Abstract
Background
The purpose of this study was to assess the status of glycaemic control and associated factors among patients with type 2 diabetes mellitus patients.
Methods
This was a hospital-based cross-sectional descriptive study of 326 patients with type 2 diabetes at the Ho Municipal and Teaching Hospitals. The adequate sample size was calculated using Yamane formula N/1 + Ne2, with 95 % confidence interval, 5 % margin of error and 10 % non-response rate and a sample size of 326 was determined. Using the sampling frame of patients chart, systematic random sampling technique was used to select the study participants. Glycaemic level was assessed using fasting blood glucose (FBG) readings. A poor glycaemic control was when an average of three months blood glucose level was above 130 mg/dl (7 mm/L). Data was analysed using STATA version 15.0.
Results
Out of 310 patients who participated in the study, more than two-thirds (76.1 %) had poor glycaemic control. Patients who use combination of oral medication and insulin (AOR = 3.67, 95 % CI: 1.34–8.74), patients with diabetes for 16 years or more (AOR = 4.67, 95 % CI: 2.44–9.29), patients who did not practised diabetes self-care activities (AOR = 4.32, 95 % CI: 2.82–9.31) and patients with complications were (AOR = 2.47, 95%CI: 1.45–8.66) more likely to have poor glycaemic control. Age, employment, diabetes education, comorbidities, diabetes self-care activities, treatment type, complications, resident and duration of diabetes were significantly associated with poor glycaemic control.
Conclusion
Based on this findings, teaching and counselling provided by nurses, physicians, dietitians and pharmacists should focus on improving adherence to diabetes self-care activities to attain good glycaemic control.
Keywords: Cross-sectional study, Glycaemic control, Fasting blood glucose type 2 diabetes mellitus, Ghana
1. Background
Diabetes is a public health challenge. It is no longer a disease of the metropolis but rather in less developed areas where people develop diabetes sooner, get sick quicker, and die earlier [1]. The prevalence rate of diabetes mellitus has increased cross the world however, there are regional variations. Africa would experience the highest increase in diabetes cases of 134 % by 2045 compared to 15 % increase in Europe [2]. Globally, 537 million people lived with diabetes and 90–95 % of these cases are type 2 diabetes. About 6.7 million people died from diabetes in 2021 alone, and more than 80 % of these deaths occurred in developing countries [2]. At least USD 966 billion was spent on diabetes in 2021 resulting in 9 % of total health expenditure on adults. The global prevalence rate of diabetes in 2021 was 10.8 %, and 4.5 % in Africa, while the prevalence rate of diabetes in Ghana was 6.9 %, a 35 % higher than the prevalent rate in African. Efforts over the years by Ghana Health Service (GHS) with support from World Health Organization (WHO) to provide comprehensive diabetes teaching and counselling and the development of National Policy on Non-communicable diseases with key objectives focused on diabetes [[3], [4]] however, diabetes cases continue to rise with its attendance complications.
About 90–95% of cases of diabetes are Type 2 diabetes mellitus (T2DM) [2]. Diabetes prevention and management is more than medication adherence; nutrition management, blood glucose monitoring, regular exercise, foot care have all been found to significantly decrease the occurrence and progress of adverse effects of diabetes [[5], [6], [7]]. Earlier researches revealed that poor glycaemic control increase the risk of diabetes complication in patients requiring more care with related healthcare expenditure and increasing complications and premature deaths [[8], [9], [10]]. The level of poor glycaemic control in the population is important. A previous findings in Ethiopia [7] and South Africa [11], in 2016 revealed that 70.9 % and 83.8 % of patients with T2DM had poor glycaemic control, and 72.7 % of patients with T2DM seeking healthcare at Mettu hospital in Southwest Ethiopia [12], also had poor glycaemic control. Also, previous studies in Kenya [13] and Nigeria [14] in 2016 found that 81.6 % and 55 % of patients with diabetes had poor glycaemic control. Previous studies in Ethiopia, Kenya and Bangladesh revealed that poor glycaemic control is linked with increased risk of retinopathy, neuropathy, kidney failure, amputation and cardiovascular disease [5,7,13]. Additionally, the possible reasons accounting for this included limited time, absent of appropriate guidelines for patients and caregivers, lack of awareness, inadequate resources, poor adherence to diet and lack of education [5,7,12,14].
Based on this it was concluded that optimal glycaemic control among patients with T2DM prevents and delay the progression of complications, reduce healthcare expenditure, slow down the development of comorbidities and promote health-related quality of life [8,15]. This indicated the urgent need to focus on glycaemic control to achieve a reduction in the proportion of patients with poor glycaemic control as good glycaemic control remains paramount in delaying the progress and prevention of complications [7,16]. Despite, the increasing cases of type 2 diabetes in Ghana, available evidence showed that there is little or no data on glycaemic control, a key factor in preventing and delaying the onsets of diabetes complications. Availability of such data would contribute substantially towards effective diabetes healthcare services delivery in Ghana. This study assessed the status of glycaemic levels and associated factors in people living with type 2 diabetes.
2. Methods and participants
2.1. Study design, period, and area
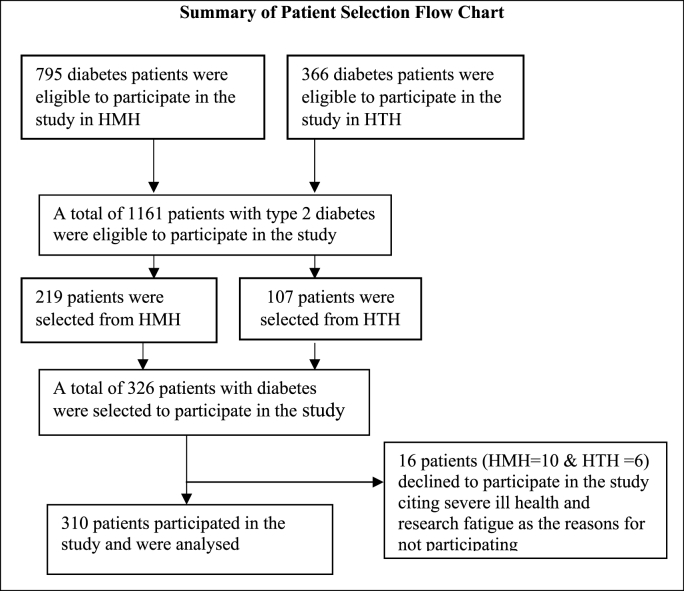
This was a hospital-based cross-sectional descriptive study conducted in the diabetes unit of the Ho Municipal and Ho Teaching Hospitals in the city of Ho in South-eastern Ghana from June 1 to 30 September 2022. Ho is about 156 km from Accra capital city of Ghana. The hospitals serve more than eight hundred thousand outpatients and inpatients yearly. The hospital serves as referral centre for medical, surgical, gynaecological and paediatric cases. The international diabetes Federation (IDF), guidelines were followed for diagnosis and classification of diabetes into type 1 and type 2 diabetes this study. The study was conducted among type 2 diabetes patients aged ≥18 years who were accessing healthcare at the diabetes unit of the hospital for at least 12 months and regular follow up to the hospital prior to the commencement of the study. The adequate sample size was determined using Yamane formula N/1 + Ne2 [17]. The total population of the type 2 diabetes patients who were regularly following up to the hospital for healthcare services were 1161. Using the Yamane formula with 95 % confidence interval, 5 % margin of error and 10 % non-response rate, a sample size of 326 was determined and the patient selection flow chart is summarised in Fig. 1. Using sampling frame of diabetes patients chart, systematic random sampling technique was used to recruit the study participants.
Fig. 1.
Summary of flowchart record selection, 2022.
2.2. Variables of the study and measurement
Glycaemic control was classified as good or poor. An average of at least three months fasting blood glucose readings between 80 and 130 mg/dl was define as good glycaemic control while blood glucose level of >130 mg/dl or 70 mg/dl was considered poor glycaemic control. The glycaemic levels were taken from patients charts. This classification of glycaemic control was based on the American Diabetes Association guideline [18], which has used to assess glycaemic control in Ethiopia [6,12]. The independent variables included socio-demographic and clinical data variables: diabetes education, diabetes duration, treatment type, body max index (BMI), smoking status, alcohol drinking, family history of diabetes; and socio-demographic variables: age, sex, level of education, marital status, employment, income, resident and religion. Standardised techniques were used to collect body measurements [19]. Participants' heights were measured and recorded to the nearest metre using a tape measure. Participants were instructed to stand with their backs against a wall, barefoot, with their heels touching. The participants' weights were recorded in kilogrammes as they were weighed while unsupported and standing barefoot. Using Quetelet's formula (weight (kg)/height (m2)), BMI was computed. It was then defined and categorised in accordance with World Health Organization criteria (underweight 18.5 kg/m2, normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, obese 30.0–39.9 kg/m2, and morbidly obese 40 kg/m2) [20]. Diabetes education was operationally defined as individuals who have had education from healthcare providers on diabetes self-care activities and the causes, signs, and symptoms and effects of diabetes after they were diagnosed with diabetes, were classified as having adequate diabetes education, and vice versa [21].
Continuous variable age was categorised into intervals of 10. Sex, marital status, religion, and educational level were collected as categorical variables, and this was maintained during the analysis. Sex was categorised into male and female and marital status was categorised into single (unmarried), married, divorced and widow/widower. Educational attainment was classified as no formal education, primary level education, Junior high school education (3 years of education and students who passed exit exam called Basic Education Certificate Examination (BECE) qualified for senior high school education. The senior high school education (3 years of secondary education) and tertiary education. The residents of the respondents were classified as rural and urban based on the Ghana Statistical Service classification [22]. Socioeconomic status was measured using income levels. The respondents were asked about their employment and how much they earned at the end of the month. These income levels were used to classify the respondents as very poor, poor, better, less poor, and least poor. Respondents who earn up to GH500.00 were classified as very poor. The daily minimum wage for Ghanaians in 2022 was GH13.53, equivalent to US$ 1. GH500.00 was just a little over the monthly minimum wage. The respondents who earned between GH¢ 501.00 and 1000.00 were classified as poor; those who earned GH¢1001–1500 were classified as better; those who earned GH¢1501–2000 were classified as less poor; and the respondents whose income levels were GH¢ 2001 and above were classified as least. Employment was categorised into unemployed, public sector, and private sector and self-employed. Religion was categorised as Christians, Moslem and Traditional African Religion (TAR). Six of the questions were dichotomous and 1 for yes answer and 0 for no answer for diabetes education, comorbidities, complication, have personal glucometer, practised self-diabetes care and family history of diabetes. Treatment type was categorised into taking only oral medications, insulin and both oral and insulin. Sociodemographic factors were assessed with structured questionnaire using one-on-one interview to collect the data with CSEntry app for data collection.
2.3. Validating methods of outcome
Preceding of checking fasting blood sugar using glucometer, the participant was asked to confirm if he/she had no caloric intake for at least 8 h immediately preceding to the hospital. This was repeated each time the participant visited the hospital. The baseline FBG was taken at the 3 consecutive times the participant visited the hospital. The glycaemic status was categorised as poor glycaemic control when >130 or <70 mg/dl based on the American Diabetes Association guideline [18].
2.4. Statistical analysis
Descriptive statistics was used to report the findings and bivariate and multivariable logistic regression model were conducted to identify the variables associated with poor glycaemic control. A p-value of less than 0.25 was used to select variables for multiple logistic regression [7]. We checked multicollinearity of the variable using variance inflation factor (VIF) and there was none. A P-value of ≤0.05 was statistically significant in the model. The Data was analysed with STATA software version 15.0 and crude and adjusted odds were used to summaries the findings.
Ethics approval
The study was approval by Ghana Health Service Ethic Review Committee (GHS-ERC: 003/03/22). A written permission was obtain from Ho Municipal and Ho Teaching Hospitals for the conduct of this study and informed consent was obtained from all subjects for this research. Individuals were informed that they could withdraw from the study at any time they feel to do so. The research methods were carried out in accordance with the principle of the Declaration of Helsinki and in accordance with the ethical guidelines and regulations of GHS-ERC.
3. Results
3.1. Socio-demographic and clinical characteristics of the respondents
326 patients with diabetes were recruited, however, sixteen declined to participate in the study as presented in Table 1 with socio-demographic information of the respondents. In total, 310 patients with diabetes were analysed making 95 % response rate. The respondents aged between 25 and 85 years with a mean age of 57.8 ± 9.8 years old. Majority of respondents were females (65.8 %) and close to one-third (31.3 %) of the participants were within the age group 56–65 years. Most of the participants were married (62.6 %) and about one-third of the respondents had junior high level education. Additionally, (62.3 %) of the respondents lived in urban areas and (32.6 %) of the respondents earned no income and (95.2 %) of the respondents were Christians. More than one-third (42.6 %) of the respondents were self-employed.
Table 1.
Socio-demographic of patients with type 2 diabetes mellitus accessing healthcare at Ho Municipal and Ho Teaching Hospitals in Ho, Ghana, 2022 (N = 310).
| Variables | Frequency n (%) |
|---|---|
| Age group (years) | |
| 25–35 | 13 (4.2) |
| 36–45 | 42 (13.6) |
| 46–55 | 76 (24.5) |
| 56–65 | 97 (31.3) |
| 66–75 | 67 (21.6) |
| 76–85 | 15 (4.8) |
| Sex | |
| Male | 106 (34.2) |
| Female | 204 (65.8) |
| Marital status | |
| Not married | 24 (7.7) |
| Married | 194 (62.6) |
| Divorced | 26 (8.4) |
| Widow | 66 (21.3) |
| Education | |
| No formal education | 27 (8.7) |
| Primary | 24 (7.7) |
| JHS | 142 (45.8) |
| SHS | 34 (11) |
| Tertiary | 83 (26.8) |
| Employment | |
| Unemployed | 123 (39.6) |
| Public sector employed | 43 (13.9) |
| Private sector employed | 12 (3.9) |
| Self-employed | 132 (42.6) |
| Resident | |
| Rural | 117 (37.7) |
| Urban | 193 (62.3) |
| Monthly income | |
| GH¢ No income | 101 (32.6) |
| GH ¢1-500 | 48 (15.5) |
| GH ¢501-1000 | 74 (23.9) |
| GH ¢1001-1500 | 42 (13.5) |
| GH ¢ 1501-2000 | 18 (5.8) |
| ≥2001 | 27 (8.7) |
| Religion | |
| Christians | 295 (95.2) |
| Muslims | 14 (4.5) |
| Traditional Africa Religion | 1 (0.3) |
GH¢ 13.53 = US$ 1.
Also Table 2 showed clinical factors of the participants accessing healthcare at HMH and HTH. Regarding the duration of diabetes, more than one-third (41 %) of respondents have been living with diabetes for 1–5years and the mean duration of diabetes was 7.4 ± 4.2 years. Regarding medication use, two-thirds (63.2 %) of respondents use only oral medicines and (26.8 %) use both oral medication and insulin. Regarding having glucometer at home, more than two-thirds (73.2 %) of the respondents have no glucometer at home and also more than two-third (71 %) of the respondents have diabetes complications and most of the respondents have diabetic retinopathy (eye damage). Overwhelming majority (88 %) of the respondents have diabetes education and 87 % of the respondents indicated they practice diabetes self-care activities. The average BMI of the participants was 28.3 (±4.49) kg/m2, and close to half (47.4 %) of the participants were overweight and more than one-fourth (28.7 %) of the respondents were obese and only 22.3 % had normal BMI. Regarding family history of diabetes more than two-thirds (69.4) of the respondents have no family history of diabetes. Overwhelming majority (79 %) of the participants do not smoke. In terms of alcohol drinking (93.5 %) of the participants do not drink alcohol. Regarding self-reported exercise activities, only one-fifth (20 %) of the respondents exercised at least 40 min for 3–5 days a week while majority (80 %) of the respondents never intentionally engaged in exercise activities.
Table 2.
Clinical characteristics of patients with type 2 diabetes mellitus accessing healthcare at Ho Municipal and Ho Teaching Hospitals in Ho, Ghana, 2022 (N = 310).
| Clinical characteristics, n = 310 | Frequency n (%) |
|---|---|
| Duration of diabetes | |
| 1–5 years | 127 (41) |
| 6–10 years | 114 (36.8) |
| 11–15 years | 43 (13.9) |
| 16 years + | 26 (8.3) |
| Treatment type | |
| Oral medication | 196 (63.2 %) |
| Insulin | 31 (10) |
| Oral medication + insulin | 83 (26.8) |
| Have glucometer at home | |
| Yes | 83 (26.8) |
| No | 227 (73.2) |
| Ever had diabetes complication | |
| Yes | 220 (71) |
| No | 90 (29) |
| Had any diabetes co-morbidity | |
| Yes | 217 (70) |
| No | 93 (30) |
| Ever had Diabetes education | |
| Yes | 273 (88) |
| No | 37 (12) |
| Practises diabetes self-care | |
| Yes | 270 (87) |
| No | 40 (13) |
| BMI | |
| Underweight | 5 (1.6) |
| Normal | 69 (22.3) |
| Overweight | 147 (47.4) |
| Obese | 89 (28.7) |
| Family history | |
| Yes | 95 (30.6) |
| No | 215 (69.4) |
| Alcohol drinking | |
| Drinker | 20 (6.5) |
| Non-drinker | 290 (93.5) |
| Smoking status | |
| Smoker | 9 (3) |
| Ex-smokers | 56 (18) |
| Non-smoker | 245 (79) |
| Self-reported exercise activities | |
| 3–5 days at least 40 min a week | 62 (20) |
| No regular exercise | 248 (80) |
3.2. Poor glycaemic control and its predictors in T2DM patients
Poor glycaemic control was found in 236 (76.1 %) respondents. Age, sex, being employment, earning income, resident, and type of treatment, diabetes complication, diabetes education, diabetes duration, BMI, comorbidities and diabetes self-care activities were selected for multiple logistic regression model. Sex, residency, taking both oral medication and insulin, being educated on diabetes, being self-employed, being overweight/obese, having diabetes complications, having diabetes comorbidities, living with diabetes for 1–5 years and over 16 years were statistically significant in the bivariate logistic analysis. In multivariable logistic regression, statistically significant differences were found in poor glycaemic control to age, employment, resident, income, treatment type, diabetes complication, diabetes education, diabetes self-care activities and diabetes duration.
The multiple logistic regression analysis was presented in Table 3 with both sociodemographic and clinical factors associated with poor glycaemic control. The association of poor glycaemic control among participants between the ages of 56–65 years were 3 times (AOR = 3.12, 95%CI: 1.45–9.37) more likely to have poor glycaemic control compared with respondents in the 25–35 year group. Also respondents in the ≥66 age group were 4 times (AOR = 4.34, 95%CI: 1.38–8.23) more likely to have poor glycaemic control compared to their counterparts in the 25–35 age group. As the age of the respondents increase the likelihood to have poor glycaemic control also increase. Income also had significant effect on glycaemic control such that patients who earned GH¢1–500 a month were 38 % times (AOR = 0.62, 95 % CI: 0.23–0.54) less likely to have poor glycaemic control compared with those patients who earned no income. Also, patients who earned GH¢ 501–1000 a month were 88 % times (AOR = 0.12, 95 % CI: 0.17–0.86) less likely to have poor glycaemic control compared to patients who earned no income. Similarly, duration of diabetes have significant effects on poor glycaemic control among patients with type 2 diabetes such that patients living with diabetes for 6–10 years were 2 times (AOR = 2.5, 95 % CI: 1.70–3.19) more likely to have poor glycaemic control compared to patients living with diabetes less than 5years. Also, patients living with diabetes for 16 years and above were 4 times (AOR = 4.67, 95 % CI: 2.44–9.29) more likely to have poor glycaemic control compare to patients living with type 2 diabetes less than 5years. Furthermore, non-alcohol drinkers in this study were 55 % times (AOR = 0.45, 95%CI: 1.96–7.35) less likely to have poor glycaemic control compared to their counters who drink alcohol. The results also revealed that patients who engage in regular exercise for 3–5 days for at least 30–40 min a week were 5 times (AOR = 5.67,95%CI: 3.72–8.40) more likely to have poor glycaemic control compared to patients who do not intentionally engage in regular exercise.
Table 3.
Factors associated with glycaemic control among type 2 diabetes mellitus patients accessing healthcare at the Diabetes Clinics of Ho Municipal and Ho Teaching Hospitals, Ghana, 2022. (n = 310).
| Variables | Good glycaemic control | Poor glycaemic control | Crude OR (95%CI) | Adjusted OR (95%CI) | P-value |
|---|---|---|---|---|---|
| Age | |||||
| 25–35 | 0 | 13 | 1 | ||
| 36–45 | 9 | 33 | 0.16 (0.27–0.87) | 2.23 (1.44–6.32) | 0.34 |
| 46–55 | 12 | 64 | 0.14 (0.30–0.65) | 2.83 (2.11–7.86) | 0.10 |
| 56–65 | 21 | 76 | 0.16 (0.38–0.66) | 3.12 (1.45–9.37) | 0.01 |
| ≥66 | 32 | 50 | 0.49 (0.12–1.90) | 4.34 (1.38–8.23) | 0.04 |
| Resident | |||||
| Urban | 53 | 140 | 1 | ||
| Rural | 21 | 96 | 0.52 (0.25–1.89) | 0.23 (0.56–2.92) | 0.42 |
| Income | |||||
| GH¢ 0 | 34 | 67 | 1 | ||
| GH¢ 1-500 | 10 | 38 | 0.18 (0.24–1.35) | 0.62 (0.23–0.54) | 0.04 |
| GH¢ 501-1000 | 9 | 65 | 0.12 (0.17–0.86) | 0.12 (0.17–0.86) | 0.03 |
| GH¢ 1001-1500 | 10 | 32 | 0.26 (0.40–1.75) | 0.26 (0.40–1.75) | 0.16 |
| GH¢1501-2000 | 4 | 14 | 0.25 (0.23–2.66) | 0.45 (0.23–2.66) | 0.25 |
| GH¢ 2001-2500 | 7 | 20 | 0.25 (0.22–2.81) | 0.35 (0.22–2.81) | 0.26 |
| Duration of diabetes | |||||
| 1–5 years | 29 | 98 | 1 | ||
| 6–10 years | 28 | 86 | 1.50 (0.70–3.19) | 2.54 (1.70–3.19) | 0.02 |
| 11–15 years | 9 | 34 | 0.86 (0.31–2.36) | 3.22 (0.97–5.36) | 0.77 |
| 16 years + | 8 | 18 | 2.30 (1.42–6.11) | 4.67 (2.44–9.29) | 0.04 |
| Family history | |||||
| Yes | 40 | 55 | 1 | ||
| No | 34 | 181 | 0.61 (0.75–3.38) | 0.72 (0.89–5.64) | 0.24 |
| Alcohol drinking | |||||
| Drinker | 8 | 12 | 1 | ||
| Non-drinker | 66 | 224 | 0.34 (1.54–6.33) | 0.45 (1.96–7.35) | 0.30 |
| Smoking status | |||||
| Smokers/Ex-smokers | 25 | 40 | 1 | ||
| Non-smoker | 49 | 196 | 0.76 (2.61–7.68) | 0.86 (2.61–8.83) | 0.21 |
| Self-reported exercise | |||||
| 3–5 days 30 min a week | 48 | 14 | 1 | ||
| No regular exercise | 26 | 222 | 4.32 (3.81–9.47) | 5.67 (3.72–8.40) | 0.42 |
| Treatment type | |||||
| Oral medication | 57 | 139 | 1 | ||
| Insulin | 5 | 26 | 0.27 (0.79–0.96) | 2.13 (0.87–6.33) | 0.04 |
| Oral medication and insulin | 12 | 71 | 0.58 (0.25–1.36) | 3.67 (1.34–8.74) | 0.02 |
| Had diabetes complication | |||||
| Yes | 49 | 178 | 1 | ||
| No | 25 | 58 | 0.47 (0.21–1.59) | 0.78 (1.45–8.66) | 0.5 |
| Have diabetes comorbidity | |||||
| Yes | 46 | 171 | |||
| No | 28 | 65 | 0.23 (0.34–1.79) | 0.56 (0.86–2.56) | 0.05 |
| Had Diabetes education | |||||
| Yes | 62 | 211 | |||
| No | 12 | 25 | 0.42 (0.15–1.12) | 0.67 (0.87–2.43) | 0.05 |
| Diabetes self-care | |||||
| Yes | 62 | 211 | |||
| No | 12 | 25 | 2.20 (1.36–9.34) | 4.32 (2.82–9.31) | 0.03 |
| BMI | |||||
| Underweight/normal | 17 | 57 | 1 | ||
| Overweight/obese | 57 | 179 | 1.26 (0.57–2.76) | 3.26 (1.64–8.83) | 0.55 |
Additionally, the type of medication patients use had effect on poor glycaemic control such that patients who use insulin only were 2 times (AOR = 2.13, 95 % CI: 0.87–6.33) more likely to have poor glycaemic control compared with those patients who use oral medications only. Also, patients who used combination of oral medication and insulin were 3 times (AOR = 3.67, 95 % CI: 1.34–8.74) more likely to have poor glycaemic control compared to patients who used only oral medication. The results also shows that diabetes complication had influence on poor glycaemic control such that patients with diabetes complication were 2 times (AOR = 2.47, 95%CI: 1.45–8.66) more likely to have poor glycaemic control compared with those without diabetes complications. Diabetes education was significantly associated with poor glycaemic control such that patients who had no diabetes education were 33 % (AOR = 0.67, 95 % CI: 0.87–2.43) less likely to have poor glycaemic control compared to patients who had diabetes education. Diabetes comorbidities had significant effect on poor glycaemic control such that patients with no comorbidities were 44 % times (AOR = 0.56, 95 % CI: 0.86–2.56) less likely to have poor glycaemic control compared to the patients with comorbidities. Finally, the results showed that diabetes self-care activities had significant effects on poor glycaemic control where patients who did not practised diabetes self-care were 4 times (AOR = 4.32, 95 % CI: 2.82–9.31) more likely to have poor glycaemic control compared to those patients who practiced diabetes self-care activities.
4. Discussions
The overarching objective of diabetes management is to achieve good glycaemic levels. We have examined the extent to which poor glycaemic control was associated with socio-demographic and clinical factors assessed among type 2 diabetes patients in Ho. The results of this study showed that (76.1 %) of patients with type 2 diabetes have poor glycaemic control. Previous studies had reported poor glycaemic control of 72 % in Malaysia, 62 % in Bangladesh [5], 72.1 % in Myanmar, 74.9 % in Saudi Arab [15], 74 % reported in Cameroon and Guniea [8] and Ethiopia 70.9 % [7], 71.4 % [6] 70.8 % [23]. However, the findings of this study was not in tandem with a study in Palestine 80.5 %[9], Kenya 81.6 %[13], Ethiopia 81.9 % [24] and South Africa 83 % [11] as the results of these earlier studies reported higher proportions compared to the present study. Similarly, the poor glycaemic control reported in the present study was far above those reported in Nigeria 55 % [14], Zambia 61.3 % [25], Turkey 67.5 % [26] and Ethiopia 64 % and 65 % [27,28] in the earlier studies. The possible explanation for the differences in the poor glycaemic control could be the variations in clinical care given by the healthcare professions in this jurisdictions, recall bias, study designs, characteristics of the respondents, and the type of treatment facility and how glycaemic control was assessed at each of these health facilities as reported earlier studies [6,14]. This finding is consistent with the previous studies as they have all reported poor glycaemic control in the majority of the respondents ranged from 55 % in Nigeria to 83 % in South Africa. The poor glycaemic control in the present study was more than many of the reported poor glycaemic control in Malaysia and Ethiopia. However, the findings of this revealed a greater difference in the proportion of poor glycaemic control reported in the global north compared to sub-Sahara Africa [16]. A study in Germany and the United States reported 45 % and 12.9 % of poor glycaemic control respectively [16]. This might be probably due to knowledge deficit in the diabetes self-care activities and management practises of the healthcare professionals among others as reported in the earlier studies [5,29]. There is the need for healthcare providers to intensify teaching and counselling at diabetes clinics to improve glycaemic control in sub-Sahara Africa to close the knowledge gap in this regard. The findings of this revealed a greater different in the proportion of poor glycaemic control reported in the global north compared to sub-Sahara Africa [14,16].
Patients with diabetes more than five years were less likely to have good glycaemic control compared to their counterparts who lived with diabetes less than five years. The result is in consonant with earlier studies in Ethiopia, Saudi Arabia, South Africa, Malaysia, Palestine and Jordan [6,7,9,11,15,23,30]. The possible explanation could be the as the individuals lived with diabetes for long period, their immune is compromised further such that as patients live long with the illness they develop comorbidities which also contribute to poor glycaemic control. Also, there was a significant difference observed in glycaemic control and the type of treatment used by the patients with T2DM.
Patients who used insulin only were more likely to have poor glycaemic control compared to patients who used oral medications only. The results further revealed that patients using combination of insulin and oral medications were 3 times less likely to have good glycaemic control compared to patients who used oral medications only. This result is in tandem with earlier findings in Ethiopia, Malaysia, Zambia, South Africa [6,7,11,24,25]. It is therefore important for healthcare providers to carefully identify the treatment option that work for each patients and educate them to adhere to it to attain good glycaemic control rather than changing and combining multiple treatment options. This is because even though there are teaching and counselling sessions in many of diabetes clinics by physicians, nurses, dietitians and pharmacist, the impact of these strategies did not seem to reflect in this population. The study also found that, patients with no diabetes comorbidities were 44 % less likely to have poor glycaemic control compared to patients with type 2 diabetes who have comorbidities.
These comorbidities leads to worsening glycaemic control levels as a results of added anxiety and stress of these comorbidities alongside the existing challenges in the management of type 2 diabetes. This finding is in consonant with earlier findings in Ethiopia, Kenya and Turkey [6,7,13,26]. There is the need to focus on managing diabetes related-diseases to ensure that those conditions are managed well alongside the diabetes itself to achieve optimal glycaemic control. Also, significant difference of poor glycaemic control was observed in patients who do not engaged in diabetes self-care activities compared to patients who practise self-care activities. Earlier studies have found that effective self-care activities could help attain optimal glycaemic control. This call for education at primary healthcare facilities by public health officials to encourage patients with diabetes to practise self-care activities with combination of clinical procedures. This is consistent with earlier findings in Kenya, Ethiopia, Jordan and the United State of America [13,16,24,30,31].
5. Limitations
It was wealthy to note that in spite of the significant contribution of this study, it was a cross-sectional study confided in two public hospitals in Ho and therefore the interpretation of the results should be done with caution. Also, there might be possible errors in the review of patients chart and recall bias on the parts of patients. Furthermore, the use of average fasting blood glucose for 3 months to determine glycaemic control might either leads to underestimate or overestimate of proportion of poor glycaemic control even though this was standard procedure use in previous studies [6,32].
6. Conclusion
Generally glycaemic control was poor among the study participants. The age, employment status, diabetes education, comorbidities, diabetes self-care practices, treatment type, diabetes complications, income, resident and duration of diabetes were significantly associated with glycaemic control. Healthcare providers should focus much on glycemic control in the management of diabetes since it is paramount in the management of diabetes. Healthcare providers should teach and counsel patients with diabetes adhered to diabetes self-care activities to improve glycemic control.
Ethics approval and consent to participate
The study was approval by Ghana Health Service Ethic Review Committee (GHS-ERC: 003/03/22). A written permission was obtain from Ho Municipal and Ho Teaching Hospitals for the conduct of this study and informed consent was obtained from all subjects for this study. The name of the study participants were not registered for the assurance of confidentiality. Individuals were informed that they could withdraw from the study at any time they feel to do so. The research methods were carried out in accordance with the principle of the Declaration of Helsinki and in accordance with the ethical guidelines and regulations of GHS-ERC.
Consent for publication
Not applicable.
Availability of data and materials
The author confirms that all data generated or analysed during this study are included in this published article. However the recruitment procedure of the participants has been added as a supporting document.
Funding
The authors declare that no funds, was received for this study.
CRediT authorship contribution statement
Stanley Kofi Alor: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. Irene M. Akwo Kretchy: Supervision, Validation. Franklin N. Glozah: Supervision, Validation. Philip Baba Adongo: Supervision, Validation, Visualization.
Declaration of competing interest
The authors declare that they have no competing interest.
Acknowledgements
The authors wish to thank all patients with diabetes who participated in this study for their time and sharing their experience with us as well as data collectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2023.100265.
Abbreviations
- BMI
body mass index
- FBG
fasting blood glucose
- IDF
International Diabetes Federation
- GHS
Ghana Health Service
- HMH
Ho Municipal Hospital
- HTH
Ho Teaching Hospital
- WHO
World Health Organization
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.IDF . 2019. International diabetes federation atlas- 9th Edition_2019. [PubMed] [Google Scholar]
- 2.IDF . tenth ed. 2021. IDF diabetes atlas; p. 2021. [Google Scholar]
- 3.GHS . Ghana Health Service; 2021. District health information management system 2. [Google Scholar]
- 4.MOH . 2022. National policy for non-communicable diseases. [Google Scholar]
- 5.Afroz A., Ali L., Karim M.N., Alramadan M.J., Alam K., Magliano D.J., Billah B. Glycaemic control for people with type 2 diabetes mellitus in Bangladesh-an urgent need for optimization of management plan. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gebermariam A.D., Tiruneh S.A., Ayele A.A., Tegegn H.G., Ayele B.A., Engidaw M. Level of glycemic control and its associated factors among type II diabetic patients in debre tabor general hospital, northwest Ethiopia. Metabolism Open. 2020;8 doi: 10.1016/j.metop.2020.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kassahun T., Eshetie T., Gesesew H. Factors associated with glycemic control among adult patients with type 2 diabetes mellitus: a cross-sectional survey in Ethiopia. BMC Res Notes. 2016;9(1):1–6. doi: 10.1186/s13104-016-1896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camara A., Baldé N.M., Sobngwi-Tambekou J., Kengne A.P., Diallo M.M., Tchatchoua A.P., Kaké A., Sylvie N., Balkau B., Bonnet F. Poor glycemic control in type 2 diabetes in the South of the Sahara: the issue of limited access to an HbA1c test. Diabetes Res Clin Pract. 2015;108(1):187–192. doi: 10.1016/j.diabres.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Radwan M., Elsous A., Al-Sharif H., Abu Mustafa A. Glycemic control among primary care patients with type 2 diabetes mellitus in the Gaza Strip, Palestine. Therapeutic advances in endocrinology metabolism Open. 2018;9(1):3–14. doi: 10.1177/2042018817742070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tekalegn Y., Addissie A., Kebede T., Ayele W. Magnitude of glycemic control and its associated factors among patients with type 2 diabetes at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adeniyi O.V., Yogeswaran P., Longo-Mbenza B., Ter Goon D., Ajayi A.I. Cross-sectional study of patients with type 2 diabetes in OR Tambo district, South Africa. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheleme T., Mamo G., Melaku T., Sahilu T. Glycemic control and its predictors among adult diabetic patients attending Mettu karl referral hospital, southwest Ethiopia: a prospective observational study. Diabetes Therapy. 2020;11(8):1775–1794. doi: 10.1007/s13300-020-00861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nduati N.J., Simon K., Eva N., Lawrence M. Factors associated with glycemic control among type 2 diabetes patients attending Mathari National Teaching Hospital, Nairobi Kenya. Endocrinol diabetes. 2016;3(6):1–11. [Google Scholar]
- 14.Ufuoma C., Godwin Y.D., Kester A.D., Ngozi J.C. Determinants of glycemic control among persons with type 2 diabetes mellitus in Niger Delta. Sahel Med J. 2016;19(4):190. [Google Scholar]
- 15.Alzaheb R.A., Altemani A.H. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes, Metab Syndrome Obes Targets Ther. 2018:15–21. doi: 10.2147/DMSO.S156214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali M.K., McKeever Bullard K., Imperatore G., Barker L., Gregg E.W. Characteristics associated with poor glycemic control among adults with self-reported diagnosed diabetes—National Health and Nutrition Examination Survey, United States, 2007–2010. MMWR Morb Mortal Wkly Rep. 2012;61(2):32–37. [PubMed] [Google Scholar]
- 17.Yamane . evanston and london and john weather hill; new york: 1967. Statistics: an introductory analysis harper and row. [Google Scholar]
- 18.ADA 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement_1):S13–S28. doi: 10.2337/dc19-S002. [DOI] [PubMed] [Google Scholar]
- 19.Daya R., Bayat Z., Raal F. Effects of diabetes mellitus on health-related quality of life at a tertiary hospital in South Africa: a cross-sectional study. S Afr Med J. 2016;106(9):918–928. doi: 10.7196/SAMJ.2016.v106i9.9899. [DOI] [PubMed] [Google Scholar]
- 20.WHO . Fact Sheets; 2010. A healthy lifestyle -WHO recommendations. [Google Scholar]
- 21.Lin Yang X., Yin G., Lin S. Diabetes self-care activities and health-related quality-of-life of individuals with type 1 diabetes mellitus in shantou, China. J Int Med Res. 2016;44(1):147–156. doi: 10.1177/0300060515597933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GSS . Ghana Statistics Service; 2010. Population & housing census: National analytical report. 2013. [Google Scholar]
- 23.Fiseha T., Alemayehu E., Kassahun W., Adamu A., Gebreweld A. Factors associated with glycemic control among diabetic adult out-patients in Northeast Ethiopia. BMC Res Notes. 2018;11:1–6. doi: 10.1186/s13104-018-3423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hailu E., Mariam W.H., Belachew T., Birhanu Z. Self-care practice and glycaemic control amongst adults with diabetes at the Jimma University Specialized Hospital in south-west Ethiopia: a cross-sectional study. African Journal of Primary Health Care Family Medicine. 2012;4(1):1–6. [Google Scholar]
- 25.Musenge E.M., Manankov A., Mudenda B., Michelo C. Glycaemic control in diabetic patients in Zambia. Pan African medical journal. 2014;19(1) doi: 10.11604/pamj.2014.19.354.5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kayar Y., Ilhan A., Kayar N.B., Unver N., Coban G., Ekinci I., Eroglu H. Relationship between the poor glycemic control and risk factors, life style and complications. Biomed Res. 2017;28(4):1581–1586. [Google Scholar]
- 27.Gebrehiwot T., Jemal H., Dawit T. Non-adherence and associated factors among type 2 diabetic patients at Jimma University Specialized Hospital, Southwest Ethiopia. J Med Sci. 2013;13(7):576–584. [Google Scholar]
- 28.Teklay G., Hussien J., Tesfaye D. Non-adherence and associated factors among type 2 diabetic patients at Jimma University Specialized Hospital, Southwest Ethiopia. Med Sci. 2013;13(7):578–584. [Google Scholar]
- 29.Boye K.S., Lage M.J., Thieu V., Shinde S., Dhamija S., Bae J.P. Obesity and glycemic control among people with type 2 diabetes in the United States: a retrospective cohort study using insurance claims data. J Diabetes Complicat. 2021;35(9) doi: 10.1016/j.jdiacomp.2021.107975. [DOI] [PubMed] [Google Scholar]
- 30.Khattab M., Khader Y.S., Al-Khawaldeh A., Ajlouni K. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complicat. 2010;24(2):84–89. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Bukhsh A., Khan T.M., Nawaz M.S., Ahmed H.S., Chan K.G., Lee L.-H., Goh B.-H. Association of diabetes-related self-care activities with glycemic control of patients with type 2 diabetes in Pakistan. Patient Prefer Adherence. 2018;12:2377. doi: 10.2147/PPA.S177314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ADA Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Supplement 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The author confirms that all data generated or analysed during this study are included in this published article. However the recruitment procedure of the participants has been added as a supporting document.