Abstract
Context.
Rapid onsite evaluation (ROSE) is critical in determining sample adequacy and triaging cytology samples. Although fine-needle aspiration biopsy (FNAB) is the primary method of initial tissue sampling in Tanzania, ROSE is not practiced.
Objective.
To investigate the performance of ROSE in determining cellular adequacy and providing preliminary diagnoses in breast FNAB in a low-resource setting.
Design.
Patients with breast masses were recruited prospectively from the FNAB clinic at Muhimbili National Hospital. Each FNAB was evaluated by ROSE for overall specimen adequacy, cellularity, and preliminary diagnosis. The preliminary interpretation was compared to the final cytologic diagnosis and histologic diagnosis, when available.
Results.
Fifty FNAB cases were evaluated, and all were adequate for diagnosis on ROSE and final interpretation. Overall percentage of agreement (OPA) between preliminary and final cytologic diagnosis was 86%, positive percentage of agreement (PPA) was 36%, and negative percentage of agreement (NPA) was 100% (κ = 0.5, P < .001). Twenty-one cases had correlating surgical resections. OPA between preliminary cytologic and histologic diagnoses was 67%, PPA was 22%, and NPA was 100% (κ = 0.2, P = .09). OPA between final cytologic and histologic diagnoses was 95%, PPA of 89%, and NPA of 100% (κ = 0.9, P = <.001).
Conclusions.
False positive rates of ROSE diagnoses for breast FNAB are low. While preliminary cytologic diagnoses had a high false negative rate, final cytologic diagnoses had overall high concordance with histologic diagnoses. Therefore, the role of ROSE for preliminary diagnosis should be considered carefully in low-resource settings and may need to be paired with additional interventions to improve pathologic diagnosis.
Breast cancer (BC) is the second leading cause of cancer-related mortality among women in Tanzania, with a majority of patients (63%–84%) presenting with advanced disease.1-4 Tanzania has a hierarchical health care structure in which the vast majority of specialized cancer care, including pathology, is concentrated in a few referral hospitals and select zonal hospitals, leading to significant delays in cancer diagnosis and treatment.5,6 Critically, BC survival rates begin to erode when delays to treatment are greater than 3 months.7 While core needle biopsy has increasingly replaced fine-needle aspiration biopsy (FNAB) in high-resource settings, this service is prohibitively expensive and not readily available in many resource-constrained settings. Therefore, there is an urgent need to evaluate techniques that have potential to increase access to and expedite BC diagnosis, such as FNAB and rapid onsite evaluation (ROSE), and to demonstrate their feasibility and relevance in low-resource settings.
FNAB is a minimally invasive and cost-effective tissue sampling method that has been successfully deployed in resource-constrained settings and has been demonstrated to have high sensitivity rates (92%–98%) and positive predictive values (95%–100%) in diagnosing palpable malignant breast masses.8-14 One major advantage of FNAB is the ability to perform ROSE. ROSE is a technique that allows a pathologist to check the cellularity and adequacy of FNAB samples, and it has been shown to improve adequacy rates and reduce the number of passes; it also allows for triage and collection for ancillary studies, such as cultures, flow cytometry, or immunohistochemistry.12,15-17 In addition, when ROSE is performed by a trained pathologist, a preliminary diagnosis can be rendered at the bedside, which helps to optimize patient triage and expedite referrals for only those who need additional specialized cancer workup or care but also reduces the number of repeat procedures.15,16 A major limitation of FNAB and ROSE is that diagnostic accuracy is contingent on formal training in sampling technique and highly dependent on the skills of the operator, and requires access to a microscope.18 Although numerous studies have evaluated the impact of ROSE on adequacy rates,12,15-17 few studies have evaluated the use of ROSE in resource-constrained settings.19 Therefore, we investigated the performance of ROSE in determining cellular adequacy and providing preliminary diagnoses in breast FNAB in a low-resource setting.
MATERIALS AND METHODS
Study Setting and Design
We performed a prospective study of patients presenting to the FNAB clinic at Muhimbili National Hospital (MNH) in Dar es Salaam, Tanzania, with a palpable breast lump from February 2020 to June 2021. Patients were recruited consecutively. MNH is the largest national referral hospital and is the public teaching hospital for Muhimbili University of Health and Allied Sciences (MUHAS) in Tanzania. All suspected cancer cases referred to MNH must be evaluated for pathologic confirmation by a pathologist at the MNH Central Pathology Laboratory prior to referral for treatment. MNH has a well-established FNAB clinic where more than 50 patients are seen weekly. Prior to this study, ROSE and specimen triage for ancillary testing was not routinely performed at any medical facility in Tanzania. The study received approvals from the institutional review boards at MUHAS (DA.287/298/01.A/) and the University of California, San Francisco (UCSF) (17-22963).
Study Population
All patients aged 18 years or older who were referred to the MNH FNAB clinic with a palpable breast mass were invited to participate. Patients who were pregnant or lactating, had a prior diagnosis of BC, or had bilateral masses were ineligible. The aims of the study, risks and benefits of participation, and sampling procedure were explained by a clinical research coordinator and a pathology resident. Patients were provided with written informed consent in Swahili, the native language of Tanzania. Clinical data were collected in the FNAB clinic by a clinical research coordinator using a data collection sheet, which included age, sex, mass laterality, and clinical tumor size.
Training in FNAB and ROSE
All 4 study pathologists who performed FNAB and ROSE completed a 3-year residency program in anatomic pathology at MUHAS. During residency, the trainees each performed more than 600 palpation-guided FNABs in clinic under the supervision of an attending pathologist and more than 100 image-guided FNABs, which were jointly performed by a radiologist and pathologist. All study pathologists also participated in 2 intensive ultrasound-guided FNAB workshops, which were hosted by pathologists and radiologists from MUHAS and UCSF in 2017 and 2019 in Dar es Salaam, Tanzania.20 The workshops were initially led by cytopathologists and breast radiologists from UCSF. The second workshop included not only UCSF faculty, but also 3 MUHAS pathologists as instructors. One of the study pathologists participated in an intensive 1-month supervised rotation in the UCSF Department of Pathology, where she refined her skills in ROSE, and observed FNAB by cytopathology experts. The remaining pathologists received a 3-day hands-on training on ROSE in the MNH FNAB clinic led by a UCSF faculty member prior to the launch of the study. Because of travel restrictions related to the COVID-19 pandemic, follow-up trainings with an observed competency assessment could not be conducted. The pathologist who received intensive training at UCSF assisted the other study pathologists with ROSE when needed.
Toluidine Blue Stain
Toluidine blue was selected for rapid staining after a situational analysis of the cytology workflow at MNH by the study pathologists. MNH primarily uses alcohol-fixed smears for cytologic diagnosis and does not use air-dried smears. Toluidine blue was also selected for its speed (staining time of approximately 5 seconds), and low cost.21-24 Toluidine blue stain was prepared in the cytology laboratory by mixing 0.5 g of toluidine blue O technical-grade powder (Sigma-Aldrich Inc, catalog number T3260-25G), 20 mL of 95% ethanol, and 80 mL of distilled water. The toluidine blue solution was then aliquoted into a plastic dropper bottle for use in the clinic.
Specimen Acquisition
FNAB of the breast masses were performed by a pathologist using a 23-gauge needle and a 10-mL syringe holder, and the initial aspirate was used to prepare 4 direct smears fixed in 95% ethanol. One slide was selected for ROSE, stained using 2 or 3 drops of toluidine blue and then cover-slipped and blotted dry. After ROSE, the coverslip was removed from the slides, which were reimmersed into 95% ethanol. If the sample was considered insufficient for diagnosis, then repeat passes were performed. Overall sample adequacy, cellularity, and a preliminary diagnosis were documented for each case. A sample was considered adequate if the pathologist performing the FNAB and ROSE felt that the sample had sufficient tissue to render a diagnosis and corresponded to their clinical breast examination. Each case was then categorized as nondiagnostic, benign, indeterminate, suspicious for malignancy, or malignant. All smears were then submitted for Papanicolaou staining and routine diagnosis as per MNH protocol.
Cytologic and Histologic Diagnosis
The cases were reviewed by the pathologist who performed the FNAB and a final cytologic diagnosis was rendered. The final cytologic diagnoses were then compared to the preliminary diagnoses. For cases in which the preliminary and final cytologic diagnoses were discordant, the slides were reviewed by 2 study pathologists, and the reason for discordance was documented. A subset of patients received surgical excision of the breast mass. In these cases, the correlating preliminary cytologic diagnosis, final cytologic diagnosis, and histologic diagnosis were compared. For cases in which there was a discordance between diagnoses, the FNAB slides and surgical excision hematoxylin-eosin slides were reviewed by 2 study pathologists. Of note, the International Academy of Cytology Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology is not currently part of standard procedures at MNH.12
Chart Review
To capture any potential nondiagnostic samples and to determine the performance characteristics of FNAB on breast masses without ROSE, we conducted a review of the pathology database for 100 consecutive FNABs at MNH from February 2022 to December 2022. Cases from patients younger than 18 years or with bilateral masses were excluded, leaving 91 eligible cases. Data on the patient’s age and sex, mass laterality, clinical tumor size, cellularity of the smears, the final cytologic diagnosis, and any corresponding surgical resections were documented.
We decided to select a timeframe during 2022 for several reasons. Prior to February 2020, the pathology department did not render “nondiagnostic” interpretations for FNAB samples. Instead, if the sample was acellular or consisted of only blood, then the case was held until the patient returned for a repeat FNAB and a diagnosis was rendered based on the repeat sample. However, during spring 2020, the anatomic pathology department began a quality improvement initiative to improve turnaround times for all pathology samples. Therefore, pathologists stopped holding cases and were willing to categorize FNABs as nondiagnostic. In addition, ROSE was discontinued for all cases after June 2021, after the pilot period ended, and routine clinical procedures resumed. The primary differences between the study procedures and MNH’s routine clinical practice is that only a single FNAB pass is performed unless a lesion is cystic, in which a second pass is performed after a single attempt at drainage. For every case, 3 direct smears are made, fixed in 95% ethanol, and stained with Papanicolaou stain. Aside from the 4 study pathologists, an additional 4 staff pathologists who did not receive study-specific training performed FNABs and signed out cytopathology cases at MNH. Of note, the criteria at MNH for rendering a nondiagnostic diagnosis is not well defined and is up to the discretion of the pathologist. It may include cases that are acellular or consist of only blood, or cases in which the sample did not correlate with the clinical presentation or physical examination.
Statistical Analysis
Deidentified data were electronically transcribed from the data collection sheets and stored in a secure web-based REDCap database. Frequency distribution of socio-demographic, clinico-pathological variables, ROSE, and final cytologic and histologic diagnoses were assessed when available. The relationship between specimen cellularity and the use of ROSE was assessed using the Fisher exact test. Measure of agreement was determined using the Cohen κ coefficient. Performance characteristics, including positive percentage of agreement (PPA), negative percentage of agreement (NPA), and overall percentage of agreement (OPA) were evaluated with their 95% confidence interval (CI). All the statistical analyses were performed using the statistical computing software SPSS, version 28 (SPSS Inc, Chicago, Illinois).
RESULTS
A total of 50 patients were recruited from the FNAB clinic at MNH and had ROSE performed. A majority of patients were female (48 of 50, 96%), and the median age was 33 years (range, 18 to 75 years old). Most of the patients presented with masses that ranged from 2 cm to 5 cm in greatest diameter (37 of 50, 74%) (Table 1). During ROSE, the FNAB samples were assessed for specimen adequacy, cellularity, and preliminary diagnosis. All samples were adequate for diagnosis, with most cases showing high cellularity (27 of 50, 54%) and only a few with low cellularity (4 of 50, 8%) (Table 1). For the FNAB cases performed without ROSE, most of the patients were female (86 of 91, 94%), and the median age was 41 years (range, 18 to 89 years old). Most patients presented with a mass that was less than 2 cm (69 of 91, 75%) (Table 1).
Table 1.
Characteristics of Study Population and Specimen Cellularity
| Characteristic | FNAB Performed With ROSE (N = 50), No. (%) |
FNAB Performed Without ROSE (N = 91), No. (%) |
|---|---|---|
| Sex | ||
| Male | 2 (4) | 5 (6) |
| Female | 48 (96) | 86 (94) |
| Age groups | ||
| <35 | 31 (62) | 34 (37) |
| >35 to ≤49 | 8 (16) | 27 (30) |
| >50 | 11 (22) | 30 (33) |
| Laterality | ||
| Left | 29 (58) | 42 (46) |
| Right | 21 (42) | 49 (54) |
| Estimated clinical tumor size | ||
| <2 cm | 2 (4) | 8 (9) |
| >2 cm to ≤5 cm | 37 (74) | 35 (38) |
| >5 cm | 11 (22) | 34 (37) |
| Unknown | — | 14 (16) |
| FNAB specimen cellularitya | ||
| Low | 4 (8) | 14 (16) |
| Moderate | 19 (38) | 42 (46) |
| High | 27 (54) | 32 (35) |
| Unknown | — | 3 (3) |
Abbreviations: FNAB, fine-needle aspiration biopsy; ROSE, rapid onsite evaluation.
P = .10.
For FNAB with ROSE, among preliminary diagnoses, 46 of 50 cases (92%) were reported as benign on ROSE, and 4 of 50 cases (8%) were reported as malignant and/or suspicious for malignancy. The distribution of preliminary cytologic diagnoses is presented in Table 2. After final cytologic review, 38 of 50 cases (76%) were categorized as benign, 2 of 50 cases (4%) as atypical, and 10 of 50 cases (20%) as malignant and/or suspicious for malignancy. The distribution of final cytologic diagnoses is listed in Table 2. Overall, there were 21 cases that underwent surgical excision, of which 12 of 21 (57%) were benign, 1 of 21 (5%) was a benign phyllodes tumor and categorized as atypical in this study, and 8 of 21 cases (38%) were invasive ductal carcinoma. The distribution of final histologic diagnoses is listed in Table 2. For FNAB without ROSE, 55 of 91 cases (60%) were categorized as benign, 1 of 91 cases (1%) as atypical, and 35 of 91 cases (39%) as malignant and/or suspicious for malignancy. The distribution of final cytologic diagnoses is listed in Table 2. Overall, there were 15 cases that underwent surgical excision, of which 5 of 15 (33%) were benign, and 10 of 15 cases (67%) were invasive ductal carcinoma. The distribution of final histologic diagnoses is listed in Table 2.
Table 2.
Distribution of Preliminary Cytologic Diagnosis (ROSE) (n = 50), Final Cytology Diagnosis (n = 50), and Final Histologic Diagnoses (n = 21)
| FNAB Performed With ROSE, No. (%) |
FNAB Performed Without ROSE |
|
|---|---|---|
| Preliminary Diagnosis by ROSE | N = 50 | |
| Benign | ||
| Adipose tissue | 1 (2) | — |
| Acute mastitis | 3 (6) | — |
| Benign breast cyst | 2 (4) | — |
| Benign, not otherwise specified | 9 (18) | — |
| Epidermal inclusion cyst | 1 (2) | — |
| Fibroadenoma | 25 (50) | — |
| Fibrocystic disease | 2 (4) | — |
| Gynecomastia | 1 (2) | — |
| Intraductal papilloma | 1 (2) | — |
| Lipoma | 1 (2) | — |
| Suspicious for malignancy/malignant | ||
| Suspicious for malignancy | — | — |
| Adenocarcinoma | 4 (8) | — |
| Final Cytologic Diagnosis | N = 50 | N = 91 |
| Benign | ||
| Acute mastitis | 2 (4) | 5 (5) |
| Chronic mastitis | — | 6 (7) |
| Benign breast cyst | 1 (2) | — |
| Benign ductal proliferation | 2 (4) | 2 (2) |
| Epidermal inclusion cyst | 1 (2) | 1 (1) |
| Fat necrosis | 2 (4) | 2 (2) |
| Fibroadenoma | 22 (44) | 25 (28) |
| Fibrocystic change | 4 (8) | 10 (11) |
| Granulomatous mastitis | 2 (4) | 1 (1) |
| Galactocele | — | 1 (1) |
| Gynecomastia | 2 (4) | 2 (2) |
| Atypical | ||
| Atypical ductal cells | 2 (4) | 1 (1) |
| Suspicious for malignancy/malignant | ||
| Suspicious for adenocarcinoma | 2(4) | 17 (19) |
| Adenocarcinoma | 8(16) | 18 (20) |
| Final Histologic Diagnosis | N = 21 | N = 15 |
| Benign | ||
| Fat necrosis | 2 (10) | — |
| Fibroadenoma | 8 (38) | 2 (13) |
| Breast adenoma | — | 1 (7) |
| Fibrocystic disease | 2 (10) | — |
| Gynecomastia | — | 2 (13) |
| Atypical | ||
| Benign phyllodes tumor | 1 (5) | — |
| Malignant | ||
| Invasive ductal carcinoma | 8 (38) | 10 (67) |
Abbreviations: FNAB, fine-needle aspiration biopsy; ROSE, rapid onsite evaluation.
The preliminary cytologic, final cytologic, and histologic diagnoses were all compared, when available. For FNABs with ROSE, the OPA between preliminary and final cytologic diagnoses was 86% (43 of 50) (95% CI, 74%–93%), the PPA was 36% (4 of 11) (95% CI, 11%–69%), and the NPA was 100% (39 of 39) (95% CI, 91%–100%) (κ = 0.5, P < .001) (Table 3). Of the concordant cases, 39 of 43 (91%) cases were benign, while 4 of 43 cases (9%) showed abnormal cytology on final cytologic review, which included any case that was categorized indeterminate, suspicious for malignancy, or malignant. Of the discordant cases, 7 were preliminarily categorized as benign, but were subsequently categorized as atypical (1 of 7; 14%), suspicious for malignancy (2 of 7; 29%), or adenocarcinoma (4 of 7; 57%) on final cytologic diagnosis, and carcinoma on based on histologic review. One FNAB was diagnosed as fibroadenoma both on preliminary and final cytologic interpretation but was revealed to be a benign phyllodes tumor after surgical resection. All of the discrepant cases demonstrated either moderate or high specimen cellularity (Table 4).
Table 3.
Comparison of Preliminary Cytologic Diagnosis by Rapid Onsite Evaluation and Final Cytologic Diagnosis (N = 50)
| Preliminary Cytologic Diagnosis |
Concordance, % |
|||
|---|---|---|---|---|
| Benign | Abnormal | |||
| Final Cytologic Diagnosis | ||||
| Benign | 38 | 0 | 100 | κ = 0.4 (P < .001) |
| Abnormal | 8 | 4 | 33 | |
Table 4.
Cases With Discrepant Diagnoses Between Preliminary Cytologic Diagnosis, Final Cytologic Diagnosis, and/or Final Histologic Diagnosis
| Case No. |
Preliminary Cytologic Diagnosis |
Final Cytologic Diagnosis |
Cytologic Specimen Cellularity |
Histologic Diagnosis |
Reason for Discrepancy |
|---|---|---|---|---|---|
| FNAB performed with ROSE | |||||
| 1 | Adipose tissue | Adenocarcinoma | Moderate | IDC | Tumor cells not present on ROSE slide |
| 2 | Fibroadenoma | Suspicious for malignancy | High | IDC | Interpretation error |
| 3 | Fibroadenoma | Suspicious for malignancy | High | N/A | Interpretation error |
| 4 | Fibroadenoma | Atypical ductal cells | High | Invasive papillary carcinoma | Interpretation error |
| 5 | Fibrocystic change | Adenocarcinoma | Moderate | IDC | Interpretation error |
| 6 | Benign | Adenocarcinoma | Moderate | IDC | Interpretation error |
| 7 | Benign | Adenocarcinoma | High | IDC | Interpretation error |
| 8 | Benign | Atypical ductal cells | Moderate | Invasive papillary carcinoma | Interpretation error |
| 9 | Fibroadenoma | Fibroadenoma | High | Benign phyllodes tumor | Interpretation error |
| FNAB performed without ROSE | |||||
| 1 | — | Chronic mastitis | Moderate | IDC | Sampling, no tumor on cytology slides |
| 2 | — | Fat necrosis | Moderate | IDC | Sampling, only a few atypical cells on cytology slides |
Abbreviations: FNAB, fine-needle aspiration biopsy; IDC, invasive ductal carcinoma; N/A, not available; ROSE, rapid onsite evaluation.
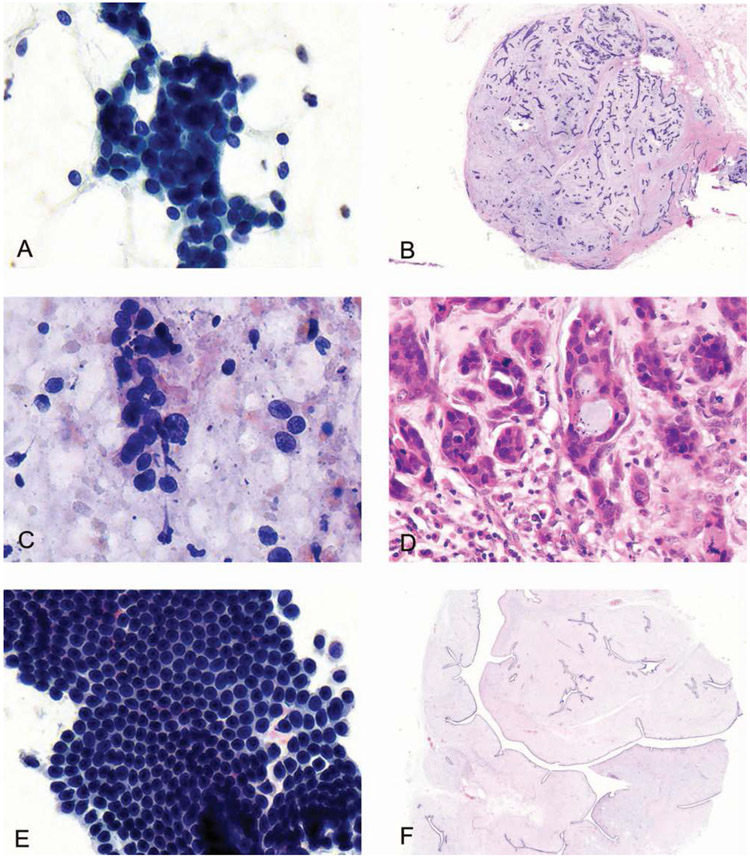
For samples with ROSE, there were 21 cases that underwent surgical excision. The OPA between the ROSE diagnosis and histologic diagnosis was 67% (14 of 21) (95% CI, 43%–85%), the PPA between the FNAB diagnostic category between ROSE and histology was 22% (2 of 9) (95% CI, 2.8%–60%), and the NPA was 100% (12 of 12) (95% CI, 74%–100%), (κ = 0.3, P < .09) (Figure, A through D; Table 5). When the final cytologic diagnoses and histologic diagnoses were compared, there was 1 discordant case in which a benign phyllodes tumor was diagnosed as a fibroadenoma on cytology (Figure, E and F). Therefore, the OPA between the final cytologic diagnosis and histology in the cohort with ROSE was 95% (20 of 21) (95% CI, 76%–100%), the PPA was 89% (8 of 9) (95% CI, 52%–100%), and the NPA was 100% (12 of 12) (95% CI, 74%–100%) (κ = 0.9, P < .001) (Table 5).
A, The cytologic smear shows a cohesive cluster of ductal cells admixed with myoepithelial cells in a staghorn pattern and stripped bipolar nuclei in the background. Stromal fragments were also identified (not shown). The preliminary and final cytologic diagnosis were fibroadenoma. B, The corresponding incisional biopsy shows a circumscribed lesion with both ductal and stromal proliferation, compatible with a fibroadenoma. C, The cytologic smear reveals discohesive ductal cells with atypia, some forming ducts, and a loss of myoepithelial cells. The preliminary diagnosis was suspicious for carcinoma, while the final cytologic diagnosis was highly suggestive for ductal carcinoma; tissue biopsy recommended. D, The corresponding excision shows infiltrating ductal carcinoma. E, The cytologic smear was highly cellular with branching and flat sheets of cohesive ductal cells admixed with myoepithelial cells as well as fragments of stroma. The preliminary and final cytologic diagnosis was fibroadenoma. F, The corresponding resection showed a predominantly stromal proliferation without atypia arranged in large slitlike spaces, consistent with a benign phyllodes tumor (Papanicolaou, original magnification ×400 [A, C, and E]; hematoxylin-eosin, original magnifications ×200 [B], ×400 [D], and ×35 [F]).
Table 5.
Comparison of Preliminary and Final Cytologic Diagnosis in FNAB Cases Performed With ROSE and Final Histologic Diagnosis (N = 21)
| Preliminary Cytologic Diagnosis |
||||
|---|---|---|---|---|
| Benign | Abnormal | Concordance, % | ||
| Final Histologic Diagnosis | ||||
| Benign | 12 | 0 | 100 | κ = 0.25 (P = .09) |
| Abnormal | 7 | 2 | 22 | |
| Final Cytologic Diagnosis |
||||
| Benign | Abnormal | Concordance, % | ||
| Benign | 12 | 0 | 100 | κ = 0.9 (P < .001) |
| Abnormal | 1 | 8 | 89 | |
Abbreviations: FNAB, fine-needle aspiration biopsy; ROSE, rapid onsite evaluation.
For samples without ROSE, there were 15 cases that underwent surgical resection. Overall, there were 2 discrepant cases between the final cytologic and final histologic interpretations. One case that was diagnosed as chronic mastitis and another case as fat necrosis were both revealed to be invasive ductal carcinoma on excision. Therefore, the OPA between the final cytologic diagnosis and histology in the cohort with ROSE was 87% (13 of 15) (95% CI, 60%–98%), the PPA was 80% (8 of 10) (95% CI, 44%–97%), and the NPA was 100% (5 of 5) (95% CI, 48%–100%) (κ = 0.7, P < .001) (Table 6).
Table 6.
Comparison of Final Cytologic Diagnosis in Fine-Needle Aspiration Biopsy Performed Without Rapid Onsite Evaluation and Final Histologic Diagnosis (N = 15)
| Final Cytologic Diagnosis |
||||
|---|---|---|---|---|
| Benign | Abnormal | Concordance, % | ||
| Final Histologic Diagnosis | ||||
| Benign | 5 | 0 | 100 | κ = 0.7 (P < .001) |
| Abnormal | 2 | 8 | 80 | |
DISCUSSION
In Tanzania, there is an urgent need to develop high-quality cytopathology services that are reliable, accurate, efficient, cost effective, and appropriate for the local context.25 Moreover, the need to assess the utility of ROSE and FNAB in the triage and diagnosis of breast masses aligns with the recently launched World Health Organization global BC initiative, which aims to reduce global BC mortality by increasing access to timely and early diagnosis.26,27 Cytopathology is able to deliver rapid accurate diagnoses with minimal equipment and less laboratory infrastructure; however, its utility remains limited by the skill of the operator and the need for highly specialized training.28,29 While leveraging ROSE to improve adequacy rates can help to optimize resources and reduce the need for repeat procedures, the ability to provide an immediate diagnosis has the potential to expedite patient management.19,30-32
FNAB continues to be one of the primary methods for tissue sampling and pathologic diagnosis of breast masses in low- and middle-income countries. Breast FNAB has been shown to be highly accurate when performed by a trained operator.9 The addition of ROSE has been shown to have several benefits, including decreasing the number of needle passes made and increasing rates of specimen adequacy, which in turn may reduce the number of repeat procedures, permitting triage for ancillary studies, and allowing for preliminary diagnosis, which can potentially expedite referral for more specialized cancer care.12,16,19,32-34 However, the impact of ROSE can be highly variable because cytology practices vary broadly across institutions and resource settings. Differences include types of cytologic sample preparation, who performs the FNAB, who performs ROSE, and whether telecytology is used.35 For example, one study from Australia found a statistically significant difference in the nondiagnostic rates between breast FNAB with and without ROSE (4% versus 17%),12 while an earlier meta-analysis found no statistically significant impact of ROSE on nondiagnostic rates but noted that the impact of ROSE depended on the non-ROSE adequacy rate.16 Although there have been a few studies showing that ROSE diagnoses have overall high rates of agreement with final cytologic and histologic diagnoses in high-resource settings, the literature on ROSE in sub-Saharan Africa is primarily limited to evaluating its use in thoracic biopsies in South Africa.36-39 Therefore, we conducted this study to investigate the potential role and performance of ROSE in FNAB of breast masses at a large national referral hospital in Tanzania.
All FNAB with ROSE samples in this study had adequate cellularity for final cytologic diagnosis, with only 8% of cases showing low cellularity. This observation is similar to a previous study conducted in India, which found that ROSE using toluidine blue increased adequacy rates from 86% to 98%.34 Due to limitations in workflow and documentation practices, we were unable to compare the adequacy rates of breast FNAB prior to the pilot implementation of ROSE. In a chart review of cases after the end of the pilot period there were also no cases that were categorized as nondiagnostic. However, there were 6 of 91 cases (7%) of “chronic mastitis” for masses that ranged from 2 to 4 cm, one of which was revealed to be invasive ductal carcinoma on excision. Although it is unclear if the remainder of the cases truly represent chronic mastitis, there is also a strong possibility that they represent nondiagnostic samples since they do not correlate with the clinical presentation. Moreover, the diagnosis of chronic mastitis is never made in the FNAB cases with ROSE. This difference may be because the nondiagnostic category is not well defined in the routine clinical practice at MNH. Some pathologists use the nondiagnostic category for FNAB only when cases contain only blood or are acellular, while others may use it if the tissue in the slide does not correlate with the clinical presentation.
In contrast to prior studies, the accuracy of ROSE for rendering preliminary diagnoses in this study was fair. One study at a large academic center in the United States retrospectively reviewed 1649 ROSE diagnoses from a range of anatomic sites and found that overall there were only 15 of 1649 cases (0.9%) in which the diagnosis rendered by ROSE and final cytologic diagnosis differed.40 Additional studies evaluating ROSE in the breast have also shown good agreement between the preliminary and final cytologic diagnoses, with overall major discrepancy rates ranging from 0.3% to 1.5%, false negative rates ranging from 0.9% to 13.5%, and false positive rates ranging from 0% to 1.9%.12,35,41-43 Moreover, a large study from Japan reported a Cohen κ of 0.99 between preliminary and final cytologic diagnoses.41 However, in our data set, the false negative rates of the preliminary cytologic diagnoses were notably higher than in the reported literature, ranging from 64% when compared to the final cytologic diagnosis to 78% when compared to cases with final histologic result. These findings suggest that while preliminary cytologic diagnosis can be reliable in high-resource settings in which ROSE is routinely practiced, systematic and dedicated training is needed to become proficient in rendering preliminary diagnoses on ROSE. Although a train-the-trainer model was adopted in which one pathologist was identified as a master trainer and received intensive training with immediate feedback from cytopathologists who had expertise in ROSE, the remaining pathologists received only a short period of training by the expert pathologist.
Most of the false negative cases were a result of interpretation error in which a mass was preliminarily interpreted as benign breast lesions, such as fibroadenoma or fibrocystic change, but was accurately recognized as adenocarcinoma on final review. Only 1 case was a result of sampling, in which the slide used for ROSE consisted of fibroadipose tissue while the other slides contained adenocarcinoma, which in retrospect should have been called nondiagnostic at the time of ROSE, but was fortunately adequate for diagnosis on final review. Although it is unclear how the smears for this case were prepared, it highlights that expelling the contents onto a single slide and splitting the material as opposed to expelling a distinct droplet of material on each slide may improve the accuracy of ROSE and reduce sampling errors.
Similar to prior studies, a low false positive rate was observed for both preliminary and final cytologic diagnoses in this cohort. This finding suggests that while the pathologists were well trained in cytopathologic interpretation with Papanicolaou-stained slides, additional longitudinal training is needed to achieve proficiency in interpreting slides stained with toluidine blue during ROSE. Another reason for a subset of the discrepancies may be that only 1 slide was selected for ROSE, which may not have been representative of the breast mass. Of note, another false negative was a mass that was diagnosed as fibroadenoma both at the time of ROSE and on final cytologic review; however, histologic examination confirmed a diagnosis of a benign phyllodes tumor. The cytologic smears demonstrated folded clusters of epithelial cells admixed with myoepithelial cells with fragments of normocellular stroma. The inability to reliably distinguish between fibroadenoma and benign phyllodes tumor on FNABs is a well-known diagnostic challenge.35,44-50 Both are biphasic, low-grade fibroepithelial lesions that consist of epithelial and stromal fragments in a background of myoepithelial cells, and low-grade phyllodes tumors do not necessarily demonstrate hypercellular stroma on FNAB. In this setting, correlation with clinical and radiologic findings is required to determine whether or not the tumor warrants excision. Therefore, in combination with clinical findings, an immediate preliminary diagnosis of malignancy by FNAB is highly accurate and sufficient to triage the patient to more specialized cancer care. Preliminary benign diagnoses by ROSE are unreliable in this setting, and final cytologic diagnoses are highly accurate when clinical presentation is taken into account.
Interestingly, while most (74%) of the masses were clinically estimated to measure approximately 2 to 5 cm, the majority of cases were benign on final cytologic and histologic diagnosis. The predominance of benign lesions may be attributable to the age distribution, since most of the patients in this study were less than 35 years old. Fibroadenomas, fibrocystic change, fat necrosis, acute mastitis, and granulomatous mastitis all presented as masses that were greater than 2 cm. Therefore, large breast masses in this setting should not be assumed to be breast carcinoma, especially in young women.
Limitations of this study include the small sample size and inability to conduct follow-up training sessions in ROSE and to conduct competency assessments as initially planned due to travel restrictions imposed during the COVID-19 pandemic. Because ROSE is new to and not yet integrated into the standard clinical workflow at MNH, study participants were accrued prospectively. Unfortunately, the pandemic also reduced the volume of patients seeking evaluation in the FNAB clinic, and all research activities involving direct clinical care were suspended by our group for several months in 2020. Therefore, the total number of participants in this study was lower than anticipated. In addition, multiple pathologists performed the ROSE, and skill levels were variable. For this study, the performance of each individual pathologist was not evaluated. However, the high false negative rate indicates that a more systematic and longitudinal approach needs to be adopted. Other limitations include our inability to collect data on adequacy rates prior to the implementation of this pilot study, a difference in how adequacy was defined by the study versus in clinical practice at MNH, variable documentation in the medical records, and also the low number of cases with surgical follow up. This study also only evaluated the use of toluidine blue, which was selected based on a local situational analysis in which it was determined to be the most cost-effective and rapid stain that also best fit into the clinical workflow at MNH. However, other stains may be considered for ROSE including modified Romanowsky and rapid Papanicolaou, as well as rapid hematoxylin-eosin stains.51 The selection of a stain for ROSE should be based on a local assessment to determine which rapid stain may be most suitable, including ability to reliably procure the stain, cost, and ability to integrate the stain into the clinical workflow.
In summary, the diagnostic impact of ROSE for evaluation of breast masses in low-resource setting showed all FNAB samples were adequate for evaluation with the majority having moderate to high cellularity.
Although there was poor agreement between the ROSE diagnosis and final cytologic and histologic diagnoses, there was excellent agreement between the final cytologic and histologic diagnoses for FNAB cases with ROSE. Moreover, the cohort with ROSE did not miss any overtly malignant cases and had a smaller proportion of cases with low cellularity, though not statistically significant, while the cohort without ROSE missed 2 cases of invasive ductal carcinoma. If the cases categorized as chronic mastitis are assumed to be nondiagnostic, then cases without ROSE have an unsatisfactory rate of up to 7%. The overall agreement between FNAB cases with ROSE was modestly better than cases without ROSE to the final histologic diagnoses, but the sample size is too small to make definitive conclusions.
Therefore, ROSE may reduce the number of false negative and nondiagnostic cases. However, larger studies are needed to further elucidate the impact of ROSE in a low-resource setting. More importantly this study reveals the need for methodical and longitudinal training for ROSE in settings where it is not routinely practiced prior to consideration for integration into routine clinical workflows. Our data demonstrate that ROSE is feasible in a low-resource setting and can be used to determine adequacy and help triage a subset of patients. However, immediate diagnosis should be practiced cautiously, and the performance should be reevaluated after additional training and more systematic educational interventions are implemented. The results of this study will continue to inform what the most effective interventions are to reduce disparities in BC diagnosis.
We would like to thank Atuganile Malango, MD, MMed; Caroline Ngimba, MD, MMed; and Advera Ngaiza, MD, MMed, from the Central Pathology Laboratory at Muhimbili National Hospital in Dar es Salaam, Tanzania (MNH) for collaboration in building capacity for fine-needle aspiration training at MNH and for performing fine-needle aspiration biopsies for this study.
Acknowledgments
This project was supported by the Fogarty International Center of the National Institutes of Health under award numbers D43TW009343 and D43TW011598, and the University of California Global Health Institute (A.H.K.); the National Cancer Institute under award number K08CA263299 (D.L.N.); and the University of California San Francisco Global Cancer Program, Helen Diller Family Comprehensive Cancer Center.
Kimambo, Vuhahula, and Ng receive research support from Cepheid, Inc. The funding is not related to this manuscript.
Footnotes
This work was previously presented as a poster at the United States and Canadian Academy of Pathology Annual Meeting; March 16-21, 2019; National Harbor, Maryland.
Contributor Information
Asteria H. Kimambo, Department of Pathology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Edda A. Vuhahula, Department of Pathology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Godfrey S. Philipo, Department of Epidemiology and Biostatistics, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Beatrice P. Mushi, Department of Epidemiology and Biostatistics, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Elia J. Mmbaga, Department of Epidemiology and Biostatistics, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; Department of Community Medicine and Global Health, University of Oslo, Oslo, Norway.
Katherine Van Loon, Division of Hematology and Oncology in the Department of Medicine, Helen Diller Family Comprehensive Cancer Center.
Dianna L. Ng, Helen Diller Family Comprehensive Cancer Center; Department of Pathology, University of California, San Francisco; Department of Pathology at Memorial Sloan Kettering Cancer Center, New York, New York.
References
- 1.Burson AM, Soliman AS, Ngoma TA, et al. Clinical and epidemiologic profile of breast cancer in Tanzania. Breast Dis. 2010;31(1):33–41. doi: 10.3233/BD-2009-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amadori D, Serra P, Bravaccini S, et al. Differences in biological features of breast cancer between Caucasian (Italian) and African (Tanzanian) populations. Breast Cancer Res Treat. 2014;145(1):177–183. doi: 10.1007/s10549-014-2903-0 [DOI] [PubMed] [Google Scholar]
- 3.Mwakigonja AR, Lushina NE, Mwanga A. Characterization of hormonal receptors and human epidermal growth factor receptor-2 in tissues of women with breast cancer at Muhimbili National Hospital, Dar es salaam, Tanzania. Infect Agents Cancer. 2017;12:60. doi: 10.1186/s13027-017-0170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rambau P, Chalya P, Manyama M, Jackson K. Pathological features of breast cancer seen in Northwestern Tanzania: a nine years retrospective study. BMC Res Notes. 2011;4:214. doi: 10.1186/1756-0500-4-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rambau PF. Pathology practice in a resource-poor setting: Mwanza, Tanzania. Arch Pathol Lab Med. 2011;135(2):191–193. doi: 10.1043/1543-2165-135.2.191 [DOI] [PubMed] [Google Scholar]
- 6.Breast Cancer Initiative 2.5 (BCI2.5). Tanzania Breast Health Care Assessment 2017: An Assessment of Breast Cancer Early Detection, Diagnosis and Treatment in Tanzania; 2017. https://www.fredhutch.org/content/dam/www/research/divisions/public-health-sciences/epidemiology/bci-25/knowledge-assessment/Tanzania-Breast-Health-Care-Assessment-2017.pdf. Accessed January 22, 2023. [Google Scholar]
- 7.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1 [DOI] [PubMed] [Google Scholar]
- 8.Thomas J Role of cytopathology in cancer control in low-resource settings: sub-Saharan Africa’s perspective. Int Health. 2011;3(1):3–6. doi: 10.1016/j.inhe.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Yu YH, Wei W, Liu JL. Diagnostic value of fine-needle aspiration biopsy for breast mass: a systematic review and meta-analysis. BMC Cancer. 2012;12(l):41. doi: 10.1186/1471-2407-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong J, Ly A, Arpin R, Ahmed Q, Brachtel E. Breast fine needle aspiration continues to be relevant in a large academic medical center: experience from Massachusetts General Hospital. Breast Cancer Res Treat. 2016;158(2):297–305. doi: 10.1007/s10549-016-3886-9 [DOI] [PubMed] [Google Scholar]
- 11.Farras Roca JA, Tardivon A, Thibault F, et al. Diagnostic performance of ultrasound-guided fine-needle aspiration of nonpalpable breast lesions in a multidisciplinary setting: the Institut Curie’s experience. Am J Clin Pathol. 2017;147(6):571–579. doi: 10.1093/ajcp/aqx009 [DOI] [PubMed] [Google Scholar]
- 12.Wong S, Rickard M, Earls P, Arnold L, Bako B, Field AS. The International Academy of Cytology Yokohama System for Reporting Breast Fine Needle Aspiration Biopsy Cytopathology: a single institutional retrospective study of the application of the system categories and the impact of rapid onsite evaluation. Acta Cytol. 2019;63(4):280–291. doi: 10.1159/000500191 [DOI] [PubMed] [Google Scholar]
- 13.Hoda RS, Brachtel EF. International Academy of Cytology Yokohama System for Reporting Breast Fine-Needle Aspiration Biopsy Cytopathology: a review of predictive values and risks of malignancy. Acta Cytol. 2019;63(4):292–301. doi: 10.1159/000500704 [DOI] [PubMed] [Google Scholar]
- 14.Montezuma D, Malheiros D, Schmitt FC. Breast fine needle aspiration biopsy cytology using the newly proposed IAC Yokohama System for Reporting Breast Cytopathology: the experience of a single institution. Acta Cytol. 2019. Feb 15:1–6. doi: 10.1159/000492638 [DOI] [PubMed] [Google Scholar]
- 15.Nasuti JF, Gupta PK, Baloch ZW. Diagnostic value and cost-effectiveness of on-site evaluation of fine-needle aspiration specimens: review of 5,688 cases. Diagn Cytopathol. 2002;27(1):1–4. doi: 10.1002/dc.10065 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt RL, Witt BL, Lopez-Calderon LE, Layfield LJ. The influence of rapid onsite evaluation on the adequacy rate of fine-needle aspiration cytology: a systematic review and meta-analysis. Am J Clin Pathol. 2013;139(3):300–308. doi: 10.1309/AJCPEGZMJKC42VUP [DOI] [PubMed] [Google Scholar]
- 17.Hamill J, Campbell ID, Mayall F, Basrtlett ASJR, Darlington A. Improved breast cytology results with near patient FNA diagnosis. Acta Cytol. 2002;46(1):19–24. doi: 10.1159/000326710 [DOI] [PubMed] [Google Scholar]
- 18.Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93(4):263–268. doi: 10.1002/cncr.9040 [DOI] [PubMed] [Google Scholar]
- 19.Philipo GS, Vuhahula E, Kimambo A, Mmbaga EJ, Van Loon K, Ng DL. Feasibility of fine-needle aspiration biopsy and rapid on-site evaluation for immediate triage in breast cancer screening in Tanzania. JCO Global Oncology. 2021;(7):146–152. doi: 10.1200/GO.20.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng DL, Vuhahula E, Zhang L, et al. Efficacy of an intensive, ultrasound-guided fine-needle aspiration biopsy training workshop in Tanzania. J Glob Oncol. 2018;(4):1–9. doi: 10.1200/JGO.18.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal P, Toi PC, Subramaniam H, Apoorva Lakshmi S. Prospective comparison of cytological specimen adequacy assessment by different rapid staining techniques for rapid on-site evaluation in fine needle aspiration cytology and their cost-effectiveness. Diagn Cytopathol. 2019;47(5):469–474. doi: 10.1002/dc.24139 [DOI] [PubMed] [Google Scholar]
- 22.Saba K, Niazi S, Bukhari MH, Imam SF. Use of supravital toluidine blue staining to improve the efficiency of fine-needle aspiration cytology reporting in comparison to Papanicolaou stain. Pak J Med Sci. 2015;31(5):1146–1151. doi: 10.12669/pjms.315.8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewer E, Schmitt AM. Ultrafast toluidine blue staining for rapid on-site evaluation of cytological smears. Acta Cytol. 2020;64(4):375–377. doi: 10.1159/000505254 [DOI] [PubMed] [Google Scholar]
- 24.Rausch T, Rochat M-C, Moret M, Braunschweig R, Mihaescu A. Modified technique of toluidine blue staining in rapid on-site evaluation. Diagn Cytopathol. 2012;40(9):847–848. doi: 10.1002/dc.21753 [DOI] [PubMed] [Google Scholar]
- 25.Yadav K, Cree I, Field A, Vielh P, Mehrotra R. Importance of cytopathologic diagnosis in early cancer diagnosis in resource-constrained countries. JCO Glob Oncol. 2022;8:e2100337. doi: 10.1200/GO.21.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duggan C, Trapani D, Ilbawi AM, et al. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol. 2021;22(11):1632–1642. doi: 10.1016/S1470-2045(21)00462-9 [DOI] [PubMed] [Google Scholar]
- 27.Anderson BO, Ilbawi AM, Fidarova E, et al. The Global Breast Cancer Initiative: a strategic collaboration to strengthen health care for non-communicable diseases. Lancet Oncol. 2021;22(5):578–581. doi: 10.1016/S1470-2045(21)00071-1 [DOI] [PubMed] [Google Scholar]
- 28.Ljung BM, Drejet A, Chiampi N, et al. Diagnostic accuracy of fine-needle aspiration biopsy is determined by physician training in sampling technique. Cancer. 2001;93(4):263–268. coi: 10.1002/cncr.9040 [DOI] [PubMed] [Google Scholar]
- 29.Field AS. Training for cytotechnologists and cytopathologists in the developing world. Cytopathology. 2016;275):313–316. doi: 10.1111/cyt.12372 [DOI] [PubMed] [Google Scholar]
- 30.Gui GP, Allum WH, Perry NM, et al. One-stop diagnosis for symptomatic breast disease. Ann R Coll Surg Engl. 1995;77(1):24–27. [PMC free article] [PubMed] [Google Scholar]
- 31.Delaloge S, Bonastre J, Borget I, et al. The challenge of rapid diagnosis in oncology: diagnostic accuracy and cost analysis of a large-scale one-stop breast clinic. Eur J Cancer. 2016;66:131–137. doi: 10.1016/j.ejca.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 32.Ly A, ono JC, Hughes KS, Pitman MB, Balassanian R. Fine-needle aspiration biopsy of palpable breast masses: patterns of clinical use and patient experience. J Natl Compr Canc Netw. 2016;14(5):527–536. doi: 10.6004/jnccn.2016.0061 [DOI] [PubMed] [Google Scholar]
- 33.Schmidt RL, Kordy MA, Howard K, Layfield LJ, Hall BJ, Adler DG. Risk-benefit analysis of sampling methods for fine-needle aspiration cytology: a mathematical modeling approach. Am J Clin Pathol. 2013;139(3):336–344. doi: 10.1309/AJCPEAKR4MO2GQBO [DOI] [PubMed] [Google Scholar]
- 34.Ammanagi AS, Dombale VD, Patil SS. 0n-site toluidine blue staining and screening improves efficiency of fine-needle aspiration cytology reporting. Acta Cytol. 2012;56(4):347–351. doi: 10.1159/000338725 [DOI] [PubMed] [Google Scholar]
- 35.Torous VF, Lopez SH, Xu C, Sweeney BJ, Pitman MB. Performance of rapid on-site evaluation in breast fine-needle aspiration biopsies: identifying areas of diagnostic challenge. Acta Cytol. 2022;66(1):1–13. doi: 10.1159/000518579 [DOI] [PubMed] [Google Scholar]
- 36.Brundyn K, Koegelenberg CFN, Diacon AH, et al. Transbronchial fine needle aspiration biopsy and rapid on-site evaluation in the setting of superior vena cava syndrome. Diagn Cytopathol. 2013;41(4):324–329. doi: 10.1002/dc.21857 [DOI] [PubMed] [Google Scholar]
- 37.Koegelenberg CFN, Diacon AH, Irusen EM, et al. The diagnostic yield and safety of ultrasound-assisted transthoracic biopsy of mediastinal masses. Respiration. 2011;81(2):134–141. doi: 10.1159/000322005 [DOI] [PubMed] [Google Scholar]
- 38.Diacon AH, Schuurmans MM, Theron J, et al. Utility of rapid on-site evaluation of transbronchial needle aspirates. Respiration. 2005;72(2):182–188. doi: 10.1159/000084050 [DOI] [PubMed] [Google Scholar]
- 39.Mfokazi A, Wright CA, Louw M, et al. Direct comparison of liquid-based and smear-based cytology with and without rapid on-site evaluation for fine needle aspirates of thoracic tumors. Diagn Cytopathol. 2016;44(5):363–368. doi: 10.1002/dc.23447 [DOI] [PubMed] [Google Scholar]
- 40.Geisler DL, Nestler RJ, Mosley BL, et al. Accuracy of definitive rapid on-site evaluation cytopathology diagnoses: assessment of potentially critical diagnoses as a quality assurance measure. J Am Soc Cytopathol. 2022;11(3):133–141. doi: 10.1016/j.jasc.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 41.Sakuma T, Mimura A, Tanigawa N, Taksamizu R, Morishima H, Matsunami N. Rapid on-site cytologic examination of 1500 breast lesions using the modified Shorr’s stain. Breast Cancer. 2015;22(3):280–286. doi: 10.1007/s12282-013-0479-x [DOI] [PubMed] [Google Scholar]
- 42.Bofin AM, Lydersen S, Isaksen C, Hagmar BM. Interpretation of fine needle aspiration cytology ofthe breast: a comparison of cytological, frozen section, and final histological diagnoses. Cytopathology. 2004;15(6):297–304. doi: 10.1111/j.1365-2303.2004.00159.x [DOI] [PubMed] [Google Scholar]
- 43.Liew PL, Liu TJ, Hsieh MC, et al. Rapid staining and immediate interpretation of fine-needle aspiration cytology for palpable breast lesions: diagnostic accuracy, mammographic, ultrasonographic and histopathologic correlations. Acta Cytol. 2010;55(1):30–37. doi: 10.1159/000320869 [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy S, Ashfaq R, Shin HJC, Sneige N. Distinction of phyllodes tumor from fibroadenoma. Cancer Cytopathol. 2000;90(6):342–349. doi: [DOI] [PubMed] [Google Scholar]
- 45.El Hag IA, Aodah A, Kollur SM, Attallah A, Mohamed AAE, Al-Hussaini H. Cytological clues in the distinction between phyllodes tumor and fibroadenoma. Cancer Cytopathol. 2010;118(1):33–40. doi: 10.1002/cncy.20057 [DOI] [PubMed] [Google Scholar]
- 46.Dusenbery D, Frable WJ. Fine needle aspiration cytology of phyllodes tumor. Potential diagnostic pitfalls. Acta Cytol. 1992;36(2):2l5–22l. [PubMed] [Google Scholar]
- 47.Scolyer RA, McKenzie PR, Achmed D, Lee CS. Can phyllodes tumours of the breast be distinguished from fibroadenomas using fine needle aspiration cytology? Pathology. 2001;33(4):437–443. doi: 10.1080/00313020120083151 [DOI] [PubMed] [Google Scholar]
- 48.Veneti S, Manek S. Benign phyllodes tumour vs fibroadenoma: FNA cytological differentiation. Cytopathology. 2001;12(5):321–328. doi: 10.1046/j.1365-2303.2001.00334.x [DOI] [PubMed] [Google Scholar]
- 49.Maritz RM, Michelow PM. Cytological criteria to distinguish phyllodes tumour of the breast from fibroadenoma. Acta Cytol. 2017;61(6):418–424. doi: 10.1159/000477573 [DOI] [PubMed] [Google Scholar]
- 50.Tummidi S, Kothari K, Agnihotri M, Naik L, Sood P. Fibroadenoma versus phyllodes tumor: a vexing problem revisited! BMC Cancer. 2020;20(1):648. doi: 10.1186/s12885-020-07129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koshikawa T, Kyotake A, Tsukamoto R, Itoh T. Standard and rapid stains in fine-needle aspiration cytology and rapid on-site evaluation with a short column (standard and rapid stains in fine-needle aspiration cytology and rapid on-site evaluation). In: Kakudo K, ed. Thyroid FNA Cytology: Differential Diagnoses and Pitfalls. Singapore: Springer; 2019:505–509. doi: 10.1007/978-981-13-1897-9_66 [DOI] [Google Scholar]