Abstract
Purpose:
Although visual impairment (VI) has been associated with worse cognitive performance among older adults, the temporal relationship between the 2 remains subject to debate. Our objective was to investigate the longitudinal impact of VI on cognitive function and vice versa.
Design:
Retrospective, time-to-event study.
Participants:
National Health and Aging Trends Study (NHATS) participants from 2011 to 2018 cycles.
Methods:
A total of 10 676 participants aged 65 years and older were included. Cox proportional hazards regression models evaluated the impact of baseline VI on subsequent dementia and impact of baseline dementia on subsequent VI. Models were adjusted for potential confounding variables, including demographics, clinical comorbidities, and hearing and physical function limitations.
Main Outcome Measures:
Hazard ratio (HR) for incident dementia among participants with baseline self-reported VI and HR for incident VI among participants with baseline dementia.
Results:
Of the 10 676 participants included in the analysis, approximately 40% were aged 65–74 years, 40% were aged 75–84 years, and the remaining 20% were aged 85 years and older. The majority were female (59%), and 68% self-identified as non-Hispanic White. Among participants with normal cognitive status at baseline, subsequent dementia was observed in 1753 (16%), and among participants with normal self-reported vision at baseline, subsequent VI was reported in 2371 (22%). In adjusted regression models, participants with baseline VI had higher likelihood of developing dementia over subsequent follow-up (HR, 2.3; 95% confidence interval [CI], 2.0–2.6; P < 0.001). Likewise, participants with baseline dementia had a higher likelihood of developing self-reported VI over time (HR, 2.5; 95% CI, 2.2–2.8; P < 0.001).
Conclusions:
Self-reported VI in the US Medicare population is associated with greater dementia likelihood over time, and dementia is similarly associated with greater VI likelihood over time. Associations are likely multifactorial and bidirectional and could be explained by intervening variables in the path from VI to dementia, or vice versa, or by common risk factors for pathological processes in both eyes and brain. These findings suggest the need for early identification of older adults with visual compromise and consideration of visual disability in the cognitively impaired.
Keywords: Aging, Cognitive dysfunction, Dementia, Low vision, Visual impairment
As the life expectancy in developed countries increases, the prevalence of diseases of aging, such as dementia (including Alzheimer’s disease) and visual dysfunction, also increase.1–3 One in 10 patients age 65 years and older are currently living with Alzheimer’s disease, and 1 in 28 are affected by blindness and vision loss by age 40 years and older.1,4,5 Prior cross-sectional investigations have suggested that an association exists between visual and cognitive functions, but longitudinal reports have offered mixed evidence.6–25 A 2018 American Geriatrics Society and National Institute on Aging report noted that comorbid sensory—specifically vision and hearing—and cognitive impairments occur more frequently than accounted for by chance alone. Potential mechanisms explaining a causative relationship are varied but include the possibility of shared common risk factors, for instance, vascular disease.26 Alternatively, vision loss may cause reduced social engagement, with fewer cognitively simulating activities and higher rates of depression,27,28 or extra cognitive effort may be necessary to interpret and respond to visual information among those with low vision, further taxing cognitive systems, and increasing dementia. Vision loss also may be a symptom of dementia resulting from neuropathology along the visual pathway.29 Although cognitive impairment and dementia are not the same entities, they do fall along the spectrum of shared disease pertaining to a decline in cognitive performance. Studies use different metrics to make a determination of cognitive impairment or dementia, but in general, dementia indicates a more severe, global pathology that affects multiple cognitive domains and interferes with activities of daily living. A deeper understanding of the relationship between dementia and vision loss will be critical when addressing the growing population of older adults affected by each.
We recently demonstrated that visual impairment (VI), both subjectively reported and objectively measured, appears to be directly linked with cognitive dysfunction among older adults in the United States.22 Findings from the Women’s Health Initiative database further support the notion that VI and cognitive decline are temporally related; however, as with other studies showing this association, conclusions are restricted in generalizability and limited by small sample sizes.30–32 Understanding how visual and cognitive dysfunction respectively affect one another over time is important for public health and patient care. This includes determining the potential utility of VI screening for older patients at high risk for developing dementia.
The aim of this study was 2-fold: to investigate the longitudinal impact of self-reported VI on dementia and to investigate the longitudinal impact of dementia on visual function. We hypothesized that there is an increased risk of developing dementia among subjects with VI and that individuals with dementia may be more likely to have VI. Using a large, nationally representative sample of Medicare beneficiaries from the National Health and Aging Trends Study (NHATS), we sought to analyze the association between self-reported VI and dementia over time.
Methods
Study Sample
We used data from the NHATS 2011–2018 cycles. The NHATS, sponsored by the National Institute on Aging, consists of a nationally representative sample of Medicare beneficiaries aged 65 years and older residing in the contiguous United States, for whom in-person interviews are conducted annually to document changes over time.33 The initial NHATS participant cohort was created in 2011 and refreshed in 2015 to replenish participants who dropped out because of death or other reasons. All participants were surveyed annually through 2018. The NHATS was designed to study functioning in later life and includes longitudinal data on both visual functioning and cognitive impairment.
Variable Selection
Visual impairment was assessed using responses to self-reported questionnaires. Respondents were categorized as having any subjective VI (impairment at distance or near), distance impairment specifically (inability to “recognize someone across the street” or “watch television across the room”), and near impairment specifically (inability to “read newspaper print”). Each was queried on the basis of best-corrected vision. Probable or possible dementia was classified per NHATS protocol.34–36 The AD8 (Alzheimer’s Disease Research Center, Washington University, St. Louis, MO) 8-item questionnaire was administered to participants to assess memory, temporal orientation, judgment, and function. Additional domain-based cognitive tests included orientation (date and naming of the president and vice president), memory (immediate and delayed word recall), and executive functioning (clock drawing). Respondents were classified as probable dementia based on a physician diagnosis, AD8 score ≥2, or cognitive test performance ≤1.5 standard deviations from the mean for at least 2 of 3 domains (memory, orientation, and executive functioning). Possible dementia was determined by impairment in 1 cognitive domain. Respondents who did not meet the criteria of probable or possible dementia were considered to have no dementia.
Potential confounding variables used as covariates in the analysis included self-reported functional impairment (hearing and physical function). Demographic and socioeconomic status variables were also determined from self-report, including age, sex, race/ethnicity, education level, and annual household income. Age was reported in 5-year intervals starting from age 65 years, with age 90 years and more comprising the oldest category. Information on annual income was imputed from data collected in 2011, 2013, 2015, and 2017 based on NHATS protocol.37 Because no income data were obtained for the years 2012, 2014, 2016, and 2018, we assumed stable income from the previous year. General health conditions and behaviors were likewise self-reported, including smoking status and history of diabetes, hypertension, coronary heart disease, myocardial infarction, and stroke.
Statistical Analysis
The NHATS database is composed of publicly available, nonidentifiable information, and this study was determined to be exempt by the Stanford University Institutional Review Board. The described research adhered to the tenets of the Declaration of Helsinki. This is a retrospective study using de-identified subject details. Informed consent was not obtained. All analyses were performed using STATA/SE, version 13.1 (StataCorp LP). Descriptive statistics were used to characterize the study groups at baseline, comparing the subgroups with versus without dementia and with versus without VI, respectively.
Given that data were obtained from annual surveys, time-to-event (survival) analysis was performed by constructing discrete time Cox proportional hazards regression models adjusted for clinical risk factors and hearing and physical function impairments treated as time-varying covariates. For our primary analyses, we evaluated the impact of baseline impairment on subsequent outcomes. To assess the influence of baseline VI on subsequent dementia incidence (model 1), we evaluated a cohort with no dementia at baseline. We determined the adjusted hazard ratio (HR) for the likelihood of developing probable/possible dementia over time for respondents with versus without baseline subjective VI (not counting VI that developed after baseline). To assess the reverse association (influence of baseline dementia on subsequent VI incidence, model 2), we evaluated a cohort with no subjective VI at baseline. In this analysis, our primary outcome was self-reported VI, and the predictor variable of interest was presence of probable/possible dementia at baseline (not counting dementia that developed after baseline). Both models were sequentially adjusted for demographics and socioeconomic status, followed by clinical risk factors including diabetes, hypertension, coronary heart disease, and myocardial infarction. The fully adjusted models accounted for these variables and the added effects of functional hearing or physical limitations. “Don’t know” and “Refuse” responses were treated as missing values and excluded from the analysis.
We also performed sensitivity analyses in which each of our predictor variables of interest was respectively treated as time-varying, that is, also counting incident VI (model 1) or incident dementia (model 2) that developed after baseline, in addition to prevalent impairment present before baseline. Therefore, these sensitivity analyses included some participants who had VI (model 1) or dementia (model 2) for a shorter period of time than in our primary analyses. Two-sided P < 0.05 was considered statistically significant for all analyses.
Results
Study Sample and Population Characteristics
From 2011 to 2018, a total of 10 676 unique participants were surveyed by NHATS. Baseline demographics for subjects enrolled during the initial recruitment phase in 2011 and those recruited during the database refresh in 2015 are reported in Table 1. In total, 13.0% of participants were age 85 to 89 years, and 10.9% were age 90 years and more; the remainder of the sample was approximately equally divided among 4 age groups between age 65 and 84 years (19% of the cohort in each). More than half of participants (59.0%) were female, and the racial-ethnic distribution was non-Hispanic White (68.4%), Black (21.1%), Mexican/Hispanic (5.7%), and other (2.9%). There was a similar distribution across education levels; however, lower income levels were disproportionately represented (particularly those with annual household income ≤$25 000, comprising 34.3% of the cohort). Self-reported physical function limitations were present in 59.1% of the cohort, and hearing impairment was present in 13.9%.
Table 1.
Demographic and Health Comorbidity Characteristics of the NHATS Study Populations
| Characteristic | NHATS | Dementia Assessmentb | Visual Impairment Assessmentb | ||||
|---|---|---|---|---|---|---|---|
| Total Study Sample (n=10,676)a | Yes (n=752) | No (n=9,094) | P Value | Yes (n=997) | No (n=8,769) | P Value | |
| Age, n (%) | |||||||
| 65–69 years | 2,052 (19.22) | 29 (3.86) | 2,001 (22) | <0.001 | 141 (14.14) | 1,883 (21.47) | <0.001 |
| 70–74 years | 2,075 (19.44) | 59 (7.85) | 1,996 (21.62) | 124 (12.44) | 1,897 (21.63) | ||
| 75–79 years | 2,017 (18.89) | 121 (16.09) | 1,811 (19.91) | 159 (15.95) | 1,763 (20.10) | ||
| 80–84 years | 1,979 (18.54) | 168 (22.34) | 1,659 (18.24) | 175 (17.55) | 1,633 (18.62) | ||
| 85–89 years | 1,392 (13.04) | 180 (23.94) | 995 (10.94) | 195 (19.56) | 967 (11.03) | ||
| 90+ years | 1,161 (10.87) | 195 (25.93) | 662 (7.28) | 203 (20.36) | 626 (7.14) | ||
| Gender, n (%) | |||||||
| Female | 6,297 (58.98) | 466 (61.97) | 5,195 (57.13) | 0.010 | 659 (66.10) | 4,956 (56.52) | <0.001 |
| Race/Ethnicity, n (%) | |||||||
| Non-Hispanic White | 7,306 (68.43) | 419 (57.55) | 6,288 (70.30) | <0.001 | 572 (58.13) | 6,084 (70.62) | <0.001 |
| Non-Hispanic Black | 2,254 (21.11) | 204 (28.02) | 1,883 (21.05) | 264 (26.83) | 1,806 (20.96) | ||
| Hispanic | 613 (5.74) | 75 (10.30) | 516 (5.77) | 114 (11.59) | 474 (5.50) | ||
| Other | 308 (2.88) | 30 (4.12) | 258 (2.88) | 34 (3.46) | 251 (2.91) | ||
| Education, n (%) | |||||||
| <12th grade | 2,399 (22.47) | 311 (43.62) | 2,088 (23.33) | <0.001 | 398 (40.74) | 1,977 (22.95) | <0.001 |
| High school graduate | 2,640 (24.73) | 205 (28.75) | 2,435 (27.20) | 266 (27.23) | 2,352 (27.30) | ||
| Some college | 2,429 (22.75) | 111 (15.57) | 2,318 (25.90) | 198 (20.27) | 2,218 (25.75) | ||
| College graduate and beyond | 2,196 (20.57) | 86 (12.06) | 2,110 (23.57) | 115 (11.77) | 2,068 (24.00) | ||
| Annual household income, n (%) | |||||||
| ≤$25,000 | 3,661 (34.29) | 428 (66.36) | 3,233 (44.53) | <0.001 | 538 (67.50) | 3,078 (43.73) | <0.001 |
| >$25,000 to ≤$45,000 | 1,828 (17.12) | 116 (17.98) | 1,712 (23.58) | 145 (18.19) | 1,667 (23.69) | ||
| >$45,000 to ≤$65,000 | 985 (9.23) | 55 (8.53) | 930 (12.81) | 54 (6.78) | 924 (13.13) | ||
| >$65,000 | 1,432 (13.41) | 46 (7.13) | 1,386 (19.09) | 60 (7.53) | 1,369 (19.45) | ||
| Smoking status, n (%) | |||||||
| Never | 4,887 (45.78) | 423 (56.93) | 4,464 (49.13) | <0.001 | 529 (53.38) | 4,313 (49.24) | 0.042 |
| Former | 4,196 (39.30) | 284 (38.22) | 3,912 (43.06) | 396 (39.96) | 3,771 (43.05) | ||
| Current | 746 (6.99) | 36 (4.85) | 710 (7.81) | 66 (6.66) | 675 (7.71) | ||
| Diabetes, n (%) | 2,543 (23.82) | 215 (28.63) | 2,328 (25.61) | 0.070 | 328 (32.90) | 2,190 (24.99) | <0.001 |
| Hypertension, n (%) | 6,601 (61.83) | 536 (71.47) | 6,065 (66.80) | 0.009 | 723 (72.59) | 5,830 (66.59) | <0.001 |
| Coronary heart disease, n (%) | 1,801 (16.87) | 210 (28.11) | 1,591 (17.53) | <0.001 | 268 (27.02) | 1,508 (17.23) | <0.001 |
| Myocardial infarction, n (%) | 1,481 (13.87) | 148 (19.73) | 1,333 (14.68) | <0.001 | 185 (18.59) | 1,272 (14.53) | 0.001 |
| Stroke, n (%) | 1,098 (10.28) | 211 (28.13) | 887 (9.77) | <0.001 | 212 (21.31) | 868 (9.91) | <0.001 |
| Hearing impairment, n (%) | 1,483 (13.89) | 254 (34.23) | 1,229 (13.53) | <0.001 | 316 (31.89) | 1,143 (13.05) | <0.001 |
| Physical limitation, n (%) | 6,312 (59.12) | 683 (90.82) | 5,629 (61.93) | <0.001 | 871 (87.36) | 5,371 (61.28) | <0.001 |
| Dementia, n (%) | 752 (7.04) | -- | -- | -- | 251 (25.18) | 481 (5.49) | <0.001 |
| Visual impairment, n (%) | 997 (9.34) | 251 (34.29) | 746 (8.26) | <0.001 | -- | -- | -- |
Percentages may not add up to 100% due to missing values for total study cohort: race/ethnicity (195, 2%), education (1012, 9%), income (2770, 26%), smoking (847, 8%), diabetes (836, 8%), hypertension (846, 8%), coronary heart disease (855, 8%), myocardial infarction (847, 8%), stroke (847, 8%), hearing impairment (853, 8%), physical limitation (835, 8%), dementia (830, 8%), visual impairment (910, 9%).
At baseline, 7% of the study sample was classified as having probable or possible dementia, and 9.3% had subjective VI at distance or near. Over the follow-up period, 16.4% (n = 1753) of the participants developed new dementia, and 22.2% (n = 2371) developed new subjective VI. Compared with those with no dementia, participants with baseline dementia were significantly more likely to be older, female, and of minority race/ethnicity, and to have less education and lower income. They were also significantly more likely to report no smoking history but positive histories for hypertension, coronary heart disease, myocardial infarction, stroke, hearing impairment, and physical function limitations. Likewise, compared with participants without VI, those with baseline VI were also more likely to be older, female, and nonwhite, to have lower socioeconomic status, to report nonsmoking history, and to be affected by other systemic comorbidities. Participants with VI were also more frequently affected by diabetes compared with those without VI, whereas there was no statistically significant difference in prevalence of diabetes for participants with dementia versus no dementia at baseline.
Influence of Visual Impairment on Dementia Incidence
Figure 1 presents Kaplan–Meier curves indicating change in the proportion of participants without incident dementia, stratified on the basis of visual function at baseline and followed over the study period. More participants with baseline VI developed dementia over time; the cumulative proportion without incident dementia in participants without self-reported VI at baseline was 0.83 (95% confidence interval [CI], 0.82–0.84) versus 0.44 (95% CI, 0.40–0.47) in those with self-reported VI. In Cox proportional hazards regression (Table 2), self-reported VI was significantly associated with more than 4-fold greater likelihood of subsequent dementia (HR, 4.4; 95% CI, 3.9–4.8; P < 0.001). After full model adjustment for demographic variables, clinical risk factors, and hearing and physical functioning impairments, the likelihood of dementia was more than 2-fold higher among participants with baseline VI (HR, 2.3; 95% CI, 2.0–2.6; P < 0.001). Sensitivity analyses that also included incident cases of VI not present at baseline yielded similar results but of lower magnitude (10%–20% increased hazard of dementia in adjusted models, Table S1, available at www.aaojournal.org). Similar results were obtained regardless of whether distance VI or near VI was reported.
Figure 1.
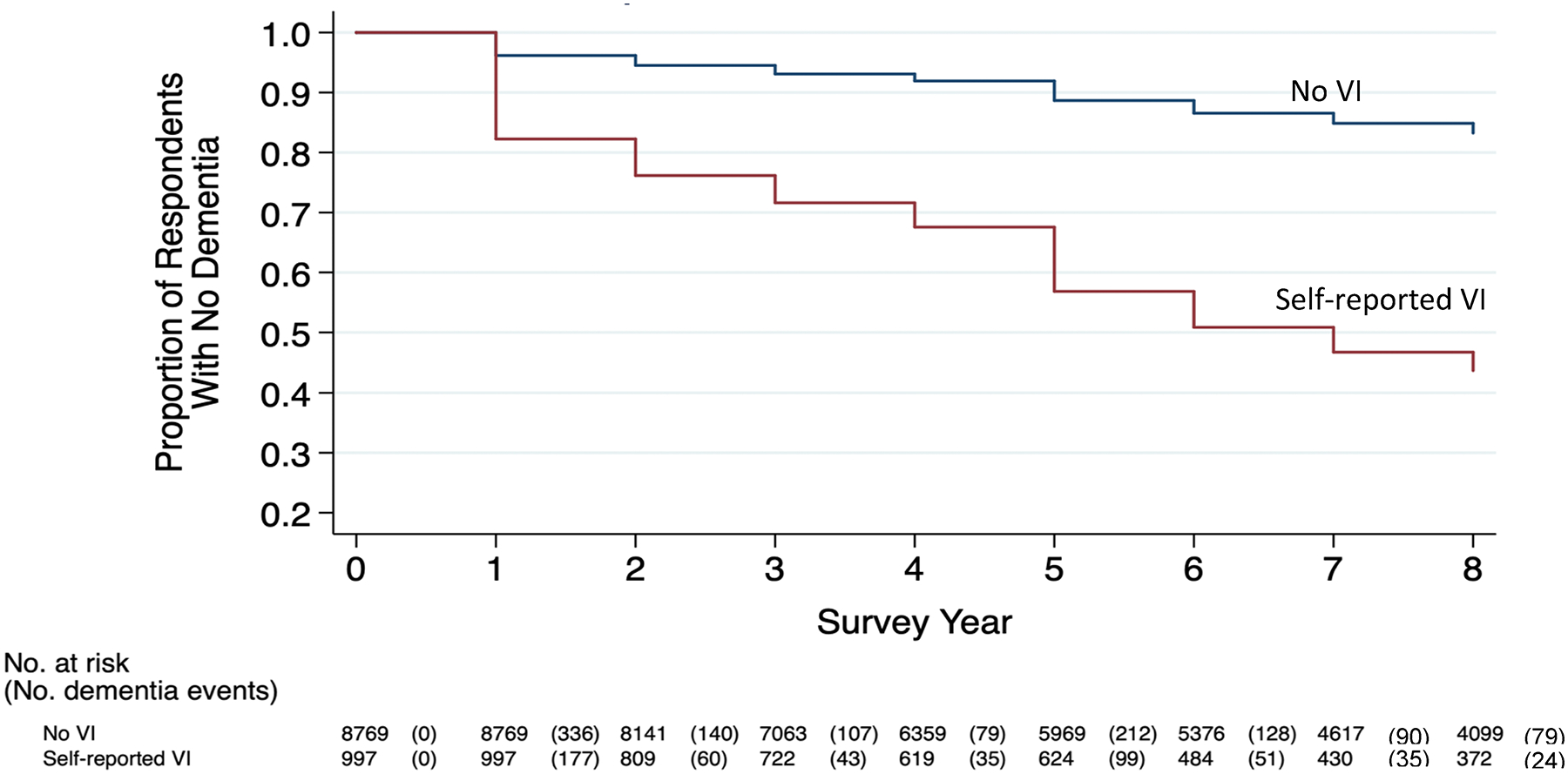
Cumulative number of incident dementia cases over time among participants with and without baseline visual impairment (VI), 2011–2018.
Table 2.
Time to Event Analysis for Development of Dementia Based on Vision Status
| Self-reported visual impairment | HR | 95% CI | P value |
|---|---|---|---|
| Unadjusted | 4.4 | (3.9 – 4.8) | <0.001 |
| Adjusteda | |||
| Demographics + SES | 2.7 | (2.4 – 3.0) | <0.001 |
| + Clinical risk factors | 2.5 | (2.2 – 2.8) | <0.001 |
| + Hearing and physical function impairments | 2.3 | (2.0 – 2.6) | <0.001 |
| Self-reported distance visual impairment | HR | 95% CI | P value |
| Unadjusted | 4.3 | (3.9 – 4.9) | <0.001 |
| Adjusteda | |||
| Demographics + SES | 2.6 | (2.3 – 3.0) | <0.001 |
| + Clinical risk factors | 2.5 | (2.2 – 2.8) | <0.001 |
| + Hearing and physical function impairments | 2.3 | (2.0 – 2.6) | <0.001 |
| Self-reported near visual impairment | HR | 95% CI | P value |
| Unadjusted | 4.2 | (3.7 – 4.8) | <0.001 |
| Adjusteda | |||
| Demographics + SES | 2.4 | (2.1 – 2.8) | <0.001 |
| + Clinical risk factors | 2.2 | (1.9 – 2.6) | <0.001 |
| + Hearing and physical function impairments | 2.0 | (1.8 – 2.4) | <0.001 |
Models adjusted for demographics and socioeconomic status (age, gender, race/ethnicity, education level, and annual household income), adding clinical risk factors (smoking status, diabetes, HTN, CHD, MI, and stroke), and finally including hearing and physical function impairments. Clinical risk factors and hearing and physical function impairments were treated as time varying covariates.
HR: hazard ratios for risk of developing dementia
HRs are reported with 95% confidence intervals (CI). Two-sided p-values <0.05 were deemed statistically significant.
Influence of Dementia on Visual Impairment Incidence
The reverse relationship was also present: a greater number of participants with baseline dementia developed VI over time. Figure 2 illustrates change in the proportion of participants without incident self-reported VI over the study period, stratified on the basis of the presence of dementia at baseline. The cumulative proportion of participants who did not develop incident self-reported VI among those without dementia at baseline was 0.76 (95% CI, 0.74–0.77) compared with 0.26 (95% CI, 0.23–0.29) among those with baseline dementia. Cox proportional hazard regression models (Table 3) demonstrates greater likelihood of developing VI over time among participants who had dementia at baseline, with more than 4-fold higher odds in unadjusted models (HR, 4.4; 95% CI, 4.1–4.9; P < 0.001) and more than 2-fold higher odds in models adjusted for demographics, socioeconomic status, systemic comorbidities, and hearing and physical function limitations (HR, 2.5; 95% CI, 2.2–2.8; P < 0.001). Sensitivity analyses also including incident dementia not present at baseline yielded similar results but of lower magnitude (20% increased hazard of VI in adjusted models, Table S2, available at www.aaojournal.org).
Figure 2.
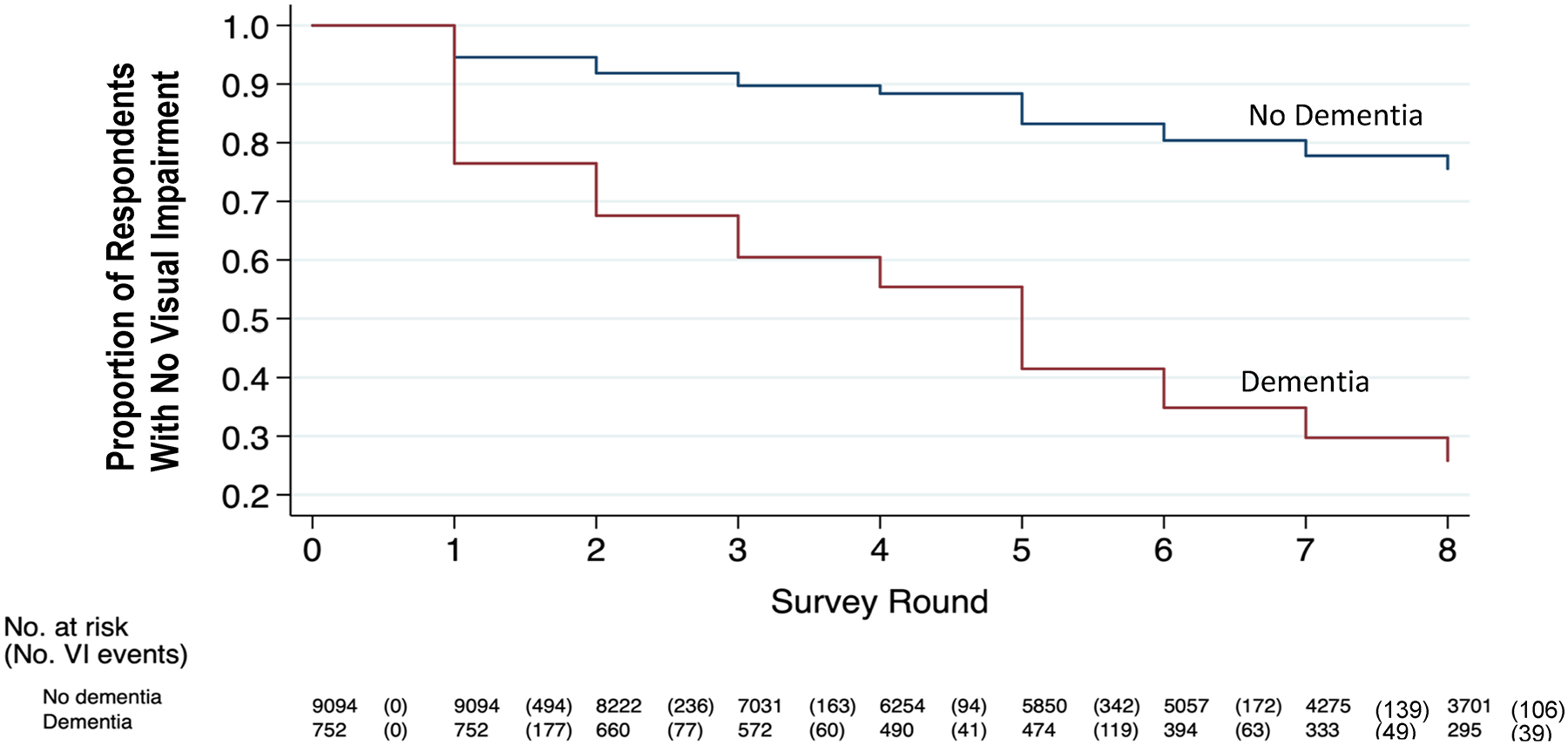
Cumulative number of incident visual impairment (VI) cases over time among participants with and without baseline dementia, 2011–2018.
Table 3.
Time to Event Analysis for Development of Visual Impairment Based on Dementia Status
| Probable/possible dementia | HR | 95% CI | P value |
|---|---|---|---|
| Unadjusted | 4.4 | (4.1 – 4.9) | <0.001 |
| Adjusteda | |||
| Demographics + SES | 2.8 | (2.5 – 3.1) | <0.001 |
| + Clinical risk factors | 2.7 | (2.4 – 3.0) | <0.001 |
| + Hearing and physical function impairments | 2.5 | (2.2 – 2.8) | <0.001 |
Models adjusted for demographics and socioeconomic status (age, gender, race/ethnicity, education level, and annual household income), adding clinical risk factors (smoking status, diabetes, HTN, CHD, MI, and stroke), and finally including hearing and physical function impairments. Clinical risk factors and hearing and physical function impairments were treated as time varying covariates.
HR: hazard ratios for risk of developing visual impairment
HRs are reported with 95% confidence intervals (CI). Two-sided p-values <0.05 were deemed statistically significant.
Discussion
Using time-to-event analysis, our study demonstrates a strong temporal association between VI and dementia over 8 years of follow-up in a representative sample of US Medicare beneficiaries. When fully adjusted for potential confounding variables, including demographics, socioeconomic status, clinical comorbidities, hearing impairment, and physical function limitations, we identified an approximately 2-fold increased likelihood in both directions: a higher likelihood of developing new dementia when VI was present at baseline and a higher likelihood of developing new VI when dementia was present at baseline. These findings have important implications for public health and examination recommendations in older adults.
This study provides a unique longitudinal analysis of VI and cognitive dysfunction encompassing a representative sample of the US Medicare population and evaluating bidirectional associations. The results generalize our recent findings from the Women’s Health Initiative to the wider population of older adults in the United States and expand upon our prior work showing a significant association between VI and worse cognitive performance by introducing the element of time.22,32 We also provide more robust data with multiple repeated measures over time to bolster data from other international studies, supporting the hypothesis that vision and cognition appear to be longitudinally associated.25,28,38,39
Vision screening has yet to become public policy because of insufficient evidence supporting an improvement in visual acuity or clinical outcomes, as concluded by the 2016 US Preventive Services Task Force.22,40 However, the US Preventive Services Task Force recommendations are for the general public of older adults and do not specifically address the needs of vulnerable populations, such as patients with cognitive impairment who may be at higher risk of preventable or treatable VI. A 2017 study reported 37% of participants with dementia in a long-term care facility had measurable VI, approximately half of which was correctable with a simple refraction.41 There is mounting interest in developing vision screening protocols for the cognitively impaired, reflected by a growing body of work in the literature. In Canada, for example, Campos et al42 reviewed tools to screen vision in long-term care residents with dementia to identify those who could benefit from intervention, and Kergoat et al43 are attempting to develop specific vision screening tools targeting older individuals with dementia. In Europe, Hooper et al44 have reported on an international study testing the feasibility of implementing hearing and vision sensory interventions in patients with dementia.
Despite research on the topic, the direction of causality between self-reported VI and cognitive function remains unclear. Prior studies have suggested many mechanisms for a causative relationship between low vision and impaired cognition. For instance, VI may interfere with engagement in cognitively stimulating activities and reduce socialization, in turn increasing risk of dementia. Visual impairment may also lead to neuronal atrophy, directly compromising cognitive function. Alternatively, perhaps neuronal dysfunction or other common risk factors such as vascular disease or environmental factors are common causes for both dementia and VI, and VI may be an early symptom of dementia. Finally, people with VI may require higher cognitive resource allocation to perceive visual sensory input, leaving fewer cognitive resources to process other tasks, thereby increasing cognitive load or strain, and hastening the onset of dementia.27,31,45
In the reverse direction, dementia may directly or indirectly cause VI. Impaired central visual perception and processing caused by dementia may manifest as impaired vision, reflecting not necessarily eye pathology, but rather, an inability to effectively interpret or express visual input. As well, older adults with dementia may be more likely to have untreated VI from eye disease. We have previously demonstrated that patients with dementia are less likely to be seen by any eye care provider, including an ophthalmologist, and less likely to have cataract surgery even if they are seen by an ophthalmologist.46 Especially with the suggested relationship between VI and increased likelihood of subsequent cognitive impairment, this latter possibility is particularly concerning. Untreated VI could potentially worsen or hasten cognitive decline among patients with existing dementia, an area that warrants further research.
Our findings have implications for the care of older adults in both the primary care and eye care settings. Primary care providers, geriatricians, and neurologists may consider a lower threshold for referring cognitively impaired patients to eye care specialists to be evaluated for reversible VI such as refractive error and cataracts. This offers an opportunity for quick intervention with new spectacles or cataract surgery, widely accepted as safe and effective.47–49 Nonreversible eye disease may also benefit from eye care. For example, it is possible that by improving vision in treatable diseases, for instance, via administration of intravitreal injections for wet age-related macular degeneration or diabetic macular edema, we may be able to reduce the risk of further cognitive decline. Conversely, there may be a role for ophthalmologists to advocate for dementia screening or geriatrics/neurology referrals during the evaluation of visually impaired patients, especially for those whose poor visual acuity is inadequately explained by a complete ophthalmic examination and testing. An acute stroke in vascular dementia, for example, could present with subjective VI. Future research on causal relationships between VI and cognition as well as the impact of specific cognitive and ophthalmic interventions will provide important clinical information to benefit patients most vulnerable to these impairments and help to prospectively define guidelines for screening.
Study Limitations
Our results are limited by the subjective nature of VI in this analysis, determined by self-report in the NHATS survey and potentially subject to recall or other biases. Formal vision assessment and objective measurements were not performed because of resource constraints nor were specific causes of vision loss identified. However, other recent analyses, including data from the Health, Aging, and Body Composition Study and Salisbury Eye Evaluation Study, respectively, found significant associations between objectively measured VI and cognitive test performance, which support our findings linking VI and dementia.50,51 Furthermore, we believe subjective VI is a valuable measure of participants’ functional vision, which is highly relevant to an aging population. Subjective VI is also more practical for potential screening in nonophthalmic clinics, such as in primary care or geriatric offices. That our study demonstrates a positive association using subjective vision assessment highlights its potential use for application in these settings. Furthermore, NHATS features a unique longitudinal design incorporating a representative US population with clinical information as well as survey and testing data, a rarity among large national health databases, especially in older populations. This importantly allows for the assessment of temporal relationships. Priority was given to determination of dementia status via a rigorous, multifaceted approach with contributions from physician diagnosis and formal cognitive function tasks. Despite this robust methodology, we acknowledge the potential for misclassification, ascertainment, or sampling biases. For example, participants with higher educational attainment or greater cognitive reserve may have performed better on cognitive testing despite the presence of dementia and being incorrectly classified with nondementia. Although NHATS is a large, population-based study, only 7% of the study sample was classified with probable or possible dementia at baseline, compared with population estimates from the 2010 US Census suggesting a prevalence of 11% to 12% for Alzheimer’s disease.52 However, inclusion of physician diagnosis and assessment of multiple cognitive domains bolster the clinical relevance of the NHATS dementia classification, and potential underdetection of dementia would be expected to bias our results away from finding a significant relationship between dementia and VI (such that our results may actually underestimate the relationship).
In conclusion, this study provides evidence for a longitudinal, bidirectional association between self-reported VI and dementia. Given the results of this nationally representative study and the temporal associations demonstrated, vision screening in the cognitively impaired and cognitive assessments in the visually impaired, when conducted in a timely fashion, may help mitigate the development of comorbid disease that can be devastating to older individuals. Future studies should investigate the pathway between cognition and VI prospectively, determine optimal timelines for screening high-risk populations, and continue to innovate geriatric care to identify and intervene upon comorbid VI and cognitive dysfunction in older populations.
Supplementary Material
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): S.P.: Personal fees – Acumen LLC and Verana Health; Grants – National Institutes of Health.
Supported by the Stanford University School of Medicine Medical Scholars Fund to S.P.C. and A.D.A., and by funding from the National Eye Institute (P30-026877) and Research to Prevent Blindness, Inc, to S.P.
Abbreviations and Acronyms:
- CI
confidence interval
- HR
hazard ratio
- NHATS
National Health and Aging Trends Study
- VI
visual impairment
Footnotes
Supplemental material available at www.aaojournal.org.
Presented at the American Academy of Ophthalmology Annual Meeting, San Francisco, California, November 13–15, 2020.
HUMAN SUBJECTS: Human subjects were not included in this study. The NHATS database is composed of publicly available, nonidentifiable information, and this study was determined to be exempt by the Stanford University Institutional Review Board. All research adhered to the tenets of the Declaration of Helsinki. This is a retrospective study using de-identified subject details. Informed consent was not obtained.
No animal subjects were used in this study.
References
- 1.Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. https://www.alz.org/media/Documents/alzheimers-facts-and-figures_1.pdf; 2020. Accessed September 2, 2020.
- 2.Tielsch JM, Sommer A, Witt K, et al. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–290. [DOI] [PubMed] [Google Scholar]
- 3.Evans JR, Fletcher AE, Wormald RP, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: results from the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2002;86:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Bienias JL, Aggarwal NT, et al. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemons TE, Rankin MW, McBee WL. Age-Related Eye Disease Study Research Group. Cognitive impairment in the Age-Related Eye Disease Study: AREDS report no. 16. Arch Ophthalmol. 2006;124:537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitson HE, Cousins SW, Burchett BM, et al. The combined effect of visual impairment and cognitive impairment on disability in older people. J Am Geriatr Soc. 2007;55: 885–891. [DOI] [PubMed] [Google Scholar]
- 8.Baker ML, Wang JJ, Rogers S, et al. Early age-related macular degeneration, cognitive function, and dementia: the Cardiovascular Health Study. Arch Ophthalmol. 2009;127:667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ong SY, Cheung CY, Li X, et al. Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study. Arch Ophthalmol. 2012;130:895–900. [DOI] [PubMed] [Google Scholar]
- 10.Woo SJ, Park KH, Ahn J, et al. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119:2094–2101. [DOI] [PubMed] [Google Scholar]
- 11.Mangione CM, Seddon JM, Cook EF, et al. Correlates of cognitive function scores in elderly outpatients. J Am Geriatr Soc. 1993;41:491–497. [DOI] [PubMed] [Google Scholar]
- 12.Dupuis K, Pichora-Fuller MK, Chasteen AL, et al. Effects of hearing and vision impairments on the Montreal Cognitive Assessment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:413–437. [DOI] [PubMed] [Google Scholar]
- 13.Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 1996;51: 317–330. [DOI] [PubMed] [Google Scholar]
- 14.Rait G, Fletcher A, Smeeth L, et al. Prevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the community. Age Ageing. 2005;34:242–248. [DOI] [PubMed] [Google Scholar]
- 15.Bowen ME, Edgar DF, Hancock B. The Prevalence of Visual Impairment in People with Dementia (the PrOVIDe study): a cross-sectional study of people aged 60–89 years with dementia and qualitative exploration of individual, carer and professional perspectives. Southampton (UK): NIHR Journals Library; 2016. July. [PubMed] [Google Scholar]
- 16.Pham TQ, Kifley A, Mitchell P, Wang JJ. Relation of age-related macular degeneration and cognitive impairment in an older population. Gerontology. 2006;52:353–358. [DOI] [PubMed] [Google Scholar]
- 17.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 18.Garin N, Olaya B, Lara E, et al. Visual impairment and multimorbidity in a representative sample of the Spanish population. BMC Public Health. 2014;14:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Court H, McLean G, Guthrie B, et al. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada Y, Denkinger MD, Onder G, et al. Dual sensory impairment and cognitive decline: the results from the Shelter Study. J Gerontol A Biol Sci Med Sci. 2016;71:117–123. [DOI] [PubMed] [Google Scholar]
- 21.Ward ME, Gelfand JM, Lui LY, et al. Reduced contrast sensitivity among older women is associated with increased risk of cognitive impairment. Ann Neurol. 2018;83:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michalowsky B, Hoffmann W, Kostev K. Association between hearing and vision impairment and risk of dementia: results of a case-control study based on secondary data. Front Aging Neurosci. 2019;11:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rovner BW, Casten RJ, Leiby BE, Tasman WS. Activity loss is associated with cognitive decline in age-related macular degeneration. Alzheimers Dement. 2009;5:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies-Kershaw HR, Hackett RA, Cadar D, et al. Vision impairment and risk of dementia: findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2018;66: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 26.Whitson HE, Cronin-Golomb A, Cruickshanks KJ, et al. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: Sensory Impairment and Cognitive Decline in Older Adults. J Am Geriatr Soc. 2018;66:2052–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laitinen A, Koskinen S, Harkanen T, et al. A nationwide population-based survey on visual acuity, near vision, and self-reported visual function in the adult population in Finland. Ophthalmology. 2005;112:2227–2237. [DOI] [PubMed] [Google Scholar]
- 28.Hong T, Mitchell P, Burlutsky G, et al. Visual impairment and depressive symptoms in an older Australian cohort: longitudinal findings from the Blue Mountains Eye Study. Br J Ophthalmol. 2015;99:1017–1021. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong RA, Syed AB. Alzheimer’s disease and the eye. Ophthalmic Physiol Opt. 1996;16(Suppl 1):S2–8. [DOI] [PubMed] [Google Scholar]
- 30.Rogers MA, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol. 2010;171:728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nael V, Peres K, Dartigues JF, et al. Vision loss and 12-year risk of dementia in older adults: the 3C cohort study. Eur J Epidemiol. 2019;34:141–152. [DOI] [PubMed] [Google Scholar]
- 32.Tran EM, Stefanick ML, Henderson VW, et al. Association of visual impairment with risk of incident dementia in a women’s health initiative population. JAMA Ophthalmol. 2020;138: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Health and Aging Trends Study. NHATS User Guide. https://www.nhats.org/researcher/nhats/methods-documentation?id=user_guide. Accessed August 28, 2020.
- 34.National Health and Aging Trends Study. Dementia Classification. https://www.nhats.org/scripts/documents/DementiaTechnicalPaperJuly_2_4_2013_10_23_15.pdf; 2013. Accessed September 5, 2020.
- 35.National Health and Aging Trends Study. Addendum to classification of persons by dementia status in the National Health and Aging Trends Study for rounds 2–5. https://www.nhats.org/sites/default/files/inline-files/NHATS%20Dementia%20Classification%20Addendum%20for%20Follow-up%20Rounds.pdf; 2015. Accessed September 5, 2020.
- 36.National Health and Aging Trends Study. STATA programming statements for construction of dementia classification in the National Health and Aging Trends Study. https://www.nhats.org/scripts/documents/NHATS_Addendum_to_Technical_Paper_5_STATA_Programming_Statements_Jul2013.pdf; 2013. Accessed August 25, 2020.
- 37.National Health and Aging Trends Study. Income Imputation. https://www.nhats.org/sites/default/files/inline-files/NHATS_Round_5_Income_Imputation.pdf; 2016. Accessed August 28, 2020.
- 38.Hong T, Mitchell P, Burlutsky G, et al. Visual impairment, hearing loss and cognitive function in an older population: longitudinal findings from the Blue Mountains Eye Study. PLoS One. 2016;11:e0147646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elyashiv SM, Shabtai EL, Belkin M. Correlation between visual acuity and cognitive functions. Br J Ophthalmol. 2014;98:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chou R, Dana T, Bougatsos C, et al. Screening for impaired visual acuity in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:915–933. [DOI] [PubMed] [Google Scholar]
- 41.Chriqui E, Law C, Kergoat MJ, et al. Visual impairment in older institutionalised Canadian seniors with dementia. Ophthalmic Physiol Opt. 2017;37:225–233. [DOI] [PubMed] [Google Scholar]
- 42.Campos JL, Hobler F, Bitton E, et al. Screening for vision impairments in individuals with dementia living in long-term care: a scoping review. J Alzheimers Dis. 2019;68:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kergoat H, Law C, Chriqui E, et al. Tool for screening visual acuity in older individuals with dementia. Am J Alzheimers Dis Other Demen. 2017;32:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hooper E, Simkin Z, Abrams H, et al. Feasibility of an intervention to support hearing and vision in dementia: the SENSE-Cog Field Trial. J Am Geriatr Soc. 2019;67:1472–1477. [DOI] [PubMed] [Google Scholar]
- 45.Ivers RQ, Mitchell P, Cumming RG. Sensory impairment and driving: the Blue Mountains Eye Study. Am J Public Health. 1999;89:85–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pershing S, Goldstein MK, Henderson VW, et al. Receipt of eye care services among Medicare Beneficiaries with and without dementia. Ophthalmology. 2020;127:1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heemraz BS, Lee CN, Hysi PG, et al. Changes in quality of life shortly after routine cataract surgery. Can J Ophthalmol. 2016;51:282–287. [DOI] [PubMed] [Google Scholar]
- 48.Gray CS, Karimova G, Hildreth AJ, et al. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. J Cataract Refract Surg. 2006;32:60–66. [DOI] [PubMed] [Google Scholar]
- 49.Lundstrom M, Stenevi U, Thorburn W. Quality of life after first- and second-eye cataract surgery: five-year data collected by the Swedish National Cataract Register. J Cataract Refract Surg. 2001;27:1553–1559. [DOI] [PubMed] [Google Scholar]
- 50.Swenor BK, Wang J, Varadaraj V, et al. Vision impairment and cognitive outcomes in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2019;74:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng DD, Swenor BK, Christ SL, et al. Longitudinal associations between visual impairment and cognitive functioning: The Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2018;136:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


