However, they were lacking in lysine, as reported previously by Day (2013) and Herreman et al. (2020). In contrast, the legume proteins had a high proportion of lysine but were lower in sulphur-containing AAs. Their combination in a blend could be used to improve protein quality. For example, products made with cereal proteins can be enriched with legume proteins, or vice versa, to provide nutritional advantages (Chiang et al., 2021; Monnet et al., 2019).
The predominant AA in the plant proteins was glutamic acid, which was higher than in the meat samples. Animal proteins provide essential AA in balanced proportions, while plant proteins gave sub-optimal ratios with slightly higher leucine and phenylalanine contents but lower lysine and methionine contents (Day, 2013; Herreman et al., 2020). Jiménez-Munoz, Tavares, and Corredig (2021) mentioned that food products using a single plant protein would not be sufficient to provide an appropriate dietary source of amino acids, even at high concentrations. Hence, complementary protein sources must be combined to achieve a good AA profile to design future foods using plant proteins for nutritional characteristics comparable to animal protein. The subsequent section used mathematical optimisation models to obtain several mixed plant protein combinations to attain AA profiles similar to animal proteins.
3.2. Numerical optimisation results
This section presented the numerical optimisation results for chicken breast meat. The results for the other three animal protein sources (i.e., beef, salmon, and pork) can be found in the supplementary materials. The presented results were generated from three different scenarios. In Scenario 1, SPC-1, SPC-2, WG, PPI, MBPI and CPI were used as candidates from the plant protein powders. In Scenario 2, SPC-1, SPC-2, PPI, CPI, CPC, MBPI, FBP, LP, RPI and WG were all used as candidates. In Scenario 3, the amount of WG, which served as the basis for protein texturisation, should be at least 10% w/w. It was noted that due to the flexibility of the mathematical optimisation model, the above three scenarios could be easily solved in a single programme with slight modification on some input parameters.
In Table 5a–c, formulas for replicating the AA profile of chicken breast meat were presented under different scenarios with various optimisation models. One can see the necessity of introducing more plant protein powders and optimisation criteria to obtain formulas with more varieties. Table 5a shows that with six plant protein powders, there were six trivial formulas. However, the number of trivial formulas was reduced to three when ten plant protein powders were used (Table 5b). A constraint on the minimum amount of WG was also introduced, where seven trivial formulas were obtained. This was unsurprising since such constraint reduced the volume of the convex cone spanned by the plant protein powders. As a result, the ‘projection’ of the target AA profile had a more considerable change to be an edge of the convex cone. The necessity of introducing more optimisation criterion to obtain non-trivial formulas was also apparent. For instance, in Table 5c, only one non-trivial formula was obtained in the first six plain models. After introducing the ‘ratio’ and ‘relative’ models, ten non-trivial candidate formulas were generated for further exploration with texture factors.
Table 5a.
Portion of plant protein powders for replicating the total amino acid profile of chicken breast meat under different optimisation criterion (Scenario 1).
| Plant protein1 |
Optimisation criterion | |||||
|---|---|---|---|---|---|---|
| SPC-1 | SPC-2 | WG | PPI | MBPI | CPI | |
| 0 | 0 | 0 | 0.324 | 0.676 | 0 | chebyshev_dist |
| 0 | 0 | 0 | 1 | 0 | 0 | cos_sim∗ |
| 0 | 0 | 0 | 0.976 | 0.024 | 0 | euclidean_dist |
| 0.223 | 0 | 0 | 0.777 | 0 | 0 | manhattan_dist |
| 0.223 | 0 | 0 | 0.777 | 0 | 0 | min_all_defi |
| 0 | 0 | 0 | 1 | 0 | 0 | min_greatest_defi∗ |
| 0 | 0 | 0 | 0 | 0 | 1 | ratio_cheby_dist∗ |
| 0 | 0.921 | 0.079 | 0 | 0 | 0 | ratio_cos_sim |
| 0 | 0.145 | 0 | 0 | 0 | 0.855 | ratio_euclidean_dist |
| 0 | 1 | 0 | 0 | 0 | 0 | ratio_manhattan_dist∗ |
| 1 | 0 | 0 | 0 | 0 | 0 | ratio_min_all_defi |
| 0 | 0.928 | 0.072 | 0 | 0 | 0 | ratio_min_greatest_defi |
| 0 | 0.449 | 0.073 | 0 | 0 | 0.478 | relative_chebyshev_dist |
| 1 | 0 | 0 | 0 | 0 | 0 | relative_cos_sim∗ |
| 1 | 0 | 0 | 0 | 0 | 0 | relative_euclidean_dist∗ |
| 0 | 0.6205 | 0 | 0.3795 | 0 | 0 | relative_manhattan_dist |
| 0 | 0.245 | 0 | 0.755 | 0 | 0 | relative_min_all_defi |
| 0 | 0 | 0.128 | 0 | 0 | 0.872 | relative_min_greates_defi |
Optimisation criteria leading to trivial formulas: only use single plant protein.
Abbreviation: SPC-1, soy protein concentration (brand 1); SPC-2, soy protein concentration (brand 2); WG, wheat gluten; PPI, pea protein isolate; MBPI, mung bean protein isolate; CPI, chickpea protein isolate.
Table 5b.
Portion of plant protein powders for replicating the total amino acid profile of chicken breast meat under different optimisation criterion (Scenario 2).
| Plant protein1 |
Optimisation criterion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPC-1 | SPC-2 | WG | PPI | MBPI | CPC | FBP | CPI | LP | RPI | |
| 0 | 0 | 0 | 0.324 | 0.676 | 0 | 0 | 0 | 0 | 0 | chebyshev_dist |
| 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | cos_sim∗ |
| 0 | 0 | 0 | 0.976 | 0.024 | 0 | 0 | 0 | 0 | 0 | euclidean_dist |
| 0.223 | 0 | 0 | 0.777 | 0 | 0 | 0 | 0 | 0 | 0 | manhattan_dist |
| 0.223 | 0 | 0 | 0.777 | 0 | 0 | 0 | 0 | 0 | 0 | min_all_defi |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | min_greatest_defi∗ |
| 0 | 0 | 0 | 0 | 0.122 | 0.312 | 0 | 0 | 0 | 0.566 | ratio_cheby_dist |
| 0.61 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.39 | ratio_cos_sim |
| 0.347 | 0 | 0 | 0 | 0.136 | 0 | 0 | 0 | 0 | 0.517 | ratio_euclidean_dist |
| 0.714 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.286 | ratio_manhattan_dist |
| 0 | 0 | 0 | 0 | 0 | 0 | 0.939 | 0 | 0.061 | 0 | ratio_min_all_defi |
| 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ratio_min_greatest_defi∗ |
| 0.542 | 0 | 0 | 0 | 0 | 0.354 | 0 | 0 | 0 | 0.105 | relative_chebyshev_dist |
| 0.936 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.064 | relative_cos_sim |
| 0.919 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.081 | relative_euclidean_dist |
| 0 | 0.6205 | 0 | 0.3795 | 0 | 0 | 0 | 0 | 0 | 0 | relative_manhattan_dist |
| 0 | 0.245 | 0 | 0.755 | 0 | 0 | 0 | 0 | 0 | 0 | relative_min_all_defi |
| 0 | 0 | 0 | 0 | 0 | 0.905 | 0 | 0 | 0 | 0.095 | relative_min_greates_defi |
Optimisation criteria leading to trivial formulas: only use single plant protein.
Abbreviation: SPC-1, soy protein concentration (brand 1); SPC-2, soy protein concentration (brand 2); WG, wheat gluten; PPI, pea protein isolate; MBPI, mung bean protein isolate; CPC, chickpea protein concentrate; FBP, faba bean protein; CPI, chickpea protein isolate; LP, lentil protein; RPI, rice protein isolate.
Table 5c.
Portion of plant protein powders for replicating the total amino acid profile of chicken breast meat under different optimisation criterion (Scenario 3).
| Plant protein1 |
Optimisation criterion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPC-1 | SPC-2 | WG | PPI | MBPI | CPC | FBP | CPI | LP | RPI | |
| 0 | 0 | 0.1 | 0 | 0.9 | 0 | 0 | 0 | 0 | 0 | chebyshev_dist∗ |
| 0 | 0 | 0.1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | cos_sim∗ |
| 0 | 0 | 0.1 | 0.668 | 0.232 | 0 | 0 | 0 | 0 | 0 | euclidean_dist |
| 0 | 0 | 0.1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | manhattan_dist∗ |
| 0 | 0 | 0.1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | min_all_defi∗ |
| 0 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0.9 | 0 | min_greatest_defi∗ |
| 0 | 0 | 0.1 | 0 | 0.022 | 0.38 | 0 | 0 | 0 | 0.498 | ratio_cheby_dist |
| 0.579 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.321 | ratio_cos_sim |
| 0.298 | 0 | 0.1 | 0 | 0.15 | 0 | 0 | 0 | 0 | 0.451 | ratio_euclidean_dist |
| 0.671 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.229 | ratio_manhattan_dist |
| 0 | 0 | 0.1 | 0 | 0 | 0 | 0.833 | 0 | 0.067 | 0 | ratio_min_all_defi |
| 0 | 0 | 0.1 | 0 | 0 | 0 | 0.9 | 0 | 0 | 0 | ratio_min_greatest_defi∗ |
| 0.428 | 0 | 0.1 | 0 | 0 | 0.443 | 0 | 0 | 0 | 0.028 | relative_chebyshev_dist |
| 0.9 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | relative_cos_sim |
| 0.881 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.019 | relative_euclidean_dist |
| 0 | 0.399 | 0.1 | 0.501 | 0 | 0 | 0 | 0 | 0 | 0 | relative_manhattan_dist |
| 0 | 0 | 0.1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | relative_min_all_defi∗ |
| 0 | 0 | 0.1 | 0 | 0 | 0.879 | 0 | 0 | 0 | 0.021 | relative_min_greates_defi |
Optimisation criteria leading to trivial formulas: only use single plant protein except for wheat gluten, which served as the base.
Abbreviation: SPC-1, soy protein concentration (brand 1); SPC-2, soy protein concentration (brand 2); WG, wheat gluten; PPI, pea protein isolate; MBPI, mung bean protein isolate; CPC, chickpea protein concentrate; FBP, faba bean protein; CPI, chickpea protein isolate; LP, lentil protein; RPI, rice protein isolate.
In Table 6a, Table 6b, Table 6ca–c, the AA profiles of replicas under different scenarios were compared with various optimisation criterion with the target chicken breast meat AA profile. Some observations and discussions were listed as follows.
-
1)
All plant-based replicas had a deficiency in total essential AA. This was due to the fundamental difference between animal sources and plant protein powders. Plant protein generally contains less essential AA than animal protein (Gorissen et al., 2018).
-
2)
Among essential AAs, those having more significant deficiencies were leucine, lysine, and methionine (Gorissen et al., 2018).
-
3)
In all formulas, WG was used with a minimal amount (10% serving as the base). The reason was that WG has the lowest amount of essential AA in total and almost every kind of essential AA. Thus, using more WG would make the AA profile of the replica further from the target animal source AA profile. Consequently, the models always used a minimal amount of WG. This was the natural consequence of the AA profile optimisation procedure. On the other hand, WG helped to improve the fibre formation and texture of HMMA (Gasparre, van den Berg, Oosterlinck and Sein, 2022); in some cases, these aspects may be preferred.
Table 6a.
Calculated total amino acid profile comparison between chicken breast meat and its replica under different optimisation criterion (per 100g protein) for Scenario 1.
| Optimisation criterion | Threonine | Valine | Isoleucine | Leucine | Phenylalanine | Lysine | Histidine | Methionine | Non-essential TAA |
|---|---|---|---|---|---|---|---|---|---|
| Chicken breast meat | 4.621 | 5.232 | 4.998 | 8.213 | 4.204 | 10.257 | 3.327 | 2.969 | 56.178 |
| chebyshev_dist | 3.293 | 5.523 | 4.938 | 8.861 | 6.654 | 6.841 | 2.803 | 1.494 | 59.593 |
| cos_sim∗ | 3.891 | 5.375 | 5.046 | 8.640 | 5.716 | 7.188 | 2.531 | 1.364 | 60.250 |
| euclidean_dist | 3.870 | 5.38 | 5.042 | 8.648 | 5.749 | 7.176 | 2.541 | 1.368 | 60.226 |
| manhattan_dist | 3.919 | 5.233 | 5.002 | 8.487 | 5.630 | 7.055 | 2.574 | 1.432 | 60.668 |
| min_all_defi | 3.919 | 5.233 | 5.002 | 8.487 | 5.630 | 7.055 | 2.574 | 1.432 | 60.668 |
| min_greatest_defi∗ | 3.891 | 5.375 | 5.046 | 8.640 | 5.716 | 7.188 | 2.531 | 1.364 | 60.250 |
| ratio_cheby_dist | 3.570 | 4.916 | 4.889 | 8.397 | 6.712 | 6.681 | 2.589 | 1.734 | 60.512 |
| ratio_cos_sim∗ | 4.056 | 4.717 | 4.744 | 7.904 | 5.270 | 5.613 | 2.707 | 1.723 | 63.266 |
| ratio_euclidean_dist | 3.658 | 4.898 | 4.880 | 8.336 | 6.502 | 6.580 | 2.614 | 1.732 | 60.798 |
| ratio_manhattan_dist∗ | 4.180 | 4.794 | 4.827 | 7.981 | 5.266 | 5.984 | 2.759 | 1.722 | 62.487 |
| ratio_min_all_defi | 4.018 | 4.735 | 4.848 | 7.952 | 5.331 | 6.590 | 2.724 | 1.670 | 62.132 |
| ratio_min_greatest_defi | 4.066 | 4.723 | 4.751 | 7.910 | 5.270 | 5.644 | 2.711 | 1.723 | 63.202 |
| relative_chebyshev_dist | 3.774 | 4.780 | 4.780 | 8.108 | 5.961 | 5.972 | 2.630 | 1.729 | 62.267 |
| relative_cos_sim∗ | 4.018 | 4.735 | 4.848 | 7.952 | 5.331 | 6.590 | 2.724 | 1.670 | 62.132 |
| relative_euclidean_dist∗ | 4.018 | 4.735 | 4.848 | 7.952 | 5.331 | 6.590 | 2.724 | 1.670 | 62.132 |
| relative_manhattan_dist | 4.078 | 4.999 | 4.904 | 8.213 | 5.425 | 6.409 | 2.679 | 1.596 | 61.698 |
| relative_min_all_defi | 3.962 | 5.233 | 4.992 | 8.479 | 5.606 | 6.893 | 2.587 | 1.452 | 60.798 |
| relative_min_greates_defi | 3.447 | 4.775 | 4.747 | 8.219 | 6.535 | 5.990 | 2.527 | 1.734 | 62.026 |
Optimisation criteria leading to trivial formulas: only use single plant protein.
Table 6b.
Calculated total amino profile comparison between chicken breast meat and its replica under different optimisation criterion (per 100g protein) for Scenario 2.
| Optimisation criterion | Threonine | Valine | Isoleucine | Leucine | Phenylalanine | Lysine | Histidine | Methionine | Non-essential TAA |
|---|---|---|---|---|---|---|---|---|---|
| Chicken breast meat | 4.621 | 5.232 | 4.998 | 8.213 | 4.204 | 10.257 | 3.327 | 2.969 | 56.178 |
| chebyshev_dist | 3.293 | 5.523 | 4.938 | 8.861 | 6.654 | 6.841 | 2.803 | 1.494 | 59.593 |
| cos_sim∗ | 3.891 | 5.375 | 5.046 | 8.640 | 5.716 | 7.188 | 2.531 | 1.364 | 60.250 |
| euclidean_dist | 3.870 | 5.380 | 5.042 | 8.648 | 5.749 | 7.176 | 2.541 | 1.368 | 60.226 |
| manhattan_dist | 3.919 | 5.233 | 5.002 | 8.487 | 5.630 | 7.055 | 2.574 | 1.432 | 60.668 |
| min_all_defi | 3.919 | 5.233 | 5.002 | 8.487 | 5.630 | 7.055 | 2.574 | 1.432 | 60.668 |
| min_greatest_defi∗ | 4.167 | 4.844 | 4.547 | 7.935 | 5.409 | 7.459 | 2.505 | 0.826 | 62.309 |
| ratio_cheby_dist | 3.579 | 5.495 | 4.532 | 8.439 | 6.090 | 4.699 | 2.526 | 2.333 | 62.308 |
| ratio_cos_sim | 3.897 | 5.147 | 4.618 | 8.107 | 5.408 | 5.262 | 2.599 | 2.122 | 62.840 |
| ratio_euclidean_dist | 3.720 | 5.398 | 4.548 | 8.295 | 5.674 | 4.839 | 2.586 | 2.254 | 62.684 |
| ratio_manhattan_dist | 3.929 | 5.038 | 4.679 | 8.066 | 5.388 | 5.615 | 2.632 | 2.002 | 62.652 |
| ratio_min_all_defi | 3.856 | 4.734 | 4.431 | 8.009 | 4.756 | 7.028 | 2.796 | 0.837 | 63.553 |
| ratio_min_greatest_defi∗ | 3.836 | 4.727 | 4.423 | 8.014 | 4.714 | 7.000 | 2.815 | 0.838 | 63.634 |
| relative_chebyshev_dist | 3.827 | 4.910 | 4.801 | 8.151 | 5.840 | 6.266 | 2.643 | 1.814 | 61.749 |
| relative_cos_sim | 3.998 | 4.803 | 4.810 | 7.978 | 5.344 | 6.372 | 2.703 | 1.744 | 62.248 |
| relative_euclidean_dist | 3.993 | 4.820 | 4.800 | 7.984 | 5.347 | 6.316 | 2.698 | 1.763 | 62.278 |
| relative_manhattan_dist | 4.078 | 4.999 | 4.904 | 8.213 | 5.425 | 6.409 | 2.679 | 1.596 | 61.698 |
| relative_min_all_defi | 3.962 | 5.233 | 4.992 | 8.479 | 5.606 | 6.893 | 2.587 | 1.452 | 60.798 |
| relative_min_greates_defi | 3.583 | 4.999 | 4.829 | 8.392 | 6.600 | 6.349 | 2.572 | 1.838 | 60.838 |
Optimisation criteria leading to trivial formulas: only use single plant protein.
Table 6c.
Calculated total amino acid profile comparison between chicken breast meat and its replica under different optimisation criterion (per 100g protein) for Scenario 3.
| Optimisation criterion | Threonine | Valine | Isoleucine | Leucine | Phenylalanine | Lysine | Histidine | Methionine | Non-essential TAA |
|---|---|---|---|---|---|---|---|---|---|
| Chicken breast meat | 4.621 | 5.232 | 4.998 | 8.213 | 4.204 | 10.257 | 3.327 | 2.969 | 56.178 |
| chebyshev_dist∗ | 2.967 | 5.416 | 4.775 | 8.770 | 6.925 | 6.136 | 2.850 | 1.574 | 60.587 |
| cos_sim∗ | 3.763 | 5.219 | 4.919 | 8.476 | 5.677 | 6.597 | 2.488 | 1.401 | 61.461 |
| euclidean_dist | 3.558 | 5.270 | 4.882 | 8.552 | 5.998 | 6.478 | 2.581 | 1.446 | 61.236 |
| manhattan_dist∗ | 3.763 | 5.219 | 4.919 | 8.476 | 5.677 | 6.597 | 2.488 | 1.401 | 61.461 |
| min_all_defi∗ | 3.763 | 5.219 | 4.919 | 8.476 | 5.677 | 6.597 | 2.488 | 1.401 | 61.461 |
| min_greatest_defi∗ | 4.012 | 4.741 | 4.470 | 7.841 | 5.401 | 6.840 | 2.464 | 0.917 | 63.314 |
| ratio_cheby_dist | 3.530 | 5.257 | 4.464 | 8.246 | 5.993 | 4.399 | 2.455 | 2.276 | 63.380 |
| ratio_cos_sim | 3.777 | 4.982 | 4.552 | 7.984 | 5.394 | 4.966 | 2.559 | 2.048 | 63.737 |
| ratio_euclidean_dist | 3.585 | 5.249 | 4.481 | 8.189 | 5.686 | 4.534 | 2.548 | 2.183 | 63.545 |
| ratio_manhattan_dist | 3.806 | 4.885 | 4.606 | 7.948 | 5.376 | 5.279 | 2.588 | 1.942 | 63.571 |
| ratio_min_all_defi | 3.735 | 4.643 | 4.367 | 7.907 | 4.821 | 6.458 | 2.723 | 0.927 | 64.418 |
| ratio_min_greatest_defi∗ | 3.713 | 4.635 | 4.359 | 7.912 | 4.775 | 6.427 | 2.744 | 0.928 | 64.507 |
| relative_chebyshev_dist | 3.670 | 4.753 | 4.742 | 8.066 | 5.948 | 6.003 | 2.593 | 1.738 | 62.488 |
| relative_cos_sim | 3.877 | 4.643 | 4.741 | 7.858 | 5.330 | 6.059 | 2.661 | 1.676 | 63.155 |
| relative_euclidean_dist | 3.871 | 4.663 | 4.730 | 7.865 | 5.334 | 5.996 | 2.656 | 1.698 | 63.189 |
| relative_manhattan_dist | 3.878 | 4.987 | 4.832 | 8.213 | 5.497 | 6.117 | 2.579 | 1.544 | 62.353 |
| relative_min_all_defi∗ | 3.763 | 5.219 | 4.919 | 8.476 | 5.677 | 6.597 | 2.488 | 1.401 | 61.461 |
| relative_min_greates_defi | 3.477 | 4.824 | 4.765 | 8.256 | 6.549 | 6.068 | 2.536 | 1.757 | 61.768 |
Optimisation criteria leading to trivial formulas: only use single plant protein except for wheat gluten, which served as the base.
3.3. Extruded mixed protein matrices
3.3.1. Validation of the mathematical optimised model
Six HMMA formulations (F1 to F6) were chosen to be produced via extrusion. The AA composition of the extruded mixed protein matrices was compared with the values obtained from the optimised replicate formula design and presented as a percentage difference in Table 7. The difference in the values between the actual AA composition of the extruded mixed protein matrices and those from the optimised replicate formula design ranged from −12.59 (methionine in F2) to 16.41% (lysine in F6). These discrepancies could be due to the extrusion process, where AA content was affected by the feed moisture, pressure, temperature, and other parameters (Nadeesha Dilrukshi, Torrico, Brennan and Brennan, 2022). Alonso, Grant, Dewey, and Marzo (2000) reported that extrusion significantly lowered the methionine content of peas by 34%. This phenomenon was also observed in extruded field beans and soybeans. The authors attributed this to the formation of cross-linkages or Maillard reactions between AA and reducing sugars, thereby reducing the amount of available AA.
Table 7.
Measured protein content and total amino acid composition of chicken breast meat and selected extruded mixed protein matrices, including percentage difference of amino acid composition in selected extruded mixed protein matrices against the mathematically optimised amount.
| Optimisation criterion (sample) | Protein content (%) | Total amino acids (mg/100 mg protein) (% difference against the mathematically optimised amount) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Threonine | Valine | Isoleucine | Leucine | Phenylalanine | Lysine | Histidine | Methionine | Total NEAA | ||
| Chicken breast meat | 30.74 ± 0.63 a | 3.85 ± 0.18 c | 4.36 ± 0.14 d | 4.16 ± 0.13 c | 6.84 ± 0.23 d | 3.50 ± 0.14 b | 8.54 ± 0.44 b | 2.77 ± 0.14 c | 2.47 ± 0.09 c | 46.81 ± 1.56 c |
| ratio_cos_sim (F1) | 33.02 ± 0.06 c | 2.44 ± 0.02 b (−1.26) | 2.91 ± 0.01 a (1.28) | 2.90 ± 0.02 a (0.48) | 4.80 ± 0.03 a (−0.18) | 3.20 ± 0.03 a (−0.26) | 3.64 ± 0.17 a (6.64) | 1.67 ± 0.01 ab (1.27) | 0.93 ± 0.01 a (−11.07) | 38.35 ± 0.41 ab (0.60) |
| relative_chebyshev_dist (F2) | 31.82 ± 0.25 b | 2.49 ± 0.02 b (−0.37) | 3.16 ± 0.02 ab (−0.27) | 3.16 ± 0.02 ab (−0.27) | 5.31 ± 0.02 bc (−1.16) | 3.87 ± 0.04 c (−2.11) | 4.38 ± 0.61 a (10.62) | 1.76 ± 0.04 ab (0.98) | 1.00 ± 0.02 a (−12.59) | 41.12 ± 0.15 b (0.48) |
| relative_manhattan_dist (F3) | 36.42 ± 0.28 e | 2.45 ± 0.08 b (−5.47) | 3.13 ± 0.12 ab (−1.50) | 3.08 ± 0.14 ab (−1.03) | 5.18 ± 0.17 abc (−0.63) | 3.55 ± 0.11 b (3.25) | 4.62 ± 0.73 a (13.56) | 1.68 ± 0.03 ab (−1.27) | 0.90 ± 0.05 a (−11.67) | 38.91 ± 0.85 abc (1.11) |
| ratio_euclidean (F4) | 38.78 ± 0.05 f | 2.49 ± 0.04 b (1.81) | 3.51 ± 0.11 c (−1.21) | 3.10 ± 0.05 ab (3.66) | 5.55 ± 0.12 c (1.66) | 3.84 ± 0.09 c (2.77) | 3.58 ± 0.09 a (12.34) | 1.80 ± 0.03 b (5.93) | 1.34 ± 0.06 b (−9.40) | 40.61 ± 0.88 bc (2.69) |
| relative_manhattan_dist (F5) | 34.90 ± 0.31 d | 2.51 ± 0.10 b (6.96) | 3.24 ± 0.10 b (−6.64) | 3.13 ± 0.10 ab (−2.70) | 5.35 ± 0.20 bc (−5.03) | 3.63 ± 0.16 bc (−8.21) | 4.60 ± 0.44 a (7.73) | 1.71 ± 0.09 ab (0.69) | 0.90 ± 0.03 a (−5.24) | 40.81 ± 1.57 bc (−1.84) |
| euclidean_dist (F6) | 39.19 ± 0.50 f | 2.16 ± 0.03 a (−9.19) | 3.15 ± 0.02 ab (2.90) | 2.91 ± 0.04 a (−1.99) | 5.12 ± 0.08 ab (1.59) | 3.66 ± 0.06 bc (8.39) | 4.37 ± 0.50 a (16.41) | 1.61 ± 0.05 a (1.43) | 0.87 ± 0.02 a (−8.27) | 37.56 ± 0.46 a (3.18) |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| F-value | 227.77 | 124.34 | 82.96 | 75.80 | 62.52 | 15.20 | 38.15 | 104.98 | 431.81 | 28.90 |
Data are presented as the mean and standard deviation of three replicates.
Values bearing different lowercase letters under the same column were significantly different (p ≤ 0.05) according to Tukey's posthoc test.
Consequently, the change in lysine content indicated the extent of thermal treatments (Alonso et al., 2000). On the contrary, Cargo-Froom et al. (2022) explained that subjecting legumes to moisture, heat, and pressure during extrusion could make more sulphur-containing AA available in the sample, thus allowing more to be captured during AA digestion and analysis. Meanwhile, Osen et al. (2015) reported no significant change in amino acid contents and attributed this to the protective effect of feed moisture on amino acid as it can lower the shear stress and dispersion of mechanical energy in the extruder during the extrusion process.
3.3.2. Protein content and amino acid compositions of chicken breast meat and mixed protein matrices
Table 7 also describes the protein content and total AA composition (mg/100 mg protein) of chicken breast meat and selected extruded mixed protein matrices. All HMMA had significantly higher protein content than chicken breast meat. However, the total amount of essential and non-essential AA of the HMMA was still lower (Table 7). Due to the difference in peptide bond stability within the protein, acid hydrolysis of protein to amino acids was hypothesised to have not been completed (Chiang et al., 2021). Strong intermolecular bonds, such as disulphide and hydrophobic bonds, between proteins could hinder acid access to hydrolyse the protein backbones (Dai et al., 2019). Globulin, consisting of 7S and 11S oligomeric proteins, is the predominant protein fraction in legumes (Carbonaro and Nucara, 2022). While the ratio of 7S:11S present in legumes is dependent on species type, Wu et al. (2020) reported that a higher amount of 11S ratio resulted in protein aggregates with higher surface hydrophobicity and disulphide bond content. This could explain the higher resistance of the HMMA to acid hydrolysis compared to chicken.
Furthermore, cereal proteins (e.g. RPI) are deficient in lysine, whereas legume proteins are lacking in sulphur-containing AAs such as methionine and cysteine (Sá et al., 2020). These trends were reflected in Table 7, where F4 (containing RPI) had the least lysine content while F1, F2, F3, F5, and F6 (all containing mainly legume proteins) had much lower methionine content than F4 and chicken breast meat. This suggests that while mathematical programming could optimise the AA profile of HMMA by generating plant protein combinations, it is still incomparable to animal proteins as plant proteins are inherently lower in some of the essential AA contents.
The sum of essential and non-essential AA of the HMMA ranged between 60.84 and 66.25 mg/100 mg protein, with F1 and F2 having the lowest and highest values, respectively (Table 7). As mentioned previously, the amount of globulin present is one factor that might improve intermolecular bonding between proteins due to higher surface hydrophobicity and disulphide bond content. Soy, pea, chickpea, and mung bean protein comprise around 90%, 65%–80%, 33.11%–52.16% and 62% globulin, respectively. As such, F1, which contains mostly SPC, might have the highest globulin content and, thus, the highest surface hydrophobicity and disulphide bond content. This might have provided F1 with the highest resistance to hydrolysis. Conversely, F2, which contains chickpea protein, likely has the least globulin and weaker intermolecular bonds.
Deficiencies in essential AA could reduce the nutritional values of plant crops to 50–75% compared to a diet comprising a balanced level of AA (Galili and Amir, 2013). The lack of some essential AA such as leucine, lysine, and methionine might have contributed to lower anabolic properties of plant proteins. In the presence of a limiting essential AA, the remaining AA will not be rightly used for protein synthesis but instead will be deaminated, oxidised, and irreversibly eliminated (Berrazaga et al., 2019). Thus, in this case, utilising different combinations of plant protein and then fortifying formulations with free AA is one practical approach to help improve the anabolic properties of plant-based products (Kouw et al., 2022). Kouw et al. (2022) reported in a study involving 24 healthy and young male participants that ample consumption of protein (40 g) in the form of a lysine-enriched plant protein product increased muscle protein synthesis rates to a similar extent as an isonitrogenous amount of chicken. Likewise, Engelen et al. (2007) demonstrated that compared to a soy diet, supplementation of branched-chain AA (i.e., valine, isoleucine, and leucine) to the soy meal reduced splanchnic protein synthesis, while improving peripheral (e.g., muscle) protein synthesis in healthy elderly.
3.3.3. Visual observation and colour analysis
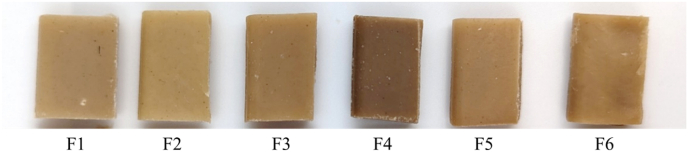
Fig. 1.
Visual images of selected extruded mixed protein matrices.
Table 8.
Colourimetric values, moisture content, pH and specific mechanical energy (SME) of selected extruded mixed protein matrices.
| Optimisation criterion (sample) | L* | a* | b* | Moisture (%) | pH | SME (kJ/kg) |
|---|---|---|---|---|---|---|
| ratio_cos_sim (F1) | 48.95 ± 0.95 c | 1.04 ± 0.01 a | 13.30 ± 0.35 a | 52.51 ± 1.08 | 6.96 ± 0.02 b | 133.60 ± 8.17 d |
| relative_chebyshev_dist (F2) | 53.93 ± 0.73 d | 1.55 ± 0.23 b | 18.16 ± 0.40 c | 53.93 ± 1.73 | 6.98 ± 0.03 b | 87.64 ± 7.96 a |
| relative_manhattan_dist (F3) | 48.15 ± 0.69 c | 2.44 ± 0.26 c | 16.36 ± 0.15 b | 51.18 ± 1.06 | 7.29 ± 0.01 d | 119.55 ± 8.03 c |
| ratio_euclidean (F4) | 44.29 ± 0.35 a | 4.36 ± 0.06 e | 14.27 ± 0.44 a | 52.02 ± 0.76 | 6.84 ± 0.02 a | 120.91 ± 8.39 c |
| relative_manhattan_dist (F5) | 48.53 ± 0.49 c | 3.40 ± 0.06 d | 16.38 ± 0.46 b | 53.51 ± 0.68 | 7.09 ± 0.02 c | 107.14 ± 5.86 b |
| euclidean_dist (F6) | 46.26 ± 0.64 b | 4.54 ± 0.08 e | 18.19 ± 0.36 c | 51.51 ± 0.62 | 7.09 ± 0.02 c | 101.89 ± 0.00 b |
| p-value | 0.000 | 0.000 | 0.000 | 0.050 | 0.000 | 0.000 |
| F-value | 70.78 | 288.75 | 83.35 | 3.22 | 212.10 | 65.98 |
Data are presented as the mean and standard deviation of three replicates.
Values bearing different lowercase letters under the same column were significantly different (p ≤ 0.05) according to Tukey's posthoc test.
All HMMA appeared to be brown (Fig. 1). Among the six formulations, F4 was observed to be the darkest in appearance (Fig. 1), while its L* value was the lowest (Table 8). Their a* and b* values were significantly higher and lower, respectively. While the extrusion process could result in colour changes due to the Maillard reaction, Hwang et al. (2011) showed that browning intensity could be affected by the type of AA and reducing sugar present. Sugar systems containing alanine, tryptophan, and asparagine resulted in Maillard reaction products with the highest browning intensity (Hwang et al., 2011). Indeed, RPI, present in F4, had the highest protein and alanine content (Table 4). Rice protein was also known to be hydrophobic and rich in amide-containing amino acids such as asparagine (Paraman et al., 2007).
3.3.4. Moisture content and pH
Table 8 describes the moisture content and pH of the HMMA. Although the moisture content of the feed was set to 55% w/w, moisture was still lost to a limited extent during the extrusion process. Moisture loss could have occurred along the extrusion flow or when the extrudate exited the cooling die. The amount of water vapour flashed off at the cooling die upon exit of the extrudate was dependent on the amount of water vapour entrapped in the starch matrix of the mixture (Mohamad Mazlan et al., 2019). Nevertheless, the moisture content of the extrudates between F1 and F6 was not significantly different (Table 8).
The pH of the extrudates ranged from 6.84 to 7.29, with F4 and F3 having the lowest and highest pH values, respectively (Table 8). The results found in this study were similar to the pH range (6.98–7.13) reported by Chiang et al. (2019).
3.3.5. Specific mechanical energy (SME)
SME represents the energy transferred to the feed material during extrusion and can be used to establish extrusion conditions (Feng and Lee, 2014). It assists in the optimisation of process conditions to produce better quality extruded products while improving energy efficiency (Kantrong et al., 2018). The SME during the extrusion of F1 to F6 was calculated and reported in Table 8.
Among the six formulations, SME was the highest and lowest during the extrusion of F1 and F2, respectively (Table 8). SME can be affected by factors such as screw speed, barrel temperature, and the characteristics of the raw materials (Kantrong et al., 2018). Process conditions like rotational screw speed and temperature were kept constant during the extrusion process in this study. This suggested that using different raw materials (i.e., plant protein powders) at different water feeds (to achieve approximately 55% moisture content) significantly affects the SME value. A protein dough could form when protein and water were mixed in the extruder due to protein-water interactions and/or protein-water-protein interactions (Lee et al., 2022). Lee et al. (2022) elaborated that due to the lower water affinity of RPI than soy protein isolate (SPI), replacing SPI with RPI lowered the dough's elasticity and increased the mixture's mass flow rate. As a result, lower screw force was used, and the residence time of the mixture in the barrel was shortened. This signified that the mixture was subjected to a lower amount of mechanical energy while the extent of protein denaturation was limited, causing a lower level of texturisation in the extrudate.
4. Conclusion
For producing HMMA via the extrusion process, mathematical optimisation was used to generate combinations of plant protein powders at different compositions (F1 – F6) to mimic the AA composition of chicken breast meat. Slight discrepancies in AA content between the calculated and actual HMMA values existed as the extrusion process could have influenced the amount of AA available for capture and analysis. As plant proteins naturally contain less of some essential AA, the resulting HMMA produced still had lower AA contents than chicken. Strong intermolecular bonds (e.g., disulphide and hydrophobic bonds) present in the globulin fraction might also have hindered the completion of protein acid hydrolysis during AA composition analysis, lowering the AA content detected in the HMMA compared to chicken breast meat. The use of different plant proteins also affected the final product's colour; F4, containing RPI, appeared the darkest. This was likely due to the high alanine and asparagine content that could form Maillard reaction products with the highest browning intensity. Lastly, SME values were mainly altered by the plant proteins' characteristics, as differences in water affinity could affect the dough's elasticity, screw force used and residence time of the mixture in the extruder barrel. To conclude, this study demonstrated the possibility of using mathematical programming to generate optimised plant protein combinations that best matched an animal source, such as chicken breast meat. To further improve the AA composition of HMMA, fortifying formulations with free AA could be employed. The structural, textural and chemical properties of the HMMA derived in this study will be explored in subsequent work.
CRediT authorship contribution statement
Xin Yi Hua: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Yushen Long: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Dayna Shu Min Ong: Methodology, Formal analysis, Investigation. Alicia Hui Ping Theng: Methodology, Formal analysis, Investigation. Jing K. Shi: Methodology, Formal analysis, Investigation. Raffael Osen: Writing – review & editing. Min Wu: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition. Jie Hong Chiang: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was funded by the A*STAR BMRC (Biomedical Research Council) on SFS-2 IAF-PP Future Foods: Alternative Proteins: H20H8a002 awarded to Jie Hong Chiang and Min Wu. The authors thank Professor Christiani Jeyakumar Henry and Dr Sumanto Haldar for their input and support.
Handling Editor: Professor A.G. Marangoni
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2023.100648.
Contributor Information
Min Wu, Email: wumin@i2r.a-star.edu.sg.
Jie Hong Chiang, Email: chiang_jie_hong@sifbi.a-star.edu.sg.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Andersson J.A.E., Gillis J., Horn G., Rawlings J.B., Diehl M. CasADi – a software framework for non-linear optimization and optimal control. Mathematical Programming Computation. 2019;11(1):1–36. doi: 10.1007/s12532-018-0139-4. [DOI] [Google Scholar]
- Alonso R., Grant G., Dewey P., Marzo F. Nutritional assessment in vitro and in vivo of raw and extruded peas (Pisum sativum L.) J. Agric. Food Chem. 2000;48(6):2286–2290. doi: 10.1021/jf000095o. [DOI] [PubMed] [Google Scholar]
- Barkholt V., Jensen A.L. Amino acid analysis: determination of cysteine plus half-cystine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal. Biochem. 1989;177(2):318–322. doi: 10.1016/0003-2697(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Bedoya M.G., Montoya D.R., Tabilo-Munizaga G., Pérez-Won M., Lemus-Mondaca R. Promising perspectives on novel protein food sources combining artificial intelligence and 3D food printing for food industry. Trends Food Sci. Technol. 2022;128:38–52. doi: 10.1016/j.tifs.2022.05.013. [DOI] [Google Scholar]
- Berrazaga I., Micard V., Gueugneau M., Walrand S. The role of the anabolic properties of plant- versus animal-based protein sources in supporting muscle mass maintenance: a critical review. Nutrients. 2019;11(8) doi: 10.3390/nu11081825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro M., Nucara A. Legume proteins and peptides as compounds in nutraceuticals: a structural basis for dietary health effects. Nutrients. 2022;14(6) doi: 10.3390/nu14061188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargo-Froom C.L., Newkirk R.W., Marinangeli C.P.F., Shoveller A.K., Ai Y., Columbus D.A. The effects of extrusion on nutrient content of Canadian pulses with a focus on protein and amino acids. Can. J. Anim. Sci. 2022;103(1):44–58. doi: 10.1139/cjas-2022-0088. [DOI] [Google Scholar]
- Chiang J.H., Loveday S.M., Hardacre A.K., Parker M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019;19 doi: 10.1016/j.foostr.2018.11.002. [DOI] [Google Scholar]
- Chiang J.H., Tay W., Ong D.S.M., Liebl D., Ng C.P., Henry C.J. Physicochemical, textural and structural characteristics of wheat gluten-soy protein composited meat analogues prepared with the mechanical elongation method. Food Struct. 2021;28 doi: 10.1016/j.foostr.2021.100183. [DOI] [Google Scholar]
- Choton S., Gupta N., Bandral J.D., Anjum N., Choudary A. Extrusion technology and its application in food processing: a review. Pharm. Innov. 2020;9(2):162–168. [Google Scholar]
- Colombo S.M., Mazal X. Investigation of the nutritional composition of different types of salmon available to Canadian consumers. Journal of Agriculture and Food Research. 2020;2 doi: 10.1016/j.jafr.2020.100056. [DOI] [Google Scholar]
- Dai L., Reichert C.L., Hinrichs J., Weiss J. Acid hydrolysis behavior of insoluble protein-rich fraction extracted from Chlorella protothecoides. Colloids Surf. A Physicochem. Eng. Asp. 2019;569:129–136. doi: 10.1016/j.colsurfa.2019.02.064. [DOI] [Google Scholar]
- Daszkiewicz T., Bąk T., Denaburski J. Quality of pork with a different intramuscular fat (IMF) content. Pol. J. Food Nutr. Sci. 2005;55(1):31–36. [Google Scholar]
- Day L. Proteins from land plants – potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013;32(1):25–42. doi: 10.1016/j.tifs.2013.05.005. [DOI] [Google Scholar]
- De Marchi M., Costa A., Pozza M., Goi A., Manuelian C.L. Detailed characterization of plant-based burgers. Sci. Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-81684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelen M.P.K.J., Rutten E.P.A., De Castro C.L.N., Wouters E.F.M., Schols A.M.W.J., Deutz N.E.P. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am. J. Clin. Nutr. 2007;85(2):431–439. doi: 10.1093/ajcn/85.2.431. [DOI] [PubMed] [Google Scholar]
- Feng Y., Lee Y. Effect of specific mechanical energy on digestion and physical properties of extruded rice-based snacks. Food Nutr. Sci. 2014;5:1818–1827. doi: 10.4236/fns.2014.519196. [DOI] [Google Scholar]
- Galili G., Amir R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality. Plant Biotechnol. J. 2013;11(2):211–222. doi: 10.1111/pbi.12025. [DOI] [PubMed] [Google Scholar]
- García-Leal J., Espinoza Pérez A.T., Vásquez Ó.C. Towards the sustainable massive food services: an optimization approach. Soc. Econ. Plann. Sci. 2023;87 doi: 10.1016/j.seps.2023.101554. [DOI] [Google Scholar]
- Gasparre N., van den Berg M., Oosterlinck F., Sein A. High-moisture shear processes: molecular changes of wheat gluten and potential plant-based proteins for its replacement. Molecules. 2022;27(18) doi: 10.3390/molecules27185855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorissen S.H.M., Crombag J.J.R., Senden J.M.G., Waterval W.A.H., Bierau J., Verdijk L.B., van Loon L.J.C. Protein content and amino acid composition of commercially available plant-based protein isolates. Amino Acids. 2018;50(12):1685–1695. doi: 10.1007/s00726-018-2640-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni C., Chiarelli D.D., Luciano A., Ottoboni M., Perpelek S.N., Pinotti L., Rulli M.C. Global assessment of natural resources for chicken production. Adv. Water Resour. 2021;154 doi: 10.1016/j.advwatres.2021.103987. [DOI] [Google Scholar]
- Hassoun A., Aït-Kaddour A., Abu-Mahfouz A.M., Rathod N.B., Bader F., Barba F.J.…Regenstein J. The fourth industrial revolution in the food industry—Part I: industry 4.0 technologies. Crit. Rev. Food Sci. Nutr. 2022:1–17. doi: 10.1080/10408398.2022.2034735. [DOI] [PubMed] [Google Scholar]
- He J., Evans N.M., Liu H., Shao S. A review of research on plant-based meat alternatives: driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020;19(5):2639–2656. doi: 10.1111/1541-4337.12610. [DOI] [PubMed] [Google Scholar]
- Henderson J.W., Brooks A. Retrieved from Agilent Technologies, Inc.; USA: 2010. Improved Amino Acid Methods Using Agilent ZORBAX Eclipse Plus C18 Columns for a Variety of Agilent LC Instrumentation and Separation Goals.https://www.agilent.com/cs/library/applications/5990-4547EN.pdf [Google Scholar]
- Henderson J.W., Ricker R.D., Bidlingmeyer B.A., Woodward C. Retrieved from Agilent Technologies, Inc.; USA: 2000. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids. Amino Acid Analysis Using Zorbax Eclipse-AAA Columns and the Agilent 1100 HPLC.https://www.agilent.com/cs/library/chromatograms/59801193.pdf [Google Scholar]
- Herreman L., Nommensen P., Pennings B., Laus M.C. Comprehensive overview of the quality of plant- and animal-sourced proteins based on the digestible indispensable amino acid score. Food Sci. Nutr. 2020;8(10):5379–5391. doi: 10.1002/fsn3.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G.E., Kim J.H., Ahn S.J., Lee C.H. Changes in meat quality characteristics of the sous-vide cooked chicken breast during refrigerated storage. Korean J Food Sci Anim Resour. 2015;35(6):757–764. doi: 10.5851/kosfa.2015.35.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I.G., Kim H.Y., Woo K.S., Lee J., Jeong H.S. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011;126(1):221–227. doi: 10.1016/j.foodchem.2010.10.103. [DOI] [Google Scholar]
- Jensen I.-J., Eilertsen K.-E., Otnæs C.H., Mæhre H.K., Elvevoll E.O. An update on the content of fatty acids, dioxins, PCBs and heavy metals in farmed, escaped and wild atlantic salmon (Salmo salar L.) in Norway. Foods. 2020;9(12) doi: 10.3390/foods9121901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrong H., Charunuch C., Limsangouan N., Pengpinit W. Influence of process parameters on physical properties and specific mechanical energy of healthy mushroom-rice snacks and optimization of extrusion process parameters using response surface methodology. J. Food Sci. Technol. 2018;55(9):3462–3472. doi: 10.1007/s13197-018-3271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesse-Guyot E., Allès B., Brunin J., Fouillet H., Dussiot A., Mariotti F.…Baudry J. Nutritionally adequate and environmentally respectful diets are possible for different diet groups: an optimized study from the NutriNet-Santé cohort. Am. J. Clin. Nutr. 2022;116(6):1621–1633. doi: 10.1093/ajcn/nqac253. [DOI] [PubMed] [Google Scholar]
- Kouw I.W.K., Pinckaers P.J.M., Le Bourgot C., van Kranenburg J.M.X., Zorenc A.H., de Groot L.C.P.G.M.…van Loon L.J.C. Ingestion of an ample amount of meat substitute based on a lysine-enriched, plant-based protein blend stimulates postprandial muscle protein synthesis to a similar extent as an isonitrogenous amount of chicken in healthy, young men. Br. J. Nutr. 2022;128(10):1955–1965. doi: 10.1017/S0007114521004906. [DOI] [PubMed] [Google Scholar]
- Kumar S. Meat Analogs “Plant based alternatives to meat products: their production technology and applications”. Crit. Rev. Food Sci. Nutr. 2016 doi: 10.1080/10408398.2016.1196162. 00-00. [DOI] [PubMed] [Google Scholar]
- Lee J.-S., Oh H., Choi I., Yoon C.S., Han J. Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT. 2022;157 doi: 10.1016/j.lwt.2021.113056. [DOI] [Google Scholar]
- Leonard W., Zhang P., Ying D., Fang Z. Application of extrusion technology in plant food processing byproducts: an overview. Compr. Rev. Food Sci. Food Saf. 2020;19(1):218–246. doi: 10.1111/1541-4337.12514. [DOI] [PubMed] [Google Scholar]
- Ma K.K., Greis M., Lu J., Nolden A.A., McClements D.J., Kinchla A.J. Functional performance of plant proteins. Foods. 2022;11(4) doi: 10.3390/foods11040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad Mazlan M., Talib R.A., Mail N.F., Taip F.S., Chin N.L., Sulaiman R.…Mohd Nor M.Z. Effects of extrusion variables on corn-mango peel extrudates properties, torque and moisture loss. Int. J. Food Prop. 2019;22(1):54–70. doi: 10.1080/10942912.2019.1568458. [DOI] [Google Scholar]
- Monnet A.-F., Laleg K., Michon C., Micard V. Legume enriched cereal products: a generic approach derived from material science to predict their structuring by the process and their final properties. Trends Food Sci. Technol. 2019;86:131–143. doi: 10.1016/j.tifs.2019.02.027. [DOI] [Google Scholar]
- Nadeesha Dilrukshi H.N., Torrico D.D., Brennan M.A., Brennan C.S. Effects of extrusion processing on the bioactive constituents, in vitro digestibility, amino acid composition, and antioxidant potential of novel gluten-free extruded snacks fortified with cowpea and whey protein concentrate. Food Chem. 2022;389 doi: 10.1016/j.foodchem.2022.133107. [DOI] [PubMed] [Google Scholar]
- Osen R. Technische Universität München. 2017. Texturization of pea protein isolates using high moisture extrusion cooking.https://mediatum.ub.tum.de/1356359 [Google Scholar]
- Osen R., Toelstede S., Eisner P., Schweiggert-Weisz U. Effect of high moisture extrusion cooking on protein–protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015;50(6):1390–1396. doi: 10.1111/ijfs.12783. [DOI] [Google Scholar]
- Paraman I., Hettiarachchy N.S., Schaefer C. Glycosylation and deamidation of rice endosperm protein for improved solubility and emulsifying properties. Cereal Chem. 2007;84(6):593–599. doi: 10.1094/CCHEM-84-6-0593. [DOI] [Google Scholar]
- Purchas R.W., Wilkinson B.H.P., Carruthers F., Jackson F. A comparison of the nutrient content of uncooked and cooked lean from New Zealand beef and lamb. J. Food Compos. Anal. 2014;35(2):75–82. doi: 10.1016/j.jfca.2014.04.008. [DOI] [Google Scholar]
- Sá A.G.A., Moreno Y.M.F., Carciofi B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020;97:170–184. doi: 10.1016/j.tifs.2020.01.011. [DOI] [Google Scholar]
- Schuster R. Determination of amino acids in biological, pharmaceutical, plant and food samples by automated precolumn derivatization and high-performance liquid chromatography. J Chromatogr. 1988;431(2):271–284. doi: 10.1016/s0378-4347(00)83096-0. [DOI] [PubMed] [Google Scholar]
- Sheibani E., Dabbagh Moghaddam A., Sharifan A., Afshari Z. Linear programming: an alternative approach for developing formulations for emergency food products. J. Sci. Food Agric. 2018;98(4):1444–1452. doi: 10.1002/jsfa.8612. [DOI] [PubMed] [Google Scholar]
- Sprague M., Fawcett S., Betancor M.B., Struthers W., Tocher D.R. Variation in the nutritional composition of farmed Atlantic salmon (Salmo salar L.) fillets with emphasis on EPA and DHA contents. J. Food Compos. Anal. 2020;94 doi: 10.1016/j.jfca.2020.103618. [DOI] [Google Scholar]
- Szenderák J., Fróna D., Rákos M. Consumer acceptance of plant-based meat substitutes: a narrative review. Foods. 2022;11(9) doi: 10.3390/foods11091274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpicer A., Onopiuk A., Barczak M., Kurek M. The optimization of a gluten-free and soy-free plant-based meat analogue recipe enriched with anthocyanins microcapsules. LWT. 2022;168 doi: 10.1016/j.lwt.2022.113849. [DOI] [Google Scholar]
- Tan T., Abbas F., Azhar M. Characterization of the ribose-induced maillard reaction in minced chicken and minced pork: a potential means of species differentiation. Int. Food Res. J. 2012;19(2) [Google Scholar]
- Torrissen O., Olsen R.E., Toresen R., Hemre G.I., Tacon A.G.J., Asche F.…Lall S. Atlantic salmon (Salmo salar): the “Super-Chicken” of the sea? Rev. Fish. Sci. 2011;19(3):257–278. doi: 10.1080/10641262.2011.597890. [DOI] [Google Scholar]
- Tougan P.U., Dahouda M., Salifou C.F.A., Ahounou S.G.A., Kpodekon M.T., Mensah G.A.…Karim I. Conversion of chicken muscle to meat and factors affecting chicken meat quality: a review. Int. J. Appl. Agric. Res. 2013;3(8):1–20. [Google Scholar]
- van Dooren C. A review of the use of linear programming to optimize diets, nutritiously, economically and environmentally. Front. Nutr. 2018;5 doi: 10.3389/fnut.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Delgado L.H., González-Mondragón E.G., Ramírez-Andrade J., Salazar-Govea A.Y., Santiago-Castro J.T. Oxidative stability in raw, cooked, and frozen ground beef using Epazote (Chenopodium ambrosioides L.) Meat Sci. 2020;168 doi: 10.1016/j.meatsci.2020.108187. [DOI] [PubMed] [Google Scholar]
- Witte F., Sawas E., Berger L.M., Gibis M., Weiss J., Röser A.…Terjung N. Influence of finely chopped meat addition on quality parameters of minced meat. Appl. Sci. 2022;12(20) doi: 10.3390/app122010590. [DOI] [Google Scholar]
- Wu C., Wang J., Yan X., Ma W., Wu D., Du M. Effect of partial replacement of water-soluble cod proteins by soy proteins on the heat-induced aggregation and gelation properties of mixed protein systems. Food Hydrocolloids. 2020;100 doi: 10.1016/j.foodhyd.2019.105417. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.